Published online Oct 28, 2020. doi: 10.3748/wjg.v26.i40.6295
Peer-review started: May 12, 2020
First decision: June 18, 2020
Revised: June 25, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: October 28, 2020
Processing time: 168 Days and 23.9 Hours
Ethylmalonic encephalopathy (EE) is a rare autosomal recessive metabolic disorder caused by impaired mitochondrial sulfur dioxygenase. Due to poor therapeutic effect of current conventional treatments, progressive psychomotor regression and neurological impairment usually contribute to early death in the first decade of life. Liver transplantation (LT) is emerging as a novel therapeutic option for EE; however, worldwide experience is still limited.
An 18-mo-old patient with the diagnosis of EE received a living donor liver transplant in our institution, which, to our knowledge, is the first case in Asian-Pacific countries. During 20 mo of follow-up, the longitudinal metabolic evaluations revealed a wild fluctuation in urinary EMA levels, still far beyond the normal range. Urinary 2-methylsuccinic acid levels gradually restored to normal, whereas the concentrations of urinary isobutyrylglycine and plasma C4- and C5-acylcarnitines fluctuated markedly and still remained above the reference limits. Only mild amelioration of petechiae and ecchymosis was observed, and no dramatic reversion of chronic mucoid diarrhea and orthostatic acrocyanosis occurred. Although brain magnetic resonance imaging suggested a certain improvement in basal ganglia lesions, the patient still presented developmental delay and neurologic disability.
LT may bring about a partial but not complete cure to EE. Given its definite role in defending against the devastating natural progression of EE, LT should still be considered for patients with EE in the absence of other superior therapeutic options. Early establishment of diagnosis and initiation of conventional treatment pre-transplant, timely LT, and continuous administration of metabolism-correcting medications post-transplant may be helpful in minimizing the neurologic impairment and maximizing the therapeutic value of LT in EE.
Core Tip: Liver transplantation (LT) is emerging as a novel therapeutic option for ethylmalonic encephalopathy (EE). However, worldwide experience is still limited. Our case of LT for EE, to the best of our knowledge, is the first case in Asian-Pacific countries. The case suggests that despite only a partial cure, LT does have a definite role in defending against the devastating natural progression of EE. Early establishment of diagnosis and initiation of conventional treatment pre-transplant, timely LT, and continuous administration of metabolism-correcting medications post-transplant may be helpful in minimizing the neurologic impairment and maximizing the therapeutic value of LT in EE.
- Citation: Zhou GP, Qu W, Zhu ZJ, Sun LY, Wei L, Zeng ZG, Liu Y. Compromised therapeutic value of pediatric liver transplantation in ethylmalonic encephalopathy: A case report. World J Gastroenterol 2020; 26(40): 6295-6303
- URL: https://www.wjgnet.com/1007-9327/full/v26/i40/6295.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i40.6295
Ethylmalonic encephalopathy [EE, Online Mendelian Inheritance in Man number #602473][1] is a rare autosomal recessively inherited disorder of mitochondrial metabolism caused by homozygous or compound heterozygous mutations in the ETHE1 gene, which is located on chromosome 19q13[2]. To date, more than 100 patients with EE have been reported worldwide[3] but rarely reported in China. However, based on the results of newborn screening from Jining city in 2014-2015, live-birth incidence of EE is approximately 1:25000[4]. ETHE1 deficiency results in chronic accumulation of hydrogen sulfide (H2S) and its derivative thiosulfate in multisystemic tissues such as colonic mucosa, liver, muscle, and brain[5,6]. Characteristic patients with ETHE1 disease present with (1) progressive neurological degeneration, including global neurodevelopmental delay, early-onset psychomotor regression, progressive pyramidal and extrapyramidal signs, and seizures and (2) diffuse microvasculature injury, including recurrent petechial purpura, orthostatic acrocyanosis, and chronic hemorrhagic diarrhea[6,7]. Currently, the mainstay of conservative treatment options aimed at lowering production and promoting detoxification of H2S is a methionine and isoleucine restricted diet and combined use of metronidazole (an antibiotic against enterobacterial anaerobic flora) and N-acetylcysteine (a cell-permeable precursor of the H2S scavenger glutathione)[8]. For EE patients presenting with severe encephalopathy, intravenous N-acetylcysteine infusion therapy may be more effective[9]. Since most affected individuals are compound heterozygotes resulting in unclear genotype-phenotype correlation, some patients with milder courses of EE who receive combined treatments with metronidazole and N-acetylcysteine may survive into childhood and adolescence, even though they are likely to suffer acute episodes of metabolic decompensation. Kitzler et al[10] found that during an acute episode of metabolic decompensation, continuous renal replacement therapy may help to regain metabolic control in patients with EE. Despite this, the overall outcome of this devastating disorder remains poor. Progressive clinical deterioration along with psychomotor regression usually result in death within the first few years of life.
Since 2016, Dionisi-Vici et al[3] and Tam et al[11] successively performed liver transplantation (LT) in three children with EE. Although psychomotor delay remained after liver transplant, progressive improvements of neurological function and psychomotor development and dramatic amelioration of biochemical abnormalities were observed. These results suggest that LT may be a promising therapeutic approach that can improve the prognosis of EE. However, worldwide experience is still limited, and potential benefits of LT must be weighed against the potentially high risk of this procedure. Herein we describe a case of living donor liver transplantation (LDLT) for an 18-mo-old patient with EE. To our knowledge, this is the first case in Asian-Pacific countries.
A 15-mo-old boy with the diagnosis of EE was admitted for consideration of LT.
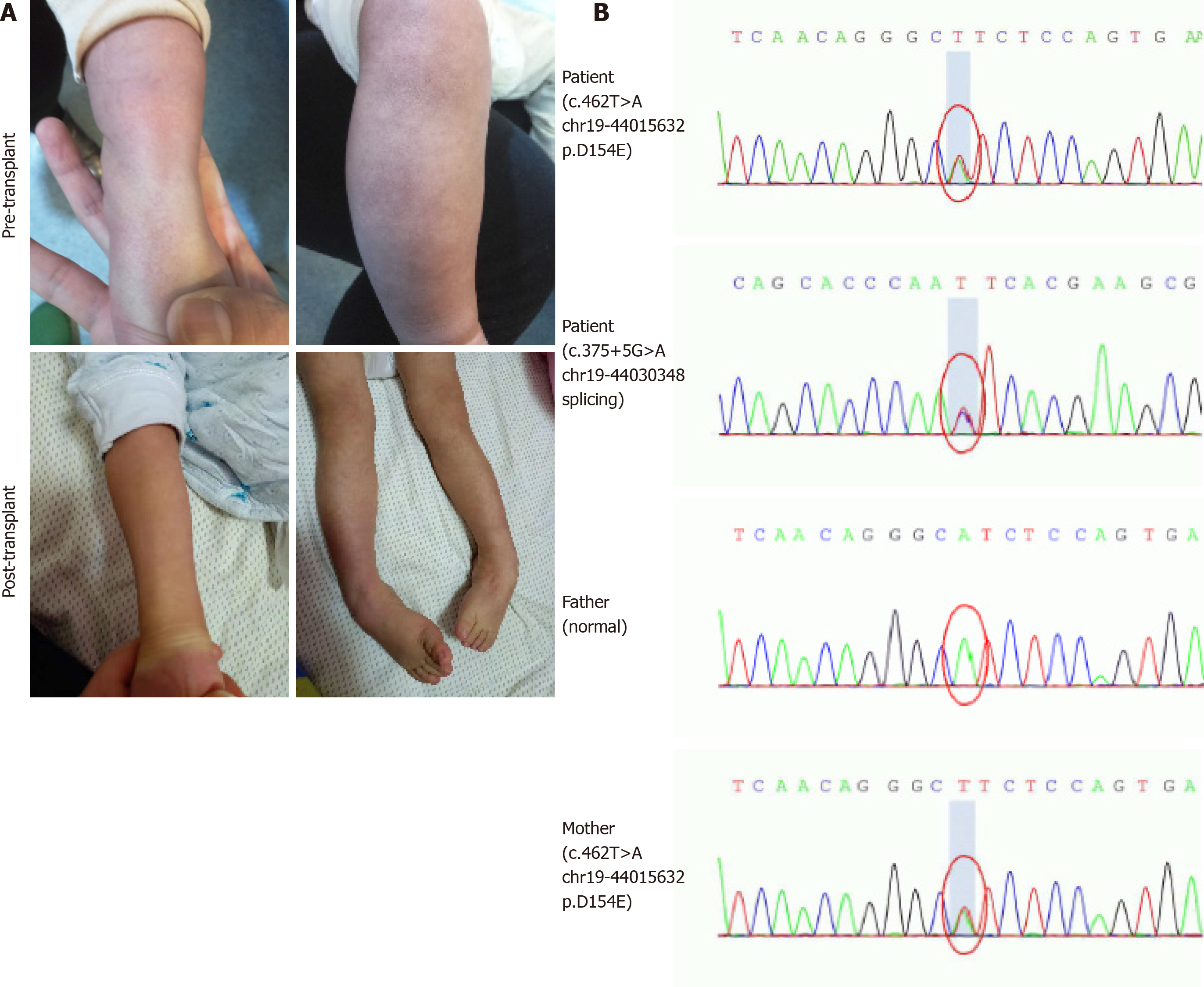
The patient was the first child of non-consanguineous parents after an uncomplicated pregnancy. He had persistent mucoid diarrhea since birth, and scattered petechiae and ecchymosis occurred in the periocular and extremities (Figure 1A). By 9 mo of age, the boy still had poor head control and no ability to sit or roll over unaided. Then, he was referred to a local children hospital. A lactose-free diet and administration of levocarnitine, benzhexol hydrochloride, coenzyme Q10, and multivitamins were initiated. However, during 3-mo follow-up, no obvious improvements in diarrhea, acrocyanosis, and developmental retardation occurred to him.
The patient had no other significant medical history.
There was no significant personal and family medical history.
The neurological examination revealed significant axial hypotonia, muscle weakness, increased deep tendon reflexes, clonus, and sustained bilateral Babinski sign.
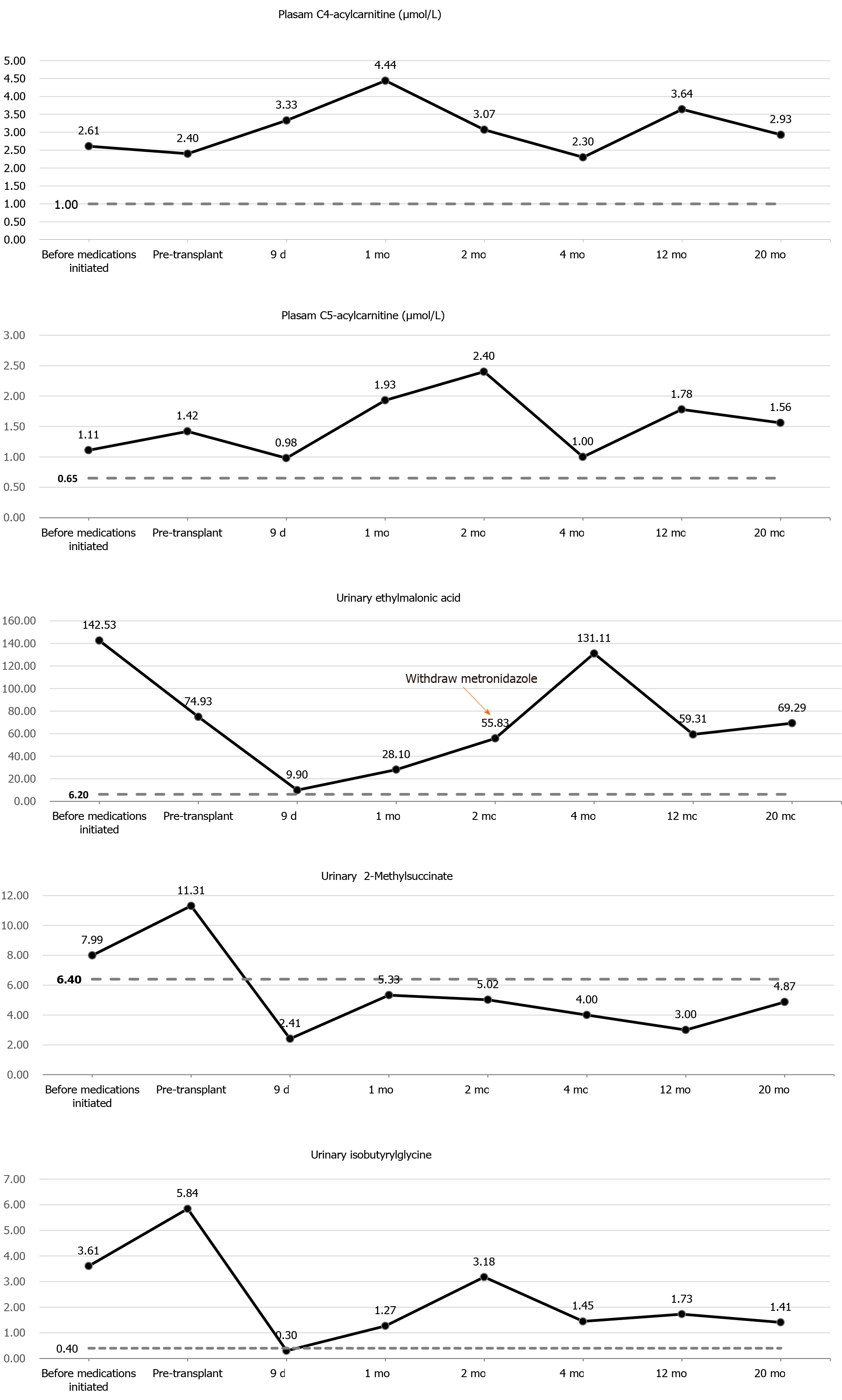
Urine organic acid profile revealed elevated EMA at 142.53 (reference range: 0.00-6.20) and a slight increased isobutyrylglycine and 2-methylsuccinic acid at 3.61 (reference range: 0.00-0.40) and 7.99 (reference range: 0.00-6.40), respectively. Plasma acylcarnitine profile revealed elevated C4 at 2.61 μmol/L (reference range: < 1.00 μmol/L) and C5 at 1.11 μmol/L (reference range: < 0.65 μmol/L) (Figure 2). Gene sequencing analysis showed compound heterozygous mutations in the ETHE1 gene, a previously reported pathogenic mutation (c.375+5G>A chr19:44030348 splicing)[2] and a novel mutation (c.462T>A chr19:44015632 p.D154E) (Figure 1B).
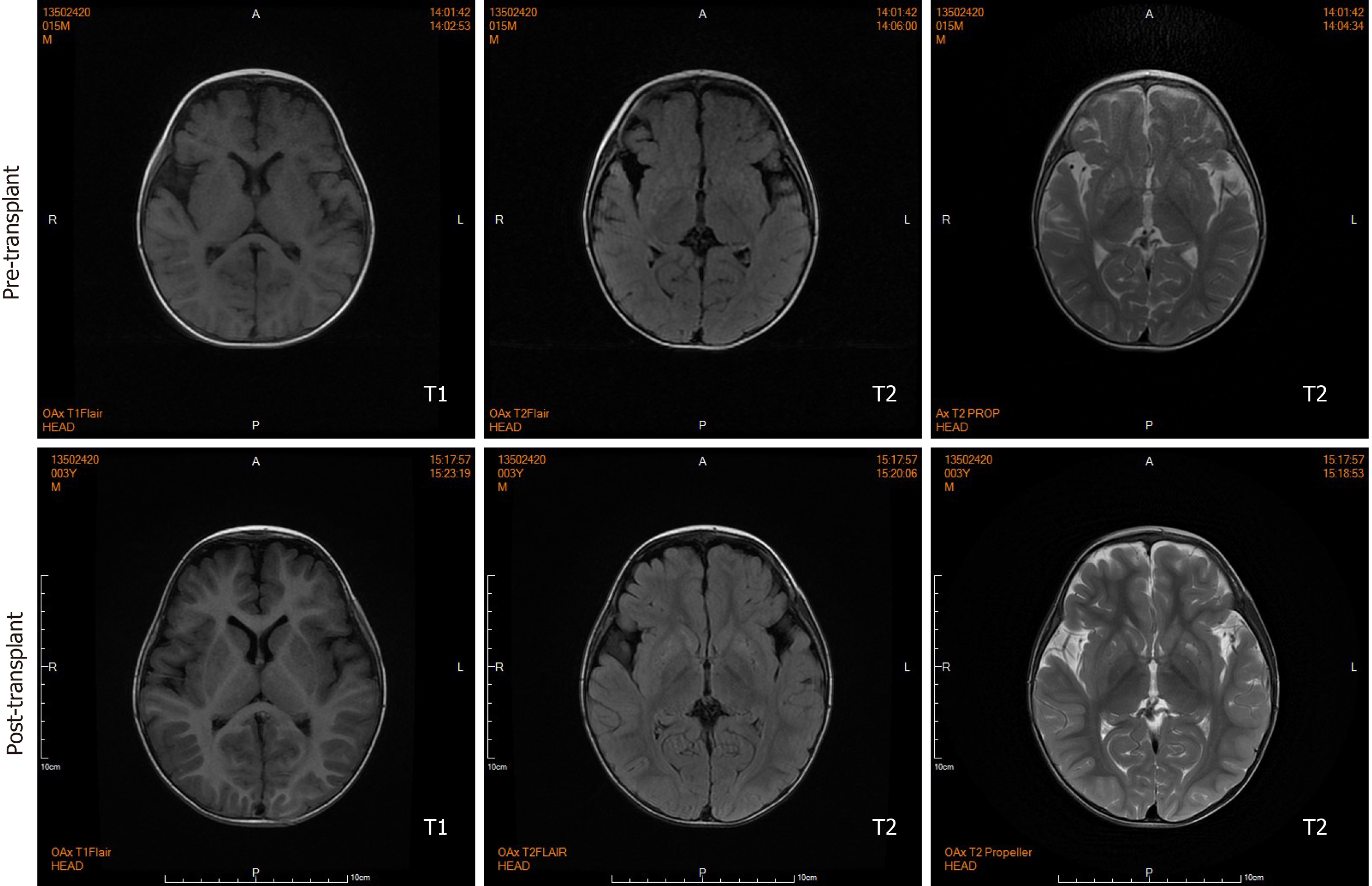
Brain magnetic resonance imaging (MRI) by our institution revealed fronto-temporal atrophy with multiple bilateral symmetrical abnormal low and high signal intensity of basal ganglia in T1- and T2-weighted images, respectively (Figure 3).
The patient was diagnosed with EE.
A sulfur-containing amino acids restricted diet and the combined use of metronidazole (30 mg/kg/d) and N-acetylcysteine (80 mg/kg/d) were started for the boy until we found that he was allergic to N-acetylcysteine (manifested by fever and rash), for which N-acetylcysteine was stopped immediately. Following 3 mo of combined medical and dietary treatments, urinary EMA levels decreased slightly, but remained elevated at 74.93, and values of isobutyrylglycine and 2-methylsuccinic acid rose to 5.84 and 11.31, respectively. Plasma acylcarnitine profile showed a slight decrease in C4 (2.40 μmol/L) and increase in C5 (1.42 μmol/L) (Figure 2). No obvious improvements were observed in his clinical conditions.
At the age of 18 mo, the boy received a LDLT with reduced-size left lateral lobe donated by his heterozygous mother under the approval of the Ethical Committee of Beijing Friendship Hospital, Capital Medical University.
The patient underwent a smooth operation, and postoperative course was uneventful. Immunosuppressive treatments consisted of tacrolimus and low-dose corticosteroids. Administration of metronidazole and levocarnitine was continued during the perioperative period, and metabolite levels decreased evidently. Urinary EMA, isobutyrylglycine, and 2-methylsuccinic acid levels decreased to 9.90, 0.30, and 2.41, respectively, on post-transplantation day 9. Plasma C4 increased and C5 decreased, and they did not return to normal levels (Figure 2). However, due to the recipient’s intolerance of metronidazole, the administration was privately discontinued by his mother 2 mo after transplantation.
During the 20-mo follow-up period, the patient was administered levocarnitine supplementation without a strict protein-restriction diet. Although his liver graft function remained constantly in the normal range, no remarkable amelioration of clinical conditions and neurological development was observed. The longitudinal evaluations disclosed a wild fluctuation in urinary EMA levels, far beyond the normal range. Urinary 2-methylsuccinic acid levels gradually restored to normal, whereas the concentrations of urinary isobutyrylglycine and plasma C4- and C5-acylcarnitines fluctuated markedly and still remained above the reference limits (Figure 2). Only mild amelioration of petechiae and ecchymosis was observed, and no dramatic reversion of chronic mucoid diarrhea and orthostatic acrocyanosis occurred (Figure 1). Although brain MRI suggested a certain improvement in basal ganglia lesions (Figure 3), developmental delay and neurologic disability continued. Now, at the age of 38 mo, the boy has a certain extent of head control, but is still unable to roll over, sit, stand, or walk by himself. He can just say several simple words, such as mama and papa. Meanwhile, the living donor recovered well with no postoperative complications during the follow-up.
EE is an inborn error of metabolism caused by a defect in the mitochondrial sulfur dioxygenase. The widely distributed metabolic deficit leads to significant accumulation of toxic metabolites in multiple organs. Patients with ETHE1 deficiency are characterized by a relentless progressive clinical deterioration, usually leading to death within the first decade of life. Current therapeutic approaches are most often supportive, having no remarkable impact on the natural history of EE[8,12].
Di Meo et al[13] found that intravenous injection of adeno-associated virus 2/8 carrying the ETHE1 gene into the livers of constitutive Ethe1−/− mice resulted in obvious amelioration of clinical phenotypes, spectacular prolongation of survival, and improvements in biochemical parameters. Based on the above findings, replacing the metabolically defective liver may help regain enzyme activity, and thus LT has been viewed as one of the most potential treatment modalities for EE. Since the first attempt in 2016, LT is emerging as a promising therapeutic option for this devastating disease[3,11]. However, the clinical outcomes of LT in our patient was not favorable. Though 20 mo have elapsed since LT, the recipient merely achieved minimal improvements in biochemical features as well as the lesions in the brain MRI without significant clinical and neurological amelioration, which compromised the promising therapeutic value of LT in EE.
Since EE is a multisystemic disease, the underlying enzymatic defect also exits in other tissues besides liver. Although the implanted liver graft containing normal ETHE1 gene expression could help regain missing enzymatic activity, the overall metabolic defect is only partially corrected, as extrahepatic synthesis and accumulation of toxic metabolites remain[6]. Illustrating this fact, after successful LT, postoperative levels of blood and urine metabolites among three previous recipients[3,11] and our patient did not decrease to the normal ranges. Nevertheless, cases including ours suggest that LT does have a definite role in defending against the devastating natural progression of EE. Moreover, satisfactory neurodevelopmental improvements and partial reversal of biochemical abnormalities were observed in those three liver transplant recipients. Our patient, by contrast, has not yet fully benefited from the promising therapeutic effects of LT. The detailed characteristics of these four patients are summarized in Table 1. It can be inferred that our patient’s unfavorable prognosis may be attributed to the late diagnosis of EE, late initiation of conventional treatment, pre-transplant administration of metronidazole alone, and post-transplant discontinuation of metronidazole. Furthermore, undefined genotype-phenotype correlation and the use of a heterozygous carrier donor in LDLT may also lead to the post-transplant suboptimal clinical outcomes of our patient.
| Ref. | Case number | Gender | Age at diagnosis | Pre-LT dietary restriction | Pre-LT medical treatment | Age at LT in mo | Type of graft | Post-LT dietary restriction | Post-LT medical treatment | Follow-up in mo | Neurodevelopmental outcomes |
| Dionisi-Vici et al[3] 2016 | 1 | Female | 7 mo | N/A | Metronidazole, N-acetylcysteine | 9 | LDLT | N/A | Carnitine, metronidazole | 12 | Striking neurological improvement, remarkable achievements in psychomotor development. |
| Tam et al[11] 2019 | 2 | Female | 13 mo | N/A | Metronidazole, N-acetylcysteine | 19 | DDLT | N/A | Carnitine, metronidazole, N-acetylcysteine | 9 | Drastically improved social interaction and language. |
| 3 | Male | 8 wk | N/A | Metronidazole, N-acetylcysteine | 13 | OLT | N/A | Metronidazole, N-acetylcysteine | 22 | No other encephalopathic episodes and slow developmental progress. | |
| Present case | 4 | Male | 9 mo | Yes (start from 15 mo) | Carnitine, metronidazole, (start from 15 m) | 18 | LDLT | No | Carnitine, metronidazole (stop 2 mo post-LT) | 20 | No obvious improvement. |
The long-term clinical prognosis, especially neurodevelopmental outcomes, of liver transplant recipients depends largely on the pre- and post-transplant metabolic control. Early establishment of diagnosis and early initiation of conventional supportive management before the onset of clinical symptoms are of paramount importance as they can control the H2S overload, thereby improving clinical outcomes and biochemical markers[14]. The early clinical manifestations and neurologic deterioration arising from EE may present soon after birth[12]. It is necessary for physicians to strengthen their understanding of EE in order to make an early and correct diagnosis for these patients. After the diagnosis and before LT, combined medical and dietary treatments should be initiated without delay. It should be emphasized that only correct medical and dietary treatments, namely the combined use of metronidazole and N-acetylcysteine and a diet restricted in sulfur-containing amino acids, can help minimize H2S toxicity. Given LT cannot be performed until optimal age and weight criteria for liver transplant are achieved, conventional treatments in newborns until transplant could therefore contribute to opportune LT. The continuous administration of metronidazole and N-acetylcysteine is also necessary after liver transplant to minimize accumulation of toxic metabolites and maximize therapeutic effect of LT. Nevertheless, heterogenous genotype-phenotype correlations may influence the residual ETHE1 enzymatic activity, severity of phenotypes, and response to conventional interventions in patients with EE, which may partially explain the difference in outcomes with LT among the present several patients. In addition, although previous study suggested that the use of asymptomatic heterozygous carrier donor in LDLT for this autosomal-recessive condition was viable with satisfactory clinical outcomes[3], ETHE1 enzyme activity within the different heterozygous carrier donors’ livers may vary. However, due to the unavailability of enzyme activity measurement technology, we did not perform donor enzyme activity assay before LT. Thus, whether the compromised therapeutic effect in our patient is associated with the use of a heterozygous carrier donor remains unclear. Notably, there was no description of dietary restriction for the three previous liver transplant recipients. Whether a diet restricted in sulfur-containing amino acids could further improve liver transplant recipients’ clinical prognosis deserves further research.
Although the reversal of neurological damage still needs to be further observed in the long run, the possibility of irreversibility of neurological disability posttransplant should always be kept in the mind of doctors and patients’ guardians. Taking into account the perioperative morbidity, transplant-related complications, and long-term immunosuppression, scrupulous preoperative clinical assessment of patient’s overall clinical conditions, especially severity of neurological damage, is necessary for the evaluation of the benefit–risk ratio. More importantly, the joint participation of a multidisciplinary team including metabolism physicians, pediatric hepatologists, pediatric neurologists, pediatric transplantation team, metabolic nutritionists, and neurorehabilitation physicians, is indispensable for the long-term management of patients with EE.
LT does have a definite role in defending against the devastating natural progression of EE. Despite only a partial cure, LT should still be considered for patients with EE in the absence of other superior therapeutic options. Early establishment of diagnosis and initiation of conventional treatment pre-transplant, timely LT, and continuous administration of metabolism-correcting medications post-transplant may be helpful in minimizing the neurologic impairment and maximizing the therapeutic value of LT in EE.
We would like to thank He EH, Xu RF and Yi ZX who contributed to ultrasound monitoring in this patient.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iliescu EL, Verran DJ S-Editor: Huang P L-Editor: Filipodia P-Editor: Wang LL
| 1. | Online Mendelian Inheritance in Man [Internet]. Available from: https://www.omim.org/entry/602473. |
| 2. | Tiranti V, D'Adamo P, Briem E, Ferrari G, Mineri R, Lamantea E, Mandel H, Balestri P, Garcia-Silva MT, Vollmer B, Rinaldo P, Hahn SH, Leonard J, Rahman S, Dionisi-Vici C, Garavaglia B, Gasparini P, Zeviani M. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am J Hum Genet. 2004;74:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Dionisi-Vici C, Diodato D, Torre G, Picca S, Pariante R, Giuseppe Picardo S, Di Meo I, Rizzo C, Tiranti V, Zeviani M, De Ville De Goyet J. Liver transplant in ethylmalonic encephalopathy: a new treatment for an otherwise fatal disease. Brain. 2016;139:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Yang CJ, Wei N, Li M, Xie K, Li JQ, Huang CG, Xiao YS, Liu WH, Chen XG. Diagnosis and therapeutic monitoring of inborn errors of metabolism in 100,077 newborns from Jining city in China. BMC Pediatr. 2018;18:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Tiranti V, Zeviani M. Altered sulfide (H(2)S) metabolism in ethylmalonic encephalopathy. Cold Spring Harb Perspect Biol. 2013;5:a011437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Di Meo I, Lamperti C, Tiranti V. Mitochondrial diseases caused by toxic compound accumulation: from etiopathology to therapeutic approaches. EMBO Mol Med. 2015;7:1257-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Viscomi C, Burlina AB, Dweikat I, Savoiardo M, Lamperti C, Hildebrandt T, Tiranti V, Zeviani M. Combined treatment with oral metronidazole and N-acetylcysteine is effective in ethylmalonic encephalopathy. Nat Med. 2010;16:869-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Kılıç M, Dedeoğlu Ö, Göçmen R, Kesici S, Yüksel D. Successful treatment of a patient with ethylmalonic encephalopathy by intravenous N-acetylcysteine. Metab Brain Dis. 2017;32:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Kitzler TM, Gupta IR, Osterman B, Poulin C, Trakadis Y, Waters PJ, Buhas DC. Acute and Chronic Management in an Atypical Case of Ethylmalonic Encephalopathy. JIMD Rep. 2019;45:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Tam A, AlDhaheri NS, Mysore K, Tessier ME, Goss J, Fernandez LA, D'Alessandro AM, Schwoerer JS, Rice GM, Elsea SH, Scaglia F. Improved clinical outcome following liver transplant in patients with ethylmalonic encephalopathy. Am J Med Genet A. 2019;179:1015-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Boyer M, Sowa M, Di Meo I, Eftekharian S, Steenari MR, Tiranti V, Abdenur JE. Response to medical and a novel dietary treatment in newborn screen identified patients with ethylmalonic encephalopathy. Mol Genet Metab. 2018;124:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Di Meo I, Auricchio A, Lamperti C, Burlina A, Viscomi C, Zeviani M. Effective AAV-mediated gene therapy in a mouse model of ethylmalonic encephalopathy. EMBO Mol Med. 2012;4:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Govindaraj P, Parayil Sankaran B, Nagappa M, Arvinda HR, Deepha S, Jessiena Ponmalar JN, Sinha S, Gayathri N, Taly AB. Child Neurology: Ethylmalonic encephalopathy. Neurology. 2020;94:e1336-e1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |