Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4557
Peer-review started: March 29, 2020
First decision: April 26, 2020
Revised: May 6, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: August 14, 2020
Processing time: 131 Days and 21.5 Hours
At present, minimally invasive endoscopic treatment is mostly used for patients with actively bleeding Dieulafoy’s lesions, , as it has the advantages of minimal trauma, short operation time and good hemostatic effect, although bleeding can easily recur postoperatively. Recently, extensive gastric cuneiform resection has been advocated for use in these patients because the constant-diameter artery follows a long path to the gastric mucosa.
A 47-year-old man was admitted to the hospital for repeated hematemesis and black stool, and he was diagnosed with Dieulafoy’s disease. We chose a method that not only simulates surgical gastric cuneiform resection but also reduces trauma. We performed enlarged local endoscopic full-thickness resection of the gastric wall and abdominal constant-diameter artery and sutured the gastric wall. Postoperative follow-up showed that the constant-diameter artery had been resected from the gastric wall, which was confirmed to have no blood flow signals by endoscopic ultrasonography.
Endoscopic full-thickness resection of the gastric wall and abdominal constant-diameter artery with suturing of the gastric wall has demonstrated potential as a new treatment for Dieulafoy's disease.
Core tip: Gastric Dieulafoy’s disease, also known as gastric submucosal constant-diameter arterial malformation, can occur in any part of the digestive tract but is most common in the esophagus and within 6 cm of the gastroesophageal junction. The main symptoms of this disease are recurrent vomiting and tar-like stool. In severe cases, patients can develop hemorrhagic shock, and the mortality rate is high. We performed enlarged local endoscopic full-thickness resection of the gastric wall and abdominal constant-diameter artery with suturing of the gastric wall. We report a case diagnosed as Dieulafoy’s disease. This is the first case of Dieulafoy's disease treated by this method.
- Citation: Yu S, Wang XM, Chen X, Xu HY, Wang GJ, Ni N, Sun YX. Endoscopic full-thickness resection to treat active Dieulafoy's disease: A case report. World J Gastroenterol 2020; 26(30): 4557-4563
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4557.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4557
Gastric Dieulafoy’s disease, also known as gastric submucosal constant-diameter arterial malformation, can occur in any part of the digestive tract but is most common in the esophagus and within 6 cm of the gastroesophageal junction. The main symptoms of this disease are recurrent vomiting and tar-like stool. In severe cases, patients can develop hemorrhagic shock, and the mortality rate is high. Minimally invasive endoscopic treatment is mostly used, as it has the advantages of minimal trauma, short operation time and good hemostatic effect. Endoscopic treatment is simple and minimally traumatic, but bleeding can easily recur postoperatively. Recently, extensive gastric cuneiform resection has been advocated for use in these patients because the constant-diameter artery follows a long path to the gastric mucosa.
We performed enlarged local endoscopic full-thickness resection (EFR) of the gastric wall and abdominal constant-diameter artery with suturing of the gastric wall. Postoperative follow-up showed that the vascular lesion had been resected from the gastric wall, which was confirmed to have no blood flow signals by endoscopic ultrasonography.
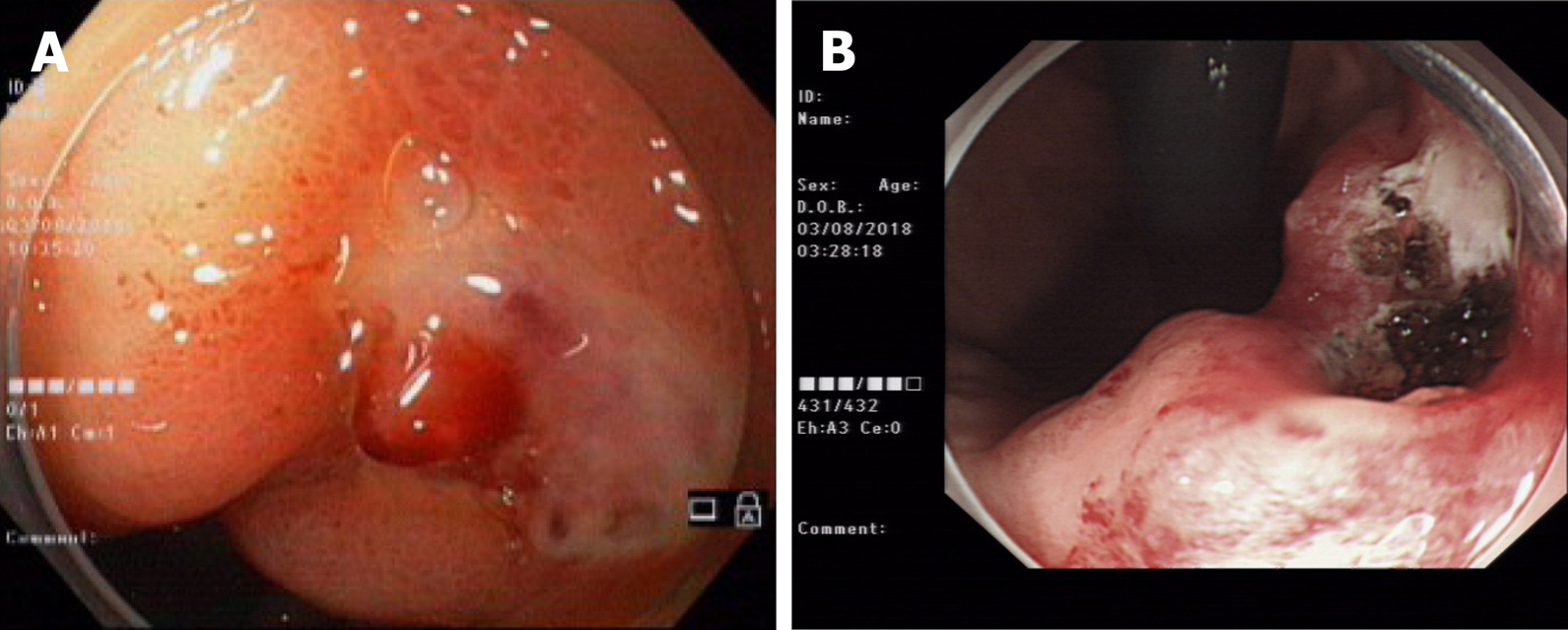
A 47-year-old male was admitted to the hospital due to abdominal pain for 1 wk, vomiting and black stool. Before admission, the patient had no symptoms, such as middle and upper abdominal distension, pain, fever, vomiting, and diarrhea. Three hours before admission, the patient suddenly defecated black stool twice, accompanied by vomiting dark red blood twice (approximately 500 mL), dizziness, and fatigue; thus, he made an emergency visit to our hospital. One year ago, the patient had been admitted to our hospital for vomiting dark blood. Painless gastroscopy suggested a focal mucosal ulcer on the posterior wall along the curvature of the gastric body, and blood clots could be seen. Dieulafoy's disease was considered. The wound was closed with a metal clip after high-frequency electrocoagulation (Figure 1). No rebleeding occurred, and the patient was discharged from the hospital.
The patient had no history of hypertension, coronary heart disease, diabetes, smoking or drinking, and denied a similar family history.
The examination on admission revealed the following: Body temperature, 36.7°C; pulse, 80 beats/min; respiration, 20 times/min; blood pressure, 93/64 mmHg; acute illness, an anemic appearance, a clear mind, conjunctiva, pale nail beds, normal heart and lung findings, a soft abdomen, upper abdominal tenderness, no muscle tension or rebound pain, negative mobility, and normal intestinal sounds.
The routine blood test results showed a hemoglobin level of 82 g/L, a positive result for fecal occult blood and normal findings regarding coagulation, liver and kidney function, electrolytes, and tumor markers.
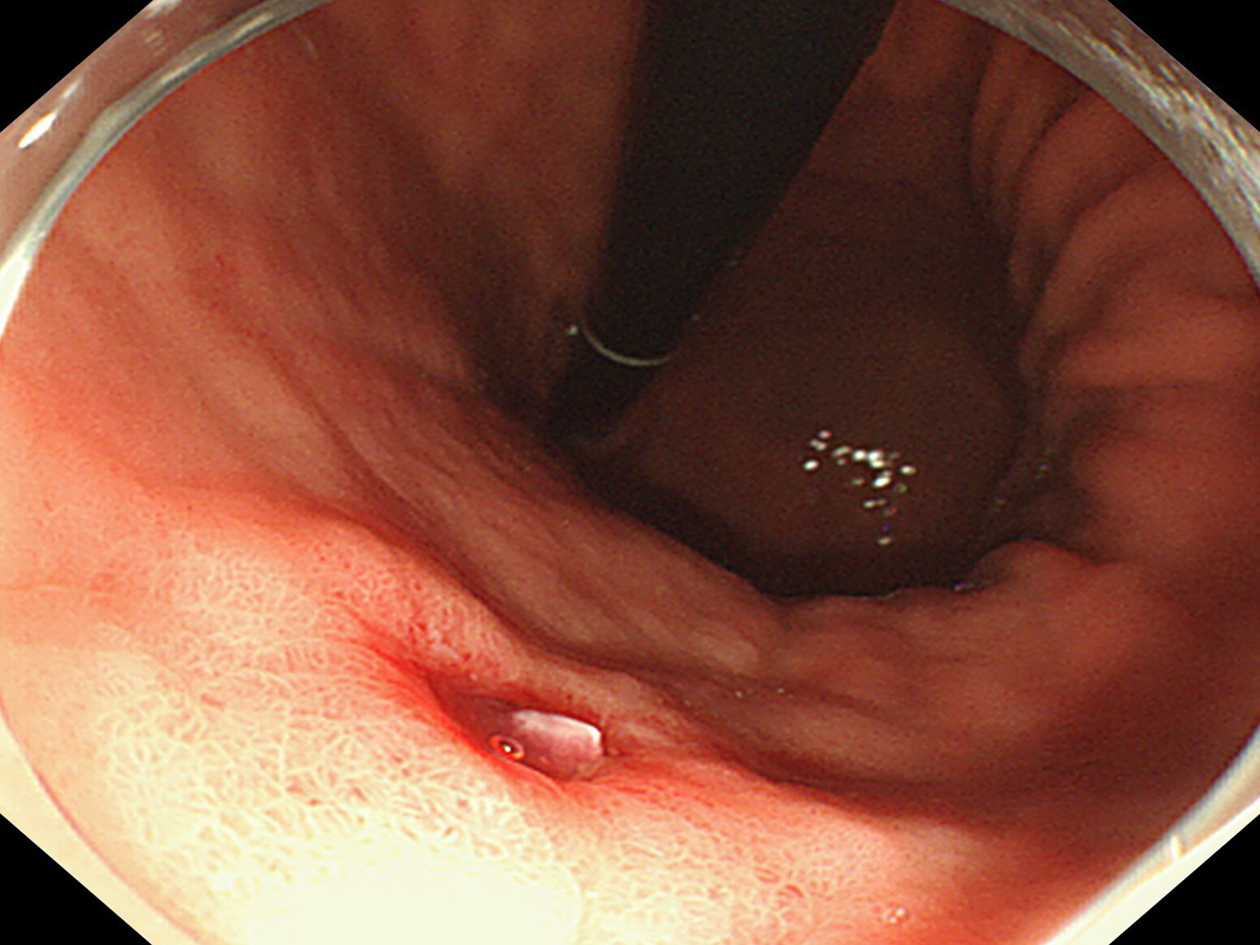
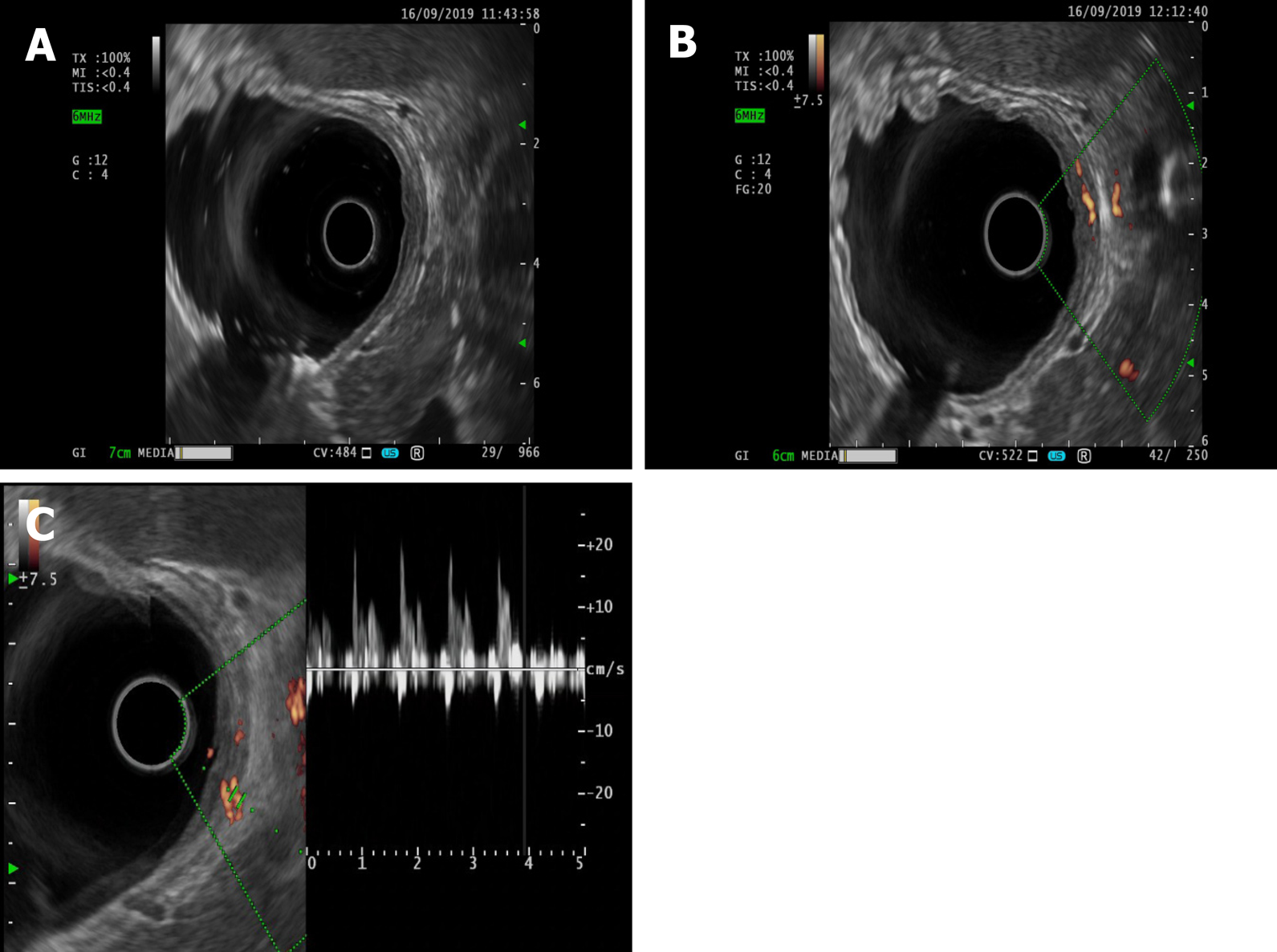
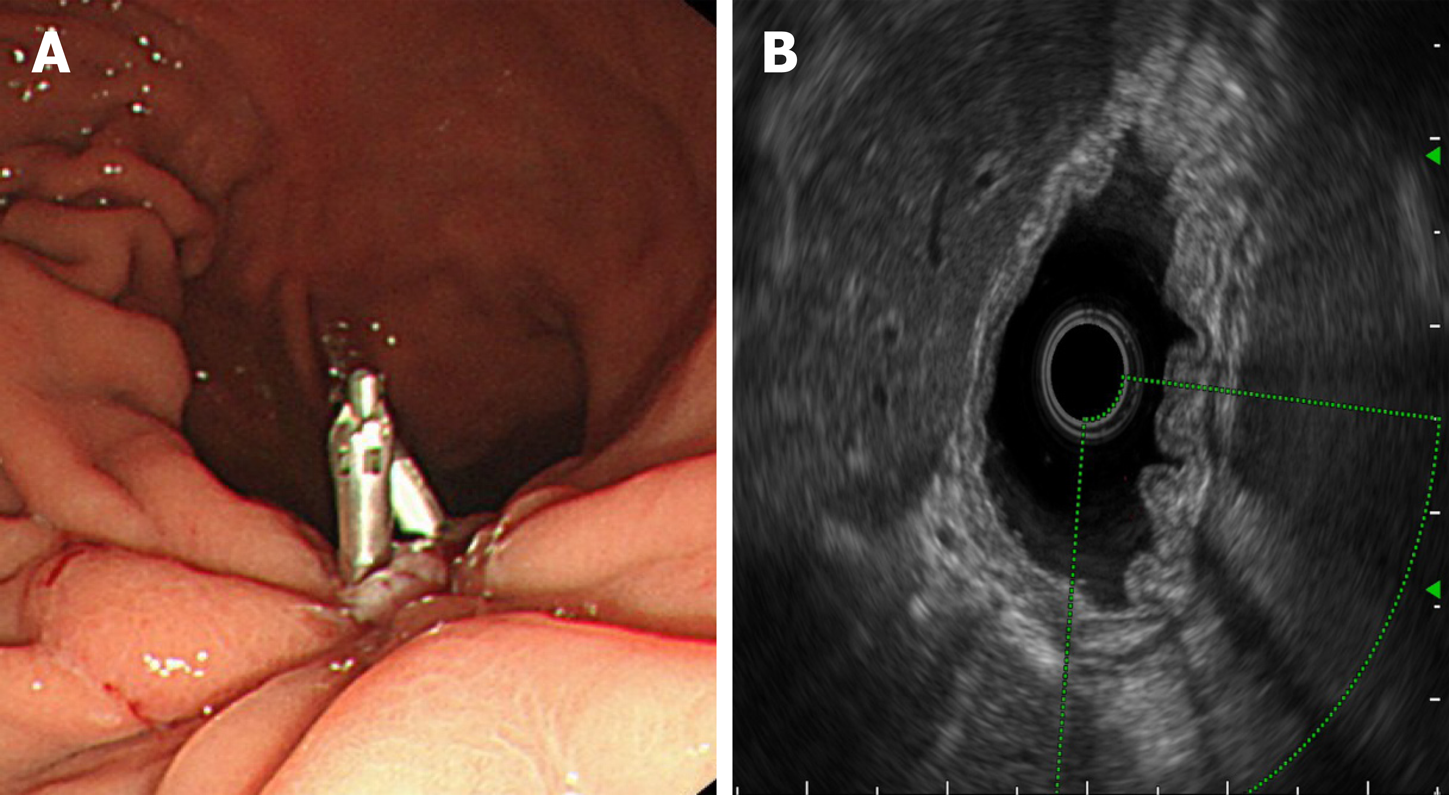
Electrocardiography and chest radiography were performed. Abdominal color Doppler ultrasound suggested a left renal cyst. Painless gastroscopy suggested a focal mucosal ulcer on the posterior wall along the curvature of the gastric body, and blood clots could be seen (Figure 2). Endoscopic ultrasonography revealed that the ulcer on the posterior wall of the small curvature of the stomach was approximately 0.8 cm x 1.0 cm. The structure of the first four layers of the gastric wall was not clear. There were no obvious tubular structures, but blood flow signals could be seen, and pulse Doppler showed an arterial blood flow signal; the serosa was complete (Figure 3).
Dieulafoy's disease.
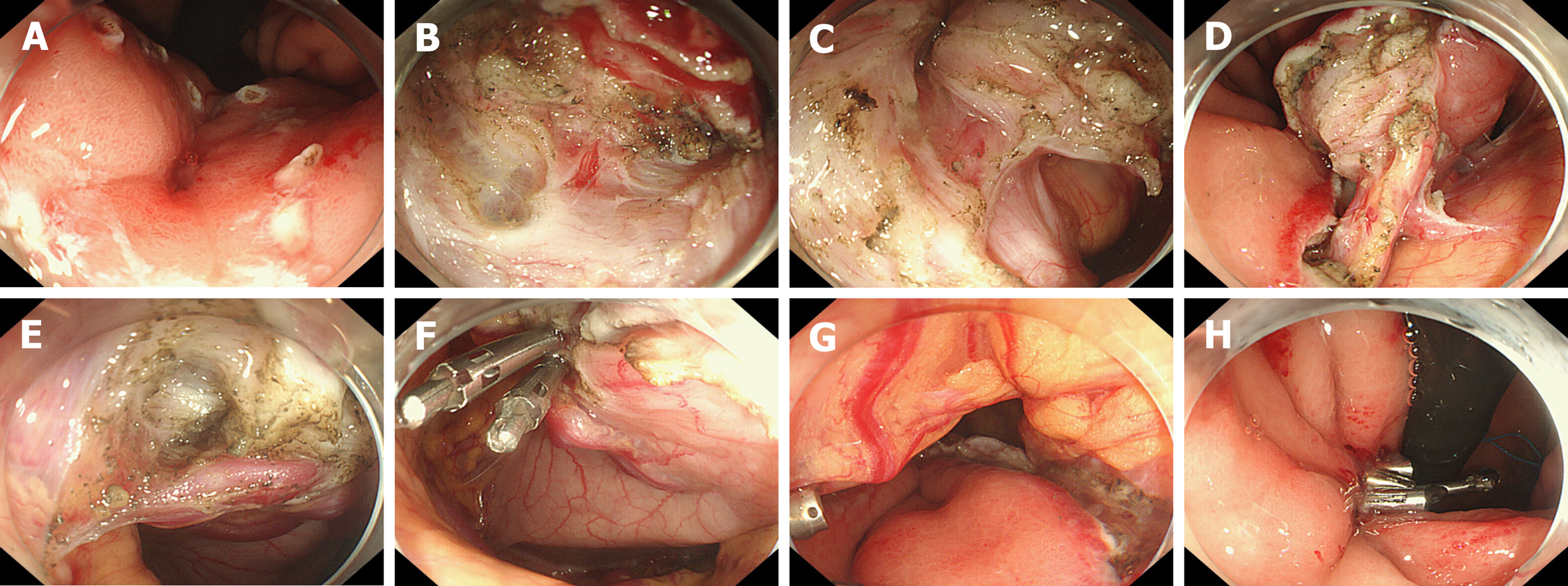
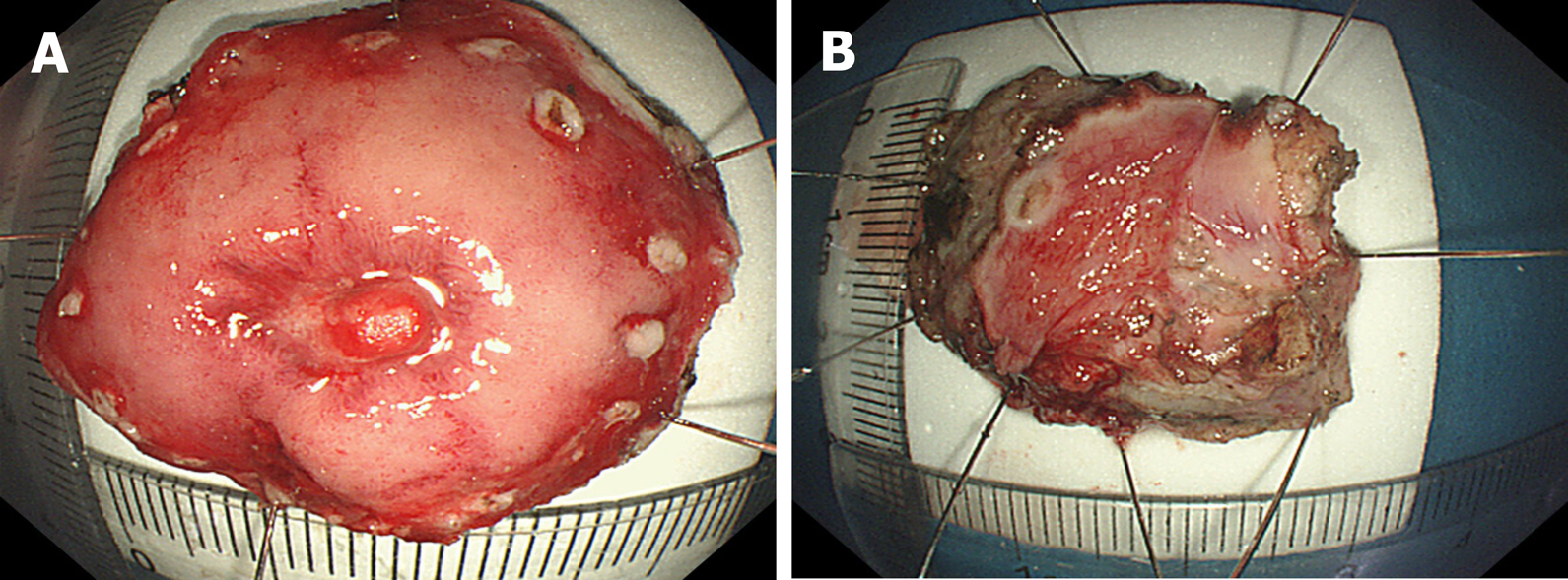
Surgery was planned and contraindications were evaluated. The patient signed an informed consent form for EFR. .EFR was performed under endotracheal intubation and anesthesia. The procedures were as follows (Figure 4 and 5): (1) A DualKnife (KD-650L, Olympus, Japan) was used to mark a 1 cm margin around the lesion one week before surgery, and a disposable needle (NM-200U-0423 Olympus Japan) was used to inject fluid into the marking lateral submucous membrane to form a liquid pad; (2) The lateral side of the marking was cut layer by layer from the gastric wall to the abdominal cavity, and the small blood vessels were separated by ele-ctrocoagulation; (3) The large blood vessels on the serosal surface of the lesion were fully exposed with the use of soft tissue clamps (ROCC-D-26-195, Nanjing Minimally Invasive, China); (4) After carefully separating the soft tissue around the large vessels with the DualKnife, a disposable hemostatic clip (M00522610, Poko National Medical, United States) was placed in the medial serosa of the abdominal cavity, and the blood vessels were severed at the serosal surface. After achieving complete hemostasis, the lesion was completely resected; (5) Several soft tissue clips and nylon sutures (HX-20Q-1, MAJ-254, Olympus, Japan) were used to close the wound; and (6) A gastrointestinal decompression tube was inserted.
After the operation, the patient fasted from water, and gastrointestinal decompression, acid suppression, protection of the gastric mucosa, intravenous nutrition, anti-inflammation, and hemostasis were administered. The patient was discharged from the hospital without bleeding, abdominal pain, and infection.
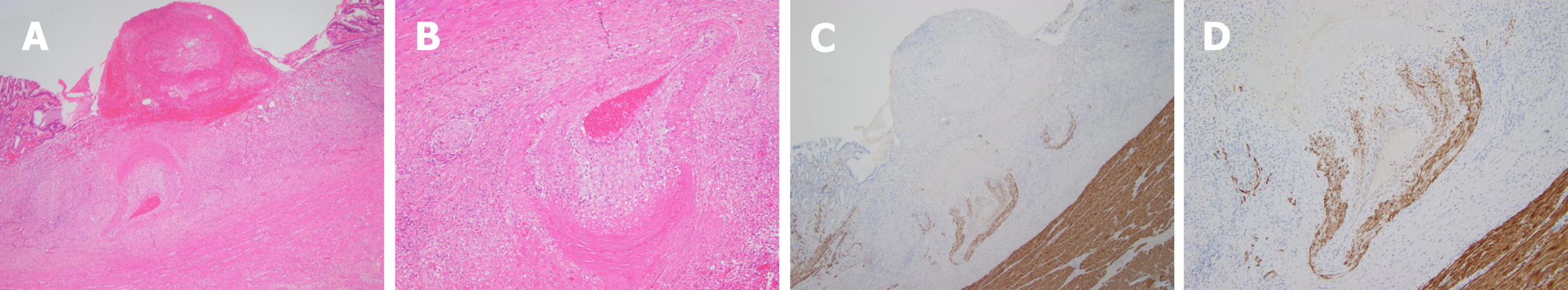
Postoperative pathology revealed gastric Dieulafoy’s disease (Figure 6). Two months after the operation, the wound was re-examined by gastroscopy, and no blood flow signals or tubular echogenicity were observed in the gastric wall (Figure 7).
Gastric Dieulafoy’s disease, also known as gastric submucosal constant-diameter arterial malformation, can occur in any part of the digestive tract but is most common in the esophagus and within 6 cm of the gastroesophageal junction[1]. The main symptoms of this disease are recurrent vomiting and tar-like stool. In severe cases, patients can develop hemorrhagic shock, and the mortality rate is high.
In our patient with Dieulafoy’s disease, normal gastric mucosa was observed, no varicose veins were observed on the mucosal surface, and only the active bleeding point was found in the small curvature of the gastric cardia. Careful observation of this area revealed superficial erosion, with mucosal defects and rash-like protuberances, and while passive or active bleeding from the surface was observed, the surrounding mucosa was normal. If bleeding stops, blood clots may be attached to the mucosal surface, and the bleeding points can be found by wiping the clots away with an absorbent gelatin sponge. In some patients, arterioles protrude into the gastric cavity through superficial defects in the gastric mucosa and exhibit active bleeding. If superficial, actively bleeding lesions in the gastric mucosa are observed, Dieulafoy’s disease can be considered[2]. Emergency gastroscopy is the first choice and the most intuitive diagnostic method, and preliminary treatment can be provided according to the findings, followed by angiography, which can be used as an independent diagnostic method or a remedial method when the findings of the endoscopic examination are negative[3]. This examination should be carried out in the case of active bleeding, which can be considered when the amount of bleeding is ≥ 0.5 mL/min. During the period of intermittent bleeding, the diagnostic rate of angiography is low.
At present, for patients with actively bleeding Dieulafoy’s lesions, minimally invasive endoscopic treatment is mostly used, as it has the advantages of minimal trauma, short operation time and good hemostatic effect. When endoscopic treatment is ineffective or bleeding occurs again, surgery should be selected accordingly. The surgical methods applied include subtotal resection of the proximal stomach and local cuneiform resection. Extensive gastric cuneiform resection is currently advocated in such patients. If endoscopic therapy fails or if the patient cannot tolerate surgery, angiography and embolization can be selected[4]. However, embolization requires superselective catheterization of the left gastric artery. Angiography can be used to determine the point of bleeding without affecting collateral vessels and can be performed if the vital signs are stable and there is sufficient time for embolization. This technique is often performed, but the success rate is low.
Endoscopic treatment is simple and minimally traumatic, but bleeding can easily recur postoperatively. Recently, extensive gastric cuneiform resection has been advocated for use in these patients because the constant-diameter artery follows a long path to the gastric mucosa. With resection, the cause of the disease can be ruled out, the recurrence of bleeding can be avoided, and the final diagnosis can be established by pathological examination of the resected specimen. However, because of the high degree of trauma, this surgery is not often applied. Therefore, we performed enlarged local ERF of the gastric wall and abdominal constant-diameter artery with suturing of the gastric wall. This method not only simulates surgical gastric cuneiform resection but also reduces trauma. This approach has promise as a new treatment for Dieulafoy's disease. Instead of performing endoscopic submucosal dissection for the treatment of static Dieulafoy’s disease, as reported by Yang et al[5] and Chen et al[6], as the patient in this case had an active lesion similar to a submucosal eminence of the gastric stroma, we gradually dissected and locally expanded the range from the mucous membrane to the abdominal cavity to find, suture and detach the source of the constant-diameter artery to the abdominal cavity so that the gastric wall could be completely removed by extensive local resection of the gastric wall. This approach avoids recurrence. There are also risks associated with full-thickness resection, such as pneumoperitoneum, bleeding, abdominal infection and damage to adjacent tissue or organs. Due to the routine use of carbon dioxide gas during the operation, abdominal puncture is needed after the operation to exhaust pneumothorax or pneu-moperitoneum, and attention should be paid to the treatment of exposed blood vessels. The serosal surface of the large blood vessels is particularly important. Adequate treatment of blood vessels is required to avoid serosal surface bleeding. Sufficient washing of the gastrointestinal mucosa preoperatively and the prophylactic use of antibiotics before resection of the focus to remove gastrointestinal fluid, as well as wound closure as soon as possible to reduce the operation time, can prevent abdominal infection. Endoscopic physicians with experience in EFR and natural orifice transluminal endoscopic surgery are required for this treatment. However, the branches of Dieulafoy's lesions pass through the submucosa to the mucosal layer with a constant diameter and then turn back and twist to the serosal surface, resulting in acute angles or vertical vascular loops. Therefore, how much areas of the gastric wall should be resected in the process of endoscopic surgery, with the bleeding focus as the center, to completely remove the constant-diameter artery, needs to be confirmed in more cases. We attempted to detect arterial signals on endoscopic ultrasonography and detect the shape of the artery by injecting methylene blue and other stains into the blood vessel under endoscopic ultrasonography to completely remove the lesion with the smallest surgical range and reduce the surgical trauma. The results showed that the lesion could be completely resected with the smallest surgical range and that the surgical trauma could be reduced.
EFR is a novel treatment for Dieulafoy's disease. The method simulates surgical gastric wedge resection and has value in reducing trauma, removing Dieulafoy's lesions and avoiding recurrent bleeding. However, at the same time, due to certain risks, this method needs to be performed by an experienced endoscopic physician. The safety and efficacy of this method requires validation by further large-sample studies, and we will conduct further prospective studies after a full assessment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hansel S, Souza JLS S-Editor: Ma YJ L-Editor: MedE-Ma JY P-Editor: Zhang YL
| 1. | Kasapidis P, Georgopoulos P, Delis V, Balatsos V, Konstantinidis A, Skandalis N. Endoscopic management and long-term follow-up of Dieulafoy's lesions in the upper GI tract. Gastrointest Endosc. 2002;55:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Saleh R, Lucerna A, Espinosa J, Scali V. Dieulafoy lesion: the little known sleeping giant of gastrointestinal bleeds. Am J Emerg Med. 2016;34:2464.e3-2464.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Baxter M, Aly EH. Dieulafoy's lesion: current trends in diagnosis and management. Ann R Coll Surg Engl. 2010;92:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Jeon HK, Kim GH. Endoscopic Management of Dieulafoy's Lesion. Clin Endosc. 2015;48:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Yang L, Cao HL, Wang SN, Zhang YJ, Jiang K, Wang BM. Endoscopic submucosal dissection in the treatment of gastric dieulafoy lesion at rest: a case report. Zhonghua Xiaohua Neijing Zazhi. 2018;35:599-560. [DOI] [Full Text] |
| 6. | Chen X, Cao H, Wang S, Wang D, Xu M, Piao M, Wang B. Endoscopic submucosal dissection for silent gastric Dieulafoy lesions mimicking gastrointestinal stromal tumors: Report of 7 cases-a case report series. Medicine (Baltimore). 2016;95:e4829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |