Published online Jul 7, 2021. doi: 10.3748/wjg.v27.i25.3940
Peer-review started: January 22, 2021
First decision: February 28, 2021
Revised: March 2, 2021
Accepted: April 21, 2021
Article in press: April 21, 2021
Published online: July 7, 2021
Processing time: 164 Days and 16.3 Hours
Schwannomas, also known as neurinomas, are tumors that derive from Schwann cells. Gastrointestinal schwannomas are extremely rare, but the stomach is the most common site. Gastric schwannomas are usually asymptomatic. Endoscopy and imaging modalities might offer useful preliminary diagnostic information. However, to diagnose schwannoma, the immunohistochemical positivity for S-100 protein is essential, whereas CD117, CD34, SMA, desmin, and DOG-1 are negative.
A 45-year-old female was found to have a gastric mass during a medical examin
Immunohistochemical staining is essential for the diagnosis of schwannoma. Endoscopic full-thickness resection is an effective treatment method for gastric schwannoma.
Core Tip: Schwannomas can occur in any part of the digestive tract but are most common in the stomach. Gastric schwannomas are typically asymptomatic, and it is difficult to make a precise preoperative diagnosis. The final diagnosis of schwannoma is based on immunohistochemical staining. We performed endoscopic full-thickness resection and endoscopic purse-string suture. We report a case diagnosed with gastric schwannoma.
- Citation: Lu ZY, Zhao DY. Gastric schwannoma treated by endoscopic full-thickness resection and endoscopic purse-string suture: A case report. World J Gastroenterol 2021; 27(25): 3940-3947
- URL: https://www.wjgnet.com/1007-9327/full/v27/i25/3940.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i25.3940
Schwannomas, which are also known as neurinomas, were first described in 1910 by Verocay and are rarely observed in the gastrointestinal tract[1,2]. The stomach is the site with the highest incidence of schwannomas in the gastrointestinal tract[3]. Gastric schwannomas are typically asymptomatic, and the most common symptoms are stomachache, abdominal mass, and gastrointestinal hemorrhage[4,5].
Although endoscopy and imaging modalities, such as computed tomography (CT), magnetic resonance imaging, positron emission tomography-CT, might offer useful preliminary diagnostic information, it is still difficult to achieve the precise preo
In our case, the schwannoma was discovered incidentally by abdominal CT. Gastroscopy and endoscopic ultrasonography (EUS) were then performed. Endoscopic full-thickness resection and endoscopic purse-string suture were performed. Finally, the diagnosis of gastric schwannoma was confirmed by histological, immunohistochemical, and gene mutational investigations.
A 45-year-old female had a gastric mass during a medical examination.
A 45-year-old female visited a local hospital for a regular health examination without any symptoms. The patient had an abdominal CT scan, which revealed a rounded mass arising from the greater curvature of the gastric body, suggesting a gastro
There was no other significant medical history. The patient had no history of prior gastroenterological symptoms. There was no relevant history including past inter
The patient had no history of smoking or drinking alcohol. Her occupation was a housewife. There was no relevant family history.
All vital signs of the patient were stable, and physical examination revealed no noteworthy positive sign.
The levels of the tumor markers AFP, CA125, and CEA were in the normal range. Blood tests, fecal examinations, and coagulation function all demonstrated normal results.
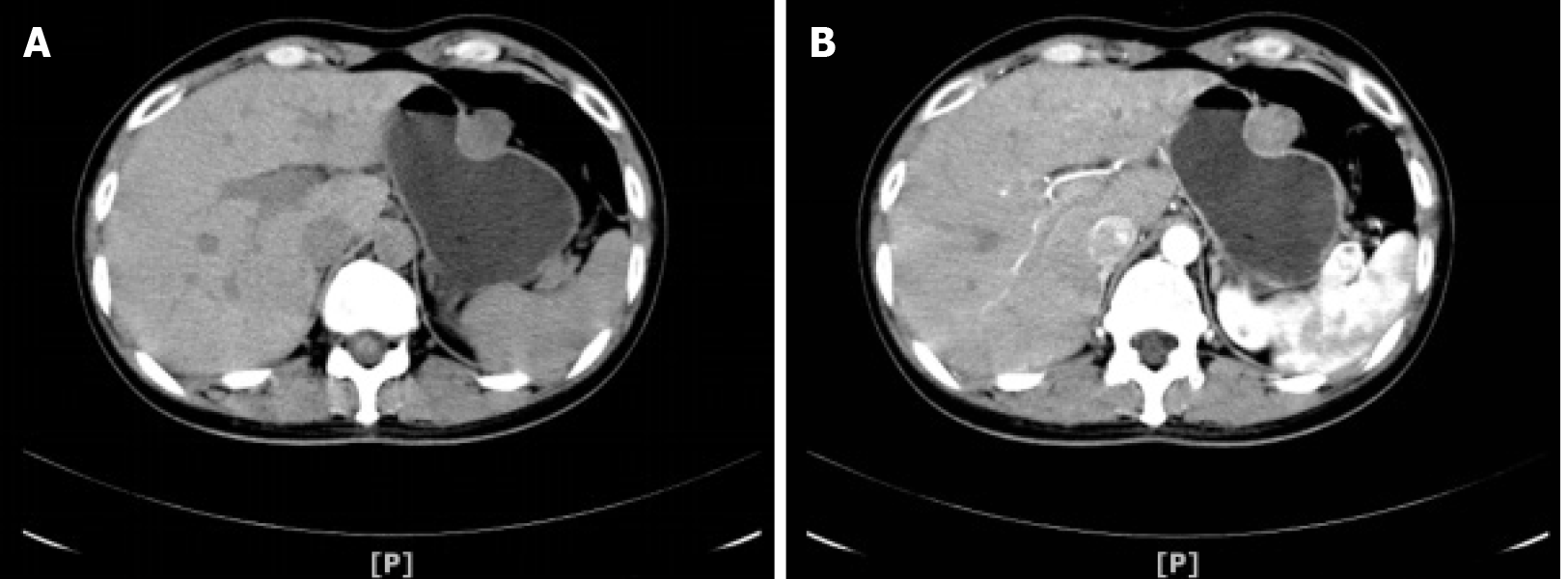
Nine days before admission, the patient underwent abdominal CT scanning for a medical examination, and it revealed a 24.9 mm × 23.9 mm rounded mass arising from the greater curvature of the gastric body with slight internal contrast enhancement (Figure 1), suggesting a GIST as a likely diagnosis. No enlarged pericolic lymph nodes were observed.
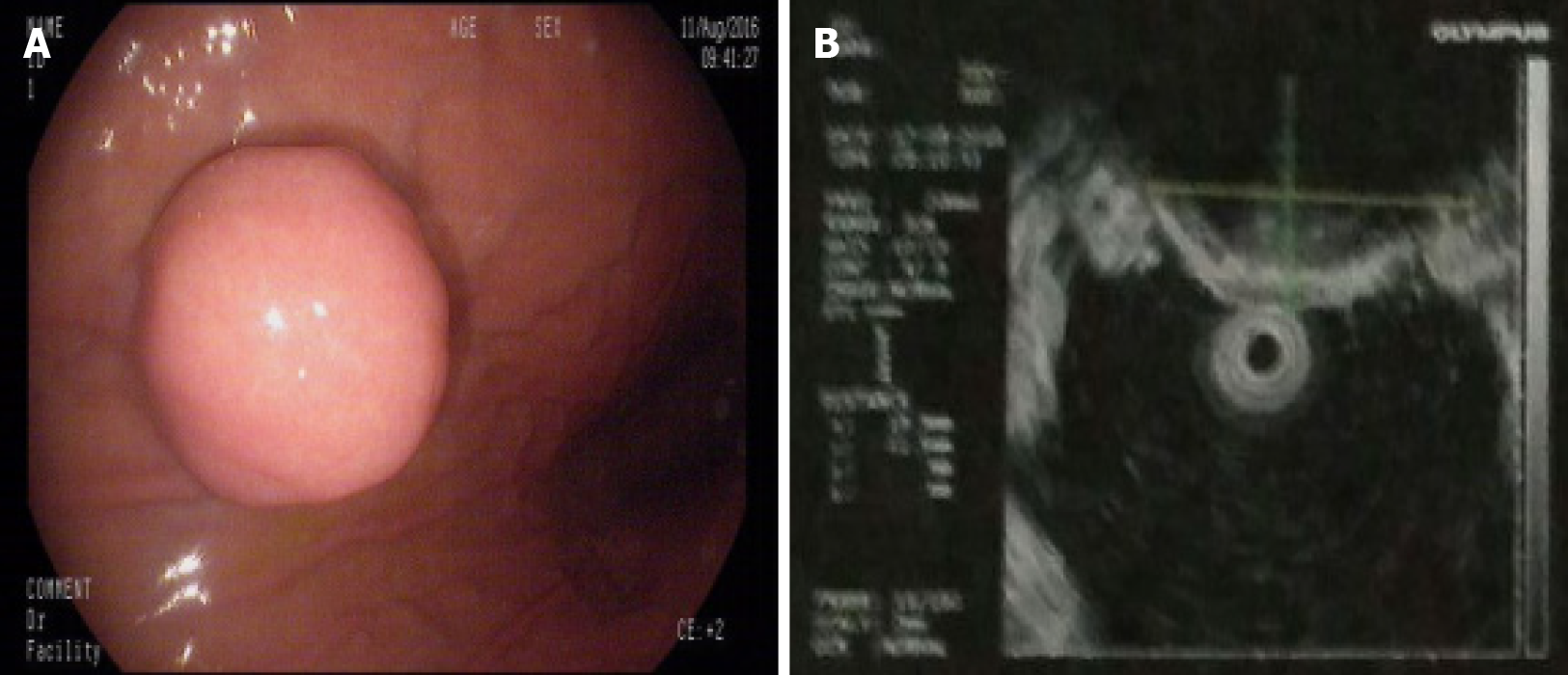
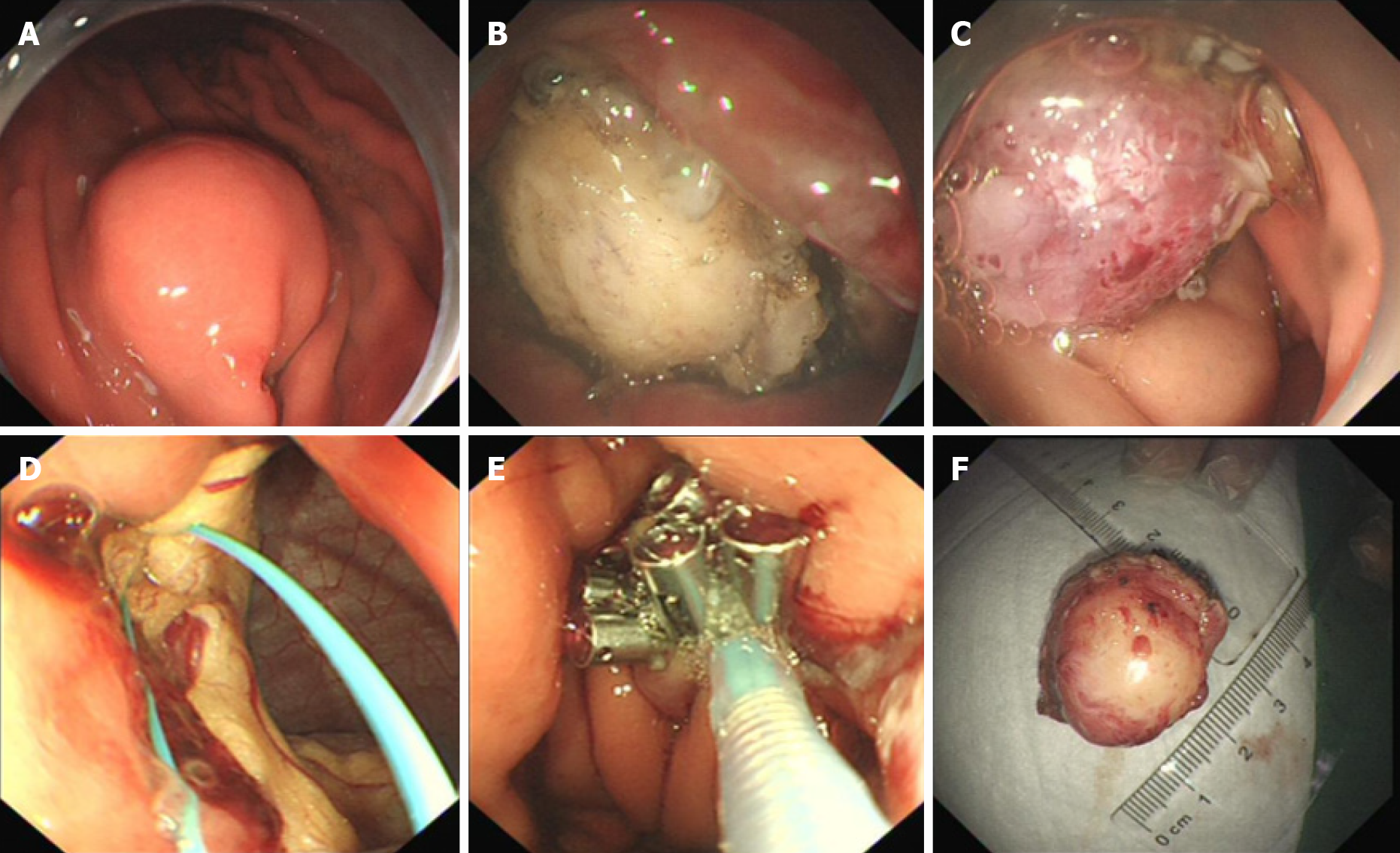
Gastroscopy demonstrated a 2.0 cm × 1.8 cm hemispherical protrusion lesion of the gastric body, and EUS revealed hypoechoic and homogeneous echo lesions originating from the muscularis propria (Figure 2).
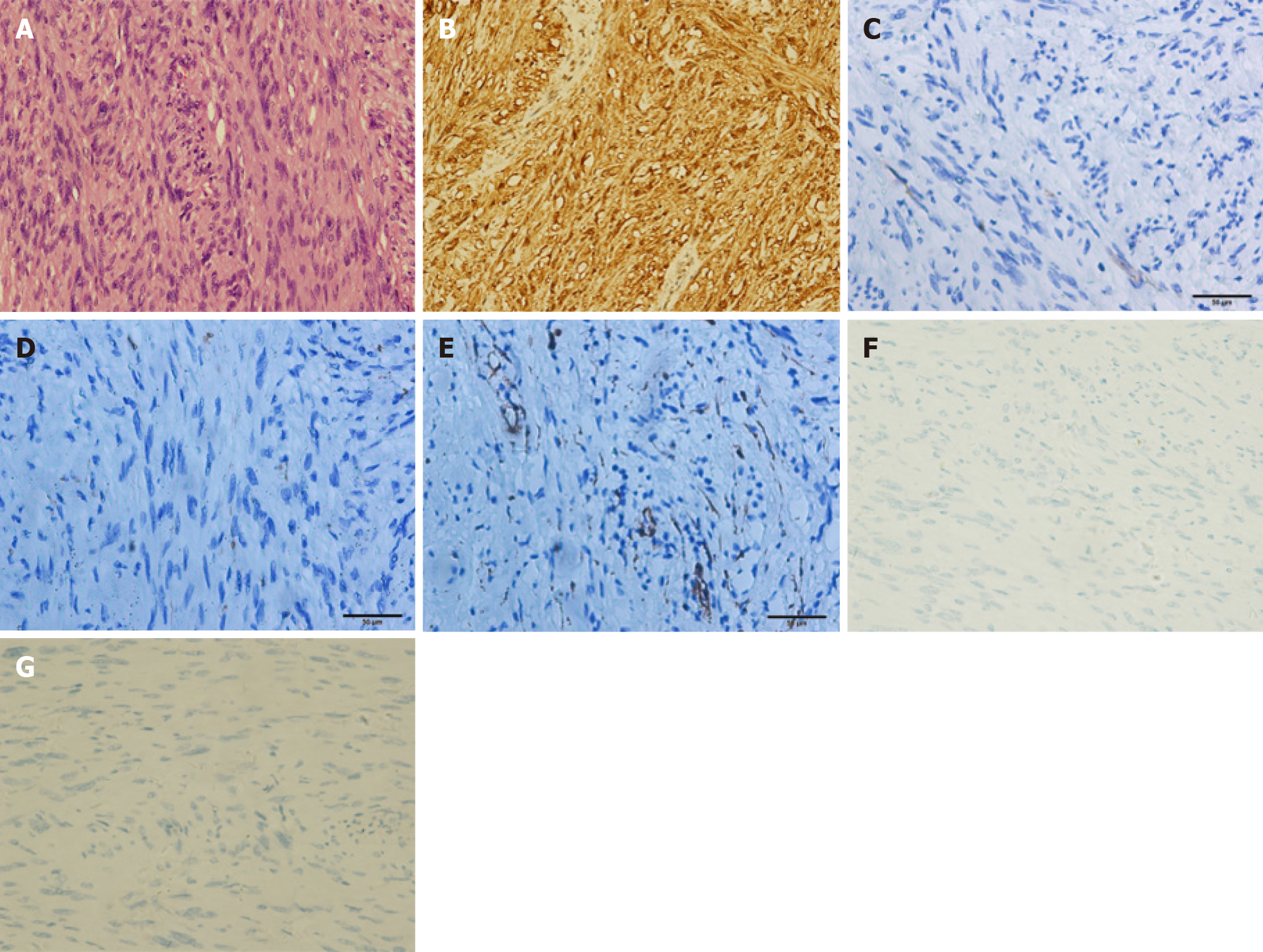
Pathological analysis and immunohistochemical staining (Figure 3) confirmed the diagnosis of gastric schwannoma through positivity for S-100 protein, whereas CD117, CD34, α-SMA, desmin, and DOG-1 were negative.
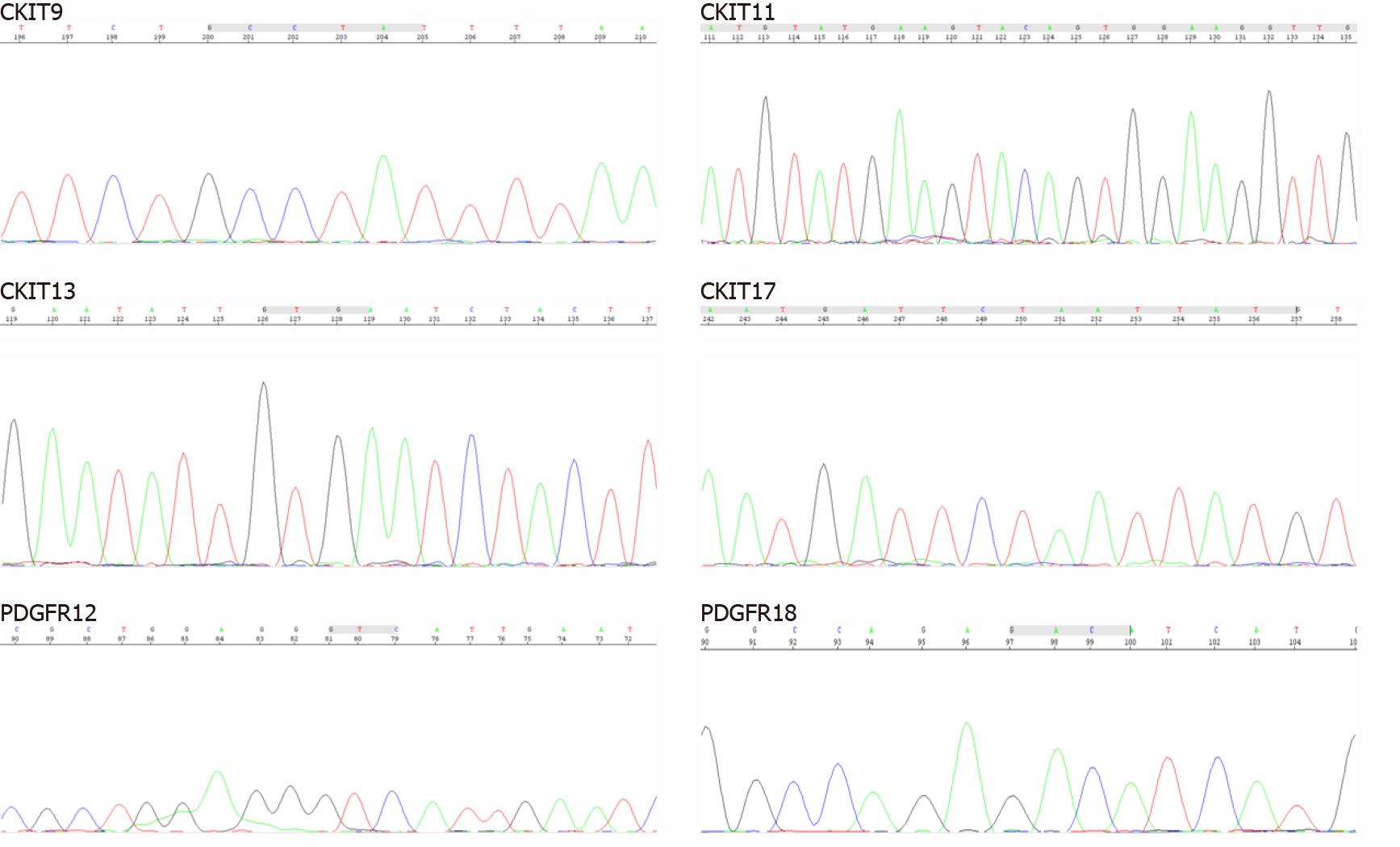
To provide evidence for the differential diagnosis of GIST, we performed a mutational detection of the c-Kit and PDGFRA genes (Figure 4), and the results showed that no mutations were detected in the sample.
The final diagnosis of the presented case was gastric schwannoma.
Endoscopic full-thickness resection and endoscopic purse-string suture were performed (Figure 5). Postoperatively, acid suppression, hemostasis, protection of the gastric mucosa, and nutritional support were administered.
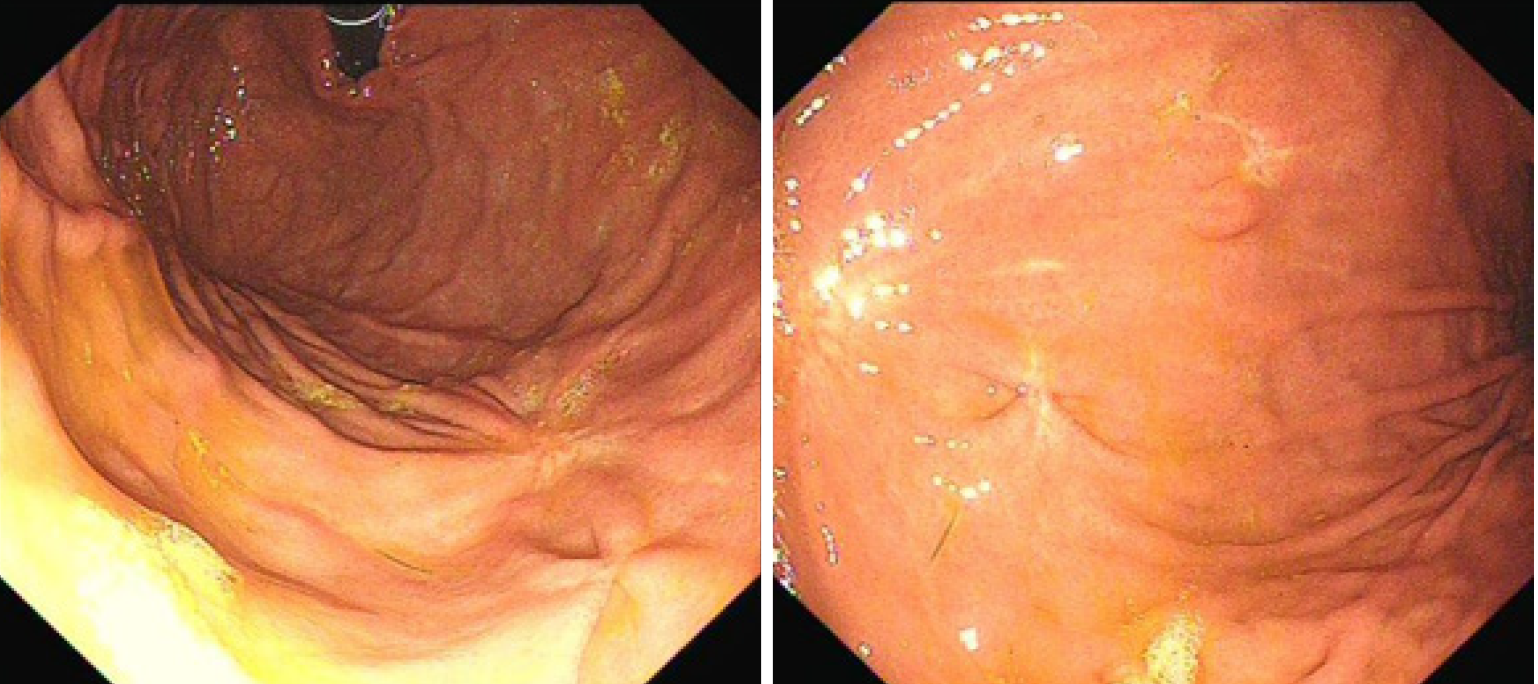
The patient was well recovered and was discharged on her seventh day post operation. The patient was followed up for 16 mo after the operation. Gastroscopy was performed (Figure 6), and the results indicated that the incision recovered well.
Schwannomas are tumors originating from Schwann cells that usually affect the subcutaneous tissue of the distal limbs[9]. Schwannomas of the gastrointestinal tract represent approximately 3% of all mesenchymal tumors of the gastrointestinal tract[10]. In the gastrointestinal tract, the stomach is the site with the highest incidence of schwannomas followed by the colon[11]. The small intestine and esophagus are the most infrequently affected sites[12,13]. Gastric schwannomas account for 0.2% of all gastric tumors[9].
Diagnostic methods for gastric schwannomas, such as endoscopy, EUS, CT, magnetic resonance imaging, and positron emission tomography, have recently been proposed. On endoscopy, gastric schwannomas appear as elevated submucosal masses, with or without a central ulcer[14]. Endoscopic biopsy is not as effective as expected, as it can lead to false negative results[9]. On EUS evaluation, a rounded submucosal mass, a well-defined margin, heterogeneous hypoechogenicity or isoechogenicity, and deficiency of cystic change and calcification are significant for the diagnosis of gastric schwannoma[15-17].
Previous studies demonstrated that gastric schwannomas showed well-demarcated masses that are heterogeneous or homogeneous contrast enhancement on CT[14,18]. Ji et al[19] and Wang et al[20] reported that homogeneous progressive enhancement on dynamic CT was a characteristic finding of gastric schwannoma. On magnetic resonance imaging examination, the signal intensity of most gastric schwannomas is low to medium on T1-weighted images and high on T2-weighted images[21]. Recently, several cases of gastric schwannoma that were found with an increased uptake of fluorodeoxyglucose on positron emission tomography were reported[22]. Even with the above modern imaging modalities, it is still difficult to achieve the precise preoperative diagnosis of gastric schwannomas.
In our case, the schwannoma was discovered incidentally by abdominal CT, suggesting GIST as a likely diagnosis. Gastroscopy and EUS provided the same primary diagnosis. The tumor was misdiagnosed as a GIST until the immunohistochemical findings and mutational analysis were revealed.
Gastric schwannomas are almost uniformly benign without recurrence or metastasis, and no malignant variant was found in previous follow-up studies[6,8]. The optimal treatment for gastric schwannoma is surgical resection, which should follow the same principles with GISTs[23].
However, in recent years, therapies for gastric submucosal tumor resection have rapidly developed, and less invasive endoscopic techniques, such as snare polype
In our case, the gastric schwannoma was treated by EFTR. To close the gastric perforation, endoscopic purse-string suture was performed. The patient had an uneventful recovery with no major complications.
Gastric schwannomas are relatively rare. Even with endoscopy and modern imaging modalities, the precise preoperative diagnosis of gastric schwannomas remains difficult. The final diagnosis of schwannoma is based on pathological and immunohistochemical examination. Gastric schwannomas are almost always benign, and patients with this type of tumor often have a favorable prognosis. Surgical resection is the optimal treatment for gastric schwannoma. Recently, minimally invasive techniques such as EFTR have been more widely considered and employed and are a safe and feasible treatment for gastric schwannomas.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naem A S-Editor: Zhang H L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Baek SJ, Hwangbo W, Kim J, Kim IS. A case of benign schwannoma of the ascending colon treated with laparoscopic-assisted wedge resection. Int Surg. 2013;98:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Melvin WS, Wilkinson MG. Gastric schwannoma. Clinical and pathologic considerations. Am Surg. 1993;59:293-296. [PubMed] |
| 3. | Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Pu C, Zhang K. Gastric schwannoma: a case report and literature review. J Int Med Res. 2020;48:300060520957828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Mekras A, Krenn V, Perrakis A, Croner RS, Kalles V, Atamer C, Grützmann R, Vassos N. Gastrointestinal schwannomas: a rare but important differential diagnosis of mesenchymal tumors of gastrointestinal tract. BMC Surg. 2018;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 6. | Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Wu X, Li B, Zheng C, He X. Clinical Characteristics and Surgical Management of Gastrointestinal Schwannomas. Biomed Res Int. 2020;2020:9606807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Hong X, Wu W, Wang M, Liao Q, Zhao Y. Benign gastric schwannoma: how long should we follow up to monitor the recurrence? Int Surg. 2015;100:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Lin CS, Hsu HS, Tsai CH, Li WY, Huang MH. Gastric schwannoma. J Chin Med Assoc. 2004;67:583-586. [PubMed] |
| 10. | Hou YY, Tan YS, Xu JF, Wang XN, Lu SH, Ji Y, Wang J, Zhu XZ. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Braumann C, Guenther N, Menenakos C, Junghans T. Schwannoma of the colon mimicking carcinoma: a case report and literature review. Int J Colorectal Dis. 2007;22:1547-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kitada M, Matsuda Y, Hayashi S, Ishibashi K, Oikawa K, Miyokawa N. Esophageal schwannoma: a case report. World J Surg Oncol. 2013;11:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Fukushima N, Aoki H, Fukazawa N, Ogawa M, Yoshida K, Yanaga K. Schwannoma of the Small Intestine. Case Rep Gastroenterol. 2019;13:294-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Hong HS, Ha HK, Won HJ, Byun JH, Shin YM, Kim AY, Kim PN, Lee MG, Lee GH, Kim MJ. Gastric schwannomas: radiological features with endoscopic and pathological correlation. Clin Radiol. 2008;63:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Jung MK, Jeon SW, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Gastric schwannomas: endosonographic characteristics. Abdom Imaging. 2008;33:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Okai T, Minamoto T, Ohtsubo K, Minato H, Kurumaya H, Oda Y, Mai M, Sawabu N. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom Imaging. 2003;28:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Zhong DD, Wang CH, Xu JH, Chen MY, Cai JT. Endoscopic ultrasound features of gastric schwannomas with radiological correlation: a case series report. World J Gastroenterol. 2012;18:7397-7401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol. 2005;184:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Ji JS, Lu CY, Mao WB, Wang ZF, Xu M. Gastric schwannoma: CT findings and clinicopathologic correlation. Abdom Imaging. 2015;40:1164-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Wang W, Cao K, Han Y, Zhu X, Ding J, Peng W. Computed tomographic characteristics of gastric schwannoma. J Int Med Res. 2019;47:1975-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Takeda M, Amano Y, Machida T, Kato S, Naito Z, Kumita S. CT, MRI, and PET findings of gastric schwannoma. Jpn J Radiol. 2012;30:602-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Ohno T, Ogata K, Kogure N, Ando H, Aihara R, Mochiki E, Zai H, Sano A, Kato T, Sakurai S, Oyama T, Asao T, Kuwano H. Gastric schwannomas show an obviously increased fluorodeoxyglucose uptake in positron emission tomography: report of two cases. Surg Today. 2011;41:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Williamson JM, Wadley MS, Shepherd NA, Dwerryhouse S. Gastric schwannoma: a benign tumour often mistaken clinically, radiologically and histopathologically for a gastrointestinal stromal tumour--a case series. Ann R Coll Surg Engl. 2012;94:245-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Zhai YQ, Chai NL, Li HK, Lu ZS, Feng XX, Zhang WG, Liu SZ, Linghu EQ. Endoscopic submucosal excavation and endoscopic full-thickness resection for gastric schwannoma: five-year experience from a large tertiary center in China. Surg Endosc. 2020;34:4943-4949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 25. | Jain D, Mahmood E, Desai A, Singhal S. Endoscopic full thickness resection for gastric tumors originating from muscularis propria. World J Gastrointest Endosc. 2016;8:489-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |