Published online Nov 14, 2020. doi: 10.3748/wjg.v26.i42.6614
Peer-review started: August 12, 2020
First decision: August 22, 2020
Revised: August 29, 2020
Accepted: September 10, 2020
Article in press: September 10, 2020
Published online: November 14, 2020
Processing time: 92 Days and 20.3 Hours
Although previous studies have confirmed the feasibility of magnetic compression anastomosis (MCA), there is still a risk of long-term anastomotic stenosis. For traditional MCA devices, a large device is associated with great pressure, and eventually increased leakage.
To develop a novel MCA device to simultaneously meet the requirements of pressure and size.
Traditional nummular MCA devices of all possible sizes were used to conduct ileac anastomosis in rats. The mean (± SD) circumference of the ileum was 13.34 ± 0.12 mm. Based on short- and long-term follow-up results, we determined the appropriate pressure range and minimum size. Thereafter, we introduced a novel “fedora-type” MCA device, which entailed the use of a nummular magnet with a larger sheet metal.
With traditional MCA devices, the anastomoses experienced stenosis and even closure during the long-term follow-up when the anastomat was smaller than Φ5 mm. However, the risk of leakage increased when it was larger than Φ4 mm. On comparison of the different designs, it was found that the “fedora-type” MCA device should be composed of a Φ4-mm nummular magnet with a Φ6-mm sheet metal.
The diameter of the MCA device should be greater than 120% of the enteric diameter. The novel “fedora-type” MCA device controls the pressure and optimizes the size.
Core Tip: To address some of the deficiencies in the current magnetic compression anastomosis (MCA) model, we explored the optimal size and pressure of the MCA device for intestinal anastomosis in rats. We found that the suggested diameter of the MCA device should be larger than 120% of the enteric diameter to avoid stenosis. Further, we developed a novel “fedora-type” MCA device for the current model, using a Φ4-mm nummular magnet with a Φ6-mm sheet metal. This model safely formed anastomosis and ensured long-term anastomosis. This novel anastomat controlled pressure and optimized the size, thus meeting our stipulated requirements.
- Citation: Chen H, Ma T, Wang Y, Zhu HY, Feng Z, Wu RQ, Lv Y, Dong DH. Fedora-type magnetic compression anastomosis device for intestinal anastomosis. World J Gastroenterol 2020; 26(42): 6614-6625
- URL: https://www.wjgnet.com/1007-9327/full/v26/i42/6614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i42.6614
Since Obora et al[1] used magnetic compression anastomosis (MCA) to successfully reconstruct vessels for the first time in 1978, MCA has been proven to be capable of compressing and penetrating various tissues[2]. Thus, MCA has been applied in many scenarios, especially for conditions in the digestive tract, such as esophageal[3-5], intestinal[6-8], gastrointestinal[9-11], biliary-intestinal[12-14], and pancreas-intestinal anastomoses[15]. However, research has shown that there is a risk of long-term anastomotic stenosis and even closure after MCA[15-20]; this eventually restricted further clinical application of MCA.
Therefore, effective and reliable MCA must satisfy all of the following criteria: Appropriate pressure, safe formation of anastomosis without leakage in the short-term follow-up, adequate size, and avoidance of anastomotic stenosis or closure in the long-term follow-up. Unfortunately, previous studies mostly focused on the formation of anastomosis[10,21,22], and thus long-term outcomes were neglected. Conversely, for traditional MCA devices, the compression force was positively correlated with the size. Thus, larger anastomosis was associated with a higher risk of leakage[22].
Thus, for MCA, there are three uncertainties that require clarification. First, the minimum initial size of anastomosis needs to be determined for reconstruction of the digestive tract of a certain size. Second, the suitable compression pressure range to form anastomosis without leakage needs to be determined for the particular tissue to be anastomosed. Third, clarity is required to determine the most effective design of a novel MCA device to simultaneously meet the compression pressure and size requirements.
To address these gaps, we designed the following two experiments. First, based on the anatomical characteristics of the rat intestine, we used traditional nummular MCA devices of all possible sizes to conduct ileac side-to-side anastomosis. Based on the short-term follow-up results, we determined the appropriate pressure range required for MCA. According to the long-term follow-up results, we confirmed the minimum size required to avoid anastomotic stenosis or closure. Second, based on the results of the former experiment, we introduced a novel design concept, known as the “fedora-type,” to the MCA device to simultaneously meet the requirements of both pressure and size, so that stable anastomosis could be formed.
All experimental protocols were approved by the Committee on the Ethics of Animal Experiments of Xi'an Jiaotong University (No. XJTULAC2020-1281). This research was conducted based on the guidelines for the Care and Use of Laboratory Animals from Xi'an Jiaotong University Health Science Center. A total of 105 male Sprague-Dawley rats weighing 240-260 g were obtained from the Experimental Animal Center, Xi'an Jiaotong University, Xi'an, China. The circumference of the intestine was measured for each rat during the operation, and the mean (± standard deviation, SD) was 13.34 ± 0.12 mm. All rats were anesthetized by isoflurane inhalation and were commonly treated pre- and post-operation. Postoperative complications and survival rates were observed.
Sixty rats were divided into four groups (groups 1.1-1.4), with 15 rats in each group. Traditional nummular MCA devices with different sizes were used in each group. As shown in Figure 1A, the MCA device involved a pair of nummular magnets (parent and daughter parts, NdFeB and N45). The diameters of the MCA devices in groups 1.1-1.4 were 3, 4, 5, and 6 mm, respectively, and the corresponding mean (± SD) compression pressures were 54.56 ± 1.40, 126.07 ± 1.38, 147.56 ± 3.42, and 152.60 ± 2.67 kPa, respectively.
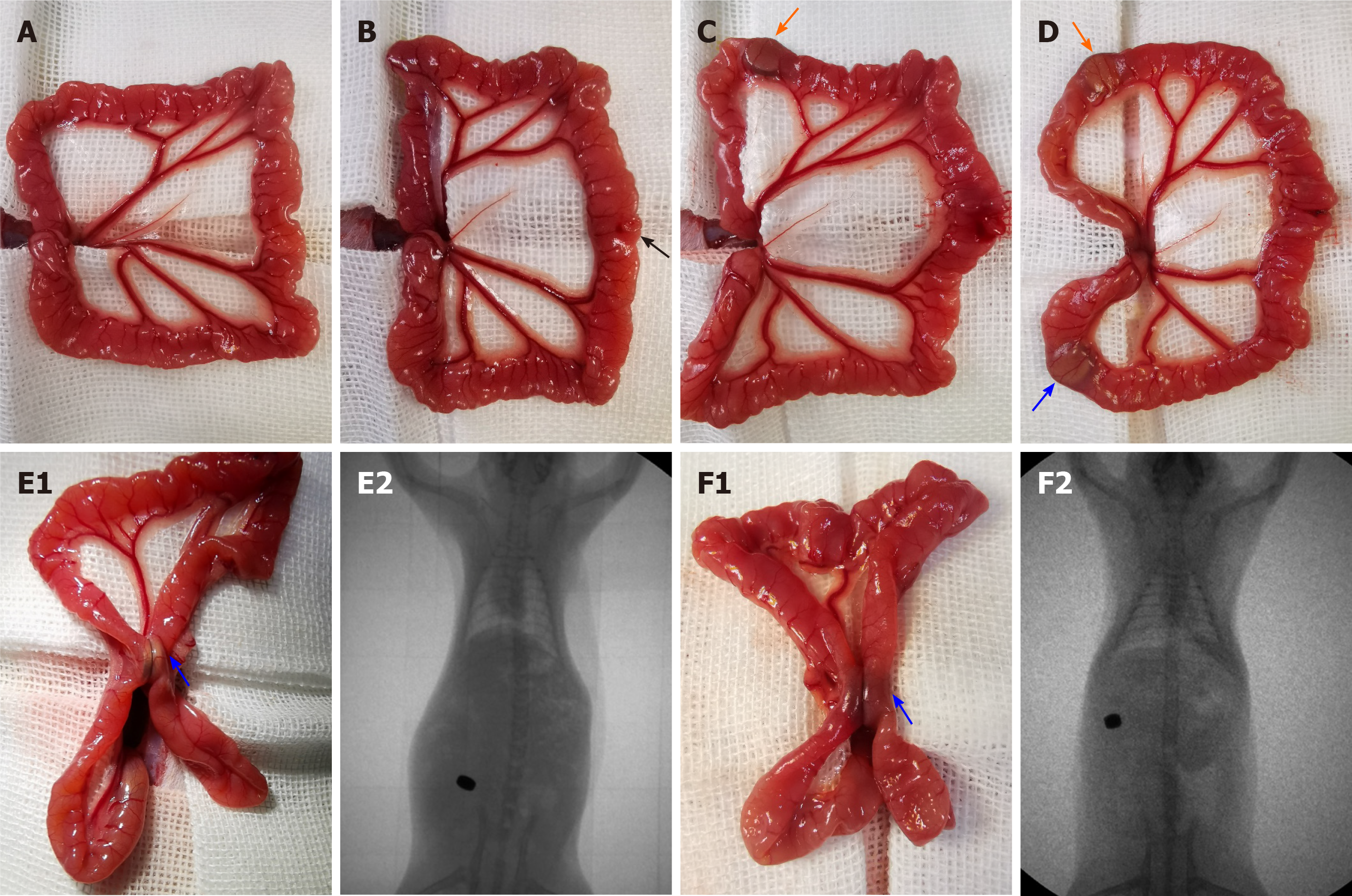
After anesthesia, a 3-cm midline incision was made, and the small intestine was removed and covered with sterile gauze in normal warm saline. Then, a 6-mm incision was made 12 cm distal to the cecum. Afterwards, the parent and daughter parts of the MCA device were inserted into the intestine from the incision, reaching 6 cm proximal and distal to the incision, respectively. After adjusting the locations of the magnets, they were gently coupled to compress the ileum wall. The incisions made in the intestine and abdominal wall were sutured (Figure 2).
Forty-five rats were randomly divided into three groups (groups 2.1-2.3) with 15 rats in each group. Based on experiment 1, a self-made “fedora-type” MCA device with different designs was adopted in each group. This device also consisted of parent and daughter parts. Each part involved a nummular magnet (NdFeB, N45) and a larger sheet metal (Ti6Al4V), just like a fedora cap, as shown in Figure 1B and C. The nummular magnets for all the groups were Φ4 mm, and the sheet metals for groups 2.1-2.3 were Φ4, Φ5, and Φ6 mm, respectively. Additionally, the mean (± SD) compression pressures for the different groups were 126.07 ± 1.38, 80.69 ± 0.88, and 56.03 ± 0.61 kPa, respectively.
The surgical procedure used was the same as that described in experiment 1.
X-ray fluoroscopy was conducted to confirm the accurate coupling of daughter and parent parts immediately after the operation (Figure 2E2 and F2). Routine X-rays were performed every day to verify the device’s movement and stable coupling in the digestive tract until the devices were discharged.
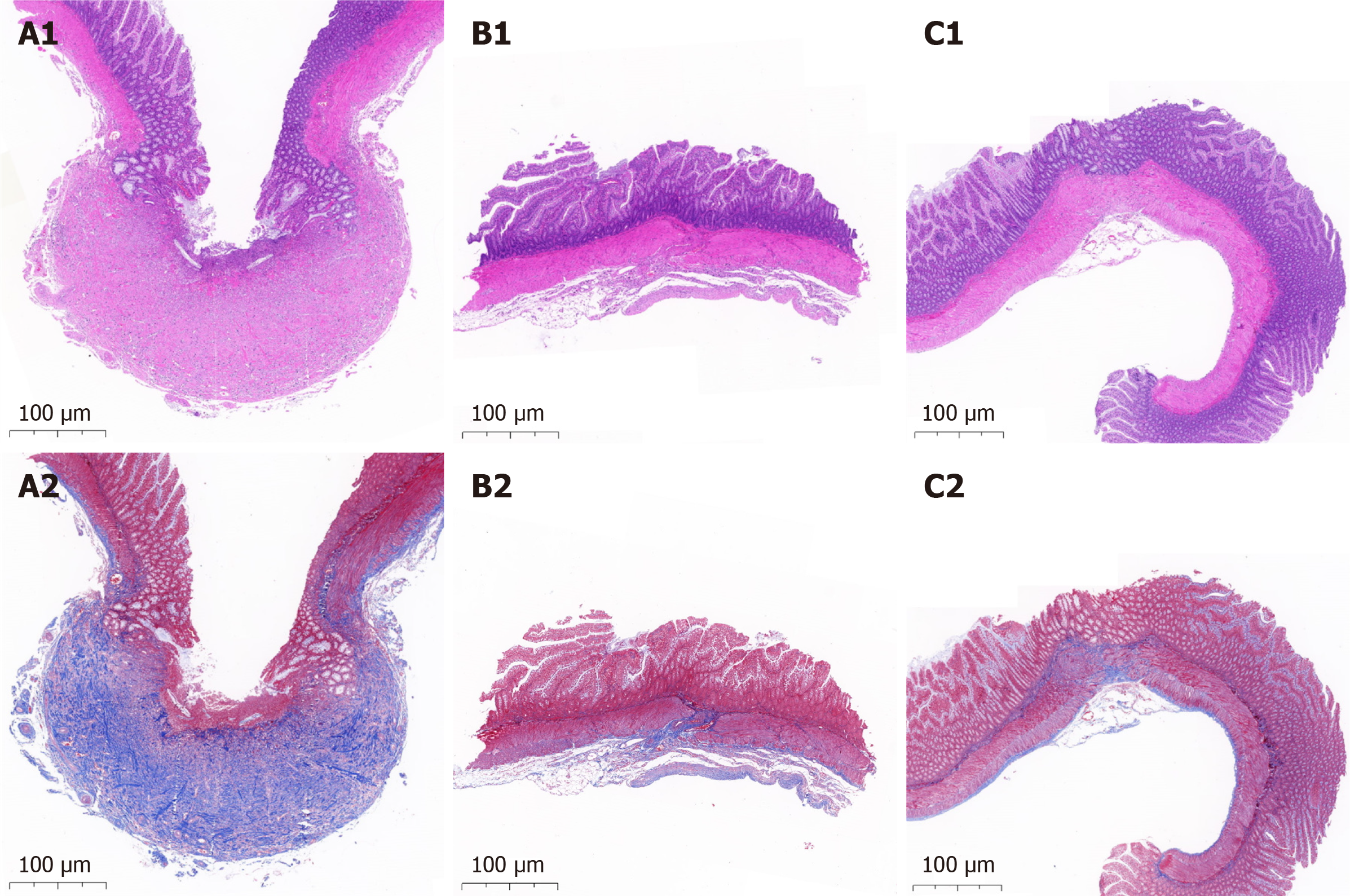
On postoperative days 30, 90, and 180, five rats in each group were euthanized to collect the anastomotic tissue specimens. The gross appearance of specimens was assessed based on a widely accepted scale, as shown in supplementary Table 1[23]. The sizes of the anastomosis were measured and analyzed using ImageJ_v1.8.0. The mechanical properties were evaluated based on bursting pressure using a self-made manometer. The histological morphology of ileac stomas was evaluated using Masson’s trichrome staining and hematoxylin and eosin (HE) staining.
SPSS Statistics Software version 23.0 (IBM Corporation, Armonk, NY, United States) was used for all analyses. Categorical variables are reported as numbers and proportions, and were compared using Chi-squared or nonparametric tests as appropriate. Normal continuous variables are reported as the mean ± SD and were compared using analysis of variance tests. Abnormal variables are reported as medians [interquartile range (IQR)] and were compared using nonparametric tests. All hypothesis tests were two-sided, and P values < 0.05 were considered statistically significant. The significance levels (α) for post hoc tests were adjusted accordingly.
Survival rate, expulsion time, and bursting pressure: No notable difficulties were encountered, and blood loss during the surgical procedure was minimal. There were no significant differences in the survival rates between the groups (groups 1.1-1.4, 93.3%, 100%, 73.3%, and 73.3%; P = 0.083) (Table 1). However, the combined survival rate for groups 1.1 and 1.2 was significantly higher than that of groups 1.3 and 1.4 (96.7% vs 73.3%, P = 0.026).
| Group 1.1 | Group 1.2 | Group 1.3 | Group 1.4 | P value | |
| Survival rate | 93.3% (14/15) | 100% (15/15) | 73.3% (11/15) | 73.3 (11/15) | 0.083 |
| Discharge time (d) | 3 (IQR 3-4) | 3 (IQR 3-4) | 2 (IQR 1-4) | 2.5 (IQR 2-3) | 0.002 |
| Adhesion score | |||||
| 0 | 92.9% (13/14) | 86.7% (13/15) | 27.3% (3/11) | 27.3% (3/11) | < 0.001 |
| 1 | 7.1% (1/14) | 13.3% (2/15) | 27.3% (3/11) | 9.1 (1/11) | |
| 2 | 0 (0/14) | 0 (0/15) | 9.1% (1/11) | 27.3% (3/11) | |
| 3 | 0 (0/14) | 0 (0/15) | 18.2% (2/11) | 18.2% (2/11) | |
| 4 | 0 (0/14) | 0 (0/15) | 18.2% (2/11) | 18.2% (2/11) | |
| Circumference of anastomotic stomas (mm) | |||||
| 30 d | 2.47 ± 0.18 | 8.84 ± 0.31 | 13.54 ± 0.31 | 15.98 ± 0.73 | < 0.001 |
| 90 d | 1.20 ± 0.18 | 5.90 ± 0.27 | 13.73 ± 0.49 | 16.43 ± 0.30 | < 0.001 |
| 180 d | 0.35 ± 0.19 | 2.07 ± 0.37 | 13.24 ± 0.68 | 16.33 ± 0.37 | < 0.001 |
| Bursting pressure (mmHg) | |||||
| 30 d | 247.64 ± 10.78 | 245.18 ± 7.77 | 242.90 ± 11.56 | 205.725 ± 8.06 | 0.032 |
| 90 d | 264.55 ± 7.87 | 269.46 ± 9.30 | 261.47 ± 9.72 | 256.03 ± 15.63 | 0.830 |
| 180 d | 263.32 ± 10.85 | 258.62 ± 10.19 | 261.08 ± 12.06 | 265.05 ± 11.26 | 0.978 |
Routine X-ray fluoroscopy showed that all traditional nummular MCA devices coupled tightly after operation. The larger devices appeared to require shorter expulsion time. The median expulsion times were 3 (IQR 3-4), 3 (IQR 3-4), 2 (IQR 1-3), and 2.5 (IQR 2-3) d for groups 1.1-1.4, respectively (P = 0.002) (Table 1).
The bursting pressure for group 1.4 was lower than that in the other groups on the 30th postoperative day (P = 0.032) (Table 1). There was no significant difference in the bursting pressure between any of the groups on postoperative days 90 and 180 (Table 1).
Size of anastomosis: On postoperative days 30 and 90, it was observed that as the size of the MCA device increased, the circumference of the anastomosis increased (P < 0.008, adjusted α = 0.008). On the 180th postoperative day, the circumference also increased with size, with the exception of that in group 1.1 when compared to group 1.2 (group 1.1 vs group 1.2, P = 0.044; P < 0.008 for other comparisons; adjusted α = 0.008) (Table 1).
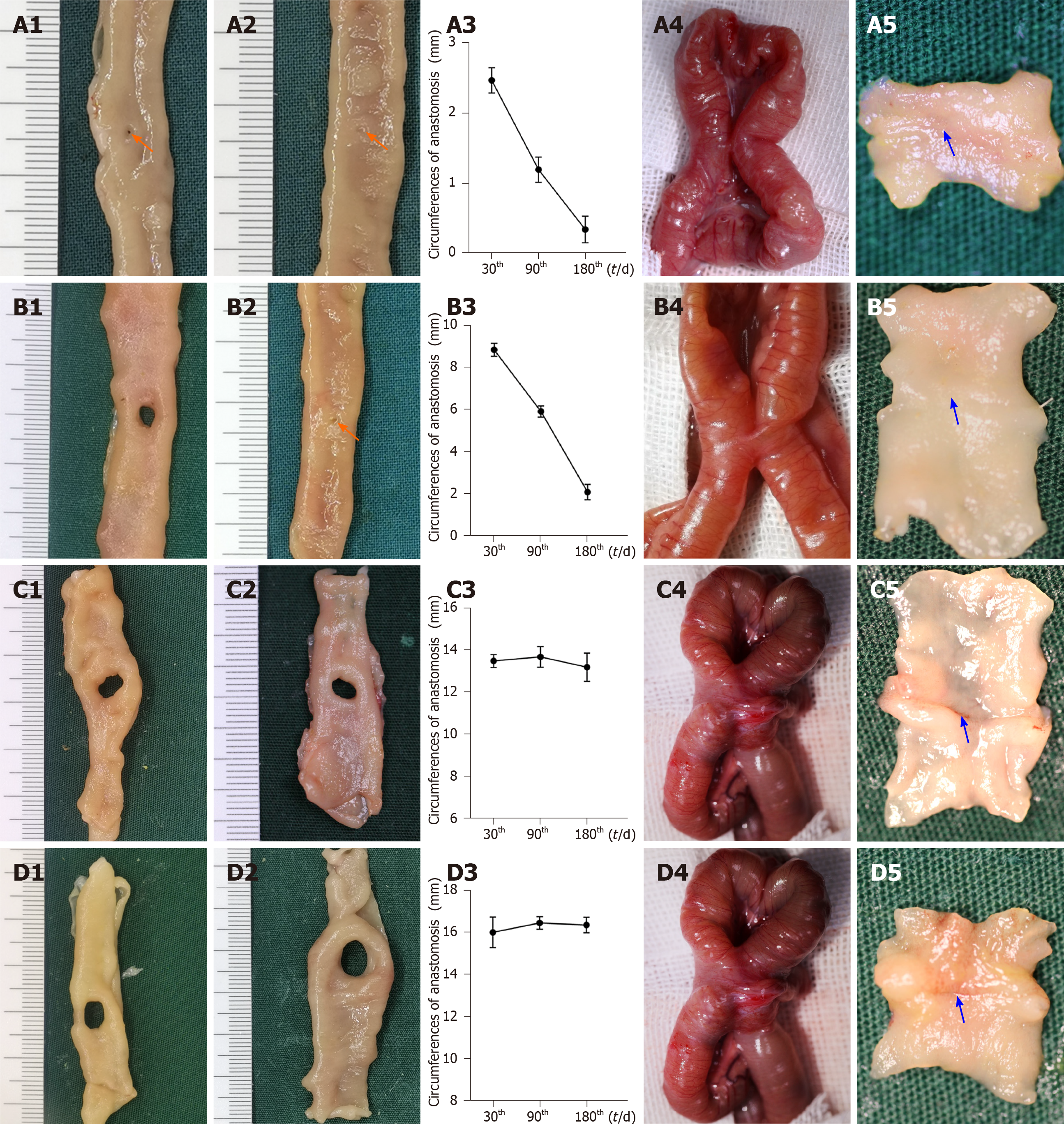
For the smaller groups (groups 1.1 and 1.2), the anastomosis circumferences decreased as time progressed (group 1.1: 2.47 ± 0.18, 1.20 ± 0.18, and 0.35 ± 0.19 mm for postoperative days 30, 90, and 180, respectively, P < 0.001; group 1.2: 8.84 ± 0.31, 5.90 ± 0.27, and 2.07 ± 0.37 mm for postoperative days 30, 90, and 180, respectively, P < 0.017, adjusted α = 0.017) (Figure 3A1-A3 and B1-B3) In group 1.1, the anastomoses were nearly closed by the 90th postoperative day. In group 1.2, closure of anastomoses occurred by the 180th postoperative day. As for the larger groups (groups 1.3 and 1.4), no significant differences in the circumference were found between the different time points (group 1.3, P = 0.811; group 1.4, P = 0.830) (Figure 3C1-C3 and D1-D3).
Morphological analysis: On the 30th postoperative day, the gross appearance of the anastomoses in the smaller groups was better than that in the larger groups. In groups 1.1 and 1.2, the anastomoses were clean and intact, and the mucosa was smooth and flat without any ulcers or erosions (Figure 3A4, A5, B4, and B5). However, the adhesion around the anastomoses was severe in groups 1.3 and 1.4, and the mucosa was not smooth and flat (Figure 3C4, C5, D4, and D5). As shown in Table 1, the adhesion scores for groups 1.3 and 1.4 were significantly higher than those in groups 1.1 and 1.2, respectively (P < 0.008 for both, adjusted α = 0.008).
The histological morphology showed that the serosal, submucosal, and mucosal layers were interrupted by scar tissue in the larger groups (Figure 4A1 and A2). However, it was continuous in the smaller groups (Figure 4B1 and B2).
Survival rate, expulsion time, and bursting pressure: The surgical procedures went well for all of the different fedora-type MCA devices used. After the operation, X-ray fluoroscopy showed that the daughter and parent parts for all the fedora-type MCA devices were tightly coupled. There was no significant difference in the survival rates (groups 2.1-2.3: 93.33%, 100%, and 93.33%, respectively, P = 0.434) or expulsion time (groups 2.1-2.3: 3 (IQR 3-3.25), 4 (IQR 2-5), and 4 (IQR 3-5) d, respectively, P = 0.175) between different fedora-type MCA devices. Additionally, there was no significant difference in the bursting pressure based on the different fedora-type MCA devices used (Table 2).
| Group 2.1 | Group 2.2 | Group 2.3 | P value | |
| Survival rate | 93.3% (14/15) | 100% (15/15) | 93.3% (14/15) | 0.434 |
| Discharge time (d) | 3 (IQR 3-3.25) | 4 (IQR 2-5) | 4 (IQR 3-5) | 0.175 |
| Adhesion score | ||||
| 0 | 85.7% (12/14) | 86.7% (13/15) | 85.7% (12/14) | 0.985 |
| 1 | 7.1% (1/14) | 13.3% (2/15) | 7.1% (1/14) | |
| 2 | 7.1% (1/14) | 0 (0/15) | 0 (0/14) | |
| 3 | 0 (0/14) | 0 (0/15) | 7.1% (1/14) | |
| 4 | 0 (0/14) | 0 (0/15) | 0 (0/14) | |
| Circumference of anastomotic stomas (mm) | ||||
| 30 d | 8.04 ± 0.62 | 13.10 ± 0.43 | 15.85 ± 0.47 | < 0.001 |
| 90 d | 5.36 ± 0.32 | 13.56 ± 0.58 | 16.20 ± 0.52 | < 0.001 |
| 180 d | 2.45 ± 0.67 | 13.57 ± 0.47 | 16.42 ± 0.31 | < 0.001 |
| Bursting pressure (mmHg) | ||||
| 30 d | 242.80 ± 8.90 | 239.32 ± 9.18 | 250.88 ± 7.71 | 0.634 |
| 90 d | 259.14 ± 7.42 | 267.00 ± 9.38 | 261.14 ± 12.01 | 0.842 |
| 180 d | 258.35 ± 14.46 | 260.82 ± 11.78 | 265.85 ± 14.07 | 0.972 |
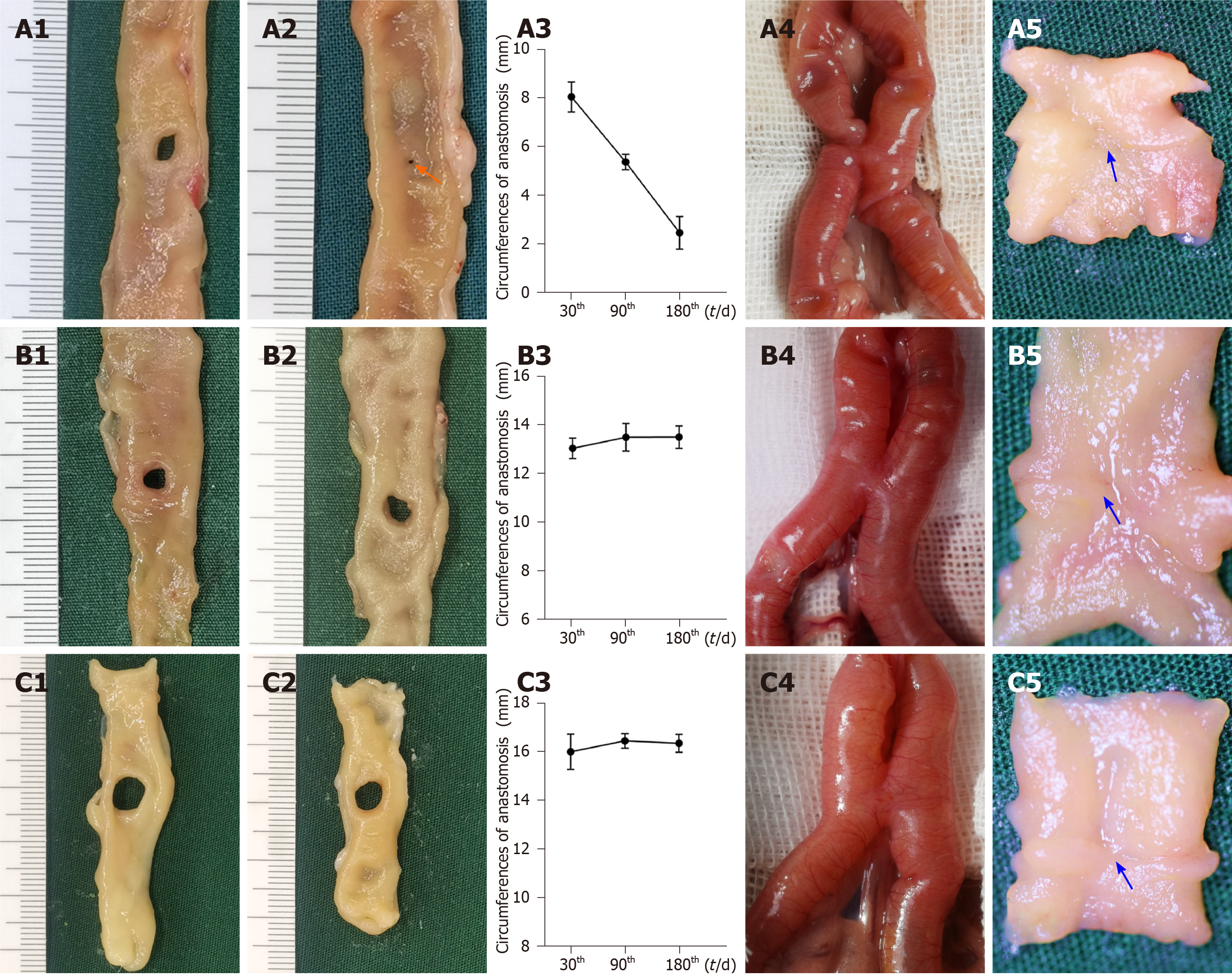
Size of anastomosis: On the 30th, 90th, and 180th postoperative days, the larger fedora-type MCA devices had a larger anastomosis circumference (P < 0.017 for all, adjusted α = 0.017) (Table 2). Based on the findings from the former experiment, the circumferences of the anastomoses in the smaller fedora-type MCA device (group 2.1) decreased as time progressed (8.04 ± 0.62 mm, 5.36 ± 0.32 mm, and 2.45 ± 0.67 mm for postoperative days 30, 90, and 180, respectively; P < 0.017 for all, adjusted α = 0.017), and the stomas were nearly closed by the 180th postoperative day (Figure 5A1-A3). There were no significant differences in the circumference at the different postoperative time points for the large fedora-type MCA devices (group 2.2: P = 0.749; group 2.3: P = 0.712) (Figure 5B1-B3 and C1-C3).
Morphological analysis: On the 30th postoperative day, the gross appearance of anastomoses in all groups did not significantly differ. The anastomoses were clean and intact for all designs of the fedora-type MCA devices on the 30th postoperative day, and all the mucosae were smooth and flat, without any ulcers or erosions (Figure 5A4, A5, B4, B5, C4, and C5). As shown in Table 2, the difference in the adhesion score between the groups was not significant (P = 0.985). The HE and Masson’s trichrome staining in all groups showed that the serosal, submucosal, and mucosal layers were continuous (Figure 4C1 and C2).
Although previous studies have confirmed the feasibility of MCA in animal experiments[24-26] and clinical practice[27-29], there is still a risk of anastomotic stenosis or even closure in the long run after MCA[15-17]. One interesting finding regarding MCA is the correlation between the size of anastomosis and the MCA device. Therefore, in the current study, traditional nummular MCA devices with different sizes were used to explore the suitable size and pressure for MCA. However, for traditional MCA devices, as the pressure increased, the size also increased[22]. Larger MCA devices increased the risk of leakage; therefore, we developed a novel “fedora-type” MCA device to allow for a large size but low pressure. Each part of the fedora-type MCA device had a nummular magnet with a larger sheet metal. After comparison, the optimal design for the fedora-type MCA device was that with a Φ4-mm nummular magnet and a Φ6- mm sheet metal.
The anastomat influenced the outcome of MCA in terms of pressure and size. The pressure affects the ischemic necrosis speed of the compressed tissue. If this speed surpasses the healing of anastomotic tissue, leakage could occur[21,22]. However, if the pressure is too low, dissociation of the MCA device might occur[10]. Furthermore, the importance of size is embodied in the following two aspects. First, if the size is too small, the anastomosis would narrow or even close with time; this is perhaps due to the insufficient shunt. Conversely, if the size is too large, placement and discharge of the anastomat will be difficult[30]. Thus, pressure influenced the short-term outcome of anastomotic formation for MCA, while size influenced the long-term outcome of anastomotic stenosis or closure for MCA. The existing limited basic work regarding MCA devices has mostly been focused on the effect of pressure, with a relatively short-term follow-up period (no more than 3 mo)[10,21,22]. These previous studies have ignored the importance of the size, which required subgroups and long-term follow-up. However, anastomotic stenosis or closure was identified as the real challenge for MCA devices in the gut[15,16,20].
To our knowledge, this is the first study to simultaneously explore the optimal size and pressure of traditional nummular MCA devices for intestinal anastomosis in the rat model, with a 6-mo follow-up period. The rat model simplified the subgroups. Thus, all sizes of traditional MCA devices were explored; this was crucial to investigate the relationship between anastomat, gut sizes, and anastomotic stenosis. This study showed that 5-6 mm was the optimal size range for ileac side-to-side anastomosis in the rat model. When the size was smaller than 5 mm, the anastomosis formed was small, and anastomotic stenosis or closure occurred in the long-term follow-up. While the size reached up to 7 mm, it was difficult to insert it into the intestine. In the current model, the mean (± SD) circumference of the intestine was 13.34 ± 0.12 mm, meaning that the diameter was approximately 4.2 mm. Thus, we speculated that the size of the MCA device should be larger than 120% of the enteric diameter, otherwise the anastomosis stoma would not receive sufficient shunt. This would result in stenosis or closure in the long-term follow-up. Unfortunately, the size was only approximately 58%-66% of the enteric diameter in a previously published study[6-8]. This study also demonstrated that 54.56 ± 1.40 kPa to 126.07 ± 1.38 kPa was the optimal compression pressure range, in accordance with previously published studies.
Although we determined the optimal size and pressure, they were almost impossibly achieved by traditional MCA devices, which were either of large or small size and achieved high or low pressure, respectively. The high pressure increased the risk of leakage, while the small size caused anastomotic stenosis or even closure. Devices that were large in size and led to a low amount of pressure were the ideal design for MCA devices in the gut. Therefore, we developed a novel MCA device to meet these parameters, which we called a “fedora-type” MCA device. Both parts of the novel anastomat consisted of a nummular magnet and a larger sheet metal. This allowed for control of the compression pressure by adjustment of the magnet, and for optimal size by allowing for the sheet metal to be changed. The novel design broke the internal connection between size and compression pressure in MCA devices and allowed for a large size and low pressure. Of all the different designs for the fedora-type MCA device used, the Φ4-mm nummular magnet with a Φ6-mm sheet metal could safely form anastomosis after operation and ensure long-term stability. It should be noted that the pressure produced by this design was almost the same as that of the Φ3-mm traditional nummular MCA device, which was the smallest one used in the first experiment in this study (54.56 ± 1.40 kPa vs 56.03 ± 0.61 kPa). However, the circumference of anastomosis at 6 mo was comparable to that of the Φ6- mm traditional MCA device (16.33 ± 0.37 mm vs 16.42 ± 0.31 mm, P = 0.893). This confirmed that the anastomotic stenosis was associated with the size of the MCA device, instead of the pressure.
This study was subject to several limitations that merit consideration. These results are only applicable to rats; models in larger animals and further clinical trials are needed to test this hypothesis and guide clinical application. Although some results of the current work cannot be directly translated into clinical practice, such as the size of MCA device, other results would provide important guidance for further clinical application. For example, with an adequate number of animals, we demonstrated that the diameter of MCA device should be greater than 120% of the enteric diameter to ensure the stability of intestinal anastomosis. In this study, the anastomotic specimens at postoperative days 30, 90, and 180 were analyzed. The anastomotic specimens from a longer follow-up duration might be more convincing. However, we suspect that if the anastomosis remained stable for 6 mo, stenosis would rarely occur.
To address some of the deficiencies in the current MCA model, we explored the optimal size and pressure of the MCA device for intestinal anastomosis in rats. We found that the suggested diameter of the MCA device should be larger than 120% of the enteric diameter to avoid stenosis. Then, we developed a novel “fedora-type” MCA device for the current model, using a Φ4-mm nummular magnet with a Φ6-mm sheet metal. This model safely formed anastomosis and ensured long-term anastomosis. This novel anastomat controlled pressure and optimized the size, thus meeting our stipulated requirements for a large size and small force device.
The feasibility of magnetic compression anastomosis (MCA) has been confirmed by previous studies; however, there is still a risk of long-term anastomotic stenosis. In fact, anastomat influences the outcome of MCA in terms of pressure and size. High pressure increases the risk of leakage, while small size causes anastomotic stenosis or even closure. One defect of traditional MCA lies in the correlation between the size of anastomosis and the MCA device. For traditional MCA devices, a large size has represented large pressure, eventually leading to increased leakage, meaning “large size & large force”.
Studies have shown that there is a risk of long-term anastomotic stenosis and even closure after MCA; this has restricted further clinical application of MCA.
This study aimed to explore the optimal size and pressure of the MCA device for intestinal anastomosis in rats. Thereafter, a novel MCA device (“fedora-type” MCA device) was developed to simultaneously meet the requirements of pressure and size.
We designed the following two experiments. First, based on the anatomical characteristics of rat intestines, we used traditional nummular MCA devices with all possible sizes to conduct ileac side-to-side anastomosis. Based on the short-term results, we determined the appropriate pressure range required for MCA. According to the long-term results, we confirmed the minimum size required to avoid anastomotic stenosis or closure. Second, based on the results of the former experiment, we introduced a novel design concept, referred to as the “fedora-type” MCA device, to simultaneously meet the requirements of both pressure and size, so that stable anastomosis could be formed.
The optimal size range was 5-6 mm for ileac side-to-side anastomosis in the rat model (the diameter of the MCA device should be within 120%-140% of the enteric diameter). When the size was smaller than 5 mm, anastomotic stenosis or closure occurred. This study also demonstrated that 54.56 ± 1.40 kPa to 126.07 ± 1.38 kPa was the optimal compression pressure range. Traditional MCA cannot meet both of these requirements. This newly developed “fedora-type” MCA device consisted of a nummular magnet and a larger sheet metal. This allowed for control of the compression pressure by adjustment of the magnet, and for optimal size by allowing for the sheet metal to be changed. The novel design broke the internal connection between size and compression pressure in MCA devices and allowed for a large size and low pressure. Of all the different designs for the fedora-type MCA device used, the Φ4 mm nummular magnet with a Φ6 mm sheet metal could safely form anastomosis after operation and ensure long-term stability.
The diameter of the MCA device should be larger than 120% of the enteric diameter to avoid stenosis. This novel anastomat controlled pressure and optimized the size respectively, thus meeting our stipulated requirements for a large size and small force device. The “fedora-type” MCA device for this model, using a Φ4 mm nummular magnet with a Φ6 mm sheet metal, safely formed anastomosis and ensured long-term anastomosis.
Models in larger animals and further clinical trials are needed to test this hypothesis and guide clinical application.
The authors thank all the staff at the National Local Joint Engineering Research Center for Precision Surgery & Regenerative Medicine for their help and provision of facilities to conduct this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Harrison MR S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Obora Y, Tamaki N, Matsumoto S. [Nonsuture microvascular anastomosis using magnet rings (author's transl)]. Neurol Med Chir (Tokyo). 1980;20:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Lv Y, Shi Y; Scientific Committee of the First International Conference of Magnetic Surgery. Xi'an consensus on magnetic surgery. Hepatobiliary Surg Nutr. 2019;8:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Noh M, Mooney DP, Trumper DL. Magnet-Assisted Hydraulic Bougienage for Correction of Long-Gap Esophageal Atresia. IEEE Trans Biomed Eng. 2018;65:2178-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Oetzmann von Sochaczewski C, Lindner A, Heimann A, Balus A, Patel VH, Harrison MR, Muensterer OJ. Beyond Magnamosis: A Method to Test Sutureless Esophageal Anastomotic Devices in Living Swine by Creating an Esophageal Bypass Loop for Natural Oral Nutrition. J Laparoendosc Adv Surg Tech A. 2019;29:852-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Muensterer OJ, Sterlin A, Oetzmann von Sochaczewski C, Lindner A, Heimann A, Balus A, Dickmann J, Nuber M, Patel VH, Manfredi MA, Jennings RW, Smithers CJ, Fauza DO, Harrison MR. An experimental study on magnetic esophageal compression anastomosis in piglets. J Pediatr Surg. 2020;55:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Lebares CC, Graves CE, Lin MY, Fidelman N, Cello J, Harrison MR, Rogers S. Endoscopic Magnetic Compression Anastomosis For Small Bowel Bypass in a High Operative Risk Setting. Surg Laparosc Endosc Percutan Tech. 2019;29:e84-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Toselli L, Martinez-Ferro M, Cervio G, Kwiat D, Imamura-Ching J, Graves CE, Gaston B, Harrison M. Magnetic Compression Anastomosis (Magnamosis) for Functional Undiversion of Ileostomy in Pediatric Patients. J Laparoendosc Adv Surg Tech A. 2017;27:1314-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Machytka E, Bužga M, Zonca P, Lautz DB, Ryou M, Simonson DC, Thompson CC. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc. 2017;86:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | An Y, Zhang Y, Liu H, Ma S, Fu S, Lv Y, Yan X. Gastrojejunal anastomosis in rats using the magnetic compression technique. Sci Rep. 2018;8:11620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Lambe T, Ríordáin MG, Cahill RA, Cantillon-Murphy P. Magnetic compression in gastrointestinal and bilioenteric anastomosis: how much force? Surg Innov. 2014;21:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Diaz R, Davalos G, Welsh LK, Portenier D, Guerron AD. Use of magnets in gastrointestinal surgery. Surg Endosc. 2019;33:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Kawabata H, Hitomi M, Inoue N, Kawakatsu Y, Okazaki Y, Miyata M. Intraductal Ultrasonography as a Local Assessment Before Magnetic Compression Anastomosis for Obstructed Choledochojejunostomy. Gastroenterology Res. 2017;10:255-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Saito R, Tahara H, Shimizu S, Ohira M, Ide K, Ishiyama K, Kobayashi T, Ohdan H. Biliary-duodenal anastomosis using magnetic compression following massive resection of small intestine due to strangulated ileus after living donor liver transplantation: a case report. Surg Case Rep. 2017;3:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Matsuura R, Ueno T, Tazuke Y, Tanaka N, Yamanaka H, Takama Y, Nakahata K, Yamamichi T, Maeda N, Osuga K, Yamanouchi E, Okuyama H. Magnetic compression anastomosis for postoperative biliary atresia. Pediatr Int. 2017;59:737-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Liu XM, Li Y, Xiang JX, Ma F, Lu Q, Guo YG, Yan XP, Wang B, Zhang XF, Lv Y. Magnetic compression anastomosis for biliojejunostomy and pancreaticojejunostomy in Whipple's procedure: An initial clinical study. J Gastroenterol Hepatol. 2019;34:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Qiao W, Shi A, Ma F, Yan X, Duan J, Wu R, Li D, Lv Y. Further Development of Magnetic Compression for Gastrojejunostomy in Rabbits. J Surg Res. 2020;245:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Ellebaek MBB, Qvist N, Rasmussen L. Magnetic Compression Anastomosis in Long-Gap Esophageal Atresia Gross Type A: A Case Report. European J Pediatr Surg Rep. 2018;6:e37-e39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Woo R, Wong CM, Trimble Z, Puapong D, Koehler S, Miller S, Johnson S. Magnetic Compression Stricturoplasty For Treatment of Refractory Esophageal Strictures in Children: Technique and Lessons Learned. Surg Innov. 2017;24:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Fan C, Zhang H, Yan X, Ma J, Wang C, Lv Y. Advanced Roux-en-Y hepaticojejunostomy with magnetic compressive anastomats in obstructive jaundice dog models. Surg Endosc. 2018;32:779-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Liu XM, Yan XP, Zhang HK, Ma F, Guo YG, Fan C, Wang SP, Shi AH, Wang B, Wang HH, Li JH, Zhang XG, Wu R, Zhang XF, Lv Y. Magnetic Anastomosis for Biliojejunostomy: First Prospective Clinical Trial. World J Surg. 2018;42:4039-4045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Zhao G, Ma J, Yan X, Li J, Ma F, Wang H, Liu Y, Lv Y. Optimized force range of magnetic compression anastomosis in dog intestinal tissue. J Pediatr Surg. 2019;54:2166-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Xue F, Guo HC, Li JP, Lu JW, Wang HH, Ma F, Liu YX, Lv Y. Choledochojejunostomy with an innovative magnetic compressive anastomosis: How to determine optimal pressure? World J Gastroenterol. 2016;22:2326-2335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Dziki AJ, Duncan MD, Harmon JW, Saini N, Malthaner RA, Trad KS, Fernicola MT, Hakki F, Ugarte RM. Advantages of handsewn over stapled bowel anastomosis. Dis Colon Rectum. 1991;34:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Bai J, Huo X, Ma J, Lv Y, Yan X. Magnetic compression technique for colonic anastomosis in rats. J Surg Res. 2018;231:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Gao Y, Wu RQ, Lv Y, Yan XP. Novel magnetic compression technique for establishment of a canine model of tracheoesophageal fistula. World J Gastroenterol. 2019;25:4213-4221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 26. | Bruns NE, Glenn IC, Craner DR, Schomisch SJ, Harrison MR, Ponsky TA. Magnetic compression anastomosis (magnamosis) in a porcine esophagus: Proof of concept for potential application in esophageal atresia. J Pediatr Surg. 2019;54:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Li Y, Sun H, Yan X, Wang S, Dong D, Liu X, Wang B, Su M, Lv Y. Magnetic compression anastomosis for the treatment of benign biliary strictures: a clinical study from China. Surg Endosc. 2020;34:2541-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Kubo M, Wada H, Eguchi H, Gotoh K, Iwagami Y, Yamada D, Akita H, Asaoka T, Noda T, Kobayashi S, Nakamura M, Ono Y, Osuga K, Yamanouchi E, Doki Y, Mori M. Magnetic compression anastomosis for the complete dehiscence of hepaticojejunostomy in a patient after living-donor liver transplantation. Surg Case Rep. 2018;4:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Parlak E, Eminler AT, Koksal AS, Toka B, Uslan MI, Sokmensuer C, Guven M. A new method for lumen restoration in a patient with aphagia: Oro-oesophageal through-the-scope magnetic compression anastomosis. Clin Otolaryngol. 2019;44:1214-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Zhao G, Yan X, Ma L, Liu W, Zhang J, Guo H, Liu Y, Lv Y. Biomechanical and Performance Evaluation of Magnetic Elliptical-Ring Compressive Anastomoses. J Surg Res. 2019;239:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |