Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.2994
Peer-review started: February 4, 2021
First decision: March 28, 2021
Revised: April 9, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: June 14, 2021
Processing time: 118 Days and 18.6 Hours
More than 90% of cases of hepatocellular carcinoma (HCC) occurs in patients with cirrhosis, of which hepatitis B virus and hepatitis C virus are the leading causes, while the tumor less frequently arises in autoimmune liver diseases. Advances in understanding tumor immunity have led to a major shift in the treatment of HCC, with the emergence of immunotherapy where therapeutic agents are used to target immune cells rather than cancer cells. Regulatory T cells (Tregs) are the most abundant suppressive cells in the tumor microenvironment and their presence has been correlated with tumor progression, invasiveness, as well as metastasis. Tregs are characterized by the expression of the transcription factor Foxp3 and various mechanisms ranging from cell-to-cell contact to secretion of inhibitory molecules have been implicated in their function. Notably, Tregs amply express checkpoint molecules such as cytotoxic T lymphocyte-associated antigen 4 and programmed cell-death 1 receptor and therefore represent a direct target of immune checkpoint inhibitor (ICI) immunotherapy. Taking into consideration the critical role of Tregs in maintenance of immune homeostasis as well as avoidance of autoimmunity, it is plausible that targeting of Tregs by ICI immunotherapy results in the development of immune-related adverse events (irAEs). Since the use of ICI becomes common in oncology, with an increasing number of new ICI currently under clinical trials for cancer treatment, the occurrence of irAEs is expected to dramatically rise. Herein, we review the current literature focusing on the role of Tregs in HCC evolution taking into account their opposite etiological function in viral and autoimmune chronic liver disease, and we discuss their involvement in irAEs due to the new immunotherapies.
Core Tip: Hepatocellular carcinoma (HCC) is the fifth most common cancer with poor prognosis despite significant improved diagnostic and therapeutic strategies. Most of HCC occurs in cirrhotic patients, with hepatitis B and C viruses being leading causes, Recent studies showed that HCC development and progression are associated with a unique immune response profile of the liver microenvironment where CD4+CD25+ Foxp3 regulatory T-cells (Tregs) play a crucial role through their immunosuppressive role. We discuss the role of Tregs in chronic liver diseases as well as in HCC initiation and the role of immunotherapy to enhance anti-tumor immune response by blocking Treg activity.
- Citation: Granito A, Muratori L, Lalanne C, Quarneti C, Ferri S, Guidi M, Lenzi M, Muratori P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J Gastroenterol 2021; 27(22): 2994-3009
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/2994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.2994
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most frequent cause of cancer-related death worldwide, with more than 900000 new cases and more than 800000 deaths in 2020[1].
HCC accounts for nearly 90% of primary liver cancers and is a leading world health problem. The incidence of HCC rises sharply with age in all populations, achieving a peak at age 70 and it is increasing in most countries representing the dominant cause of mortality in cirrhotic patients[2-4].
Worldwide, chronic viral hepatitis has been reported as the leading risk factors for HCC development, although in high-income areas HCC related to non-alcoholic fatty liver disease is increasing due to the rising prevalence of metabolic disorders[5-7].
In contrast, vaccination and treatment for hepatitis B virus (HBV) infection, prevention campaigns for sexual and iatrogenic transmission of hepatitis B (HBV) and hepatitis C virus (HCV), and the introduction of effective HCV antiviral agents are reducing the burden of chronic viral liver disease[8-11].
HCC arises almost exclusively in the setting of chronic liver inflammation and, unlike the elevated risk associated with chronic viral (HBV and HCV) infections, it has been reported that the tumor is less common in liver cirrhosis caused by autoimmune liver diseases[12-15]. However, it is known that, regardless of etiology, cirrhosis per se represents a precancerous condition leading to an increased risk of HCC[15,16].
Indeed, irrespective of etiology, a typical sequence of chronic necroinflammation, compensatory liver regeneration, induction of liver fibrosis and subsequent cirrhosis often precedes hepatocarcinogenesis. HCC is a prototypical inflammation-driven tumor arising on the backdrop of liver cirrhosis. The evidence of an immune-rich contexture of the HCC microenvironment has inspired several studies in recent years that have further defined profile and crucial pathogenetic role of immune cells in tumor development[17].
The liver is a central immunomodulator that ensures organ and systemic protection while maintaining immunotolerance. Deregulation of this tightly controlled liver immunological network is a hallmark of chronic liver disease and HCC[18].
Recently, within the cell subset characterizing the HCC immune microenvironment, a key role has been highlighted for CD4+ CD25+ regulatory T cells (Tregs), which are crucially implicated in both pathogenesis of chronic liver diseases and development and spread of HCC[19,20].
In this review we examine the evidence that has recently accumulated on the different role that these cells play in chronic viral liver diseases (HBV and HCV) and autoimmune liver diseases, and their function in the tumor microenvironment (TME) characterizing HCC. We also discuss the possible implications for emerging immunotherapies and the potential risks of immune-mediated liver toxicity from this treatment[21].
It is well established that cirrhosis represents the most significant risk factor for the development of HCC[22]. Historically, it has been reported that the risk of HCC in cirrhosis due to viral causes is higher than in other non-viral etiologies, however a precise comparison of the incidence of HCC in various chronic liver diseases, especially in cirrhosis, has only recently been evaluated[23,24].
In a recent meta-analysis it was shown that the annual incidence of HCC in chronic liver diseases and the ratio of HCC incidence in non-cirrhotic/cirrhotic stages has the following etiological hierarchy: HCV-related disease 0.68% to 4.81% (7.07-fold, P < 0.001), HBV-related liver disease 0.37% to 3.23% (8.73 fold, P < 0.001), primary biliary cholangitis (PBC) (pre-cirrhotic vs Scheuer’s III-IV stage) 0.26 to 1.79% (6.88-fold, P < 0.001), NASH 0.03% to 1.35% (45-fold, P < 0.001), autoimmune hepatitis (AIH) 0.19% to 0.53% (2.79-fold, P = 0.03), and that the incidence of HCC is markedly increased (2.79- fold to 45-fold) in the cirrhotic stage compared with the non-cirrhotic stage, regardless of etiology[24]. Thus, it is confirmed that there is a significant difference in HCC incidence between viral and autoimmune liver diseases, with the lowest risk in the latter even when in the cirrhotic stage.
Worldwide, about 54% of cases can be ascribed to HBV infection (affecting 400 million people globally) while 31% can be associated with HCV infection (affecting 170 million people), leaving about 15% attributable to other causes[25].
Incidence of HCC in autoimmune liver disease is less definitively established. In a recent systematic review of 25 published cohorts, a total of 6.528 AIH patients with a median follow-up of 8 years were evaluated for the incidence of HCC. The pooled incidence rate was 3.1 per 1.000 person-years in AIH patients that tripled in those with cirrhosis[26,27].
PBC-related cirrhosis has also been reported as a potential HCC risk factor. In a study of 273 PBC-related cirrhotic patients, followed for 3 years, the incidence rate was 5.9%, significantly higher in males with stage III/IV disease than in females[28].
In a systematic review of 17 studies, including 16.368 patients seen between 1984 and 2011, compared with the general population, PBC patients exhibited a significantly higher risk of HCC (pooled risk ratio 18.80; 95% confidence interval: 10.81-26.79)[29].
Factors associated with an increased risk of HCC development in chronic liver disease have been only partially defined. However, chronic inflammation has been reported as a crucial mechanism for the development of HCC[30-32].
In this respect, it has been reported that in HBV and HCV cirrhotic patients, transaminase serum level is one of the predictive factors for the development of HCC[32]. Similar findings have been reported in AIH as persistent elevation of serum transaminases was reported to be associated with development of HCC hence supporting the prominent pathogenic role of chronic inflammation. Other emerged risk factors included cirrhosis ≥ 10 years, portal hypertension, and immunosuppressive therapy ≥ 3 years[33].
The risk factors of HCC usually lead to a unresolving inflammatory response and necrosis resulting in tissue damage which in turn drives the sequential development of regeneration, fibrosis, cirrhosis, and eventually HCC[34,35]. In parallel, immune cells within the premalignant environment produce a wide range of cytokines, growth factors, chemokines, prostaglandins, and proangiogenic factors, contributing to an environment that supports hepatocyte transformation and promotes their survival through activation of anti-apoptotic pathways, neoangiogenesis and inhibition of immune surveillance[36].
It has been recently established that the carcinogenic process is aided by a host of immuno-related factors intrinsically linked to cell infiltrate, chemokines and their receptors that foster cell survival and proliferation[37].
In this regard, it has increasingly gained relevance to fully define the immunological characteristics of liver immune microenvironment.
The liver can be considered as an “immunological” organ, housing a wide range of resident immune cells performing key functions in preserving organ homeostasis[38].
As a result of its intrinsic role in detoxification, the liver is repeatedly exposed to external agents, including dietary products or commensal bacteria derived from the intestine via the portal vein, as well as infectious microorganisms arising from the systemic circulation via the arterial vein. Therefore, immune surveillance in the organ is extremely dynamic.
Resident innate immune cells comprising macrophages or Kupffer cells, natural killer (NK) cells, NKT cells, and dendritic cells (DCs) are recognized as the most predominant sentinels in the liver[39]. In addition, tissue resident memory T cells, which are normally homing cells without recirculating and which readily attack pathogens at the site of infection, are also implicated[40]. However, a key role appears to be played by resident Tregs that are highly specialized in preserving tissue tolerance[19,41]. Tregs are a subset of T lymphocytes that regulate the immune response by suppressing the proliferation and cytokines production of effector T lymphocytes[42,43].
In 2003, the forkhead box transcription factor foxp3 was identified as a specific marker of Tregs, and its expression was found crucial for their suppressive activity[44-46].
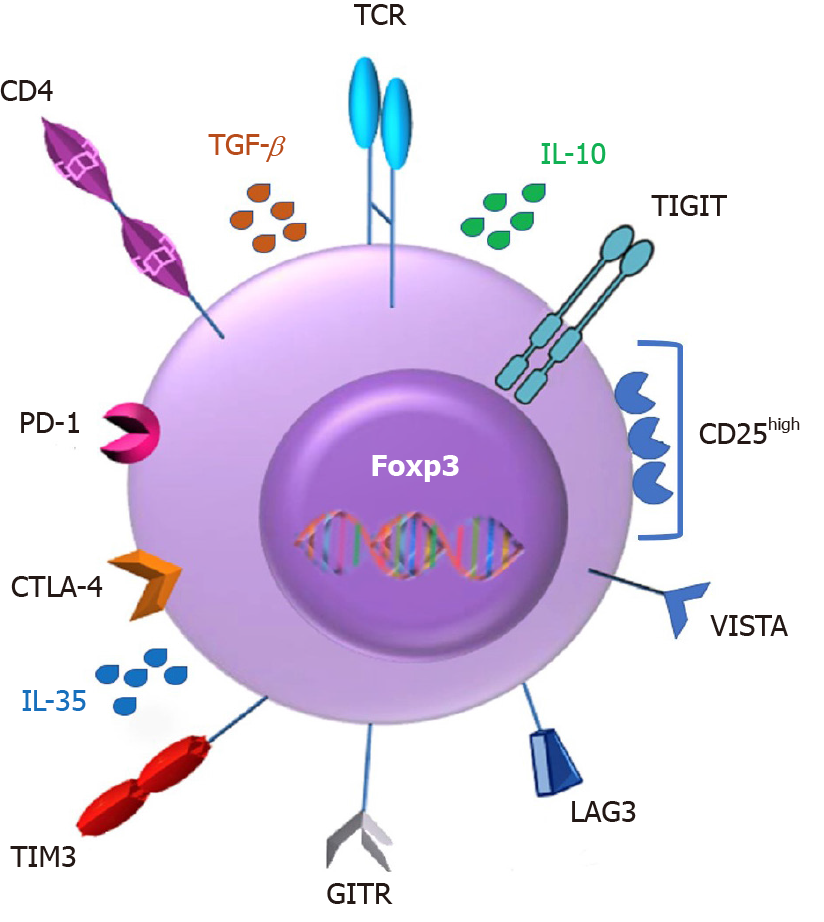
Tregs arise in the thymus, constitutively express high levels of the interleukin (IL)-2 receptor (IL-2R) α chain (CD25), cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), and glucocorticoid-induced TNF receptor family-related gene (GITR), accounting for 5% to 10% of peripheral CD4+ T cells[47,48].
They play a critical role in mediating immunological self-tolerance by suppressing self-reactive T lymphocytes[44]. Tregs have been proposed to operate both through core mechanisms of suppression, including IL-2 deprivation and CTLA-4–mediated downregulation of costimulatory molecules on antigen presenting cells (APCs), and by diverse context-dependent mechanisms, including the secretion of cytokines[49-52]. Earlier studies have confirmed that Tregs mediate suppressive effects in vivo mainly through the production of inhibitory cytokines such as IL-10, IL-35 and transforming growth factor β (TGF-β)[53-55].
With the discovery of Tregs and the understanding of their immunosuppressive role, evidences have been accumulated that this cell population is decisively implicated in the pathogenesis of various conditions such as chronic viral and autoimmune liver diseases as well as HCC[56].
In particular, CD4+CD25+ Tregs are thought to contribute to the impaired immune response during chronic HBV and HCV infection. Patients with chronic HBV infection are characterized by increased percentage of CD4+CD25+ Tregs in their peripheral blood and a significant accumulation of these cells in the liver, with a positive correlation between their frequency and serum HBV DNA load[57-60]. Similarly, in patients with persistent HCV infection it has been reported an increased frequency of CD4+CD25+ Tregs in the blood and in the liver[61-65]. Taken together, these data prove that chronic HBV and HCV infections are immunologically characterized by a host immune response suppression driven by Tregs.
On the contrary, autoimmune liver diseases are related to both numerical and functional defect of CD4+CD25+ Tregs, to the extent that therapeutic interventions aimed at restoring an adequate number and function of these cells are followed by a remission of the autoimmune inflammatory activity[66,67].
These findings have thus outlined a pattern of chronic inflammation characterized by a diametrically opposed liver immune phenotype in chronic viral and autoimmune diseases, the former being characterized by a predominance of Tregs exerting an immunosuppressive effect that hinders the antiviral response and infection eradication, the latter by a significant numerical and functional deficiency of Tregs that do not adequately suppress self-reactive lymphocytes[68-70].
While it has been widely reported that TME characterizing HCC during chronic viral liver disease is dominated by a marked Treg infiltration likely in continuity with the conditions favoring chronic infection, the expression pattern of Tregs in the TME supporting HCC development in autoimmune liver disease is not as well known[71,72].
This finding would be relevant for the assessment of potential adverse events related to emerging immunotherapies that cause a decline in Treg number and function and are therefore associated with the risk of triggering autoimmune disorders, as we discuss below.
Tumor infiltrating lymphocytes represent the host immune response to cancer and comprise CD8+ cytotoxic T lymphocytes (CTLs) and NK cells as favorable anti-tumor responders, and CD4+ CD25+ Tregs as immunosuppressors.
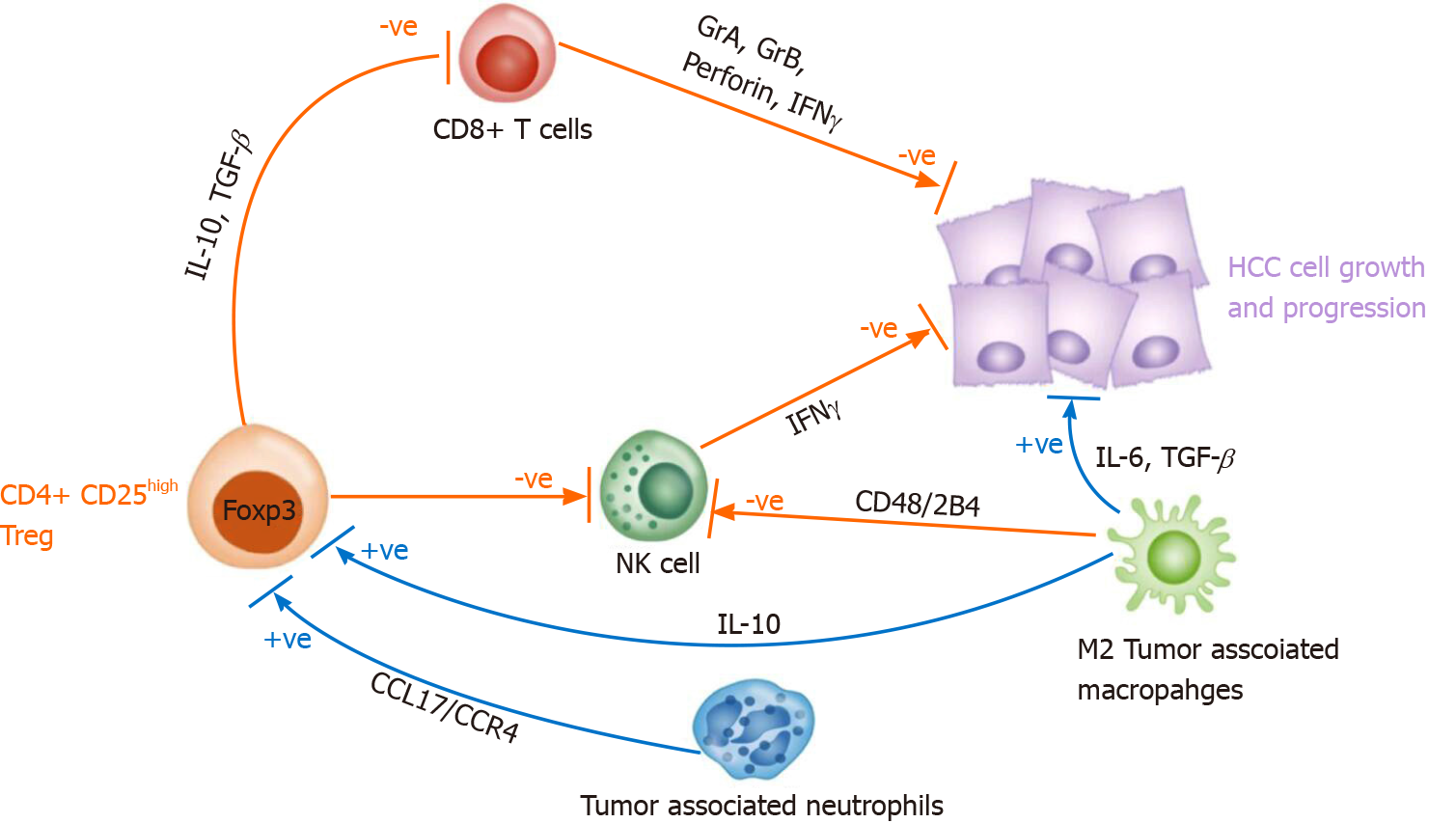
Recently, many studies have shown that the TME plays a major role in HCC initiation and progression[73]. Lymphocytes contribute to the TME through immunity and inflammation. CD8+ CTLs can directly kill target cells by releasing granules including membrane-lytic materials such as perforin and granzymes (granzyme A and B) in acquired immune responses, thus covering a crucial role in anti-tumor immunity. As a matter of fact, a large presence of CD8+ CTL infiltrating tumor tissue is closely associated with a better prognosis[74,75]. However, despite T cell infiltration, HCC develops and spreads as a result of a depletion of pro-inflammatory T cells and a significant accumulation of Tregs[76-78].
T-cell exhaustion is characterized by reduced responses to stimulation, impaired cytokine production, decreased proliferation and reduced toxicity. Such immune profile is hallmarked by over-expression of co-inhibitory receptors such as CTLA-4 and programmed cell-death 1 receptor (PD-1).
An exhausted state of circulating and intratumoral CD8+ T cells is associated with a worse prognosis in HCC patients[79,80].
Exhaustion inside the TME is dominated by the inhibitory cytokine environment rich in IL-10 and TGF-β released by the Treg, both prohibiting CTLs and TH1 CD4+ T cells activation[81,82].
In addition, tumor associated neutrophils (by secreting CCL17 and CCR4) and M2 tumor-associated macrophages (by secreting IL-10) can induce CD4+ CD25+ Tregs thereby indirectly supporting tumor growth and progression[83,84] (Figure 1).
A significant advance in the field of immunotherapy has now reached with the use of immune checkpoint inhibitors (ICIs), which are antagonistic antibodies that inhibit key immune regulatory molecules (checkpoint molecules), such as CTLA-4, PD-1, and its ligand PD-L1, which suppresses T cell effector function under physiological conditions[85].
Tregs are among the most prevalent suppressor cells in TME and their presence has been related to tumor progression, invasiveness, and metastasis. Their regulatory function involves a broad spectrum of immune cells besides T cells, including macrophages, DCs, neutrophils, NK cells, T cells, and innate lymphoid cells[86-88].
There are many factors favoring Treg enrichment in the TME. Experimental evidences implicate the Treg recruitment within the tumor mass through chemokines produced by cancer cells and, specifically, HCC cells have been shown to secrete CCL5, CCL22 and CCL28 chemokines mediating Treg accumulation (Table 1)[89-100].
| Treg function in HCC | Ref. | |
| Molecule | Target | |
| IL-10 | IL-10R | Marra and Tacke[90] |
| IL-35 | IL-12Rβ2 | Shen et al[91] |
| TGF-β | TGF-βR | Fu et al[92] |
| CTLA-4 | CD80/CD86 | Chen et al[93] |
| CD39-CD73 | ATP | Chen et al[94] |
| IL-2 Rα (CD 25) | IL-2 | Li et al[95] |
| LAG3 | MHC class IImolecules | Cabrera et al[96] |
| Treg recruitment in HCC | Ref. | |
| Molecule | Receptor | |
| CCL22 | CCR4 | Li et al[97] |
| CCL5 | CCR5 | Cheng et al[98] |
| CCL28 | CCR3, CCR10 | Singh et al[99] |
The mechanisms through which Tregs induce suppression of proliferation, activation and function of immune effector cells have been well studied. Firstly, they modulate the activity of APCs by engaging inhibitory co-stimulatory receptors on their surface and in this way, signaling between APCs and T cells is impaired or abolished[101,102].
On a similar line, they down-regulate the expression of CD40, CD80 and CD86 on DCs[103]. Second, Tregs, through the secretion of inhibitory cytokines (e.g., IL-10, IL-35, TGF-β), repress the activity of immune cells[104].
Of major interest, Tregs express a panel of chemokine receptors and surface molecules such as CTLA4, PD-1 and others, thus potentially making them a very direct target of ICI immunotherapy (Figure 2).
Advances in understanding tumor immunity have resulted in a significant shift in the HCC treatment, with the emergence of immunotherapy where therapeutic interventions are used to target immune cells rather than cancer cells.
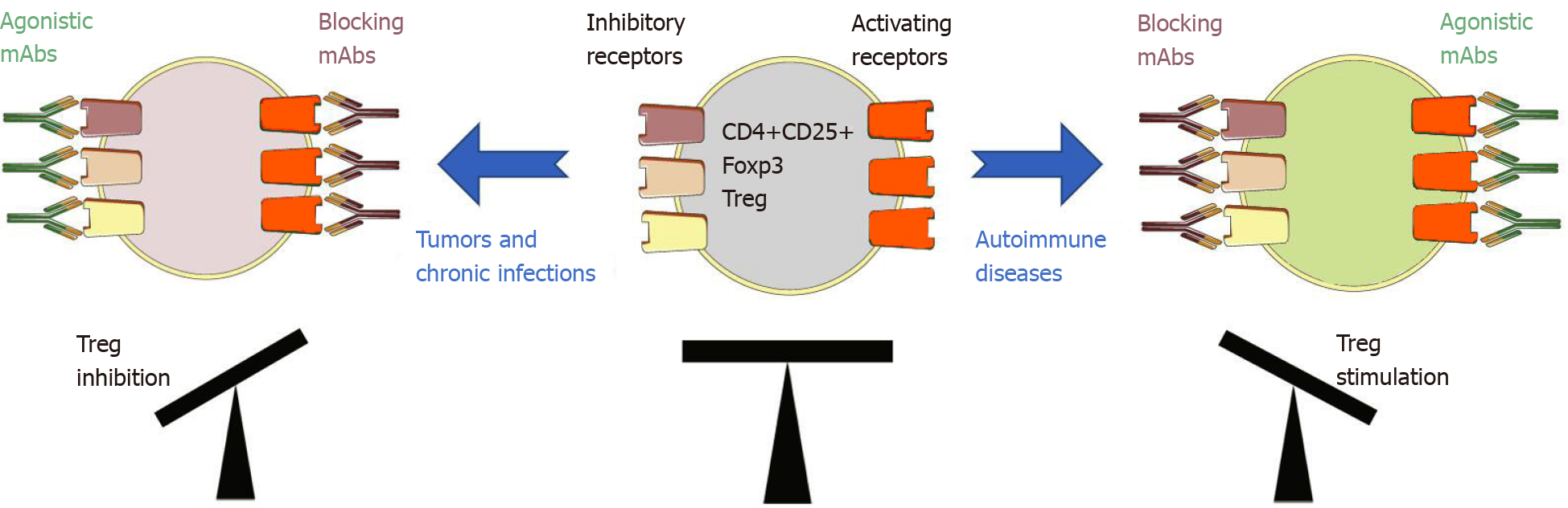
Tregs abundantly express both co-inhibitory and co-stimulatory molecules at levels that are likely dependent on the TME. Modulating their function through stimulation of inhibitory receptors and inhibition of activating receptors could therefore result in a decrease of the TME immunosuppressive profile resulting ultimately in an enhanced antitumor immune response (Figure 3).
In support of this therapeutic approach, treatment with blocking antibodies for PD-1 (nivolumab, pembrolizumab, sintilimab, penpulimab, camrelizumab, toripalimab, spartalizumab, tislelizumab), programmed death ligand 1 (durvalumab, avelumab, atezolizumab), and CTLA-4 (tremelimumab, ipilimumab) have reported promising results in HCC treatment[105].
Since CTLA-4 is constitutively expressed on Tregs, its specific deletion is associated with a marked reduction of their suppressive ability potentially resulting in a severe T cell mediated autoimmune disease[106].
Resulting in a break of balance in the immune system, ICI treatments can give rise to a broad spectrum of serious autoimmune manifestations, including liver injury, reported in recent studies as immune-related adverse events (irAEs) and potentially limiting or precluding HCC treatments[107,108].
Unfortunately, clinical studies that include in their objectives the assessment of immune cell populations, particularly Tregs, are still too scarce, thus leaving this potentially relevant issue unresolved. Clinical trials based on ICI agents as treatment for HCC are reported in Table 2.
| Clinical trials identifier | Official title | Phase | Secondary outcome | Intervention/treatment | Status |
| NCT04518774 | The safety assessment of ex vivo expanded allogeneic γδT cells in hepatocellular carcinoma patients in phase 1 clinical trial | I | Number and phenotype of γδT cells in peripheral blood | Patients will receive 3 cycles of ex vivo expanded allogeneic γδT cells treatments, at four-weeks' intervals, each cycle has 2 infusions. Ex vivo expanded γδT cells are transfused to patients in a dosage escalated manner (dose escalation, 1 × 107, 3 × 107, 9 × 107 per kg of body weight) | Recruiting |
| NCT03841201 | IMMUNIB: An open-label, single-arm phase II study of immunotherapy with nivolumab in combination with lenvatinib for advanced stage | II | Immune cell infiltrates FOXP3 expression | Lenvatinib peroral qd (8 mg for patients with body weight < 60 kg and 12 mg for patients with body weight ≥ 60 kg); Nivolumab i.v. q2w (240 mg fixed dose IV) max. 36 cycles | Recruiting |
| NCT04777708 | Pilot feasibility study of intratumoral BO-112 in combination with pembrolizumab for advanced hepatocellular carcinoma | I | Intratumoral CD4+, CD8+ expression and cluster of differentiation 56 (CD56+) expression (natural killer cells) | Patients receive pembrolizumab IV over 30 min on day 1 of odd number cycles. Patients also receive BO-112 by intratumoral injection on day 1, 8, and 15 of cycle 1, and day 15 of subsequent cycles. Treatment repeats every 3 wk for up to 17 cycles in the absence of disease progression or unacceptable toxicity | Not yet recruiting |
| NCT04721132 | An open-label, phase II, pre-operative study of atezolizumab plus bevacizumab for resectable hepatocellular carcinoma | II | To measure baseline and longitudinal changes of immune infiltration including CD8/regulatory T cell ratio and CD68+ density, and fibrosis stage | Patients receive atezolizumab IV over 30-60 min and bevacizumab IV over 30-90 min on day 1. Treatment repeats every 21 d for up to 3 cycles in the absence of disease progression or unacceptable toxicity. Patients then undergo surgery during week 12 | Recruiting |
| NCT00396682 | Elimination of CD4+CD25+ regulatory T cells in patients with advanced hepatocellular carcinoma after treatment with cyclophosphamide | I | Function and Phenotype of CD4+CD25+ regulatory T cells | Cyclophosphamide 150 mg to 250 mg to 350 mg | Completed |
The development of irAEs, potentially affecting multiple organs, following loss of self-tolerance, has been widely reported[109,110].
Among the possible adverse events, of particular relevance is the development of liver toxicity as it could cause worsening of liver function in patients who almost always have underlying preexisting chronic liver disease[107].
Hepatic toxicity associated with ICI is characterized by elevation of liver parameters values. The pattern of liver enzymes elevation is defined by the increase of alanine aminotransferase (ALT) or alkaline phosphatase (ALP) alone above a specific threshold or by the ratio of serum ALT to ALP levels [R value = [ALT/upper normal level (UNL)]/(ALP/UNL)] and can be categorized as hepatocellular (ALT ≥ 5-fold above UNL or R > 5), mixed (R > 2 to < 5), or cholestatic (ALP ≥ 2 fold above UNL or R < 2)[111].
The pattern of ICI-related liver toxicity is heterogenous since it may be cytolytic, cholestatic or mixed, although ICI-related cholestasis seems to be rarer[107].
In HCC patients receiving ICIs the incidence of liver toxicity ranges according to the type of drug and the dose received. It has been reported that liver toxicity is more frequent in HCC patients receiving anti-CTLA-4 therapies. In HCC patients receiving the anti-PD-1 antibody nivolumab (CHECKMATE 040 trial) and in those receiving another anti-PD-1 antibody pembrolizumab (KEYNOTE-224 trial), ALT elevation of any grade and of grade ≥ 3 was found in 15% and 6% (nivolumab) vs 9% and 4% (pembrolizumab), respectively[112,113].
Differently, therapy with the anti-CTLA-4 antibody tremelimumab was associated with an ALT elevation of any grade and of grade ≥ 3 in 19% and 9% of patients, respectively[114].
Interestingly, during HCC clinical studies, HCV and HBV positive patients exhibited a reduction in viral load during immunotherapy, more pronounced with anti-CTLA-4 agents, thus suggesting that the treatment induced immunological shift has favorable effect on antiviral response[112-116].
No definitive data has been reported about patients with autoimmune liver diseases treated with ICIs. However, in most of the HCC clinical trial, pre-existing autoimmune diseases was a contraindication for enrollment in light of previous data demonstrating that immunotherapies might trigger a flare-up of pre-existing autoimmune disease or the onset of additional immune-related disease[117-119].
No data are available concerning genetic and autoantibody profile of patients before starting ICI treatments, however autoantibodies such as antinuclear and anti-smooth muscle have been reported in patients after ICI-induced liver toxicity onset.
A management protocol for patients experiencing liver toxicity due to ICI treatment administered for non-liver tumors has been proposed and is based on corticosteroids or mycophenolate mofetil/tacrolimus (for patients not improving under corticosteroids), and ursodeoxycholic acid for those with a predominant cholestasis[120].
Increasing understanding of the immunologic mechanisms that characterize the TME of HCC has led to a better insight into the pathogenesis of HCC and its link to chronic inflammation and cirrhosis. As well as, the different prevalence in viral vs non-viral liver diseases, particularly autoimmune, confirms the key role of the liver immune microenvironment.
Although the precise pathogenetic mechanisms of irAEs remain largely undefined, several processes have been proposed to be involved in the development of irAEs such as genetic factors, gut microbiome, epitope spreading, and cross-presentation of neoantigens[121,122]. A thorough evaluation on the role of Tregs in the pathogenesis of irAEs is crucial. Since cancer and autoimmunity constitute two sides of the same coin, it is perhaps not surprising that when we manipulate the immune system to treat cancer through the use of checkpoint therapy, we inevitably unbalance the vital mechanisms that regulate self-tolerance, inducing a number of irAEs. This is, at least in part, related to the impairment of Treg homeostasis, which is crucial for maintaining immune tolerance[123].
Currently used ICIs may also target Tregs, since several checkpoint molecules including CTLA4 and PD-1 are highly expressed on their surface, therefore it is possible that the development of irAEs may be in part attributed to the Treg destabilization. Consistent with this line, it has been demonstrated that anti-CTLA4 disrupts the crosstalk between Foxp3 Tregs and antigen-presenting cells to promote autoimmunity[124]. In light of the effects of immunotherapy on the enhancement of the immune response, it should be investigated whether in cases of HCC occurring in patients with pre-existing autoimmune liver and non-liver diseases these therapies are safe and not potentially hepatotoxic, since presently this information is lacking due to the exclusion of patients with autoimmune diseases from clinical trials. Of interest, other approaches to target the immunosuppressive effect of Tregs are ongoing. Given the TGF-β mediated inhibitory role in HCC development and progression, studies are ongoing to assess how to target TGF-β. Galunisertib, an inhibitor of TGF-β, is currently in a phase II clinical trial for HCC patients[125,126].
Due to the potential effect of immunotherapies on Tregs and the possible effect of triggering autoimmune disorders, it is desirable that in future HCC trials the autoantibody profile, as well as genetic background (HLA) and change in the percentage of peripheral Treg during therapy be monitored to assess whether these variables predict the risk of hepatotoxicity or extrahepatic autoimmune disorders development.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Association for The Study of Liver Diseases, No. 10678.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen W, Shen L S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | International Agency for Research on Cancer. Global Cancer Observatory. [cited 10 Febraury 2021]. In: International Agency for Research on Cancer [Internet]. Available from: https://gco.iarc.fr/today/home. |
| 2. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264-1273. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2499] [Article Influence: 192.2] [Reference Citation Analysis (2)] |
| 3. | White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017; 152: 812-820. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (1)] |
| 4. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 771] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 5. | Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651-5661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 650] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 7. | Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, Sumpter K, Stewart S, Rose J, Hudson M, Manas D, Reeves HL. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 432] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 8. | Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, Sood GK. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL, Morishima C, Lindsay KL, Lok AS; HALT-C Trial Group. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Santi V, Buccione D, Di Micoli A, Fatti G, Frigerio M, Farinati F, Del Poggio P, Rapaccini G, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F, Giannini EG, Caturelli E, Chiaramonte M, Bernardi M, Trevisani F. The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J Hepatol. 2012;56:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis. 2015;61:730-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | Rigopoulou EI, Dalekos GN. Current Trends and Characteristics of Hepatocellular Carcinoma in Patients with Autoimmune Liver Diseases. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21357] [Article Influence: 2135.7] [Reference Citation Analysis (3)] |
| 14. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 708] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 15. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3222] [Article Influence: 460.3] [Reference Citation Analysis (1)] |
| 16. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6019] [Article Influence: 859.9] [Reference Citation Analysis (3)] |
| 17. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 734] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 18. | Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 779] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 19. | Oo YH, Sakaguchi S. Regulatory T-cell directed therapies in liver diseases. J Hepatol. 2013;59:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Granito A, Muratori P, Ferri S, Pappas G, Quarneti C, Lenzi M, Bianchi FB, Muratori L. Diagnosis and therapy of autoimmune hepatitis. Mini Rev Med Chem. 2009;9:847-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Kole C, Charalampakis N, Tsakatikas S, Vailas M, Moris D, Gkotsis E, Kykalos S, Karamouzis MV, Schizas D. Immunotherapy for Hepatocellular Carcinoma: A 2021 Update. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1789] [Article Influence: 85.2] [Reference Citation Analysis (2)] |
| 23. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4079] [Article Influence: 582.7] [Reference Citation Analysis (6)] |
| 24. | Tarao K, Nozaki A, Ikeda T, Sato A, Komatsu H, Komatsu T, Taguri M, Tanaka K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019;8:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 25. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1490] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 26. | Tansel A, Katz LH, El-Serag HB, Thrift AP, Parepally M, Shakhatreh MH, Kanwal F. Incidence and Determinants of Hepatocellular Carcinoma in Autoimmune Hepatitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2017; 15: 1207-1217. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Lleo A, de Boer YS, Liberal R, Colombo M. The risk of liver cancer in autoimmune liver diseases. Ther Adv Med Oncol. 2019;11:1758835919861914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Jones DE, Metcalf JV, Collier JD, Bassendine MF, James OF. Hepatocellular carcinoma in primary biliary cirrhosis and its impact on outcomes. Hepatology. 1997;26:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Liang Y, Yang Z, Zhong R. Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology. 2012;56:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N, Totsuka S. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. 1999;86:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, Lu SN, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL–HBV) Study Group. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011; 141: 1240-1248, 1248.e1-1248. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 32. | Ishikawa T, Ichida T, Yamagiwa S, Sugahara S, Uehara K, Okoshi S, Asakura H. High viral loads, serum alanine aminotransferase and gender are predictive factors for the development of hepatocellular carcinoma from viral compensated liver cirrhosis. J Gastroenterol Hepatol. 2001;16:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am J Gastroenterol. 2008;103:1944-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Shi JH, Line PD. Effect of liver regeneration on malignant hepatic tumors. World J Gastroenterol. 2014;20:16167-16177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 35. | Li H, Zhang L. Liver regeneration microenvironment of hepatocellular carcinoma for prevention and therapy. Oncotarget. 2017;8:1805-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 37. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11263] [Article Influence: 489.7] [Reference Citation Analysis (2)] |
| 38. | Ficht X, Iannacone M. Immune surveillance of the liver by T cells. Sci Immunol. 2020;5:eaba2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Lian M, Selmi C, Gershwin ME, Ma X. Myeloid Cells and Chronic Liver Disease: a Comprehensive Review. Clin Rev Allergy Immunol. 2018;54:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJW, Burton AR, Stegmann KA, Schurich A, Swadling L, Gill US, Male V, Luong T, Gander A, Davidson BR, Kennedy PTF, Maini MK. IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med. 2017;214:1567-1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 41. | Piconese S, Cammarata I, Barnaba V. Viral hepatitis, inflammation, and cancer: A lesson for autoimmunity. J Autoimmun. 2018;95:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1907] [Cited by in RCA: 1955] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 43. | Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 446] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 44. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5853] [Article Influence: 266.0] [Reference Citation Analysis (0)] |
| 45. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6077] [Cited by in RCA: 6380] [Article Influence: 290.0] [Reference Citation Analysis (0)] |
| 46. | Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337-342. |
| 47. | McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1063] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 48. | Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1292] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 49. | Wing JB, Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 51. | Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113-10118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 558] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 52. | von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 770] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 53. | Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 359] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 54. | Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1466] [Cited by in RCA: 1560] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 55. | Chen X, Du Y, Lin X, Qian Y, Zhou T, Huang Z. CD4+CD25+ regulatory T cells in tumor immunity. Int Immunopharmacol. 2016;34:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 56. | Zheng MH, Gu DN, Braddock M, Leishman AJ, Jin C, Wen JS, Gong YW, Chen YP. CD4+ CD25+ regulatory T cells: a therapeutic target for liver diseases. Expert Opin Ther Targets. 2008;12:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, Tang Z, Fu YX, Wang FS. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 359] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 58. | Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 59. | Yang G, Liu A, Xie Q, Guo TB, Wan B, Zhou B, Zhang JZ. Association of CD4+CD25+Foxp3+ regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int Immunol. 2007;19:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008;123:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 61. | Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 430] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 63. | Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsäcker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860-7867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 64. | Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852-7859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 65. |
Granito A, Maldini MC, Muratori P, Chiesa D, Bassi M et al Increased percentage of CD4+CD25high T cells in chronic hepatitis C patients correlates with higher viremia levels.
|
| 66. | Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, Hussain MJ, Ma Y, Lenzi M, Mieli-Vergani G, Bianchi FB, Vergani D, Muratori L. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 67. | Longhi MS, Meda F, Wang P, Samyn M, Mieli-Vergani G, Vergani D, Ma Y. Expansion and de novo generation of potentially therapeutic regulatory T cells in patients with autoimmune hepatitis. Hepatology. 2008;47:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Bonilla CM, McGrath NA, Fu J, Xie C. Immunotherapy of hepatocellular carcinoma with infection of hepatitis B or C virus. Hepatoma Res. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Chaoul N, Mancarella S, Lupo L, Giannelli G, Dituri F. Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Lapierre P, Lamarre A. Regulatory T Cells in Autoimmune and Viral Chronic Hepatitis. J Immunol Res. 2015;2015:479703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 72. | Kurebayashi Y, Kubota N, Sakamoto M. Immune microenvironment of hepatocellular carcinoma, intrahepatic cholangiocarcinoma and liver metastasis of colorectal adenocarcinoma: Relationship with histopathological and molecular classifications. Hepatol Res. 2021;51:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Bian J, Lin J, Long J, Yang X, Lu X, Sang X, Zhao H. T lymphocytes in hepatocellular carcinoma immune microenvironment: insights into human immunology and immunotherapy. Am J Cancer Res. 2020;10:4585-4606. [PubMed] |
| 74. | Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 75. | Ding W, Xu X, Qian Y, Xue W, Wang Y, Du J, Jin L, Tan Y. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2018;97:e13301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 76. | Chiou SH, Sheu BC, Chang WC, Huang SC, Hong-Nerng H. Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol. 2005;67:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 891] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 78. | Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 79. | Ma J, Zheng B, Goswami S, Meng L, Zhang D, Cao C, Li T, Zhu F, Ma L, Zhang Z, Zhang S, Duan M, Chen Q, Gao Q, Zhang X. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 80. | Tauber C, Schultheiss M, Luca R, Buettner N, Llewellyn-Lacey S, Emmerich F, Zehe S, Price DA, Neumann-Haefelin C, Schmitt-Graeff A, Hofmann M, Thimme R. Inefficient induction of circulating TAA-specific CD8+ T-cell responses in hepatocellular carcinoma. Oncotarget. 2019;10:5194-5206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019-10026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 82. | Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2593] [Cited by in RCA: 2463] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 83. | Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016; 150: 1646-1658. e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 610] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 84. | Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 85. | Tovoli F, De Lorenzo S, Trevisani F. Immunotherapy with Checkpoint Inhibitors for Hepatocellular Carcinoma: Where Are We Now? Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3892] [Article Influence: 228.9] [Reference Citation Analysis (0)] |
| 87. | Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1343] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 88. | Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol. 2013;10:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 89. | Ren L, Yu Y, Wang L, Zhu Z, Lu R, Yao Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget. 2016;7:75763-75773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 90. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology 2014; 147: 577-594. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 625] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 91. | Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Fu YP, Yi Y, Cai XY, Sun J, Ni XC, He HW, Wang JX, Lu ZF, Huang JL, Cao Y, Zhou J, Fan J, Qiu SJ. Overexpression of interleukin-35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Br J Cancer. 2016;114:767-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol Med. 2019;25:1010-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 94. | Chen X, Du Y, Hu Q, Huang Z. Tumor-derived CD4+CD25+regulatory T cells inhibit dendritic cells function by CTLA-4. Pathol Res Pract. 2017;213:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 95. | Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 96. | Cabrera R, Ararat M, Eksioglu EA, Cao M, Xu Y, Wasserfall C, Atkinson MA, Liu C, Nelson DR. Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol. 2010;72:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Li FJ, Zhang Y, Jin GX, Yao L, Wu DQ. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8(+) T cell in HCC patients. Immunol Lett. 2013;150:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 98. | Cheng X, Wu H, Jin ZJ, Ma D, Yuen S, Jing XQ, Shi MM, Shen BY, Peng CH, Zhao R, Qiu WH. Up-regulation of chemokine receptor CCR4 is associated with Human Hepatocellular Carcinoma malignant behavior. Sci Rep. 2017;7:12362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 99. | Singh SK, Mishra MK, Rivers BM, Gordetsky JB, Bae S, Singh R. Biological and Clinical Significance of the CCR5/CCL5 Axis in Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 100. | Xue D, Zheng Y, Wen J, Han J, Tuo H, Liu Y, Peng Y. Role of chemokines in hepatocellular carcinoma (Review). Oncol Rep. 2021;45:809-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 101. | Caridade M, Graca L, Ribeiro RM. Mechanisms Underlying CD4+ Treg Immune Regulation in the Adult: From Experiments to Models. Front Immunol. 2013;4:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Maj T, Wang W, Crespo J, Zhang H, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, Lyssiotis C, Liu JR, Kryczek I, Zou W. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 558] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 103. | Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, Thompson S, Chen X, Andreansky S, Katsanis E. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. 2007;56:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 104. | Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, Mempel TR. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. 2014;124:2425-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 105. | Lai E, Astara G, Ziranu P, Pretta A, Migliari M, Dubois M, Donisi C, Mariani S, Liscia N, Impera V, Persano M, Tolu S, Balconi F, Pinna G, Spanu D, Pireddu A, Saba G, Camera S, Musio F, Puzzoni M, Pusceddu V, Madeddu C, Casadei Gardini A, Scartozzi M. Introducing immunotherapy for advanced hepatocellular carcinoma patients: Too early or too fast? Crit Rev Oncol Hematol. 2021;157:103167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 106. | Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2013] [Cited by in RCA: 2243] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 107. | De Martin E, Michot JM, Rosmorduc O, Guettier C, Samuel D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep. 2020;2:100170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 108. | McGonagle D, Bragazzi NL, Amital H, Watad A. Mechanistic classification of immune checkpoint inhibitor toxicity as a pointer to minimal treatment strategies to further improve survival. Autoimmun Rev. 2020;19:102456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1566] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 110. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3131] [Article Influence: 447.3] [Reference Citation Analysis (0)] |
| 111. | European Association for the Study of the Liver. ; Clinical Practice Guideline Panel: Chair:; Panel members; EASL Governing Board representative:. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 663] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 112. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3296] [Article Influence: 412.0] [Reference Citation Analysis (1)] |
| 113. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1891] [Article Influence: 270.1] [Reference Citation Analysis (0)] |
| 114. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 639] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 115. | Ravi S, Spencer K, Ruisi M, Ibrahim N, Luke JJ, Thompson JA, Shirai K, Lawson D, Bartell H, Kudchadkar R, Gunter NT, Mehnert JM, Lipson EJ. Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: a multicenter, retrospective case series. J Immunother Cancer. 2014;2:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 116. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 749] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 117. | Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, Champiat S, Aspeslagh S, Haroche J, Albiges L, Massard C, Girard N, Dalle S, Besse B, Laghouati S, Soria JC, Mateus C, Robert C, Lanoy E, Marabelle A, Lambotte O. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 118. | Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med. 2018;168:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 119. | Tison A, Quéré G, Misery L, Funck-Brentano E, Danlos FX, Routier E, Robert C, Loriot Y, Lambotte O, Bonniaud B, Scalbert C, Maanaoui S, Lesimple T, Martinez S, Marcq M, Chouaid C, Dubos C, Brunet-Possenti F, Stavris C, Chiche L, Beneton N, Mansard S, Guisier F, Doubre H, Skowron F, Aubin F, Zehou O, Roge C, Lambert M, Pham-Ledard A, Beylot-Barry M, Veillon R, Kramkimel N, Giacchero D, De Quatrebarbes J, Michel C, Auliac JB, Gonzales G, Decroisette C, Le Garff G, Carpiuc I, Vallerand H, Nowak E, Cornec D, Kostine M; Groupe de Cancérologie Cutanée; Groupe Français de Pneumo-Cancérologie; and Club Rhumatismes et Inflammations. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019;71:2100-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 120. | De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A, Laghouati S, Robert C, Marabelle A, Guettier C, Samuel D. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 121. | Day D, Hansen AR. Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. BioDrugs. 2016;30:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 122. | June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med. 2017;23:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 123. | Sekai I, Hagiwara S, Watanabe T, Kudo M. A case with hepatic immune-related adverse events caused by nivolumab exhibiting impaired accumulation of regulatory T cells. Clin J Gastroenterol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Alissafi T, Banos A, Boon L, Sparwasser T, Ghigo A, Wing K, Vassilopoulos D, Boumpas D, Chavakis T, Cadwell K, Verginis P. Tregs restrain dendritic cell autophagy to ameliorate autoimmunity. J Clin Invest. 2017;127:2789-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 125. | Eli Lilly and Company. A Randomized Phase 2 Study of LY2157299 Versus LY2157299 - Sorafenib Combination Versus Sorafenib in Patients With Advanced Hepatocellular Carcinoma. [cited 10 Febraury 2021]. In: ClinicalTrials.gov [Internet]. Indianapolis (IN): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02178358?term=Galunisertib&cond=Hepatocellular+Carcinoma&draw=2&rank=3 ClinicalTrials.gov Identifier: NCT00417417. |
| 126. | Giannelli G, Mikulits W, Dooley S, Fabregat I, Moustakas A, ten Dijke P, Portincasa P, Winter P, Janssen R, Leporatti S, Herrera B, Sanchez A. The rationale for targeting TGF-β in chronic liver diseases. Eur J Clin Invest. 2016;46:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |