Published online Dec 14, 2020. doi: 10.3748/wjg.v26.i46.7416
Peer-review started: September 18, 2020
First decision: October 17, 2020
Revised: October 30, 2020
Accepted: November 13, 2020
Article in press: November 13, 2020
Published online: December 14, 2020
Processing time: 87 Days and 7.3 Hours
It is important to differentiate benign and malignant focal liver lesions (FLLs) accurately. Despite the wide use and acceptance of shear wave elastography (SWE), its value for assessing the elasticity of FLLs and differentiating benign and malignant FLLs is still investigational. Previous studies of SWE for FLLs used mean elasticity as the parameter to reflect the stiffness of FLLs. Considering the inhomogeneity of tumor stiffness, maximal elasticity (Emax) might be the suitable parameter to reflect the stiffness of FLLs and to differentiate malignant FLLs from benign ones.
To explore the value of SWE with Emax in differential diagnosis of solid FLLs.
We included 104 solid FLLs in 95 patients and 50 healthy volunteers. All the subjects were examined using conventional ultrasound (US) and virtual touch tissue quantification(VTQ) imaging. A diagnosis of benign or malignant FLL was made using conventional US. Ten VTQ values were acquired after 10 consecutive measurements for each FLL and each normal liver, and the largest value was recorded as Emax.
There were 56 cases of malignant FLLs and 48 cases of benign FLLs in this study. Emax of malignant FLLs (3.29 ± 0.88 m/s) was significantly higher than that of benign FLLs (1.30 ± 0.46 m/s, P < 0.01) and that of livers in healthy volunteers (1.15 ± 0.17 m/s, P < 0.01). The cut-off point of Emax was 1.945, and the area under the curve was 0.978. The sensitivity and specificity of Emax were 92.9% and 91.7%, respectively, higher (but not significantly) than those of conventional US (80.4% for sensitivity and 81.3% for specificity). Combined diagnosis of conventional US and Emax using parallel testing improved the sensitivity to 100% with specificity of 75%.
SWE is a convenient and easy method to obtain accurate stiffness information of solid FLLs. Emax is useful for differential diagnosis of FLLs, especially in combination with conventional US.
Core Tip: Shear wave elastography (SWE) has been used with promising results in the assessment of liver fibrosis and in the differential diagnosis of thyroid and breast nodules. However, its value for the differential diagnosis between malignant and benign focal liver lesions (FLLs) is still investigational. In this study, instead of the common parameter (mean elasticity), we used maximal elasticity (Emax) as the parameter to explore the value of SWE in the differential diagnosis of FLLs. Our results showed that Emax is useful for differential diagnosis of FLLs, especially in combination with conventional ultrasound.
- Citation: Zhang HP, Gu JY, Bai M, Li F, Zhou YQ, Du LF. Value of shear wave elastography with maximal elasticity in differentiating benign and malignant solid focal liver lesions. World J Gastroenterol 2020; 26(46): 7416-7424
- URL: https://www.wjgnet.com/1007-9327/full/v26/i46/7416.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i46.7416
Focal liver lesions (FLLs) are common. Accurate differential diagnosis is important for treatment and assessment of prognosis[1]. Conventional ultrasound (US), with the advantages of real-time imaging, no radiation, and low cost, is the first choice for the detection and diagnosis of FLLs[2]. The diagnostic efficiency, however, is not as good as that of computed tomography (CT) and magnetic resonance imaging (MRI).
With the development of new US techniques, especially the application of microbubbles and US elastography (UE), the diagnostic efficiency of US has been improved rapidly[3,4]. The value of contrast-enhanced US (CEUS) for the differential diagnosis of solid FLLs has been confirmed and the diagnostic efficiency of CEUS is comparable to or even better than that of contrast-enhanced CT[5].
UE is a useful tool that can provide elasticity information of tissue; a different physical property other than acoustic impedance. UE, especially shear wave elastography (SWE), which can provide elastic information quantitatively, is widely used and with promising results in the differential diagnosis of thyroid and breast nodules[6,7]. The value of SWE in the assessment of liver fibrosis is significant too[8]. However, the value of SWE for the differential diagnosis between benign and malignant FLLs is still investigational according to the guidelines of the World Federation for Ultrasound in Medicine and Biology for liver UE[9]. Previous studies of SWE for FLLs have usually used mean elasticity (Emean) as the parameter to reflect the stiffness of FLLs[10-12]. However, as tumors (especially malignant tumors) usually have inhomogeneous stiffness, maximal elasticity (Emax) has been confirmed as the best performing SWE feature for breast cancers[13]. The diagnostic value of Emax for FLLs has not yet been confirmed.
Virtual touch tissue quantification (VTQ) imaging is one kind of point SWE (pSWE) that can determine the stiffness of the tissue in a small region of interest (ROI) and be shown on screen as a VTQ value (m/s). In this study, we used VTQ imaging with Emax as the parameter to measure the stiffness of FLLs and to explore the value of SWE with Emax in the differential diagnosis of FLLs.
This study was designed prospectively and approved by the Ethics Committee of Shanghai First People’s Hospital. Written informed consent was obtained from every patient before US examination.
Between July and December 2017, patients in the Department of General Surgery at the hospital were included if they met the following criteria: (1) Presence of one or more solid FLLs with a minimum diameter > 1 cm and a maximum depth < 8 cm shown on conventional US; (2) Patients could follow the instructions of the operator and control their breath well; and (3) VTQ imaging done successfully with 10 VTQ values after 10 consecutive measurements. The exclusion criteria were: (1) Known history of any liver surgery; (2) Known history of chemotherapy, radiotherapy, or other treatment of liver tumor; and (3) Without definite diagnosis proven pathologically or by at least two imaging methods (CEUS together with contrast-enhanced CT and/or MRI in 1 wk before or after VTQ imaging). We included 104 solid FLLs in 95 patients (57 men and 38 women; aged 22-79 years; mean age 50.9 years). Fifty normal volunteers (aged 18-65 years; mean age 47.2 years) who had normal hepatic function, normal a-fetoprotein, no previous medical history of any systemic diseases, and no intrahepatic lesions examined by conventional US were included in this study as a control.
All the conventional US examinations were performed by a radiologist with 16 years’ experience in conventional US. An Acuson S2000 diagnostic US system (Siemens Medical Solutions, Mountain View, CA, United States) with a transabdominal convex 6C1 probe was used. The subjects were all instructed to fast for at least 8 h before the US examinations.
For the volunteers, a thorough hepatic US scan was used for the exclusion of any liver lesions, including FLLs and diffuse hepatic diseases.
For the patients, conventional US was used for the detection of solid FLLs. The location, size, shape, boundary, and echogenicity of the lesion were observed. A diagnosis as benign or malignant was made and recorded. The diagnostic values of conventional US were assessed.
The same US equipment was used for VTQ imaging after conventional US examination by another radiologist with 16 years’ experience in conventional US and 5 years’ experience in UE.
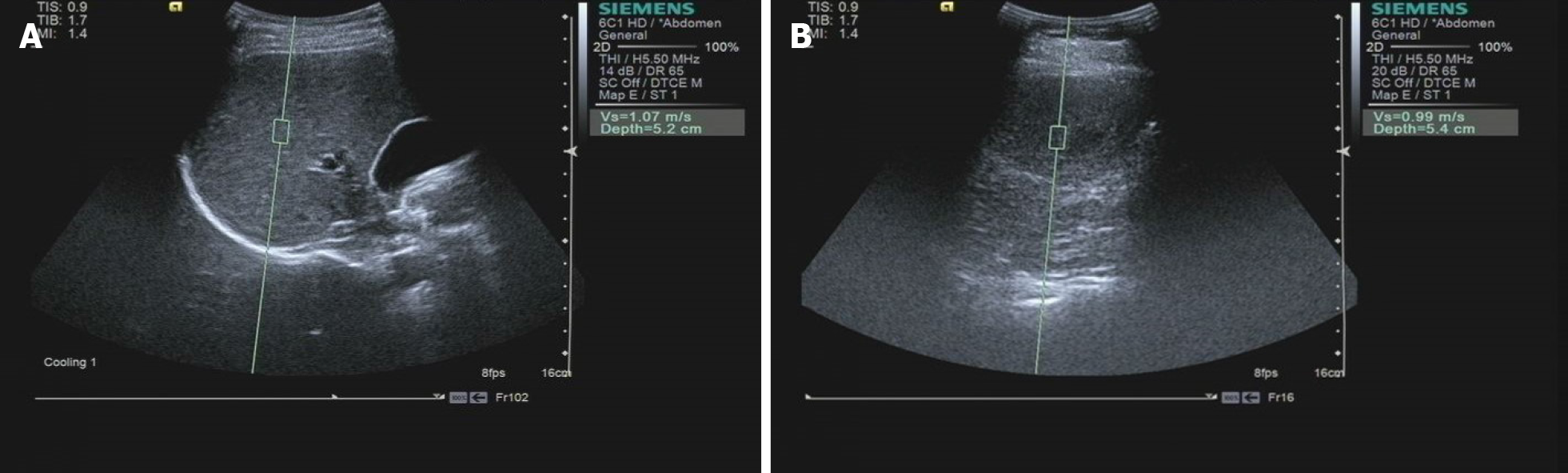
The volunteers were asked to assume a supine or left-lateral position. The probe was positioned on the skin gently with no pressure, and the volunteers were asked to hold their breath to avoid the effect of breath movement. The ROI (with fixed size as 10 mm × 5 mm) was placed at a depth of 4-6 cm, and tubular structures, such as portal veins, hepatic veins, and intra hepatic bile ducts, were carefully avoided (Figure 1A). After 10 consecutive measurements, 10 VTQ values were acquired. The largest VTQ value was recorded as Emax; the coefficient of variation (CV = mean/standard deviation) of the VTQ values was calculated and recorded.
The patients were also asked to hold their breath to avoid the effect of breath movement. The probe was positioned on the skin gently, and the ROI was placed inside the targeted FLL (Figure 1B). After 10 consecutive measurements, the largest value was recorded as Emax. The cut-off point of Emax was calculated. The diagnostic values of Emax were assessed and compared with those of conventional US.
Parallel combined diagnosis of conventional US and Emax was used to improve diagnostic sensitivity. If a lesion was diagnosed as malignant by either conventional US or Emax, the result of combined diagnosis was malignant, and the result of combined diagnosis was benign when a lesion was diagnosed as benign by both conventional US and Emax.
SPSS version13.0 software (IBM Corporation, Chicago, IL, United States) was used for statistical analysis. P < 0.05 was considered statistically significant. The data of Emax were presented as mean ± standard deviation and compared using analysis of variance and least-significant difference method. The cut-off point of Emax was calculated by a receiver operating characteristic curve. The diagnostic values of conventional US, Emax, and combined diagnosis were assessed in terms of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy. The sensitivity and the specificity of Emax were compared with those of conventional US using McNemar’s χ2 test.
There were 56 malignant and 48 benign solid FLLs. Among the malignant FLLs, 25 were hepatocellular carcinoma (HCC) and 31 were metastatic hepatic carcinoma (MHC). Among the benign solid FLLs, 35 were hemangioma, 10 were focal nodular hyperplasia (FNH), and three were regenerative nodules (RNs).
Eleven malignant FLLs were misdiagnosed as benign, and nine benign FLLs were misdiagnosed as malignant using conventional US (Table 1). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 80.4%, 81.3%, 83.3%, 78.0%, and 80.8%, respectively (Table 2).
| Conventional ultrasound | Emax | |||
| Benign | Malignant | Benign | Malignant | |
| Benign, n = 48 | 39 (81.3) | 9 (18.8) | 44 (91.7) | 4 (8.3) |
| Malignant, n = 56 | 11 (19.6) | 45 (80.4) | 4 (7.1) | 52 (92.9) |
| Diagnostic methods | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy |
| Conventional US | 80.4 | 81.3 | 83.3 | 78.0 | 80.8 |
| Emax | 92.9 | 91.7 | 92.9 | 91.7 | 92.3 |
| Combination of conventional US and Emax | 100 | 75.0 | 82.4 | 100 | 88.5 |
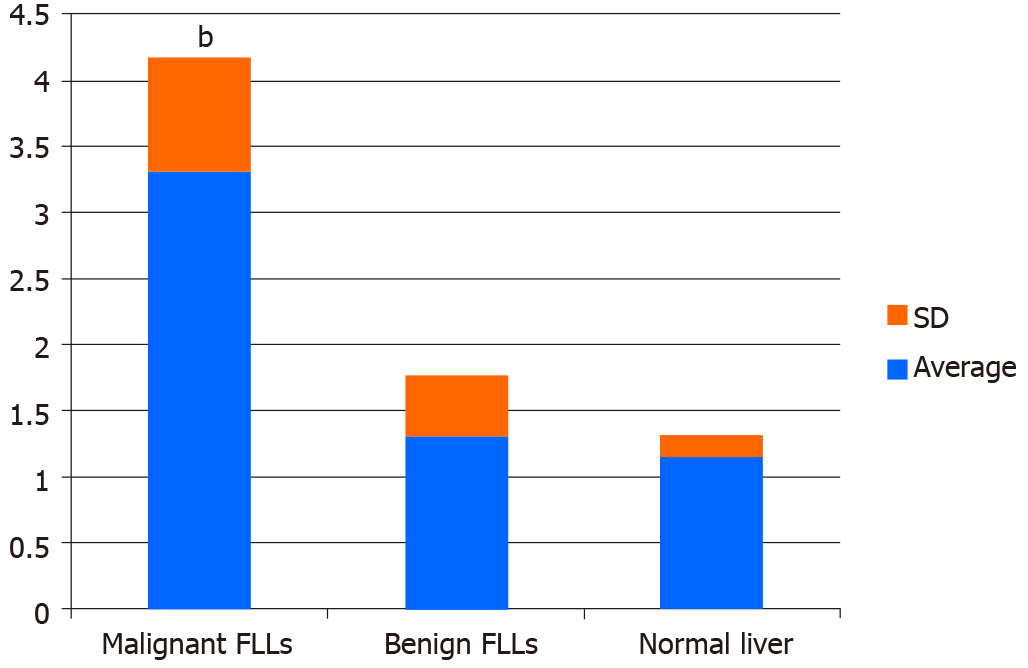
The comparison of Emax among normal livers, benign FLLs, and malignant FLLs is shown in Figure 2. Emax values of the normal livers in volunteers were 1.15 ± 0.17 m/s and ranged from 0.79 to 1.43 m/s. CV values of the VTQ values in each volunteer ranged from 4.5% to 14.6%. Emax values of the benign FLLs were 1.30 ± 0.46 m/s. Emax values of the malignant FLLs were 3.29 ± 0.88 m/s. There were significant differences among Emax of normal livers, benign FLLs, and malignant FLLs (F = 216.304, P < 0.01). Further multiple comparisons showed significant differences between Emax values of the malignant and benign FLLs (P < 0.01) and between Emax values of the malignant FLLs and normal livers (P < 0.01). There was no significant difference between Emax values of the benign FLLs and normal liver.
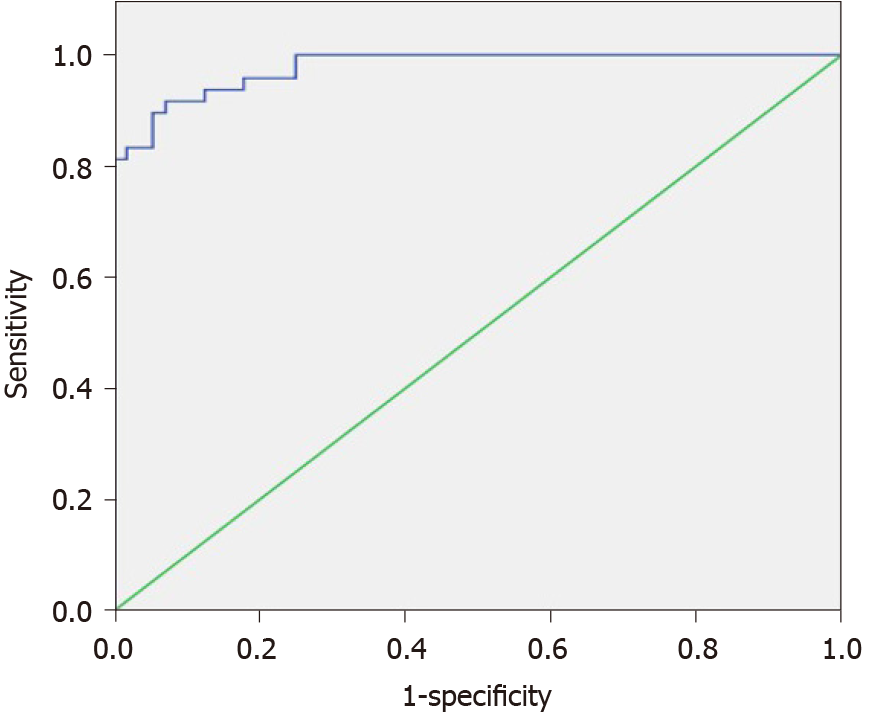
The cut-off point of Emax was 1.945, and area under the curve (AUC) was 0.978 (Figure 3). Using Emax > 1.945 for diagnosis as malignant, four malignant FLLs (one HCC and three MHCs) were misdiagnosed as benign and four benign FLLs (two hemangiomas, one FNH, and one RN) were misdiagnosed as malignant (Table 1), with sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 92.9%, 91.7%, 92.9%, 91.7%, and 92.3%, respectively (Table 2). Although the sensitivity and specificity of Emax were higher than those of conventional US (92.9% vs 80.4% and 91.7% vs 81.3%), the differences were not statistically significant.
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of combined diagnosis of conventional US and Emax using parallel test were 100%, 75%, 82.4%, 100%, and 88.5%, respectively (Table 2).
We explored the value of SWE with Emax as a parameter in the differential diagnosis of solid FLLs. Our results show that SWE is a convenient and easy method that can provide accurate stiffness information of solid FLLs, and Emax is useful for the differential diagnosis of FLLs.
SWE, including VTQ imaging as pSWE, has been proven to be a useful and accurate method for the assessment of liver stiffness[14-16]. In this study, 50 volunteers with normal hepatic function and without any intrahepatic lesion were included as controls. Our results showed that CVs of the VTQ values in each volunteer were between 4.5% and 14.6%, which proved that VTQ imaging could provide reliable and reproducible quantitative information.
Although the value of SWE for solid FLLs has not yet been determined definitively, some studies have used VTQ imaging to differentiate between benign and malignant FLLs[10,11]. Akdoğan et al[10] reported that there were significant differences in VTQ values between malignant and benign FLLs. A VTQ value of 2.32 m/s was used as a cut-off value to differentiate malignant liver masses from benign ones, and the sensitivity, specificity, and AUC were 0.93, 0.60, and 0.826, respectively. Sun et al[11] also showed that VTQ values of malignant tumors were significantly higher than those of benign tumors, with a cut-off of 1.60 m/s and AUC of 0.851. This diagnostic efficiency was not good enough to meet clinical requirements. In these two studies, Emean, the performance of which was not as good as Emax for breast cancer, was used as the parameter to assess the stiffness of each FLL. In our study, we used Emax as the parameter, and a promising result was achieved (sensitivity 92.9%, specificity 91.7%, and AUC 0.978).
Although shear stiffness is an important feature of malignant tumors[17-19], there were still four malignant FLLs among the 56 that were misdiagnosed as benign in our study. One confusing result in our study was that for a patient with multiple liver metastases from breast cancer, the Emax values of three targeted metastases were not similar (4.01, 4.31, and 1.73, respectively). One probable reason was that tumor size may have affected its stiffness. There may be other reasons for the inconsistency and misdiagnosis and further studies are needed.
Conventional US is the prerequisite and foundation of SWE. The sensitivity and specificity of conventional US were 80.4% and 81.3%, respectively, in our study. When combined with Emax, the sensitivity was improved to 100% with no false-negative results. This reduced significantly the rate of missed diagnosis and avoided delay of further diagnosis and treatment for malignant FLLs. Compared with CEUS, VTQ imaging is easier to operate and interpret, and cheaper, with no risk of allergy caused by contrast agents. With all these advantages and excellent diagnostic efficiency, it makes VTQ imaging a good choice for the differential diagnosis of FLLs.
There were some limitations to our study. First, the sample number was not large enough for the comparison of Emax among different pathological types of FLLs. Second, because of the technical limitation of VTQ imaging, only FLLs with a minimum diameter > 1 cm and a maximum depth < 8 cm could be assessed. The ROI could not be placed directly in the area with highest stiffness of a tumor. Further studies with a larger sample number and improved techniques are needed to confirm our results.
In conclusion, SWE is a convenient and easy method that can provide accurate stiffness information of solid FLLs. Emax is useful for the differential diagnosis of FLLs, and combined with conventional US, the diagnostic efficiency is improved.
Shear wave elastography (SWE), which could reflect tissue stiffness quantitatively, is the technologic leap of ultrasound (US) and is playing a more and more important role clinically. SWE is a convenient and cheap method with good repeatability and without any risk of radiation. The values of SWE in the differential diagnosis of thyroid and breast nodules and the assessment of liver fibrosis are significant. However, its value for the differential diagnosis between malignant and benign focal liver lesions (FLLs) was not widely accepted yet.
The World Federation for Ultrasound in Medicine and Biology guidelines 2018 did not recognize the value SWE for the differential diagnosis between malignant and benign FLLs. Previous studies about SWE application in liver usually used mean elasticity as the parameter. Considering the inhomogeneity of the stiffness of FLLs, maximal elasticity (Emax) might be the suitable parameter to reflect the stiffness of FLLs and to differentiate malignant FLLs from benign ones. So, it was necessary to explore the value of SWE with Emax in differential diagnosis of solid FLLs.
We aim to explore the value of SWE with Emax in differential diagnosis of FLLs.
This study included 104 solid FLLs and 50 healthy volunteers, who were examined using conventional US and SWE. Coefficient of variation (CV) of virtual touch tissue quantification (VTQ) values in each volunteer was calculated after 10 consecutive measurements for each liver. Each lesion was diagnosed as benign or malignant using conventional US by a radiologist with 16 years’ experience in US. The largest VTQ value was recorded as Emax after 10 consecutive measurements for each FLL. The cut-off point of Emax was calculated by a receiver operating characteristic curve. The diagnostic efficiencies of conventional US, Emax, and combined test was calculated and compared.
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of conventional US were 80.4%, 81.3%, 83.3%, 78.0%, and 80.8%, respectively. CV of the VTQ values in each volunteer ranged from 4.5% to 14.6%. Emax of malignant FLLs (3.29 ± 0.88 m/s) was significantly higher than that of benign FLLs (1.30 ± 0.46 m/s, P < 0.01) and that of livers in healthy volunteers (1.15 ± 0.17 m/s, P < 0.01). The cut-off point of Emax was 1.945, and the area under the curve was 0.978. The sensitivity and specificity of Emax were 92.9% and 91.7%, higher (but not significantly) than those of conventional US. Combined diagnosis of conventional US and Emax using parallel testing improved the sensitivity to 100% with specificity of 75%.
SWE is a convenient and easy method that can provide accurate stiffness information of solid FLLs. Emax is useful for the differential diagnosis of malignant and benign FLLs, and combined with conventional US, the diagnostic efficiency is improved.
In this study, we demonstrated the value of SWE with Emax in differential diagnosis of FLLs. Prospective study with large numbers of patients and different kinds of FLLs will be needed to confirm the results. The application of two-dimensional SWE may be more convenient and accurate for differentiating FLLs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dueland S, Pratschke S, Sumi K S-Editor: Huang P L-Editor: Filipodia P-Editor: Liu JH
| 1. | Fu J, Wang H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018;412:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 2. | Minami Y, Minami T, Hagiwara S, Ida H, Ueshima K, Nishida N, Murakami T, Kudo M. Ultrasound-ultrasound image overlay fusion improves real-time control of radiofrequency ablation margin in the treatment of hepatocellular carcinoma. Eur Radiol. 2018;28:1986-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Săftoiu A, Gilja OH, Sidhu PS, Dietrich CF, Cantisani V, Amy D, Bachmann-Nielsen M, Bob F, Bojunga J, Brock M, Calliada F, Clevert DA, Correas JM, D'Onofrio M, Ewertsen C, Farrokh A, Fodor D, Fusaroli P, Havre RF, Hocke M, Ignee A, Jenssen C, Klauser AS, Kollmann C, Radzina M, Ramnarine KV, Sconfienza LM, Solomon C, Sporea I, Ștefănescu H, Tanter M, Vilmann P. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med. 2019;40:425-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 4. | Battaglia V, Cervelli R. Liver investigations: Updating on US technique and contrast-enhanced ultrasound (CEUS). Eur J Radiol. 2017;96:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, Lyshchik A, Dietrich CF, Willmann JK, Vezeridis A, Sirlin CB. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol. 2017;23:280-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Du YR, Ji CL, Wu Y, Gu XG. Combination of ultrasound elastography with TI-RADS in the diagnosis of small thyroid nodules (≤10 mm): A new method to increase the diagnostic performance. Eur J Radiol. 2018;109:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Fernandes J, Sannachi L, Tran WT, Koven A, Watkins E, Hadizad F, Gandhi S, Wright F, Curpen B, El Kaffas A, Faltyn J, Sadeghi-Naini A, Czarnota G. Monitoring Breast Cancer Response to Neoadjuvant Chemotherapy Using Ultrasound Strain Elastography. Transl Oncol. 2019;12:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Zhuang Y, Ding H, Zhang Y, Sun H, Xu C, Wang W. Two-dimensional Shear-Wave Elastography Performance in the Noninvasive Evaluation of Liver Fibrosis in Patients with Chronic Hepatitis B: Comparison with Serum Fibrosis Indexes. Radiology. 2017;283:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 373] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 10. | Akdoğan E, Yılmaz FG. The role of acoustic radiation force impulse elastography in the differentiation of benign and malignant focal liver masses. Turk J Gastroenterol. 2018;29:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Sun XL, Yao H, Men Q, Hou KZ, Chen Z, Xu CQ, Liang LW. Combination of acoustic radiation force impulse imaging, serological indexes and contrast-enhanced ultrasound for diagnosis of liver lesions. World J Gastroenterol. 2017;23:5602-5609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Ronot M, Di Renzo S, Gregoli B, Duran R, Castera L, Van Beers BE, Vilgrain V. Characterization of fortuitously discovered focal liver lesions: additional information provided by shearwave elastography. Eur Radiol. 2015;25:346-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, Ohlinger R, Mendelson EB, Balu-Maestro C, Locatelli M, Tourasse C, Cavanaugh BC, Juhan V, Stavros AT, Tardivon A, Gay J, Henry JP, Cohen-Bacrie C; BE1 Investigators. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 590] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 14. | Potthoff A, Attia D, Pischke S, Kirschner J, Mederacke I, Wedemeyer H, Manns MP, Gebel MJ, Rifai K. Influence of different frequencies and insertion depths on the diagnostic accuracy of liver elastography by acoustic radiation force impulse imaging (ARFI). Eur J Radiol. 2013;82:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040-3053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Ding H, Ma JJ, Wang WP, Zeng WJ, Jiang T, Huang BJ, Chen SY. Assessment of liver fibrosis: the relationship between point shear wave elastography and quantitative histological analysis. J Gastroenterol Hepatol. 2015;30:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Venkatesh SK, Yin M, Glockner JF, Takahashi N, Araoz PA, Talwalkar JA, Ehman RL. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008;190:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Vogl TJ, Martin SS, Johnson AA, Haas Y. Evaluation of MR elastography as a response parameter for transarterial chemoembolization of colorectal liver metastases. Eur Radiol. 2020;30:3900-3907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Serai SD, Obuchowski NA, Venkatesh SK, Sirlin CB, Miller FH, Ashton E, Cole PE, Ehman RL. Repeatability of MR Elastography of Liver: A Meta-Analysis. Radiology. 2017;285:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |