Published online Jan 28, 2020. doi: 10.3748/wjg.v26.i4.448
Peer-review started: October 16, 2019
First decision: November 22, 2019
Revised: December 5, 2019
Accepted: January 2, 2020
Article in press: January 2, 2020
Published online: January 28, 2020
Processing time: 93 Days and 10.7 Hours
Hepatic artery stenosis is a complication of orthotopic liver transplant occurring in 3.1%-7.4% of patients that can result in graft failure and need for re-transplantation. Endovascular therapy with angioplasty and stenting has been used with a high degree of technical success and good clinical outcomes, but tortuous hepatic arteries present a unique challenge for intervention. Suitable stents for this application should be maneuverable and conformable while also exerting adequate radial force to maintain a patent lumen.
Herein we report our experience with a neurovascular Wingspan stent system in a challenging case of recurrent hepatic artery stenosis and discuss the literature of stenting in tortuous transplant hepatic arteries.
Wingspan neurovascular stent is self-expanding, has good conformability, and adequate radial resistance and as such it could be added to the armamentarium of interventionalists in the setting of a tortuous and stenotic transplant hepatic artery.
Core tip: Endovascular therapy of hepatic artery stenosis using angioplasty and stenting can be performed by interventional radiologists and has good outcomes and safety record, reducing the need for surgical revascularization or re-transplantation. The Wingspan neurovascular stent is a new self-expanding stent that has good conformability, maneuverability and adequate radial resistance for this application.
- Citation: Barahman M, Alanis L, DiNorcia J, Moriarty JM, McWilliams JP. Hepatic artery stenosis angioplasty and implantation of Wingspan neurovascular stent: A case report and discussion of stenting in tortuous vessels. World J Gastroenterol 2020; 26(4): 448-455
- URL: https://www.wjgnet.com/1007-9327/full/v26/i4/448.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i4.448
Hepatic artery stenosis (HAS) is a possible complication of orthotopic liver transplant (OLT) occurring in 3.1%-7.4% of patients[1-4]. HAS may progress to hepatic artery thrombosis (HAT), limiting graft perfusion and causing liver dysfunction and biliary ischemia. Traditionally, HAS was treated surgically with revascularization or re-transplantation, but endovascular treatments have become more commonplace in the last 3-4 decades. Hepatic angiography can definitively diagnose HAS and allows simultaneous treatment using percutaneous transluminal angioplasty (PTA), stenting, or a combination of both. However, significant arterial tortuosity and kinking can occur in a subgroup of patients, associated with poorer technical success, poorer patient outcomes, and a higher complication rate[2,5-8]. Stent placement may improve outcomes in these patients, but stent delivery and deployment in tortuous anatomy can be difficult. New neurovascular stents have recently become available that may suit this application well as they are intended for use in tortuous intracranial anatomy. In a recent case of a patient with HAS after OLT, we utilized a neurovascular Wingspan stent (Stryker, Kalamazoo, MI, United States) to treat a tight stenosis around a hairpin bend. Neurointerventional devices have been used for the treatment of visceral aneurysms[9], but so far no reports are available for their use in stenotic visceral arteries. To our knowledge this is the first report of successful implantation of the Wingspan neurovascular stent in a transplant hepatic artery.
Nausea, vomiting, weakness, and lethargy.
A 47-year-old woman with a history of alcoholic cirrhosis status post OLT and a complex post-transplant course had elevated liver function tests (LFTs) 69 d post-transplant.
The patient has a history of decompensated cirrhosis secondary to alcohol use underwent OLT at our institution. Her Model for End-stage Liver Disease score was 33, and she received a liver allograft from a 61-year-old deceased donor who died from complications of a cerebrovascular accident. The arterial anastomosis was formed between the donor celiac axis and a branch patch of the recipient’s proper hepatic and gastroduodenal arteries. Initial graft function was good. Post-transplant course was complicated by failure to thrive, pneumonia with respiratory failure, intractable nausea and vomiting, and chronic kidney disease on dialysis. She remained an inpatient during her convalescence, and on POD #69 she developed newly elevated LFTs.
Vitals: within normal limits; General: Alert, oriented, and cooperative; Neck: Tracheostomy in place, healing well; Heart: Regular rate and rhythm, no murmurs; Lungs: Clear to auscultation, mildly decreased lung sounds at bases; Abdomen: Soft, non-tender, bowel sounds normal, no masses, no organomegaly, chevron incision. Gastrojejunostomy tube in place; Extremities: No edema, pulses normal; Skin: Skin color, texture, and turgor are normal.
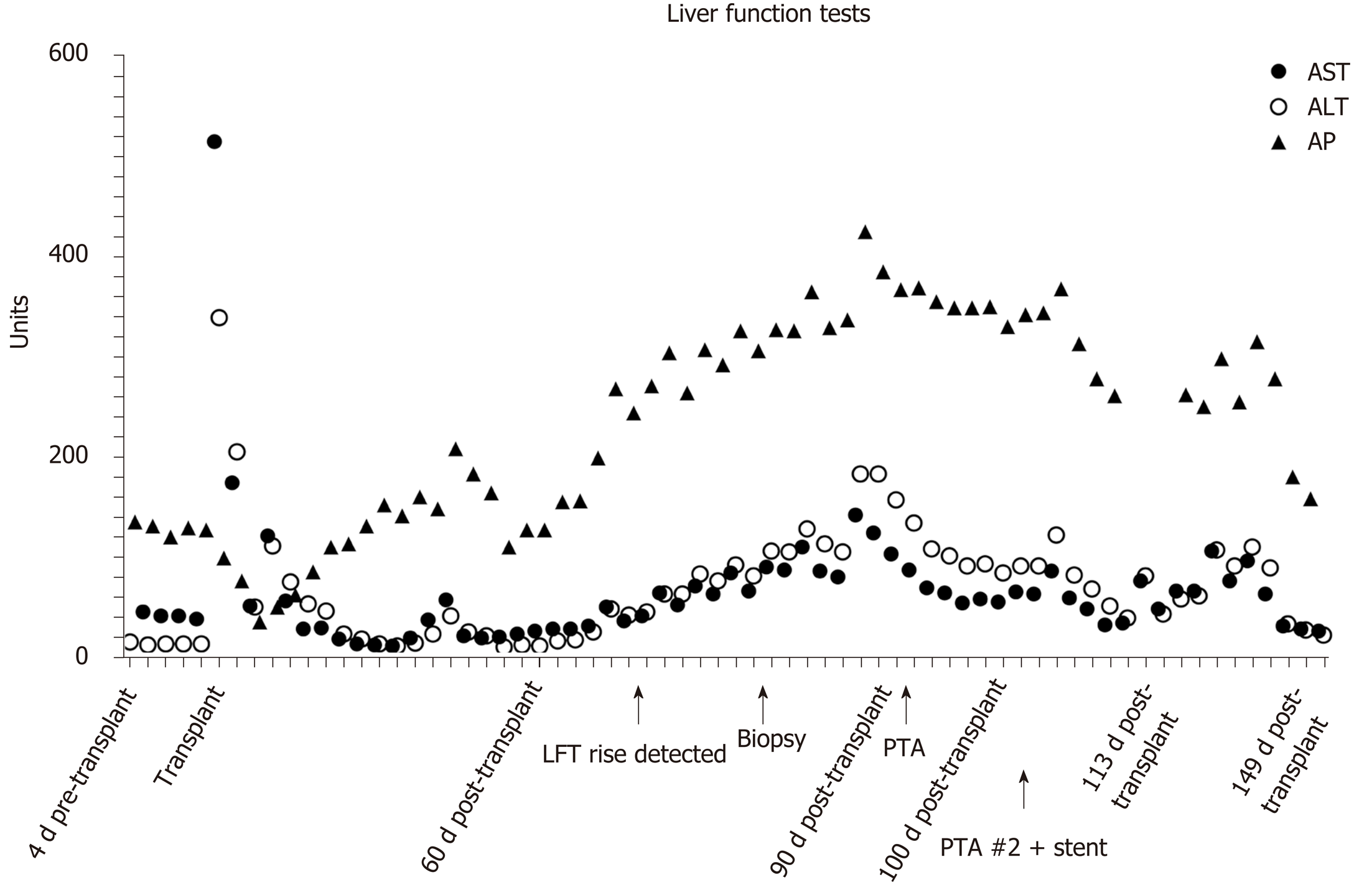
At 69 d post-transplant, the patient’s LFTs became elevated (Figure 1).
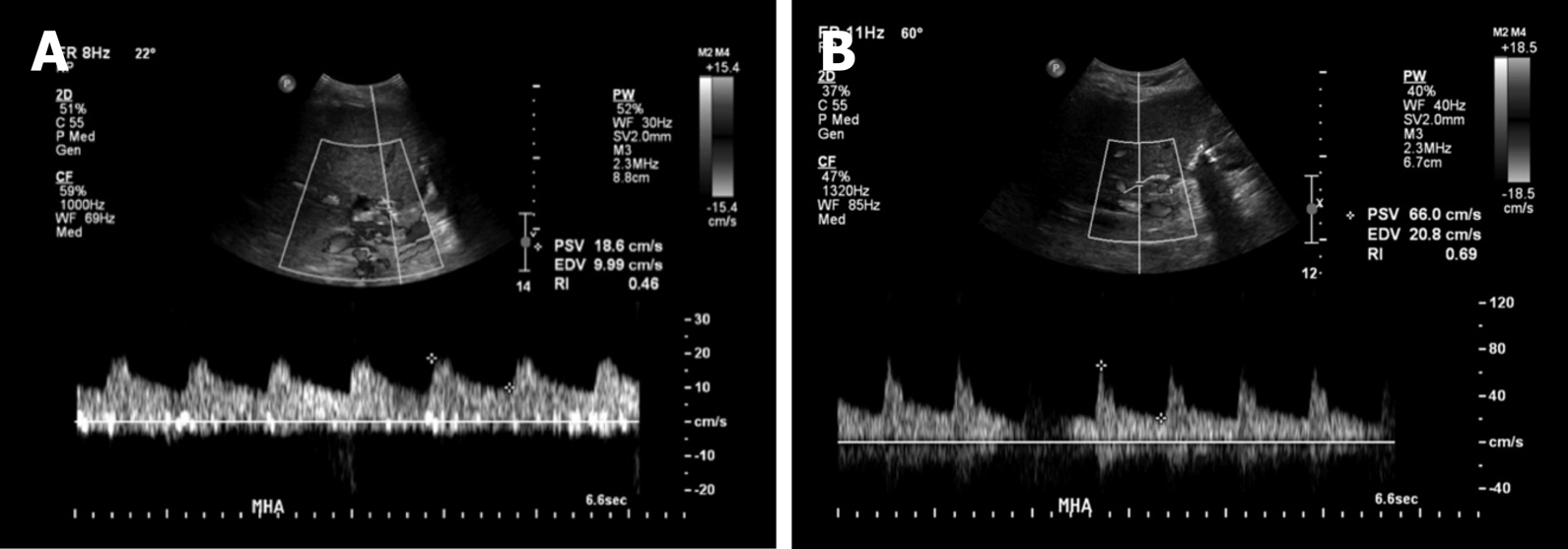
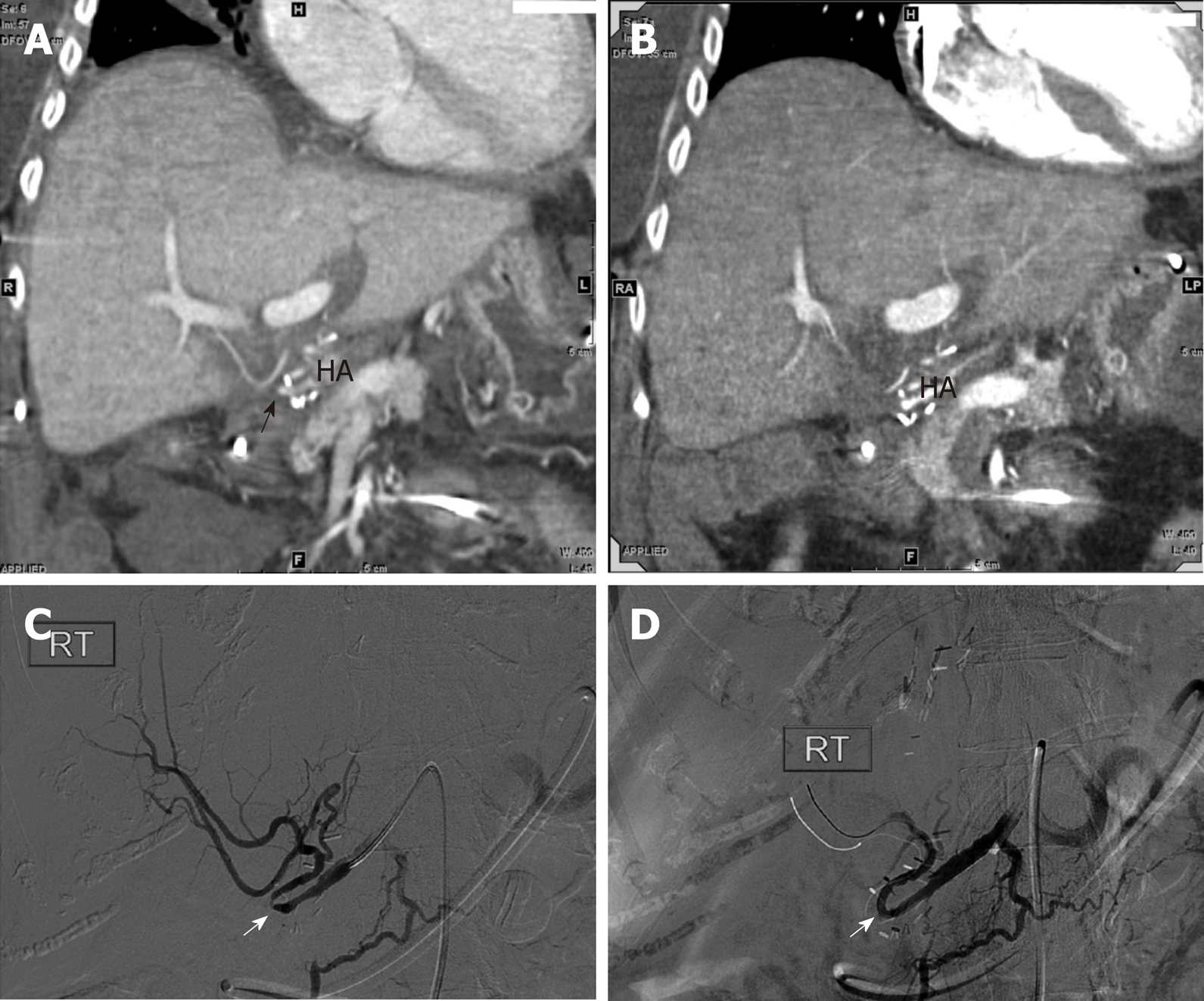
Initial Doppler ultrasound (DUS) showed patent vasculature, but was notable for a subtle parvus et tardus waveform and relatively low resistive indices throughout the hepatic artery, ranging from 0.4 to 0.5 (Figure 2A). A liver biopsy showed no evidence of acute T-cell mediated rejection, biliary obstruction, or ischemic injury. Ultimately, a computed tomography (CT) angiogram of the abdomen and pelvis performed on post-transplant day 90 identified a diminutive and irregular proper hepatic artery, which appeared smaller when compared to 1 mo prior (Figure 3A and B).
History of liver transplant with elevated LFTs and imaging findings concerning for recurrence of HAS.
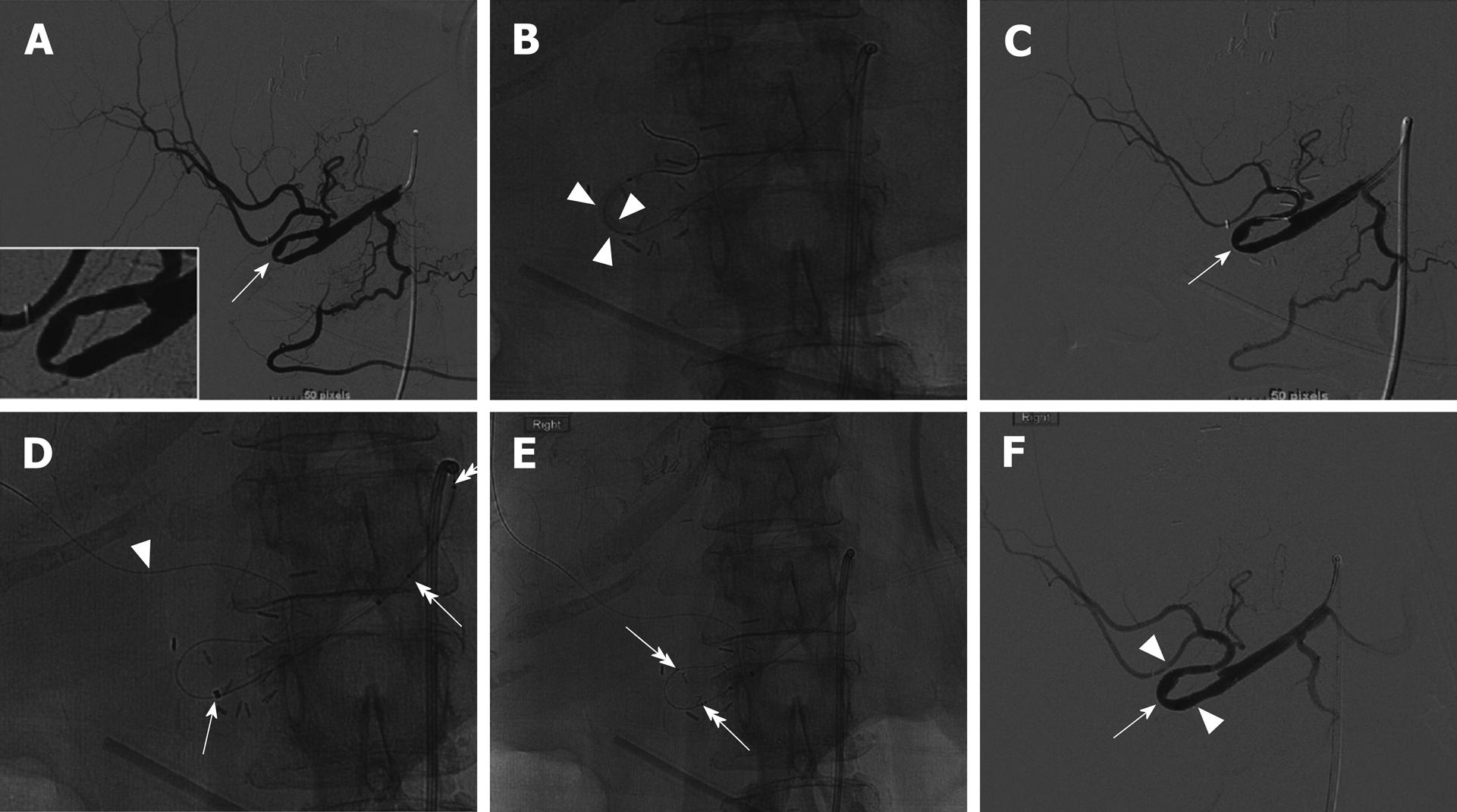
On post-transplant day 91, a hepatic artery angiogram was performed via right common femoral artery access, which revealed an approximately 70% stenosis around a hairpin bend in the hepatic artery. Low-pressure angioplasty using 1:1 sizing was performed using a 3 mm × 20 mm Sterling balloon (Boston Scientific, Marlborough, MA, United States), which relieved the stenosis and improved flow (Figure 3C and D). The patient’s LFTs down-trended from post-transplant days 92 to 99, but then resumed an uptrend (Figure 1), and re-stenosis was suspected. The patient returned to the angiography suite on post-transplant day 100, and angiography from right common femoral access demonstrated recurrence of an approximately70% stenosis around the same hairpin bend (Figure 4A). Repeat PTA was performed using a 3 mm × 20 mm Coyote balloon (Boston Scientific, Marlborough, MA, United States) over a 014 BMW wire (Abbott, Santa Clara, CA, United States) through a 7Fr RDC guide catheter (Terumo, Somerset, NJ, United States). This angioplasty improved the stenosis (Figure 4B and C), but given the failure of the prior angioplasty in the same location, a decision was made to stent the stenotic area. The patient was anticoagulated with 3000 units of IV heparin. Coaxial support proximal to the lesion was achieved by passing a Navien 058 Intracranial Support catheter (Covidien Vascular Therapies, Mansfield, MA, United States) into the hepatic artery through the 7Fr RDC guide catheter. Access across the stenosis was achieved using a 0.014 Synchro Support Pre-Shaped exchange-length guidewire (Stryker, Kalamazoo, MI, United States) and a Prowler Select Plus microcatheter (Codman Neurovascular, Raynham, MI, United States). Due to the small size and tortuosity of the vessel, we considered coronary and neurovascular stents. We chose a 4.5 mm × 20 mm Wingspan neurovascular self-expanding bare metal stent (Stryker, Kalamazoo, MI, United States) to closely approximate the size of the artery proximal and distal to the stenosis. The microcatheter was removed over the microwire, and the Wingspan stent delivery system was advanced across the lesion and deployed (Figure 4D and E). No post-implantation balloon dilation was performed. Post intervention angiogram showed marked improvement in the luminal diameter with brisk flow of contrast through the stent and into the right and left hepatic arteries (Figure 4F).
The patient’s LFTs declined immediately post procedure and have been normal at 2 mo follow-up (Figure 1). Her LFTs remained stable except for a period of 4 d (post-transplant days 114-118) during which she was re-admitted for an unrelated illness. Post procedure the patient was placed on a heparin drip to bridge to warfarin and a single antiplatelet agent (aspirin). DUS performed 1 day and 15 d post-intervention demonstrated improved hepatic arterial waveforms with normal peak systolic velocities and resistive indices (Figure 2B). Two months later, the patient is well and remains on warfarin and aspirin.
Endovascular procedures are now a common option for treatment of HAS in liver transplant patients. The first cases reported in 1989-1990[5,10] usd PTA with good technical success and clinical benefit. However, vessel tortuosity and arterial kinking were identified as significant impediments to intervention because they prevented crossing of the stenosis[5]. Even with more modern techniques and devices, vessel tortuosity remains an important factor in procedure success and clinical outcome. Saad et al[6] showed in a retrospective study of 42 patients undergoing intervention for HAS that the technical success rate for angioplasty in vessels with kinks vs without kinks was substantially lower (14% vs 94%) and the complication rate was higher (29% vs 10%). They recommended avoiding endovascular treatment in kinked vessels and treating instead with surgical revascularization, an opinion which has been echoed by others[7]. In another study, vessel tortuosity prevented technical success in 10% of patients[8]. In the case described here, we encountered a stenosis with significant vessel tortuosity (a tight hairpin bend), which failed initial angioplasty but was successfully treated with neurovascular stent placement.
Stenting in the transplant hepatic artery is described in a number of case studies. A case series of 4 patients treated for HAS (n = 2) and HAT (n = 2) using Wallstents and Palmaz stents (Cordis, Warren, NJ, United States) showed patency at 18-25 mo follow-up with no need for re-transplantation[11]. A retrospective study of a series of 14 patients who received coronary stents for HAS showed 78% stent patency at 12 mo[12]. A separate case report also described the use of a coronary stent for HAS, which was successful at 4 mo follow-up[13]. A single-institution, retrospective study of stenting in 30 HAS patients showed primary patency of 90% at 1 year[7]. A number of larger studies evaluated stenting compared to angioplasty alone. A meta-analysis of 263 liver transplants in 257 patients who underwent 147 PTA and 116 stent placements showed equivalent technical success, complications, patency, survival, and requirement for reintervention. The authors concluded that the use of stents should be based on anatomical considerations such as kinked vessels or in cases where PTA is attempted but results in restenosis shortly thereafter[14]. A single-center retrospective study of 42 patients treated with either PTA alone (n = 17) or primary stenting (n = 25) showed patency at 12 mo of 40% vs 78% and a time to reintervention of 51 d vs 105.8 d, suggesting better outcomes for primary stenting[15]. The long-term outcomes of endovascular treatment were evaluated in a single-center retrospective study of 30 HAS patients treated with PTA + stent (90%) or PTA alone (10%). This study showed restenosis in 33% of all treated patients and combined patency at 12 mo of 68% that was maintained at 5 years[8].
The complications of PTA and stenting in HAS have been well described. A retrospective single-center study in a large transplant center examined 79 patients with HAS who were treated with PTA alone (33%) or stenting (66%). Major complications occurred in 7.5% of cases and consisted of arterial dissection or rupture. Complications were treated endovascularly in 6 of 8 cases, but these patients had a much higher rate of progression to HAT. Severe vessel tortuosity was a risk factor for complications, present in 75% of those with complications, but only in 34.6% of those without complications[2].
Stenting the transplant hepatic artery results in robust clinical outcomes, has an acceptable complication rate, and is recommended especially in tortuous vasculature. However, vessel tortuosity increases the technical challenge of the procedure in several ways: (1) Crossing a stenosis with the stent deployment system is more technically challenging; (2) The stent must be flexible enough to track around tortuous vessels and conform to the vessel tortuosity; and (3) The stent must have adequate radial force to maintain patency of the stenosis. Thus far, all studies in this field have used coronary stents or Wallstents[8,11-15] and some authors recommend balloon-expandable stents for better deployment control[15].
Additional stent options may address the challenges of stent use in tortuous anatomy. In our case, a self-expanding Wingspan neurovascular stent was used. The Wingspan stent has a high radial force and low bending moment[16] compared to other neurovascular stents, which allows it to cross and conform to tortuous vessels but also resist stenotic lesions. The Wingspan is available in a variety of sizes appropriate for this application from 2.5-4.5 mm diameter and 9-20 mm length. The stent diameter is sized to exceed the diameter of a normal patent vessel by 0.5-1 mm[17]. To our knowledge the use of Wingspan stents has not been reported previously in extra-cranial applications. However, there is precedent for using neurointerventional stents in visceral arterial applications with good outcomes[9]. In intracranial applications, the Wingspan stent has been used with good safety and efficacy[18-20].
Post procedure management varies widely between centers but generally includes a course of anti-coagulation and/or anti-platelet therapy and imaging of the treated vessel for assessment of re-stenosis. Recent reports advocate the use of dual-antiplatelet therapy for 3-6 mo[7,15] followed by low dose aspirin indefinitely. For monitoring of patency, one approach has been contrast CT at 3 mo and DUS at 1 d, 1 mo, 6 mo, and yearly thereafter[8].
HAS is an important complication of liver transplantation that is associated with significant morbidity and potential for graft loss. The use of endovascular therapies has significantly reduced the need for surgical revascularization and re-transplantation. Stenting in addition to PTA offers safe, durable outcomes but is technically challenging and prone to complications in tortuous vessels. Our experience with using the Wingspan neurovascular stent that is self-expanding, has good conformability, and adequate radial resistance indicates that this stent could be added to the armamentarium of interventionalists in the setting of a tortuous and stenotic transplant hepatic artery.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Corresponding Author's Membership in Professional Societies: American Society of Interventional Radiology (Fellow), Western Angiographic and Interventional Society (Fellow).
P-Reviewer: Faggioli G, Ren J S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL
| 1. | da Silva RF, Raphe R, Felício HC, Rocha MF, Duca WJ, Arroyo PC, Palini GL, Vasquez AM, Miquelin DG, Reis LF, Silva AA, da Silva RC. Prevalence, treatment, and outcomes of the hepatic artery stenosis after liver transplantation. Transplant Proc. 2008;40:805-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Goldsmith LE, Wiebke K, Seal J, Brinster C, Smith TA, Bazan HA, Sternbergh WC. Complications after endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg. 2017;66:1488-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Vidjak V, Novačić K, Matijević F, Kavur L, Slavica M, Mrzljak A, Filipec-Kanižaj T, Leder NI, Škegro D. Percutaneous Endovascular Treatment for Hepatic Artery Stenosis after Liver Transplantation: The Role of Percutaneous Endovascular Treatment. Pol J Radiol. 2015;80:309-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Hamby BA, Ramirez DE, Loss GE, Bazan HA, Smith TA, Bluth E, Sternbergh WC. Endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg. 2013;57:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Abad J, Hidalgo EG, Cantarero JM, Parga G, Fernandez R, Gomez M, Colina F, Moreno E. Hepatic artery anastomotic stenosis after transplantation: treatment with percutaneous transluminal angioplasty. Radiology. 1989;171:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Saad WE, Davies MG, Sahler L, Lee DE, Patel NC, Kitanosono T, Sasson T, Waldman DL. Hepatic artery stenosis in liver transplant recipients: primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol. 2005;16:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Sarwar A, Chen C, Khwaja K, Malik R, Raven KE, Weinstein JL, Evenson A, Faintuch S, Fisher R, Curry MP, Ahmed M. Primary Stent Placement for Hepatic Artery Stenosis After Liver Transplantation: Improving Primary Patency and Reintervention Rates. Liver Transpl. 2018;24:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Rajakannu M, Awad S, Ciacio O, Pittau G, Adam R, Cunha AS, Castaing D, Samuel D, Lewin M, Cherqui D, Vibert E. Intention-to-treat analysis of percutaneous endovascular treatment of hepatic artery stenosis after orthotopic liver transplantation. Liver Transpl. 2016;22:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Murray TÉ, Brennan P, Maingard JT, Chandra RV, Little DM, Brooks DM, Kok HK, Asadi H, Lee MJ. Treatment of Visceral Artery Aneurysms Using Novel Neurointerventional Devices and Techniques. J Vasc Interv Radiol. 2019;30:1407-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Castaneda F, So SK, Hunter DW, Castaneda-Zuniga WR, Amplatz K. Reversible hepatic transplant ischemia: case report and review of literature. Cardiovasc Intervent Radiol. 1990;13:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Cotroneo AR, Di Stasi C, Cina A, De Gaetano AM, Evangelisti R, Paloni F, Marano G. Stent placement in four patients with hepatic artery stenosis or thrombosis after liver transplantation. J Vasc Interv Radiol. 2002;13:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Huang M, Shan H, Jiang Z, Li Z, Zhu K, Guan S, Qian J, Chen G, Lu M, Yang Y. The use of coronary stent in hepatic artery stenosis after orthotopic liver transplantation. Eur J Radiol. 2006;60:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Shaikh F, Solis J, Bajwa T. Hepatic artery stenosis after liver transplant, managed with percutaneous angioplasty and stent placement. Catheter Cardiovasc Interv. 2007;69:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Rostambeigi N, Hunter D, Duval S, Chinnakotla S, Golzarian J. Stent placement versus angioplasty for hepatic artery stenosis after liver transplant: a meta-analysis of case series. Eur Radiol. 2013;23:1323-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Le L, Terral W, Zea N, Bazan HA, Smith TA, Loss GE, Bluth E, Sternbergh WC. Primary stent placement for hepatic artery stenosis after liver transplantation. J Vasc Surg. 2015;62:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Krischek O, Miloslavski E, Fischer S, Shrivastava S, Henkes H. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg. 2011;54:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Bai WX, Gao BL, Li TX, Wang ZL, Cai DY, Zhu LF, Xue JY, Li ZS. Wingspan stenting can effectively prevent long-term strokes for patients with severe symptomatic atherosclerotic basilar stenosis. Interv Neuroradiol. 2016;22:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Zhao T, Zhu WY, Xiong XY, Li J, Wang L, Ding HY, Wei F, Zhou Y, Gong ZL, Cheng SY, Liu Y, Shuai J, Yang QW. Safety and Efficacy of Wingspan Stenting for Severe Symptomatic Atherosclerotic Stenosis of the Middle Cerebral Artery: Analysis of 278 Continuous Cases. J Stroke Cerebrovasc Dis. 2016;25:2368-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Li TX, Gao BL, Cai DY, Wang ZL, Zhu LF, Xue JY, Bai WX, He YK, Li L. Wingspan Stenting for Severe Symptomatic Intracranial Atherosclerotic Stenosis in 433 Patients Treated at a Single Medical Center. PLoS One. 2015;10:e0139377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Hanel RA, Woo H, Rasmussen PA, Hopkins LN, Masaryk TJ, McDougall CG. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |