Published online Sep 28, 2020. doi: 10.3748/wjg.v26.i36.5484
Peer-review started: June 12, 2020
First decision: July 25, 2020
Revised: July 27, 2020
Accepted: September 4, 2020
Article in press: September 4, 2020
Published online: September 28, 2020
Processing time: 103 Days and 19 Hours
Recently, a technique has been developed to use magnetic resonance enterography (MRE) for the evaluation of small bowel motility. The hypothesis was that assessment of the motility index (MI) should reflect differences in motility between clinical conditions.
To aim of the present observational, cross-sectional study was to evaluate the use of the MI in daily clinical practice.
All consecutive patients aged 18-70 years who were referred for MRE at the Department of Radiology during a 2-year period were asked to participate. Healthy volunteers were included as controls. MRE was prepared and conducted in accordance with clinical routines. On the day of examination, all the participants had to complete the visual analog scale for irritable bowel syndrome (IBS) and IBS-symptom severity scale. Maps of MI were calculated from dynamic MR images. ANOVA was used to evaluate differences in MI between groups, classified as healthy, Crohn’s disease, ulcerative colitis, IBS, other assorted disorders and dysmotility. Logistic and linear regression were applied to the MI values. All medical records were scrutinized for medical history.
In all, 224 examinations were included (inclusion prevalence 76.3%), with 22 controls and 202 patients. There was a significant difference in the MI of the jejunum (P = 0.021) and terminal ileum (P = 0.007) between the different groups. The MI was inversely associated with the mural thickness of the terminal ileum in men (P < 0.001) and women (P = 0.063) after adjustments, and tended to be lower in men than in women (P = 0.056). Subjectively observed reduction of motility on MRI was accomplished by reduced MI of terminal ileum in men (P < 0.001) and women (P = 0.030). In women, diarrhea was inversely associated with the MI of the jejunum (P = 0.029), and constipation was positively associated with the MI of the terminal ileum (P = 0.039).
Although MIs differ across diseases, a lower MI of the terminal ileum is mainly associated with male sex and an increased mural thickness. Symptoms are weakly associated with the MI.
Core Tip: Motility index (MI) of the terminal ileum measured by magnetic resonance enterography (MRE) was inversely associated with mural thickness, especially in men. The tendency of lower MI in men than in women, could possibly be explained by higher weight in men. In women, diarrhea was inversely associated with MI of jejunum, and constipation was positively associated with MI of terminal ileum. There were differences in MI of jejunum and terminal ileum between healthy controls and other assorted diseases. MIs in the jejunum and ileum revealed few findings. It seems essential to develop additional techniques to identify true motility patterns according to MRE.
- Citation: Månsson S, Ekberg O, Ohlsson B. Motility index measured by magnetic resonance enterography is associated with sex and mural thickness. World J Gastroenterol 2020; 26(36): 5484-5497
- URL: https://www.wjgnet.com/1007-9327/full/v26/i36/5484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i36.5484
Symptoms in the gastrointestinal tract are nonspecific and independent of the etiology of the disease. Magnetic resonance enterography (MRE) is therefore often used in daily clinical practice to diagnose or exclude the presence of structural lesions related to inflammatory bowel disease (IBD) in patients with gastrointestinal symptoms[1]. Dysmotility of the small bowel may cause similar symptoms as IBD, but is difficult to diagnose since methods to analyze motility are expensive, not easily available and invasive. In the absence of proper investigation, patients with gastrointestinal dysmotility and pain are therefore often diagnosed with irritable bowel syndrome (IBS) and treated with dietary advice and psychological treatment when no structural findings are present on MRE[2,3].
Different techniques have recently been developed to use MR imaging (MRI) for the evaluation and quantification of motility[4]. The motility index (MI) is a quantifier based on displacement mapping. A decreased MI has been suggested to reflect reduced motility in patients with active Crohn’s disease (CD), due to inflammatory activity[5-8]. We previously performed a retrospective MRI study, and the findings revealed a lower MI of the terminal ileum of patients with small bowel CD than in healthy controls[9]. We confirmed this in a cross-sectional study, where we showed that the MIs in the ileum and terminal ileum differed between healthy controls and patients with active CD; we also found an inverse correlation between mural thickness and the MI[10].
However, there is a need for improved diagnostic tools to correctly diagnose patients with enteric neuromuscular pathology (independent of inflammatory activity) at an earlier stage to be able to provide appropriate health care[11] rather than having these patients wait for several years until a correct diagnosis is made[12].
Our hypothesis was that the MI could be used to identify enteric neuromuscular pathology in patients with gastrointestinal symptoms. To test this hypothesis, we consecutively included all patients undergoing MRE during a 2-year period and evaluated the use of MRI to assess motility in daily practice. On the day of examination, each participant had to complete the validated visual analog scale for IBS (VAS-IBS) and IBS-symptom severity scale (IBS-SSS). Medical records were examined to collect data about medical history, examinations and final diagnoses. The aim of the present study was to evaluate the usefulness and potential of the MI in a large cohort of unselected patients and healthy controls to examine whether the MI could be helpful to identify altered motility patterns in addition to morphological MRE changes and clinical characteristics. In particular, the aim was to examine the association between the MI and basal characteristics and gastrointestinal symptoms independent of the diagnosis.
This study was performed in accordance with the Helsinki Declaration and approved by the Ethics Review Board at Lund University (No. 2016/330, date of approval 4 August 2016). All patients and healthy volunteers gave their written informed consent before inclusion.
During a 2-year time period, all consecutive patients who were referred for MRE were invited to participate in the present study. A letter including information about the study and an invitation to participate was sent to the patients, along with the calling to the examination. Both the MRE and the preparation before the examination were performed in accordance with standard clinical routines. At the time point of examination, all subjects were asked to sign the agreement to participate and to complete the VAS-IBS and the IBS-SSS to assess their symptoms. All medical records were examined by a specialist in gastroenterology (BO) to collect information about medical history, including previous examinations and treatments as well as final diagnoses made by the patients´ regular physicians. Healthy volunteers underwent the same procedure regarding MRE and the completion of the questionnaires, but no medical records were checked. Bowel motility was assessed both subjectively by a radiologist and by calculation of MI derived from regions of interest (ROIs) of transferred MRIs.
All consecutive patients aged 18-70 years, referred for MRE at the Department of Radiology, Skåne University Hospital, Malmö, Sweden, between 1 October 2016 and 1 October 2018, were invited to take part in the study. The only exclusion criteria were the inability to understand the Swedish language and contraindications for the MRI examination. All medical records were examined to collect information about medical history, previous examinations, histopathology, laboratory analyses, medical and surgical treatment and final diagnoses made by the patient´s regular physicians after all information was evaluated. CD and ulcerative colitis (UC) were categorized as active diseases when there were signs of actual inflammation on endoscopy or MRE, i.e., increased mural thickness, inflammatory lesions or ulcers, and/or signs of inflammatory activity in blood, plasma or feces. Patients without any signs of active inflammation on endoscopy or MRE and with blood and feces analyses within reference values were classified as inactive disease.
Healthy controls were volunteers who were recruited by means of advertisement (n = 13). These volunteers completed the VAS-IBS and IBS-SSS to confirm that they were healthy and had no gastrointestinal symptoms. A second subset of controls (n = 9) were patients typically seen in the clinic for the investigation of iron deficiency or anemia to exclude gastrointestinal bleeding. If all clinically performed investigations were normal, if there were no signs of gastrointestinal disorder, and if no intestinal symptoms were indicated by the results of the VAS-IBS or IBS-SSS, these patients were classified as healthy controls.
This questionnaire has been validated to assess the most common gastrointestinal symptoms experienced by the respondent during the previous 2 wk[13]. The VAS-IBS has been further validated to assess symptoms over time[14]. The symptoms were measured on a scale from 0-100 mm and include abdominal pain, diarrhea, constipation, bloating and flatulence, vomiting and nausea, psychological well-being and intestinal symptoms’ influence on daily life, where 0 represents a complete lack of problems and 100 represents severe problems. The item assessing psychological well-being has been found to be strongly correlated with the positive and negative aspects of psychological well-being, anxiety in close relations, self-esteem and coping skills[15]. The scales were inverted from the original version[13].
The IBS-SSS consists of four questions answered on VAS, where scores close to 0 mm suggest “no symptoms”, and scores close to 100 mm suggest “severe symptoms”. The questions assessed abdominal pain, abdominal distension, satisfaction with bowel habits and the impact of bowel habits on daily life; in addition, there was a question about the number of days with abdominal pain during the last 10 d. The maximum achievable score is 500. Scores < 75 are classified as normal, scores between 75 and 174 suggest mild disease, scores 175-299 suggest moderate disease and scores ≥ 300 suggest severe disease[16].
MRE was performed on a standard 1.5 Tesla (T) MRI-scanner (Symphony, Siemens, Healthineers, Erlangen, Germany). The patients and healthy volunteers were allowed to eat breakfast and lunch the day before, drinking was allowed during the evening, and fasting was required after midnight. Four Toilax® tablets (Toilax®, bisacodyl, Orion Pharma, Danderyd, Finland) were taken the day before examination, and a Toilax microenema was taken in the morning. At the hospital, the patient drank up to 1800 mL of MovPrep® (MoviPrep®, polyethylene glycol electrolyte solution, Norgine, Solna, Sweden), starting 45 min before the MRI examination. The examination started typically around 10 am. Patients were examined using a standard MRI protocol including T2-weighted imaging, true fast imaging with steady precession (TrueFISP) motility imaging and T1-weighted 2D gradient echo. Before T1-weighted imaging, butylscopolamin (Buscopan®, 20 mg/mL, 40 mg; Sanofi AB, Stockholm, Sweden) was administered given intravenously to paralyze of the small intestine. Then, intravenous gadolinium contrast medium was given at a “double-dose” (Dotarem®, 279.3 mg/mL, 0.2 mmol/kg body weight, Guerbert, Roissy, France). The examination was performed with the patient in the prone position, and with a total duration of approximately 30 min. Healthy volunteers underwent TrueFISP motility imaging and T1-weighted 2D gradient echo, but they did not receive butylscopolamin or gadolinium.
For the evaluation of intestinal motility, coronal TrueFISP images were acquired dynamically during free breathing. Fifty image frames were acquired of an 8-mm-thick slice over 21 s (imaging parameters in Table 1). The whole bowel was covered by using 10-14 slices from the ventral to dorsal direction of the abdomen. A radiologist with > 40 years of experience in the field (OE) who was blinded to the MI subjectively evaluated the visible motility of the duodenum, jejunum, ileum, terminal ileum and colon, using the same dynamic TrueFISP series as used for the calculation of MI. Motility scores ranged from 1-4, where 1 = normal motility, 2 = reduced motility, 3 = akinetic motility (not moving at all) and 4 = increased motility. The maximal thickness of the wall in the different segments of the small bowel and colon was measured (in mm) at maximal bowel lumen distention using electronic calipers on the TrueFISP images.
| Parameters | Values |
| Repetition time | 3.26 ms |
| Echo time | 1.63 ms |
| Pixel size | 2.5 mm × 1.8 mm |
| Slice thickness | 8 mm |
| Flip angle | 50 degrees |
| Parallel imaging acceleration | 2 |
| Number of averages | 1 |
| Number of frames | 50 |
| Time between frames | 409 ms |
| Duration of acquisition | 21 s |
TrueFISP images were retrieved as digital imaging and communications in medicine (DICOM) files from the picture archiving and communication system (PACS), anonymized and transferred to Motilent, London, United Kingdom, for motility analysis.
The motility in the jejunum, ileum and terminal ileum was quantified by using the method described by Menys et al[5]. The dynamic series for each subject were processed with a previously validated registration software designed for the assessment of bowel motility (GIQuant, version 2.0; Motilent)[6]. Briefly, for each slice, the software generates an anatomical reference image and deformation fields describing the displacement of each time frame relative the reference image. The MI, in arbitrary units (au), is then calculated pixel-by-pixel as the standard deviation over time of the deformation fields, with a score of zero representing no motility.
Regions of interest (ROIs) were drawn manually in a blinded manner in the reference image using Osirix Imaging Software for DICOM images (V.4.1.2 32-bit) for Macintosh. One ROI was drawn for each segment of jejunum, ileum and terminal ileum, using the slice that best visualized the given segment. The ROI was placed to include as much of each segment as possible, without including the stomach or colon, and both normal and diseased bowels were included. The terminal ileum was defined as the last 10 cm of the small bowel. For those patients who had undergone ileum resection before MRE, the neoterminal ileum replaced the terminal ileum in the ROI placement. If the quality of the images was poor, or the segment was difficult to visualize, the image was classified as missing value. After drawing each ROI, it was copied from the reference image to the corresponding MI map and the mean and standard deviation within the ROI was noted.
The hypothesis raised was as follows: the assessment of the MI in an unselected cohort of patients with gastrointestinal symptoms could be used to differentiate between diagnoses and identify patients with gastrointestinal dysmotility due to neuromuscular pathology. Statistical calculations were performed in SPSS© for Windows (release 25.0; IBM). The mean values of the MI from the ROIs of jejunum, ileum and terminal ileum were used in the calculations. The MI was normally distributed, whereas almost all other values were not. Therefore, MIs were analyzed using Student’s t-test or ANOVA with post hoc Bonferroni correction, whereas other parameters were analyzed using the Mann-Whitney U-test and Kruskal-Wallis test. Spearman’s correlation test was used to examine correlations, and Fisher’s exact test was used to examine dichotomous variables. Logistic regression was performed with sex as the dependent variable and the MI, weight and logarithmic values of mural thickness as independent variables. Linear regression was used with the MI as the dependent variable and symptoms (categorized into quartiles), weight and logarithmic values of mural thickness as independent variables. Variables that showed statistically significant differences in the unadjusted regression were then calculated together in an adjusted model. There was difference in the MI of the terminal ileum between men and women (P < 0.013). Therefore, calculations were performed both with all subjects together and stratified by sex. Values are presented as the mean ± SD, median (interquartile range), β values and 95% confidence interval (CI) or odds ratio (OR) and 95%CI. P < 0.05 was considered statistically significant. Calculations without statistical significance were not reported in the text.
In total, 279 MRE examinations were performed during the study period. Of these, 213 agreed to participate (inclusion prevalence: 76.3%). Since all subjects were referred for a clinical MRE, they did not have to explain why they did not want to participate in the present observational study. Data were also collected from 13 healthy volunteers, resulting in data from 226 examinations. One patient who was examined twice was excluded due to being younger than 18 years old. Thus, data from 224 examinations were analyzed. A total of 22 participants were controls, including 12 men (54.5%), and the median age in this group was 30.0 years old (25.0-52.6). A total of 200 were patients (two patients examined twice leading to 202 examinations) who had CD (n = 134), IBS (n = 34), UC (n = 15), other assorted diseases (n = 15) or diagnosed dysmotility (n = 4). Eighty-nine patients (66.4%) with CD and 5 patients (33.3%) with UC had an active disease according to clinical examinations at the time point of the MRE. Patients with other assorted diseases had different diseases that were not classified as IBD, IBS or a defined dysmotility disorder (Table 2). The diseases included diabetes mellitus (n = 3), invagination or ileus (n = 3), gastrointestinal stromal tumors (n = 2), Parkinson’s disease (n = 2), obesity surgery (n = 1), Yersinia colitis (n = 1), malrotation (n = 1), esophagus malignancy (n = 1) and sarcoma (n = 1). The dysmotility group included two patients with chronic intestinal pseudo-obstruction, one patient with esophageal and gastric dysmotility and one patient with suspected enteric dysmotility that was under further investigation. The distribution of sex, but not age and weight, differed between the disease groups (Table 2). The mural thickness was 2 (2-2) mm in all disease groups, and differed significantly between CD and all other disease groups in the terminal ileum (data not shown). The mural thickness of the terminal ileum was correlated with weight in men (r = 0.213, P = 0.038) but not in women (r = 0.048, P = 0.664).
| Controls, n = 22 | Crohn’s disease, n = 134 | IBS, n = 34 | Ulcerative colitis, n = 15 | Other, n = 15 | Dysmotility, n = 4 | P value | |
| Men/women | 12 (54.5)/10 | 75 (56.0)/59 | 8 (23.5)/26 | 8 (53.3)/7 | 13 (86.7)/2 | 1 (25.0)/3 | 0.001 |
| (45.5) | (44.0) | (76.5) | (46.7) | (13.3) | (75.0) | ||
| Age (yr) | 30 (25-52) | 35 (28-48) | 34 (24-44) | 35 (25-44) | 45 (30-67) | 43 (37-63) | 0.130 |
| Weight (kg) | 65 (55-89) | 73 (61-85) | 70 (55-84) | 79 (54-90) | 76 (66-93) | 66 (64-70) | 0.639 |
| Missing value | 2 | 4 | 9 | 5 | 2 | ||
| Abdominal pain | 0 (0-0) | 25 (0-51) | 71 (54-82) | 0 (0-35) | 66 (24-80) | 80 (16-99) | < 0.001 |
| Days of pain | 0 (0-0) | 29 (0-71) | 61 (23-100) | 21(0-21) | 36 (7-100) | 50 (0- | < 0.001 |
| Diarrhea | 3 (0-14) | 41 (8-70) | 54 (20-76) | 43 (22-51) | 50 (10-67) | 42 (2-90) | 0.003 |
| Constipation | 5 (0-14) | 12 (0-46) | 37 (4-62) | 20 (0-52) | 50 (7-68) | 90 (74-96) | 0.002 |
| Bloating | 0 (0-7) | 22 (0-47) | 51 (26-63) | 0 (0-38) | 0 (0-46) | 82 (18-99) | < 0.001 |
| Vomiting and Nausea | 2 (0-16) | 6 (0-25) | 25 (2-65) | 0 (0-8) | 18 (3-49) | 75 (65-95) | < 0.001 |
| Flatulence | 6 (0-26) | 46 (25-68) | 63 (48-89) | 23 (8-54) | 21 (5-73) | 76 (66-94) | < 0.001 |
| Satisfaction with bowel habits | 10 (7-20) | 50 (22-76) | 54 (28-80) | 44 (13-57) | 62 (18-71) | 86 (63-100) | < 0.001 |
| Influence on daily life | 5 (0-15) | 51 (25-75) | 77 (58-88) | 50 (36-60) | 49 (22-66) | 100 (80-100) | < 0.001 |
| Psychological well-being | 8 (5-17) | 36 (14-77) | 48 (20-71) | 7 (2-42) | 52 (5-76) | 100 (94- | < 0.001 |
| Total IBS-SSS | 16 (10-72) | 172 (88-301) | 317 (234-351) | 157 (114-194) | 182 (90-313) | 318 (146- | < 0.001 |
| Missing value | 6 | 69 | 7 | 10 | 10 | 2 |
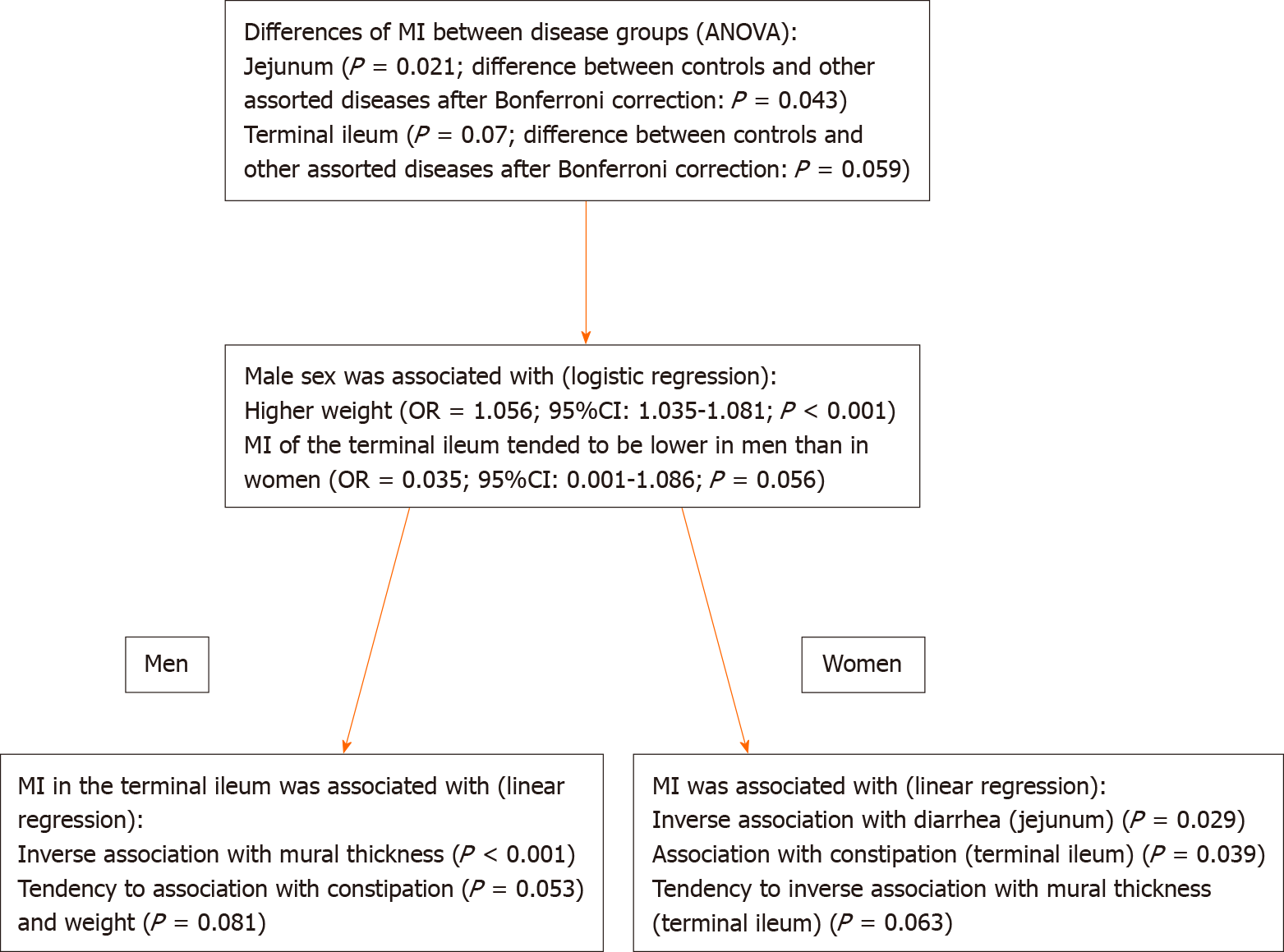
There was a significant difference in the MI of the jejunum and terminal ileum between groups according to ANOVA (Table 3), such that control patients had a higher MI of the jejunum (P = 0.043) and a tendency to higher MI of the terminal ileum (P = 0.059) than patients with other assorted disorders (Figure 1). When stratifying for sex, there were no differences in the MI between the different disease groups (data not shown).
| Controls, n = 22 | Crohn’s disease, n = 134 | IBS, n = 34 | Ulcerative colitis, n = 15 | Other, n = 15 | Dysmotility, n = 4 | P valueANOVA | |
| Jejunum | 0.36 ± 0.08 | 0.32 ± 0.09 | 0.33 ± 0.07 | 0.30 ± 0.10 | 0.27 ± 0.07 | 0.39 ± 0.07 | 0.021 |
| Missing | 1 | 1 | 0 | 0 | 2 | 0 | |
| Men | 0.34 ± 0.10 | 0.34 ± 0.08 | 0.35 ± 0.08 | 0.29 ± 0.09 | 0.27 ± 0.08 | 0.39 ± -#- | |
| Women | 0.37 ± 0.05 | 0.31 ± 0.09 | 0.33 ± 0.06 | 0.30 ± 0.12 | 0.28 ± 0.06 | 0.39 ± 0.09 | |
| P value sex | 0.405 | 0.085 | 0.365 | 0.761 | 0.778 | 0.957 | |
| Ileum | 0.42 ± 0.06 | 0.38 ± 0.09 | 0.40 ± 0.06 | 0.38 ± 0.09 | 0.36 ± 0.08 | 0.39 ± 0.03 | 0.200 |
| Missing | 1 | 1 | 0 | 0 | 2 | 0 | |
| Men | 0.43 ± 0.54 | 0.38 ± 0.09 | 0.37 ± 0.05 | 0.38 ± 0.11 | 0.36 ± 0.08 | 0.36 ± -#- | |
| Women | 0.42 ± 0.06 | 0.37 ± 0.09 | 0.40 ± 0.07 | 0.38 ± 0.06 | 0.41 ± 0.01 | 0.40 ± 0.03 | |
| P value sex | 0.898 | 0.487 | 0.251 | 0.940 | 0.399 | 0.384 | |
| Terminal ileum | 0.34 ± 0.10 | 0.27 ± 0.11 | 0.32 ± 0.09 | 0.32 ± 0.08 | 0.22 ± 0.05 | 0.34 ± 0.02 | 0.007 |
| Missing | 3 | 19 | 2 | 4 | 5 | 0 | |
| Men | 0.31 ± 0.09 | 0.26 ± 0.11 | 0.27 ± 0.07 | 0.35 ± 0.09 | 0.22 ± 0.05 | 0.36 ± -#- | |
| Women | 0.38 ± 0.12 | 0.29 ± 0.11 | 0.33 ± 0.10 | 0.29 ± 0.07 | 0.25 ± -#- | 0.34 ± 0.03 | |
| P value sex | 0.180 | 0.241 | 0.110 | 0.270 | 0.637 | 0.645 |
Subjectively observed reduction of motility of the terminal ileum (n = 46, 20.5%), independent of disease category, was accomplished by reduced MI in both men (0.20 ± 0.09 au vs 0.29 ± 0.10 au, P < 0.001) and women (0.26 ± 0.12 au vs 0.32 ± 0.10 au, P = 0.030) compared with observed normal motility.
There were no differences in the MI between sexes in each disease group separately (Table 3). Among all disease groups, men had higher weights and greater mural thicknesses of the ileum and terminal ileum than women, whereas men had a lower MI of the terminal ileum than women (Table 4).
| Women, n = 107 | Men, n = 117 | P value | |
| Age (yr) | 37.00 (27.00-47.00) | 35.00 (27.50-50.00) | 0.353 |
| Weight (kg) | 66.88 ± 16.84 | 79.93 ± 17.04 | < 0.001 |
| Missing value | 18 | 17 | |
| Mural thickness (mm) | |||
| Jejunum | 2 (2-2) | 2 (2-2) | 0.135 |
| Missing value | 2 | ||
| Ileum Missing value | 2 (2-2) | 2 (2-2) 2 | 0.031 |
| Terminal ileum | 2 (2-2) | 2 (2-4.5) | 0.044 |
| Missing value | 5 | 4 | |
| Motility index (au) | |||
| Jejunum | 0.32 ± 0.08 | 0.33 ± 0.08 | 0.542 |
| Missing value | 1 | 3 | |
| Ileum | 0.39 ± 0.08 | 0.38 ± 0.08 | 0.808 |
| Missing value | 1 | 3 | |
| Terminal ileum | 0.31 ± 0.11 | 0.27 ± 0.10 | 0.013 |
| Missing value | 16 | 7 |
To further examine the differences between sexes, a logistic regression was performed, which showed that male sex was associated with a lower MI of the terminal ileum (P = 0.014) and higher weight (P < 0.001) but not with the mural thickness of the terminal ileum (P = 0.130). After adjustment, male sex remained associated with higher weight (OR = 1.056; 95%CI: 1.035-1.081; P < 0.001) and the MI of the terminal ileum tended to be lower in men than in women (OR = 0.035; 95%CI: 0.001-1.086; P = 0.056) (Figure 1).
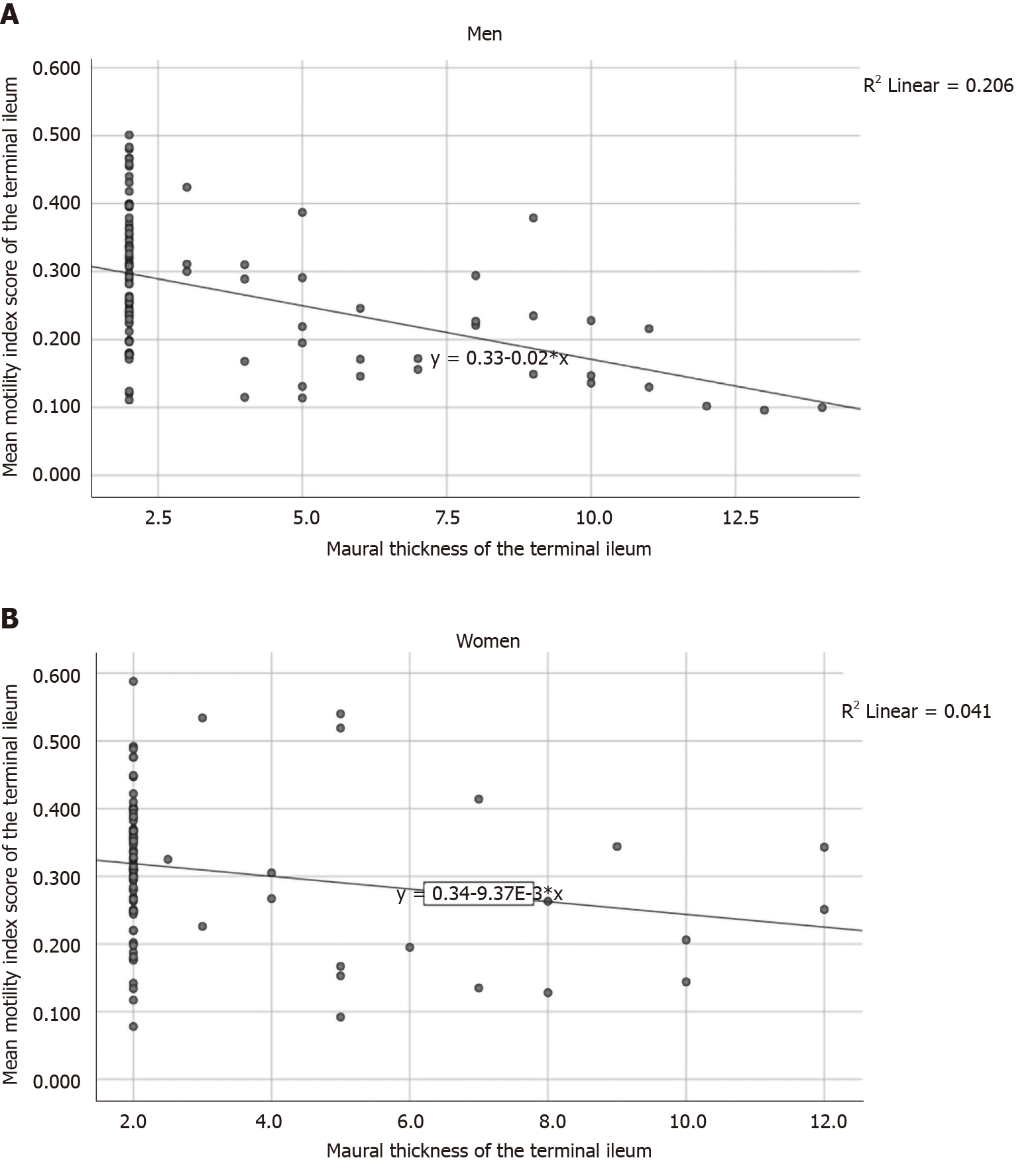
In men, the MI of the terminal ileum was inversely associated with mural thickness (r = -0.449, P < 0.001; β: -0.077; 95%CI: -0.107 to -0.047, P < 0.001) (Figure 2A) and weight (r = -0.258, P = 0.019; β: -0.001; 95%CI: -0.003 to 0.000, P = 0.044). Additionally, there was an inverse correlation between weight and the MI of the ileum in simple correlation analysis; however, this correlation was not observed in the linear regression (r = -0.216, P = 0.034; β: -0.001; 95%CI: -0.002–0.000, P = 0.116).
In women, the MI of the terminal ileum tended to be inversely associated with mural thickness (r = -0.204, P = 0.055; β: -0.044; 95%CI: -0.089-0.000, P = 0.050) (Figure 2B).
There were statistically significant differences in symptoms between controls and patients in each disease group (Table 2). These differences were still present when the analyses were stratified by sex (data not shown).
The MI of the jejunum was inversely correlated with diarrhea in women (r = -0.237, P = 0.031), and this association was still significant in the unadjusted linear regression (β: 0.000; 95%CI: -0.001-0.000; P = 0.029).
The MI of the terminal ileum was inversely correlated with constipation in men (r = -257, P = 0.021), and this association was observed also in the linear regression (β: -0.025; 95%CI: -0.047- -0.003; P = 0.027). In contrast, the MI of the terminal ileum was positively correlated with constipation in women (r = 0.246, P = 0.035), and this association remained significant in a linear regression model (β: 0.023; 95%CI: 0.003-0.043; P = 0.022).
After adjusting for variables that were significantly associated with the MI of the terminal ileum in men, i.e., weight, mural thickness and constipation, the inverse association between the MI and mural thickness remained (β: -0.073: 95%CI: -0.109 to -0.036, P < 0.001), whereas constipation (β: -0.021; 95%CI: -0.043-0.000; P = 0.053) and weight tended to be significantly associated after adjustments (β: -0.001; 95%CI: -0.003-0.000, P = 0.081). After adjusting for mural thickness and constipation among women, the positive association between the MI of the terminal ileum and constipation was still statistically significant (β: 0.021; 95%CI: 0.001-0.041; P = 0.039), and the MI and mural thickness tended to be inversely associated (β: -0.044; 95%CI: -0.090-0.002; P = 0.063) (Figure 1).
There were no correlations between albumin and C-reactive protein levels in plasma, hemoglobin and leukocytes in blood and calprotectin in feces and the MI of the jejunum, ileum or terminal ileum (data not shown).
The main finding of the present study was the inverse association between the MI of the terminal ileum and mural thickness, especially in men. The tendency of lower MI of the terminal ileum in men than in women could possibly be explained by men having higher weights. In women, diarrhea was inversely associated with the MI of the jejunum and constipation was positively associated with the MI of the terminal ileum. There were differences in the MI of the jejunum and the terminal ileum between healthy controls and other assorted diseases.
The associations, or tendency of associations, between a lower MI and increased mural thickness, male sex and higher weight suggest that the MI not only assesses the function of the small bowel but also largely represents morphological signs. Previous publications have shown that the reduced motility induced by active CD may reflect signs of increased inflammation in the bowel wall, as indicated by the inverse correlation between the MI and mural thickness in the terminal ileum[5-8]. However, the sensitivity and specificity of the MI are lower than those of other inflammatory parameters, e.g., elevated fecal calprotectin and increased mural thickness, to differ between active and inactive CD[10]. Regarding UC, no difference in MI could be identified between active and inactive disease[17]. Since previous studies have used smaller sample sizes, they have not been observed the differences between sexes that we observed herein[9,10]. Likewise, the importance of weight has also not been described previously. Increased weight leads to the storage of adipose tissue, which is mainly deposited as subcutaneous adipose tissue and visceral adipose tissue; men have more visceral adipose tissue and women have more subcutaneous adipose tissue[18]. Therefore, the bowel wall may also be thicker, especially in men, and there may be fat deposits in the submucosa and serosa, which could explain the correlation between weight and mural thickness. Another option is that a third factor leads to the correlations between weight and mural thickness. For example, patients with acute relapse of their IBD are treated with corticosteroids, which can increase weights[19].
Although correlations between constipation and the MI of the terminal ileum were found in both sexes, the associations were in opposite directions, and the differences were weakened after adjusting for weight and mural thickness. The etiology of gastrointestinal symptoms in patients without morphological abnormalities may be difficult to elucidate. Therefore, IBS has been defined as a functional bowel disease without organic findings, to describe and visualize these patients[2]. Enteric neurogenic and/or myogenic pathology may lead to objectively measurable dysmotility from the esophagus to the distal colon[20,21]. However, gastrointestinal symptoms are seldom associated with objective measurements of gastrointestinal dysmotility, e.g., gastroparesis[22], and VAS scores of symptoms do not help clinicians differentiate between IBS and other dysmotility disturbances[23]. Common imaging techniques such as computer tomography or endoscopy are not useful for diagnosing dysmotility. Instead, examinations of the motor function and/or histopathology of bowel samples are necessary to make a correct diagnosis[20]. The different treatments for IBS and dysmotility[3,11] may lead to consequences for the patients[24]; therefore, it is essential to identify patients with dysmotility and give them a correct diagnosis to be able to appropriately treat them.
Full-thickness biopsies of the bowel are sometimes used to diagnose dysmotility[12]. However, there is a risk of bowel perforation and anesthetic complications. Both autonomic and neurophysiological tests, as well as skin biopsies, may be useful for diagnosing gastrointestinal dysmotility[24]. Nevertheless, it seems important to find new methods for the identification of patients with dysmotility. Since MRE is widely used at an early stage in many patients, a method to utilize this examination for motility studies could also be valuable. However, there was no difference in the MI between controls and the few dysmotility patients in the present study. Further, no difference was observed between healthy and patients with CD or UC, although dysmotility is often found in IBD[25]. The inverse associations between diarrhea and the MI of the jejunum and the positive association between constipation and the MI of the terminal ileum may be explained by effects on the mural thickness, e.g., a bowel loaded with feces may lead to stretching of the bowel, resulting in thinner bowel walls, whereas an empty bowel may have thicker walls. Thus, the effects may be mechanical and do not prove any functional mechanism. The weak correlations between objective findings in different tests and gastrointestinal symptoms may be related to visceral hypersensitivity, rather than dysmotility[26]. Chronic pain sensation is not due to organic disorders, but may rather be dependent on impaired pain modulation at a central level[27,28].
After Bonferroni correction, we observed differences in the MI between healthy controls and patients with other assorted disorders. These patients suffered from severe pathology such as ileus, malrotation or major gastrointestinal surgery. Furthermore, some of them suffered from diabetes or Parkinson’s disease, and these diseases are associated with a high prevalence of gastrointestinal dysmotility[22]. It is possible that these patients should be included in the dysmotility group, but they had not undergone a proper motility examination. Therefore, they were not classified as part of the dysmotility group. Differences between controls and patients with other assorted disorders may be due to the male dominance in the latter group; the difference may not exist in a sample with equal sex distribution.
There are differences in gut transit time between sexes, such that women had longer transit times throughout the whole gastrointestinal tract than men[29]. Furthermore, more women suffer from dysmotility than men[30]. A lower MI is assumed to represent reduced motility[7,8], which is consistent with the finding in the present study that a reduced MI was observed in areas of visibly reduced motility. Thus, the clinical role of a lower MI in men than in women remains to be determined.
Almost all significant associations involving the MI were in the terminal ileum, with no significant associations in the ileum. This may be explained by the vast majority of patients with CD having localized disease in the terminal ileum[10]. Altogether, it seems of low value to assess the MI of the jejunum and ileum[9,10]. This is remarkable for a method that was developed to assess motility, especially since neurogenic abnormalities have been described in longer, also uninvolved, segments in patients with IBD[31]. Lower MI is supposed to reflect less motility[7,8], but dysmotility may also be caused by increased, disorganized motility[19], which must be considered in methods assessing motility.
The strength of the present study was the large cohort from a clinical center. The limitations were that we have not considered the medical treatment provided to the patients. However, in previous reports on active and inactive disease, no effect on the MRI were observed by treatment or results of laboratory analyses[10,17]. Although we examined a large study cohort, the subjects were in different disease groups, which could affect the findings, as up to six different groups were compared. When we chose only to compare controls and CD patients, there were still weak differences between the groups[10]. Although as many as 134 examinations represented CD, sex differences were not observed in that cohort[10]. Thus, it is valuable to recruit a larger cohort to examine sex and weight differences. Weight did not differ between disease groups, but was associated with the MI. One limitation of the study is that interdigestive patterns or migrating motor complexes, which are studied in antroduodenal manometry, could not be analyzed by MRE; in empty bowels, the bowel wall is difficult to visualize, and ROIs can therefore not be drawn. Another limitation is the short duration of the dynamic MRI series used for MI calculation, which captured only 21 s of the diurnal motility. A longer measurement duration is necessary to obtain representative MI values. Further, this study was only performed at a single center, which may be a weakness.
Taken together, the present results indicate that it is essential to develop additional techniques to identify motility patterns by MRE. This method should be compared with other established modalities such as antroduodenal manometry and wireless capsules[32], to evaluate motility and identify the best metric and cut off values in both sexes. The MI should be less affected by mural thickness, sex and weight or classified by proper criteria for different weights and sexes, before being introduced in daily clinical practice.
In conclusion, MIs differ across diseases, but a lower MI of the terminal ileum is mainly associated with male sex and an increased mural thickness. Symptoms are weakly associated with the MI.
Symptoms in the gastrointestinal tract are common and nonspecific. Magnetic resonance enterography (MRE) is therefore often used to diagnose or exclude the presence of structural lesions related to inflammatory bowel disease (IBD). If MRE does not show any organic lesions, the patients are suspected to be healthy or are diagnosed as irritable bowel syndrome (IBS). Dysmotility of the small bowel is observed both as a primary disease and secondary to several common diseases such as diabetes and neurological diseases. Dysmotility may cause similar symptoms as IBD, but dysmotility is difficult to diagnose and the condition is often over-looked. In the absence of proper investigation, which are often invasive and not easily available, patients with gastrointestinal dysmotility may be without any treatment.
Studies have described that MRE also can be used to assess motility by calculating motility index (MI), based on displacement mapping. If MRE could be used also to assess gastrointestinal motility, patients with dysmotility could possibly be identified earlier in the disease course, and selected patients could be referred to further examinations. Thus, patients with gastrointestinal dysmotility could be treated appropriately.
The objective of the present study was to evaluate the usefulness and potential of the MI in a large cohort of unselected patients and healthy controls to examine whether the MI could be helpful to identify also altered motility patterns in addition to morphological MRE changes. The main focus was to examine the association between the MI and basal characteristics and gastrointestinal symptoms.
All consecutive patients referred for MRE during a 2-year period were asked to participate. Healthy volunteers were included as controls. MRE was prepared and conducted in accordance with clinical routines. All the participants had to complete the visual analog scale for IBS and IBS-symptom severity scale to assess gastrointestinal symptoms and all medical records were scrutinized. Maps of MI were calculated from dynamic MR images. ANOVA was used to evaluate differences in MI between groups, classified as healthy, Crohn’s disease, ulcerative colitis, IBS, other assorted disorders and dysmotility. Logistic and linear regression were applied to the MI values.
There was a difference in MI between the disease groups in jejunum and terminal ileum (P = 0.021 and P = 0.07), which was explained by a difference between controls and other assorted diseases (P = 0.043 and P = 0.059). The weight was higher in men than in women (OR = 1.056; 95%CI: 1.035-1.081; P < 0.001), and MI of the terminal ileum tended to be lower in men than in women (OR = 0.035; 95%CI: 0.001-1.086; P = 0.056). In men, MI of the terminal ileum was inversely associated with increased mural thickness (P < 0.001). There was a tendency to association between MI and constipation (P = 0.053) and weight (P = 0.081). In women, MI of the jejunum was inversely associated with diarrhea (P = 0.029) and MI of the terminal ileum was associated with constipation (P = 0.039). There was a tendency to inverse association between MI of the terminal ileum and mural thickness (P = 0.063).
Although MIs differ across diseases, the most important findings of the present study are that a lower MI of the terminal ileum is mainly associated with male sex and an increased mural thickness. Symptoms are weakly associated with the MI.
Before introduction of this technique in the daily clinical practice, the MI should be less affected by mural thickness, sex and weight or classified by proper criteria for different weights and sexes. Additional techniques probably need to be developed to identify motility patterns by MRE, less influenced by basal characteristics. Furthermore, this method should be compared with other established modalities such as antroduodenal manometry and wireless capsules to evaluate motility.
We want to thank all the staff at the Department of Imaging and Function, Skåne University Hospital, Malmö; Alex Menys at Motilent for constructing Motility maps; and Julia Dreja for calculating the regions of interest.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Swedish Society of Gastroenterology; and Meeting of Members of UEG.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Sweden
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chisthi MM, Li Q S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Khatri G, Coleman J, Leyendecker JR. Magnetic Resonance Enterography for Inflammatory and Noninflammatory Conditions of the Small Bowel. Radiol Clin North Am. 2018;56:671-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1896] [Article Influence: 210.7] [Reference Citation Analysis (3)] |
| 3. | Camilleri M. Management Options for Irritable Bowel Syndrome. Mayo Clin Proc. 2018;93:1858-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | de Jonge CS, Smout AJPM, Nederveen AJ, Stoker J. Evaluation of gastrointestinal motility with MRI: Advances, challenges and opportunities. Neurogastroenterol Motil. 2018;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Menys A, Atkinson D, Odille F, Ahmed A, Novelli M, Rodriguez-Justo M, Proctor I, Punwani S, Halligan S, Taylor SA. Quantified terminal ileal motility during MR enterography as a potential biomarker of Crohn's disease activity: a preliminary study. Eur Radiol. 2012;22:2494-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Odille F, Menys A, Ahmed A, Punwani S, Taylor SA, Atkinson D. Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn Reson Med. 2012;68:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Bickelhaupt S, Pazahr S, Chuck N, Blume I, Froehlich JM, Cattin R, Raible S, Bouquet H, Bill U, Rogler G, Frei P, Boss A, Patak MA. Crohn's disease: small bowel motility impairment correlates with inflammatory-related markers C-reactive protein and calprotectin. Neurogastroenterol Motil. 2013;25:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Menys A, Makanyanga J, Plumb A, Bhatnagar G, Atkinson D, Emmanuel A, Taylor SA. Aberrant Motility in Unaffected Small Bowel is Linked to Inflammatory Burden and Patient Symptoms in Crohn's Disease. Inflamm Bowel Dis. 2016;22:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Åkerman A, Månsson S, Fork FT, Leander P, Ekberg O, Taylor S, Menys A, Ohlsson B. Computational postprocessing quantification of small bowel motility using magnetic resonance images in clinical practice: An initial experience. J Magn Reson Imaging. 2016;44:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Dreja J, Ekberg O, Leander P, Månsson S, Ohlsson B. Volumetric analysis of small bowel motility in an unselected cohort of patients with Crohn's disease. Neurogastroenterol Motil. 2020;e13909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Di Nardo G, Karunaratne TB, Frediani S, De Giorgio R. Chronic intestinal pseudo-obstruction: Progress in management? Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Hammar O, Ohlsson B, Veress B, Alm R, Fredrikson GN, Montgomery A. Depletion of enteric gonadotropin-releasing hormone is found in a few patients suffering from severe gastrointestinal dysmotility. Scand J Gastroenterol. 2012;47:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Bengtsson M, Ohlsson B, Ulander K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Bengtsson M, Persson J, Sjölund K, Ohlsson B. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol Nurs. 2013;36:188-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Bengtsson M, Ohlsson B. The brief Visual Analogue Scale for Irritable Bowel Syndrome questionnaire can be used to evaluate psychological well-being in patients with irritable bowel syndrome. Eur J Intern Med. 2013;24:e82-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1223] [Article Influence: 43.7] [Reference Citation Analysis (1)] |
| 17. | Tufvesson H, Dreja J, Ekberg O, Leander P, Månsson S, Ohlsson B. Quantified small bowel motility in patients with ulcerative colitis and gastrointestinal symptoms: a pilot study. Acta Radiol. 2020;284185120946713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1831] [Cited by in RCA: 2142] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 19. | Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 323] [Article Influence: 40.4] [Reference Citation Analysis (1)] |
| 20. | Wingate D, Hongo M, Kellow J, Lindberg G, Smout A. Disorders of gastrointestinal motility: towards a new classification. J Gastroenterol Hepatol. 2002;17 Suppl:S1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Keller J, Bassotti G, Clarke J, Dinning P, Fox M, Grover M, Hellström PM, Ke M, Layer P, Malagelada C, Parkman HP, Scott SM, Tack J, Simren M, Törnblom H, Camilleri M; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15:291-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Faraj J, Melander O, Sundkvist G, Olsson R, Thorsson O, Ekberg O, Ohlsson B. Oesophageal dysmotility, delayed gastric emptying and gastrointestinal symptoms in patients with diabetes mellitus. Diabet Med. 2007;24:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Bengtsson M, Hammar O, Mandl T, Ohlsson B. Evaluation of gastrointestinal symptoms in different patient groups using the visual analogue scale for irritable bowel syndrome (VAS-IBS). BMC Gastroenterol. 2011;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Ohlsson B, Dahlin LB, Englund E, Veress B. Autonomic and peripheral neuropathy with reduced intraepidermal nerve fiber density can be observed in patients with gastrointestinal dysmotility. Clin Case Rep. 2020;8:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Abdalla SM, Kalra G, Moshiree B. Motility Evaluation in the Patient with Inflammatory Bowel Disease. Gastrointest Endosc Clin N Am. 2016;26:719-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W, Van der Merwe S, Mols R, Ghesquière B, Cirillo C, Kortekaas I, Carmeliet P, Peetermans WE, Vermeire S, Rutgeerts P, Augustijns P, Hellings PW, Belmans A, Vanner S, Bulmer DC, Talavera K, Vanden Berghe P, Liston A, Boeckxstaens GE. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2016;150:875-87.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 27. | Rapps N, van Oudenhove L, Enck P, Aziz Q. Brain imaging of visceral functions in healthy volunteers and IBS patients. J Psychosom Res. 2008;64:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 541] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 29. | Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Syed AR, Wolfe MM, Calles-Escandon J. Epidemiology and Diagnosis of Gastroparesis in the United States: A Population-based Study. J Clin Gastroenterol. 2020;54:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 31. | Villanacci V, Bassotti G, Nascimbeni R, Antonelli E, Cadei M, Fisogni S, Salerni B, Geboes K. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol Motil. 2008;20:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Grønlund D, Poulsen JL, Sandberg TH, Olesen AE, Madzak A, Krogh K, Frøkjaer JB, Drewes AM. Established and emerging methods for assessment of small and large intestinal motility. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |