Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5849
Peer-review started: May 21, 2020
First decision: May 29, 2020
Revised: June 10, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: October 14, 2020
Processing time: 144 Days and 22 Hours
Liver cirrhosis is a significant source of morbidity and mortality worldwide. The disease is usually indolent and asymptomatic early in its course while many cirrhotic patients are diagnosed late when severe complications occur. A major challenge is to diagnose advanced fibrosis as early as possible, using simple and non-invasive diagnostics tools. Thrombocytopenia represents advanced fibrosis and portal hypertension (HTN) and most non-invasive scores that predict liver fibrosis incorporate platelets as a strong risk factor. However, little is known about the association between longitudinal changes in platelet counts (PTC), when still within the normal range, and the risk of cirrhosis.
To explore whether platelet counts trajectories over time, can predict advanced liver fibrosis across the different etiologies of liver diseases.
A nested case-control study utilizing a large computerized database. Cirrhosis cases (n = 5258) were compared to controls (n = 15744) matched for age and sex at a ratio of 1:3. All participants had multiple laboratory measurements prior to enrollment. We calculated the trends of PTC, liver enzymes, bilirubin, international normalized ratio, albumin and fibrosis scores (fibrosis-4 and aspartate transaminase-to-platelet ratio index) throughout the preceding 20 years prior to cirrhosis diagnosis compared to healthy controls. The association between PTC, cirrhosis complications and fibrosis scores prior to cirrhosis diagnosis was investigated.
The mean age in both groups was 56 (SD 15.8). Cirrhotic patients were more likely to be smokers, diabetic with chronic kidney disease and had a higher prevalence of HTN. The leading cirrhosis etiologies were viral, alcoholic and fatty liver disease. The mean PTC decreased from 240000/μL to 190000/μL up to 15 years prior to cirrhosis diagnosis compared to controls who’s PTC remained stable around the values of 240000/μL. This trend was consistent regardless of sex, cirrhosis etiology and was more pronounced in patients who developed varices and ascites. Compared to controls whose values remained in the normal range, in the cirrhosis group aspartate aminotransferase and alanine aminotransferase, increased from 40 U/L to 75 U/L and FIB-4 increased gradually from 1.3 to 3 prior to cirrhosis diagnosis. In multivariable regression analysis, a decrease of 50 units in PTC was associated with 1.3 times odds of cirrhosis (95%CI 1.25-1.35).
In the preceding years before the diagnosis of cirrhosis, there is a progressive decline in PTC, within the normal range, matched to a gradual increase in fibrosis scores.
Core tip: Cirrhosis is usually asymptomatic thus often diagnosed late when complications occur. Most non-invasive hepatic fibrosis scores (fibrosis-4, aspartate transaminase-to-platelet ratio index) incorporate platelets as a strong risk factor. However, the association between platelets average trends within the normal range and the risk of cirrhosis development is unknown. We found that up to 15 years before the diagnosis of cirrhosis, a progressive decline in platelet counts occurs, within the normal limits along with a gradual incline in FIB-4. The decline in platelet counts may alert of an early liver disease and may enable early therapeutic and preventive interventions before complications occur.
- Citation: Gotlieb N, Schwartz N, Zelber-Sagi S, Chodick G, Shalev V, Shibolet O. Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis. World J Gastroenterol 2020; 26(38): 5849-5862
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5849.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5849
Liver cirrhosis is an important public health concern and a significant cause of morbidity and mortality worldwide. The global prevalence of cirrhosis ranges from 4.5% to 9.5% of the general population and is likely to increase due to the aging of hepatitis C virus (HCV) patients and rise in non-alcoholic fatty liver disease (NAFLD)[1,2]. In 2017, Cirrhosis caused more than 1.32 million deaths globally, compared with less than 899000 deaths in 1990. Most of the cases were secondary to decompensated liver disease[3]. Chronic liver disease (CLD) is usually indolent and asymptomatic early in its course, thus many cirrhotic patients are diagnosed late, when manifestations of portal hypertension (HTN) such as variceal bleeding, ascites or hepatocellular carcinoma (HCC) appear. Early diagnosis of cirrhosis is important in order to enroll patients into HCC surveillance programs and offer therapeutic interventions to halt or reverse disease progression. Nearly 1.5% of patients with cirrhosis remain undiagnosed throughout life, therefore, better diagnostics tools using laboratory and imaging modalities are needed[4].
Patients with advanced liver disease and cirrhosis may present changes in laboratory values such as thrombocytopenia, hypoalbuminemia, abnormal clotting function, anemia, and changes in hepatocellular and cholestatic liver enzymes. Thrombocytopenia (platelet count < 150000/μL) is one of the most common abnormalities in patients with cirrhosis, seen in up to 78% of cirrhotic patients[5]. Thrombocytopenia carries important prognostic information in terms of the presence of cirrhosis, portal hypertensive complications, hepatocellular carcinoma, post-liver resection and the post-transplant course[6]. Indeed, there is a correlation between the degree of thrombocytopenia and the stage and severity of liver disease; severe thrombocytopenia (< 50000/μL) is a poor prognostic factor associated with significant morbidity, indicating an advanced liver disease with established portal HTN[7,8]. This strong association has been corroborated by a study indicating that liver diseases is the underlying cause of thrombocytopenia in 58% of outpatients from all hospital departments[9].
The pathogenesis of thrombocytopenia in CLD and liver cirrhosis is multifactorial. Possible causes include splenic sequestration of platelets, suppression of platelet production in the bone marrow, decreased thrombopoetin production in the liver and an autoimmune mediated destruction[5]. Additionally, platelets actively participate in pathophysiologic processes in the liver, resulting in fibrosis and cirrhosis; previous studies including animal models showed that platelets have a major role in liver inflammation via interactions with the hepatic sinusoidal endothelium and myeloid cells, inducing diverse hepatic processes ranging from liver repair and regeneration to necroinflammation and fibrosis[10,11]. Additionally, studies hypothesized that circulating platelet-neutrophil aggregates can induce neutrophil activation, thus driving end organ damage in patients with cirrhosis. Indeed, various liver diseases are associated with neutrophil recruitment; these include cholestatic liver injury, alcoholic hepatitis, drugs and chemical-induced injury[12].
While the association between thrombocytopenia and cirrhosis is well-established, little is known about the association between subtle changes in platelet counts over time and the long-term risk of cirrhosis development. Few previous studies have shown that platelet counts may start to fall earlier in the course of NAFLD and HCV induced liver diseases[13,14]. Additionally, platelet counts have been incorporated into non-invasive tools for the diagnosis of liver fibrosis and cirrhosis. Among others, these are the aspartate aminotransferase-to-platelet ratio index (APRI), fibrosis-4 (FIB-4) score and NAFLD fibrosis score which are used to assess the presence of liver fibrosis[15,16]. However, the platelet values in the aforementioned scores and studies were taken as a single value at a single time point. No study has tested the association between platelet trends within the normal range and cirrhosis incidence. Current computerized systems allow the collection of big data sets and enable the detection of subtle platelet changes, decades prior to the diagnosis of liver cirrhosis. Subtle trends in laboratory results are now being incorporated in machine learning algorithms which utilize artificial intelligence to generate predictive models more effectively than conventional methods, through detection of hidden patterns within large data sets.
In this study we aimed to explore whether platelet counts trajectories over time can advance the diagnosis of early liver disease and its predictive ability across the different etiologies of cirrhosis, in parallel to different fibrosis scores. In addition, we aimed to test the association between platelets decline and portal HTN complications (variceal bleeding, ascites, hepatic encephalopathy, HCC) among cirrhotic patients.
A nested case-control study with diagnosed cirrhosis patients and matched controls, utilizing the Maccabi Health Services (MHS) database was performed. MHS is a 2.3-million-member state-mandated health services organization, representing 25% of the local population of Israel[17]. MHS's data are automatically collected and include information regarding all diagnoses, comorbidities, hospitalizations, emergency department visits, physician visits, outpatient specialist visits, purchase of medications, laboratory tests and radiologic imaging results. MHS’s database was established and collects data from 1998, with a 99% members’ retention rate which enables a unique opportunity to assess the long-term trends in laboratory results. All biochemical assessments are performed by a single laboratory that maintains a quality management system, as required and using the same standard laboratory methods[18]. The data are automatically and continuously updated, and are not dependent on active reporting by physicians.
Cases included all cirrhotic patients aged 18 to 80 years diagnosed between 2001 and 2018 using the International Classification of Diseases, 9th Revision (ICD-9) codes (Supplementary table 1). The first diagnosis was defined as the index date.
Controls were hepatic disease-free MHS members, matched for age, sex and birth country at a ratio of 1:3. Sampling date in the control group was matched to the cirrhosis diagnosis date. All study patients were required to have at least three PTC measurements prior to index date.
Patients with known etiologies for thrombocytopenia other than cirrhosis (various diseases and medications), as indicated in the medical record, were excluded from analysis (ICD-9 codes in Supplementary Tables 2 and 3).
Cirrhosis etiology (viral, autoimmune/cholestatic, NAFLD) as well as data regarding the complications of cirrhosis and portal HTN (hepatocellular carcinoma, ascites, varices, hepatic encephalopathy, splenomegaly) were all based on ICD-9 codes (Supplementary Table 4). We calculated the longitudinal trends of PTC as well the following laboratory parameters throughout the preceding 20 years prior to cirrhosis diagnosis compared to healthy controls: Complete blood count, bilirubin (total), liver enzymes [aspartate aminotransferase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT) and alkaline phosphatase], coagulation tests [prothrombin time (PT/INR), aPTT] and albumin were recorded (normal ranges are presented in Supplementary Table 5). APRI and FIB-4 were calculated for each patient according to the previously described formulas[15].
The study was approved by the MHS institutional review board (IRB). Since this is a retrospective study in which we used coded (anonymized) administrative data from electronic medical records, exemption from informed consent was granted by the IRB committee.
The statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, United States). Significance was set at P < 0.05. Categorical variables are presented using frequencies and percent. Continuous variables are presented using mean (standard deviation) [median, interquartile range]. The non-parametric locally weighted scatterplot smoothing was used for the presentation of the PTC (as well as other laboratory measurements) throughout 15 years period. For the cirrhosis group, the measurements were prior to the cirrhosis diagnosis and for the control group, the measurements were prior to the sampling year of each individual. Multivariable logistic regression was performed using PROC GENMOD utilizing general estimation equation methodology for correlated data (i.e., several platelets measurements for each subject). The model included the platelets measurements, as well as the time gaps (in years) of each measurement from the diagnosis/sample year for the cirrhosis and control respectively. The number of measurements was also included in the model. Adjusted odds ratio as well as 95%CI were used to display the association between the study groups and the potential risk factors.
Characteristics of study population are presented in Table 1. The mean age in both groups was 56 (SD 15.8) and 54% were females. Most patients (25.7%) were diagnosed with cirrhosis in the years 2009-2012 while the least (14%) were diagnosed earlier between 2001 to 2004. Co-morbid conditions are presented in Table 2. Cases were more likely to be smokers (OR = 1.5; 95%CI: 1.39-1.6) as well as to be diagnosed with diabetes (OR = 1.17; 95%CI: 1.02-1.33), chronic kidney disease (CKD, OR = 1.24; 95%CI: 1.09-1.4) and tended to have higher prevalence of HTN (OR = 1.15; 95%CI: 0.96-1.37).
| Cirrhosis (n = 5258) | Control (n = 15774) | |
| Age (yr) | 55.91 (15.83), (56, 17-99) | 56.04 (16.43), (57, 17-108) |
| 17-30 | 338 (6.43) | 1014 (6.43) |
| 30-40 | 633 (12.04) | 1899 (12.04) |
| 40-50 | 929 (17.67) | 2787 (17.67) |
| 50-60 | 1215 (23.11) | 3645 (23.11) |
| 60-70 | 1124 (21.38) | 3372 (21.38) |
| 70-80 | 731 (13.9) | 2193 (13.9) |
| 80+ | 288 (5.48) | 864 (5.48) |
| Gender | ||
| Female | 2866 (54.51) | 8598 (54.51) |
| Male | 2392 (45.49) | 7176 (45.49) |
| Country of birth | ||
| Africa and Middle East | 273 (5.19) | 819 (5.19) |
| America | 73 (1.39) | 219 (1.39) |
| Asia | 19 (0.36) | 57 (0.36) |
| Europe | 2334 (44.39) | 7002 (44.39) |
| Israel | 2425 (46.12) | 7275 (46.12) |
| Data N/A | 134 (2.55) | 402 (2.55) |
| Diagnosis/sampling year | ||
| 2001-2004 | 732 (13.92) | 2196 (13.92) |
| 2005-2008 | 1263 (24.02) | 3789 (24.02) |
| 2009-2012 | 1351 (25.69) | 4053 (25.69) |
| 2013-2015 | 1068 (20.31) | 3204 (20.31) |
| 2016-2018 | 844 (16.05) | 2532 (16.05) |
| Cirrhosis | Control | OR | 95%CI | ||
| Low | Up | ||||
| Platelets measurements | 15.59 (14.36) | 10.69 (10.33) | 1.033 | 1.031 | 1.036 |
| Platelets measurements: 3-10 | 2510 (47.74) | 10482 (66.45) | 1 | ||
| 11-20 | 1409 (26.8) | 3395 (21.52) | 1.73 | 1.61 | 1.87 |
| 21+ | 1339 (25.47) | 1897 (12.03) | 2.95 | 2.72 | 3.20 |
| Diabetes | 326 (6.2) | 847 (5.37) | 1.17 | 1.02 | 1.33 |
| HTN | 170 (3.23) | 447 (2.83) | 1.15 | 0.96 | 1.37 |
| CKD | 390 (7.42) | 961 (6.01) | 1.24 | 1.09 | 1.4 |
| Dyslipidemia | 915 (17.4) | 2843 (18.02) | 0.96 | 0.88 | 1.04 |
| Smoking | 1563 (29.73) | 3470 (22) | 1.5 | 1.398 | 1.609 |
Of the cases with known etiology (n = 2058), the most common etiology for liver disease was viral infection (48%) followed by alcoholic liver disease (ALD, 24%) and NAFLD (20%). A total of 2768 cases had complications of liver cirrhosis, including splenomegaly (14.5%), varices (10.5%), ascites (8.5%) and hepatic encephalopathy (5.6%). Portal vein thrombosis was documented in 1.3% of cases and 10 patients (0.19%) had HCC (Table 3).
| Frequency | Percent | |
| Etiology | ||
| ALD | 488 | 9.28 |
| Viral | 987 | 18.77 |
| Autoimmune | 159 | 3.02 |
| Wilson | 9 | 0.17 |
| NAFLD | 413 | 7.85 |
| Complications | ||
| Ascites | 450 | 8.56 |
| Varices | 551 | 10.48 |
| HE | 294 | 5.59 |
| HCC | 10 | 0.19 |
| SBP | 118 | 2.24 |
| Portal HTN | 511 | 9.72 |
| Splenomgally | 764 | 14.53 |
| PVT | 70 | 1.33 |
In both groups, the mean time gap between the first PTC and the diagnosis/sampling year was similar (7.57-7.68 years) and the mean number of platelets measurements increased gradually from 2.5 (SD 2.1) to 6.4 (SD 5.7) close to the diagnosis date.
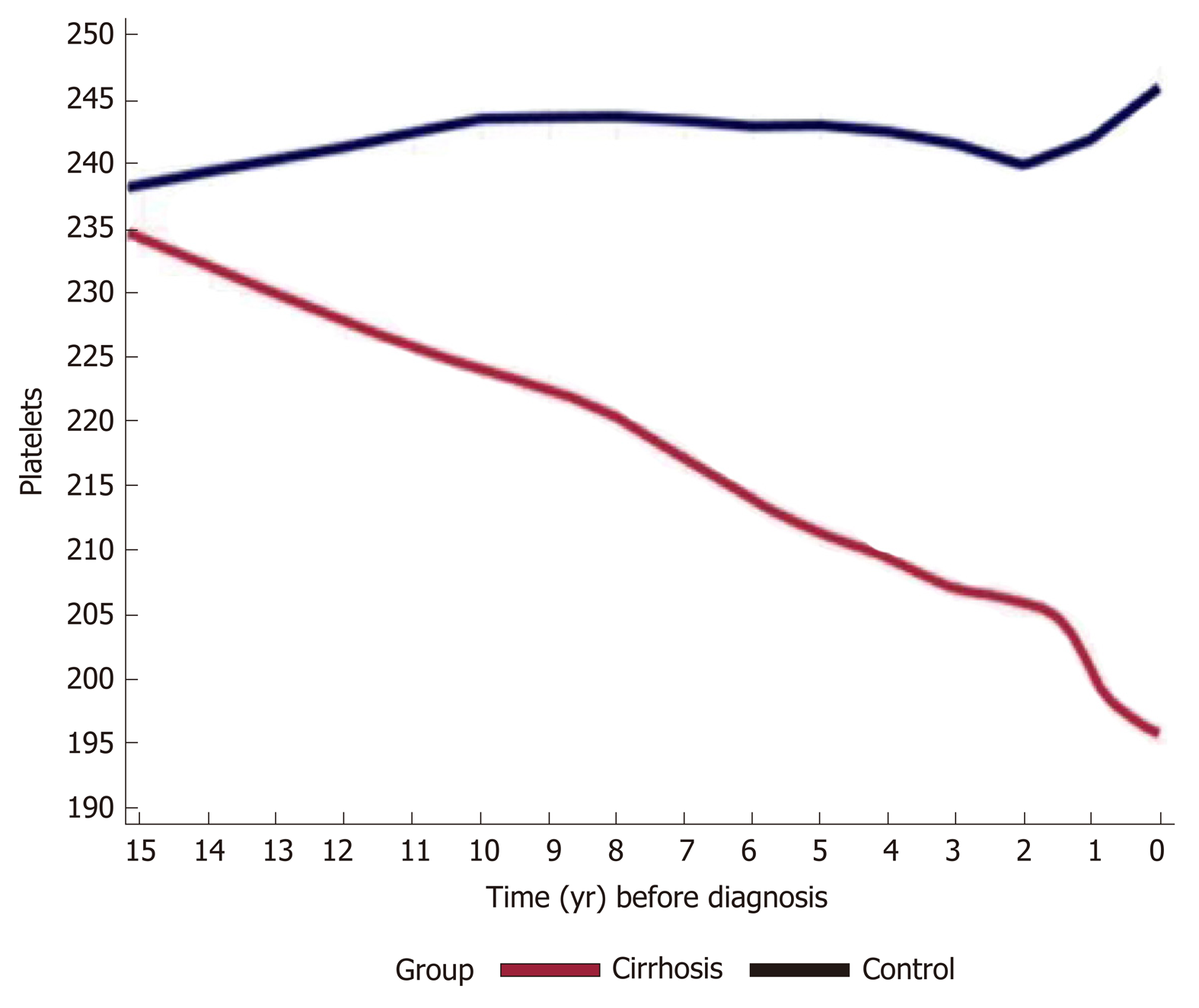
The platelets trends along the study years (total 250646 platelets measurements) stratified by the study groups are presented in Figure 1. The mean PTC in the cirrhosis group decreased from 240000/μL starting 15 years prior to cirrhosis diagnosis to approximately 190000/μL close to the diagnosis date. In the control group, the PTC remained stable throughout the years (240-250000/μL). In addition, for each subject in both groups, the mean PTC was calculated per year; the difference in mean PTC per year between groups is presented in Supplementary Table 6.
Males had lower baseline PTC in both groups (Supplementary figure 1). In the cirrhosis group, males had a mean PTC of 210000 decreasing to 170000/μL vs females, with a mean number of 250000 decreasing to 215000/μL. In the control group, the same pattern was observed: males had a mean PTC of 225-230000/μL compared to females with ranges of 250000/μL. However, despite the sex differences, the trend of gradual decrease in PTC prior the diagnosis of cirrhosis was seen in both sexes. Additional sub-grouping was performed in order to assess whether age had a modifying effect within each sex. Among younger patients (17-40), PTC was constant in both males and females throughout the years, while among older patients, a trend of gradual decrease in PTC was seen in both sexes prior to cirrhosis diagnosis.
In contrast, there was no significant change in hemoglobin levels in both groups (range of 12.8-13.4 g/dL). But, there was a steep decrease in white blood cell (WBC) counts prior the diagnosis of cirrhosis compared to controls, which had an increase of these values (Supplementary figures 2 and 3).
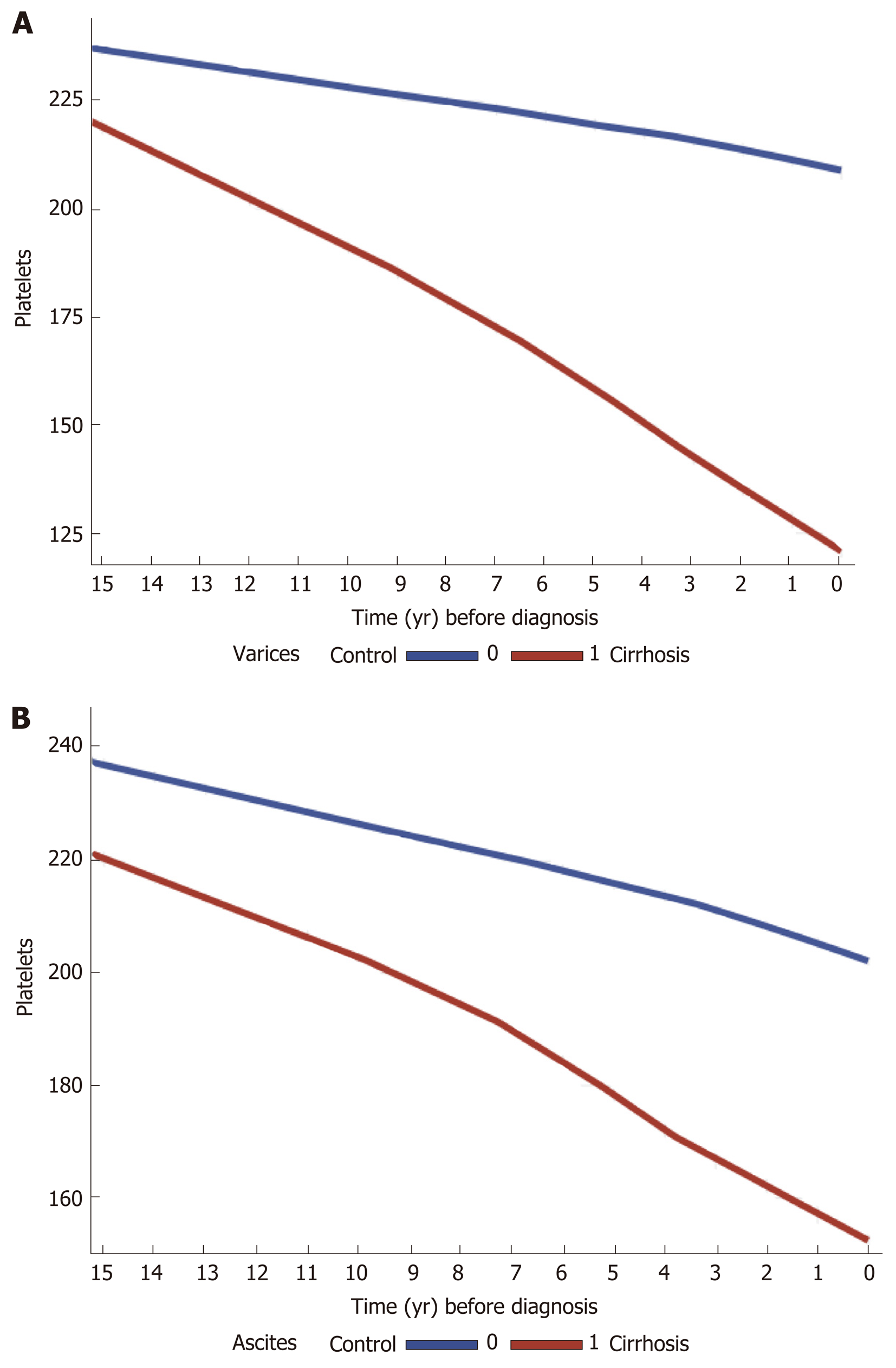
In the cirrhosis group, the trend in PTC was calculated and compared among the most common etiologies of liver cirrhosis in the cohort (viral and ALD). There was a gradual decrease in PTC prior to cirrhosis diagnosis, within the normal ranges in both etiologies. However, cirrhotic patients with ALD had lower mean platelets levels compared to those with viral liver disease, starting 15 years prior to cirrhosis diagnosis (230-180000/μL vs 200-170000/μL) (Supplementary figure 4A and B). Stratification of cases by complications of cirrhosis and portal HTN revealed a steeper decrease in PTC in cirrhotic patients who had esophageal varices, ascites and hepatosplenomegaly compared to cirrhotic patients with no such complications (Figure 2A and B).
The trends of liver enzymes during the years prior to the diagnosis of cirrhosis (or sampling year for the control group) were calculated (Supplementary Figures 5-8). Compared to controls, whose enzymes levels remained stable and within the normal range, there was a gradual increase in both ALT and AST in cirrhotic patients during the 15 years preceding the diagnosis of liver cirrhosis, whose mean levels were both above the normal range: ALT increased from 50 U/L 15 years prior diagnosis to 75 U/L close to the diagnosis date; AST increased from 40 U/L to 70 U/L close to the diagnosis date.
Similarly, a gradual increase in both cholestatic enzymes could be seen during the years prior to the diagnosis of cirrhosis: Alkaline phosphatase increased from 75 U/L 15 years prior diagnosis to 135 U/L close to the diagnosis dates; GGT increased from 60 U/L to 200 U/L close to the diagnosis date. As for the control group, there was a gradual mild increase in both enzymes over the years.
Regarding markers of the synthetic functions of the liver, a gradual increase in bilirubin levels occurred within the normal range, in the cirrhosis group compared to controls. Albumin levels decreased in both groups, but remained within the normal range (Supplementary Figure 9).
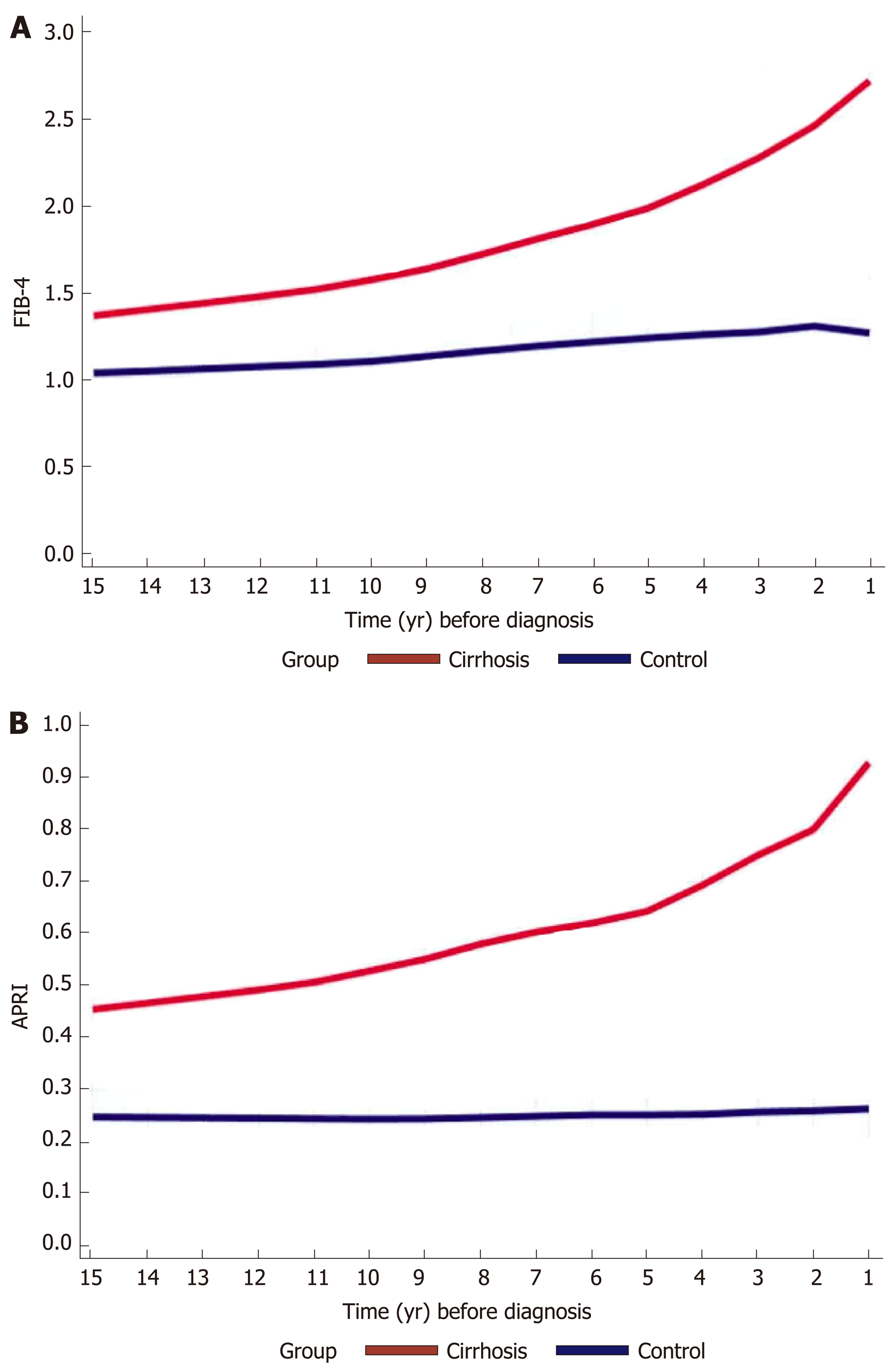
We calculated FIB-4 and APRI for both cirrhotics and controls. In the preceding years before cirrhosis diagnosis, FIB-4 and APRI increased gradually, ranging from 1.3 to 3 and 0.48 to 0.93 respectively, compared to controls whose scores either increased minimally or remained stable respectively throughout the years (Figure 3A and B).
Since cases were matched with controls by age, gender, birth country and time period (i.e. cirrhosis diagnosis year and the sampling year for the control group), these factors were adjusted by selection. After adjusting for the platelet’s measurements time points and the number of measurements, we found that for every 50 units decrease in the platelets, the odds of cirrhosis increase by 1.3-fold (95%CI: 1.25-1.35).
Based on a large, well-characterized cohort, the results of this nested case-control study indicate that liver cirrhosis is characterized by a longitudinal decrement in platelet counts, within the normal limits, that may start 15 years prior to diagnosis. This trend is consistent regardless of sex, etiology of liver disease and observed after the age of 40.
In the cirrhosis group, more patients had diabetes mellitus (DM), CKD, a tendency for HTN and were smokers compared to controls. The relationship between these metabolic factors and CLD or cirrhosis is well established. However, their effect on PTC is unclear. We did not find a clear relationship between the presence of DM or HTN and lower PTC in the literature. The exact pattern of PTC in patients with CKD is controversial but several studies revealed a decrease in PTC and platelets dysfunction in renal failure[19-21]. The trend of PTC decrease was consistent in both sexes although males had generally lower PTC values compared to females among both cases and controls. The literature on thrombocytopenia in liver disease does not show clear gender predominance so we can assume that this difference, in both groups, is physiological[22-25].
We compared other laboratory parameters that are related to CLD and portal HTN and examined the trends in the years prior to the diagnosis of cirrhosis. ALT, AST and GGT where above the upper normal limit (UNL) in the years preceding the diagnosis of cirrhosis, markedly so in the last two years before diagnosis, compared to controls in which liver enzymes were in the normal range. In both groups, there was a slight gradual increase in bilirubin and a slight decrease in albumin. These laboratory changes are probably physiological; several studies show small but incremental increases in bilirubin and a fall in serum albumin concentration that occurs with increasing age[26-29]. There was no change in the hemoglobin levels, while WBC decreased in cirrhotics compared to controls.
Due to the nature of the study we cannot ascertain why specific blood tests were taken and if liver disease was suspected by the treating physician which triggered more laboratory testing. Compared to the liver enzymes that were above to UNL and should have theoretically alerted the treating physician to the presence of liver disease, at any given time or visit, the PTC during this period decreased within the normal range and were thus easy to miss.
The most common etiologies for cirrhosis in our cohort were viral hepatitis, ALD and NAFLD. The trend of decrease in PTC in our study was consistent regardless the etiology of liver cirrhosis. It was previously suggested, that different etiologies may be associated with different hepatic damage mechanisms relating to platelets which play a role in the induction of hepatic fibrosis[30,31]. A previous study in NAFLD, showed that PTC were decreased in cirrhotics over a 5 year follow up compared to healthy controls[14]. Additionally, a negative correlation between the PTC and the severity of liver fibrosis in NAFLD patients has been demonstrated; a linear decrease of the PTC was correlated with increasing histological fibrosis stage. A similar trend occurs in chronic HBV and HCV infection; previous studies showed that liver fibrosis in HCV patients, assessed by biomarkers and FibroScan®, was negatively correlated with PTC. Moreover, patients with advanced liver fibrosis had significantly lower PTC[32-36].
The platelets decrease trend in our study was most pronounced in patients with complications of liver cirrhosis and portal HTN with the steepest decline in patients who had varices. Studies show that together with liver and spleen stiffness measurements, PTC correlates with significant portal HTN and particularly in the presence of varices. The risk of having varices increases with decreasing PTC and have been used to assess the presence of varices non-invasively[37-40].
Our results suggest for the first time that the platelets trends can be used for the prediction of liver cirrhosis regardless the underlying etiology. In multivariate regression analysis, we found that for every 50 units decrease in the PTC, the odds of cirrhosis were 1.3 times higher. When looking at the normal range of PTC, a gradual decrease in PTC, still within the normal range, from 380 to 180 over time would signify 5.2- fold increase in the risk of being diagnosed with cirrhosis compared to individuals with no change in the PTC. Indeed, the progression to cirrhosis usually takes years to develop and may be missed due to lack of clinical symptoms or laboratory aberrations before significant portal HTN appears.
Diagnosis and staging of liver fibrosis are vital part of the clinical management of CLD of any etiology as it is associated with poor outcomes. Although liver biopsy is recommended as the gold standard for the diagnosis and staging of fibrosis, due to its invasive nature and other disadvantages, indirect assessments of liver fibrosis have been developed and are widely used. These include blood-based biomarkers (APRI, FIB-4, enhanced liver fibrosis, Fibro Test) and image-based techniques (US, transient elastography, shear wave elastography, Magnetic resonance elastography) as well as innovative methods that uses combined modalities including advanced magnetic resonance imaging sequences like diffusion-weighted magnetic resonance imaging and genetic testing[41,42].
Fibrosis risk scores have been developed based on readily available clinical and laboratory parameters that are simple to use at point of care, and can be implemented into computerized medical systems. However, current risk scores have several limitations; they incorporate PTC in the formulation, however, they do not consider progressive, longitudinal changes in PTC and use a single platelets value each time they are used[15,16,43-45].
Among others, FIB-4 have been most extensively studied and validated in diverse populations for the prediction of advanced fibrosis. Two cut-off values were defined; FIB-4 score ≤ 1.3 can be regarded as having a low risk for advanced fibrosis while score > 3.25 represents advanced fibrosis or cirrhosis. It was published previously, that intermediate FIB-4 values of 1.45–3.25 have negative predictive value of 89% for excluding advanced fibrosis and patients in this range would require a liver biopsy to assess the fibrosis stage. Thirty to forty percent of patients have an indeterminate score, and in these cases, additional testing is needed[35,46].
In our study, we show that along with the increase in AST (above the ULN) and age, a longitudinal PTC decrease before the diagnosis of cirrhosis, still within the normal range was associated in high FIB-4 and APRI scores, which were mostly in the range of 1.4-3.25, reaching values compatible with advanced fibrosis. Together with the intermediated values of FIB-4, the longitudinal PTC decrease, even within the normal levels, may reflect progressing fibrosis and can predict cirrhosis development. These combined changes may be picked up by computers and alert the treating physician of an ongoing liver disease before advanced fibrosis takes place, enabling therapeutic and preventing measures.
We acknowledge several limitations of this study. The main limitation is the retrospective nature of the study, with its built-in weaknesses of data collection and selection bias. Due to the nature of the study, we could not know why specific data was ordered/collected; especially which circumstances have led to the diagnosis of cirrhosis. Additionally, clinical events may have been missed or only partly followed up, so that the diagnosis of cirrhosis could have been missed or not recorded. Additionally, we could not look at radiology or endoscopic results of each patient in order to identify signs of CLD, cirrhosis and portal HTN. All diagnoses were made exclusively according to ICD-9 codes. However, although we might have missed a large number of undiagnosed cirrhotic patients, our sample is large and representative enough to offer sound observations.
The number of platelets measurements and distribution in the preceding years before cirrhosis/sampling date was not equal. Cirrhotic patients had more PTC generally with the highest platelets measurements close to the diagnosis date. We hypothesize that there was a recognizable change in the medical condition of the patient which lead to more frequent tests. Due to the nature of this study, this information is not available. However, adjustment for the number and timing of testing did not attenuate the association.
Other limitations should also be noted. A relatively large proportion of patients in the cirrhosis group had missing data regarding the etiology of cirrhosis. However, this should not have an effect on the general observation.
This study also holds important strengths. We present longitudinal changes in PTC, compared to previous studies in which PTC were presented as a single measurement in a certain point of time. By using continuous and repeated measurements for the same individual, this method represented dynamic changes in laboratory data that indicated a trend before the diagnosis of cirrhosis. This method could potentially be used for longitudinal assessment of fibrosis regression following therapeutic interventions such as antiviral therapy or life style changes for NAFLD.
The recent interest in Big Data Mining, which is aimed at identifying patterns that are often unrecognizable during routine clinical management, enabled us to use the MHS database which offers high-quality data from electronic medical records, automatic data capture, and a central laboratory. The large number of members in this insurance group enabled the inclusion of a large study population both overall and in matched groups during a long period of time. Patients with various diseases (hematological/viral etc) and medications that could affect the PCT were excluded from the study so that the change in PTC could be attributed with high probability to the ongoing liver disease. Furthermore, our cohort represents a cohort of cirrhotic patients in a community and likely avoids selection bias seen in cohorts from tertiary referral centers.
Years before the diagnosis of liver cirrhosis is made there is a progressive decline in platelet counts, within the normal range, matched to a gradual increase in fibrosis scores. These changes may be identified by machine learning algorithms and alert the treating physicians of an early liver disease and may enable early therapeutic and preventive interventions before serious complications occur.
Liver cirrhosis is usually asymptomatic early in its course. Many cirrhotic patients are diagnosed late when severe complications occur. A major challenge is to diagnose advanced fibrosis as early as possible, using simple and non-invasive diagnostics tools. Thrombocytopenia (platelet count < 150000/μL) on the background of chronic liver disease of any etiology represents advanced fibrosis and portal hypertension. As such, platelets have been incorporated in most non-invasive scores that predict liver fibrosis as a strong risk factor.
As opposed to thrombocytopenia which is associated with advanced fibrosis, little is known about the association between longitudinal changes in platelet counts (PTC), when still within the normal range, and the risk of cirrhosis.
To explore whether big data analysis of PTC trajectories over time, can predict advanced liver fibrosis and cirrhosis complications across the different etiologies of liver diseases.
A nested case-control study with diagnosed cirrhosis patients and matched controls, utilizing the Maccabi Health Services database was performed. The trends of PTC, liver enzymes, bilirubin, international normalized ratio, albumin and fibrosis scores [fibrosis-4 (FIB-4) and aspartate transaminase-to-platelet ratio index] throughout the preceding 20 years prior to cirrhosis diagnosis were calculated and compared to healthy controls. The association between PTC, cirrhosis complications and fibrosis scores prior to cirrhosis diagnosis was investigated.
Cirrhosis cases (n = 5258) were compared to controls (n = 15744) matched for age and sex at a ratio of 1:3. The leading cirrhosis etiologies were viral, alcoholic and fatty liver disease. The mean PTC decreased from 240000/μL to 190000/μL up to 15 years prior to cirrhosis diagnosis compared to controls who’s PTC remained stable around the values of 240000/μL. This trend was consistent regardless of sex, cirrhosis etiology and was more pronounced in patients who developed varices and ascites. Compared to controls whose values remained in the normal range, in the cirrhosis FIB-4 increased gradually from 1.3 to 3 prior to cirrhosis diagnosis. Additionally, in multivariable regression analysis, a decrease of 50 units in PTC was associated with 1.3 times odds of cirrhosis (95%CI: 1.25-1.35).
This study indicates that a progressive decline in platelet counts, within the normal range, is associated with a gradual increase in fibrosis scores, starting up to 15 years before the diagnosis of cirrhosis.
Progressive PTC decline in the preceding years before the diagnosis of liver cirrhosis, when still within the normal limits, may be identified by machine learning algorithms and alert the treating physicians of an early liver disease and may enable early therapeutic and preventive interventions before serious complications occur.
The author is grateful to the staffs in Maccabi Health Services for their valuable assistance with this work.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Israel
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd El-Razek A, Hunasanahalli Giriyappa V S-Editor: Zhang H L-Editor: A P-Editor: Zhang YL
| 1. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 503] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 2. | Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733-746, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1080] [Cited by in RCA: 1008] [Article Influence: 201.6] [Reference Citation Analysis (4)] |
| 4. | Graudal N, Leth P, Mårbjerg L, Galløe AM. Characteristics of cirrhosis undiagnosed during life: a comparative analysis of 73 undiagnosed cases and 149 diagnosed cases of cirrhosis, detected in 4929 consecutive autopsies. J Intern Med. 1991;230:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Giannini EG. Review article: thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006;23:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Sigal SH, Sherman Z, Jesudian A. Clinical Implications of Thrombocytopenia for the Cirrhotic Patient. Hepat Med. 2020;12:49-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 8. | Poordad F. Review article: thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther. 2007;26 Suppl 1:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Hancox SH, Smith BC. Liver disease as a cause of thrombocytopenia. QJM. 2013;106:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Ramadori P, Klag T, Malek NP, Heikenwalder M. Platelets in chronic liver disease, from bench to bedside. JHEP Rep. 2019;1:448-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Chauhan A, Adams DH, Watson SP, Lalor PF. Platelets: No longer bystanders in liver disease. Hepatology. 2016;64:1774-1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Sturgeon JP, Manakkat Vijay GK, Ryan J, Bernal W, Shawcross DL. Could abnormal neutrophil-platelet interactions and complex formation contribute to oxidative stress and organ failure in cirrhosis? Hepatology. 2015;62:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Dai CY, Ho CK, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Yu ML, Chuang WL. Hepatitis C virus viremia and low platelet count: a study in a hepatitis B & C endemic area in Taiwan. J Hepatol. 2010;52:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Liu F, Zhou H, Cao L, Guo Z, Dong C, Yu L, Wang Y, Liu C, Qiu J, Xue Y, Liu X, Xu Y. Risk of reduced platelet counts in patients with nonalcoholic fatty liver disease (NAFLD): a prospective cohort study. Lipids Health Dis. 2018;17:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 681] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 16. | Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, Janssen HL, Lampertico P, Lau D, Bornstein JD, Schall RE, Dinh P, Yee LJ, Martins EB, Lim SG, Loomba R, Petersen J, Buti M, Marcellin P. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Chodick G, Epstein S, Shalev V. Secular trends in testosterone- findings from a large state-mandate care provider. Reprod Biol Endocrinol. 2020;18:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Fund N, Ash N, Porath A, Shalev V, Koren G. Comparison of Mortality and Comorbidity Rates Between Holocaust Survivors and Individuals in the General Population in Israel. JAMA Netw Open. 2019;2:e186643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Gafter U, Bessler H, Malachi T, Zevin D, Djaldetti M, Levi J. Platelet count and thrombopoietic activity in patients with chronic renal failure. Nephron. 1987;45:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Chaban R, Cole P, Naito K. Simulated septal deviations. Arch Otolaryngol Head Neck Surg. 1988;114:413-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Lambert MP. Platelets in liver and renal disease. Hematology Am Soc Hematol Educ Program. 2016;2016:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Eicher JD, Lettre G, Johnson AD. The genetics of platelet count and volume in humans. Platelets. 2018;29:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Bonaccio M, Di Castelnuovo A, Costanzo S, De Curtis A, Donati MB, Cerletti C, de Gaetano G, Iacoviello L; Moli-sani Investigators. Age- and sex-based ranges of platelet count and cause-specific mortality risk in an adult general population: prospective findings from the Moli-sani study. Platelets. 2018;29:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006;16:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Biino G, Gasparini P, D'Adamo P, Ciullo M, Nutile T, Toniolo D, Sala C, Minelli C, Gögele M, Balduini CL. Influence of age, sex and ethnicity on platelet count in five Italian geographic isolates: mild thrombocytopenia may be physiological. Br J Haematol. 2012;157:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Rosenthal P, Pincus M, Fink D. Sex- and age-related differences in bilirubin concentrations in serum. Clin Chem. 1984;30:1380-1382. [PubMed] |
| 27. | Boland BS, Dong MH, Bettencourt R, Barrett-Connor E, Loomba R. Association of serum bilirubin with aging and mortality. J Clin Exp Hepatol. 2014;4:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Gom I, Fukushima H, Shiraki M, Miwa Y, Ando T, Takai K, Moriwaki H. Relationship between serum albumin level and aging in community-dwelling self-supported elderly population. J Nutr Sci Vitaminol (Tokyo). 2007;53:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Veering BT, Burm AG, Souverijn JH, Serree JM, Spierdijk J. The effect of age on serum concentrations of albumin and alpha 1-acid glycoprotein. Br J Clin Pharmacol. 1990;29:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B, Merkler D, Odermatt B, Bader M, Graf R, Clavien PA, Hegazy AN, Löhning M, Harris NL, Ohashi PS, Hengartner H, Zinkernagel RM, Lang KS. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Iannacone M, Sitia G, Ruggeri ZM, Guidotti LG. HBV pathogenesis in animal models: recent advances on the role of platelets. J Hepatol. 2007;46:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Joo EJ, Chang Y, Yeom JS, Lee YG, Ryu S. Hepatitis B infection is associated with an increased incidence of thrombocytopenia in healthy adults without cirrhosis. J Viral Hepat. 2017;24:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Imajo K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Vinholt PJ, Hvas AM, Nielsen C, Söderström AC, Sprogøe U, Fialla AD, Nybo M. Reduced platelet activation and platelet aggregation in patients with alcoholic liver cirrhosis. Platelets. 2018;29:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Shao LN, Zhang ST, Wang N, Yu WJ, Chen M, Xiao N, Duan Y, Pan LZ, Song WQ, Xia YX, Zhang L, Qi N, Liu M, Zhou SH. Platelet indices significantly correlate with liver fibrosis in HCV-infected patients. PLoS One. 2020;15:e0227544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Petta S, Sebastiani G, Bugianesi E, Viganò M, Wong VW, Berzigotti A, Fracanzani AL, Anstee QM, Marra F, Barbara M, Calvaruso V, Cammà C, Di Marco V, Craxì A, de Ledinghen V. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 38. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2291] [Article Influence: 229.1] [Reference Citation Analysis (3)] |
| 39. | Marot A, Trépo E, Doerig C, Schoepfer A, Moreno C, Deltenre P. Liver stiffness and platelet count for identifying patients with compensated liver disease at low risk of variceal bleeding. Liver Int. 2017;37:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Razek AA, Massoud SM, Azziz MR, El-Bendary MM, Zalata K, Motawea EM. Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging. 2015;40:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Altamirano J, Qi Q, Choudhry S, Abdallah M, Singal AK, Humar A, Bataller R, Borhani AA, Duarte-Rojo A. Non-invasive diagnosis: non-alcoholic fatty liver disease and alcoholic liver disease. Transl Gastroenterol Hepatol. 2020;5:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Besheer T, Elalfy H, Abd El-Maksoud M, Abd El-Razek A, Taman S, Zalata K, Elkashef W, Zaghloul H, Elshahawy H, Raafat D, Elemshaty W, Elsayed E, El-Gilany AH, El-Bendary M. Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J Gastroenterol. 2019;25:1366-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2:100067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 44. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3561] [Article Influence: 187.4] [Reference Citation Analysis (0)] |
| 45. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3245] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 46. | de Oliveira AC, El-Bacha I, Vianna MV, Parise ER. Utility and limitations of APRI and FIB4 to predict staging in a cohort of nonselected outpatients with hepatitis C. Ann Hepatol. 2016;15:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |