Published online Nov 28, 2020. doi: 10.3748/wjg.v26.i44.7061
Peer-review started: August 31, 2020
First decision: September 30, 2020
Revised: October 10, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: November 28, 2020
Processing time: 88 Days and 1 Hours
Uric acid is the end product of purine metabolism. Previous studies have found that serum uric acid (SUA) levels are associated with the total cancer risk. However, due to the dual effect of uric acid on cancer, the relationship between the SUA levels and most specific-site cancer remains unclear.
To investigate the associations between the SUA levels and incidence of hepatobiliary-pancreatic cancer.
In this prospective cohort study, 444462 participants free of cancer from the UK Biobank were included. The SUA levels were measured at baseline, and the incidence of hepatobiliary-pancreatic cancer was determined by contacting the cancer registry. The hazard ratios (HRs) and 95% confidence intervals (CIs) between the SUA levels and hepatobiliary-pancreatic cancer were investigated using multiple adjusted Cox regression models adjusted for potential confounders.
In total, 920 participants developed liver, gallbladder, biliary tract or pancreatic cancer during a median of 6.6 yrs of follow-up. We found that the HR of pancreatic cancer in the highest SUA group was 1.77 (95%CI: 1.29-2.42) compared with that in the lowest group. After stratifying by gender, we further found that SUA was associated with an increased risk of pancreatic cancer only among the females (highest quartile vs lowest quartile HR 2.04, 95%CI: 1.35-3.08). Among the males, the SUA levels were positively associated with the gallbladder cancer risk (highest quartile vs lowest quartile HR 3.09, 95%CI: 1.28-7.46), but a U-shaped association with the liver cancer risk was observed (P-nonlinear = 0.03).
SUA is likely to have gender-specific effects on hepatobiliary-pancreatic cancer. High SUA levels are a risk factor for pancreatic cancer in females and gallbladder cancer in males. A U-shaped association with the liver cancer risk was identified.
Core Tip: Serum uric acid has an effect on hepatobiliary-pancreatic cancer, specifically when stratified by gender. In males, high uric acid level is a risk factor for gallbladder cancer and has a U-shape association with liver cancer risk. In females, uric acid is positively associated with the risk of pancreatic cancer. In clinical and public health practice, management of either high or low uric acid levels may contribute to the prevention of hepatobiliary-pancreatic cancer.
- Citation: Huang CF, Huang JJ, Mi NN, Lin YY, He QS, Lu YW, Yue P, Bai B, Zhang JD, Zhang C, Cai T, Fu WK, Gao L, Li X, Yuan JQ, Meng WB. Associations between serum uric acid and hepatobiliary-pancreatic cancer: A cohort study. World J Gastroenterol 2020; 26(44): 7061-7075
- URL: https://www.wjgnet.com/1007-9327/full/v26/i44/7061.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i44.7061
Hepatobiliary-pancreatic (HBP) cancer includes liver cancer, biliary tract cancer, gallbladder cancer and pancreatic cancer[1-3]. The number of new cases of HBP cancer worldwide in 2018 was approximately 1.85 million, accounting for 10% of all newly diagnosed cancer cases and resulting in a great financial burden[4,5]. Due to the large number of HBP cancer cases, from the perspective of prevention[6,7], identifying high-risk populations has become an urgent public health issue[8-10].
Serum uric acid (SUA) is the final product of purine nucleotides that are ingested or endogenously synthesized and mainly metabolized by the liver[11]. Because of its function of inhibiting reactive oxygen species formation, SUA was considered a protective factor against cancer[12], and studies have indicated that elevated SUA was associated with low cancer mortality[13,14]. However, subsequent experiments revealed that SUA was associated with inflammatory mediators, which act as cancer-promoting factors[15,16]. A meta-analysis conducted in 2019 that included eight cohort studies investigating cancer incidence, and SUA suggested that high SUA levels increased the risk of all cancers[17].
However, few previously published studies have focused on the SUA levels and the incidence of cancer at specific sites, and none of these studies highlighted HBP cancer. Therefore, we conducted this study to evaluate the associations between SUA and the HBP cancer risk based on the UK Biobank cohort.
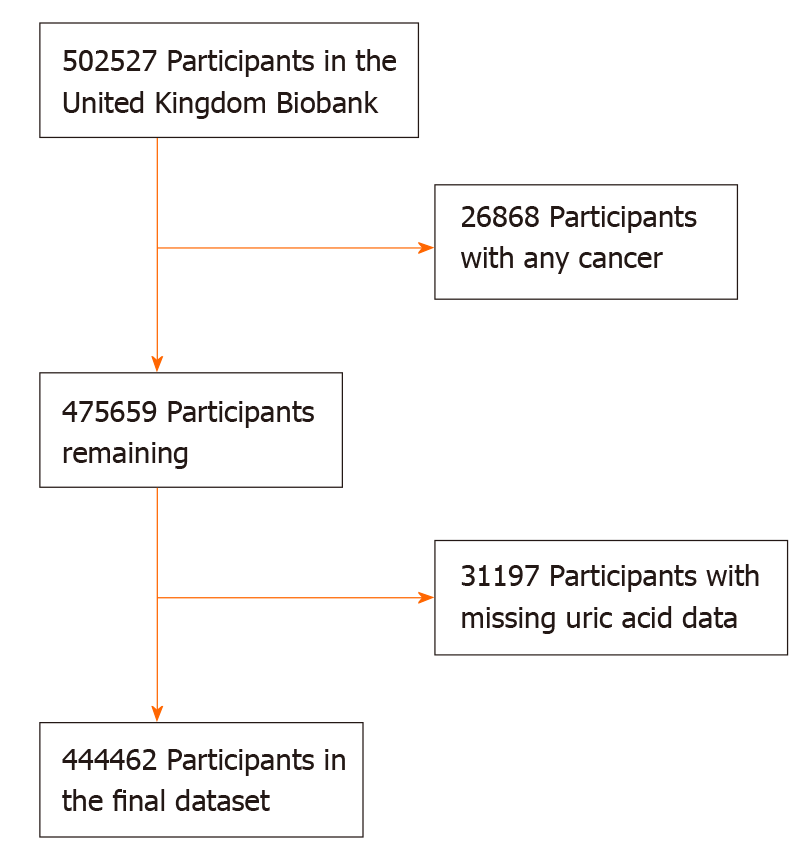
The UK Biobank is a national and international health resource with over 500000 participants aged 40-69 years recruited from all over the United Kingdom from 2006-2010. More details of the UK Biobank are available elsewhere[18]. The UK Biobank has received ethical approval from the North West Multi-centre Research Ethics Committee, the England and Wales Patient Information Advisory Group and the Scottish Community Health Index Advisory Group. All participants provided written informed consent. In this analysis, we excluded 26868 participants with any cancer prior to recruitment (except for non-melanoma skin cancer 10th revision of the International Classification of Diseases C44) and 31197 participants with missing SUA data (Figure 1). Eventually, 444462 participants were included in the final analysis and were grouped by quartiles of SUA (Q1-Q4).
The baseline characteristics were collected by self-completed touch-screen questionnaires, computer-assisted interviews and physical measurements. The data retrieved for the analysis included age, gender, education, ethnic group, family history of cancer, annual household income and lifestyle habits such as fruit and vegetable intake (more than five portions or not), alcohol consumption, smoking status, physical activity (categorized according to the standard International Physical Activity Questionnaire guidelines[19] as high, moderate or low) and body mass index [body mass index (BMI), calculated as weight divided by height squared, kg/m2). Approximately 45 mL of blood and 9 mL of urine were collected to measure specific biomarkers by using the latest analytical methods in a dedicated facility in Stockport. The samples were stored separately for the subsequent detection and stored at -80 °C and -180 °C[20]. SUA was measured by a Beckman Coulter AU5800 (BC, United States) using enzymatic determination (Uricase PAP).
Information regarding the cancer diagnoses in the UK Biobank is provided by the National Health Service (NHS) Digital and Public Health England for participants residing in England and Wales and the NHS Central Register for participants residing in Scotland. The general classification of the cancer cases was based on the 10th revision of the International Classification of Diseases codes. The primary outcomes in this study were liver cancer (C22), gallbladder cancer (C23), biliary tract cancer (C24) and pancreatic cancer (C25).
The baseline characteristics are presented as numbers (percentages) for the categorical variables and means (standard deviations) for the continuous variables. Cox regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of the association between SUA and HBP cancer. The potential confounders were adjusted gradually in three models. In model 1, we adjusted for the general demographic characteristics (gender, age, education, ethnic group and family history of cancer). Then, we further adjusted for lifestyle factors (alcohol intake, smoking status, annual household income, physical activity, fruit and vegetable intake) in model 2. Because obesity is closely related to the SUA levels and cancer risk, in model 3, we adjusted for BMI separately in addition to the variables included in model 2. The potential nonlinear associations between the SUA levels and the HBP cancer risk were investigated by fitting restricted cubic splines in a fully adjusted Cox regression model. In addition, considering the large gender difference in the distribution of SUA, we also conducted a gender-stratified analysis.
A sensitivity analysis was performed to verify the stability of our results by excluding participants with less than 2 yrs of follow-up from the fully adjusted Cox regression models. All statistical analyses were conducted by using R software (version 3.5.0, R Foundation for Statistical Computing, Vienna, Austria).
This study included 444462 participants (Tables 1 and 2). There were fewer males in the quartiles with higher SUA levels and fewer females in the quartiles with lower SUA levels. As the SUA level quartiles increased, the participants tended to be older, have a higher BMI, consume more alcohol, consume less fruit and vegetables and have fewer college or university degrees.
| Group | Q1 | Q2 | Q3 | Q4 |
| SUA level (mg/dL) | (1.50, 4.20) | (4.20, 5.09) | (5.09, 6.06) | (6.06, 17.90) |
| Number | 111087 | 110975 | 111241 | 111159 |
| Gender | ||||
| Male | 99225 (89.3%) | 73883 (66.6%) | 44282 (39.8%) | 21466 (19.3%) |
| Female | 11862 (10.7%) | 37092 (33.4%) | 66959 (60.2%) | 89693 (80.7%) |
| Mean age (SD) | 55.30 (8.20) | 56.90 (8.00) | 57.50 (7.97) | 57.70 (8.00) |
| White European | 104975 (94.5%) | 104411 (94.1%) | 104447 (93.9%) | 104475 (94.0%) |
| Current smokers | 11629 (10.5%) | 11 95 (10.6%) | 12154 (10.9%) | 11444 (10.3%) |
| Alcohol intake | ||||
| Over four times a week | 40852 (36.8%) | 45126 (40.7%) | 49976 (44.9%) | 57439 (51.7%) |
| Once or twice a week | 29330 (26.4%) | 29071 (26.2%) | 28787 (25.9%) | 27396 (24.6%) |
| One to three times a month | 14577 (13.1%) | 13324 (12.0%) | 1 850 (10.7%) | 9681 (8.7%) |
| Seldom or never | 26102 (23.5%) | 23203 (20.9%) | 20363 (18.3%) | 16388 (14.7%) |
| Fruit and vegetable intake | ||||
| Yes | 47673 (42.9%) | 44146 (39.8%) | 40049 (36.0%) | 35593 (32.0%) |
| No | 63175 (56.9%) | 66545 (60.0%) | 70869 (63.7%) | 75234 (67.7%) |
| Physical activity | ||||
| High | 36219 (32.6%) | 36706 (33.1%) | 36948 (33.2%) | 36029 (32.4%) |
| Moderate | 37562 (33.8%) | 36235 (32.7%) | 36318 (32.6%) | 36115 (32.5%) |
| Low | 14838 (13.4%) | 16003 (14.4%) | 17162 (15.4%) | 19457 (17.5%) |
| Annual household income | ||||
| Less than £18000 | 20748 (18.7%) | 21456 (19.3%) | 21341 (19.2%) | 21536 (19.4%) |
| £18000 to £30999 | 23304 (21.0%) | 24243 (21.8%) | 24310 (21.9%) | 24159 (21.7%) |
| £31000 to £51999 | 24672 (22.2%) | 24624 (22.2%) | 25270 (22.7%) | 25168 (22.6%) |
| £52000 to £100000 | 19676 (17.7%) | 18781 (16.9%) | 19544 (17.6%) | 20555 (18.5%) |
| Greater than £100000 | 5132 (4.6%) | 4947 (4.5%) | 5211 (4.7%) | 5666 (5.1%) |
| College or University degree | 38460 (34.6%) | 36499 (32.9%) | 35860 (32.2%) | 33817 (30.4%) |
| Family history of cancer | 38048 (34.3%) | 38942 (35.1%) | 39465 (35.5%) | 39063 (35.1%) |
| Mean BMI, kg/m2 (SD) | 25.10 (4.01) | 26.80 (4.45) | 28.10 (4.62) | 29.60 (4.82) |
| Male | Female | |||||||
| Group | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| SUA level (mg/dL) | (1.50, 5.13) | (5.13, 5.87) | (5.87, 6.69) | (6.69, 17.90) | (1.50, 3.77) | (3.77, 4.42) | (4.42, 5.18) | (5.18, 12.90) |
| Number | 51349 | 51445 | 51364 | 51448 | 59639 | 59627 | 59872 | 59718 |
| Mean age (SD) | 56.90 (8.23) | 56.90 (8.23) | 57.00 (8.17) | 57.30 (8.13) | 54.60 (8.18) | 56.00 (8.05) | 57.20 (7.83) | 58.90 (7.33) |
| White European | 48082 (93.6%) | 48342 (94.0%) | 48405 (94.2%) | 48480 (94.2%) | 56435 (94.6%) | 56437 (94.7%) | 56340 (94.1%) | 55787 (93.4%) |
| Current smokers | 7983 (15.5%) | 6579 (12.8%) | 5816 (11.3%) | 5377 (10.5%) | 5759 (9.7%) | 5412 (9.1%) | 5138 (8.6%) | 4958 (8.3%) |
| Alcohol intake | ||||||||
| Over four times a week | 23146 (45.1%) | 25543 (49.6%) | 27432 (53.4%) | 29611 (57.5%) | 20964 (35.1%) | 22423 (37.6%) | 22866 (38.2%) | 21408 (35.8%) |
| Once or twice a week | 13636 (26.6%) | 13770 (26.8%) | 13308 (25.9%) | 12478 (24.3%) | 15822 (26.5%) | 15714 (26.4%) | 15444 (25.8%) | 14412 (24.1%) |
| One to three times a month | 5297 (10.3%) | 4919 (9.6%) | 4300 (8.4%) | 3749 (7.3%) | 8141 (13.7%) | 7837 (13.1%) | 7669 (12.8%) | 7520 (12.6%) |
| Seldom or never | 9124 (17.8%) | 7098 (13.8%) | 6203 (12.0%) | 5500 (10.7%) | 14598 (24.5%) | 13546 (22.7%) | 13761 (23.0%) | 16226 (27.2%) |
| Fruit and vegetable intake | ||||||||
| Yes | 16819 (32.8%) | 16373 (31.8%) | 15863 (30.9%) | 15126 (29.4%) | 26202 (43.9%) | 26292 (44.1%) | 25919 (43.3%) | 24867 (41.6%) |
| No | 34326 (66.8%) | 34910 (67.9%) | 35318 (68.8%) | 36183 (70.3%) | 33328 (55.9%) | 33228 (55.7%) | 33826 (56.5%) | 34704 (58.1%) |
| Physical activity | ||||||||
| High | 19424 (37.8%) | 19002 (36.9%) | 18382 (35.8%) | 17317 (33.7%) | 19204 (32.2%) | 18936 (31.8%) | 18096 (30.2%) | 15541 (26.0%) |
| Low | 7605 (14.8%) | 7864 (15.3%) | 8360 (16.3%) | 9204 (17.9%) | 7781 (13.0%) | 8103 (13.6%) | 8586 (14.3%) | 9957 (16.7%) |
| Moderate | 16226 (31.6%) | 16580 (32.2%) | 16858 (32.8%) | 16777 (32.6%) | 20402 (34.2%) | 20063 (33.6%) | 19842 (33.1%) | 19482 (32.6%) |
| Annual household income | ||||||||
| Less than £18000 | 10135 (19.7%) | 8851 (17.2%) | 8622 (16.8%) | 9477 (18.4%) | 10830 (18.2%) | 10982 (18.4%) | 11999 (20.0%) | 14185 (23.8%) |
| £18000 to £30999 | 11380 (22.2%) | 11083 (21.5%) | 11039 (21.5%) | 10931 (21.2%) | 12249 (20.5%) | 12676 (21.3%) | 13038 (21.8%) | 13620 (22.8%) |
| £31000 to £51999 | 12024 (23.4%) | 12537 (24.4%) | 12538 (24.4%) | 12138 (23.6%) | 13368 (22.4%) | 13137 (22.0%) | 12663 (21.2%) | 11329 (19.0%) |
| £52000 to £100000 | 9559 (18.6%) | 10489 (20.4%) | 10708 (20.8%) | 10307 (20.0%) | 10844 (18.2%) | 10200 (17.1%) | 9238 (15.4%) | 7211 (12.1%) |
| Greater than £100000 | 2471 (4.8%) | 2874 (5.6%) | 3002 (5.8%) | 2921 (5.7%) | 2839 (4.8%) | 2704 (4.5%) | 2397 (4.0%) | 1748 (2.9%) |
| College or university degree | 18240 (35.5%) | 18096 (35.2%) | 17459 (34.0%) | 15895 (30.9%) | 21019 (35.2%) | 20036 (33.6%) | 18344 (30.6%) | 15547 (26.0%) |
| Family history of cancer | 17522 (34.1%) | 17651 (34.3%) | 17911 (34.9%) | 17933 (34.9%) | 20375 (34.2%) | 20767 (34.8%) | 21329 (35.6%) | 22030 (36.9%) |
| Mean BMI, kg/m2 (SD) | 26.30 (3.90) | 27.30 (3.87) | 28.20 (3.99) | 29.60 (4.41) | 24.60 (3.82) | 25.90 (4.29) | 27.40 (4.82) | 30.30 (5.79) |
In total, 920 participants developed HBP cancer during a median of 6.6 yrs of follow-up. The risk of pancreatic cancer tended to increase with the SUA levels (adjusted HR per 1 mg/dL increase in SUA = 1.12, 95%CI: 1.04-1.21). In model 1, the HR of the pancreatic cancer risk was 1.91 (95%CI: 1.42-2.58) in the highest quartile (Q4) of SUA compared with the lowest quartile (Q1). After adjusting for potential confounders, the HR was gradually attenuated, but the association still existed in model 3. The risk of pancreatic cancer in the highest quartile of SUA increased by 77% compared with that in the lowest quartile (HR 1.77, 95%CI: 1.29-2.42, Table 3).
| Cancer | Group | Cases | Incidence1 | HR (95%CI) | ||
| Model 1 | Model 2 | Model 3 | ||||
| Liver | Q1 | 42 | 5.76 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 62 | 8.45 | 1.08 (0.72-1.60) | 1.08 (0.73-1.61) | 1.00 (0.67-1.50) | |
| Q3 | 62 | 8.58 | 0.90 (0.59-1.36) | 0.90 (0.59-1.36) | 0.79 (0.52-1.21) | |
| Q4 | 95 | 13.12 | 1.20 (0.80-1.81) | 1.18 (0.79-1.78) | 0.98 (0.64-1.49) | |
| Estimated HR (per 1 mg/dL) | 1.08 (0.97-1.19) | 1.06 (0.96-1.17) | 1.01 (0.91-1.13) | |||
| Gallbladder | Q1 | 13 | 1.78 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 15 | 2.05 | 1.02 (0.48-2.19) | 1.01 (0.47-2.18) | 0.98 (0.45-2.12) | |
| Q3 | 10 | 1.38 | 0.85 (0.37-1.97) | 0.84 (0.36-1.93) | 0.79 (0.33-1.86) | |
| Q4 | 18 | 2.49 | 1.52 (0.68-3.39) | 1.45 (0.65-3.24) | 1.32 (0.56-3.11) | |
| Estimated HR (per 1 mg/dL) | 1.09 (0.87-1.35) | 1.07 (0.86-1.33) | 1.04 (0.82-1.31) | |||
| Biliary tract | Q1 | 11 | 1.51 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 19 | 2.59 | 1.36 (0.64-2.89) | 1.37 (0.65-2.93) | 1.32 (0.61-2.89) | |
| Q3 | 27 | 3.74 | 1.64 (0.78-3.47) | 1.69 (0.80-3.58) | 1.48 (0.68-3.22) | |
| Q4 | 17 | 2.35 | 0.94 (0.41-2.15) | 0.99 (0.43-2.27) | 0.75 (0.31-1.82) | |
| Estimated HR (per 1 mg/dL) | 0.92 (0.76-1.13) | 0.94 (0.77-1.14) | 0.85 (0.69-1.05) | |||
| Pancreas | Q1 | 76 | 10.43 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 115 | 15.68 | 1.31 (0.97-1.75) | 1.31 (0.98-1.76) | 1.29 (0.96-1.73) | |
| Q3 | 155 | 21.45 | 1.68 (1.26-2.24) | 1.69 (1.27-2.26) | 1.61 (1.19-2.16) | |
| Q4 | 183 | 25.28 | 1.91 (1.42-2.58) | 1.92 (1.43-2.59) | 1.77 (1.29-2.42) | |
| Estimated HR (per 1 mg/dL) | 1.15 (1.07-1.23) | 1.15 (1.07-1.23) | 1.12 (1.04-1.21) | |||
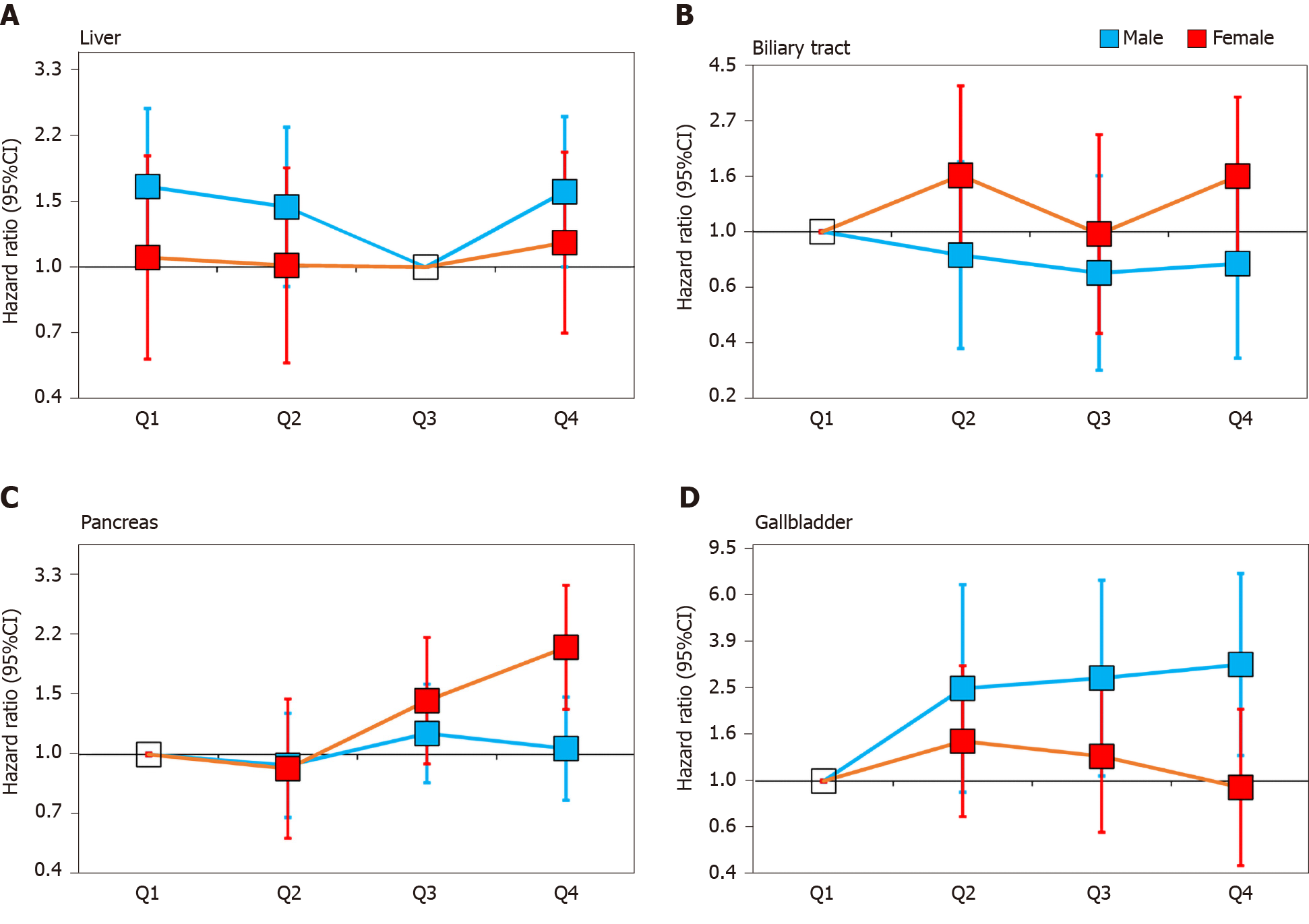
The stratified analysis results showed that SUA had different effects on HBP cancer between the males and females. In the male population, after fully adjusting for potential confounders, the risk of liver cancer decreased in the second quartile (HR 0.87, 95%CI: 0.57-1.34) and the third quartile (HR 0.61, 95%CI: 0.38-0.98) compared with that in the lowest quartile. However, in the highest quartile (HR 0.96, 95%CI: 0.63-1.46), the HR of liver cancer was increased compared with that in the third quartile (Figure 2A). In contrast to liver cancer, the risk of gallbladder cancer in the second quartile (HR 2.45, 95%CI: 0.90-6.70), the third quartile (HR 2.71, 95%CI: 1.05-6.98) and the highest quartile (HR 3.09, 95%CI: 1.28-7.46) were all higher than that in the lowest quartile (Figure 2D). The risk of biliary tract cancer and pancreatic cancer did not differ between the lowest and other quartiles (Figure 2B).
In the female population, no difference was found between the lowest quartile and the other quartiles of the SUA levels in gallbladder cancer and liver cancer. Regarding the biliary tract cancer risk, the HR of the highest quartile of SUA was 2.33 (95%CI: 1.14-4.76) compared with the lowest quartile in model 2 (Table 4). However, after additionally adjusting for BMI in model 3, the risk was attenuated (HR 1.65, 95%CI: 0.81-3.36). Regarding the pancreatic cancer risk, the risk increased by 1.33 times per 1 mg/dL SUA level in model 3 (HR 1.33, 95%CI: 1.21-1.47, Table 4). After an additional adjustment for potential confounders, the highest quartile still showed an increased risk compared with the lowest quartile (HR 2.04, 95%CI: 1.35-3.08, Figure 2C).
| Gender | Cancer | Group | Cases | Incidence1 | HR (95%CI) | ||
| Model 1 | Model 2 | Model 3 | |||||
| Male | Liver | Quartile 1 | 44 | 13.12 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 39 | 11.71 | 0.89 (0.58-1.37) | 0.92 (0.60-1.42) | 0.87 (0.57-1.34) | ||
| Quartile 3 | 29 | 8.67 | 0.65 (0.41-1.05) | 0.68 (0.42-1.08) | 0.61 (0.38-0.98) | ||
| Quartile 4 | 53 | 15.78 | 1.16 (0.78-1.73) | 1.17 (0.78-1.75) | 0.96 (0.63-1.46) | ||
| Estimated HR (per 1 mg/dL) | 1.07 (0.95-1.21) | 1.07 (0.95-1.21) | 1.01 (0.89-1.15) | ||||
| Gallbladder | Quartile 1 | 2 | 0.60 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 5 | 1.50 | 2.48 (0.48-12.80) | 2.54 (0.93-6.94) | 2.45 (0.90-6.70) | ||
| Quartile 3 | 6 | 1.79 | 2.91 (0.59-14.41) | 2.93 (1.14-7.56) | 2.71 (1.05-6.98) | ||
| Quartile 4 | 8 | 2.38 | 3.67 (0.78-17.30) | 3.56 (1.47-8.58) | 3.09 (1.28-7.46) | ||
| Estimated HR (per 1 mg/dL) | 1.20 (0.86-1.67) | 1.17 (0.85-1.60) | 1.11 (0.81-1.53) | ||||
| Biliary tract | Quartile 1 | 13 | 3.88 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 10 | 3.00 | 0.77 (0.34-1.76) | 0.80 (0.35-1.84) | 0.81 (0.35-1.88) | ||
| Quartile 3 | 9 | 2.69 | 0.69 (0.30-1.62) | 0.74 (0.32-1.75) | 0.69 (0.29-1.67) | ||
| Quartile 4 | 11 | 3.27 | 0.82 (0.37-1.83) | 0.89 (0.39-2.00) | 0.75 (0.32-1.75) | ||
| Estimated HR (per 1 mg/dL) | 0.82 (0.64-1.07) | 0.85 (0.65-1.10) | 0.80 (0.61-1.04) | ||||
| Pancreas | Quartile 1 | 68 | 20.28 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 62 | 18.61 | 0.91 (0.65-1.29) | 0.93 (0.66-1.32) | 0.93 (0.66-1.32) | ||
| Quartile 3 | 80 | 23.92 | 1.18 (0.85-1.62) | 1.20 (0.87-1.66) | 1.15 (0.83-1.60) | ||
| Quartile 4 | 76 | 22.62 | 1.06 (0.77-1.48) | 1.08 (0.77-1.50) | 1.04 (0.74-1.47) | ||
| Estimated HR (per 1 mg/dL) | 1.02 (0.93-1.12) | 1.02 (0.93-1.12) | 1.01 (0.91-1.12) | ||||
| Female | Liver | Quartile 1 | 20 | 5.06 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 21 | 5.35 | 0.87 (0.47-1.64) | 0.87 (0.47-1.64) | 0.86 (0.46-1.62) | ||
| Quartile 3 | 23 | 5.86 | 1.06 (0.59-1.91) | 1.05 (0.58-1.90) | 1.02 (0.56-1.87) | ||
| Quartile 4 | 32 | 8.21 | 1.22 (0.69-2.15) | 1.16 (0.65-2.05) | 1.09 (0.59-2.02) | ||
| Estimated HR (per 1 mg/dL) | 1.10 (0.93-1.31) | 1.08 (0.91-1.28) | 1.06 (0.88-1.28) | ||||
| Gallbladder | Quartile 1 | 6 | 1.52 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 10 | 2.55 | 1.47 (0.53-4.06) | 1.49 (0.72-3.10) | 1.47 (0.71-3.06) | ||
| Quartile 3 | 10 | 2.55 | 1.31 (0.47-3.61) | 1.30 (0.63-2.72) | 1.27 (0.61-2.65) | ||
| Quartile 4 | 9 | 2.31 | 1.02 (0.36-2.88) | 0.99 (0.46-2.10) | 0.94 (0.44-2.00) | ||
| Estimated HR (per 1 mg/dL) | 1.20 (0.86-1.67) | 1.17 (0.85-1.60) | 1.01 (0.75-1.35) | ||||
| Biliary tract | Quartile 1 | 4 | 1.01 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 8 | 2.04 | 1.77 (0.53-5.88) | 1.78 (0.80-3.97) | 1.66 (0.74-3.71) | ||
| Quartile 3 | 6 | 1.53 | 1.19 (0.33-4.22) | 1.17 (0.48-2.85) | 0.98 (0.40-2.39) | ||
| Quartile 4 | 13 | 3.34 | 2.24 (0.72-6.94) | 2.33 (1.14-4.76) | 1.65 (0.81-3.36) | ||
| Estimated HR (per 1 mg/dL) | 0.82 (0.64-1.07) | 0.85 (0.65-1.1) | 1.09 (0.81-1.48) | ||||
| Pancreas | Quartile 1 | 35 | 8.86 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 36 | 9.17 | 0.93 (0.58-1.48) | 0.93 (0.58-1.47) | 0.91 (0.57-1.44) | ||
| Quartile 3 | 64 | 16.29 | 1.51 (1.00-2.28) | 1.50 (0.99-2.27) | 1.43 (0.94-2.18) | ||
| Quartile 4 | 108 | 27.71 | 2.27 (1.54-3.34) | 2.25 (1.53-3.31) | 2.04 (1.35-3.08) | ||
| Estimated HR (per 1 mg/dL) | 1.02 (0.93-1.12) | 1.02 (0.93-1.12) | 1.33 (1.21-1.47) | ||||
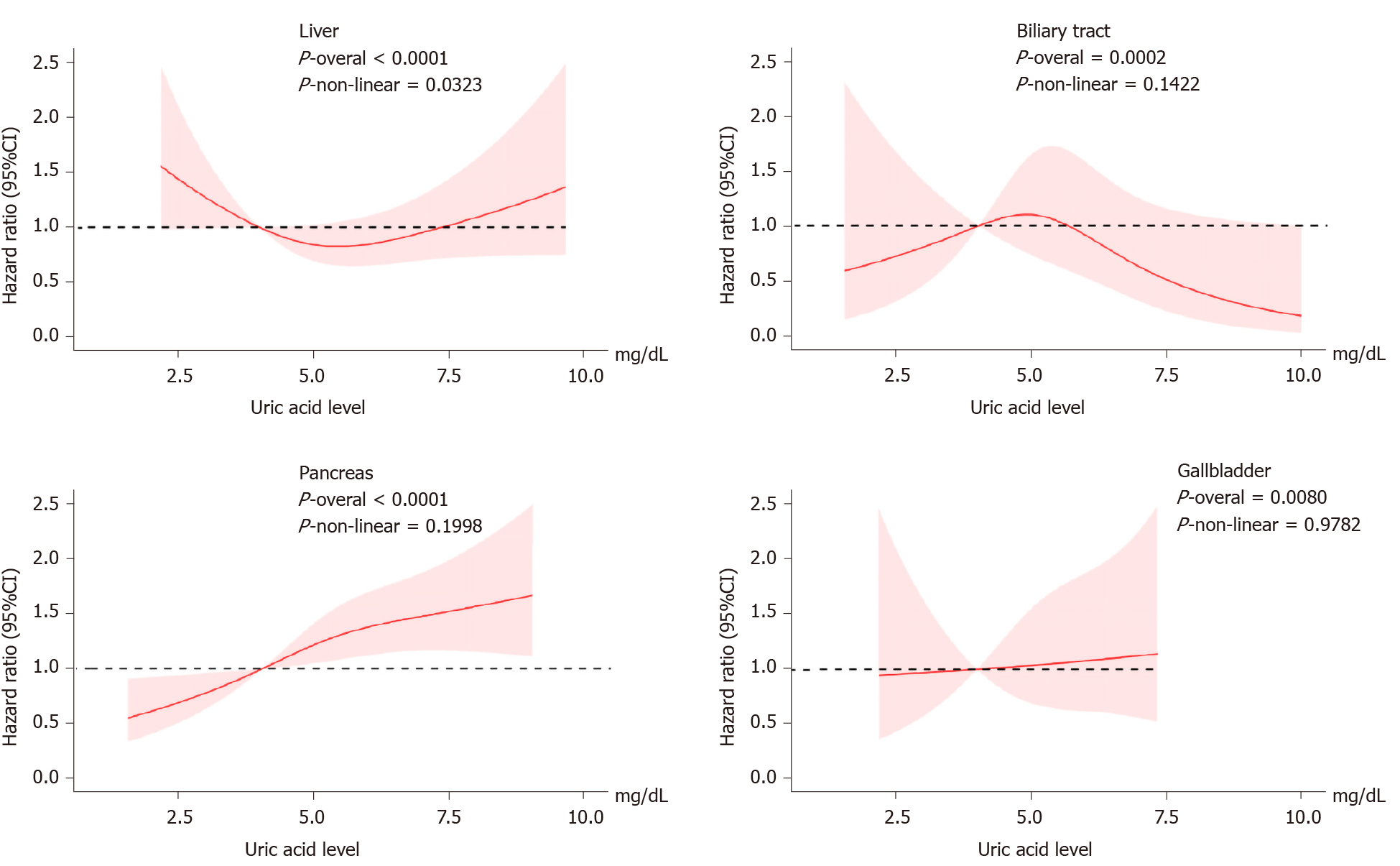
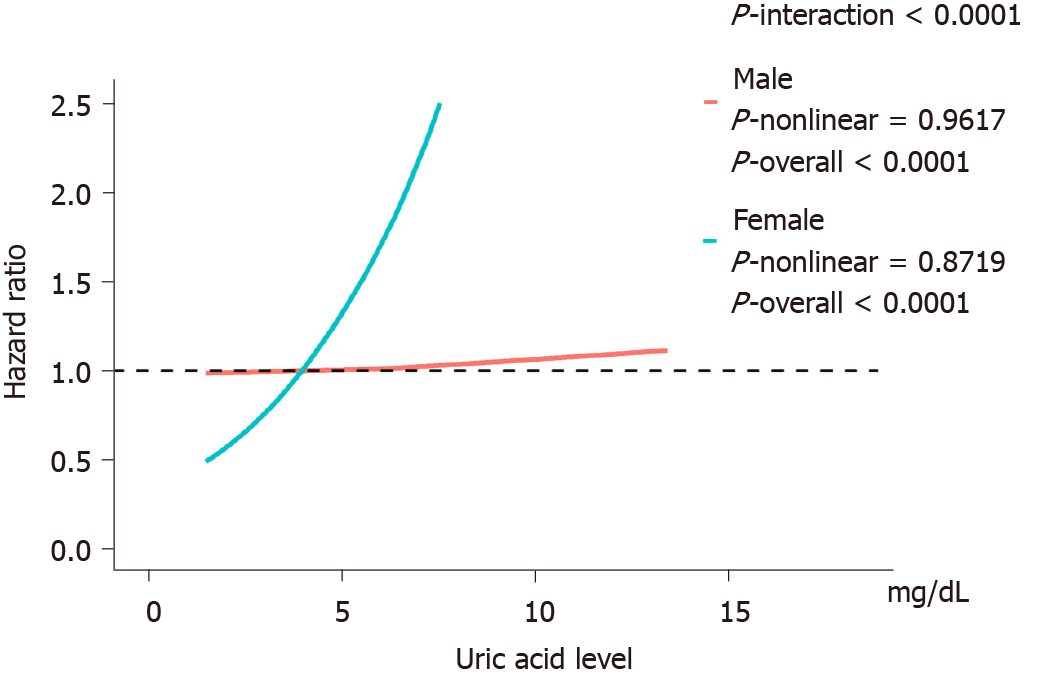
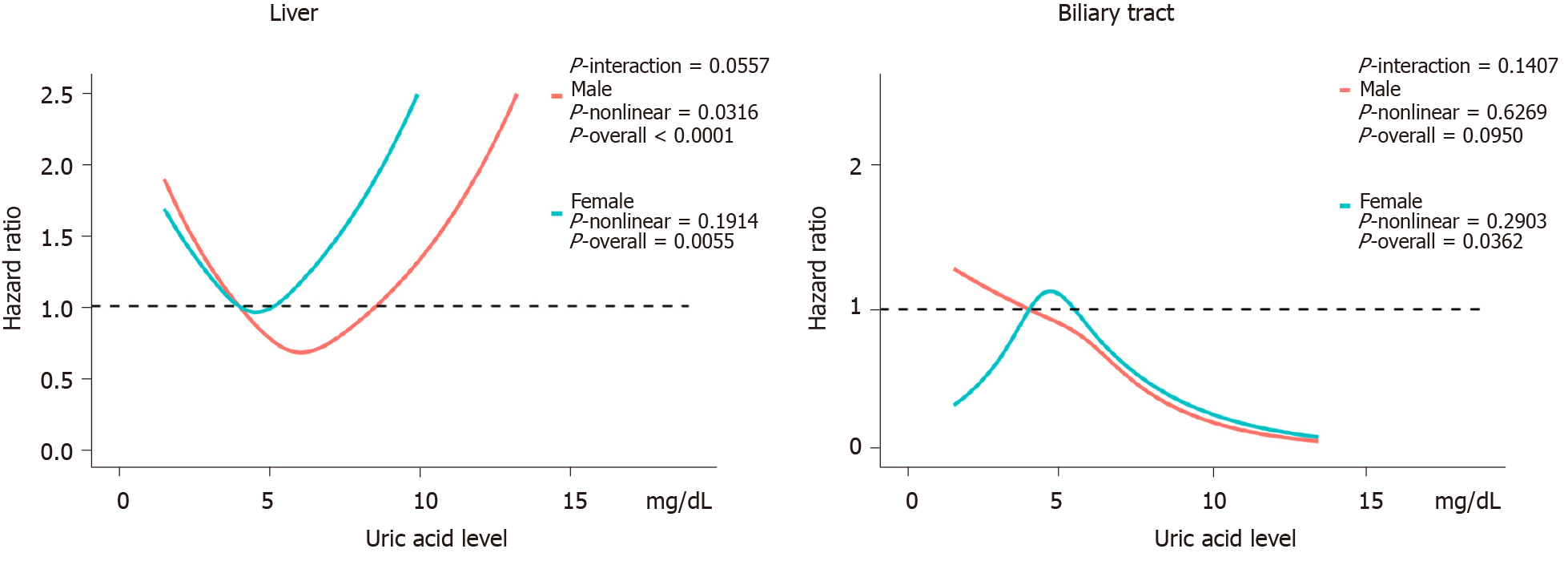
Figure 3 shows the evaluation of the potential nonlinear relationship with HBP cancer. A strong linear dose-response relationship was observed between the SUA levels and the risk of pancreatic cancer (P-nonlinear > 0.05, P-overall < 0.0001, Figure 3). After the stratification by genders, the SUA levels exhibited a linear dose-response relationship with the risk of pancreatic cancer in both the male and female populations, but the effect was much stronger in the females than in the males (P-interaction < 0.0001, Figure 4). The liver cancer risk showed a U-shaped relationship with the SUA levels (P-nonlinear < 0.05, P-overall < 0.0001, Figure 3); however, a nonlinear relationship with the risk of liver cancer was observed only in the males (P-nonlinear < 0.05, Figure 5). Additionally, regarding the SUA levels and the risk of gallbladder cancer and biliary tract cancer, a linear dose-response relationship was observed.
These results suggest that high SUA levels are associated with an increased risk of pancreatic cancer in females and gallbladder cancer in the males. Moreover, the risk of liver cancer showed a U-shaped association in the males as both too high and too low levels of SUA were associated with an increased risk. We did not observe sufficient evidence of an association between the SUA levels and biliary tract cancer.
In the sensitivity analysis, by excluding cases that were documented in the first 2 yrs, we did not observe major changes in the primary results (Tables 5 and 6).
| Cancer | Group | HR (95%CI) | ||
| Model 1 | Model 2 | Model 3 | ||
| Liver | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 1.00 (0.64-1.55) | 1.01 (0.65-1.57) | 0.92 (0.59-1.43) | |
| Quartile 3 | 0.78 (0.49-1.24) | 0.79 (0.50-1.25) | 0.68 (0.42-1.08) | |
| Quartile 4 | 1.04 (0.66-1.63) | 1.04 (0.66-1.63) | 0.82 (0.51-1.31) | |
| Gallbladder | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 1.68 (0.66-4.28) | 1.71 (0.67-4.36) | 1.63 (0.63-4.21) | |
| Quartile 3 | 1.41 (0.51-3.89) | 1.42 (0.52-3.93) | 1.31 (0.46-3.72) | |
| Quartile 4 | 2.71 (1.02-7.21) | 2.72 (1.03-7.23) | 2.40 (0.85-6.80) | |
| Biliary tract | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 1.09 (0.48-2.47) | 1.10 (0.49-2.51) | 1.12 (0.48-2.61) | |
| Quartile 3 | 1.10 (0.48-2.50) | 1.14 (0.50-2.60) | 1.08 (0.46-2.55) | |
| Quartile 4 | 0.75 (0.31-1.82) | 0.79 (0.32-1.93) | 0.68 (0.26-1.75) | |
| Pancreas | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 1.26 (0.90-1.76) | 1.26 (0.90-1.77) | 1.25 (0.89-1.76) | |
| Quartile 3 | 1.76 (1.27-2.44) | 1.77 (1.28-2.46) | 1.71 (1.22-2.40) | |
| Quartile 4 | 2.09 (1.49-2.92) | 2.11 (1.51-2.95) | 1.96 (1.38-2.80) | |
| Gender | Cancer | Group | HR (95%CI) | ||
| Model 1 | Model 2 | Model 3 | |||
| Male | Liver | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 0.88 (0.55-1.40) | 0.92 (0.57-1.46) | 0.86 (0.54-1.38) | ||
| Quartile 3 | 0.60 (0.36-1.01) | 0.62 (0.37-1.05) | 0.56 (0.33-0.94) | ||
| Quartile 4 | 0.99 (0.63-1.55) | 0.99 (0.63-1.56) | 0.81 (0.51-1.29) | ||
| Gallbladder | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 3.04 (0.32-29.21) | 3.20 (0.90-11.35) | 3.01 (0.85-10.67) | ||
| Quartile 3 | 4.92 (0.57-42.13) | 5.22 (1.78-15.28) | 4.58 (1.57-13.41) | ||
| Quartile 4 | 5.52 (0.66-45.96) | 5.85 (2.08-16.43) | 4.64 (1.65-13.03) | ||
| Biliary tract | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 0.83 (0.34-1.99) | 0.87 (0.36-2.10) | 0.88 (0.36-2.17) | ||
| Quartile 3 | 0.82 (0.34-1.97) | 0.88 (0.36-2.15) | 0.83 (0.33-2.06) | ||
| Quartile 4 | 0.70 (0.28-1.73) | 0.77 (0.30-1.93) | 0.64 (0.24-1.68) | ||
| Pancreas | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 1.04 (0.70-1.55) | 1.07 (0.72-1.59) | 1.06 (0.71-1.59) | ||
| Quartile 3 | 1.37 (0.95-1.99) | 1.41 (0.97-2.04) | 1.33 (0.91-1.95) | ||
| Quartile 4 | 1.15 (0.78-1.69) | 1.17 (0.79-1.73) | 1.11 (0.74-1.66) | ||
| Female | Liver | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 | 0.91 (0.45-1.81) | 0.92 (0.46-1.85) | 0.89 (0.44-1.78) | ||
| Quartile 3 | 0.98 (0.50-1.92) | 1.00 (0.51-1.95) | 0.92 (0.47-1.83) | ||
| Quartile 4 | 1.05 (0.55-2.00) | 1.02 (0.53-1.96) | 0.88 (0.43-1.76) | ||
| Gallbladder | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 2.06 (0.53-7.98) | 2.13 (0.55-8.24) | 2.11 (0.54-8.20) | ||
| Quartile 3 | 2.38 (0.64-8.81) | 2.47 (0.66-9.15) | 2.42 (0.64-9.15) | ||
| Quartile 4 | 2.08 (0.56-7.74) | 2.10 (0.56-7.85) | 2.02 (0.51-8.08) | ||
| Biliary tract | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 1.56 (0.46-5.33) | 1.56 (0.45-5.33) | 1.48 (0.43-5.09) | ||
| Quartile 3 | 0.80 (0.20-3.22) | 0.80 (0.20-3.23) | 0.72 (0.18-2.97) | ||
| Quartile 4 | 1.23 (0.36-4.26) | 1.29 (0.37-4.47) | 1.04 (0.27-3.96) | ||
| Pancreas | Quartile 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Quartile 2 | 0.78 (0.47-1.32) | 0.78 (0.46-1.31) | 0.77 (0.46-1.29) | ||
| Quartile 3 | 1.21 (0.77-1.92) | 1.20 (0.76-1.90) | 1.16 (0.73-1.85) | ||
| Quartile 4 | 2.07 (1.37-3.13) | 2.05 (1.35-3.10) | 1.93 (1.24-3.01) | ||
As a very common metabolite, SUA has multiple effects on the human body, and high SUA levels have been considered harmful. Previous studies have found that elevated SUA levels are associated with gout, diabetes, hypertension, hyperlipidemia, obesity[21-25] and cancer[26-28]. Kolonel et al[29] conducted a prospective cohort study including 7889 males and indicated that high SUA levels were associated with a high prostate cancer risk. Deng et al[30] included 8274 patients with type 2 diabetes from the Shanghai Diabetes Registry and found that in female diabetic patients, SUA was positively associated with the cancer risk. Another Mendelian randomization study analyzed 86210 individuals from the Copenhagen General Population Study and indicated that high SUA levels were associated with an increased cancer risk[31]. In our research, we also found a relationship between high SUA levels and an increased risk of pancreatic cancer in females and gallbladder cancer in males.
SUA has been found to be associated with inflammatory stress, which is closely related to the occurrence of cancer. Components of the inflammatory microenvir-onment, such as adiponectin, C-reactive protein, leptin and cyclooxygenase 2 (COX-2), which are closely related to SUA, were found to be associated with cancer development[15,32]. In addition, in our study, the SUA levels were positively correlated with pancreatic cancer and gallbladder cancer, and COX-2 was widely expressed in tumor tissues[33,34]. Xie et al[35] conducted an in vitro experiment examining the effect of COX-2 on the angiogenesis of pancreatic cancer cells and indicated that COX-2 was positively associated with the microvascular density, promoting pancreatic cancer cell growth. Celecoxib, a selective COX-2 inhibitor, was found to enhance the effect of chemotherapeutic drugs on pancreatic cancer and inhibit the proliferation of gallbladder cancer cells[36,37]. Ohtsubo et al[38] confirmed that SUA regulates the expression of COX-2 through XOR in in vivo and in vitro experiments, which may explain the association between SUA and cancer. Based on the evidence from previous epidemiological and experimental studies along with our findings, high SUA levels are likely to lead to an increased risk of cancer in various sites, suggesting that we should pay attention to reducing the SUA levels to reduce the risk of cancer.
Although many studies have shown that high SUA levels are a risk factor for cancer, some evidence suggests that the SUA levels should not be too low. Ames et al[12]. first proposed the hypothesis that SUA might act as a protective factor against cancer due to its antioxidant function and its function as a scavenger of singlet oxygen and free radicals. Some epidemiological studies also supported this hypothesis. Tilman et al[39] conducted a population-based study of endogenous antioxidants, including albumin, bilirubin and SUA, and indicated that a high SUA level was associated with a low risk of breast cancer and low mortality of all cancers. Patients with oral cancer and lung cancer also had lower SUA levels[40,41]. The results of a cohort study confirmed that low SUA levels were associated with lung cancer[42]. In our study, we found that as the SUA levels increased, the risk of liver cancer first exhibited a downward trend. Male participants in the third quartile had a 39% decreased risk of liver cancer (HR 0.61, 95%CI: 0.38-0.98) compared with those in the lowest quartile, possibly due to the protective function of SUA. However, as the SUA levels further increased, the risk of liver cancer also increased. In the highest quartile, the risk of liver cancer was notably higher than the risk in the third quartile, revealing a U-shaped relationship. Strasak et al[43] conducted a population-based study involving Austrian men and suggested a J-shaped effect of SUA on the risk of overall cancer incidence, which is similar to our results in liver cancer, indicating that SUA within a proper range is better in the context of liver cancer, and too low or too high levels of SUA represent a risk factor for liver cancer. Similarly, COX-2 is overexpressed during the development of liver cancer and tumor tissues, while normal liver tissues scarcely express COX-2[44,45]. Chen et al[46] showed that COX-2 was a leading factor related to liver cancer in a spontaneous liver cancer mouse model that overexpressed COX-2 specifically in the liver. As the SUA levels increase, the cancer-promoting effect of COX-2 overexpression may be stronger, leading to the U-shaped association between SUA and liver cancer.
In the gender stratified analysis, we found that only gallbladder cancer and liver cancer were associated with SUA in males, and that pancreatic cancer was related to SUA in females. This finding might be related to the reduced number of cases after the stratification as the statistical power was insufficient. In addition, we found that the risk associated with SUA in biliary tract, gallbladder and liver cancer in the female participants was generally higher than that in the male participants. Similar results were found in previous studies. A Chinese cohort study found that high SUA levels were associated with cancer risk in diabetic female patients[30]. Yan et al[47] conducted a systematic review and suggested that high SUA levels were associated with a high cancer risk, especially among females. Further analysis suggested that SUA and gender had an interactive effect on pancreatic cancer because sex hormones may lead to different sensitivity to SUA. More research is still required to reveal such gender differences.
To the best of our knowledge, this study is the first to focus on the association between SUA and HBP cancer. The main strength of our research is the large sample size. We included over 0.44 million participants in this analysis, allowing us to discover the relationship between the SUA levels and HBP cancer at multiple levels. Additionally, the UK Biobank comprehensively collected data related to established HBP cancer risk factors, allowing us to sufficiently control for potential confounders. We also investigated the potential nonlinear relationship, which provided insight into the carcinogenicity of SUA and contributes to individualized cancer prevention.
This study has limitations. First, as an observational study, we cannot confirm the causal-relationship between SUA and HBP cancer. Second, due to the limited number of cases, we were unable to conduct further stratified analyses of some variables. Third, in the UK Biobank, most included people were white Europeans, and the role of SUA in other races is unclear. More research is needed to compensate for the above limitations.
In conclusion, SUA is likely to have gender-specific effects on HBP cancer. High SUA levels represent a risk factor for gallbladder cancer in males and have a strong effect on pancreatic cancer in females. SUA levels that are too high or too low are associated with an increased risk of liver cancer in males. In clinical and public health practice, the management of either too high or too low SUA levels may contribute to the prevention of HBP cancer. Future research is required to confirm our conclusion and investigate the mechanisms underlying these associations.
In 2018, new cases of hepatobiliary-pancreatic (HBP) cancer reached 1.85 million and identifying high-risk populations has become an urgent public health issue. As one of the important metabolites of the human body, serum uric acid (SUA) is considered to be related to cancer risk, but there is controversy about its role in specific cancers.
Because of the dual effect of SUA on cancer risk, the associations between SUA levels and the HBP cancer risk remain unclear.
To evaluate the associations between SUA levels and incidence of hepatobiliary-pancreatic cancer based on the UK Biobank cohort and to investigate the gender differences.
This is a prospective cohort study from the UK Biobank. We estimated the hazard ratios and 95% confidence intervals between SUA levels and hepatobiliary-pancreatic cancer by using multiple adjusted Cox regression models adjusted for potential confounders. In addition, we also conducted a sensitivity analysis to verify the stability of our results.
We included 444462 participants free of cancer. With a median of 6.6 yrs of follow-up, 920 participants developed liver, gallbladder, biliary tract or pancreatic cancer. The risk of pancreatic cancer increases with the SUA levels; however, after the gender-stratified analysis, the association only occurred among the females. Both too high and too low SUA levels are the risk factors of liver cancer among the males. For gallbladder cancer, the positive association with SUA levels was identified among the males. Regarding biliary tract cancer, there is not sufficient evidence for biliary tract cancer and SUA levels.
SUA is likely to have gender-specific effects on HBP cancer. In clinical and public health practice, controlling SUA levels in an appropriate range may help prevent HBP cancer.
In the future, more research is needed to investigate the association between the SUA levels and other specific-site cancer risk and the underlying mechanism.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abeysekera K, Forrest E, Myojin Y, Ong J S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Cazacu IM, Singh BS, Saftoiu A, Bhutani MS. Recent developments in hepatopancreatobiliary EUS. Endosc Ultrasound. 2019;8:146-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Kovacevic B, Karstensen JG, Havre RF, Pham KD, Giovannini M, Dabizzi E, Arcidiacono P, Santo E, Sequeiros EV, Klausen P, Rift CV, Hasselby JP, Toxværd A, Kalaitzakis E, Hansen CP, Vilmann P. Initial experience with EUS-guided microbiopsy forceps in diagnosing pancreatic cystic lesions: A multicenter feasibility study (with video). Endosc Ultrasound. 2018;7:383-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Sãftoiu A, Bhutani MS, Itoi T, Arcidiacono PG, Bories E, Cazacu IM, Constantin A, Coronel E, Dietrich CF, Duda DG, Garcia JI, Hocke M, Ignee A, Jenssen C, Jinga M, Khor C, Oppong KW, Pereira S, Petrone MC, Santo E, Seicean A, Seo DW, Siyu S, Vilmann P, Waxman I, Yeaton P. Changes in tumor vascularity depicted by contrast-enhanced EUS as a predictor of prognosis and treatment efficacy in patients with unresectable pancreatic cancer (PEACE): A study protocol. Endosc Ultrasound. 2019;8:235-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55843] [Article Influence: 7977.6] [Reference Citation Analysis (132)] |
| 5. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1082] [Article Influence: 180.3] [Reference Citation Analysis (1)] |
| 6. | Jiang T, Deng Z, Li J, Tian G. Pancreatic cancer: Does it work if EUS and laser ablation get married? Endosc Ultrasound. 2018;7:207-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Cazacu IM, Udristoiu A, Gruionu LG, Iacob A, Gruionu G, Saftoiu A. Artificial intelligence in pancreatic cancer: Toward precision diagnosis. Endosc Ultrasound. 2019;8:357-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Shi T, Feng X, Jie Z. Progress and Current Status of Influenza Researches in China. J Transl Int Med. 2019;7:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Babazadeh A, Mohseni Afshar Z, Javanian M, Mohammadnia-Afrouzi M, Karkhah A, Masrour-Roudsari J, Sabbagh P, Koppolu V, Vasigala VK, Ebrahimpour S. Influenza Vaccination and Guillain-Barré Syndrome: Reality or Fear. J Transl Int Med. 2019;7:137-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Liu C, Shao Z. Aplastic Anemia in China. J Transl Int Med. 2018;6:134-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 12. | Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858-6862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1948] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 13. | Hsueh CY, Shao M, Cao W, Li S, Zhou L. Pretreatment Serum Uric Acid as an Efficient Predictor of Prognosis in Men with Laryngeal Squamous Cell Cancer: A Retrospective Cohort Study. Oxid Med Cell Longev. 2019;2019:1821969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Taghizadeh N, Vonk JM, Boezen HM. Serum uric acid levels and cancer mortality risk among males in a large general population-based cohort study. Cancer Causes Control. 2014;25:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Fini MA, Elias A, Johnson RJ, Wright RM. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Sahin IH, Hassan MM, Garrett CR. Impact of non-steroidal anti-inflammatory drugs on gastrointestinal cancers: current state-of-the science. Cancer Lett. 2014;345:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Xie Y, Xu P, Liu K, Lin S, Wang M, Tian T, Dai C, Deng Y, Li N, Hao Q, Zhou L, Dai Z, Guo H. Hyperuricemia and gout are associated with cancer incidence and mortality: A meta-analysis based on cohort studies. J Cell Physiol. 2019;234:14364-14376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6354] [Cited by in RCA: 7487] [Article Influence: 748.7] [Reference Citation Analysis (0)] |
| 19. | IPAQ Research Committee. Guidelines for data processing analysis of the International Physical Activity Questionnaire (IPAQ) - Short and long forms. 2005. Available from: http://www.ipaq.ki.se. |
| 20. | Elliott P, Peakman TC; UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 588] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 21. | Chen MY, Zhao CC, Li TT, Zhu Y, Yu TP, Bao YQ, Li LX, Jia WP. Serum uric acid levels are associated with obesity but not cardio-cerebrovascular events in Chinese inpatients with type 2 diabetes. Sci Rep. 2017;7:40009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 23. | Kuwabara M, Borghi C, Cicero AFG, Hisatome I, Niwa K, Ohno M, Johnson RJ, Lanaspa MA. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: A five-year cohort study in Japan. Int J Cardiol. 2018;261:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Mortada I. Hyperuricemia, Type 2 Diabetes Mellitus, and Hypertension: an Emerging Association. Curr Hypertens Rep. 2017;19:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 25. | Shani M, Vinker S, Dinour D, Leiba M, Twig G, Holtzman EJ, Leiba A. High Normal Uric Acid Levels Are Associated with an Increased Risk of Diabetes in Lean, Normoglycemic Healthy Women. J Clin Endocrinol Metab. 2016;101:3772-3778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Stotz M, Szkandera J, Seidel J, Stojakovic T, Samonigg H, Reitz D, Gary T, Kornprat P, Schaberl-Moser R, Hoefler G, Gerger A, Pichler M. Evaluation of uric acid as a prognostic blood-based marker in a large cohort of pancreatic cancer patients. PLoS One. 2014;9:e104730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Yue CF, Feng PN, Yao ZR, Yu XG, Lin WB, Qian YM, Guo YM, Li LS, Liu M. High serum uric acid concentration predicts poor survival in patients with breast cancer. Clin Chim Acta. 2017;473:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Shin HS, Lee HR, Lee DC, Shim JY, Cho KH, Suh SY. Uric acid as a prognostic factor for survival time: a prospective cohort study of terminally ill cancer patients. J Pain Symptom Manage. 2006;31:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Kolonel LN, Yoshizawa C, Nomura AM, Stemmermann GN. Relationship of serum uric acid to cancer occurrence in a prospective male cohort. Cancer Epidemiol Biomarkers Prev. 1994;3:225-228. [PubMed] |
| 30. | Deng Z, Gu Y, Hou X, Zhang L, Bao Y, Hu C, Jia W. Association between uric acid, cancer incidence and mortality in patients with type 2 diabetes: Shanghai diabetes registry study. Diabetes Metab Res Rev. 2016;32:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Kobylecki CJ, Afzal S, Nordestgaard BG. Plasma Urate, Cancer Incidence, and All-Cause Mortality: A Mendelian Randomization Study. Clin Chem. 2017;63:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012;47:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Maust JD, Frankowski-McGregor CL, Bankhead A, Simeone DM, Sebolt-Leopold JS. Cyclooxygenase-2 Influences Response to Cotargeting of MEK and CDK4/6 in a Subpopulation of Pancreatic Cancers. Mol Cancer Ther. 2018;17:2495-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Legan M, Luzar B, Marolt VF, Cor A. Expression of cyclooxygenase-2 is associated with p53 accumulation in premalignant and malignant gallbladder lesions. World J Gastroenterol. 2006;12:3425-3429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Xie C, Xu X, Wang X, Wei S, Shao L, Chen J, Cai J, Jia L. Cyclooxygenase-2 induces angiogenesis in pancreatic cancer mediated by prostaglandin E2. Oncol Lett. 2018;16:940-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Chen Q, Wang J, Zhang Q, Zhang J, Lou Y, Yang J, Chen Y, Wei T, Zhang J, Fu Q, Ye M, Zhang X, Dang X, Liang T, Bai X. Tumour cell-derived debris and IgG synergistically promote metastasis of pancreatic cancer by inducing inflammation via tumour-associated macrophages. Br J Cancer. 2019;121:786-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Deng M, Qin Y, Chen X, Li D, Wang Q, Zheng H, Gu L, Deng C, Xue Y, Zhu D, Wang Q, Wang J. Combination of celecoxib and PD184161 exerts synergistic inhibitory effects on gallbladder cancer cell proliferation. Oncol Lett. 2017;13:3850-3858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res. 2004;95:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Kühn T, Sookthai D, Graf ME, Schübel R, Freisling H, Johnson T, Katzke V, Kaaks R. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. 2017;117:1572-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 40. | Ara SA, Ashraf S, Patil BM. Evaluation of serum uric acid levels in patients with oral squamous cell carcinoma. Indian J Dent Res. 2016;27:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Bozkir A, Simşek B, Güngört A, Torun M. Ascorbic acid and uric acid levels in lung cancer patients. J Clin Pharm Ther. 1999;24:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Horsfall LJ, Nazareth I, Petersen I. Serum uric acid and the risk of respiratory disease: a population-based cohort study. Thorax. 2014;69:1021-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Strasak AM, Lang S, Kneib T, Brant LJ, Klenk J, Hilbe W, Oberaigner W, Ruttmann E, Kaltenbach L, Concin H, Diem G, Pfeiffer KP, Ulmer H; VHM&PP Study Group. Use of penalized splines in extended Cox-type additive hazard regression to flexibly estimate the effect of time-varying serum uric acid on risk of cancer incidence: a prospective, population-based study in 78,850 men. Ann Epidemiol. 2009;19:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Xu G, Wang Y, Li W, Cao Y, Xu J, Hu Z, Hao Y, Hu L, Sun Y. COX-2 Forms Regulatory Loop with YAP to Promote Proliferation and Tumorigenesis of Hepatocellular Carcinoma Cells. Neoplasia. 2018;20:324-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, Nakashima Y, Nakashima O, Kojiro M, Kurohiji T, Sata M. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 46. | Chen H, Cai W, Chu ESH, Tang J, Wong CC, Wong SH, Sun W, Liang Q, Fang J, Sun Z, Yu J. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017;36:4415-4426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 47. | Yan S, Zhang P, Xu W, Liu Y, Wang B, Jiang T, Hua C, Wang X, Xu D, Sun B. Serum Uric Acid Increases Risk of Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis. Mediators Inflamm. 2015;2015:764250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |