Published online Jun 7, 2021. doi: 10.3748/wjg.v27.i21.2664
Peer-review started: March 17, 2021
First decision: April 5, 2021
Revised: April 14, 2021
Accepted: May 10, 2021
Article in press: May 10, 2021
Published online: June 7, 2021
Processing time: 70 Days and 12.7 Hours
Cystic pancreatic lesions involve a wide variety of pathological entities that include neoplastic and non-neoplastic lesions. The proper diagnosis, differentiation, and staging of these cystic lesions are considered a crucial issue in planning further management. There are great challenges for their diagnostic models. In our time, new emerging methods for this diagnosis have been discovered. Endoscopic ultrasonography-guided fine-needle aspiration cytology with chemical and molecular analysis of cyst fluid and EUS-guided fine needle-based confocal laser endomicroscopy, through the needle microforceps biopsy, and single-operator cho
Core Tip: Pancreatic cystic lesions are commonly recognized with increasing frequency and have become a more common finding in clinical practice. Today, there has been an increased incidence in cystic pancreatic lesions that have in turn caused a lot of diagnostic modalities to emerge. We discuss the various methods of the diagnosis of cystic pancreatic lesions and compare these modalities.
- Citation: Okasha HH, Awad A, El-meligui A, Ezzat R, Aboubakr A, AbouElenin S, El-Husseiny R, Alzamzamy A. Cystic pancreatic lesions, the endless dilemma. World J Gastroenterol 2021; 27(21): 2664-2680
- URL: https://www.wjgnet.com/1007-9327/full/v27/i21/2664.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i21.2664
Currently, pancreatic cystic lesions (PCLs) are commonly recognized with increasing frequency and have become a more common finding in clinical practice; their detection rate is rising with the advances in imaging technology, and there is an increased incidence of detection of unsuspected small PCLs[1]. The frequency of detection of pancreatic cysts reported in the literature ranges from 0.7% to 36.7%[2,3].
They comprise a broad spectrum of entities ranging from benign cysts to potentially malignant or malignant cystic lesions[4,5]; they differ greatly in the clinical behavior and malignant potential that give these lesions a diagnostic challenge[1].
The proper diagnosis, differentiation between neoplastic and non-neoplastic PCLs, and staging of these cystic lesions are considered a crucial issue for planning further management[6]. Endoscopic ultrasonography (EUS) and transpapillary diagnosis are the two major endoscopic approaches used. EUS-guided fine-needle aspiration (FNA) cytology, EUS-guided fine needle-based confocal laser endomicroscopy (nCLE), and needle microforceps biopsy are promising methods that have been used in the differential diagnosis of mucinous and non-mucinous pancreatic cysts[7].
The great benefits of EUS in detecting small lesions, making a differential diagnosis, and tumor staging provide a great challenge as many benign and malignant cystic lesions and also inflammatory cysts have a similar endosonographic appearance. Moreover, the permission of the evaluation of cyst fluid obtained by EUS-FNA for the early diagnosis or prediction of prognosis is a major concern to increase the diagnostic accuracy for more proper management[7].
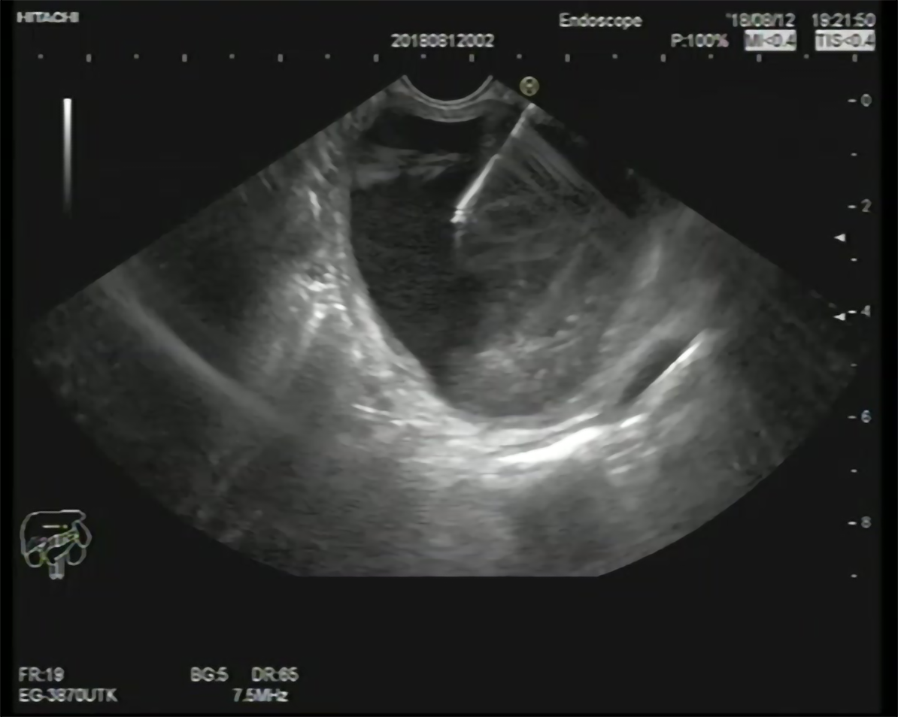
EUS-FNA enables the use of aspirated samples for cytopathology examination and biochemical analyses, which provide an opportunity to further enhance diagnosis and medical decision-making[8] (Figure 1); therefore, the addition of FNA to various imaging modalities has been associated with increasing the diagnostic accuracy of PCLs[9].
The combination of both EUS-FNA findings with cystic fluid tumor markers analysis, along with clinical, radiologic, histologic, genetic, and molecular characteristics, enhances the diagnostic accuracy of PCLs and helps to construct a novel model in the era of cystic pancreatic lesion diagnosis[10].
EUS-guided radiofrequency ablation (EUS-RFA) is now considered an interesting alternative to doing major surgery with high morbidity and mortality for small, mostly benign, pancreatic lesions[11,12].
Owing to the close proximity of the transducer of EUS to the liver, from the transgastric and transduodenal routes, EUS allows good visualization of the liver anatomy and its vasculature by providing detailed images, and this plays a significant role in detecting small-sized liver metastasis from malignant pancreatic lesions, as well as potentially obtaining EUS-FNA with higher diagnostic accuracy[13]. This can be achieved in most liver segments except segment VII and VI which are very difficult to be visualized during EUS examination.
Pancreatic cysts are classified into inflammatory fluid collections and neoplastic and non-neoplastic pancreatic cysts. They may also be related to other diseases such as polycystic kidney disease or Von Hippel Lindau disease[14].
According to the revised Atlanta classification, inflammatory fluid collections are further categorized into acute peripancreatic fluid collections, pseudocysts, acute necrotic collections, and walled-off pancreatic necrosis[15].
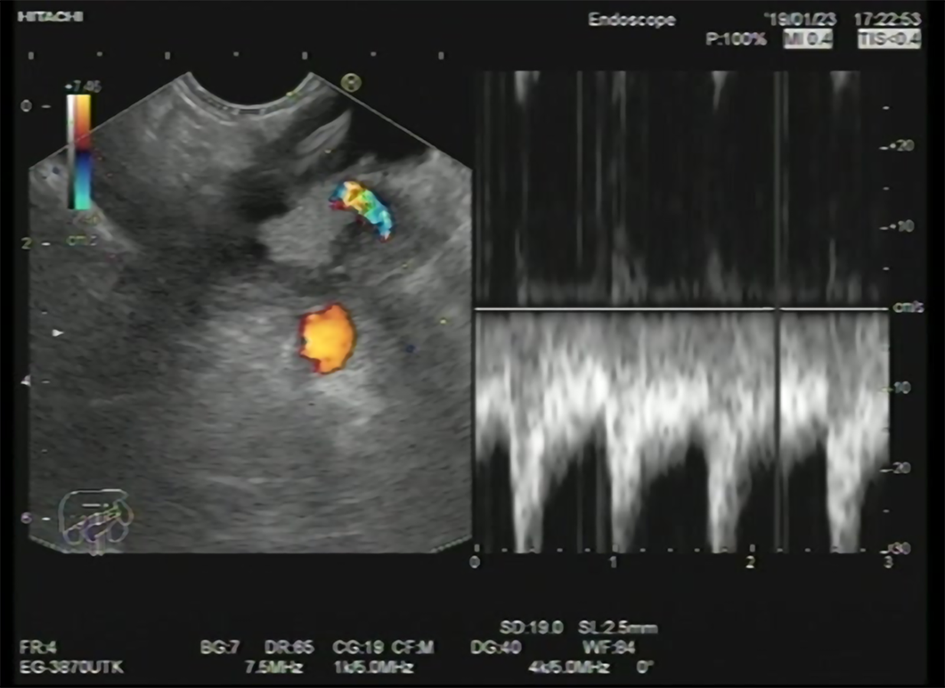
Pancreatic cysts can range from simple cysts to neoplastic ones, so proper diagnosis and management are important. Mucinous cysts (Figure 2), for example, carry unpredictable potential malignant transformation[16]. Various methods are used to diagnose the type of the cystic lesion such as sonographic appearance, cytopathological examination of the cystic fluid, and tumor markers analysis from the cystic fluid.
Some sonographic findings are indicative of malignancy, including a thick wall, septations, and the presence of mural nodules[17]. Still, sonographic appearance or cytopathological examination has a low predictive value for diagnosis[18,19]. Mucin staining, carcinoembryonic antigen (CEA), CA19-9, and amylase levels in the cystic fluid improve the diagnosis of pancreatic cysts[20-22]. A study carried out by Okasha et al[10] shows that the highest AUC was that of cystic CEA, with a cutoff value of 160 ng/mL, and that it had a sensitivity of 60.4% and a specificity of 85%. It also mentioned that the best cutoff value for cystic CA19-9 was 1318 U/mL with a sensitivity of 64.1% and a specificity of 68.1%. The cutoff value of the cyst amylase level was 5500 U/L, with 84.2% sensitivity and 37.1% specificity. The sensitivity of mucin staining in detecting mucinous cystic neoplasm (MCN) was 85.45%, and specificity was 86.05% with an accuracy of 85.87%.
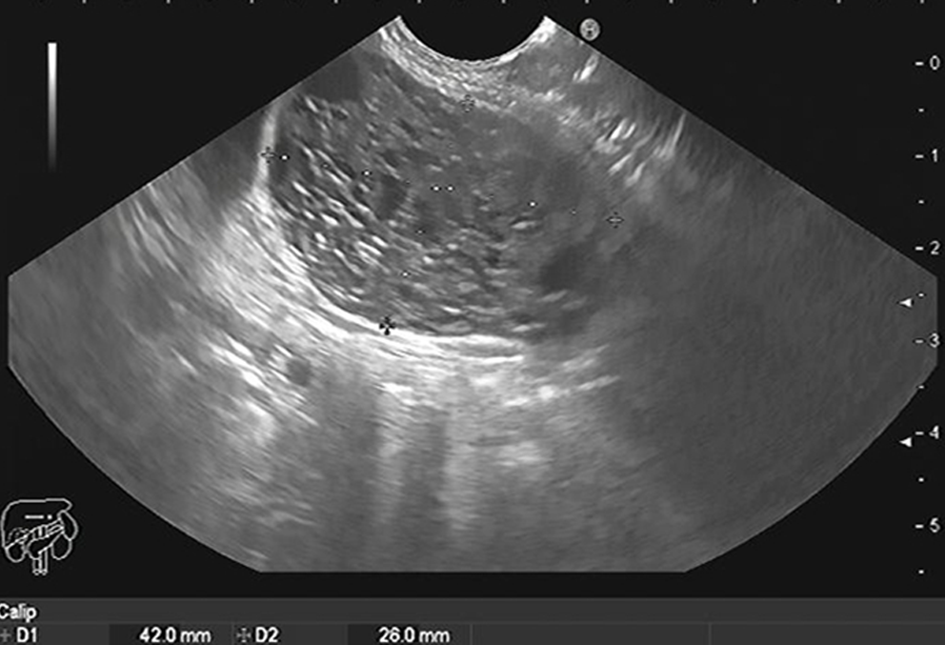
Low CEA with cuboidal epithelial cells, clear cytoplasm, and excess glycogen can diagnose serous cystic lesions (Figure 3)[23]. The presence of mucin, ovarian-like stroma with a degree of cell atypia is mainly found with mucinous pancreatic cysts[24]. Intraductal papillary mucinous neoplasm (IPMN) also produces mucin but is connected to the pancreatic duct with various degrees of dysplasia[23]. Malignant transformation in IPMN can be diagnosed by measuring cyst fluid CA19-9[25,26]. CEA > 192 ng/mL can differentiate between mucinous and non-mucinous cysts[27]. Cystic fluid amylase is helpful in diagnosing pseudocysts, especially if above > 250 IU/L[28], but cannot distinguish mucinous from non-mucinous cysts[29]. Table 1 shows different types of pancreatic cysts.
| Type of cyst | CEA | CA-19-9 | Amylase | Mucin stain |
| Inflammatory pseudocyst | Normal | ↑ | ↑ | Negative |
| Lymphoepithelial cysts | Normal | Normal | Normal | Negative |
| Serous cystic neoplasms | Normal | Normal | Normal | Negative |
| Mucinous cystic neoplasm | ↑ | ↑ | Normal | Positive |
| Intraductal papillary mucinous neoplasm | ↑/Normal | ↑/Normal | ↑/Normal | Positive |
| Solid papillary neoplasm | - | - | - | Negative |
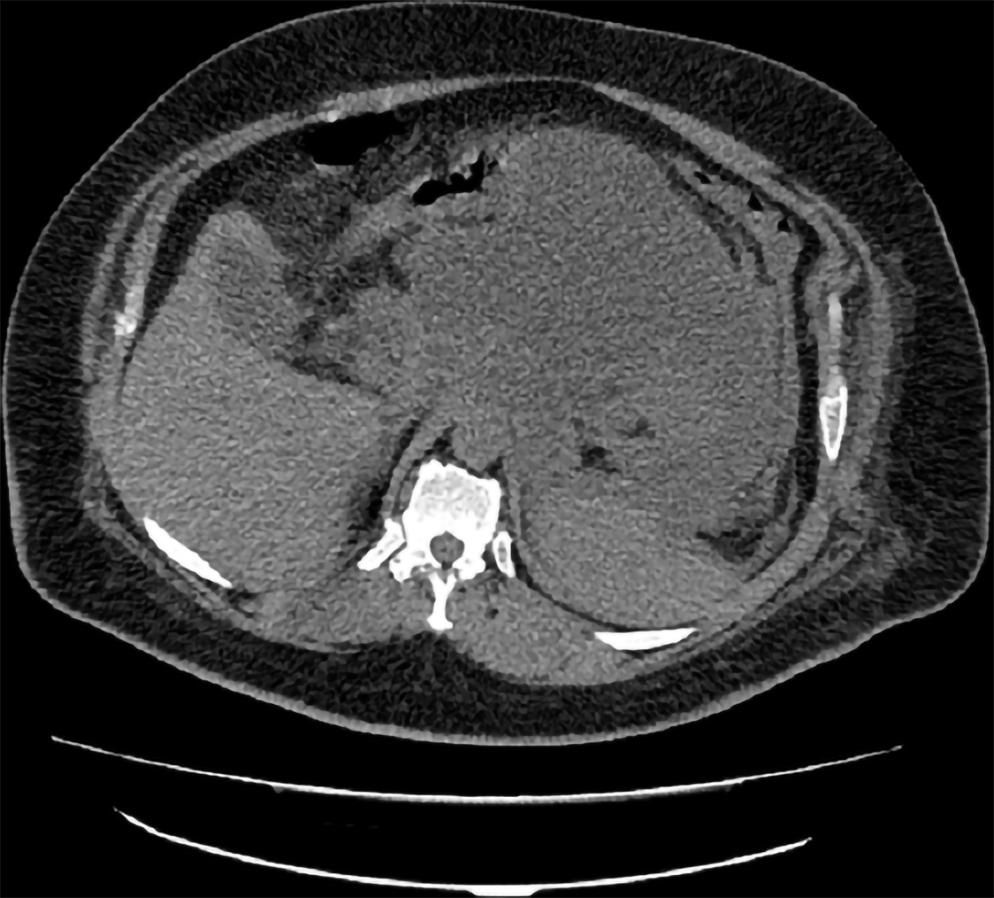
Ultrasound, computed tomography (CT) scan, and EUS are the most commonly used imaging modalities in diagnosing cystic pancreatic lesions. Still, magnetic resonance imaging (MRI), particularly T2-weighted images[30], and magnetic resonance cholangiopancreatography have better results in detecting the communication between pancreatic cysts and the pancreatic duct system, in addition to better detection for the mural nodules[31-35]. Variable data are available for comparing the accuracy of CT and MRI in diagnosing pancreatic cysts. (Figure 4). A study carried out by Visser et al[36] concluded similar accuracy for both modalities. Procacci et al[37] described successful CT diagnosis for pancreatic cysts in 60% of examined cases. Another study done by Abraham et al[38] showed that both modalities carried equal performance accuracy.
Contrast-enhanced ultrasound is one of the most used imaging techniques. According to it, pancreatic lesions are divided into type I, with unilocular cysts; type II, presented as microcystic lesions; type III, with macrocystic lesions; and, lastly, type IV, containing cystic lesions with solid components or an irregularly thickened cystic wall[39]. According to this classification, type I is mostly a pancreatic pseudocyst. Type II represents serous cystadenoma mostly. Type III includes mainly mucinous cystadenoma. Lastly, type IV is for solid pseudopapillary tumors and pancreatic masses with cystic degeneration[40].
Serous cystadenoma usually shows a honeycomb appearance in CT scan and EUS. The presence of central calcification is pathognomonic[41]. It is presented either as oligocystic or microcystic lesion[42] with low malignant potential transformation[43] and can be in any part of the pancreas.
Mucinous pancreatic cysts usually appear as unilocular lesions in a CT scan or EUS with no communication with the main pancreatic duct (MPD)[24]. They mainly appear in the body and tail of the pancreas. They have a high risk of malignant transformation [24] and generally appear in females in the fourth decade with mucin and ovarian-like stroma inside the cystic fluid. The presence of mural nodules during EUS examination favors malignant diagnosis. According to a study by Okasha et al[9], during the examination of 77 patients with PCLs, 11 out of 28 had mural nodules during EUS examination, 9 of which were proved to be mucinous cystadenocarcinoma.
IPMNs are divided into main duct, branched duct, or mixed according to the communication with the main or side branch pancreatic ducts[44]. The lesions are mostly solitary and located in the pancreatic head, but multifocal lesions can be detected[45].
Some pancreatic tumors may exhibit cystic degeneration such as solid pseudopapillary neoplasms, cystic neuroendocrine tumor, and ductal adenocarcinoma[46]. However; the most common are MCNs, IPMNs, serous neoplasms (SCNs), and pseudocysts. In most cases, IPMN is multilocular connected to the pancreatic duct and usually located at the pancreatic head, whereas MCNs are rounded with a thick wall and located at the pancreatic tail. Mural nodules can sometimes be detected in both types of cysts, such as localized epithelial protrusions, and represent high-risk stigmata for both MCNs and IPMNs, but they are not typically found in SCNs. Hence, both IPMNs and MCNs have malignant potential. By contrast, SCNs do not have a predilection toward malignant transformation. Therefore, it is important to determine cyst type and its malignant potential so as to assign a treatment strategy and follow-up plan.
For the diagnosis of cystic lesions of the pancreas, 3 approaches have been described: (1) Diagnosis by EUS that involves morphology of the cyst during EUS, EUS-FNA, EUS-guided fine nCLE, through the needle biopsy (TTNB), and contrast-enhanced harmonic EUS; (2) Endoscopic retrograde cholangiopancreatography (ERCP); and (3) Single-operator cholangioscopy/pancreatoscopy.
EUS became an indispensable tool for the diagnosis of cystic pancreatic lesions and the treatment of various nonmalignant pancreatic cysts. Nevertheless, EUS has significant importance in the early detection of small lesions, and the diagnostic accuracy of EUS imaging ranges from 40% to 96% when compared to histopathological examination of the surgical specimen[16].
In a single prospective study, the sensitivity and specificity were 56% and 45%, respectively. For EUS morphological diagnosis, by differentiating mucinous from non-mucinous cysts, the overall accuracy therefore lowered to 51%[22]. In contrast, EUS was superior to CT for detecting lesions, especially those less than 2 cm in size (83% vs 33%)[47].
In addition, EUS was equivalent to MRI in detecting communications of the PCLs to the MPD, thus differentiating IPMNs from MCNs and SCNs[48]. Furthermore, Binmoeller et al[47], in their consensus guidelines, recommended initial evaluation of IPMNs by EUS regardless of the presence or absence of high-risk stigmata or worrisome features in CT or MRI. In that study, the diagnostic accuracy was 100% when compared to ultrasound, CT, or MRI, which showed a rate of detection of malignant IPMNs at 47%, 53%, and 53%, respectively. Additional reports depicted that EUS is the modality of highest sensitivity for detecting mural nodules in malignant IPMNs[49-51].
Typically, the morphological features of cystic pancreatic lesions are not an independent factor in differentiating malignant from nonmalignant lesions. In addition, recent reports concluded that the appearance of PCLs during EUS evaluation was not sufficient in predicting their malignant potential[52,53].
In their study that included 77 patients with PCLs, Okasha et al[9] conducted FNA guided by either EUS or abdominal ultrasound for over 2 years. As a result, 28 (39.3%) had MCN, and 11 had mural nodules on EUS examination. Nine were proved to be mucinous cystadenocarcinoma, and 2 were diagnosed as IPMN, representing 81.8% of all cases of mucinous adenocarcinoma and 28.6% of all cases of IPMN, respectively. CEA was analyzed in 62 patients (80.5%). The cutoff value of 105 ng/dL showed a statistical difference between mucinous and non-mucinous lesions with a sensitivity and specificity of 80% and 77%, respectively. The reported accuracy was 78% for mucinous lesions. In addition, CA19-9 did not add much in differentiating neoplastic from non-neoplastic lesions. By contrast, the accuracy of positive mucin staining in differentiating between mucinous and non-mucinous, as well as neoplastic and non-neoplastic lesions was 91% and 80%, respectively. On the whole, the afore mentioned study concluded that cyst fluid analysis increases the yield of diagnostic accuracy of the PCL when there is insufficient clinical and poor morphological data using different imaging modalities[9].
In their study, Hawes et al[54] had depicted the essential role of EUS-FNA of the cyst fluid and its analysis regarding CEA, amylase, and cytology for the diagnosis of indeterminate cysts whose potential nature could not be predicted by imaging alone. On the other hand, cyst wall sampling using EUS-FNA may increase the diagnostic yield by 37%[55].
In the meantime, EUS-FNA shows the highest utility for high-risk stigmata for the malignant potential of PCLs, namely mural nodule or mass lesion, cyst size > 3 cm, or MPD dilation during EUS evaluation[52,56-62].
Molecular analysis of cyst fluid has been proposed to increase the diagnostic yield of PCLs. Typically, KRAS mutation analysis has increased the diagnostic accuracy of IPMNs to 81%[63]; when combined with CEA, it increased its sensitivity to 84% for mucinous cysts[64].
A committee member of the International Society of EUS Task Force conducted and drafted a multicenter survey-based study. This survey was distributed among 60 EUS experts in their centers around the world, this survey discussed the role of FNA in cystic pancreatic lesions, the analysis of cyst fluid and its role in differentiating specific types of various PCLs, and the bleeding risk that may complicate this procedure. It has been concluded that the utility of EUS diagnosis of PCLs based on the morphology is limited, but when combined with other imaging modalities such as CT or MRI, as well as FNA, it will lead to augmentation of the diagnostic yield and increased sensitivity and specificity of EUS as a diagnostic tool[7].
Confocal laser endomicroscopy (nCLE) technology has recently been added as a diagnostic tool for PCLs evaluation. It permits visualization of the epithelial lining inside the cyst. In addition, IV fluorescein can be used during EUS-nCLE to further display the blood vessels supplying the cyst as well as other structures such as mural nodules[65].
Furthermore, each cyst type has a characteristic pattern when being visualized during EUS-nCLE. For instance, IPMN has finger-like projections and branched vascularity, whereas MCNs are lined by multiple epithelial layers. Typically, SCNs appear as a densely arranged superficial network of capillary vessels, whereas pseudocysts and SPENs appear as lightly colored inflammatory cells against a dark background and nets of dark cells separated by blood vessels[66].
INSPECT, DETECT, CONTACT-1 and -2, and INDEX trials are recent studies that validated the safety and feasibility of EUS-nCLE and its ability to augment the yield diagnosis of PCLs. In the most recent CONTACT-2 study, which included 78 patients, the sensitivity for EUS-nCLE to diagnose premalignant PCLs from benign lesions was 96%, and specificity was 95%, whereas for positive predictive value, the negative predictive values were 98% and 91%, respectively[67].
By contrast, in their study that evaluated the inter-observer agreement of EUS-nCLE, Karia et al[68] concluded that the inter-observer agreement and accuracy were low and that further studies are needed to validate EUS-nCLE as an accurate diagnostic tool.
Contrast-enhanced EUS (CE EUS) can be a useful and substantial tool in differentiating between different types of pancreatic cystic neoplasms (PCNs). Recently, it has been widely applied. The CE EUS agent is a type of contrast that does not interfere with the interstitial space. As a result, it has the ability to reflect the microcirculation of tissues required for clinical correlation and diagnosis of various PCNs. Recent studies proved that CE EUS can differentiate solid pancreatic lesions of different origins. In their study, Zhong et al[69] revealed that CE EUS had an added accuracy in comparison to CT/MRI and fundamental B-mode EUS (CEEUS vs CT: 92.3% vs 76.9%; CE EUS vs MRI: 93.0% vs 78.9%; CE EUS vs fundamental B-mode EUS: 92.7% vs 84.1%) and that was consistent with previous reports.
TTNB is a method used for better tissue acquisition in pancreatic cysts, which increases the diagnostic yield. Taking a biopsy from the wall of the cystic lesion ensures a better cytological gain. TTNB was first used in 2010 by Aparicio et al[70], associated with a spyglass fiber optic probe. Then, Moray microforceps were introduced in the field and proved better histological diagnosis[71,72].
The cyst is firstly punctured by a 19-gauge EUS-FNA needle. The forceps are then introduced through the fine needle, trans-gastric or trans-abdominal, to reach the wall of the cyst. The jaws of the forceps are pushed openly against the cystic wall and then closed to be pulled back, causing tenting. Crinò et al[73] recommended 3 passes per patient for adequate tissue acquisition and one bite with a “tent sign” for effective grip by the forceps. Yang et al[74] suggested 3 bites per pass, with a total of 3 passes for each case.
Technical failure is attributed to loss of echoendoscope flexibility when both the needle and forceps are introduced or when the site of puncture is difficult. Basar et al[75] recorded failed biopsy in 9.6% of the cases. Aparicio et al[70] attributed inadequate material to clots in 10 cases and amorphous materials in another 5 investigated cases. Kovacevic et al[76] linked failure to difficult transgastric puncture and difficult manipulation with a maximally flexed echoendoscope.
ERCP is not routinely required for the evaluation of PCLs. Nevertheless, duodenoscopy may reveal some specific findings in the main-duct IPMN, such as mucinous material flowing out of the patulous pancreatic duct (Fish Mouth appearance), which is seen in 20% to 55% of patients with main-duct IPMNs and is considered a pathognomonic finding for this type of IPMN[77-80].
Pancreatoscopy is an emerging alternative for direct visualization of stones, tumors, and mucus, and when combined with an intraductal ultrasound, its accuracy in differentiating between benign and malignant IPMN reached 88% in one study[77].
ERCP-guided tissue acquisition, whether by brush cytology, biopsy specimens of fixed filling defects or strictures, or random biopsy specimens of dilated duct walls, is used for the evaluation of PCLs. Although ERCP tissue sampling has a relatively low diagnostic yield, transpapillary biopsy with standard or pediatric-sized forceps resulted in a diagnosis of 11 out of 13 patients with IPMN in one study[78].
Pancreatoscopy using a video scope with narrow-band imaging or via a fiberoptic probe has been described in several reports to acquire issue or cytology[81-84].
A mother-baby endoscope was used for a while during pancreatobiliary examination but had many problems. Single-operator cholangioscopy/pancreatoscopy has succeeded in overcoming the problems faced by the mother-baby endoscope: high cost, fragile baby scope, and absence of an irrigation channel. In addition to that, single-operator cholangioscopy/pancreatoscopy has a disposable access catheter for reusable optic probe[85]. A microforcep can be introduced to gain a specimen for histopathological examination[86,87]. A study was done to evaluate the benefit of using single-operator cholangioscopy/pancreatoscopy in diagnosing IPMN with 100% sensitivity and specificity for detecting the malignancy, as well as determining the operative excision line in 3 patients[84]. Another case report for a 74-year-old man with chronic pancreatitis and marked weight loss was diagnosed by a single-operator cholangioscopy/pancreatoscopy with IPMN[88].
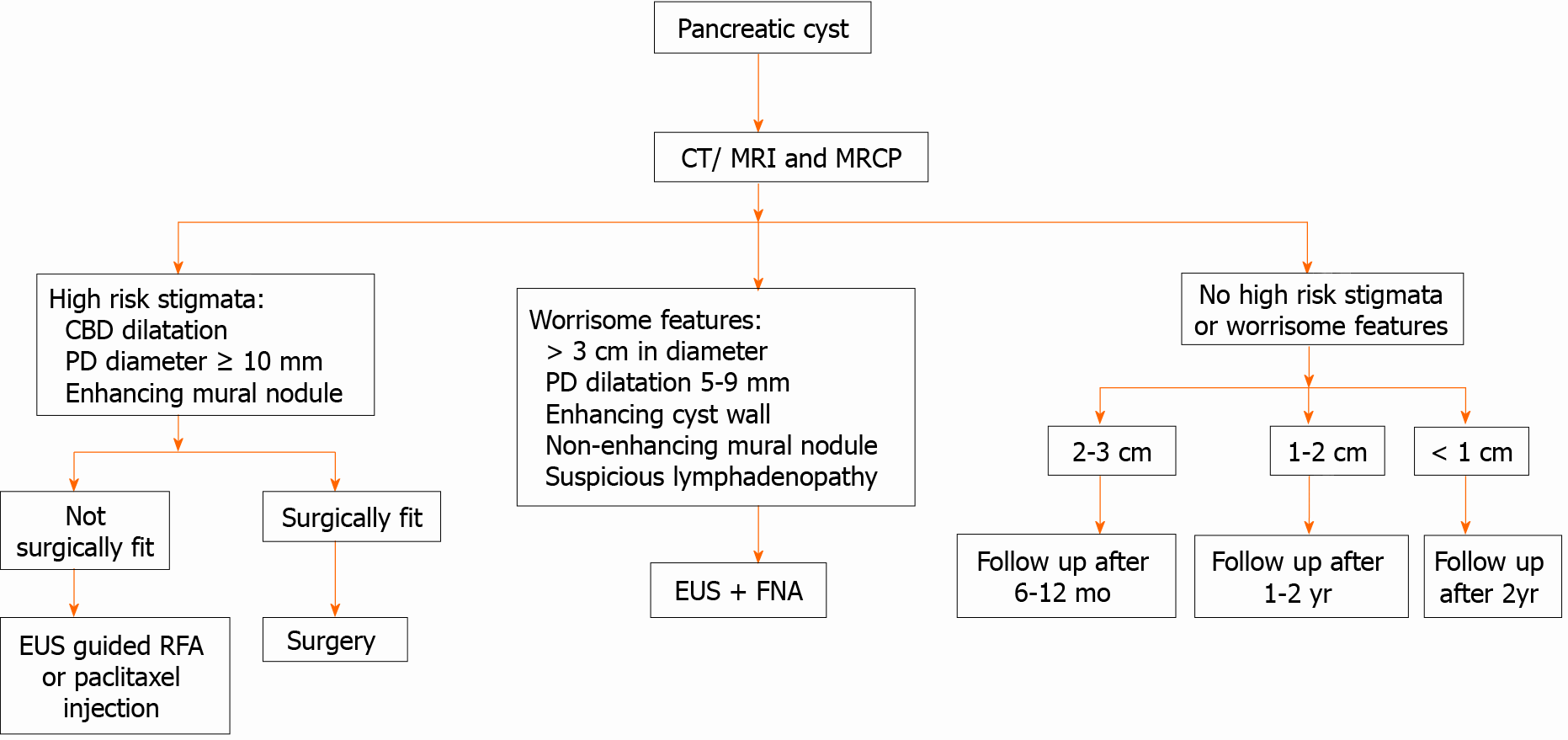
We demonstrated a simplified flow chart for the diagnosing of PCLs as shown in Figure 5.
RFA through a 19G needle using a monopolar probe has been recently described for the management of PCLs. It has been considered a safe and effective procedure that depends on thermal ablation and induces coagulative necrosis of thermosensitive pancreatic tissue[89-91].
In their study, Pai et al[91] conducted RFA ablation on 8patients, where 6 had a PCN and 2 had a cystic neuroendocrine tumor in the head region. It was concluded that EUS-RFA is a safe procedure and that the response rate demonstrated a 100% resolution of PCNs in most cases[92].
By contrast, potential adverse events may arise as a result of the effect of thermal injury, which can lead to biliary leakage and pancreatic and vascular injury[78]. Nevertheless, the data of PCNs’ EUS-RFA are limited to a few human case series and animal data, and an ongoing Phase II multicenter trial of EUS-RFA has been conducted to evaluate the outcomes of the pancreatic cyst at 12 mo following cyst RFA[93].
Testing for mutations related to pancreatic malignancies increases the diagnostic accuracy of cystic pancreatic lesions[62,94-100]. The routine molecular analysis of KRAS/GNAS mutations gives high sensitivity and specificity, reaching 90% in detecting mucinous lesions[101-104] KRAS mutations still do not grade the level of malignancy. Springer et al[105] concluded in their study that mutations in the von Hippel-Lindau (VHL) gene (3p35), with a loss of heterozygosity at gene locus on chromosome 3 and chromosome 3p aneuploidy, were detected in approximately 67% of serous cystic adenomas.
A study carried out by Arner et al[106] mentioned that “The addition of DNA molecular analysis alters the clinical management of PCLs most often when CEA levels are intermediate (45-800 ng/mL) or when no CEA concentration is available”.
GNAS mutations alone can be detected in up to 66% of mucinous neoplastic lesions, and their detection may reach 96% of cases when combined with KRAS mutations [107]. They were also found in 100% of IPMN cases with sensitivity and specificity of 89% and 100%, respectively, for the detection of a mucinous cyst[108].
Other investigated mutations include TP53 mutations, deletions in p16/CDKN2A, SMAD4, mutations in TP53, PIK3CA, and/or PTEN. They are highly sensitive for IPMN[108]. According to a systematic review by Zhang and Pitman[109], although molecular testing is not an alternative to cytological and chemical testing, it can still add value to the diagnostic yielding.
Studies using DNA analysis of cyst fluid seem to be promising but require further attention.
The last randomized clinical studies discussing the radiological and imaging modalities of PCLs are shown in Table 2[108,110-120]; however further studies are warranted not only to improve diagnostic ability but also determine a clear strategy for the use of novel endoscopic techniques for the diagnosis of PCLs.
| Ref. | Country | Sample size | Study period/design | Aim of study | Conclusion |
| Singhi et al[108], 2018 | Pittsburgh | 595 | 2014-2017/prospective | Evaluation of preoperative pancreatic cyst fluid (PCF) DNA testing | Preoperative next-generation sequencing of PCF for KRAS/GNAS mutations is highly sensitive for IPMNs and specific for mucinous PCLs. In addition, the combination of TP53/PIK3CA/PTEN alterations is a useful preoperative marker for advanced neoplasia |
| Basar et al[75], 2018 | United States | 42 | 2015-2016/retrospective | Comparison between the tissue acquisition and diagnostic tissue yield of microforceps biopsy (MFB) with cyst fluid cytology | The cyst tissue acquisition yield for MFBs was 90%. Although cytology of cyst fluid and MFB were comparable in distinguishing mucinous and nonmucinous cysts and detecting cysts at high risk for malignancy, MFB was far superior to cytology for providing a specific cyst diagnosis |
| Kovacevic et al[76], 2018 | Multicenter | 28 | NR/retrospective | Evaluation of the use of EUS-guided MFB in diagnosing pancreatic cystic lesions in a multicenter clinical setting | The use of the microforceps is feasible with acceptable rates of technical and clinical success |
| Mittal et al[110], 2018 | United States | 27 | 2016-2017/retrospective | Assessment of the technical feasibility, diagnostic yield, and safety of EUS-guided MFB for PCLs | MFBs were associated with high technical success, and an excellent safety profile and may be a useful adjunctive tool, complementing existing EUS-FNA sampling protocols for PCLs |
| Zhang et al[111], 2018 | United States | 48 | 2016-2017/retrospective | Comparing the diagnostic performance of the MFB with the current conventional analysis of PCF | PCF analysis and MFB have comparable performance in distinguishing between mucinous and non-mucinous cysts and for detecting high-risk cysts. However, MFB was found to be superior for diagnosing specific cyst subtypes, thus adding a significant value to preoperative patient management |
| Cheesman et al[112], 2019 | United States | 41 | NR/retrospective | Comparing the diagnostic outcomes and changes in clinical management resulting from MFB and nCLE use in PCL | The combination of cyst fluid cytology/chemistry along with MFB and/or nCLE results in a significantly higher rate of a specific PCL diagnosis and has a major impact on changing clinical management decisions including need for continued surveillance or surgery. MFB and/or nCLE should thus be utilized with standard cyst fluid cytology/chemistry when performing EUS-FNA of PCL |
| Crinò et al[73], 2019 | Italy | 61 | 2016-2018/prospective | Evaluation of the diagnostic yield of EUS-guided through-the-needle MFB sampling of pancreatic cystic lesions according to the number of macroscopically visible samples retrieved | Two TTNB macroscopically visible specimens reached 100% histologic adequacy and a specific diagnosis in 74% of patients. The collection of a third specimen did not add any additional information and should be avoided to possibly decrease the risk of adverse events |
| Robles-Medranda and Olmos[113], 2019 | Ecuador | 36 | 2013-2018/retrospective | Defining the role of through-the-needle technologies such as nCLE and EUS- through-the-needle MFB in the diagnosis of pancreatic cyst malignancy | EUS-through-the-needle direct intracystic MFB and nCLE improves malignancy detection in pancreatic cysts |
| Samarasena et al[114], 2019 | United States | 15 | NR/retrospective | Reporting the technical success and safety of EUS-guided through the needle biopsy (TTNB) for pancreatic cystic lesions | This technique has the potential to improve the diagnostic yield of EUS-FNA for pancreatic cystic neoplasms |
| Vestrup Rift et al[115], 2019 | Denmark | 27 | 2016-2017/retrospective | Analysis of the results of next-generation sequencing of microbiopsies from pancreatic cysts | Next-generation sequencing of microbiopsies may have the potential to improve diagnostic decision-making |
| Yang et al[116], 2019 | United States | 114 | 2016-2018/prospective | Comparing the yield of tissue acquired with EUS-guided TTNB with that of samples collected by EUS-guided FNA, and the accuracy of analysis of each sample type in the diagnosis of mucinous PCLs | TTNB collection of tissues for histologic analysis is safe and feasible, with an acquisition yield of 83.3%. Histologic analysis of samples collected by TTNB identified a larger proportion of mucinous PCLs compared with cytologic analysis of samples collected by FNA-even among samples categorized as equivocal, based on the level of carcinoembryonic antigen (CEA) |
| Wilen et al[117], 2019 | United States | 30 | 2016-2018/retrospective | Evaluation of the feasibility and added value of cyst wall biopsy using micro forceps in the diagnosis of pancreatic cysts | Cyst wall biopsy was able to make the diagnosis in 44% of cases where cytology was non-diagnostic and in 64% of cases when composite fluid markers and cytology was non-diagnostic. The high rate of histologic and IHC evaluation suggests that it offers the potential to incorporate tissue-based biomarkers in the diagnosis and management of pancreatic cysts |
| Krishna et al[118], 2020 | United States | 144 | 2015-2018/ Prospective | Comparing the accuracy of EUS with nCLE in differentiating mucinous from non-mucinous PCLs with that of measurement of CEA and cytology analysis | Analysis of cysts by nCLE identified mucinous cysts with greater accuracy than measurement of CEA and cytology analysis. EUS with nCLE can be used to differentiate mucinous from non-mucinous PCLs |
| Keane et al[119], 2019 | United Kingdom | 56 | 2014-2016/prospective | Defining the safety and efficacy of nCLE in diagnosis of indeterminate PCL | EUS-nCLE under conscious sedation in the day case setting is safe and provides additional information to standard EUS-FNA for diagnosing indeterminate PCL |
| Napoleon et al[67], 2019 | France | 78 | 2013-2016/prospective | Evaluation of the diagnostic performance of nCLE for large single non-communicating PCLs using surgical histopathology or EUS-FNA cytohistopathology as a reference diagnosis | nCLE had excellent diagnostic performance that surpassed that of CEA and EUS for the diagnosis of large single non-communicating PCLs. The nCLE procedure should be considered in patients with indeterminate PCLs to ensure a more specific diagnosis |
| Cheesman et al[120], 2020 | United States | 44 | 2016-2018/retrospective study | Comparing the diagnostic outcomes and changes in clinical management resulting from MFB and nCLE use in PCLs | MFB and nCLE led to significant improvements in specific PCL diagnosis, which in turn has major impacts in clinical management |
The real clinical challenge in the management of cystic pancreatic lesions is to identify those patients who should undergo pancreatic resection for early non-metastatic cancer and high-risk precancerous cystic lesions while appropriately observing those patients with limited or no potential for malignant transformation. In the context of many aspects of the EUS management of PCLs, available data are currently insufficient to make evidenced-based decisions.
In conclusion, by combining the use of EUS modalities with cystic fluid tumor markers, analysis constructs a novel diagnostic model for PCLs. Also, it indeed highlights that the accurate diagnosis of PCLs requires a well-experienced multidisciplinary and multimodal team approach, side by side with the integration of clinical findings, imaging, cytology, cyst fluid analysis, and molecular testing.
We would like to acknowledge our great Kasr Al-Aini Hospital, and its workers, nurse and staff members, for all the support and help in this study and throughout our careers.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozkan OF, Rompianesi G S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Abdelkader A, Hunt B, Hartley CP, Panarelli NC, Giorgadze T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch Pathol Lab Med. 2020;144:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 2. | Becker WF, Welsh RA, Pratt HS. Cystadenoma and cystadenocarcinoma of the pancreas. Ann Surg. 1965;161:845-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423-438. [PubMed] |
| 5. | Grützmann R, Niedergethmann M, Pilarsky C, Klöppel G, Saeger HD. Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Oncologist. 2010;15:1294-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Jani N, Bani Hani M, Schulick RD, Hruban RH, Cunningham SC. Diagnosis and management of cystic lesions of the pancreas. Diagn Ther Endosc. 2011;2011:478913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Kamata K, Kitano M. Endoscopic diagnosis of cystic lesions of the pancreas. Dig Endosc. 2019;31:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Ge N, Brugge WR, Saxena P, Sahai A, Adler DG, Giovannini M, Pausawasdi N, Santo E, Mishra G, Tam W, Kida M, de la Mora-Levy JG, Sharma M, Umar M, Katanuma A, Lee L, Garg PK, Eloubeidi MA, Yu HK, Raijman I, Arturo Arias BL, Bhutani M, Carrara S, Rai P, Mukai S, Palazzo L, Dietrich CF, Nguyen NQ, El-Nady M, Poley JW, Guaraldi S, Kalaitzakis E, Sabbagh LC, Lariño-Noia J, Gress FG, Lee YT, Rana SS, Fusaroli P, Hocke M, Dhir V, Lakhtakia S, Ratanachu-Ek T, Chalapathi Rao AS, Vilmann P, Okasha HH, Irisawa A, Ponnudurai R, Leong AT, Artifon E, Iglesias-Garcia J, Saftoiu A, Larghi A, Robles-Medranda C, Sun S. An international, multi-institution survey of the use of EUS in the diagnosis of pancreatic cystic lesions. Endosc Ultrasound. 2019;8:418-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Okasha HH, Ashry M, Imam HM, Ezzat R, Naguib M, Farag AH, Gemeie EH, Khattab HM. Role of endoscopic ultrasound-guided fine needle aspiration and ultrasound-guided fine-needle aspiration in diagnosis of cystic pancreatic lesions. Endosc Ultrasound. 2015;4:132-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Okasha H, E Behiry M, Ramadan N, Ezzat R, Yamany A, El-Kholi S, Ahmed G. Endoscopic ultrasound-guided fine needle aspiration in diagnosis of cystic pancreatic lesions. Arab J Gastroenterol. 2019;20:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 12. | Okasha HH, Naga YM, El Sherbiny M. EUS-guided radiofrequency ablation: Where we are? Endosc Ultrasound. 2020;9:277-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Hafez HA, Okasha HH, Hashem AM, Hassany M, El-Nahaas SM. The Role of EUS and EUS-FNA in Detection of Small Sized Liver Metastatic Lesions in Patients with Pancreatic and Gastro-Intestinal Primary Malignancy. Med J Cairo Univ. 2020;88:1219-1225. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Kim JA, Blumenfeld JD, Chhabra S, Dutruel SP, Thimmappa ND, Bobb WO, Donahue S, Rennert HE, Tan AY, Giambrone AE, Prince MR. Pancreatic Cysts in Autosomal Dominant Polycystic Kidney Disease: Prevalence and Association with PKD2 Gene Mutations. Radiology. 2016;280:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4343] [Article Influence: 361.9] [Reference Citation Analysis (45)] |
| 16. | ASGE Standards of Practice Committee; Muthusamy VR, Chandrasekhara V, Acosta RD, Bruining DH, Chathadi KV, Eloubeidi MA, Faulx AL, Fonkalsrud L, Gurudu SR, Khashab MA, Kothari S, Lightdale JR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Yang J, Cash BD, DeWitt JM. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest Endosc. 2016;84:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Gress F, Gottlieb K, Cummings O, Sherman S, Lehman G. Endoscopic ultrasound characteristics of mucinous cystic neoplasms of the pancreas. Am J Gastroenterol. 2000;95:961-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Lewis JJ, Kowalski TE. Endoscopic ultrasound and fine needle aspiration in pancreatic cancer. Cancer J. 2012;18:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Snozek CL, Mascarenhas RC, O'Kane DJ. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin Biochem. 2009;42:1585-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc. 2006;64:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 23. | Lanke G, Lee JH. Similarities and differences in guidelines for the management of pancreatic cysts. World J Gastroenterol. 2020;26:1128-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 24. | Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 25. | Wang W, Zhang L, Chen L, Wei J, Sun Q, Xie Q, Zhou X, Zhou D, Huang P, Yang Q, Xie H, Zhou L, Zheng S. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Biomed Rep. 2015;3:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Kim JR, Jang JY, Kang MJ, Park T, Lee SY, Jung W, Chang J, Shin Y, Han Y, Kim SW. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19-9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. J Hepatobiliary Pancreat Sci. 2015;22:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Al-Rashdan A, Schmidt CM, Al-Haddad M, McHenry L, Leblanc JK, Sherman S, Dewitt J. Fluid analysis prior to surgical resection of suspected mucinous pancreatic cysts. A single centre experience. J Gastrointest Oncol. 2011;2:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 30. | Fukukura Y, Fujiyoshi F, Hamada H, Takao S, Aikou T, Hamada N, Yonezawa S, Nakajo M. Intraductal papillary mucinous tumors of the pancreas. Comparison of helical CT and MR imaging. Acta Radiol. 2003;44:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 32. | Repák R, Rejchrt S, Bártová J, Malírová E, Tycová V, Bures J. Endoscopic ultrasonography (EUS) and EUS-guided fine-needle aspiration with cyst fluid analysis in pancreatic cystic neoplasms. Hepatogastroenterology. 2009;56:629-635. [PubMed] |
| 33. | Dumonceau JM, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, Aithal GP, Arcidiacono PG, Bastos P, Carrara S, Czakó L, Fernández-Esparrach G, Fockens P, Ginès À, Havre RF, Hassan C, Vilmann P, van Hooft JE, Polkowski M. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy. 2017;49:695-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 34. | Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 35. | Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM, Zaheer A, Wolfgang C, Hruban R, Marchegiani G, Fernández Del Castillo C, Brugge W, Ha Y, Kim MH, Oh D, Hirai I, Kimura W, Jang JY, Kim SW, Jung W, Kang H, Song SY, Kang CM, Lee WJ, Crippa S, Falconi M, Gomatos I, Neoptolemos J, Milanetto AC, Sperti C, Ricci C, Casadei R, Bissolati M, Balzano G, Frigerio I, Girelli R, Delhaye M, Bernier B, Wang H, Jang KT, Song DH, Huggett MT, Oppong KW, Pererva L, Kopchak KV, Del Chiaro M, Segersvard R, Lee LS, Conwell D, Osvaldt A, Campos V, Aguero Garcete G, Napoleon B, Matsumoto I, Shinzeki M, Bolado F, Fernandez JM, Keane MG, Pereira SP, Acuna IA, Vaquero EC, Angiolini MR, Zerbi A, Tang J, Leong RW, Faccinetto A, Morana G, Petrone MC, Arcidiacono PG, Moon JH, Choi HJ, Gill RS, Pavey D, Ouaïssi M, Sastre B, Spandre M, De Angelis CG, Rios-Vives MA, Concepcion-Martin M, Ikeura T, Okazaki K, Frulloni L, Messina O, Lévy P. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 36. | Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am J Roentgenol. 2007;189:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, Spoto E, Andreis IA, De Marco R, Megibow AJ. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Abraham AS, Simon B, Eapen A, Sathyakumar K, Chandramohan A, Raju RS, Joseph P, Kodiatte TA, Gowri M. Role of Cross-sectional Imaging (CT/MRI) in Characterization and Distinguishing Benign from Malignant/Potentially Malignant Cystic Lesions of Pancreas. J Clin Imaging Sci. 2020;10:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Fan Z, Yan K, Wang Y, Qiu J, Wu W, Yang L, Chen M. Application of Contrast-Enhanced Ultrasound in Cystic Pancreatic Lesions Using a Simplified Classification Diagnostic Criterion. Biomed Res Int. 2015;2015:974621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Wang Y, Fan Z, Shan J, Yan K. The Value of Contrast-Enhanced Ultrasound Classification in Diagnosis of Pancreatic Cystic Lesions. Biomed Res Int. 2019;2019:5698140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Kimura W, Moriya T, Hirai I, Hanada K, Abe H, Yanagisawa A, Fukushima N, Ohike N, Shimizu M, Hatori T, Fujita N, Maguchi H, Shimizu Y, Yamao K, Sasaki T, Naito Y, Tanno S, Tobita K, Tanaka M. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas. 2012;41:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Lewandrowski K, Warshaw A, Compton C. Macrocystic serous cystadenoma of the pancreas: a morphologic variant differing from microcystic adenoma. Hum Pathol. 1992;23:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978;69:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 311] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Distler M, Aust D, Weitz J, Pilarsky C, Grützmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int. 2014;2014:474905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Castellano-Megías VM, Andrés CI, López-Alonso G, Colina-Ruizdelgado F. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol. 2014;6:311-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Cooper CL, O'Toole SA, Kench JG. Classification, morphology and molecular pathology of premalignant lesions of the pancreas. Pathology. 2013;45:286-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Binmoeller KF, Soehendra N. Endoscopic ultrasonography in the diagnosis and treatment of pancreatic pseudocysts. Gastrointest Endosc Clin N Am. 1995;5:805-816. [PubMed] |
| 48. | Pausawasdi N, Ratanachu-Ek T. Endoscopic ultrasonography evaluation for pancreatic cysts: Necessity or overkill? Dig Endosc. 2017;29:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Kamata K, Kitano M, Kudo M, Sakamoto H, Kadosaka K, Miyata T, Imai H, Maekawa K, Chikugo T, Kumano M, Hyodo T, Murakami T, Chiba Y, Takeyama Y. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Baba T, Yamaguchi T, Ishihara T, Kobayashi A, Oshima T, Sakaue N, Kato K, Ebara M, Saisho H. Distinguishing benign from malignant intraductal papillary mucinous tumors of the pancreas by imaging techniques. Pancreas. 2004;29:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Maguchi H, Osanai M, Yanagawa N, Takahashi K, Itoh H, Katanuma A, Obara T, Kohgo Y. Endoscopic ultrasonography diagnosis of pancreatic cystic disease. Endoscopy. 1998;30 Suppl 1:A108-A110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Huang ES, Turner BG, Fernandez-Del-Castillo C, Brugge WR, Hur C. Pancreatic cystic lesions: clinical predictors of malignancy in patients undergoing surgery. Aliment Pharmacol Ther. 2010;31:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Buscaglia JM, Giday SA, Kantsevoy SV, Jagannath SB, Magno P, Wolfgang CL, Daniels JA, Canto MI, Okolo Iii PI. Patient- and cyst-related factors for improved prediction of malignancy within cystic lesions of the pancreas. Pancreatology. 2009;9:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Hawes RH, Clancy J, Hasan MK. Endoscopic ultrasound-guided fine needle aspiration in cystic pancreatic lesions. Clin Endosc. 2012;45:128-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Rogart JN, Loren DE, Singu BS, Kowalski TE. Cyst wall puncture and aspiration during EUS-guided fine needle aspiration may increase the diagnostic yield of mucinous cysts of the pancreas. J Clin Gastroenterol. 2011;45:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Atef E, El Nakeeb A, El Hanafy E, El Hemaly M, Hamdy E, El-Geidie A. Pancreatic cystic neoplasms: predictors of malignant behavior and management. Saudi J Gastroenterol. 2013;19:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | de Castro SM, Houwert JT, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Evaluation of a selective management strategy of patients with primary cystic neoplasms of the pancreas. Int J Surg. 2011;9:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Donahue TR, Hines OJ, Farrell JJ, Tomlinson JS, Eibl G, Reber HA. Cystic neoplasms of the pancreas: results of 114 cases. Pancreas. 2010;39:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Grobmyer SR, Cance WG, Copeland EM, Vogel SB, Hochwald SN. Is there an indication for initial conservative management of pancreatic cystic lesions? J Surg Oncol. 2009;100:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Sawhney MS, Al-Bashir S, Cury MS, Brown A, Chuttani R, Pleskow DK, Callery MP, Vollmer CM. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol. 2009;7:1373-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Goh BK, Tan YM, Thng CH, Cheow PC, Chung YF, Chow PK, Wong WK, Ooi LL. How useful are clinical, biochemical, and cross-sectional imaging features in predicting potentially malignant or malignant cystic lesions of the pancreas? J Am Coll Surg. 2008;206:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, Kochman ML, Foley PJ, Drebin J, Oh YS, Ginsberg G, Ahmad N, Merchant NB, Isbell J, Parikh AA, Stokes JB, Bauer T, Adams RB, Simeone DM. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? J Gastrointest Surg. 2008;12:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Bournet B, Vignolle-Vidoni A, Grand D, Roques C, Breibach F, Cros J, Muscari F, Carrère N, Selves J, Cordelier P, Buscail L. Endoscopic ultrasound-guided fine-needle aspiration plus KRAS and GNAS mutation in malignant intraductal papillary mucinous neoplasm of the pancreas. Endosc Int Open. 2016;4:E1228-E1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 65. | Durkin C, Krishna SG. Advanced diagnostics for pancreatic cysts: Confocal endomicroscopy and molecular analysis. World J Gastroenterol. 2019;25:2734-2742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Poizat F, Giovannini M. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 67. | Napoleon B, Palazzo M, Lemaistre AI, Caillol F, Palazzo L, Aubert A, Buscail L, Maire F, Morellon BM, Pujol B, Giovannini M. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis. Endoscopy. 2019;51:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 68. | Karia K, Waxman I, Konda VJ, Gress FG, Sethi A, Siddiqui UD, Sharaiha RZ, Kedia P, Jamal-Kabani A, Gaidhane M, Kahaleh M. Needle-based confocal endomicroscopy for pancreatic cysts: the current agreement in interpretation. Gastrointest Endosc. 2016;83:924-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Zhong L, Chai N, Linghu E, Li H, Yang J, Tang P. A Prospective Study on Contrast-Enhanced Endoscopic Ultrasound for Differential Diagnosis of Pancreatic Cystic Neoplasms. Dig Dis Sci. 2019;64:3616-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Aparicio JR, Martínez J, Niveiro M, Cabezas A, Ruiz F, De Madaria E, Casellas JA. Direct intracystic biopsy and pancreatic cystoscopy through a 19-gauge needle EUS (with videos). Gastrointest Endosc. 2010;72:1285-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Shakhatreh MH, Naini SR, Brijbassie AA, Grider DJ, Shen P, Yeaton P. Use of a novel through-the-needle biopsy forceps in endoscopic ultrasound. Endosc Int Open. 2016;4:E439-E442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Huelsen A, Cooper C, Saad N, Gupta S. Endoscopic ultrasound-guided, through-the-needle forceps biopsy in the assessment of an incidental large pancreatic cystic lesion with prior inconclusive fine-needle aspiration. Endoscopy. 2017;49:E109-E110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Crinò SF, Bernardoni L, Brozzi L, Barresi L, Malleo G, Salvia R, Frulloni L, Sina S, Parisi A, Remo A, Larghi A, Gabbrielli A, Manfrin E. Association between macroscopically visible tissue samples and diagnostic accuracy of EUS-guided through-the-needle microforceps biopsy sampling of pancreatic cystic lesions. Gastrointest Endosc. 2019;90:933-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | Yang D, Samarasena JB, Jamil LH, Chang KJ, Lee D, Ona MA, Lo SK, Gaddam S, Liu Q, Draganov PV. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: a multicenter study. Endosc Int Open. 2018;6:E1423-E1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Basar O, Yuksel O, Yang DJ, Samarasena J, Forcione D, DiMaio CJ, Wagh MS, Chang K, Casey B, Fernandez-Del Castillo C, Pitman MB, Brugge WR. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc. 2018;88:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Kovacevic B, Karstensen JG, Havre RF, Pham KD, Giovannini M, Dabizzi E, Arcidiacono P, Santo E, Sequeiros EV, Klausen P, Rift CV, Hasselby JP, Toxværd A, Kalaitzakis E, Hansen CP, Vilmann P. Initial experience with EUS-guided microbiopsy forceps in diagnosing pancreatic cystic lesions: A multicenter feasibility study (with video). Endosc Ultrasound. 2018;7:383-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Hara T, Yamaguchi T, Ishihara T, Tsuyuguchi T, Kondo F, Kato K, Asano T, Saisho H. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Maire F, Couvelard A, Hammel P, Ponsot P, Palazzo L, Aubert A, Degott C, Dancour A, Felce-Dachez M, O'toole D, Lévy P, Ruszniewski P. Intraductal papillary mucinous tumors of the pancreas: the preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Azar C, Van de Stadt J, Rickaert F, Devière M, Baize M, Klöppel G, Gelin M, Cremer M. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Kitagawa Y, Unger TA, Taylor S, Kozarek RA, Traverso LW. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 81. | Yamaguchi T, Shirai Y, Ishihara T, Sudo K, Nakagawa A, Ito H, Miyazaki M, Nomura F, Saisho H. Pancreatic juice cytology in the diagnosis of intraductal papillary mucinous neoplasm of the pancreas: significance of sampling by peroral pancreatoscopy. Cancer. 2005;104:2830-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Miura T, Igarashi Y, Okano N, Miki K, Okubo Y. Endoscopic diagnosis of intraductal papillary-mucinous neoplasm of the pancreas by means of peroral pancreatoscopy using a small-diameter videoscope and narrow-band imaging. Dig Endosc. 2010;22:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Arisaka Y, Moriyasu F. Initial experience of peroral pancreatoscopy combined with narrow-band imaging in the diagnosis of intraductal papillary mucinous neoplasms of the pancreas (with videos). Gastrointest Endosc. 2007;66:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Nagayoshi Y, Aso T, Ohtsuka T, Kono H, Ideno N, Igarashi H, Takahata S, Oda Y, Ito T, Tanaka M. Peroral pancreatoscopy using the SpyGlass system for the assessment of intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci. 2014;21:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 85. | Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc. 2007;65:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Sekaran A, Rao GV. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 87. | Draganov PV, Chauhan S, Wagh MS, Gupte AR, Lin T, Hou W, Forsmark CE. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012;75:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 88. | Tanaka SA, McKee JD, Conway WC. Intracystic Biopsy and Diagnosis of Intraductal Papillary Mucinous Neoplasm via SpyGlass Pancreatoscopy. Ochsner J. 2015;15:452-454. [PubMed] |
| 89. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 90. | Chaudhary S, Sun SY. Endoscopic ultrasound-guided radiofrequency ablation in gastroenterology: New horizons in search. World J Gastroenterol. 2017;23:4892-4896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 91. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 92. | Silviu UB, Daniel P, Claudiu M, Săndulescu L, Simona F, Ştefan P, Valeriu Ş, Adrian S. Endoscopic ultrasound-guided radiofrequency ablation of the pancreas: An experimental study with pathological correlation. Endosc Ultrasound. 2015;4:330-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Ofosu A, Ramai D, Adler DG. Endoscopic ultrasound-guided ablation of pancreatic cystic neoplasms: ready for prime time? Ann Gastroenterol. 2019;32:39-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Schönleben F, Allendorf JD, Qiu W, Li X, Ho DJ, Ciau NT, Fine RL, Chabot JA, Remotti HE, Su GH. Mutational analyses of multiple oncogenic pathways in intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2008;36:168-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Satoh K, Shimosegawa T, Moriizumi S, Koizumi M, Toyota T. K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas. 1996;12:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Schönleben F, Qiu W, Remotti HE, Hohenberger W, Su GH. PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbecks Arch Surg. 2008;393:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Khalid A, Pal R, Sasatomi E, Swalsky P, Slivka A, Whitcomb D, Finkelstein S. Use of microsatellite marker loss of heterozygosity in accurate diagnosis of pancreaticobiliary malignancy from brush cytology samples. Gut. 2004;53:1860-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Hamilton SR. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Digestive System. IARC Press: Lyon, 2000. |
| 99. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 100. | Z'graggen K, Rivera JA, Compton CC, Pins M, Werner J, Fernández-del Castillo C, Rattner DW, Lewandrowski KB, Rustgi AK, Warshaw AL. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226:491-8; discussion 498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Jones M, Zheng Z, Wang J, Dudley J, Albanese E, Kadayifci A, Dias-Santagata D, Le L, Brugge WR, Fernandez-del Castillo C, Mino-Kenudson M, Iafrate AJ, Pitman MB. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016;83:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 102. | Kadayifci A, Atar M, Wang JL, Forcione DG, Casey BW, Pitman MB, Brugge WR. Value of adding GNAS testing to pancreatic cyst fluid KRAS and carcinoembryonic antigen analysis for the diagnosis of intraductal papillary mucinous neoplasms. Dig Endosc. 2017;29:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Rosenbaum MW, Jones M, Dudley JC, Le LP, Iafrate AJ, Pitman MB. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol. 2017;125:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 104. | Singhi AD, Nikiforova MN, Fasanella KE, McGrath KM, Pai RK, Ohori NP, Bartholow TL, Brand RE, Chennat JS, Lu X, Papachristou GI, Slivka A, Zeh HJ, Zureikat AH, Lee KK, Tsung A, Mantha GS, Khalid A. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014;20:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 105. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 106. | Arner DM, Corning BE, Ahmed AM, Ho HC, Weinbaum BJ, Siddiqui U, Aslanian H, Adams RB, Bauer TW, Wang AY, Shami VM, Sauer BG. Molecular analysis of pancreatic cyst fluid changes clinical management. Endosc Ultrasound. 2018;7:29-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 108. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 109. | Zhang ML, Pitman MB. Practical Applications of Molecular Testing in the Cytologic Diagnosis of Pancreatic Cysts. J Mol Pathol. 2021;2:11-22. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Mittal C, Obuch JC, Hammad H, Edmundowicz SA, Wani S, Shah RJ, Brauer BC, Attwell AR, Kaplan JB, Wagh MS. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video). Gastrointest Endosc. 2018;87:1263-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 111. | Zhang ML, Arpin RN, Brugge WR, Forcione DG, Basar O, Pitman MB. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018;126:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 112. | Cheesman AR, Zhu H, Kumta NA. EUS-guided microforceps biopsy and needle-based confocal laser endomicroscopy significantly improve the diagnostic yield and have major impact on clinical management of pancreatic cystic lesions [abstract]. Gastrointest Endosc. 2019;89:AB144. |
| 113. | Robles-Medranda C, Olmos JI. EUS-through-the-needle technologies in the diagnosis and malignancy detection of pancreatic cysts: a comparative study between different technologies [abstract]. Gastrointest Endosc. 2019;89:AB608-A609. |
| 114. | Samarasena J, Yu A, Lee D, Hashimoto R, Lu Y, Thieu D, Mai D, Lee J, Chang K. EUS-guided through-the-needle biopsy for pancreatic cystic lesions. VideoGIE. 2019;4:436-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Vestrup Rift C, Melchior LC, Kovacevic B, Toxvaerd A, Klausen P, Karstensen JG, Kalaitzakis E, Storkholm J, Palnaes Hansen C, Vilmann P, Preuss Hasselby J. Next-generation sequencing of endoscopic ultrasound guided microbiopsies from pancreatic cystic neoplasms. Histopathology. 2019;75:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Yang D, Trindade AJ, Yachimski P, Benias P, Nieto J, Manvar A, Ho S, Esnakula A, Gamboa A, Sethi A, Gupte A, Khara HS, Diehl DL, El Chafic A, Shah J, Forsmark CE, Draganov PV. Histologic Analysis of Endoscopic Ultrasound-Guided Through the Needle Microforceps Biopsies Accurately Identifies Mucinous Pancreas Cysts. Clin Gastroenterol Hepatol. 2019;17:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 117. | Wilen J, Visrodia K, Lan G. The feasibility and value of cyst wall biopsy using micro forceps in the diagnosis of pancreatic cysts [abstract]. Gastrointest Endosc. 2019;89:AB605. |
| 118. | Krishna SG, Hart PA, Malli A, Kruger AJ, McCarthy ST, El-Dika S, Walker JP, Dillhoff ME, Manilchuk A, Schmidt CR, Pawlik TM, Porter K, Arnold CA, Cruz-Monserrate Z, Conwell DL. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin Gastroenterol Hepatol 2020; 18: 432-440. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 119. | Keane MG, Wehnert N, Perez-Machado M, Fusai GK, Thorburn D, Oppong KW, Carroll N, Metz AJ, Pereira SP. A prospective trial of CONfocal endomicroscopy in CYSTic lesions of the pancreas: CONCYST-01. Endosc Int Open. 2019;7:E1117-E1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Cheesman AR, Zhu H, Liao X, Szporn AH, Kumta NA, Nagula S, DiMaio CJ. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest Endosc. 2020;91:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |