Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3238
Peer-review started: October 24, 2020
First decision: November 25, 2020
Revised: December 6, 2020
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: June 21, 2021
Processing time: 236 Days and 11.1 Hours
Non-alcoholic fatty liver disease (NAFLD), is a disease spectrum characterized by fat accumulation in hepatocytes presenting as hepatic steatosis to advance disease with active hepatic inflammation, known as nonalcoholic steatohepatitis. Chronic steatohepatitis will lead to progressive hepatic fibrosis causing cirrhosis and increased risk for developing hepatocellular carcinoma (HCC). Fatty liver disease prevalence has increased at alarming rates alongside obesity, diabetes and metabolic syndrome to become the second most common cause of cirrhosis after alcohol related liver disease worldwide. Given this rise in prevalence, it is becoming increasingly more important to find non-invasive methods to diagnose disease early and stage hepatic fibrosis. Providing clinicians with the tools to diagnose and treat the full spectrum of NAFLD will help prevent known complications such as cirrhosis and HCC and improve quality of life for the patients suffering from this disease. This article discusses the utility of current non-invasive liver function testing in the clinical progression of fatty liver disease along with the imaging modalities that are available. Additionally, we summarize available treatment options including targeted medical therapy through four different pathways, surgical or endoscopic intervention.
Core Tip: Fatty liver disease rates along with obesity, diabetes and metabolic syndrome continue to increase and now is the second leading cause of cirrhosis secondary to alcohol related liver disease. The need for consistent and readily available methods to accurately diagnose and stage hepatic fibrosis becomes increasingly necessary. With an up to date armamentarium to diagnose and treat the full spectrum of non-alcoholic fatty liver disease will decrease complications such as cirrhosis and hepatocellular carcinoma and will improve the likelihood for patients to have a higher quality of life.
- Citation: Seen TK, Sayed M, Bilal M, Reyes JV, Bhandari P, Lourdusamy V, Al-khazraji A, Syed U, Sattar Y, Bansal R. Clinical indicators for progression of nonalcoholic steatohepatitis to cirrhosis. World J Gastroenterol 2021; 27(23): 3238-3248
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3238.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3238
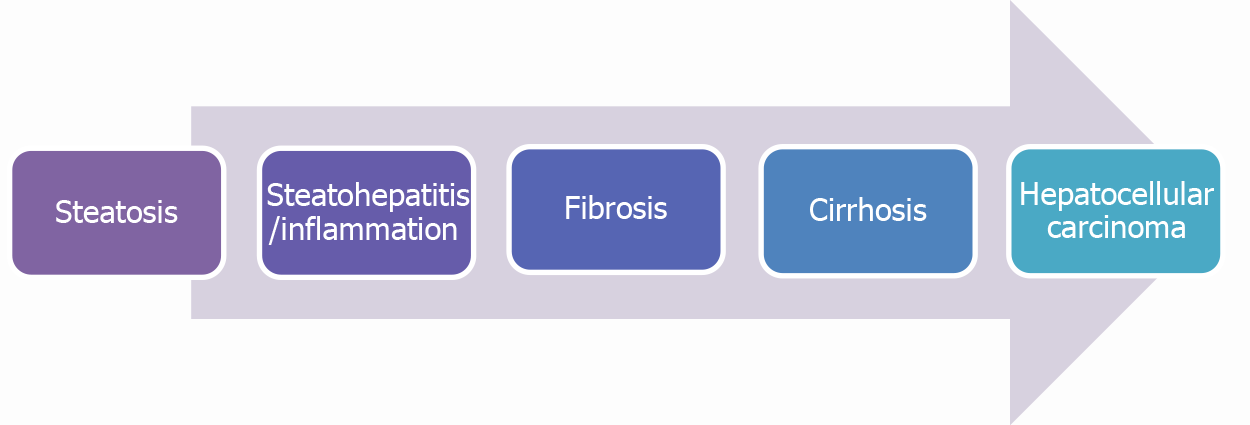
Nonalcoholic fatty liver disease (NAFLD) was first introduced by Schaffner and Thaler[1] in 1986. They assembled a group of non-alcoholic patients with liver diseases and biopsy specimens of liver pathology similar to that of alcoholic liver disease. They defined these subsets of patients as NAFLD. Over the last 20 years, the “non-alcoholic” portion of the diagnosis has been heavily criticized, as it carries an unfavorable connotation for patients that may negatively impact their overall care. In 2019, a group of international experts suggested the term metabolic (dysfunction) associated fatty liver disease “MAFLD” as a more appropriate diagnosis for NAFLD. As the underlying pathology is more related to metabolic dysfunction rather than the exclusion of alcohol[2]. Over the decades, NAFLD has grown to become the second most common cause of liver cirrhosis after alcohol related liver disease. The prevalence of NAFLD has grown every year in the United States secondary to a rise in diabetes, obesity and metabolic syndrome, with an incidence of 31% in 2012 as opposed to 18% in 1988–1991[3,4]. NAFLD refers to a spectrum of liver injury due to accumulation of triglycerides in hepatocytes presenting as a spectrum of conditions, ranging from a simple hepatic steatosis characterized by fat accumulation in the absence of hepatic inflammation to a more severe disease form characterized by active hepatic inflammation, also known as nonalcoholic steatohepatitis (NASH). Progressive hepatic inflammation will lead to cirrhosis and increase the risk of developing hepatocellular carcinoma (HCC) as shown in (Figure 1). Up to 1/3 of NAFLD patients will have NASH which is a risk factor for fibrosis progression, and approximately 40% of NASH patients will experience fibrosis progression[5]. The recent estimated annual progression of fibrosis from NAFLD is up to 0.09% with an incidence of advanced fibrosis as 70 per 1000 patients[6]. The high prevalence of NASH among the biopsied patients could be explained secondary to the indication for biopsy in these patients with elevated liver function tests (LFTs), and the data cannot be extrapolated to the subset of NAFLD patients with normal LFTs where biopsy is not performed often. In the same study, the prevalence of NASH in patients without indication for biopsy was 6.7%[5]. The annual incidence of HCC in NAFLD patients is 44% per 1000 person-years. NAFLD-related HCC amounts to about 2% to 4% of annual cases[7].
Liver biopsy is not always performed and the diagnosis is often made with available non-invasive tests including blood tests and elastography [magnetic resonance elastography (MRE), Fibroscan]. Advantages of fibroscan, other than being a non-invasive modality that helps in sequential assessment of progression or regression of steatosis/fibrosis, include elimination of sampling error experienced by liver biopsy. Liver biopsy is the gold standard in confirming the diagnosis of NAFLD and allowing accurate hepatitis fibrosis staging. The major histologic features include steatosis, lobular inflammation, and cytological ballooning; these findings help in grading and staging the disease[8,9]. Moreover, the diagnostic tests especially non-invasive fibrosis assessment testing helps to monitor NAFLD stages to prevent disease progression and diagnose cancer early. Our review article discusses the indicators that help in understanding the progression of the disease including symptomatic worsening, liver function testing, imaging, and histopathological changes.
NAFLD encompasses a spectrum of conditions which ranges from bland hepatic steatosis to steatohepatitis causing hepatic fibrosis which will lead to cirrhosis, liver failure and increase the risk of HCC. The risk factors of fatty liver disease are similar to those of metabolic syndrome which leads to insulin resistance[5]. This includes diabetes mellitus, dyslipidemia and elevated body mass index (BMI). It is important to distinguish simple hepatic steatosis, which carries very low risk of developing chronic disease and cirrhosis vs NASH which carries a risk of progressive fibrosis, cirrhosis, liver failure and HCC. Overall, one fifth of NASH patients can progress to advanced hepatic fibrosis[10-12]. Hence, the assessment of the degree of hepatic fibrosis with noninvasive diagnostic panels and imaging is important in monitoring disease progression. Several scoring systems and specialized biomarkers have been developed by combining various serologic and clinical parameters for the prediction of fibrosis in NAFLD[13-18]. Despite the advancement of many diagnostic noninvasive fibrosis assessment modalities, one fourth of advanced fibrosis NASH patients can be misclassified as mild hepatic fibrosis[14].
The mortality of NAFLD is not merely targeting the liver. The majority of NAFLD patients are at risk of developing atherosclerotic coronary artery disease carrying higher mortality rate approaching[19]. Understanding patients’ risk factors and stage of hepatic fibrosis can help predict patients’ clinical outcomes[10]. Multiple clinical indicators and serological markers of disease progression remains an area of intensive clinical and basic science research till this day (Tables 1 and 2).
| Sr. No. | Indicator | Ref. | Journal | Year | Results |
| 1 | Bilirubin | Demir et al[24] | PLoS One | 2013 | Total bilirubin was identified as a significant predictor of advanced fibrosis and used to construct the NIKEI score which can reliably exclude advanced fibrosis in subjects with NAFLD |
| Ratziu et al[25] | BMC Gastroenterol | 2006 | FibroTest which includes total bilirubin in its panel is a simple and noninvasive quantitative estimate of liver fibrosis which reliably predicts advanced fibrosis | ||
| Adams et al[11] | J Hepatol | 2005 | Hepascore, a model of 4 serum markers plus age and sex provides clinically useful information regarding different fibrosis stages among hepatitis C patients | ||
| 2 | Serum AST/ALT | Martin-Rodriguez et al[20] | Medicine (Baltimore) | 2017 | Serum ALT level is the most predictive laboratory investigation for the NAFLD. The AST-ALT Ratio (AAR) is higher in increasing liver fat content, fibrosis and other metabolic derangements like diabetes and dyslipidemia |
| Enomoto et al[21] | World J Gastroenterol | 2015 | AAR > 1 is consistent with NASH | ||
| Arora et al[22] | J Clin Exp Hepatol | 2012 | AAR > 1 may indicate the progression of NAFLD and aid in diagnosing liver fibrosis | ||
| Shah et al[17] | Clin Gastroenterol Hepatol | 2009 | The FIB-4 score composed of age, AST and ALT and platelet counts is an invasive and inexpensive method which has shown superiority to BAAT (BMI, Age, ALT, Triglycerides) and BARD (BMI, AST: ALT, Diabetes) scores in monitoring the progress of NASH | ||
| McPherson et al[18] | Eur J Gastroenterol Hepatol | 2013 | The FIB-4 score was reliable in ruling out advanced fibrosis in patients with histological evidence of NAFLD who had normal or increased levels of ALT, thus decreasing the need for invasive liver biopsy | ||
| 3 | Platelet Count | Enomoto et al[21] | World J Gastroenterol | 2015 | A reducing level of platelet count has been well documented in advancing liver diseases |
| Kawamura et al[26] | Hepatol Int | 2015 | FSN score of 17 variables including platelet count could accurately predict fibrotic stage and discriminates patients with advanced fibrosis of NASH | ||
| Kessoku et al[27] | World J Gastroenterol | 2014 | PLALA Score is a very unique scoring system as it has shown usefulness in distinguishing cirrhosis in NAFLD when compared with most fibrosis scoring systems | ||
| Abdel-Razik A et al[47] | Eur J Gastroenterol | 2016 | MPV is a noninvasive novel marker to predict advanced disease as it was increased in NASH patients and advance liver fibrosis | ||
| Cengiz et al[48] | Eur J Gastroenterol | 2015 | Red cell volume distribution width-to-platelet ratio was both correlated and able to predict liver fibrosis. It may reduce liver biopsy in NAFLD | ||
| 4 | Fasting blood glucose and glycosylated protein | Pelusi et al[49] | PLoS One | 2016 | Nonalcoholic steatohepatitis with greater degree of fibrosis was discovered in patients with insulin resistance. Type 2 diabetes in patients with NAFLD tends to drive the rate of fibrosis |
| 5 | Hyaluronic acid (hyluroante) tissue metaloproteinase | Arora et al[22] | J Clin Exp Hepatol | 2012 | European Liver Fibrosis score ELF scoring system has indicators for cellular matrix activities including Hyaluronic acid (hyluroante) tissue metalloproteinase which has been indicative of fibrosis |
| 6 | Type IV collagen | Nakamura et al[30] | J Diabetes Investig | 2013 | NAFIC Score including type IV collagen 7S and Modified NAFIC score were proven to be clinically useful in screening for NASH in NAFLD patients |
| 7 | Glycosylated Albumin to Glycosylated Hemoglobin Ratio | Hu et al[28] | World J Gastroenterol | 2014 | HOMA-IR score indicates NAFLD progression using a formula that involves insulin levels and fasting glucose to calculate insulin resistance. The score has a high sensitivity for NASH |
| Stål[29] | World J Gastroenterol | 2015 | NAFLD fibrosis score, a non- invasive score which includes the presence of diabetes or impaired fasting glucose is the most predictive of mortality in NASH as compared to NAFL patients | ||
| 8 | Prothrombin time | Assy et al[31] | World J Gastroenterol | 2005 | Increase prothrombin time is usually associated with cirrhotic changes |
| 9 | Albumin | Bazick et al[5] | Diabetes Care | 2015 | Serum albumin gets reduced in patients progressing to NASH and fibrosis from NAFLD |
| Sr. No. | Imaging modality | Ref. | Journal | Year | Results |
| 1 | Ultrasound | Sanyal[32] | Gastroenterology | 2002 | US is currently the preferred method in United States for screening asymptomatic patients with elevated liver enzymes and suspected NAFLD with sensitivity in detecting steatosis varying between 60%–94% |
| 2 | Magnetic Resonance Elastography | Iijima et al[35] | Hepatol Res | 2007 | Magnetic resonance elastography has excellent diagnostic accuracy with sensitivity and specificity of 98% and 99%, respectively, for detecting all grades of fibrosis |
| Huwart et al[36] | Gastroenterology | 2008 | Magnetic resonance elastography was associated with a higher technical success rate than US elastography | ||
| 3 | Fibroscan | Wong et al[39] | Gut | 2012 | Transient elastography had shown good results in patients with NAFLD. It is a non-invasive method of assessing liver fibrosis which can be performed at the bedside or in the outpatient clinic |
| Wong et al[40] | Hepatology | 2010 | Transient elastography had shown good results in patients with NAFLD. It is a non-invasive method of assessing liver fibrosis which can be performed at the bedside or in the outpatient clinic | ||
| Ratziu et al[43] | Gastroenterology | 2005 | Fibroscan has now been validated in NAFLD, and represents a useful tool for rapid, non-invasive assessment of liver fibrosis and determining the need for biopsy. Nonetheless, fibroscan values should be interpreted in consonance with clinical, biological, and morphological data |
NASH is a histological diagnosis characterized by hepatocytic inflammation that may progress to fibrosis. Hepatic fibrosis divided into four stages. Stage I describes as mild hepatic fibrosis, stage II moderate hepatic fibrosis, stage III moderate to severe fibrosis, and stage IV severe or advanced fibrosis. It is crucial to identify advanced fibrosis stage as these patients are at-risk to develop decompensated cirrhosis and end-stage liver disease. A number of clinical factors help clinicians to predict the likelihood of the patient progressing into devastating categories of this disease.
Liver chemistry test identify active hepatic inflammation. This includes alanine aminotransferases (ALT) and aspartate aminotransferases (AST), alkaline phosphatase (ALP) and direct and indirect bilirubin. Other laboratory data should be monitored in NASH patients are platelet count and coagulation panel, fasting blood glucose and glycosylated proteins and lipid panel. Serum hyaluronic acid tissue metalloproteinase, and type 4 collagen are serological markers help in assessing fibrosis stage.
AST and ALT: In a cross-sectional study, Martin-Rodriguez et al[20] reported that serum ALT level is the most predictive laboratory investigation for NAFLD. The AST-ALT Ratio (AAR) is higher in increased liver fat content, fibrosis, and other metabolic derangements like diabetes and dyslipidemia. Steatosis or steatohepatitis can be observed, but nevertheless patients have normal serum ALT levels[8]. An AAR > 1 is consistent with a diagnosis of NASH. This forms the basis of several other laboratory combinations that may indicate the progression of NAFLD and diagnosing liver fibrosis including BAAT (which uses BMI, age, ALT, and triglycerides), BARD (which uses BMI, AST: ALT, and diabetes), and FIB-4 scores[21,22]. The FIB-4 score is a simple, noninvasive and inexpensive test superior to BAAT and BARD scores in monitoring the progress of NASH[17]. The FIB-4 score is reliable in ruling out advanced fibrosis in patients with histological evidence of NAFLD who had normal or increased levels of ALT, thus decreasing the need for invasive liver biopsy with sensitivity 84%-94%[18].
ALP: Few subsets of NASH patients present with an isolated ALP elevation[23]. Cholestasis also has been noted on histology in NASH[23]. Elevated ALP should be accompanied by an increase in γ-glutamyltransferase (GGT) enzyme suggesting hepatic inflammation. Otherwise elevated ALP without GGT elevation are seen in pregnancy, muscular disease and bone disease such as Paget’s disease.
Bilirubin: Bilirubin is synthetic marker for liver function alongside with PT/INR. Also, it is a part of various scoring system used to estimate the degree of fibrosis. Demir et al[24] introduced the non-invasive koeln-essen-index (NIKEI) score which uses age, AST, AST/ALT ratio, and total bilirubin. In a prospective study by Ratziu et al[25], the diagnostic utility of FibroTest, a noninvasive marker of fibrosis, was determined in a sample of 170 patients with NAFLD. The FibroTest includes α-2-macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, and γ-glutamyl-transpeptidase. Ratziu concluded this simple and noninvasive quantitative estimate of liver fibrosis reliably predicts advanced fibrosis[25]. Hepascore, a combination of bilirubin, γ-glutamyl-transpeptidase, hyaluronic acid, and 2-macroglobulin together with age and sex, is an accurate and reliable panel in predicting different stages of fibrosis. However, the limitation of this study included validation of this score among only patients with hepatitis C[21]. NIKEI had superior negative predictive value for advanced fibrosis compared to the FIB-4 score (which uses age, AST, ALT, and platelet counts)[24].
Platelet count: Platelet count has great value in assessing degree of fibrosis. Thrombocytopenia occurs in cirrhosis secondary to thrombopoietin deficiency and splenic sequestration from underlying splenomegaly occurring from portal hypertension. Platelet level has been used in combination with other biochemical parameters such as in AST/platelet ratio index and FIB-4 score to monitor liver disease progression[21]. Kawamura established the fibrosis score for NASH (FSN), a new scoring system specific to the fibrotic stage of NASH[26]. FSN can accurately predict the fibrotic stage and distinguishes patients with advanced fibrosis of NASH. The platelet albumin AAR (PLALA) score is unique in that it distinguishes cirrhosis in NAFLD compared to most other fibrosis scoring systems. Each factor (platelet count < 15.3 × 104 /µL; albumin < 4 g/dL; AAR > 0.9) is awarded 1 point, and a PLALA score of 2 or 3 may be predictive of cirrhosis in patients with NAFLD[27]. The PLALA score may be an ideal scoring system for detecting cirrhosis in NAFLD patients with sufficient accuracy and simplicity for clinical use. Mean platelet volume (MPV) was elevated in NASH and advanced liver fibrosis (stages 3–4) patients, making MPV a noninvasive, novel marker to predict advanced disease. Another study looked into the performance of red cell volume distribution width-to-platelet ratio in predicting liver fibrosis in patients with NAFLD[27]. This ratio was both correlated and able to predict liver fibrosis.
Fasting blood glucose and glycosylated protein: In their observational cohort of 118 patients, assessing the clinical determinants of fibrosis progression rate in NAFLD patients with baseline and follow-up histological evaluation. Advanced fibrosis is more likely to be found in patients with underlying type 2 diabetes[28]. These patients had histological evidence of more inflammation in the fibrous portal areas in those already developing cirrhosis than those at an earlier stage of the disease. Furthermore, this study also observed that type 2 diabetes can drive fibrosis in the absence of hepatic inflammation.
Glycosylated albumin to glycosylated hemoglobin ratio: Glycosylated albumin (GA) and glycosylated hemoglobin (HbA1c), which are indicators of glycemic control, show a strong relationship with advanced liver fibrosis. The GA/HbA1c ratio, which is typically 3 in a healthy individual, is higher in liver fibrosis patients. Patients with chronic liver diseases have reduced albumin turnover resulting in an elevated level of GA. Also, they have a reduced erythrocyte lifespan which accounts for changes in the increased ratio. The GA/HbA1c ratio’s accuracy in detecting liver fibrosis might be limited by other concurrent diseases that can affect plasma and hemoglobin levels[25]. The HOMA-insulin resistance score is a somewhat rigorous and reliable scoring system that indicates NAFLD progression using a formula that involves insulin levels and fasting glucose to calculate insulin resistance[28]. The HOMA-insulin resistance score has a high sensitivity for NASH. The NAFLD fibrosis score (NFS) is a nonin
Hyaluronic acid tissue metalloproteinase: A high level of hyaluronic acid (hyalu
Type IV collagen: The FSN score, which includes type IV collagen 7S, platelet count, AST, and ALT, has been more efficient in distinguishing the advanced fibrosis stages 3–4 of NASH compared to other scoring systems including APRI (AST to platelet ratio index), NAFLD Score, FIB-4 Index, BARD, and NIKEI[26]. The nonalcoholic steatohepatitis, ferritin, insulin, and type IV collagen 7S (NAFIC) score, and modified NAFIC score were proven to be clinically useful in screening for fatty liver patients[30].
Albumin: Bazick et al[5] demonstrated that serum albumin gets reduced drastically in patients presenting with NASH. Their clinical variable could be used to guide clinical decision making about referring patients with diabetes and NAFLD to hepatolo
Prothrombin time: In a study by Assy et al[31], up to 46% of patients with NAFLD showed thrombotic risk factors. The presence of thrombotic risk factors correlated with the extent of hepatic fibrosis. This is consistent with known coagulopathy in those with altered synthetic function due to hepatic fibrosis.
Noninvasive techniques such as ultrasonography (US), computed tomography, magnetic resonance imaging, and proton magnetic resonance spectroscopy can detect hepatic steatosis but cannot reliably distinguish simple steatosis from NASH[32].
US is the preferred cost effective method in the United States for screening patients with suspected NAFLD. The findings on US include: diffuse increase in echogenicity of the liver parenchyma, hepatomegaly and vascular blunting[33]. The sensitivity of US in detecting hepatic steatosis up to 94%. The sensitivity decreases as the degree of steatosis dropped below 30%[33-35]. US cannot differentiate between simple hepatic steatosis vs NASH. Thus, laboratory serological and histological data is helpful in pointing towards NASH[22].
MRE stands for magnetic resonance elastography which is an imaging technique that combines MRI imaging with low-frequency vibrations to measure hepatic stiffness. MRE equivalent of transient elastography has recently demonstrated excellent diagnostic accuracy. It has shown a sensitivity and specificity of 98% and 99%, respectively, for detecting all grades of fibrosis[35]. Huwart et al[36] conducted a prospective blind comparison of MRE, US elastography, and APRI (AST to platelet ratio index) in a study of 141 patients who underwent liver biopsy for chronic liver disease. They found MRE was associated with a higher technical success rate than US elastography[36].
Transient Elastography (Fibroscan, Echosens, Paris, France) is a noninvasive method of assessing liver fibrosis. It can be performed at the bedside or in the outpatient clinic. It employs US-based technology to measure liver stiffness and has been validated for use in patients with chronic hepatitis C and B[37,38]. However, studies have shown good results in patients with NAFLD[39,40]. In only 5% of the cases, it has failed to show any readings. This is mostly seen in obese patients. This limits the TE’s utility in the NAFLD cohort. However, a recently introduced XL probe may reduce this problem[41]. In a meta-analysis for NASH with advanced fibrosis, pooled area under the receiver operating characteristic curve, sensitivity and specificity of NFS, and fibroscan are 0.85 (0.80-0.93), 0.90 (0.82-0.99), 0.97 (0.94-0.99), and 0.94 (0.90-0.99), 0.94 (0.88-0.99) and 0.95 (0.89-0.99), respectively[16]. Fibroscan is validated in NAFLD and represents a useful tool for rapid, noninvasive assessment of liver fibrosis and determining the need for biopsy. As this modality evaluates liver stiffness (related to fibrosis, inflammation, and portal hypertension), Fibroscan values should be interpreted in context of the morphological, biological, and clinical data.
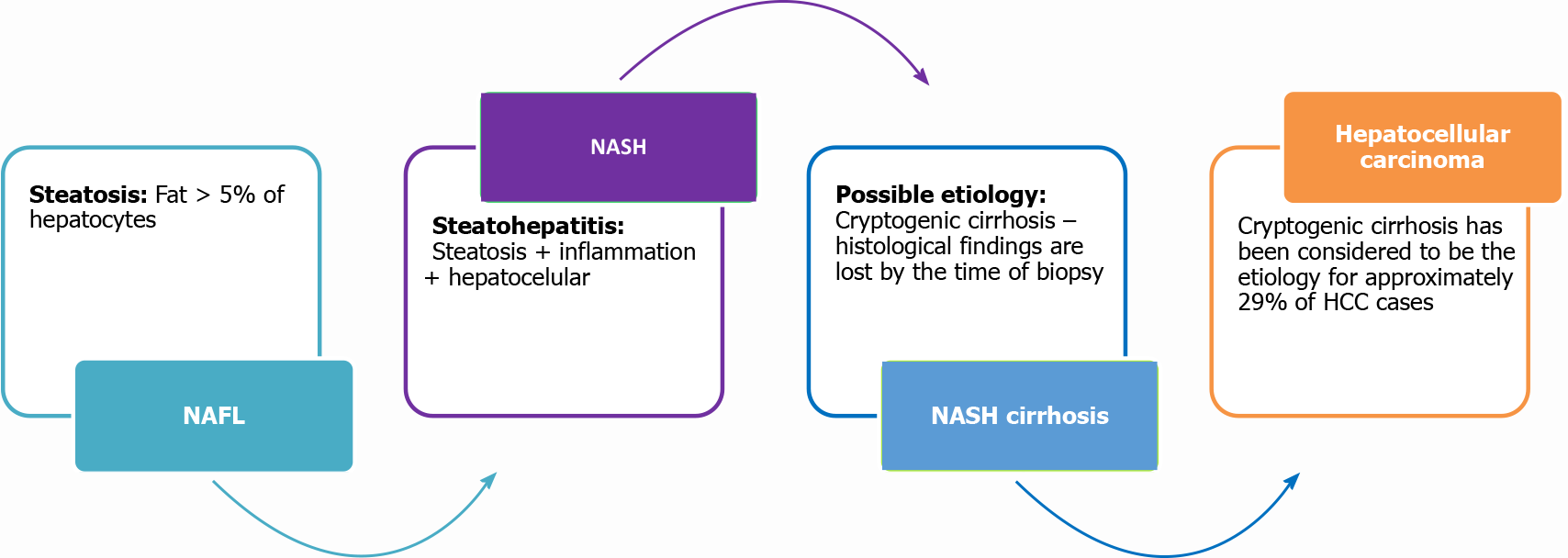
A percutaneous liver biopsy is currently the gold standard to assess hepatic fibrosis and inflammation in chronic liver disease[42]. However, liver biopsy is an invasive procedure with associated costs, complications, and inherent inaccuracy due to sampling error and inter-observer and intra-observer variability in histopathological interpretation[42,43]. Despite the criticism of liver biopsy’s associated risks, there are 3 basic histological systems that can be used to monitor the progression of NASH (Figure 2). These systems are the steatosis activity and fibrosis score, the NASH activity score, and the Brunt system that grades and stages NASH (Table 3)[28,44]. Due to the risks and limitations associated with liver biopsy, it is controversial to perform liver biopsy on every patient suspected of having NAFLD. Therefore, it cannot be considered a “screening” tool[45]. However, there are studies that support the importance of liver biopsy. An older study, by Skelly et al[46] showed that biopsy on 354 patients with abnormal liver tests-66% had fatty liver, 50% of those had steatohepatitis, and approximately 19% of the remaining biopsies had other treatable causes diagnosed by the pathology evaluation. This included autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis and alcoholic liver disease. An adequate liver biopsy, with appropriate clinical history, interpreted by a trained liver pathologist, is not only pivotal for an accurate and complete diagnosis (or exclusion) of NAFLD (or NASH), but also is optimal for obtaining detailed information regarding disease pattern, severity and fibrosis. It not only provides important information with respect to subtypes, potential future risks, possible etiology, and natural history of disease, but also sets the groundwork for future molecular studies and clinical trials, assisting clinical colleagues and patients with treatments and follow-up[47-49].
| NASH activity score | Steatosis, activity and fibrosis score | Brunt grading and staging |
| Steatosis grade 0-3 | Steatosis S0-S3 | Grade 1 (Mild) |
| Lobular inflammation 0-3 | Activity A1-A3 | Grade 2 (Moderate) |
| Ballooning 0-2 | Lobular inflammation 0-2 | Grade 3 (Severe) |
| Fibrosis 0-4 (grade 1 has subgrade A, B, C) | Ballooning 0-2 | Stages fibrosis |
| Fibrosis F0-F4 | Stage 1-4 |
NASH-related cirrhosis is the most common cause of chronic liver disease and indication for liver transplant. The increasing number of affected people imposes a strain on available organs. There are many comorbidities and risk factors implicated in NASH severity and progression to chronic liver disease.
Due to the increasing prevalence of NAFLD in the population, there is an increasing need to find non- invasive methods to diagnose and stage NAFLD. The ideal test should be reproducible, cheap, and able to diagnose full spectrum of NAFLD, predict fibrosis, and reflect changes that occur with treatment. Preliminary evaluation includes clinical presentation with consideration of comorbidities and liver function test in the blood. Noninvasive imaging such as MRE and fibroscan can provide objective measures of liver steatosis and stiffness in patients without advanced fibrosis or cirrhosis. Due to the limitations, risks and cost of liver biopsy-it cannot be used as a screening test, although is typically relied upon to confirm the diagnosis. Several different methodologies including imaging modalities, serum markers and combined tests are currently being investigated.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tovo CV S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
| 1. | Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986;8:283-298. [PubMed] |
| 2. | Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What's in a name? Liver Int. 2020;40:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 3. | Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 4. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 5. | Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Tevar AD, Clarke C, Wang J, Rudich SM, Woodle ES, Lentsch AB, Edwards ML. Clinical review of nonalcoholic steatohepatitis in liver surgery and transplantation. J Am Coll Surg. 2010;210:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:15539-15548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 255] [Cited by in RCA: 321] [Article Influence: 29.2] [Reference Citation Analysis (3)] |
| 8. | Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7539] [Article Influence: 837.7] [Reference Citation Analysis (0)] |
| 10. | Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 330] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 11. | Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 659] [Article Influence: 33.0] [Reference Citation Analysis (1)] |
| 12. | Machado MV, Cortez-Pinto H. Non-alcoholic fatty liver disease: what the clinician needs to know. World J Gastroenterol. 2014;20:12956-12980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 151] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (4)] |
| 13. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ; European Liver Fibrosis Group. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 759] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 14. | Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD, Aithal GP, Day CP, Rosenberg WM. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 548] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 15. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2284] [Article Influence: 126.9] [Reference Citation Analysis (1)] |
| 16. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 17. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1168] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 18. | McPherson S, Anstee QM, Henderson E, Day CP, Burt AD. Are simple noninvasive scoring systems for fibrosis reliable in patients with NAFLD and normal ALT levels? Eur J Gastroenterol Hepatol. 2013;25:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263-8276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 543] [Cited by in RCA: 516] [Article Influence: 64.5] [Reference Citation Analysis (6)] |
| 20. | Martin-Rodriguez JL, Gonzalez-Cantero J, Gonzalez-Cantero A, Arrebola JP, Gonzalez-Calvin JL. Diagnostic accuracy of serum alanine aminotransferase as biomarker for nonalcoholic fatty liver disease and insulin resistance in healthy subjects, using 3T MR spectroscopy. Medicine (Baltimore). 2017;96:e6770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Enomoto H, Bando Y, Nakamura H, Nishiguchi S, Koga M. Liver fibrosis markers of nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:7427-7435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 22. | Arora A, Sharma P. Non-invasive Diagnosis of Fibrosis in Non-alcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2012;2:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Pantsari MW, Harrison SA. Nonalcoholic fatty liver disease presenting with an isolated elevated alkaline phosphatase. J Clin Gastroenterol. 2006;40:633-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Demir M, Lang S, Schlattjan M, Drebber U, Wedemeyer I, Nierhoff D, Kaul I, Sowa J, Canbay A, Töx U, Steffen HM. NIKEI: a new inexpensive and non-invasive scoring system to exclude advanced fibrosis in patients with NAFLD. PLoS One. 2013;8:e58360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF, Le Bail B, de Ledinghen V, Poynard T; LIDO Study Group; CYTOL study group. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Kawamura Y, Ikeda K, Arase Y, Sorin Y, Fukushima T, Kunimoto H, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Kumada H. New discriminant score to predict the fibrotic stage of non-alcoholic steatohepatitis in Japan. Hepatol Int. 2015;9:269-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kessoku T, Ogawa Y, Yoneda M, Imajo K, Sumida Y, Eguchi Y, Fujii H, Hyogo H, Ono M, Suzuki Y, Kawaguchi T, Chayama K, Tanaka S, Fujimoto K, Anzai K, Saibara T, Sata M, Itoh Y, Nakajima A, Okanoue T; Japan Study Group of NAFLD (JSG-NAFLD). Simple scoring system for predicting cirrhosis in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:10108-10114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Hu KC, Wang HY, Liu SC, Liu CC, Hung CL, Bair MJ, Liu CJ, Wu MS, Shih SC. Nonalcoholic fatty liver disease: updates in noninvasive diagnosis and correlation with cardiovascular disease. World J Gastroenterol. 2014;20:7718-7729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 29. | Stål P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21:11077-11087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (3)] |
| 30. | Nakamura A, Yoneda M, Sumida Y, Eguchi Y, Fujii H, Hyogo H, Ono M, Suzuki Y, Kawaguchi T, Aoki N, Okanoue T, Nakajima A, Maeda S, Terauchi Y. Modification of a simple clinical scoring system as a diagnostic screening tool for non-alcoholic steatohepatitis in Japanese patients with non-alcoholic fatty liver disease. J Diabetes Investig. 2013;4:651-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Assy N, Bekirov I, Mejritsky Y, Solomon L, Szvalb S, Hussein O. Association between thrombotic risk factors and extent of fibrosis in patients with non-alcoholic fatty liver diseases. World J Gastroenterol. 2005;11:5834-5839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Sanyal AJ; American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 781] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 33. | Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol. 2007;102:2716-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320-W323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 35. | Iijima H, Moriyasu F, Tsuchiya K, Suzuki S, Yoshida M, Shimizu M, Sasaki S, Nishiguchi S, Maeyama S. Decrease in accumulation of ultrasound contrast microbubbles in non-alcoholic steatohepatitis. Hepatol Res. 2007;37:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 539] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 37. | Boursier J, de Ledinghen V, Zarski JP, Rousselet MC, Sturm N, Foucher J, Leroy V, Fouchard-Hubert I, Bertrais S, Gallois Y, Oberti F, Dib N, Calès P. A new combination of blood test and fibroscan for accurate non-invasive diagnosis of liver fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2011;106:1255-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Osakabe K, Ichino N, Nishikawa T, Sugiyama H, Kato M, Kitahara S, Hashimoto S, Kawabe N, Harata M, Nitta Y, Murao M, Nakano T, Shimazaki H, Arima Y, Suzuki K, Yoshioka K. Reduction of liver stiffness by antiviral therapy in chronic hepatitis B. J Gastroenterol. 2011;46:1324-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J, Chan FK, Chan HL. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 40. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 41. | de Lédinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int. 2010;30:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 43. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1549] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 44. | Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017;9:715-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 509] [Article Influence: 63.6] [Reference Citation Analysis (18)] |
| 45. | Nalbantoglu IL, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:9026-9037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 95] [Reference Citation Analysis (1)] |
| 46. | Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001;35:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Abdel-Razik A, Mousa N, Shabana W, Refaey M, ElMahdy Y, Elhelaly R, Elzehery R, Zalata K, Arafa M, Elbaz S, Hafez M, Awad M. A novel model using mean platelet volume and neutrophil to lymphocyte ratio as a marker of nonalcoholic steatohepatitis in NAFLD patients: multicentric study. Eur J Gastroenterol Hepatol. 2016;28:e1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Cengiz M, Ozenirler S. Comparative diagnostic accuracy of red cell distribution width-to-platelet ratio versus noninvasive fibrosis scores for the diagnosis of liver fibrosis in biopsy-proven nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Pelusi S, Petta S, Rosso C, Borroni V, Fracanzani AL, Dongiovanni P, Craxi A, Bugianesi E, Fargion S, Valenti L. Renin-Angiotensin System Inhibitors, Type 2 Diabetes and Fibrosis Progression: An Observational Study in Patients with Nonalcoholic Fatty Liver Disease. PLoS One. 2016;11:e0163069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |