Published online Apr 21, 2021. doi: 10.3748/wjg.v27.i15.1643
Peer-review started: December 22, 2020
First decision: January 27, 2021
Revised: February 4, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: April 21, 2021
Processing time: 112 Days and 21.3 Hours
In the early stage of acute pancreatitis (AP), a large number of cytokines induced by local pancreatic inflammation seriously damage the intestinal barrier function, and intestinal bacteria and endotoxins enter the blood, causing inflammatory storm, resulting in multiple organ failure, infectious complications, and other disorders, eventually leading to death. Intestinal failure occurs early in the course of AP, accelerating its development. As an alternative method to detect small intestinal bacterial overgrowth, the hydrogen breath test is safe, noninvasive, and convenient, reflecting the number of intestinal bacteria in AP indirectly. This study aimed to investigate the changes in intestinal bacteria measured using the hydrogen breath test in the early stage of AP to clarify the relationship between intestinal bacteria and acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Early clinical intervention and maintenance of intestinal barrier function would be highly beneficial in controlling the development of severe acute pancreatitis (SAP).
To analyze the relationship between intestinal bacteria change and ALI/ARDS in the early stage of SAP.
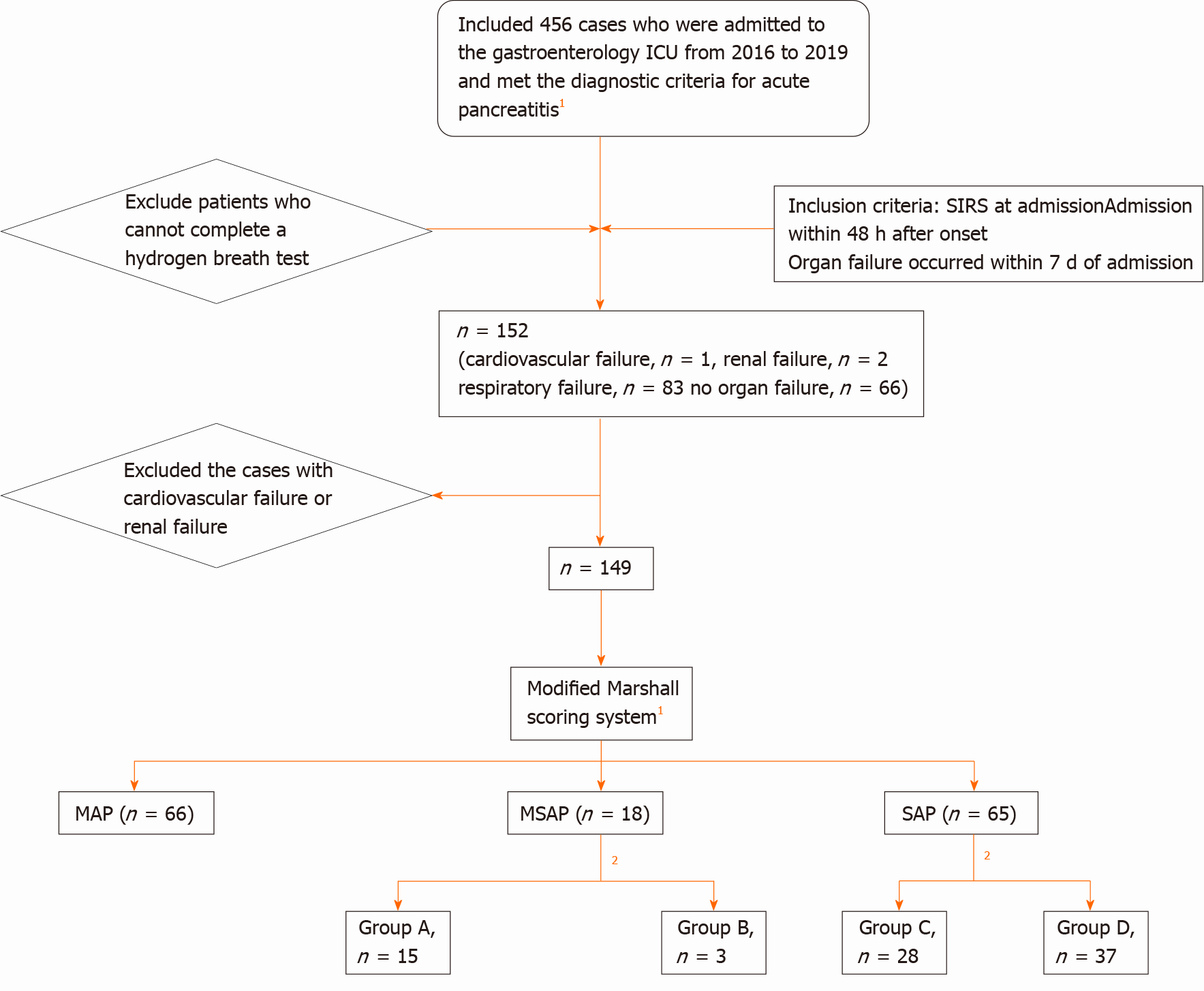
A total of 149 patients with AP admitted to the intensive care unit of the Digestive Department, Xuanwu Hospital, Capital Medical University from 2016 to 2019 were finally enrolled, following compliance with the inclusion and exclusion criteria. The results of the hydrogen breath test within 1 wk of admission were collected, and the hydrogen production rates at admission, 72 h, and 96 h were calculated. The higher the hydrogen production rates the more bacteria in the small intestine. First, according to the improved Marshall scoring system in the 2012 Atlanta Consensus on New Standards for Classification of Acute Pancreatitis, 66 patients with a PaO2/FiO2 score ≤ 1 were included in the mild AP (MAP) group, 18 patients with a PaO2/FiO2 score ≥ 2 and duration < 48 h were included in the moderately SAP (MSAP) group, and 65 patients with a PaO2/FiO2 score ≥ 2 and duration > 48 h were included in the SAP group, to analyze the correlation between intestinal bacterial overgrowth and organ failure in AP. Second, ALI (PaO2/FiO2 = 2) and ARDS (PaO2/FiO2 > 2) were defined according to the simplified diagnostic criteria proposed by the 1994 European Union Conference. The MSAP group was divided into two groups according to the PaO2/FiO2 score: 15 patients with PaO2/FiO2 score = 2 were included in group A, and three patients with score > 2 were included in group B. Similarly, the SAP group was divided into two groups: 28 patients with score = 2 were included in group C, and 37 patients with score > 2 were included in group D, to analyze the correlation between intestinal bacterial overgrowth and ALI/ARDS in AP.
A total of 149 patients were included: 66 patients in the MAP group, of whom 53 patients were male (80.3%) and 13 patients were female (19.7%); 18 patients in the MSAP group, of whom 13 patients were male (72.2%) and 5 patients were female (27.8%); 65 patients in the SAP group, of whom 48 patients were male (73.8%) and 17 patients were female (26.2%). There was no significant difference in interleukin-6 and procalcitonin among the MAP, MSAP, and SAP groups (P = 0.445 and P = 0.399, respectively). There was no significant difference in the growth of intestinal bacteria among the MAP, MSAP, and SAP groups (P = 0.649). There was no significant difference in the growth of small intestinal bacteria between group A and group B (P = 0.353). There was a significant difference in the growth of small intestinal bacteria between group C and group D (P = 0.038).
Intestinal bacterial overgrowth in the early stage of SAP is correlated with ARDS.
Core Tip: Our retrospective study included 149 patients with acute pancreatic disease. The changes in intestinal flora were detected by the hydrogen breath test. We found that the change in intestinal flora in patients with severe acute pancreatitis was related to acute respiratory distress syndrome, which can aggravate the disease. The results of the hydrogen breath test can be used as a warning signal for severe acute pancreatitis.
- Citation: Liang XY, Jia TX, Zhang M. Intestinal bacterial overgrowth in the early stage of severe acute pancreatitis is associated with acute respiratory distress syndrome. World J Gastroenterol 2021; 27(15): 1643-1654
- URL: https://www.wjgnet.com/1007-9327/full/v27/i15/1643.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i15.1643
Acute pancreatitis (AP) is a common form of acute abdomen. The mortality rate of severe AP (SAP) with early organ failure can be as high as 36%-50%[1]. The earliest SAP-related organ dysfunction occurs in the respiratory system with the main symptoms of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)[2,3]. In the early stage of AP, a large number of cytokines is induced by local pancreatitis to enter the bloodstream, causing systemic inflammatory response syndrome (SIRS) through the cytokine cascade reaction; the large number of released cytokines can seriously damage the intestinal barrier function and increase intestinal wall permeability, intestinal microbial flora imbalance, intestinal bacterial overgrowth, and intestinal bacterial translocation. When intestinal bacteria and endotoxins enter the blood, leading to multiple organ failure (multiple organ dysfunction syndrome), the end result is death[4].
The results of animal experiments have shown that when intestinal ischemia occurs in SAP, the mechanical barrier of the intestinal tract is damaged, the permeability of small intestine and colon mucosa is increased, and damage in the small intestine becomes more obvious; at that time, small intestine peristalsis decreases or even disappears, and the number of bacteria in the small intestine and colon rapidly increases, leading to small intestinal bacterial overgrowth (SIBO)[5]. Cen et al[4] established a model of intestinal motility disorder in AP rats, indicating that gastrointestinal motility disorder is the early cause of SIBO. Intestinal motility disorder is caused by neurohormone-mediated intestinal motility reduction induced by AP[6]. Escherichia coli and other bacteria grow excessively due to stagnation of substances in the cavity.
The gold standard for SIBO evaluation is quantitative culture of duodenal or jejunal aspiration under aerobic and anaerobic conditions, and a study found colony formation higher than 103 CFU/mL (colony-forming unit/mL)[7]. Because of the high cost and invasive nature of small intestinal fluid aspiration culture, the hydrogen breath test, as an alternative method for SIBO testing, has been suggested as an inexpensive, safe, and noninvasive alternative. It is widely used in the clinic as an important method to diagnose SIBO. However, the sensitivity range of the hydrogen breath test is 20%-90%, possibly because of the lack of uniform diagnostic criteria, which has been resolved by the recently published North American breath test consensus[8].
The lactose hydrogen respiration test is a noninvasive semi-qualitative test, originally designed to measure the oral-cecum passage time; it is currently used for many diseases of the digestive system, such as lactose intolerance, irritable bowel syndrome, dyspepsia, and non-alcoholic fatty liver[9,10]. The premise of the hydrogen breath test to detect SIBO is that the human metabolism does not produce hydrogen or methane. Methanogens are not bacteria, but belong to the category of archaea, mainly present in the colon, accounting for 10% of all anaerobic organisms[7]. Since the production of methane requires hydrogen metabolism, the hydrogen and methane concentrations can be simultaneously detected with the carbohydrate breath test. The lactulose hydrogen respiration test involves oral lactulose (non-absorbent sugar) to the cecum, fermentation with coliform bacteria to produce hydrogen and methane, absorption into the systemic circulation, and then exhalation to detect the concentration of hydrogen/methane in the breath; when the concentration of hydrogen/methane in the breath exceeds the baseline, a SIBO diagnosis can be reached[11].
This study aimed to examine the change in intestinal bacteria measured using the hydrogen breath test in the early stage of AP to elucidate the relationship between intestinal bacteria and ALI/ARDS. Early clinical intervention and maintenance of intestinal barrier function will be very beneficial in controlling the development of SAP.
We retrospectively analyzed the data of 456 patients with AP who met the diagnostic criteria of the 2012 "Consensus on New Atlanta Classification Standards for Acute Pancreatitis" and who were admitted to the intensive care unit (ICU) of the Department of Digestive Department, Xuanwu Hospital of Capital Medical University from 2016 to 2019. A total of 152 patients were admitted with SIRS or organ failure and could cooperate with the hydrogen breath test within 1 wk of admission. According to the 2012 "Consensus on New Atlanta Classification Standards for Acute Pancreatitis" improved Marshall scoring standard[1], 1 patient had circulatory failure, 2 patients had renal failure, 66 patients had no organ failure, and 83 patients had respiratory failure. One patient with circulatory failure and 2 patients with renal failure were excluded. A total of 149 patients were finally included. General data including age, sex, body mass index (BMI), Acute Physiology and Chronic Health Evaluation (APACHE)-II score, and mediators of inflammation were collected.
Inclusion criteria: (1) admission within 48 h after onset; (2) admission to the hospital with SIRS; (3) organ failure within 1 wk after admission; (4) age older than 18 years; and (5) clear mind, understanding ability normal, ability to communicate with the physician.
Exclusion criteria: (1) chronic pancreatitis; (2) history of immune-related diseases, immune deficiency, and AIDS; (3) malignant tumors; (4) inability to cooperate and complete the hydrogen breath test; (5) incomplete case data; or (6) circulatory failure or renal failure.
According to the improved Marshall score standard in the 2012 "Consensus on New Atlanta Classification Standards for Acute Pancreatitis"[1]: PaO2/FiO2 ≥ 301 mmHg was scored as 1 point, 201 mmHg ≤ PaO2/FiO2 ≤ 300 mmHg was scored as 2 points, 101 mmHg ≤ PaO2/FiO2 ≤ 200 mmHg was scored as 3 points, and PaO2/FiO2 ≤ 101 mmHg was scored as 4 points.
Methods: 66 patients with a PaO2/FiO2 score ≤ 1 were included in the mild AP (MAP) group, 18 patients with a PaO2/FiO2 score ≥ 2 and duration < 48 h were included in the moderately SAP (MSAP) group, and 65 patients with a PaO2/FiO2 score ≥ 2 and duration > 48 h were included in the SAP group (Figure 1).
ALI and ARDS: ALI and ARDS were defined according to the European Union Conference[12]. The simplified criteria recommended by the meeting included defining arterial hypoxia PaO2/FiO2 < 300 mmHg as ALI and PaO2/FiO2 < 200 mmHg as ARDS, excluding cardiogenic pulmonary edema. In this study, a PaO2/FiO2 score = 2 was used to define ALI, and a PaO2/FiO2 score > 2 was used to define ARDS.
Methods: The MSAP group was divided into two groups according to the PaO2/FiO2 score: 15 patients with PaO2/FiO2 score = 2 were included in group A, and 3 patients with score > 2 were included in group B. Similarly, the SAP group was divided into two groups: 28 patients with score = 2 were included in group C, and 37 patients with score > 2 were included in group D (Figure 1).
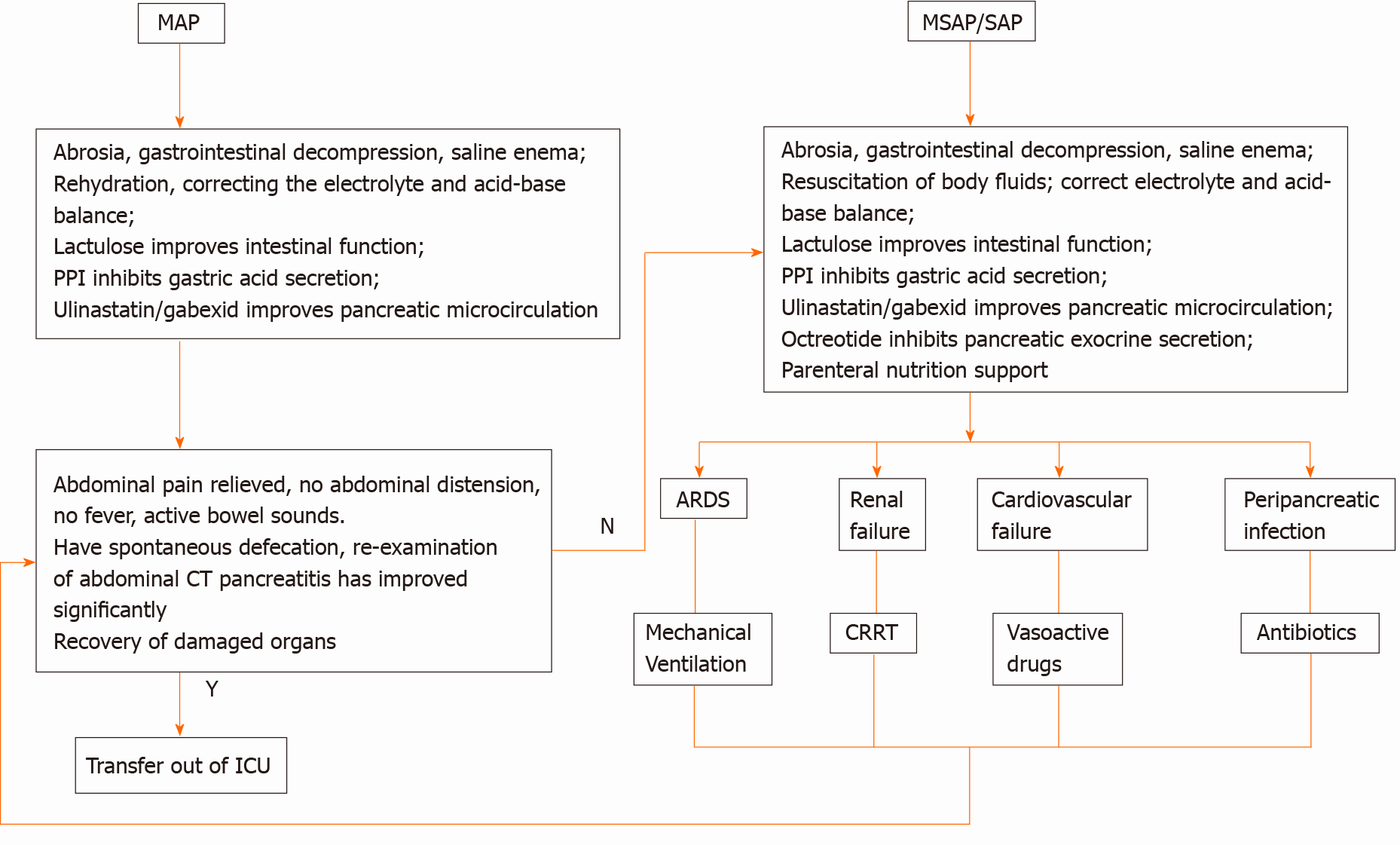
MAP: (1) abrosia, gastrointestinal decompression, saline enema; (2) fluid resuscitation, water maintenance, electrolyte and acid-base balance; and (3) lactulose to improve intestinal function, proton pump inhibitors for gastric acid secretion, ulinastatin and/or gabexate to improve pancreatic microcirculation.
MSAP/SAP: (1) abrosia, gastrointestinal decompression, saline enema; (2) fluid resuscitation, water maintenance, electrolyte and acid-base balance; and (3) lactulose to improve intestinal function, proton pump inhibitors for gastric acid secretion, ulinastatin and/or gabexate to improve pancreatic microcirculation; octreotide to inhibit pancreatic exocrine function, and parenteral nutrition support.
When ARDS appeared it was treated with a mechanical ventilator. When renal failure appeared it was treated with CRRT (continuous renal replacement therapy). Vasoactive drugs were used to increase blood pressure when circulatory failure could not be corrected. Peri-pancreatic and extra-pancreatic infections were treated with anti-infection therapy.
Outcome: After treatment, when there was no abdominal pain, abdominal distension, or fever, and when there were active bowel sounds, spontaneous defecation, and the abdominal computed tomography re-examination showed marked improvement, the patient could be discharged from the ICU (Figure 2).
Test methods: The patient remained awake and quiet during the examination and did not exercise vigorously. For the detection process, the fasting breath was collected twice, and the highest value was used as the basic exhaled hydrogen concentration (H0). The subjects were asked to quickly consume a lactulose solution including 10 g of lactulose in 100 mL of room-temperature pure water. Subjects then exhaled every 20 min 9 times (H1-H9). The hydrogen concentration in the exhalation was measured and recorded at each time point.
Data processing: Hydrogen production at admission, 72 h and 96 h was measured. The average hydrogen production rate was calculated as follows: [Hn-H(n-1)]/H0, (0< n ≤ 9).
SPSS 25.0 (IBM Corp., Armonk, NY, United States) was used for data analysis, P < 0.05 was considered statistically significant. Age, BMI, APACHE-II score, and mediators of inflammation were measured, and the data are expressed as mean ± SD; and one-way analysis of variance (ANOVA) was used to compare the two groups. The hydrogen breath test data are expressed as the average hydrogen production rate ± SD, and the repeated-measures ANOVA was used in the comparison between the two groups. The number and percentage of counting data were expressed, and the chi-square test was used for comparison between the groups.
A total of 149 patients were included: 66 patients in the MAP group, of whom, 53 patients were male (80.3%) and 13 patients were female (19.7%); 18 patients in the MSAP group, of whom 13 patients were male (72.2%) and 5 patients were female (27.8%); 65 patients in the SAP group, of whom 48 patients were male (73.8%) and 17 patients were female (26.2%) (Table 1). There was a significant difference in age, sex, and APACHE-II score among the MAP, MSAP, and SAP groups (both, P < 0.001) (Table 1).
| Variable | Total | MAP group | MSAP group | SAP group |
| Number of patients | 149 | 66 | 18 | 65 |
| Age, yr, (mean ± SD) | 42.26 ± 12.95 | 36.62 ± 9.74 | 47.17 ± 15.17 | 43.00 ± 13.95 |
| Sex (male/female) | 114/35 | 53/13 | 13/5 | 48/17 |
| BMI (kg/m2) | 28.21 ± 3.85 | 27.19 ± 3.77 | 27.98 ± 4.60 | 29.47 ± 3.77 |
| APACHE-II score | 5.92 ± 3.02 | 4.00 ± 3.23 | 6.50 ± 4.75 | 7.26 ± 4.07 |
| Mortality (%) | 0 | 0 | 0 | 0 |
There was no significant difference in interleukin (IL)-6 and procalcitonin among the MAP, MSAP, and SAP groups (P = 0.445 and P = 0.399, respectively) (Table 2). There was no significant difference in IL-6 and procalcitonin among the MAP and MSAP groups (P = 0.306 and P = 0.683, respectively) (Table 3). There was no significant difference in IL-6 and procalcitonin among the MAP and SAP groups (P = 0.879 and P = 0.311, respectively) (Table 4). There was no significant difference in IL-6 and procalcitonin among the MSAP and SAP groups (P = 0.455 and P = 0.150, respectively) (Table 5).
| Variable | Time | MAP group | MSAP group | SAP group | P value |
| IL-6 | On admission | 118.62 ± 137.66 | 98.36 ± 106.36 | 143.74 ± 174.40 | 0.445 |
| PCT | On admission | 0.46 ± 1.29 | 0.46 ± 0.74 | 0.67 ± 1.01 | 0.399 |
| 72 h | 0.35 ± 0.57 | 0.39 ± 0.49 | 0.66 ± 0.83 | ||
| 96 h | 0.54 ± 2.51 | 0.19 ± 0.12 | 0.51 ± 0.64 |
| Variable | Time | MAP group | MSAP group | P value |
| IL-6 | On admission | 118.62 ± 137.66 | 98.36 ± 106.36 | 0.306 |
| PCT | On admission | 0.46 ± 1.29 | 0.46 ± 0.74 | 0.683 |
| 72 h | 0.35 ± 0.57 | 0.39 ± 0.49 | ||
| 96 h | 0.54 ± 2.51 | 0.19 ± 0.12 |
| Variable | Time | MAP group | SAP group | P value |
| IL-6 | On admission | 118.62 ± 137.66 | 143.74 ± 174.40 | 0.879 |
| PCT | On admission | 0.46 ± 1.29 | 0.67 ± 1.01 | 0.311 |
| 72 h | 0.35 ± 0.57 | 0.66 ± 0.83 | ||
| 96 h | 0.54 ± 2.51 | 0.51 ± 0.64 |
| Variable | Time | MSAP group | SAP group | P value |
| IL-6 | On admission | 98.36 ± 106.36 | 143.74 ± 174.40 | 0.455 |
| PCT | On admission | 0.46 ± 0.74 | 0.67 ± 1.01 | 0.150 |
| 72 h | 0.39 ± 0.49 | 0.66 ± 0.83 | ||
| 96 h | 0.19 ± 0.12 | 0.51 ± 0.64 |
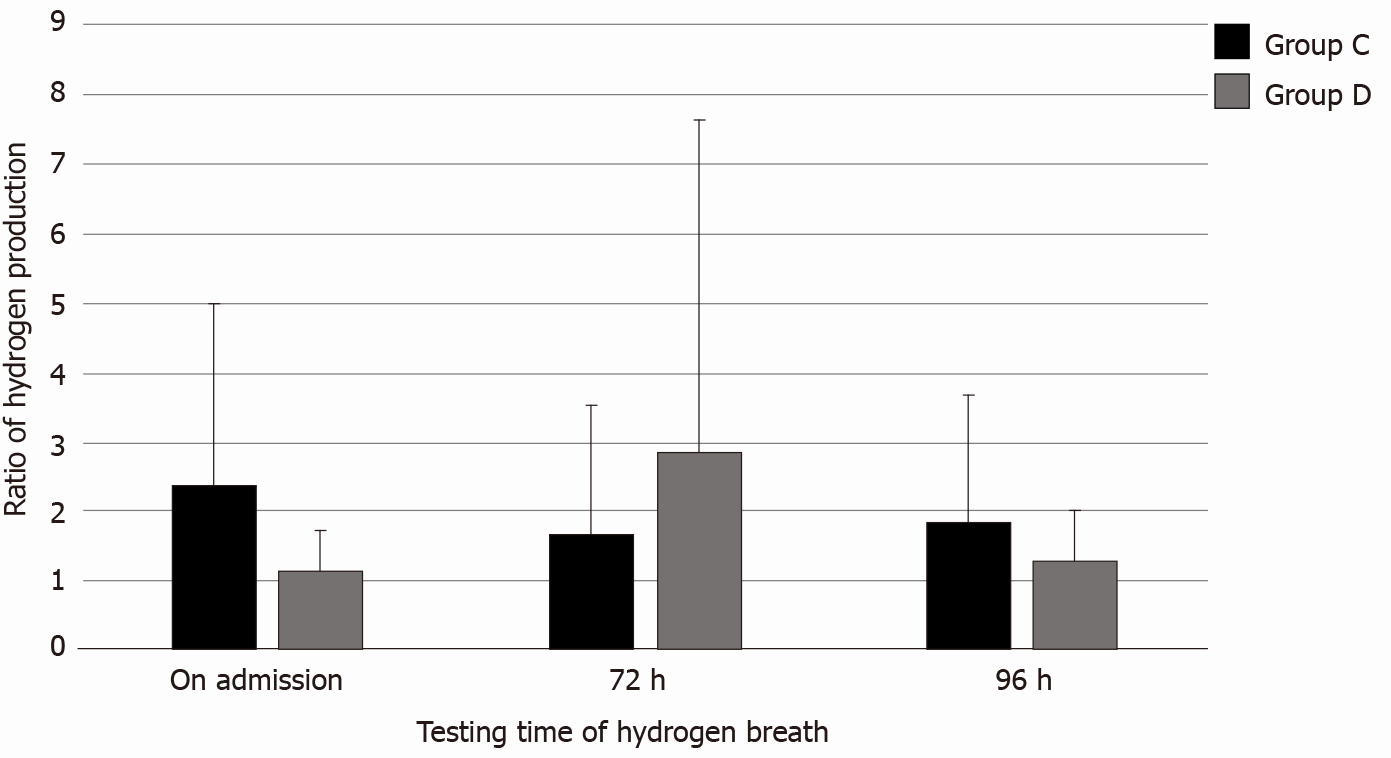
There was no significant difference in the number of intestinal bacteria among the MAP, MSAP, and SAP groups (P = 0.649) (Table 6). There was no significant difference in the number of intestinal bacteria among the MAP and MSAP groups (P = 0.196) (Table 7). There was no significant difference in the number of intestinal bacteria among the MAP and SAP groups (P = 0.494) (Table 8). There was no significant difference in the number of intestinal bacteria among the MSAP and SAP groups (P = 0.784) (Table 9). There was no significance difference in the number of intestinal bacteria and ALI/ARDS between group A and group B (P = 0.353) (Table 10). There was a significant difference in the number of intestinal bacteria and ALI/ARDS between group C and group D (P = 0.038) (Table 11, Figure 3). The changes in intestinal bacteria in the early stage of SAP were correlated with ARDS.
| Hydrogen breath test | MAP group | MSAP group | SAP group | P value |
| On admission | 1.63 ± 1.28 | 2.35 ± 2.14 | 1.68 ± 1.85 | 0.649 |
| 72 h | 1.58 ± 1.29 | 1.83 ± 2.58 | 2.13 ± 3.27 | |
| 96 h | 1.60 ± 1.22 | 1.58 ± 0.93 | 1.54 ± 1.35 |
| Hydrogen breath test | MAP group | MSAP group | P value |
| On admission | 1.63 ± 1.28 | 2.35 ± 2.14 | 0.196 |
| 72 h | 1.58 ± 1.29 | 1.83 ± 2.58 | |
| 96 h | 1.60 ± 1.22 | 1.58 ± 0.93 |
| Hydrogen breath test | MAP group | SAP group | P value |
| On admission | 1.63 ± 1.28 | 1.68 ± 1.85 | 0.494 |
| 72 h | 1.58 ± 1.29 | 2.13 ± 3.27 | |
| 96 h | 1.60 ± 1.22 | 1.54 ± 1.35 |
| Hydrogen breath test | MSAP group | SAP group | P value |
| On admission | 2.35 ± 2.14 | 1.68 ± 1.85 | 0.784 |
| 72 h | 1.83 ± 2.58 | 2.13 ± 3.27 | |
| 96 h | 1.58 ± 0.93 | 1.54 ± 1.35 |
| Hydrogen breath test | P value | MSAP group (n = 18) | |
| Group A (n = 15) | Group B (n = 3) | ||
| On admission | 0.353 | 2.59 ± 2.28 | 1.12 ± 0.04 |
| 72 h | 1.97 ± 2.81 | 1.08 ± 0.43 | |
| 96 h | 1.54 ± 0.95 | 1.82 ± 0.95 | |
| Hydrogen breath test | P value | SAP group (n = 65) | |
| Group C (n = 28) | Group D (n = 37) | ||
| On admission | 0.038 | 2.39 ± 2.61 | 1.15 ± 0.58 |
| 72 h | 1.68 ± 1.85 | 2.85 ± 4.79 | |
| 96 h | 1.84 ± 1.86 | 1.31 ± 0.71 | |
In this study, we used the hydrogen breath test to detect changes in intestinal bacteria in the early stage of AP and demonstrated that there was a correlation between ARDS and SIBO in the early stage of SAP.
This study also had some limitations. The results of the hydrogen breath test are easily affected by the number of aerogenes and the enzymatic activity of fructase. The number of gas-producing bacteria in the intestinal tract is affected by individual factors, including the environment and dietary habits, resulting in varying numbers of gas-producing bacteria among individuals. Abrosia and the use of antibiotics[13] also affect the number of gas-producing bacteria. The higher the enzymatic activity of fructase, the more gas is produced[11]. Routine lactulose treatment can improve the intestinal acid-base environment, reduce intestinal bacteria, and inhibitory activity[14]; there is also the catharsis effect of reduced intestinal bacteria; long-term lactulose treatment is equivalent to increasing the dose of lactulose in the hydrogen breath test, resulting in higher or lower results. In this study, quantitative analysis and the growth ratio were used to neutralize the influence of the basic value on the qualitative results.
As early as 2017, Zhang et al[15] and others were using the qualitative standard of the hydrogen breath test to examine the relationship between SAP and SIBO. The results showed that damage of intestinal barrier function is already present in the early stage of SAP; the positive SIBO rate was higher in patients with SAP, and SIBO mainly occurred within 72 h from onset. Finally, we found that there was a correlation between ARDS and SIBO in the early stage of SAP (P < 0.05) in line with the results of previous studies. ALI/ARDS are the earliest organ-dysfunction symptoms in SAP. In this study, it was concluded that the occurrence of ARDS was related to SIBO within 72 h of admission. Studies have shown that SAP intestinal failure appears early, consistent with the results of our study. SAP-induced ALI/ARDS is mainly due to lung injury mediated by inflammatory factors[16]. Both infectious and non-infectious factors can lead to a large number of inflammatory factors. In the early stage of AP, inflammatory factors produced by pancreatic digestion damage intestinal barrier function, leading to persistent SIRS and SIBO, with SIBO leading to harmful metabolite accumulation in the intestinal tract, especially endotoxins and pathogenic intestinal bacteria[17]; subsequently, a large number of endotoxins and bacteria enter the bloodstream, flow through the pulmonary capillaries, activate neutrophils, damage the pulmonary vascular endothelium, leading to diffuse alveolar injury and type I alveolar necrosis, and eventually to ALI/ARDS[18,19]. Zhang et al[20] found that serum endotoxins and intestinal mucosal permeability in patients with SAP were higher than in normal controls. Wang et al[3] found that lung bacteria were rich in intestine-related bacteria in mice with AP, sepsis, and ARDS. This study only showed the translocation of intestinal bacteria when sepsis and ARDS occurred simultaneously but did not explain the relationship between intestinal bacteria and ARDS when only ARDS occurred. Unfortunately, we could not locate animal experimental data on the correlation between ARDS and intestinal bacteria in the early stage of AP. The results of this clinical study showed that ARDS was correlated with intestinal bacteria, which should be further explored using animal models. However, although animal test support is lacking, in a clinical study on the relationship between the onset time and treatment time window of AP[21], the researchers concluded that the key time window for AP treatment is within 1 wk after the onset of AP; otherwise, the disease will worsen, the clinical course will be prolonged, and the mortality rate will increase; in terms of time, this study completely agrees with the previous research results. Therefore, the results of this study are of high guiding significance for clinicians who manage patients with AP. Early attention to the changes in intestinal bacteria can prevent the occurrence of SAP and high mortality.
This study also explored the correlation between IL-6 and ALI/ARDS. At present, the research results show that IL-6 is related to SAP. IL-6 can be used as an early predictor of complications[22]. The specificity of serum IL-6 > 160 pg/mL in the diagnosis of SAP was 95%[23]. Relevant animal experiments have shown that when pancreatic injury occurs, IL-6 is rapidly synthesized to play an anti-inflammatory and protective role. When IL-6 continues to be synthesized and accumulates in large quantities, it mainly plays a pro-inflammatory role, leading to the occurrence and development of SAP[22]. In a clinical study conducted by Rezaie et al[13], it was found that the content of IL-6 in the blood was positively correlated with the abundance of Enterobacteriaceae and Enterococci, but negatively correlated with the abundance of Bifidobacteria. That study also compared the characteristics of intestinal bacteria between patients with AP and healthy controls. The results showed that the intestinal bacteria of patients with AP and healthy controls differed, and the abundance of Enterobacteriaceae and Enterococci and other potentially pathogenic bacteria differed from the abundance of beneficial bacteria such as Bifidobacteria, which decreased significantly. Although the final results of this study did not show a correlation between IL-6 and SAP, it is considered that this may be related to the treatments administered after admission.
Early organ failure in SAP was found to be closely related to the intestinal bacteria. Importantly, this study also determined the timing of SIBO, which is of great guiding significance for the clinical treatment of AP.
In the early stage of acute pancreatitis (AP), a large number of cytokines induced by local pancreatic inflammation seriously destroy the intestinal barrier function, resulting in intestinal bacteria and endotoxins in the blood, causing inflammatory storms, and leading to multiple organ failure. Hydrogen breath testing is currently used for many diseases of the digestive system, such as lactose intolerance, irritable bowel syndrome, dyspepsia, and non-alcoholic fatty liver. This test, as an alternative method to detect small intestinal bacterial overgrowth (SIBO), was used to indirectly reflect the number of intestinal bacteria in AP. This study aimed to examine the change in intestinal bacteria measured using the hydrogen breath test in the early stage of AP to elucidate the relationship between intestinal bacteria and acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Early clinical intervention and maintenance of intestinal barrier function will be very beneficial in controlling the development of severe AP (SAP).
Clinical trials showed that intestinal bacteria in AP patients were different to those in healthy people and the abundance of potential pathogenic bacteria such as Enterobacteriaceae and Enterococcus increased significantly while the abundance of beneficial bacteria such as Bifidobacterium decreased significantly. Animal experiments showed that not only the changes in intestinal bacteria in patients with AP were verified, but also there was a correlation between the changes in intestinal bacteria and organ failure. However, neither of them showed a relationship between intestinal bacteria and organ failure in the early stage of AP. If we can understand the change in intestinal bacteria using the hydrogen breath test in the early stage of AP, in order to understand the relationship between intestinal bacteria and ALI/ARDS, we can intervene and maintain the stability of intestinal barrier function as soon as possible, which will be very beneficial in controlling the development of SAP.
Maintaining intestinal barrier function and reducing and preventing intestinal bacteria translocation have become the key to controlling the occurrence and development of SAP. This reduces complications in the early stage of the disease. We performed the present study to investigate the changes in organ function and intestinal bacteria in AP, and to explore the correlation between SIBO and AP organ function.
Principle of the hydrogen breath test: after taking lactulose (nonabsorbable sugar), it reaches the cecum and is fermented with coliform bacteria to produce hydrogen and methane, which are absorbed into the systemic circulation and exhaled through the lung. When the hydrogen concentration in the exhaled breath exceeds the baseline, it is diagnosed as SIBO. In this study, a portable hydrogen expiratory detector was used to detect the patients’ expired hydrogen concentration to diagnose SIBO. The change in exhaled hydrogen concentration can reflect the number of bacteria in the small intestine.
The results showed that in 37 SAP patients with ARDS, the average hydrogen production rate at admission was 1.15 ± 0.58, at 72 h was 2.85 ± 4.79 and at 96 h was 1.31 ± 0.71. In 28 SAP patients without ARDS, the average hydrogen production rate at admission was 2.39 ± 2.61, at 72 h was 1.68 ± 1.85, and at 96 h was 1.84 ± 1.86. The increase in intestinal bacteria in patients with SAP within 72 h after admission was related to the occurrence of ARDS. How to effectively maintain the stability of intestinal barrier function in the early stage of the disease and prevent the overgrowth of intestinal bacteria is a problem to be solved.
In this study, intestinal bacterial overgrowth in the early stage of SAP was associated with ARDS. Moreover, the occurrence of ARDS was related to SIBO within 72 h of admission. It is of guiding significance to maintain the stability of intestinal barrier function in the early stage in order to reduce the complications of early organ failure and late infection.
How to effectively maintain the stability of intestinal barrier function and prevent the excessive growth of intestinal bacteria in the early stage of disease, in order to reduce the occurrence and development of SAP, is the future research direction.
We thank Sun F, Professor of Medical Statistics, for advice on statistical methods and comments on the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar N, Lens S, Levick C S-Editor: Gao CC L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4337] [Article Influence: 361.4] [Reference Citation Analysis (45)] |
| 2. | Wang H, Li C, Jiang Y, Li H, Zhang D. Effects of Bacterial Translocation and Autophagy on Acute Lung Injury Induced by Severe Acute Pancreatitis. Gastroenterol Res Pract. 2020;2020:8953453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Wang C, Li Q, Ren J. Microbiota-Immune Interaction in the Pathogenesis of Gut-Derived Infection. Front Immunol. 2019;10:1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Cen ME, Wang F, Su Y, Zhang WJ, Sun B, Wang G. Gastrointestinal microecology: a crucial and potential target in acute pancreatitis. Apoptosis. 2018;23:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Li XY, He C, Zhu Y, Lu NH. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J Gastroenterol. 2020;26:2187-2193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (3)] |
| 6. | Yuan BS, Zhu RM, Braddock M, Zhang XH, Shi W, Zheng MH. Interleukin-18: a pro-inflammatory cytokine that plays an important role in acute pancreatitis. Expert Opin Ther Targets. 2007;11:1261-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Massey BT, Wald A. Small Intestinal Bacterial Overgrowth Syndrome: A Guide for the Appropriate Use of Breath Testing. Dig Dis Sci. 2021;66:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Cangemi DJ, Lacy BE, Wise J. Diagnosing Small Intestinal Bacterial Overgrowth: A Comparison of Lactulose Breath Tests to Small Bowel Aspirates. Dig Dis Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Yao CK, Tuck CJ. The clinical value of breath hydrogen testing. J Gastroenterol Hepatol. 2017;32 Suppl 1:20-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Takakura W, Pimentel M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome - An Update. Front Psychiatry. 2020;11:664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Pawłowska K, Seredyński R, Umławska W, Iwańczak B. Hydrogen excretion in pediatric lactose malabsorbers: relation to symptoms and the dose of lactose. Arch Med Sci. 2018;14:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4145] [Cited by in RCA: 4158] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 13. | Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. 2017;112:775-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 517] [Article Influence: 64.6] [Reference Citation Analysis (1)] |
| 14. | He T, Venema K, Priebe MG, Welling GW, Brummer RJ, Vonk RJ. The role of colonic metabolism in lactose intolerance. Eur J Clin Invest. 2008;38:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Zhu HM, He F, Li BY, Li XC. Association between acute pancreatitis and small intestinal bacterial overgrowth assessed by hydrogen breath test. World J Gastroenterol. 2017;23:8591-8596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Murr MM, Yang J, Fier A, Kaylor P, Mastorides S, Norman JG. Pancreatic elastase induces liver injury by activating cytokine production within Kupffer cells via nuclear factor-Kappa B. J Gastrointest Surg. 2002;6:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Wig JD, Kochhar R, Ray JD, Krishna Rao DV, Gupta NM, Ganguly NK. Endotoxemia predicts outcome in acute pancreatitis. J Clin Gastroenterol. 1998;26:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Gea-Sorlí S, Closa D. Role of macrophages in the progression of acute pancreatitis. World J Gastrointest Pharmacol Ther. 2010;1:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Gloor B, Blinman TA, Rigberg DA, Todd KE, Lane JS, Hines OJ, Reber HA. Kupffer cell blockade reduces hepatic and systemic cytokine levels and lung injury in hemorrhagic pancreatitis in rats. Pancreas. 2000;21:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Yuan C, Hua G, Tong R, Luo X, Ying Z. Early gut barrier dysfunction in patients with severe acute pancreatitis: attenuated by continuous blood purification treatment. Int J Artif Organs. 2010;33:706-715. [PubMed] |
| 21. | Thandassery RB, Yadav TD, Dutta U, Appasani S, Singh K, Kochhar R. Dynamic nature of organ failure in severe acute pancreatitis: the impact of persistent and deteriorating organ failure. HPB (Oxford). 2013;15:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Liu J, Huang L, Luo M, Xia X. Bacterial translocation in acute pancreatitis. Crit Rev Microbiol. 2019;45:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Garg PK, Singh VP. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology. 2019;156:2008-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 376] [Article Influence: 62.7] [Reference Citation Analysis (0)] |