Published online Nov 14, 2020. doi: 10.3748/wjg.v26.i42.6582
Peer-review started: August 12, 2020
First decision: September 30, 2020
Revised: October 14, 2020
Accepted: October 27, 2020
Article in press: October 27, 2020
Published online: November 14, 2020
Processing time: 93 Days and 4 Hours
Congenital vascular anomalies affecting the liver have been described in the scientific literature for decades. Understanding these malformations begins with knowledge of hepatic vascular embryology. Surgeons have applied numerous classification systems to describe both intrahepatic and extrahepatic shunts, which can confuse the reader and clinician. In our experience, focusing on one classification system for extrahepatic shunts and one for intrahepatic shunts is better. Today many patients with these shunts carry good long-term prognosis thanks to advances in imaging to better detect shunts earlier and classify them. Timely intervention by skilled radiologists and surgeons have also limited complications arising from dynamic shunts and can avoid a liver transplant. Congenital hepatic shunts are not the only vascular condition affecting the liver. Hereditary hemorrhagic telangiectasia, also known as Osler Weber Rendu syndrome, particularly type 2, may have varying severity of hepatic involvement which warrants longitudinal care from an experienced hepatologist. Lastly, congenital hemangiomas, often first identified on the skin and oral mucosa, also can affect the liver. While most will resolve in infancy and childhood, the pediatric hepatologist must understand how and when to treat persistent lesions and their complications. This article serves as a concise reference to help clinicians better care for patients with these rare conditions.
Core Tip: Hepatic shunts present from birth, hepatic hemangiomas, and hereditary hemorrhagic telangiectasia have all been described in the scientific literature over the decades. Most reviews were written by radiologists or surgeons, but none have adequately covered all these topics from the gastroenterologist’s perspective. Our review serves as a reference for most congenital vascular anomalies that present in the liver. Our goal is to provide knowledge to help clinicians understand the burden of disease of these conditions and guide management decisions.
- Citation: Schmalz MJ, Radhakrishnan K. Vascular anomalies associated with hepatic shunting. World J Gastroenterol 2020; 26(42): 6582-6598
- URL: https://www.wjgnet.com/1007-9327/full/v26/i42/6582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i42.6582
Congenital hepatoportal shunts (CHS) are rare but represent a unique entity and prognosis for pediatric patients. This review is an effort to succinctly describe various vascular anomalies and conditions associated with hepatic shunts including congenital hepatic shunts, hereditary hemorrhagic telangiectasia (HHT), and hepatic hemangiomas. We will also discuss various treatment considerations for such diseases and long-term prognosis.
When discussing vascular abnormalities, it is important to first understand the origins of the normal hepatic vasculature. Embryological development of the liver occurs between the fourth and tenth weeks of life. Hepatic tissue originates from endoderm foregut tissue. This tissue undergoes specification after exposure to fibroblast growth factor and bone morphogenic protein, followed by morphogenesis into a liver bud. This process is orchestrated by multiple complex signaling molecules including Hex and GATA6[1].
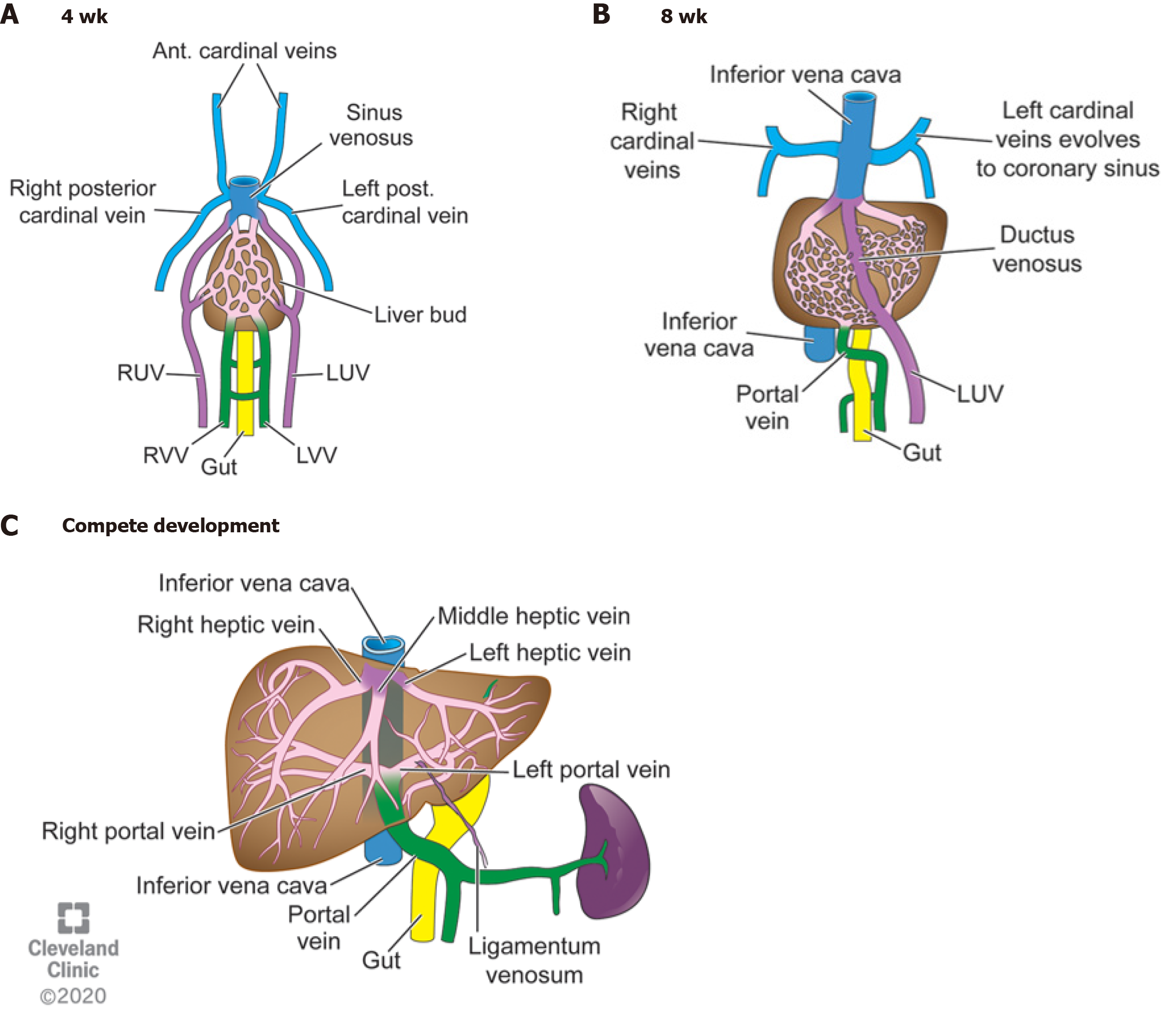
The afferent and efferent hepatic venous vasculature develops from a complex evolution of the cardinal veins, vitelline veins, and the umbilical veins. The right and left cardinal veins run vertically through the developing fetus. Both veins have cranial and caudal segments that interface at a confluence beneath the developing right atrium and on top of the primordial liver called the sinus venosus (Figure 1A). The sinus venosus also receives blood from the terminal ends of the umbilical veins and incorporates an anastomosis between the two vessels. This confluence of vessels along with drainage from developing hepatic veins will eventually develop into the inferior vena cava by the eighth week of gestation[1].
The right and left vitelline veins and their bridging anastomoses originate on the anterior surface of the yolk sac and surround the primitive foregut at four weeks gestation. The vasculature is symmetric at this stage and structured like rungs on a ladder. By the tenth week of gestation, the inferior segments of the right vitelline vein and superior portions of the left vitelline vein regress leaving an S-shaped dominant vessel that carries blood from the maturing intestines into the liver: The main portal vein (Figure 1B). Within the liver, this venous web eventually organizes to form the right and left hepatic veins. The left portal vein typically arises from one of the vitelline anastomoses.
The umbilical veins also transform from the symmetric right and left vessels which flow into the sinus venosus and directly into the liver to a single left vessel which ends in the liver parenchyma. In utero, this vessel supplies oxygenated, nutrient-rich blood to the body and forms a main intrahepatic bypass vessel through to the systemic venous drainage called the ductus venosus (DV). Approximately 40%-50% of the blood from the umbilical veins flows through the DV and onto systemic circulation. The remaining blood flow is distributed through the liver sinusoids. After birth, umbilical venous blood flow is disrupted when the umbilical cord is cut. The umbilical vein normally becomes the ligamentum teres and the DV regresses into the ligamentum venosum. This process occurs within minutes after birth but can take up to three weeks to complete.
The fully developed portal vein supplies 75% of the blood flow into the liver. It receives blood directly from the spleen, gallbladder, pancreas, and entire gastrointestinal tract, aside from the rectum, via the connecting superior and inferior mesenteric veins. The splenic vein typically flows into the superior mesenteric vein (SMV). Once in the liver, the portal vein normally divides into a left and right portal vein. The right portal vein further divides into an anterior and posterior branch (Figure 1C).
The complexity of hepatic vasculature development leads to several opportunities for abnormalities, namely from the failed closure of embryologic vessels. Other abnormalities arise from the proliferation of end vessels. This article will further review the formation, presentation, and therapeutic considerations of each of these congenital abnormalities.
A vascular shunt is any connection or orientation of blood vessels that bypass its intended organ. Congenital hepatic shunts usually present early in life either through incidental findings on imaging, on workup for liver injury secondary to the shunt itself, or work up for other causes of systemic disease either secondary to the shunt or associated with shunts. They are thought to arise from the persistence of the vitelline venous system in relation to the developing hepatic sinusoids. The incidence is roughly 1:30000-1:50000 live births. Several classification systems exist; however, the practicality of such systems has led to much debate because: (1) Anatomical characteristics can be complex; and (2) It may not make a difference clinically or with regards to management. There are two broad categories of congenital shunts: Extrahepatic where portal blood flows bypass the liver and connect to the systemic circulation, or intrahepatic where blood flow through the liver connects to the systemic circulation before it is filtered by the hepatocytes. Intrahepatic and extrahepatic shunts may overlap within the same patient. For simplification, we will only discuss two classification systems that cover most extrahepatic and intrahepatic shunts.
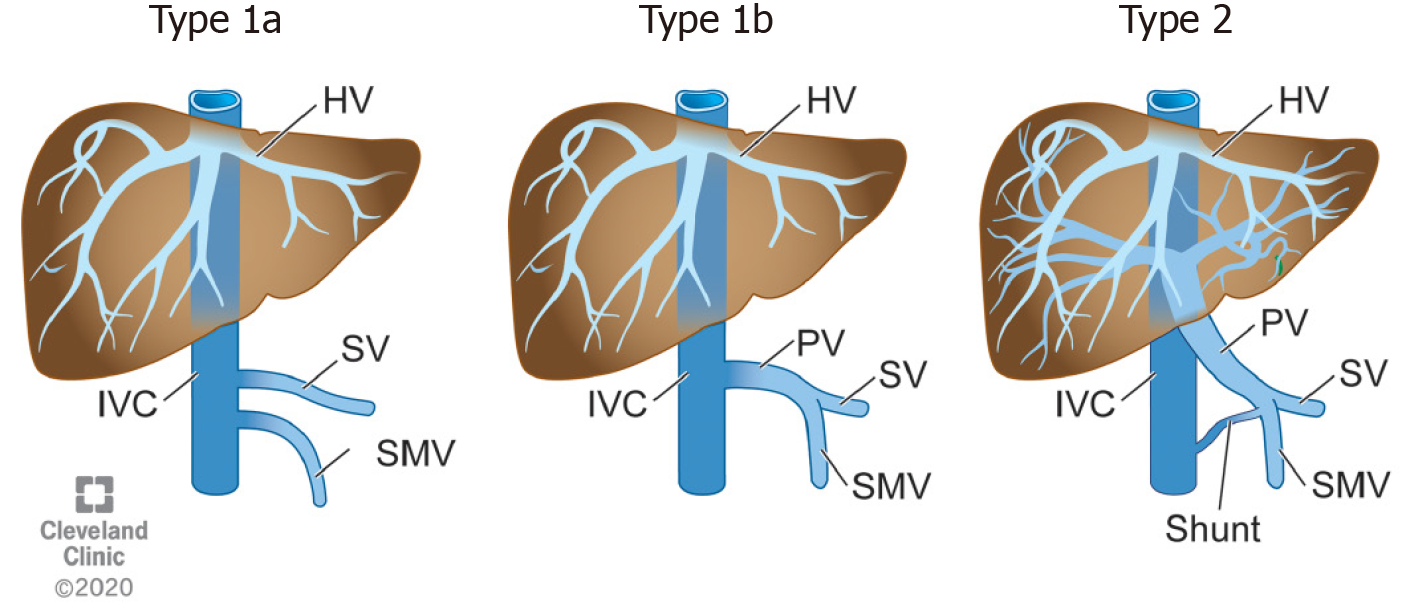
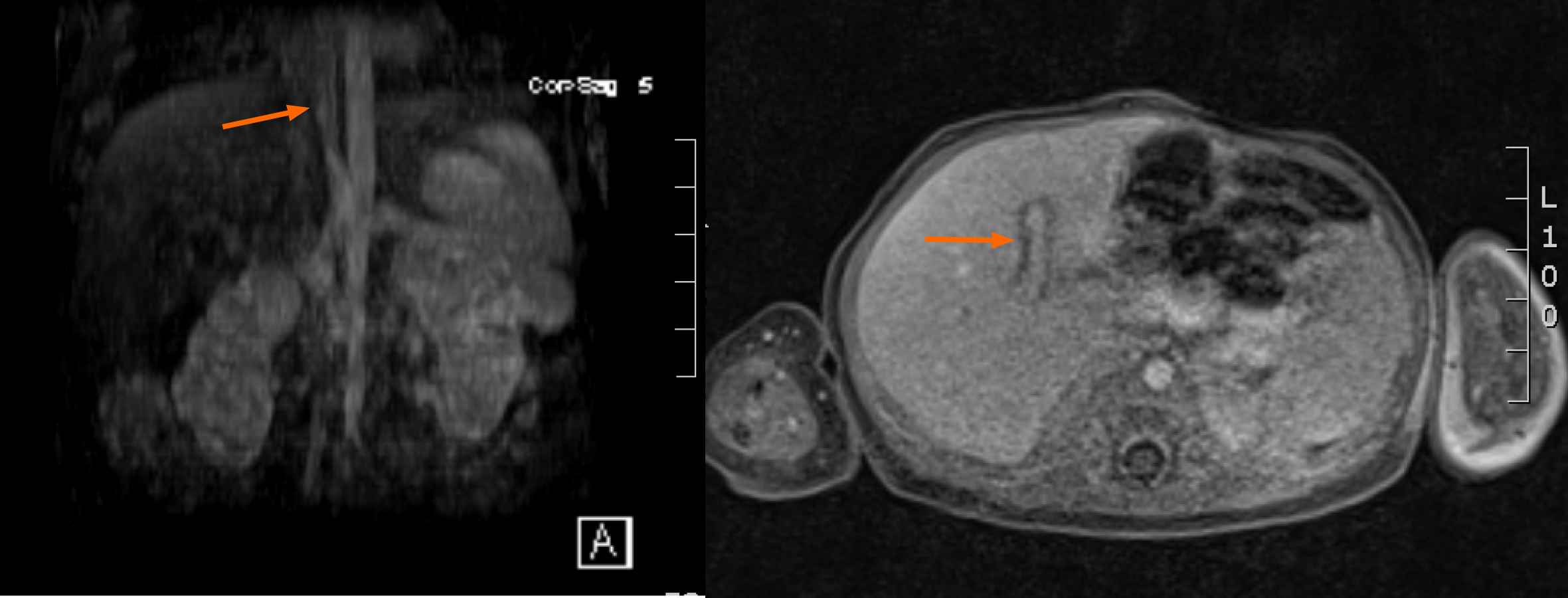
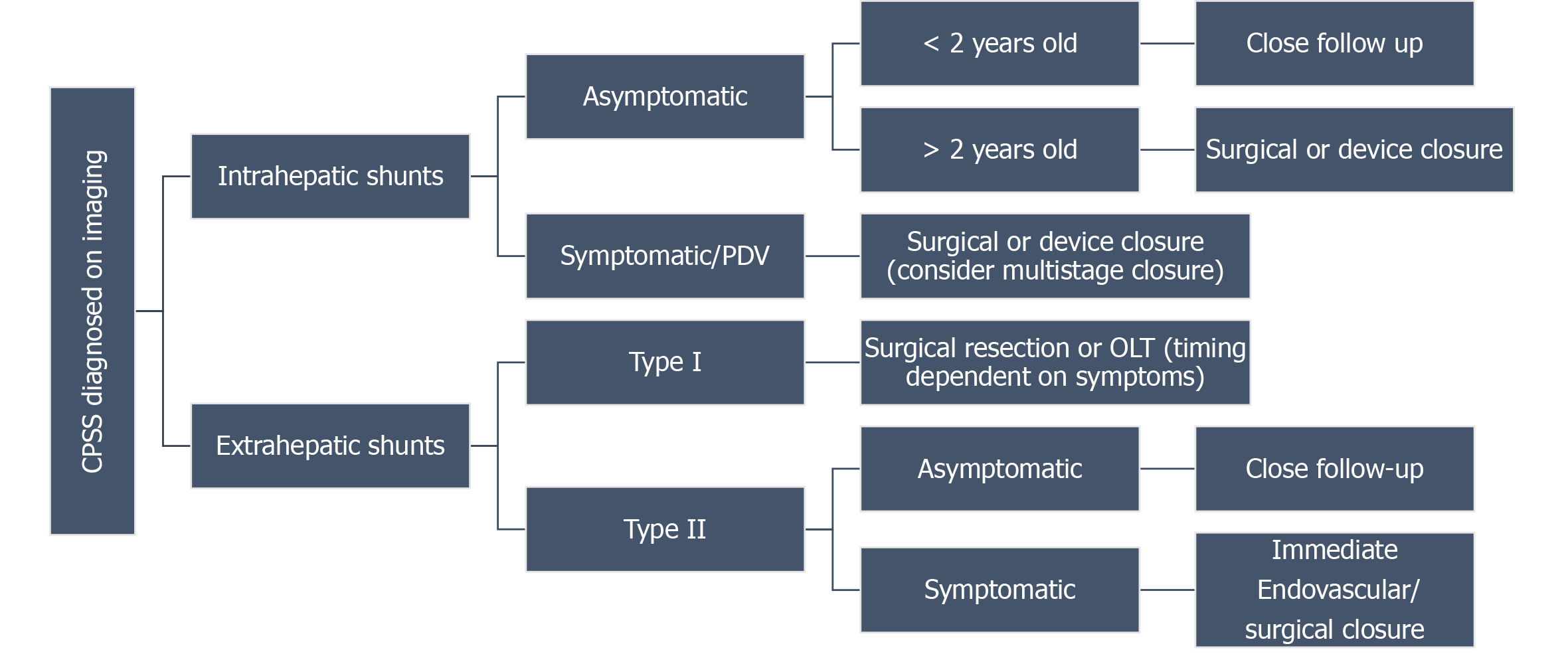
Congenital extrahepatic porto-systemic shunts (CEPSS), are best classified using Morgan and Superina’s[2] system developed in 1994 which divides these shunts into complete or partial hepatic diversion (Figure 2 and Table 1). CEPSS Type I is eponymously named Abernethy’s malformation, in honor of Dr. John Abernethy who first described the malformation in 1793[3]. In Abernethy’s malformation blood flow from the portal system bypasses the liver entirely and empties directly into the systemic venous circulation via an end-to-side anastomosis with the inferior vena cava. The portal system within the liver is, as best as can be identified, non-existent. While the pathophysiology is not completely understood, early involution of the peri duodenal vitelline plexus is the proposed mechanism for Abernethy’s malformation[4]. Diagnosis can be difficult as some cases of CEPSS Type I on initial imaging later may show hypoplastic intrahepatic portal venous flow with more invasive investigation such as cardiac catheterization and angiography. CEPSS Type I malformations can further be subcategorized depending on whether the splenic and SMV enter the systemic venous drainage separately (Type 1a) or if they converge to a common vessel before entering the systemic venous system (Type 1b) (Figure 3). CEPSS Type II malformations describe extrahepatic shunts where some of the portal flow is still intact in the presence of a smaller side-to-side anastomosis between the portal and systemic venous systems (Figure 4A-D). Persistence of the left vitelline vein leads to Type II shunts draining above the hepatic confluence or connect to the right atrium (Figure 4D).
| Morgan and Superina extrahepatic shunts |
| Type I: “Abernethy malformation” portal blood flow bypasses the liver entirely and empties into the IVC |
| The splenic vein and SMV enter the IVC separately |
| The splenic vein and SMV form common vessel before entering IVC |
| Type II: Partial shunt where an “H type” connection between the portal system and the IVC. Some portal flow to the liver is still intact |
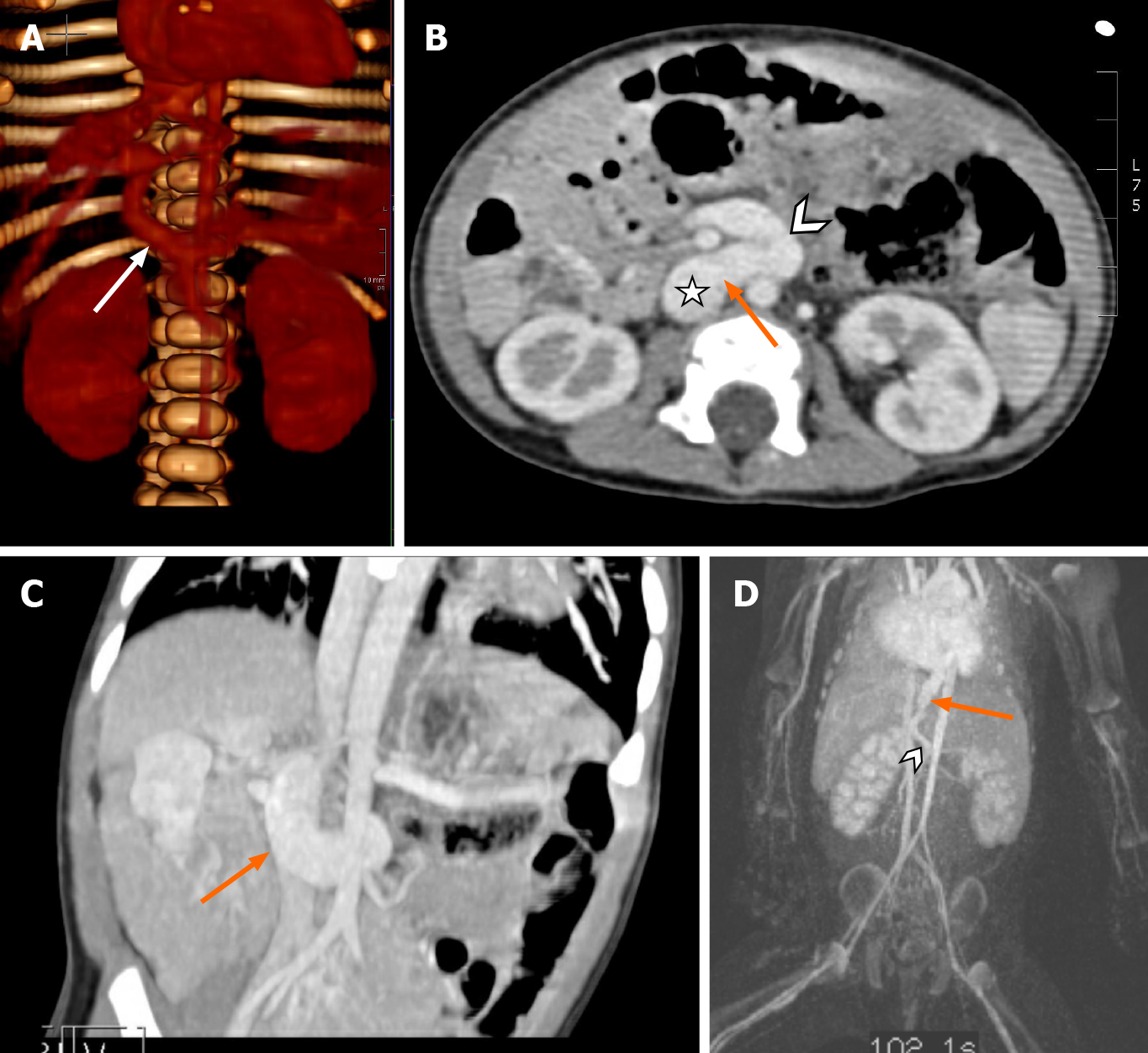
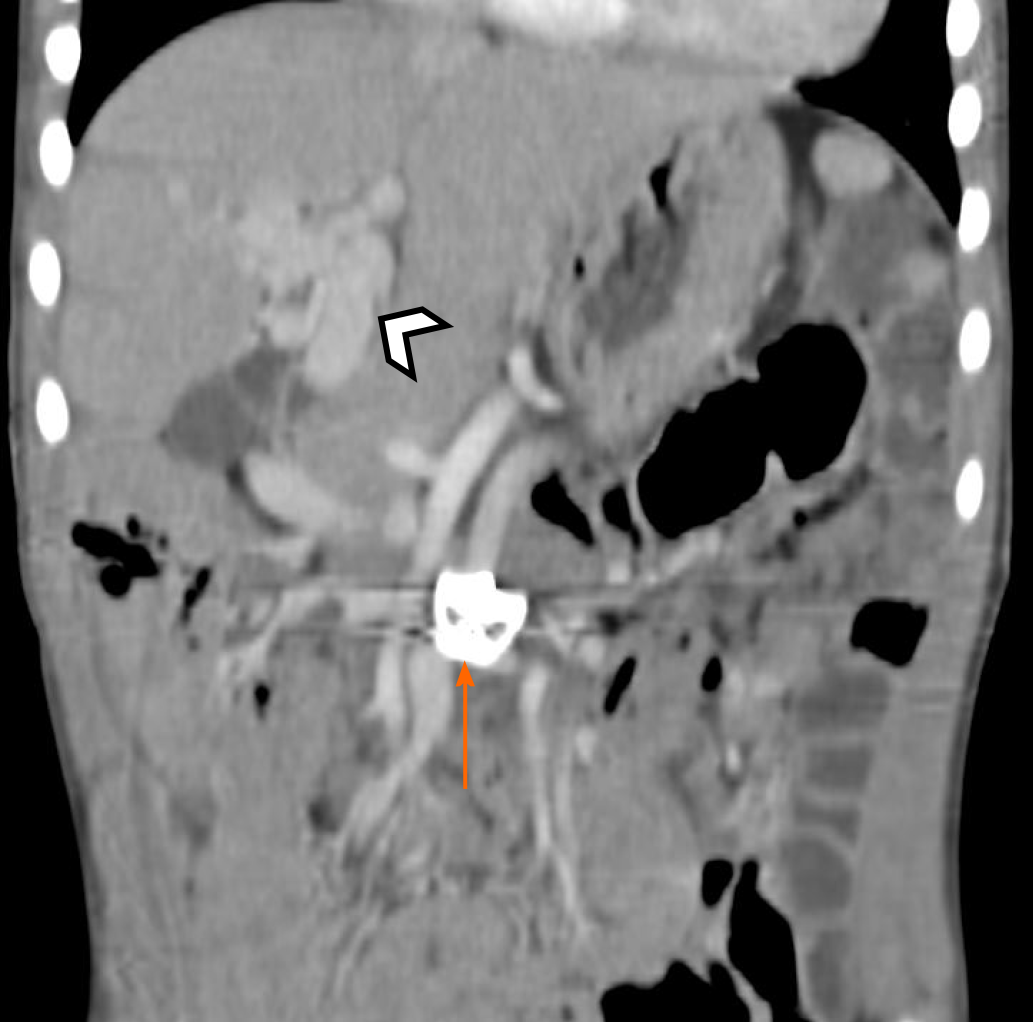
Congenital cavernous malformations are a separate extrahepatic portal malformation worth mentioning. Cavernous malformations are typically secondary to portal venous thrombus and as a response to portal hypertension, but congenital idiopathic malformations have been described. Liver vascular ultrasound may be read erroneously as antegrade flow if the collateral vessels are densely packed. It can be best identified on computed tomography (CT) angiography (Figure 5).
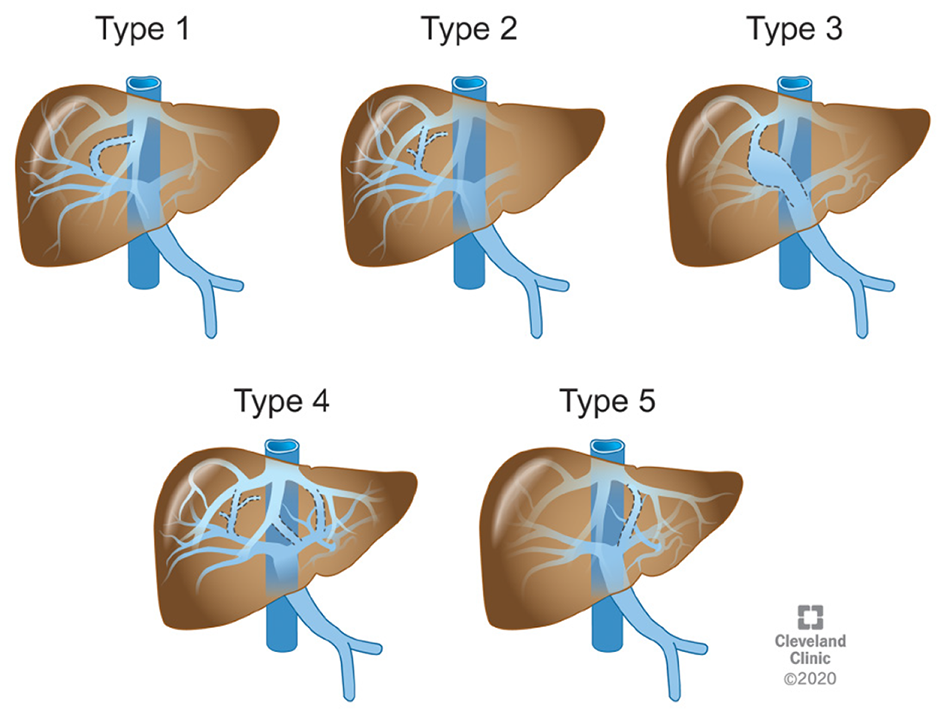
Congenital intrahepatic porto-systemic shunts (CIPSS) occur inside the liver where some normal portal venous blood flow through the liver is preserved. The embryological origins of these rare anomalies can arise from the failed fusion of the right vitellin and umbilical venous plexus which create communications between intrahepatic portal and hepatic or perihepatic veins[3,5]. CIPSS are sub-typed by location and extent of the shunt. The most widely used classification system for intrahepatic shunts was outlined by Park et al[6] in a case series from 1990. There he described 14 cases of various intrahepatic shunts (Figure 6 and Table 2). Type 1 is the most common (Figure 7). They are associated with cirrhosis if they persist but often close on their own. A patent DV is technically an intrahepatic shunt as it arises from a persistent connection between the left portal vein and a left hepatic vein via the partially involuted left umbilical vein which fails to close after birth to create the ligamentum venosum. This may be secondary to congenital heart disease causing altered hemodynamics and delayed ductal closure[7]. Patency may induce hypoplasia of the portal venous system[3].
| Park’s classification of congenital intrahepatic portosystemic shunts[5] |
| Large connection of constant diameter from the right portal vein to the intrahepatic IVC |
| Localized peripheral shunt from multiple or single communications between the peripheral branches of the portal and hepatic veins within one hepatic segment |
| An aneurism between connecting peripheral portal and hepatic veins |
| Multiple communications between peripheral and hepatic veins peripherally throughout the liver |
| Patent ductus venosus |
CHS may be found in isolation, but associations with other congenital abnormalities have been described. Several cardiac congenital anomalies are associated with CHS including atrial and ventricular septal defects, patent foramen ovale, tetralogy of Fallot, and occasionally meso and dextrocardia[4]. Situs ambiguous with malrotation and polysplenia has also been described. Other GI manifestations include malrotation, annular pancreas, and biliary atresia. About eight percent of extrahepatic shunt patients can also have polysplenia or continuation of the azygos or hemiazygos systems into the inferior vena cava[3]. There is also an increased incidence of CHS in patients with various genetic conditions such as Down’s syndrome[7]. Clinically, hepatic shunts manifest a variety of symptoms or can be indolent depending on the degree of shunting. While no longer believed to be exclusively female, CEPSS Type I are often reported with a female predominance[4]. CEPSS Type II is more male predominant. CHS reduce vital nutrition to the developing liver in utero. As 75% of the hepatic blood flow arrives via the portal vein, CHS can significantly affect liver growth and function. Liver atrophy occurs due to loss of nutrient flow to the liver as well as stimulating growth factors such as insulin and glucagon. In CHS, liver volumes can be 50%-60% of normal[8]. The body compensates for decreased portal blood flow by increasing hepatic arterial flow; however, this blood is low in nutrients, insulin, and glucagon. As a result, the cellular regenerative capacity of the liver is compromised leading to liver nodules in 20%-50% of patients[4,9]. These nodules are typically regenerative, but malignant lesions including hepatoblastoma and hepatocellular carcinoma have been described. There are currently eight reported cases of hepatoblastoma secondary to CHS in the literature as young as 17-months-old[9]. Correction of the shunt is associated with resolution of benign nodules[10].
De Vito et al[11] described histological characteristics of hepatic tissue in CHS based on a review of autopsied livers, wedge and core needle biopsies. These biopsies showed small portal venules, prominent thin walled channels, and otherwise observed portal arterio-biliary dyads suggesting atrophied venules. Increased arterial profiles were also evident, in keeping with the known compensatory mechanism of blood flow to the hepatocytes as described above. Vacuolization of hepatic nuclei, a sign of hepatocyte aging, was not evident. While the cause is not fully understood, this appears to be a result of decreased exposure to anabolic and catabolic hormones such as insulin and glucagon[12]. In the developing pediatric liver, the exposure of these hormones is likely essential to normal liver remodeling which is interrupted in patients with CHS[11].
Presentations in the neonatal period include hyperammonemia or hypergalactosemia along with elevated bile acids. Galactose is typically processed in the liver by uridine diphosphate (UDP) enzymes to be converted into glucose and stored as glycogen. Similarly, bile acids are excreted into the intestines and reabsorbed and reprocessed in the liver. In CHS, galactose rich blood partially or totally bypasses the liver leading to galactosemia without UDP enzyme deficiency. As such, CHS should remain on a differential diagnosis for galactosemia in newborns with bile acidemia[13]. Neonates may present with cholestasis. It is unclear if the cholestasis is a result of the hepatic shunting or an inciting factor which increases the resistance of blood flow through the liver favoring extrahepatic diversion.
Stigmata of chronic liver disease can be present overtime including spider nevi, digital clubbing, generalized fatigue, ascites, and growth failure. As the child ages, ongoing stress on the liver and chronic systemic exposure or toxic metabolites that have bypassed the liver will predispose the patient to hepatic encephalopathy, hepatopulmonary syndrome (HPS), or pulmonary hypertension[3,4]. Hepatic encephalopathy occurs when unprocessed ammonia produced by gut flora and absorbed by the intestines is processed into glutamine in the brain, which is neurotoxic[10]. While testing for hepatic encephalopathy in pediatrics is not well defined, monitoring ammonia levels is commonly done, especially if acute neurologic changes occur. Baseline elevated ammonia levels are seen in 66%-100% of patients with CHS[10]. While this was initially described in adults more than children, neurologic disease in children appears to be more indolent and the cause of seizures, irritability, and cognitive deficits. Hepatic encephalopathy is correlated well with shunt size[3]. Shunt ratios between 30%-60% are at increased risk of developing hepatic encephalopathy following systemic stress such as illness. If a shunt is > 60% then patients are at risk of spontaneous encephalopathy and warrants treatment[4].
HPS occurs in about 10% of patients with CHS[10]. While the cause is not completely understood, poor hepatic clearance of vasodilator substances acting on pulmonary endothelium is believed to be involved. Neonatal presentation may be subtle hypoxia. Older children will present with dyspnea on exertion and increased cardiac output. Orthodeoxia and platypnea, shown clinically as paradoxical improvement in dyspnea when transitioned to a supine position from an upright position, may also be seen in older children. Diagnosis can be made with either a “bubble” echocardiogram or technetium 99m-labeled macroaggregated albumin study. Both can measure the severity of HPS. Liver transplant remains a cornerstone of treatment for HPS for chronic liver disease[10].
Portopulmonary hypertension can occur in 13%-66% of CHS patients. It is often asymptomatic but can cause altered consciousness or syncope. It is thought to be caused by micro emboli and vasoconstrictive substances which typically are filtered by the liver. It is defined on cardiac catheterization as pulmonary artery wedge pressure of > 25 mmHg. The degree of severity does not correlate with shunt size. Early diagnosis and monitoring of portopulmonary hypertension is necessary as it is irreversible, even after shunt closure, and caries up to a 50% mortality rate[10].
Imaging is essential for the diagnosis of CHS. Non-radioactive modalities are preferred for initial investigation. Ultrasonography (US) is widely utilized in the neonatal period, which is highly specific if a portal vein is absent. A small sized liver is further evidence of compromised portal venous blood flow. The ability to apply Doppler can help provide qualitative data such as direction of blood flow within the shunt. Intrahepatic shunts will have antegrade blood flow on color Doppler. US is generally well tolerated and exposes the patient to no ionizing radiation[3,4]. Unfortunately, there is considerable operator variability and the acoustic window remains relatively small. As such, it may not highlight intrahepatic shunts and more detailed follow-up imaging with magnetic resonance angiography (MRA) or computed tomography angiography (CTA) is required.
Multidetector CTA is the gold standard for diagnosis and characterization of hepatic shunts given easy availability, rapid processing time, and detailed imaging with three-dimensional reconstruction of the hepatic vasculature. This provides the clearest picture for treatment either by a surgical team or interventional radiologist. The benefits of CT must be weighed against the increased risks of cancer from radiation exposure and nephrotoxicity secondary to contrast medium. CTA is also advantageous for patients who will not tolerate the lengthy scan time or in those with metal implants which prohibit the use of MR[4].
MRA provides excellent imaging of hepatic vasculature as well as characterize focal hepatic lesions without the associated ionizing radiation found in CT scans. Liver nodules are best defined on MR. Hyperplastic lesions are usually multiple and have increased signal intensity on T1-weighted imaging during the arterial phase and remain bright in the venous phase[4]. They vary in size from 0.5 cm to 4 cm in diameter.
For CEPSS Type II, it is helpful to determine the degree of partial shunting. Nuclear medicine may be used to measure the degree of shunting by calculating a portosystemic shunt index. In transrectal portal sintigraphy, radiolabeled 123I Iodoamphetamine is introduced into the distal colon via enema and absorbed through the inferior mesenteric vein. In patients without CHS, the isotope is taken up only by the liver. In patients with CHS, some or all of the isotopes travel through the shunt into the systemic circulation and accumulate in the lungs. By taking images of the liver and lungs, a shunt ratio is calculated thus determining the severity of the shunt. Shunt ratios > 5% are considered abnormal[4].
Diagnosis of CHS can be mostly made with the imaging modalities described above; however, there is a role for liver biopsy for patients who appear to have Abernathy’s malformation. If liver venules are present within portal triads, this may be evidence for the diagnosis of Type II CEPSS which would help management planning. Biopsies of suspicious hepatic nodules to rule out malignancy is also recommended.
CHS are rare and there are no large studies to drive treatment guidelines; however, some general principles are described here. Management of CHS first involves work up to rule out other causes and for assessment of other congenital anomalies. Shunt anatomy and severity of diverted portal venous blood flow is paramount. Before committing to intravascular or surgical management, one must first decide if or when intervention is necessary. The timing of intervention has also been greatly debated. Small type II CEPSS and many intrahepatic shunts may close spontaneously by 12 mo of age and only require close monitoring. Shunts that persist beyond 24 mo are unlikely to close spontaneously. Persistent DV and large Type II CEPSS with shunt ratio > 60% are at increased risk of developing hepatic encephalopathy and are unlikely to close spontaneously. Prophylactic closure prior to 24 mo is advised[3,14]. Symptomatic sequelae such as cardiovascular involvement, hepatic encephalopathy, or the existence of liver nodules warrant immediate treatment regardless of the shunt type. Regularly monitoring liver enzymes, alpha-fetoprotein, PT/INR, and ammonia levels as well as hepatic blood flow via ultrasound with doppler every 3-6 mo is essential. MR of liver annually is also often utilized in monitoring.
Hyperammonemia is treated by lowering the nitrogen load through diet modification and disruption of ammonia production and gut absorption. A low protein diet will limit the nitrogen load; however, this must be balanced with meeting the body’s needs for growth. We recommend a daily limit of 0.8-1.0 g/kg of dietary protein to meet nutritional needs. Co-management with a dietician is recommended. Interrupting ammonia reabsorption in the intestines can be achieved by adding lactulose, a non-absorbable sugar which acidifies the stool and promotes ammonia (NH3) conversion to non-absorbable ammonium (NH4+). Alternatively, using non-absorbable antibiotics such as rifaximin or neomycin reduces bacterial load in the intestines and stops NH3 production[4].
Intravascular closure: A definitive diagnosis of the shunt using angiography can map the vascular anatomy and assess how blood flow dynamics change with temporary shunt occlusion with the angiocatheter balloon. Sometimes, presumed CEPSS Type I are found to have open collateral vessels during temporary shunt occlusion, thus redefining them as CEPSS Type II (Figure 8A and B). Closure of Type II CEPSS as well as persistent isolated intrahepatic shunts serves to redirect portal blood flow back through the functioning liver. Portal pressure readings within the occluded vessel will determine further treatment. If portal pressure exceeds 30 mmHg, then total occlusion could cause sudden portal hypertension leading to hepatic stress and dysfunction. Therefore, a two-stage closure is recommended to allow the liver to gradually accommodate the re-routed blood flow[3] (Figure 8A-D). A variety of occlusion devices can be deployed including coils and intravascular plugs. Large shunts may be amenable to vascular plugs which can be adjusted prior to deployment for two-stage occlusion (Figure 9). Utilization in small peripheral extrahepatic shunts such as splenorenal shunts has also been described. Careful planning by skilled interventionalists is paramount. Intravascular plugs placed in short shunts with large diameter are at risk of plug migration into the systemic circulation.
Surgical management: Alternatively, laparoscopic surgical ligation may be safer for large diameter and/or short extrahepatic shunts which would make intravascular coils or plugs difficult to place. Intraoperative temporary occlusion can also be performed to assess for large hemodynamic shifts that necessitate two-stage closure. For large intrahepatic shunts which do not close spontaneously, a partial liver resection is also an option if device closure is not possible, failed, or if there is a concurrent hepatocellular mass. Shunt occlusion with N-butyl cyanoacrylate lipiodol has also been used to sclerose shunts[3,10].
Liver transplant: CEPSS Type I often requires liver transplant as there is an absence of portal vasculature through the liver. Symptomatic CEPSS Type I is a clear indication for an expedient liver transplant. Liver transplant is also reserved for severe complications such as hepatic encephalopathy, hepatoblastoma or hepatocellular carcinoma, and HPS[3,10]. Prophylactic liver transplantation in asymptomatic CHS patients has been debated and warrants careful consideration. Holding off until the inevitable pulmonary complications (PH and HPS) arise make perioperative care difficult; however, early transplantation increases the lifetime exposure to immunosuppressive medications (Figure 10). Techniques for connecting the transplanted PV to the recipient PV have different risks and benefits. End-to-end anastomosis is complicated by small bowel venous congestion. End-to-side anastomosis has less risk of small bowel congestion but may require an additional interposition venous graph to make the connection.
Patients still require regular follow-up after shunt closure. Reversal of hepatic encephalopathy and HPS is typical; however, pulmonary hypertension is unlikely to resolve following shunt occlusion but may respond to pulmonary vasodilator medication. Liver nodule regression is common. Transient portal hypertension often follows shunt occlusion. This can resolve as the liver gradually accommodates more portal blood flow, but persistent portal hypertension may spawn secondary shunts such as splenorenal shunts. Altered blood flow after shunt closure also increases the risk of portal vein thrombosis leading to portal hypertension[14]. As previously stated, migration of closure devices may theoretically occur, and patients must be made aware of this risk.
HHT is a rare autosomal dominant condition occurring in 1-2 cases per 10000, characterized by multiple angiodysplasia lesions which classically present in the skin and mucous membranes. In mucosa, they occur at the capillary level where postcapillary venules dilate and fuse with arterioles creating an arteriovenous shunt[13]. Clinical diagnostic criteria are listed[14] (Table 3). Frequent epistaxis is the most common clinical manifestation. Visceral organ involvement can occur in the liver (most common), pulmonary system, intestines, or brain and spinal cord[15]. Earlier diagnosis is on the rise following improvements in multidetector CT which can produce a clearer definition of vascular abnormalities within the organs. HHT has been categorized into two distinct types associated with distinct gene mutations with a third type currently undergoing investigation[15]. Types 1 and 2 both involved genes which control the transforming growth factor beta (TGF-beta) pathway[15]. TGF-beta signaling pathway will go on to stimulate vascular endothelial growth factor which induces vascular proliferation. The genetic mutation for type 1 is in a gene called ENG, found on chromosome 9, which codes for Endoglin, a TGF-beta receptor. Type 2 is caused by a mutation on chromosome 12 which codes for activin receptor-like kinase type 1 (ALK-1). Hepatic involvement in HHT is almost always associated with ALK-1 mutation and type 2 HHT[16,17]. There have also been cases of patients with juvenile polyposis syndrome (SMAD4 mutation) with HHT overlap, presenting with anemia, epistaxis, and pulmonary and liver telangiectasia[18]. Studies have suggested that between 15%-22% of patients with SMAD4 mutation can develop JPS-HHT overlap[18].
| Hereditary hemorrhagic telangiectasia (must have at least three of the following) |
| Recurrent spontaneous epistaxis |
| Mucocutaneous telangiectasia |
| Family history of HHT |
| Presence of visceral involvement |
Liver involvement with HHT was first proposed in the late 19th century. By the mid-20th century medicine had described three categories of HHT based on if hepatic telangiectasia were present and if the patients developed fibrosis or cirrhosis[19]. Hepatic involvement can occur in 74%-79% of patients and can be identified at an early age; however, symptoms typically do not manifest before the third decade of life[16]. Liver biopsy will show fibrosis and cord atrophy, capillary hyperplasia, and hyperplastic vascular ectasia[16]. The type and extent of the shunt can determine the involvement. Only eight percent of patients with HHT and liver involvement will become symptomatic[16].
Liver vascular malformations are unique to other telangiectasias given the three vascular pathways which interact with the liver: Hepatic arteries, hepatic veins, and portal veins. Three types of intrahepatic shunts can develop: Arteriovenous, arterioportal, and portovenous. More than one type of shunt can develop in the same patient, but one may dominate functionally[15]. Arteriovenous shunts are the most common (50%). They can classically induce hepatomegaly following congestive heart failure and pulmonary hypertension. Arterioportal shunts are less common and patients usually have arteriovenous shunting as well. Arterioportal shunts often induce portal hypertension from increased blood flow and back pressure on the portal tree. Portal hypertension, classically defined as a hepatoportal venous gradient > 10 mmHg, develops in the fifth or sixth decade of life. It presents with classic transudative ascites and varices are prone to hemorrhage. Lastly, portovenous shunts are typically only seen on microscopy in childhood but may become more prominent shunts by the fifth or sixth decade of life[15].
Complications from liver involvement typically occur in middle age. High output cardiac failure is the most common symptom and is associated with vascular malformations large enough to produce a bruit or palpable thrill in the epigastrium on exam[15]. The presentation of cardiac failure with orthopnea, dyspnea on exertion, and edema is classic. Pregnancy may be a precipitating or exacerbating event in women. Abdominal angina secondary to mesenteric artery “steal” phenomenon has also been described[15]. Portal hypertension is the second most common complication and is associated with arterioportal malformations. They can eventually cause ascites and gastric and esophageal variceal bleeding. Altered blood flow through the hepatocytes can create perfusion abnormalities. This can lead to focal nodular hyperplasia (2.9% of cases) and periportal fibrosis. Focal nodular hyperplasia and concomitant portal hypertension may be misdiagnosed as liver cirrhosis. Unlike in cirrhosis, these livers typically maintain synthetic function[15] (Table 4).
| Vascular shunts associated with HHT | Associated systemic/Hepatic manifestations |
| Arteriovenous | Hepatomegaly |
| Pulmonary hypertension | |
| High output cardiac failure | |
| Biliary ischemia/biloma | |
| Abdominal angina | |
| Arterioportal | Focal nodular hyperplasia |
| Non-cirrhotic portal hypertension | |
| Hepatic encephalopathy | |
| Portovenous | Hepatomegaly |
| Hepatic encephalopathy | |
| High output cardiac failure | |
| Non-cirrhotic portal hypertension | |
| Focal nodular hyperplasia |
Biliary disease is also well described in HHT with liver involvement. It is thought to be related to shunt induced biliary ischemia and manifests as strictures of the gallbladder neck or intra or extrahepatic bile ducts. This typically affects women in their late 30 s. Often serum alkaline phosphatase and gamma-glutamyl transferase are elevated, thus patients may be erroneously diagnosed with cholecystitis and undergo cholecystectomy. In another case series of HHT patients, three of 12 patients developed bilomas[16]. Surprisingly, each of these patients had intrahepatic arteriovenous shunting and elevated alkaline phosphatase, but with normal bilirubin. Lastly, large portovenous malformations leading to hepatic encephalopathy have been rarely reported.
Diagnosis of liver involvement typically begins with a high index of suspicion following history and exam and is confirmed with imaging. Patients with known HHT without pulmonary arterio-venous malformations (AVMs) who present with dyspnea and ascites may be in cardiac failure. Liver ultrasound with Doppler and contrast spiral CT are recommended as initial, non-invasive investigations. These tests will show evidence of intrahepatic telangiectasias and an enlarged common hepatic artery in symptomatic individuals[15]. Biliary abnormalities seen on magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP) are seen even in patients without biliary symptoms suggesting a progressive course of the disease. Cardiac catheterization and angiography are invasive but considered the gold standard for determining the shunt severity as well as the degree of heart failure if present. Portal wedge pressures can also be measured to confirm portal hypertension if present. Mesenteric steal syndrome can also be confirmed in patients presenting with abdominal pain.
Treatment of HHT is largely symptomatic control. Patients may require blood transfusions for ongoing blood loss from cutaneous bleeding. Iron deficiency is common, and supplementation is frequently required. Laser therapy may be needed for treatment of skin telangiectasias. Endoscopy typically utilizes argon plasma coagulation for gastrointestinal AVMs. Esophageal varices should be treated as they are for any other cases of portal hypertension. Liver AVMs can predispose to high output cardiac failure and may be treated with diuretics. Previously used as a compassionate care drug to treat childhood cancers, bevacizumab is an anti-VEGF antibody that has been shown to treat bleeds from cutaneous and gastrointestinal telangiectasia leading to a significantly decreased need for transfusions. Interferon has been utilized for control of cutaneous telangiectasia. In adults, hepatic arterial embolization has been described as a more of a temporizing, palliative care option in patients with arteriovenous and arterioportal shunts who failed medical management. For extensive, medically refractory disease or portovenous disease, liver transplant remains an option. This is often used for extensive hepato-biliary necrosis and or heart failure (Table 5).
| HHT hepatic involvement treatment considerations |
| Symptom control: Iron deficiency, heart failure, esophageal varices |
| Anti-VEGF antibodies (i.e., bevacizumab) |
| Hepatic arterial embolization: Typically, an adult palliative option |
| Liver transplant: In setting of extensive hepato-biliary necrosis or heart failure |
Infantile hemangiomas remain the most common tumor in neonates with a prevalence estimated at 4%-5% of all infants[20]. They are benign endothelial tumors but can lead to comorbidities based on size, location, and the number of lesions. Isolated cutaneous lesions are the most common, but visceral involvement, most commonly in the liver, is also seen with and without cutaneous lesions.
While isolated hemangiomas are common, multiple lesions are more likely to have a genetic cause and carry higher morbidity and mortality if untreated. Diffuse neonatal hemangiomas were first described in the early 1970s and 1980s, but the term suffered from ambiguity over the decades. It has meant to cover several conditions that have now been isolated through immunohistochemistry studies and better clinical characterization[21]. The term multifocal vascular hemangiomas with or without extracutaneous disease are now the preferred terminology; however, most of the literature still uses a variety of terms.
A recent publication in the Journal of Pediatrics[22] classified hemangiomas in the first year of life as either congenital or infantile as they follow different courses and have different treatment and management guidelines. Congenital hemangiomas grow in-utero and are present at birth. They are often identified on antenatal ultrasound. Lesions typically stain negative for glucose transporter 1 (GLUT-1) if biopsied. Large lesions have high vascular flow and are associated with hemodynamic instability and heart failure which may be the presenting symptom at birth. Other complications from large lesions include mild anemia, thrombocytopenia, and hypofibrinogenemia; however, these are typically transient and not as dramatic as what is seen in the Kasabach-Merritt phenomenon. Congenital hemangiomas are subcategorized into one of three patterns: Rapidly involuting congenital hemangiomas (RICH) where there is a complete self-resolution of the lesion within two years, partially involuting congenital hemangiomas where size reduces but never fully resolves, and non-involuting congenital hemangiomas where lesion size remains the same. Monitoring is the mainstay of treatment for these lesions with regular complete blood counts in the neonatal periods to assess for cytopenias and echocardiogram to monitor heart function. Hepatic lesions should be monitored with ultrasound every two weeks initially and extending the image interval by two weeks when lesion(s) are stable or start to involute. Patients should be followed for at least one year. RICH will have 80% total remission by 12 mo of age. Overall, 50% of congenital hemangiomas resolve by age five and 90% by age nine.
In contrast, infantile hemangiomas that develop in the neonatal period follow a different pattern. They typically grow over the first 6-12 mo of life. They often stain positive for GLUT-1 and are multifocal. Their progression in size through infancy means that their risk of complications increases during the first year of life compared to congenital hemangiomas. Heart failure and compartment syndrome are the most severe risk and carry a 16% mortality if not treated. Lastly, cytopenias may develop over time.
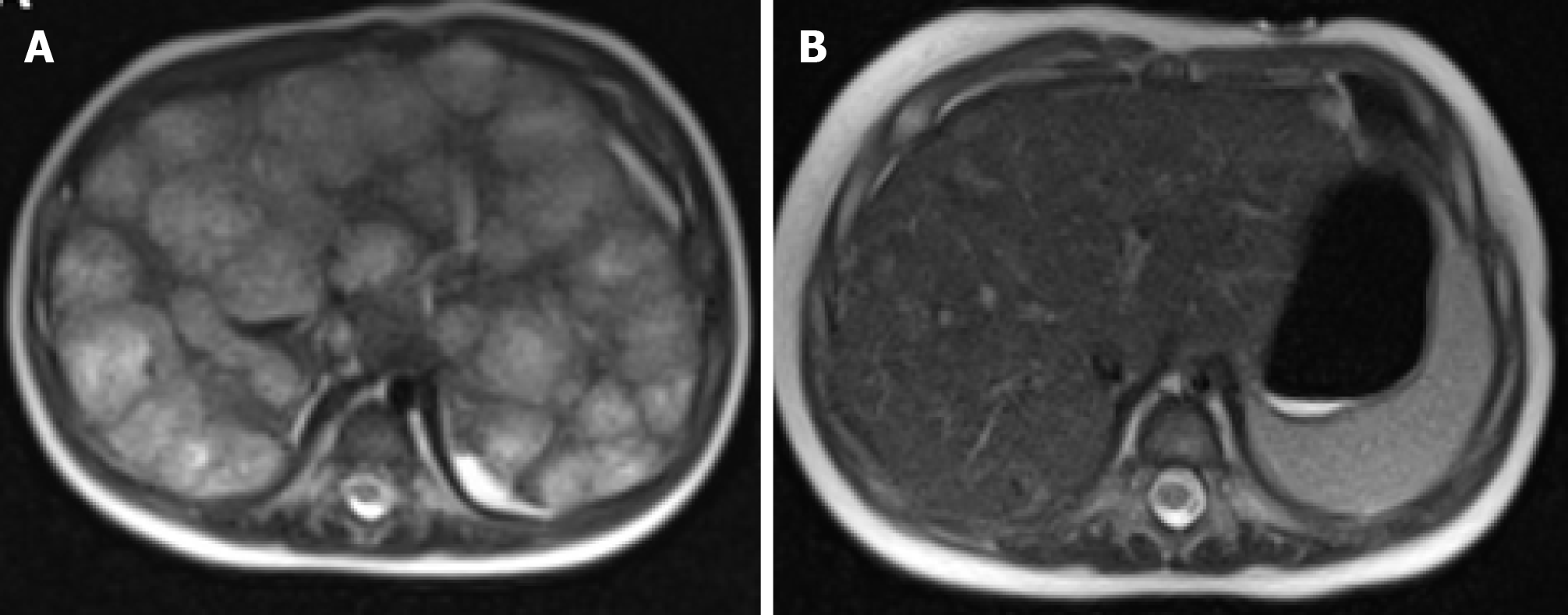
Hemangiomas presenting in the liver require thorough workup and close observation. They may be present in 0.4% to 20% of the population at any time, and between 0.4% and 7.3% based on autopsy studies[23,24]. Most are incidental findings on imaging for abdominal pain which are often unrelated to the hemangioma, particularly if it is small. Outcomes are dependent on the level of hepatic involvement. Hepatic hemangiomas can be categorized by size: Small (0-3 cm), medium, (3-10 cm), and large (greater than 10 cm)[24]. Solitary small and medium hepatic hemangiomas are more likely to behave like solitary cutaneous hemangiomas. These lesions may self-involute, while others can have high flow and persist. As such, they may be amenable to embolization via coiling or enucleation. If lesions are multifocal or diffuse, they are more reflective of infantile hepatic hemangiomatosis (IHH). IHH can be associated with high output cardiac failure, and coagulopathy depending on the level of involvement. Hepatic hemangiomatosis can either be present in the nodular or the non-nodular patterns which can be identified on CT or magnetic resonance imaging (Figure 11). The non-nodular pattern is more common overall. The latter will show coalescing ovoid low attenuation nodules measuring between 5-10 mm[25]. Contrast images may find vascular pooling within the lesion and centripetal enhancement[26]. Biopsy of these lesions will show endothelial-lined sinusoidal proliferation with erythrocyte content. Often, they are GLUT-1 positive. Of note, there has been one incidental case of diffuse hereditary hemangiomatosis in a 68-year-old adult with only liver involvement[27]. Large hemangiomas, up to 20 cm in some reports, can cause compressive symptoms causing pain and cholestasis in some cases[23].
Multifocal lymphangioendotheliomatosis with thrombocytopenia (MLT) should be on the differential for any child who presents with multiple hemangiomas. This diagnosis is distinct with notable thrombocytopenia, caused by a consumptive process, as well as the presence of lymphatic vessel endothelial receptor 1 (LYVE-1) on skin biopsies. GLUT-1 is negative in MLT. Hemangiomas are smaller 1-2 mm in diameter by comparison and grow at a slower rate than hemangiomatosis. GI bleeding is common, but liver involvement is rare. Platelet consumption is also seen in kaposiform hemangioendothelioma or tufted hemangiomas. This carries a high risk of developing Kasabach-Merritt syndrome[21].
As stated above, most isolated cutaneous hemangiomas will self-resolve without the need for medical or surgical management. Treatment considerations for the gastroenterologist are outlined here (Table 6). Large hepatic hemangiomas are associated with hypothyroidism secondary to increased type III thyronine deiodinase activity which binds and eliminates circulating T3 thyroid hormone[28]. Thyroid hormone screening on all infants with IHH is recommended and replacement is advised to prevent complications of hypothyroidism[28]. Of note, hypothyroidism on newborn screen is not typically detected in these patients.
| Treatment considerations for hepatic hemangiomas |
| Monitoring for self-involution |
| Propranolol (2-3 mg/kg/d) superior to corticosteroids or IV vincristine |
| Surgical ligation or resection of internal or complex hemangiomas |
| Enucleation for peripherally located hemangiomas |
| Artery embolization or radiofrequency ablation for emergency bleeding or in preparation for surgical intervention |
| Liver transplant for exceptionally large lesions or diffuse lesions, with severe complications such as heart failure and Kasabach-Merritt syndrome not amenable to medical management |
The prognosis of infantile hemangiomas is favorable and needs only conservative treatment; however, multiple lesions and visceral organ involvement warrant medical therapy, as high output heart failure and coagulopathy carry a high mortality if untreated[21,29]. Propranolol has been proven to be effective in the treatment of hepatic as well as cutaneous hemangiomas[28,30-32]. Meta-analysis has found it to be superior to placebo and oral steroids[33]. Commonly reported adverse events with oral propranolol include diarrhea, constipation, and bronchial hyperreactivity. Propranolol’s mechanism of action is thought to be related to regression of hemangioma cells and peripheral vasoconstriction leading to permanent involution within a couple of months (Figure 11). Daily dosing on 2 mg/kg/d is commonly utilized, but up to 3 mg/kg/d has been effective in high-risk airway and facial/orbital hemangiomas[34]. Corticosteroids and weekly IV vincristine have also been studied as a treatment, but the results are inferior to propranolol[35]. Co-involvement of a dermatologist is crucial to diagnosis and management.
Lastly, surgical, ligation, enucleation, or resection of large and or symptomatic lesions not amenable to medical therapy[23]. Enucleation is technically easier with peripherally located hepatic hemangiomas and is associated with lower morbidity when compared with resection[23]. Resection is typically reserved for centrally located lesions. Laparoscopic resection has decreased morbidity over open surgery. Artery embolization, or radiofrequency ablation have been used for management of acute bleeding or to shrink large lesions prior to surgery. Liver transplant is reserved for very large lesions with severe complications such as heart failure, or Kasabach-Merritt syndrome[23].
We have discussed various conditions that can cause congenital hepatic shunts. Many review articles have been written on these conditions separately and through the lens of various specialties such as radiological or surgical perspectives. Our goal was to create a concise review of all congenital shunts from the stance of the pediatric hepatologist. As imaging techniques and interventional therapeutics evolve, we are better able to diagnose and study these conditions. Early detection and monitoring best serve patients and clinicians in making medical management decisions.
We would like to thank Dr. Myers M, MD from The Department of Pediatric Diagnostic Radiology and Dr. Fagan T, MD from The Department of Pediatric Cardiology at the Cleveland Clinic Foundation for their assistance with reviewing and selecting radiographic images for this review.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katada K S-Editor: Huang P L-Editor: A P-Editor: Wang LL
| 1. | Harpavat S, Mclin, V. Developmental Anatomy and Physiology of the Liver and Bile Ducts. In: Wyllie R, Hyams JS, Kay M. Pediatric Gastrointestinal and Liver Disease. Philadelphia: Elsevier Health Sciences, 2015: 811-821. |
| 2. | Morgan G, Superina R. Congenital absence of the portal vein: two cases and a proposed classification system for portasystemic vascular anomalies. J Pediatr Surg. 1994;29:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 296] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Papamichail M, Pizanias M, Heaton N. Congenital portosystemic venous shunt. Eur J Pediatr. 2018;177:285-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 4. | Alonso-Gamarra E, Parrón M, Pérez A, Prieto C, Hierro L, López-Santamaría M. Clinical and radiologic manifestations of congenital extrahepatic portosystemic shunts: a comprehensive review. Radiographics. 2011;31:707-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Saxena AK, Sodhi KS, Arora J, Thapa BR, Suri S. Congenital intrahepatic portosystemic venous shunt in an infant with down syndrome. AJR Am J Roentgenol. 2004;183:1783-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Park JH, Cha SH, Han JK, Han MC. Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol. 1990;155:527-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 184] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 7. | Alkan F, Düzgün F, Yüksel H, Tarhan S, Coşkun Ş. Percutaneous embolization of congenital portosystemic venous shunt in an infant with respiratory distress. Turk J Pediatr. 2018;60:456-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Shinkai M, Ohhama Y, Nishi T, Yamamoto H, Fujita S, Take H, Adachi M, Tachibana K, Aida N, Kato K, Tanaka Y, Takemiya S. Congenital absence of the portal vein and role of liver transplantation in children. J Pediatr Surg. 2001;36:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Lautz TB, Shah SA, Superina RA. Hepatoblastoma in Children With Congenital Portosystemic Shunts. J Pediatr Gastroenterol Nutr. 2016;62:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Guérin F, Blanc T, Gauthier F, Abella SF, Branchereau S. Congenital portosystemic vascular malformations. Semin Pediatr Surg. 2012;21:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | De Vito C, Tyraskis A, Davenport M, Thompson R, Heaton N, Quaglia A. Histopathology of livers in patients with congenital portosystemic shunts (Abernethy malformation): a case series of 22 patients. Virchows Arch. 2019;474:47-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Aravinthan A, Verma S, Coleman N, Davies S, Allison M, Alexander G. Vacuolation in hepatocyte nuclei is a marker of senescence. J Clin Pathol. 2012;65:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Sakura N, Mizoguchi N, Eguchi T, Ono H, Mawatari H, Naitou K, Ito K. Elevated plasma bile acids in hypergalactosaemic neonates: a diagnostic clue to portosystemic shunts. Eur J Pediatr. 1997;156:716-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | McLin VA, Franchi Abella S, Debray D, Guérin F, Beghetti M, Savale L, Wildhaber BE, Gonzales E; Members of the International Registry of Congenital Porto-Systemic Shunts. Congenital Portosystemic Shunts: Current Diagnosis and Management. J Pediatr Gastroenterol Nutr. 2019;68:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Garcia-Tsao G. Liver involvement in hereditary hemorrhagic telangiectasia (HHT). J Hepatol. 2007;46:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Song W, Zhao D, Li H, Ding J, He N, Chen Y. Liver Findings in Patients with Hereditary Hemorrhagic Telangiectasia. Iran J Radiol. 2016;13:e31116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Sabbà C, Pasculli G, Lenato GM, Suppressa P, Lastella P, Memeo M, Dicuonzo F, Guant G. Hereditary hemorrhagic telangiectasia: clinical features in ENG and ALK1 mutation carriers. J Thromb Haemost. 2007;5:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Lin HC, Fiorino KN, Blick C, Anupindi SA. A rare presentation and diagnosis of juvenile polyposis syndrome and hereditary hemorrhagic telangiectasia overlap syndrome. Clin Imaging. 2015;39:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Bernard G, Mion F, Henry L, Plauchu H, Paliard P. Hepatic involvement in hereditary hemorrhagic telangiectasia: clinical, radiological, and hemodynamic studies of 11 cases. Gastroenterology. 1993;105:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? Pediatr Dermatol. 2008;25:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 357] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Glick ZR, Frieden IJ, Garzon MC, Mully TW, Drolet BA. Diffuse neonatal hemangiomatosis: an evidence-based review of case reports in the literature. J Am Acad Dermatol. 2012;67:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Iacobas I, Phung TL, Adams DM, Trenor CC 3rd, Blei F, Fishman DS, Hammill A, Masand PM, Fishman SJ. Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring. J Pediatr 2018; 203: 294-300. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Leon M, Chavez L, Surani S. Hepatic hemangioma: What internists need to know. World J Gastroenterol. 2020;26:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (7)] |
| 24. | Bajenaru N, Balaban V, Săvulescu F, Campeanu I, Patrascu T. Hepatic hemangioma -review-. J Med Life. 2015;8 Spec Issue:4-11. [PubMed] |
| 25. | Poirier VC, Ablin DS, Frank EH. Diffuse neonatal hemangiomatosis: a case report. AJNR Am J Neuroradiol. 1990;11:1097-1099. [PubMed] |
| 26. | Nip SY, Hon KL, Leung WK, Leung AK, Choi PC. Neonatal Abdominal Hemangiomatosis: Propranolol beyond Infantile Hemangioma. Case Rep Pediatr. 2016;2016:9803975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Batista A, Matos AP, Neta JO, Ramalho M. Diffuse Hepatic Hemangiomatosis in the Adult without Extra-hepatic Involvement: An Extremely Rare Occurrence. J Clin Imaging Sci. 2014;4:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Yeh I, Bruckner AL, Sanchez R, Jeng MR, Newell BD, Frieden IJ. Diffuse infantile hepatic hemangiomas: a report of four cases successfully managed with medical therapy. Pediatr Dermatol. 2011;28:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Agarwal S, Sharma A, Maria A. Diffuse neonatal hemangiomatosis presenting as congestive heart failure. Dermatol Pract Concept. 2017;7:66-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Ferrandiz L, Toledo-Pastrana T, Moreno-Ramirez D, Bardallo-Cruzado L, Perez-Bertolez S, Luna-Lagares S, Rios-Martin JJ. Diffuse neonatal hemangiomatosis with partial response to propranolol. Int J Dermatol. 2014;53:e247-e250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Mazereeuw-Hautier J, Hoeger PH, Benlahrech S, Ammour A, Broue P, Vial J, Ohanessian G, Léauté-Labrèze C, Labenne M, Vabres P, Rössler J, Bodemer C. Efficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosis. J Pediatr. 2010;157:340-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Meyer L, Graffstaedt H, Giest H, Truebenbach J, Waner M. Effectiveness of propranolol in a newborn with liver hemangiomatosis. Eur J Pediatr Surg. 2010;20:414-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Yang H, Hu DL, Shu Q, Guo XD. Efficacy and adverse effects of oral propranolol in infantile hemangioma: a meta-analysis of comparative studies. World J Pediatr. 2019;15:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Baselga E, Dembowska-Baginska B, Przewratil P, González-Enseñat MA, Wyrzykowski D, Torrelo A, López Gutiérrez JC, Rychłowska-Pruszyńska M, de Lucas-Laguna R, Esteve-Martinez A, Roé E, Zaim M, Menon Y, Gautier S, Lebbé G, Bouroubi A, Delarue A, Voisard JJ. Efficacy of Propranolol Between 6 and 12 Months of Age in High-Risk Infantile Hemangioma. Pediatrics. 2018;142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Theunissen CI, Smitt JH, van der Horst CM. Propranolol versus corticosteroids: what should be the treatment of choice in infantile hemangiomas? Ann Plast Surg. 2015;74:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |