Published online Jan 14, 2021. doi: 10.3748/wjg.v27.i2.224
Peer-review started: November 5, 2020
First decision: November 25, 2020
Revised: November 29, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: January 14, 2021
Processing time: 67 Days and 0.6 Hours
Acute pancreatitis is the leading cause of hospitalization for acute gastrointestinal disease worldwide. The effects of probiotics in mild acute pancreatitis have not been studied. We hypothesized that the administration of probiotics may accelerate the recovery of intestinal function and shorten the length of hospital stay (LOS) in patients with mild pancreatitis.
To investigate the value of probiotics in reducing the LOS in patients with mild acute pancreatitis.
We conducted a double-blind randomized clinical trial to evaluate the effects of probiotics administered to patients with mild acute pancreatitis at a tertiary medical center. The patients were given probiotics capsules (a mixed preparation of Bacillus subtilis and Enterococcus faecium) or placebo. The primary study endpoint was the LOS. The secondary endpoints included time to abdominal pain relief, recurrent abdominal pain, and time to successful oral feeding.
A total of 128 patients were included, with 64 patients in each arm. The severity of illness and the etiological distribution of disease were similar in the two groups. There was a significant reduction in the LOS in the probiotics treatment group vs the placebo group (5.36 ± 0.15 vs 6.02 ± 0.17 d, P < 0.05). The probiotics group was associated with a shorter time to abdominal pain relief and time to successful oral feeding (P < 0.01 for both) than the placebo group. No statistical difference was found in recurrent abdominal pain between the two groups.
The study results showed that the administration of probiotics capsules is associated with a shorter duration of hospitalization in patients with mild acute pancreatitis.
Core Tip: Acute pancreatitis is the leading cause of hospitalization for acute gastrointestinal diseases. Probiotics are widely used for intestinal diseases, although its effect on mild acute pancreatitis has not been studied. This is the first study to explore the effect of probiotics in mild pancreatitis patients. The results indicated that probiotics were associated with decreased length of hospitalization.
- Citation: Wan YD, Zhu RX, Bian ZZ, Sun TW. Effect of probiotics on length of hospitalization in mild acute pancreatitis: A randomized, double-blind, placebo-controlled trial. World J Gastroenterol 2021; 27(2): 224-232
- URL: https://www.wjgnet.com/1007-9327/full/v27/i2/224.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i2.224
Acute pancreatitis is one of the most common causes of acute abdominal pain, resulting in a huge clinical and financial burden worldwide. A nationwide retrospective analysis involving two million acute pancreatitis patients from the United States found that there was a 13.2% increase in admissions in the years 2009-2012 compared to that in 2002–2005[1]. In 2012, the annual cost of acute pancreatitis hospitalizations was about $2.6 billion[2]. Most patients (80%) with acute pancreatitis present with mild disease, which is characterized by a short hospital stay, no or few complications, and rapid resumption of oral feeding[3]. Allowing for the very low mortality rate (about 0.79%) of mild acute pancreatitis[1], shortening the length of hospital stay (LOS), and reducing medical financial expenditure have become the research priority.
More and more studies have shown that intestinal microbial communities are closely related to the occurrence and progression of gastrointestinal diseases, such as inflammatory bowel disease, irritable bowel syndrome, and colorectal cancer[4,5]. Altering the microbiota to decrease the severity of disease or even cure the disease has become a sought-after research direction. Probiotics play an important role in a variety of microecologic interventions. Probiotics were defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”[6]. The mechanisms of probiotics in the treatment of intestinal disease include improving barrier function, modulating cell-mediated and humoral immune functions, interacting with the gut microbiota through competition for nutrients, antagonism, cross-feeding, and supporting microbiota stability, etc.[7]. In a multicenter randomized, placebo-controlled trial, 298 patients predicted with severe acute pancreatitis were randomly assigned to receive a multispecies probiotic or placebo; it was found that probiotic prophylaxis did not reduce the risk of infectious complications and was associated with an increased risk of mortality[8]. Subsequently, a meta-analysis involving six trials with 536 patients showed that probiotics supplementation did not significantly affect the pancreatic infection rate, LOS, or mortality[9]. In contrast, another meta-analysis showed reduced LOS in Chinese patients with severe acute pancreatitis[10]. However, the effect of probiotics in mild acute pancreatitis has not been studied. Considering the potential benefits of probiotics in the treatment of gastrointestinal disease, we hypothesized that probiotics may accelerate the recovery of intestinal function and shorten the LOS in patients with mild pancreatitis. Thus, we conducted a randomized, double-blind, placebo-controlled trial to evaluate the effect of the administration of probiotics in patients with mild pancreatitis.
This 1:1 randomized, controlled, double-blind clinical trial was conducted at the emergency ward of a tertiary teaching hospital with 5186 beds in China from July 2017 to July 2019. The study protocol was defined in accordance with the declaration of Helsinki and was approved by the Ethics Committee of our institution. The trial was registered in the Chinese Clinical Trial Registry (ChiCTR2000030425) and is reported in accordance with the CONSORT guidelines. All patients provided written informed consent before study participation. All study authors had access to the data and approved the final manuscript.
Patients were randomly allocated to a probiotics capsules or placebo group, and probiotics capsules or placebo were given orally from study inclusion to discharge. Considering the safety and efficacy shown in other clinical research[11], we chose a probiotics commercial preparation, a mixed preparation of Bacillus subtilis and Enterococcus faecium, which has been widely used in China (Meichangan, national medicine permission number S20030087). A computer-generated random-number table was prepared by statisticians to assign patients to receive either the probiotics capsules or placebo. The random-number table was unknown to the investigators, who enrolled the patients. To ensure blinding, placebo capsules identical to the probiotics capsules were manufactured (same appearance, color, and odor). The patients, clinicians, evaluators, and statisticians were blinded to the study treatment.
The inclusion criteria[12] were patients who (1) were 18-75 years old; and (2) met two of the following three diagnostic criteria for acute pancreatitis, including typical upper abdominal pain, serum amylase and/or lipase activity more than three times, and imaging manifestations of pancreatitis. Patients who presented with any indication of severe acute pancreatitis were excluded from our study. The indications of severe acute pancreatitis included: (1) Evidence of organ failure (oxygen saturation less than 90% on room air, mean arterial pressure less than 70 mmHg, the requirement for vasopressors or inotropic support, serum creatinine greater than 2.0 mg/dL, and Glasgow coma score less than 15)[12]; (2) Suspected or confirmed infected pancreatic necrosis; (3) C-reactive protein (CRP) over 150 mg/L[8]; and (4) Acute Physiology and Chronic Health Evaluation score ≥ 8[8]. Patients were included only for the first episode of mild pancreatitis. Patients receiving probiotics therapy during the 3 mo preceding the index admission were excluded. Additionally, pregnant or breastfeeding women were not allowed to participate.
We extracted clinical data from the hospital electronic medical record systems. We collected the clinical data including basic information at admission such as sex, age, CRP, body mass index (BMI), Charlson score, disease etiology (gallstones-related, hypertriglyceridemia, alcohol-related, others, or unknown), relevant diagnostic and therapeutic interventions, and outcomes including LOS, time to successful oral feeding, rate of recurrent abdominal pain, time to abdominal pain relief, 30-d readmissions, and mortality. Successful oral feeding means that patients could take food through the mouth without any abdominal pain or other discomforts. Rate of recurrent abdominal pain means the failure rate of the patient's initial attempt to take food orally characterized as abdominal pain, nausea, vomiting, or other abdominal discomforts after the attempt. Abdominal pain relief means the time when abdominal pain symptoms completely disappeared for the first time. Previously reported adverse effects of probiotics in our study were closely monitored, including exacerbating abdominal discomfort (abdominal pain, nausea, vomiting, flatulence, or diarrhea)[13], anaphylactic reaction[14], probiotic-related infections[13], and lactic acidosis[15].
We used LOS as our primary endpoint. It is generally believed that patients with mild pancreatitis, characterized by complete abdominal pain relief, no abdominal discomfort (such as nausea and vomiting), and successfully eating solid or semi-liquid foods, could be discharged from the hospital[16]. Thus, we chose the following indexes as our secondary endpoint: Time to first abdominal pain relief, rate of recurrent abdominal pain, and time to successful oral feeding. Patients were followed for 30 d for mortality and 30-d readmissions. All these outcomes were prespecified in the protocol.
According to a previous study[16,17], assuming the median LOS of mild pancreatitis patients was 5 d, we needed 128 patients (64 in each group) to obtain 80% power for detecting a 1-d relative decrease in the LOS with the one-sided alpha risk of 0.05.
Continuous variables are expressed as the mean ± SD if they were normally distributed. Categorical variables are expressed as percentages. The Kolmogorov–Smirnov test was used to assess whether continuous data were normally distributed (P > 0.05). For continuous variables, differences between groups were tested by Student’s t-test for normally distributed data or Mann–Whitney U test for non-normally distributed data and the c2 test for categorical variables. All analyses were done according to the intention-to-treat principle. Results were considered significant at P < 0.05. Statistical analyses were carried out using SPSS version 23.0 (IBM Inc., Chicago, Illinois, United States).
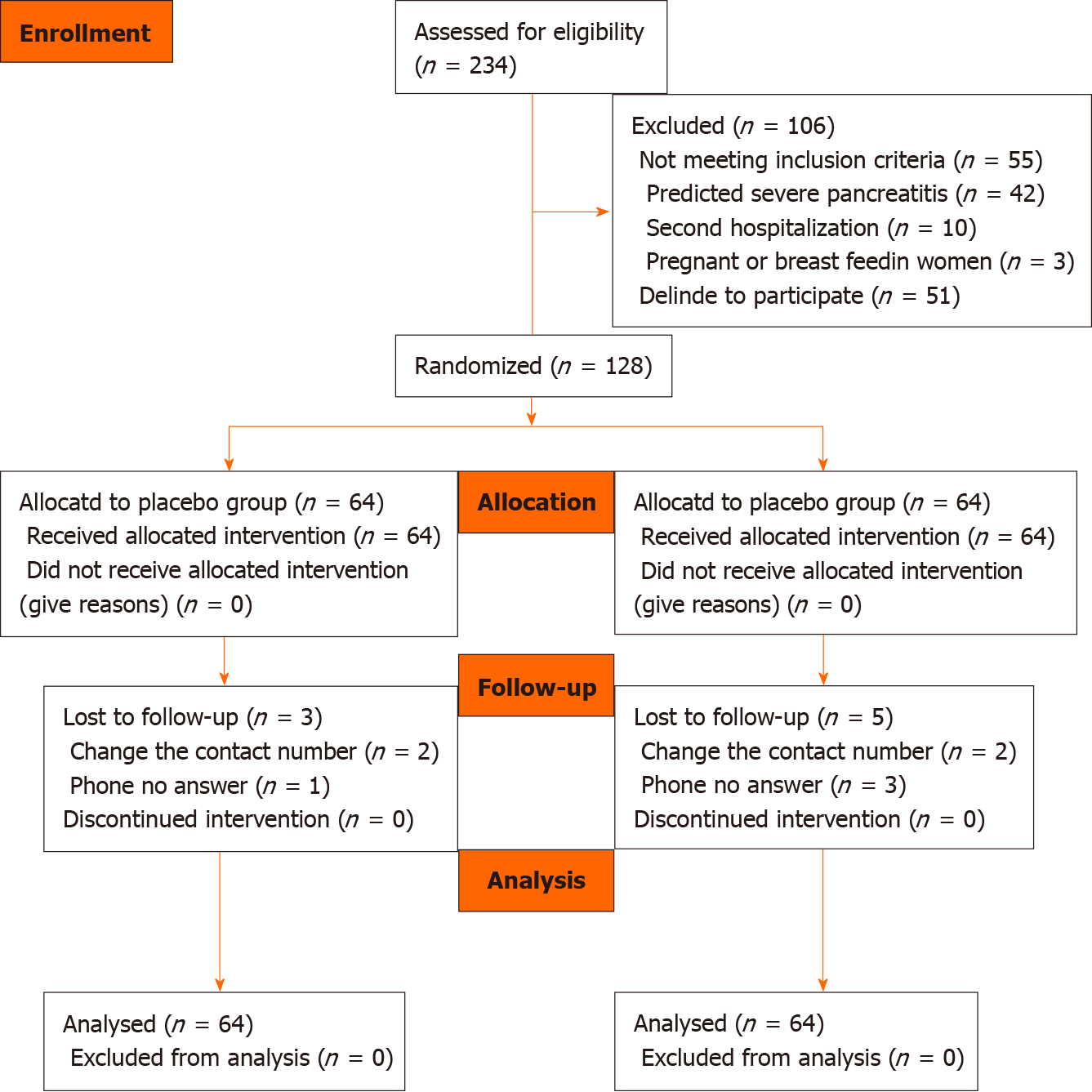
We assessed 234 patients for eligibility, and 106 were excluded. The patients were excluded for having severe acute pancreatitis, suspected or confirmed infected pancreatic necrosis, and Acute Physiology and Chronic Health Evaluation score ≥ 8. Fifty-one patients refused to participate in the study, mainly because of concerns about the safety of the drugs. The remaining 128 patients were randomized, and 64 were allocated to each group. The CONSORT standardized flow diagram shows the enrollment process (Figure 1). We followed the patients for 30 d for mortality or readmissions through telephone calls, of whom eight were lost to follow-up due to change of contact number, or non-response.
Thus, 128 patients were included in the intention-to-treat analysis. The baseline demographics of the study population are shown in Table 1. The average age of the collective group was 52.73 years, with 64.1% male. The mean CRP level was 54.9 mg/L. There were no significant differences in age, sex, BMI, CRP, Charlson score, or disease etiology distribution between the two groups. The most common etiology overall for acute pancreatitis in this study was gallstones-related (50%).
| Parameter | Probiotics (n = 64) | Placebo (n = 64) | P value |
| Age (yr) (mean ± SD) | 50.25 ± 16.79 | 54.72 ± 14.86 | 0.16 |
| Sex (female) | 24 (37%) | 22 (34%) | 0.71 |
| BMI (mean ± SD) | 25.75 ± 3.40 | 26.29 ± 2.82 | 0.33 |
| CRP (mg/L) (mean ± SD) | 57.32 ± 28.21 | 52.55 ± 24.47 | 0.31 |
| Charlson score | 3.02 ± 1.12 | 2.96 ± 0.29 | 0.32 |
| Disease etiology | |||
| Gallstones-related | 30 (46%) | 34 (53%) | 0.48 |
| Hypertriglyceridemia | 11 (17%) | 10 (16%) | 0.81 |
| Alcohol-related | 9 (14%) | 6 (9%) | 0.41 |
| Others or unknown | 12 (19%) | 12 (19%) | 1.0 |
| Time from first symptoms to admission (d) | 2 (1–4) | 3 (1–5) | 0.10 |
| Follow-up period (d) | 31.67 ± 1.97 | 31.32 ± 2.13 | 0.33 |
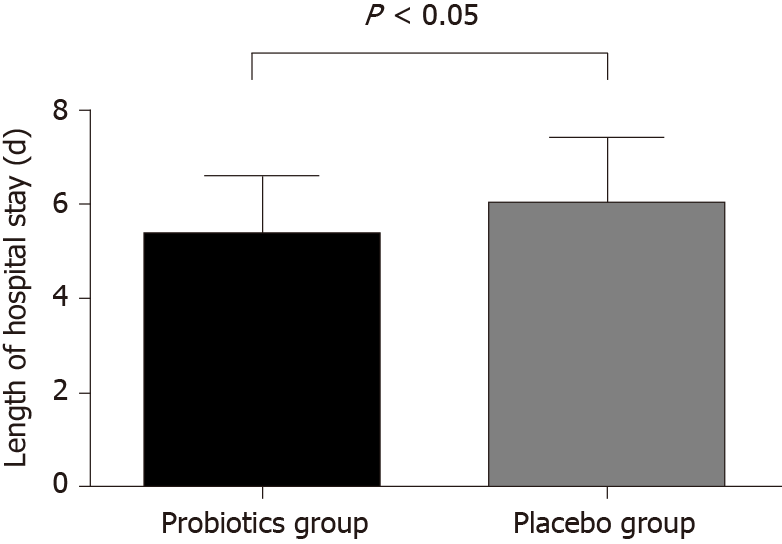
The mean LOS in the probiotics group was 5.36 ± 0.15 d, and in the placebo group was 6.02 ± 0.17 d. Patients in the probiotics group had a significantly shorter LOS than the placebo group by about 0.65 d (P < 0.05). The result are shown in Figure 2.
The study endpoints between the probiotics and placebo groups are listed in Table 2. Recurrent abdominal pain was noted in six patients in the probiotics group and nine patients in the placebo group, with no significant difference between the two groups (P = 0.41). Notably, the time to abdominal pain relief in the probiotics group was significantly shorter than that in the placebo group by about 1 d (2.98 ± 0.74 d vs 4.14 ± 1.32 d, P < 0.01). Additionally, the probiotics group had a significantly shorter time to successful oral feeding than the placebo group (4.56 ± 1.13 d vs 5.37 ± 1.13 d, P < 0.01). No patient mortality was noted in our study. One patient was re-hospitalized within 30 d of discharge because of hyperlipidemic pancreatitis, which was caused by repeated overeating in the probiotics group; and one patient in the placebo group was re-admitted for pancreatitis secondary to repeated heavy alcohol drinking.
| Probiotics (n = 64) | Placebo (n = 64) | P value | |
| Time to successful oral feeding (d, mean ± SD) | 4.56 ± 1.13 | 5.37 ± 1.13 | < 0.01 |
| Rate of recurrent abdominal pain | 6 (9%) | 9 (14%) | 0.41 |
| Time to abdominal pain relief (d, mean ± SD) | 2.98 ± 0.74 | 4.14 ± 1.32 | < 0.01 |
| 30-d readmissions | 1 (1.5%) | 1 (1.5%) | 1 |
| Mortality | 0 | 0 |
The adverse effects of probiotics administration in our study were closely monitored. The results are shown in Table 3. The most common adverse effect was exacerbating abdominal discomfort. In the probiotics group, about 18.7% (12/64) of patients experienced transient abdominal discomfort, such as abdominal pain (4.7%), flatulence (7.8%), and nausea (4.7%). The proportion was lower in that of the placebo group [14.1% (9/64)]. There was no significant difference between the two groups in the rate of exacerbating abdominal discomfort (P = 0.47). The symptoms were mild and transient despite the high abdominal discomfort rate. No anaphylactic reaction, probiotic-related infections, or lactic acidosis secondary to probiotics administration was found in our study.
| Probiotics (n = 64) | Placebo (n = 64) | P value | |
| Exacerbating abdominal discomfort | 12 | 9 | 0.47 |
| Abdominal pain | 3 | 2 | 1 |
| Nausea | 3 | 4 | 1 |
| Vomiting | 1 | 1 | 1 |
| Flatulence | 5 | 2 | 0.44 |
| Diarrhea | 0 | 0 | |
| Anaphylactic reaction | 0 | 0 | |
| Probiotic-related infections | 0 | 0 | |
| Lactic acidosis | 0 | 0 |
The present study investigated the effect of the administration of probiotics on patients with mild pancreatitis. We found that probiotics were associated with decreased LOS. The possible reasons were that probiotics may accelerate successful oral feeding and abdominal pain relief. The safety of probiotics in the treatment of mild pancreatitis in our study was also considered. Transient abdominal discomfort was the most common adverse effect found in our study, but no significant difference was found between the probiotics and placebo groups.
At present, studies on pancreatitis tend to focus on severe cases, including management of early acute inflammatory response, fluid resuscitation, and management of late infectious complications[18]. There are relatively fewer studies on mild acute pancreatitis because of its low mortality rate. However, mild acute pancreatitis accounts for up to 80% of all pancreatitis cases[3]. With the increasing pressure of medical costs due to mild acute pancreatitis, shortening the LOS and reducing the medical cost have become the primary target of acute pancreatitis treatment. At present, probiotics are mainly used to treat diarrhea, constipation, enteritis, abdominal distension, dyspepsia, and loss of appetite caused by intestinal flora imbalance. Some studies[10,19] suggested that probiotics were associated with decreased LOS, but these reports were of low quality and included a small sample of patients with severe acute pancreatitis. Besselink et al[8] conducted a study with the largest sample size of 298 randomized patients with severe acute pancreatitis; they found that probiotic prophylaxis did not reduce the risk of infectious complications and was associated with an increased risk of mortality. No difference was found in intensive care stay and hospital stay between their probiotics and non-probiotics groups.
In our randomized placebo-controlled study, probiotics were associated with a decreased LOS in patients with mild acute pancreatitis. In mild acute pancreatitis, rapid relief of abdominal pain and successful oral feeding were the main indications for hospital discharge. Based on previous gastrointestinal disease studies[20,21], we hypothesized that probiotics may improve intestinal microecology and food tolerance, reduce the inflammatory response, and consequently improve the possibility of successful oral feeding. Consistent with previous research results, our study showed accelerated successful oral feeding and abdominal pain relief after the administration of probiotics capsules consisting of Bacillus subtilis and Enterococcus faecium.
Adverse reactions to probiotics were closely observed in this study. The most frequent adverse effect was exacerbating abdominal discomfort (7%–9%), mostly experienced as flatulence. The reasons may be as follows: First, probiotic supplements are packaged as capsules that need to be taken orally with water, which contradicts the usual practice of diet prohibition before abdominal pain relief. As recommended in the 2013 International Association of Pancreatology and the American Pancreatic Association Evidence-Based Guidelines[22], and the 2018 American Gastroenterological Association Institute Guideline[23], oral feeding in mild pancreatitis can be restarted only when abdominal pain is decreasing and inflammatory markers are improving. The early administration of probiotic capsules may aggravate the gastrointestinal condition. Second, the role and target of probiotics on the intestinal flora in patients with disease states are not yet fully understood[24]. Rao et al[25] concluded that probiotics-fermented carbohydrates in the proximal small bowel induce intestinal bacterial overgrowth, resulting in increased gas output, and abdominal bloating.
The present study had several limitations. First, our results may not be generalizable to all patients with acute pancreatitis because we only included patients with mild acute pancreatitis. Although we showed that probiotics may be beneficial to patients with mild pancreatitis, the adverse reactions of probiotics should prompt clinician concern. Second, the results of this study are not generalizable to all probiotics since we only used Bacillus subtilis and Enterococcus faecium probiotic strains. At present, there are many types of probiotics with different clinical therapeutic and adverse effects that may be different or opposite to the findings of our study. Therefore, caution is advised in considering the results of this study.
In conclusion, probiotics supplementation appears to be safe and effective in reducing the length of hospitalization for patients with mild acute pancreatitis in this double-blinded randomized clinical trial. However, the sample size is small and the follow-up time is short in this study. In future studies, a randomized study with a larger sample using different types and doses of probiotics can be conducted to further clarify the role of probiotics in acute pancreatitis and other uninvestigated gastrointestinal diseases.
Acute pancreatitis is one of the most common causes of acute abdominal pain, resulting in a huge clinical and financial burden worldwide. Most patients (80%) with acute pancreatitis present with mild disease, which is characterized by a short hospital stay, no or few complications, and rapid resumption of oral feeding. Allowing for the very low mortality rate (about 0.79%) of mild acute pancreatitis, shortening the length of hospital stay (LOS), and reducing medical financial expenditure have become the research priority.
Probiotics play an important role in a variety of microecologic interventions. The effects of probiotics in mild acute pancreatitis have not been studied. Considering the potential benefits of probiotics in the treatment of gastrointestinal disease, we hypothesized that probiotics may accelerate the recovery of intestinal function and shorten the LOS in patients with mild pancreatitis. Thus, we conducted a randomized, double-blind, placebo-controlled trial to evaluate the effect of the administration of probiotics in patients with mild pancreatitis.
This study was designed to investigate the value of probiotics in reducing the LOS in patients with mild acute pancreatitis.
We conducted a double-blind randomized clinical trial to evaluate the effects of probiotics administered to patients with mild acute pancreatitis at a tertiary medical center. The patients were given probiotics capsules (a mixed preparation of Bacillus subtilis and Enterococcus faecium) or placebo. The primary study endpoint was the LOS. The secondary endpoints included time to abdominal pain relief, recurrent abdominal pain, and time to successful oral feeding.
A total of 128 patients were included, with 64 patients in each arm. The severity of illness and the etiological distribution of disease were similar in the two groups. There was a significant reduction in the LOS in the probiotics treatment group vs the placebo group (5.36 ± 0.15 vs 6.02 ± 0.17 d, P < 0.05). The probiotics group was associated with a shorter time to abdominal pain relief and time to successful oral feeding (P < 0.01 for both) than the placebo group. No statistical difference was found in recurrent abdominal pain between the two groups.
The study results showed that the administration of probiotics capsule is associated with a shorter duration of hospitalization in patients with mild acute pancreatitis.
Probiotics supplementation appears to be safe and effective in reducing the length of hospitalization for patients with mild acute pancreatitis in this double-blinded randomized clinical trial. However, the sample size is small and the follow-up time is short in this study. In future studies, a randomized study with a larger sample using different types and doses of probiotics can be conducted to further clarify the role of probiotics in acute pancreatitis and other uninvestigated gastrointestinal diseases.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Plaza MA S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The Changing Epidemiology of Acute Pancreatitis Hospitalizations: A Decade of Trends and the Impact of Chronic Pancreatitis. Pancreas. 2017;46:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, DiBonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143: 1179-1187. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1465] [Article Influence: 112.7] [Reference Citation Analysis (1)] |
| 3. | van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG; Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 4. | Nardone G, Compare D, Rocco A. A microbiota-centric view of diseases of the upper gastrointestinal tract. Lancet Gastroenterol Hepatol. 2017;2:298-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Kong F, Cai Y. Study Insights into Gastrointestinal Cancer through the Gut Microbiota. Biomed Res Int. 2019;2019:8721503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5532] [Article Influence: 502.9] [Reference Citation Analysis (2)] |
| 7. | Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 1053] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 8. | Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 858] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 9. | Gou S, Yang Z, Liu T, Wu H, Wang C. Use of probiotics in the treatment of severe acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18:R57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Tian X, Pi YP, Liu XL, Chen H, Chen WQ. Supplemented Use of Pre-, Pro-, and Synbiotics in Severe Acute Pancreatitis: An Updated Systematic Review and Meta-Analysis of 13 Randomized Controlled Trials. Front Pharmacol. 2018;9:690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Li SR, Wang HH, Wu ZY, Liu RH, Tong MH, Wang CH, Wang RL, Zhao HC, Wei W. [Efficacies of lactulose plus live combined Bacillus subtilis and Enterococcus faecium capsules in the treatment of functional constipation: a multicenter, randomized, double blind, controlled trial]. Zhonghua Yi Xue Za Zhi. 2012;92:2955-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4315] [Article Influence: 359.6] [Reference Citation Analysis (45)] |
| 13. | Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60 Suppl 2:S129-S134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 14. | Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, Huys G, Levy DD, Lutgendorff F, Mack D, Phothirath P, Solano-Aguilar G, Vaughan E. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 445] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 15. | Rao SSC, Rehman A, Yu S, Andino NM. Brain fogginess, gas and bloating: a link between SIBO, probiotics and metabolic acidosis. Clin Transl Gastroenterol. 2018;9:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Moraes JM, Felga GE, Chebli LA, Franco MB, Gomes CA, Gaburri PD, Zanini A, Chebli JM. A full solid diet as the initial meal in mild acute pancreatitis is safe and result in a shorter length of hospitalization: results from a prospective, randomized, controlled, double-blind clinical trial. J Clin Gastroenterol. 2010;44:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Riquelme F, Marinkovic B, Salazar M, Martínez W, Catan F, Uribe-Echevarría S, Puelma F, Muñoz J, Canals A, Astudillo C, Uribe M. Early laparoscopic cholecystectomy reduces hospital stay in mild gallstone pancreatitis. A randomized controlled trial. HPB (Oxford). 2020;22:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 577] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 19. | Karakan T, Ergun M, Dogan I, Cindoruk M, Unal S. Comparison of early enteral nutrition in severe acute pancreatitis with prebiotic fiber supplementation versus standard enteral solution: a prospective randomized double-blind study. World J Gastroenterol. 2007;13:2733-2737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Fam Physician. 2017;96:170-178. [PubMed] |
| 21. | Parker EA, Roy T, D'Adamo CR, Wieland LS. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018; 45: 125-134. e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1035] [Article Influence: 86.3] [Reference Citation Analysis (6)] |
| 23. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 24. | Lerner A, Shoenfeld Y, Matthias T. Probiotics: If It Does Not Help It Does Not Do Any Harm. Really? Microorganisms. 2019;7:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Rao SSC, Yu S, Tetangco EP, Yan Y. Probiotics can Cause D-Lactic Acidosis and Brain Fogginess: Reply to Quigley et al. Clin Transl Gastroenterol. 2018;9:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |