Published online Jan 21, 2020. doi: 10.3748/wjg.v26.i3.353
Peer-review started: November 23, 2019
First decision: December 23, 2019
Revised: January 7, 2020
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: January 21, 2020
Processing time: 54 Days and 0.7 Hours
Programmed cell death-1 (PD-1) inhibitor has been indicated for many types of malignancies. However, these inhibitors also cause immune-related adverse events. Hepatobiliary disorder is a phenotype of immune-related adverse event affecting 0%–4.5% of patients treated with PD-1 inhibitors. Recent studies have reported PD-1 inhibitor-related sclerosing cholangitis (SC); however, the associated clinical and pathological features are unclear.
To evaluate the clinical and pathological features of PD-1 inhibitor-related SC through a systematic review of the literature.
The review, conducted using electronic databases in PubMed, was restricted to the period from January 2014 to September 2019 and focused on case reports/series on PD-1 inhibitor-related SC published in English. We scanned the references of the selected literature to identify any further relevant studies. Six cases previously studied by us, including three that have not yet been published, were included in this review.
Thirty-one PD-1 inhibitor-related SC cases were evaluated. Median age of patients was 67 years (range, 43–89), with a male to female ratio of 21:10. The main disease requiring PD-1 inhibitor treatment was non-small cell lung cancer. Agents that caused PD-1 inhibitor-related SC were nivolumab (19 cases), pembrolizumab (10 cases), avelumab (1 case), and durvalumab (1 case). The median number of cycles until PD-1 inhibitor-related SC onset was 5.5 (range, 1–27). Abdominal pain or discomfort (35.5%, 11/31) was the most frequent symptom. Blood serum tests identified liver dysfunction with a notable increase in biliary tract enzymes relative to hepatic enzymes, and a normal level of serum immunoglobulin G4. Biliary dilation without obstruction (76.9%, 20/26), diffuse hypertrophy of the extrahepatic biliary tract (90.5%, 19/21), and multiple strictures of the intrahepatic biliary tract (30.4%, 7/23) were noted. In 11/23 (47.8%) cases, pathological examination indicated that CD8+ T cells were the dominant inflammatory cells in the bile duct or peribiliary tract. Although corticosteroids were mainly used for PD inhibitor-related SC treatment, the response rate was 11.5% (3/26).
Some clinical and pathological features of PD-1 inhibitor-related SC were revealed. To establish diagnostic criteria for PD-1 inhibitor-related SC, more cases need to be evaluated.
Core tip: This study systematically reviewed the literature on the programmed cell death-1 inhibitor-related sclerosing cholangitis. Biliary dilation without obstruction, diffuse hypertrophy of the extrahepatic biliary tract and/or multiple strictures of intrahepatic biliary tract, liver dysfunction with a notable increase in biliary tract enzymes relative to hepatic enzymes, normal level of the serum immunoglobulin G4, and a moderate to poor response to steroid therapy, and CD8+ T cell infiltration in the biliary tract were clinical and pathological features of programmed cell death-1 inhibitor-related sclerosing cholangitis.
- Citation: Onoyama T, Takeda Y, Yamashita T, Hamamoto W, Sakamoto Y, Koda H, Kawata S, Matsumoto K, Isomoto H. Programmed cell death-1 inhibitor-related sclerosing cholangitis: A systematic review. World J Gastroenterol 2020; 26(3): 353-365
- URL: https://www.wjgnet.com/1007-9327/full/v26/i3/353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i3.353
The programmed cell death-1 (PD-1) receptor is expressed on activated T cells, whereas the programmed cell death-ligand 1 (PD-L1) is overexpressed on specific types of cancer cells. When bound by PD-L1, PD-1 causes the suppression of T cell cytotoxic immune responses. This repression pathway is an essential immune prevention mechanism from host immunity and is upregulated in many malignant tumors and their surrounding microenvironment[1]. Recently, developments in immunotherapy have demonstrated efficacy for the treatment of various malignancies. PD-1 inhibitors were also indicated for many types of malignancies, such as non-small cell lung cancer, melanoma, Hodgkin lymphoma, renal cell cancer, bladder cancer, gastric cancer, and esophageal cancer[2-12]. Moreover, pembrolizumab has been indicated for solid carcinoma with mismatch repair deficiency[13,14]. Therefore, many patients with malignant disease will be treated with a PD-1 inhibitor. Although PD-1 inhibitors are beneficial for the treatment of malignancies, it has been noted that immune-related adverse events (irAEs) result from dysregulation of the host immune system[15]. Hepatobiliary disorders are irAEs that affect 0%–4.5% of patients treated with PD-1 inhibitors[16-18]. Recently, PD-1 inhibitor-related sclerosing cholangitis (SC) and its clinical features have been reported[19,20]. However, the diagnostic criteria for PD-1 inhibitor-related SC have not been clarified. We also have experience of six cases of suspected of PD-1 inhibitor-related SC.
The objective of this work was to perform a systematic review of cases of PD-1 inhibitor-related SC, and to evaluate the clinical and imaging features of PD-1 inhibitor-related SC.
We identified relevant studies in the literature by searching the databases of PubMed. The review was restricted to the period from January 2014 to September 2019 and focused on case reports or case series with PD-1 inhibitor-related SC that were published in English. The search terms consisted of the words [“Programmed cell death 1” (All Fields) and “cholangitis” (All Fields)], [“Programmed cell death ligand 1” [All Fields] AND “cholangitis” (All Fields)], [“Nivolumab”(All Fields) and “cholangitis” (All Fields)], [“Pembrolizumab” (All Fields) and “cholangitis” (All Fields)], [“Cemplimab” (All Fields) and “cholangitis” (All Fields)], [“Atezolizumab” (All Fields) and “cholangitis” (All Fields)], [“Avelumab” (All Fields) and “cholangitis” (All Fields)], and [“Durvalumab” (All Fields) and “cholangitis” (All Fields)]. We also read the reference lists of the selected studies to manually identify further relevant studies.
Articles were excluded from this review if: (1) The article was a review, basic research, commentary, or clinical study; (2) The study had insufficient information and descriptions; and (3) The full text was unavailable.
We have also investigated six cases of PD-1 inhibitor-related SC, three of which have not yet been published. We have included these three cases in this case review.
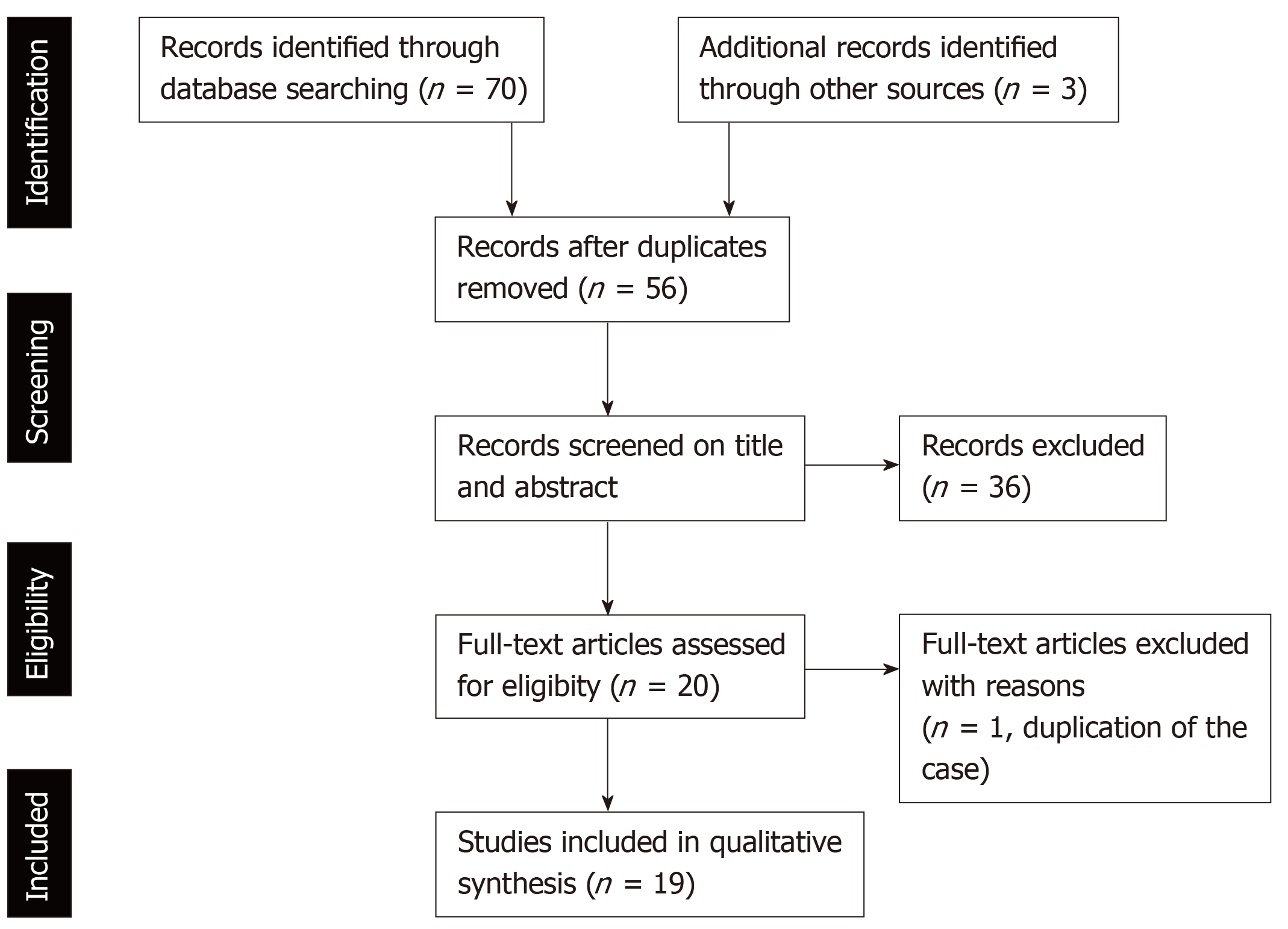
The process of the literature selection is presented in Figure 1. The literature search of the databases of PubMed identified 70 studies that met the search terms. We found an additional three relevant articles in the references of those studies. After the removal of duplicate studies, we evaluated 56 studies by screening the titles and abstracts to check that they met the search criteria. Consequently, we excluded 20 basic research studies, 3 review articles, 3 editorial letters, and 10 clinical studies. Moreover, two studies were case reports about same patient with PD-1 inhibitor-related SC; therefore, one of these studies was excluded. Finally, 19 studies, which included 5 case series and 14 case reports, were assessed in this review[19-37]. One case series reported 10 patients with hepatobiliary disorder caused by PD-1 inhibitors, which included two patients with PD-1 inhibitor-related SC[37]. With the inclusion of our three cases, a total of 31 cases of PD-1 inhibitor-related SC were evaluated.
The characteristics of patients with PD-1 inhibitor-related SC are shown in Tables 1-3. The median age at the onset of PD-1 inhibitor-related SC was 67 years (range, 43–89). PD-1 inhibitor-related SC appeared to be more prevalent in men, with a male-to-female ratio of 21:10. The patients’ primary diseases that were an adaptation disease for the treatment of PD-1 inhibitor were non-small cell lung cancer (20 cases), melanoma (4 cases), gastric cancer (3 cases), bladder cancer (2 cases), small cell lung cancer (1 case), and epithelioid mesothelioma (1 case). The agents that caused PD-1 inhibitor-related SC were nivolumab (19 cases), pembrolizumab (10 cases), avelumab (1 case), and durvalumab (1 case). The median number of cycles until onset of PD-1 inhibitor-related SC was 5.5 (range, 1–27). Abdominal pain or discomfort (35.5%, 11/31) was the most frequent symptom, followed by fever (19.4%, 6/31) and jaundice (12.9%, 4/31). Eight patients did not have any symptoms, but did have liver dysfunction (25.8%, 8/31). The median levels of total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase (ALP), and gamma-glutamyl transpeptidase were 0.75 mg/dL (range, 0.3–15.9), 129.0 U/L (range, 49–961), 125.0 U/L (range, 31–1536), 1543.0 U/L (range, 237–5066), and 452.0 U/L (range, 114–2094), respectively. Of the 13 patients tested for immunoglobulin G4 (IgG4), almost all patients were negative (92.3%, 12/13).
| Patient characteristics | Value |
| Age, median (range, yr) | 67.0 (43–89) |
| Sex, male/female | 21/10 |
| Primary disease | |
| NSCLC | 20 |
| Melanoma | 4 |
| GC | 3 |
| BC | 2 |
| SCLC | 1 |
| Epithelioid mesothelioma | 1 |
| Drugs | |
| Nivolumab | 19 |
| Pembrolizumab | 10 |
| Avelumab | 1 |
| Durvalumab | 1 |
| Atezolizumab | 0 |
| Treatment cycles until onset | 5.5 (1–27) |
| Symptoms | |
| Abdominal pain or discomfort | 11 |
| Fever | 6 |
| Jaundice | 4 |
| Vomiting | 2 |
| Appetite loss | 2 |
| Diarrhea or soft stool | 2 |
| Skin disorder | 2 |
| General fatigue | 1 |
| Backache | 1 |
| None (liver dysfunction) | 8 |
| Liver functional test | |
| T-Bil, median (range, mg/dL) | 0.75 (0.3–15.9) |
| AST, median (range, U/L) | 129.0 (49–961) |
| ALT, median (range, U/L) | 125.0 (31–1536) |
| ALP, median (range, U/L) | 1543.0 (237–5066) |
| GGT, median (range, U/L) | 452.0 (114–2094) |
| Serological test | |
| IgG, median (range, U/L) | 1230.0 (1050-1789) |
| IgA, median (range, U/L) | 297.5 (199-474.4) |
| IgM, median (range, U/L) | 64.0 (38-94) |
| IgG4, ≥ 135 U/L / < 135 U/L | 1/12 |
| Antinuclear antibody, ≥ 40 / < 40 | 7/12 |
| Imaging findings | |
| Biliary stenosis | 8 |
| Intrahepatic bile duct | 3 |
| Extrahepatic bile duct | 1 |
| Multiple | 4 |
| Absence | 15 |
| Biliary dilation | |
| Presence/Absence | 20 / 6 |
| Hypertrophy of the biliary tract | 20 |
| Diffuse | 19 |
| Gallbladder | 1 |
| Absence | 1 |
| Pathological findings | |
| Liver | 15 |
| Inflammation | 15 |
| Biliary or peribiliary tract | 14 |
| -CD 8+ T cells dominant | 8 |
| Lobular hepatitis | 2 |
| Bile duct | 8 |
| Inflammation | 8 |
| -CD 8+ T cells dominant | 2 |
| Gallbladder | 2 |
| Inflammation | 2 |
| -CD 8+ T cells dominant | 1 |
| Therapy | |
| Corticosteroid | 26 |
| UDCA | 13 |
| MMF | 6 |
| Tacrolimus | 1 |
| Bezafibrate | 1 |
| Response to steroid therapy | |
| Good | 3 |
| Moderate | 15 |
| Poor | 8 |
| Case | Ref. | Age | Sex | Primary disease | Drug | Cycles until onset | Symptoms |
| 1 | Gelsomino et al[21] | 79 | M | NSCLC | Nivolumab | 4 | Itching, jaundice |
| 2 | Kawakami et al[19] | 64 | M | NSCLC | Nivolumab | 9 | Fever, abdominal discomfort |
| 3 | Kawakami et al[19] | 73 | F | NSCLC | Nivolumab | 6 | Fever, vomiting, abdominal discomfort, diarrhea |
| 4 | Kawakami et al[19] | 82 | F | NSCLC | Nivolumab | 12 | Fever, general fatigue |
| 5 | Kashima et al[22] | 63 | M | NSCLC | Nivolumab | 24 | Epigastric pain, soft stool |
| 6 | Doherty et al[23] | 49 | F | Melanoma | Pembrolizumab | 1 | Jaundice |
| 7 | Doherty et al[23] | 59 | F | Melanoma | Nivolumab | 3 | None (liver dysfunction) |
| 8 | Doherty et al[23] | 76 | M | epithelioid mesothelioma | Pembrolizumab | 1 | Jaundice |
| 9 | Cho et al[24] | 69 | M | NSCLC | Avelumab | 21 | Right upper abdominal discomfort |
| 10 | Hamoir et al[25] | 71 | M | NSCLC | Nivolumab | NA (11 mo) | None (liver dysfunction) |
| 11 | Kuraoka et al[26] | 69 | M | NSCLC | Nivolumab | 3 | Pruritic rash, liver dysfunction |
| 12 | Ogawa et al[27] | 73 | M | Melanoma | Pembrolizumab | NA (3 mo) | None (liver dysfunction) |
| 13 | Kono et al[28] | 69 | F | GC | Nivolumab | 2 | Jaundice |
| 14 | Noda-Narita et al[29] | 57 | F | NSCLC | Nivolumab | NA (12 mo) | Abdominal pain |
| 15 | Sawada et al[30] | 76 | M | GC | Nivolumab | 4 | None (liver dysfunction) |
| 16 | Tallec et al[31] | 56 | F | NSCLC | Nivolumab | 16 (9 mo) | Myalgia, skin thickening |
| 17 | Oda et al[32] | 43 | M | GC | Nivolumab | 1 | Fever, tachycardia, appetite loss, malaise |
| 18 | Koya et al[33] | 66 | M | SCLC | Pembrolizumab | 5 | Epigastric pain |
| 19 | Fouchard et al[34] | 52 | M | NSCLC | Nivolumab | 8 | Abdominal pain |
| 20 | Fouchard et al[34] | NA | M | NSCLC | Durvalumab (+ tremelimumab) | 4 | Fever, abdominal pain |
| 21 | Fouchard et al[34] | 61 | M | NSCLC | Pembrolizumab | 17 | None (liver dysfunction) |
| 22 | Cǎlugǎreanu et al[35] | 43 | F | Melanoma | Nivolumab | 27 | Epigastralgia, anorexia, |
| 23 | Anderson et al[36] | 67 | M | NSCLC | Nivolumab | 8 | Right upper abdominal pain |
| 24 | Zen et al[37] | 68 | M | NSCLC | Pembrolizumab | NA (5.5 mo) | Abdominal pain, vomiting |
| 25 | Zen et al[40] | 67 | M | NSCLC | Pembrolizumab | NA (1 mo) | Fever, malaise |
| 26 | Our case | 61 | M | BC | Pembrolizumab | 5 | Fever |
| 27 | Our case | 89 | M | BC | Pembrolizumab | 4 | None (liver dysfunction) |
| 28 | Our case | 63 | M | NSCLC | Pembrolizumab | 7 | None (liver dysfunction) |
| 29 | Our case | 55 | M | NSCLC | Nivolumab | 11 | Abdominal pain |
| 30 | Our case | 81 | F | NSCLC | Nivolumab | 25 | Backache |
| 31 | Our case | 82 | F | NSCLC | Nivolumab | 2 | None (liver dysfunction) |
| Case | T-Bil/AST/ALT/ALP/GGT/IgG4 | Biliary stenosis /dilation | Hypertrophy of biliary tract | Pathological findings | Treatment (Dosage) | Steroid response |
| 1 | Grade 4/NA/Grade 3; Grade 3/Grade 4/NA | NA | NA | Liver: CD8+ T cells infiltration in bile duct | mPSL (1 mg/kg), + UDCA (15 mg/kg) | Moderate |
| 2 | 0.7/142/144; 1769/902/normal | -/+ | Diffuse | Liver: CD8+ and CD4+ T cells infiltration in Glisson’s capsule | PSL (0.5 mg/kg) | Poor |
| 3 | 3.8/89/101; 1947/804/normal | -/+ | Diffuse | NA | PSL (0.5 mg/kg), Biliary drainage | Moderate |
| 4 | 0.8/108/70; 2996/813/normal | -/+ | Diffuse | Liver: CD8+ and CD4+ T cells infiltration in Glisson’s capsule | Biliary drainage | - |
| 5 | NA/88/92; 1543/NA/NA | Distal bile duct/+ | Diffuse | Bile duct: Interstitial fibrosis, neutrophils infiltration in mucosa | PSL (2 mg/kg) Biliary drainage | Moderate |
| 6 | NA/961/1536; 237/2094/NA | NA/- | NA | Liver: Severe steatohepatitis, absence of bile ducts | 1st PSL (1 mg/kg), 2nd PSL + UDCA (NA) + MMF (2 g) | Poor |
| 7 | NA/NA/>300; >1000/NA/NA | NA | NA | Liver: Degenerative bile duct atypia and periductal fibrosis | 1st PSL (1 mg/kg), 2nd PSL + UDCA (NA) | Poor |
| 8 | NA/NA/>500; >700/NA/NA | NA | NA | Liver: Attenuated bile duct, cellular and canalicular cholestasis in parenchyma | mPSL (2 mg/kg) + cholestyramine (NA) + MMF (1 g) + UDCA (NA) | Poor |
| 9 | 0.6/Grade 1/Grade 1; Grade 2/Grade 2/NA | -/+ | Diffuse | NA | mPSL (1 mg/kg) | Moderate |
| 10 | Normal/129/135; 558/984/NA | Multiple/- | None | Liver: CD8+ T cell infiltration in the periportal zone and cholangitis | mPSL (0.5 mg/kg), + UDCA (10 mg/kg) | Good |
| 11 | NA/NA/NA; NA/NA/NA | -/+ | Diffuse | Bile duct: Inflammatory cells and lymphocytes infiltration in epithelium | 1st PSL (60 mg), 2nd mPSL (500 mg) | Poor |
| 12 | NA/58/77; 1111/461/NA | Multiple/+ | Diffuse | Bile duct: Destruction of epithelium, fibrosis with CD8+ T cell infiltration in submucosa | Discontinuation of Pembrolizumab | - |
| 13 | 15.9/454/NA; 5066/NA/20.2 | Intrahepatic bile duct/- | Gall bladder | NA | Biliary drainage | - |
| 14 | NA/NA/NA; 1065/304/normal | -/+ | Diffuse | NA | UDCA (300 mg) | - |
| 15 | 0.8/69/68; 2427/252/41.0 | -/+ | NA | Liver: Eosinophil, CD8+, and CD4+ T cell infiltration in the portal tract. Eosinophil infiltration in the epithelial linings of the bile duct | PSL (0.5 mg/kg), + UDCA (NA) | Good |
| 16 | Normal/272/516; 615/442/NA | NA | Diffuse | NA | Corticosteroid (NA) | Good |
| 17 | 3.7/49/31; 598/151/90 | -/- | NA | Liver: CD8+ T cells and macrophage infiltration in bile duct | 1st PSL (1 mg/kg), 2nd m PSL (1 g), 3rd PSL + MMF (2 g) | Poor |
| 18 | 1.1/313/296; 2241/868/normal | Intrahepatic bile duct/+ | Diffuse | Liver: CD8+ T cell and eosinophil infiltration in the periportal zones, Bile duct: CD8+ T cell infiltration and fibrosis in submucosa | 1st UDCA (900 mg) + bezafibrate (400 mg), 2nd m PSL (0.5 g) followed by PSL (1 mg/kg), Biliary drainage | Poor |
| 19 | Normal/>100/>100; >900/>500/NA | -/+ | NA | Gall bladder: Inflammatory cell infiltration | PSL (0.5 mg/kg) + UDCA (NA), Cholecystectomy | Moderate |
| 20 | NA/>100/>300; >800/>1700/normal | -/+ | NA | Gall bladder: CD8+ T cell infiltration | PSL (120 mg) + UDCA (NA), Cholecystectomy | Moderate |
| 21 | Normal/NA/>100; >400/>1400/NA | NA | NA | NA | PSL (1 mg/kg) | Moderate |
| 22 | Normal/52/126; 545/1007/NA | Multiple/+ | NA | Liver: CD3+ and CD8 T cell infiltration in the bile duct | PSL (1 mg/kg) | Moderate |
| 23 | NA/>300/NA; 793/NA/NA | Multiple/+ | Diffuse | Liver: Fibrosis and inflammation in the portal tract, lobular inflammation, mild macrovesicular steatosis | mPSL (NA) following PSL (50 mg), MMF (NA), Tacrolimu (NA)s | Poor |
| 24 | 0.5/67/68; 2107/279/59 | NA/- | Diffuse | Liver: Cholangiopathologic change, CD8/CD4 ratio 12:7, Bile duct: Lymphocyte, eosinophil and plasma cell infiltration | PSL (50 mg) | Moderate |
| 25 | 1.2/198/233; 1540/332/78 | NA/- | Diffuse | Liver: Lobular hepatitis with cholangiopathic change, CD8/CD4 ratio 17:2 | PSL (40 mg) | Moderate |
| 26 | 0.3/91/65; 1683/159/80.4 | -/+ | Diffuse | Bile duct: Inflammatory cell infiltration | PSL (1 mg/kg) + UDCA (600 mg) | Moderate |
| 27 | 0.4/245/124; 1245/114/352 | -/+ | Diffuse | Bile duct: Neutrophil and lymphocyte infiltration | UDCA (600 mg) | - |
| 28 | 0.6/184/254; 1783/452/128 | -/+ | Diffuse | Bile duct: Inflammatory cell infiltration | PSL (1 mg/kg) + UDCA (600 mg) | Moderate |
| 29 | 0.3/64/245; 1328/448/67.3 | Intrahepatic bile duct/+ | Diffuse | NA | mPSL (2 mg/kg) + MMF (2 g) | Moderate |
| 30 | 1.3/284/248; 3029/1070/NA | -/+ | Diffuse | NA | mPSL (2 mg/kg) + MMF (2 g), Biliary drainage | Moderate |
| 31 | 0.7/294/85; 4635/829/NA | -/+ | Diffuse | Liver: Lymphocyte infiltration in Glisson’s capsule, hydropic degeneration of hepatocytes | mPSL (1.6 mg/kg) | Moderate |
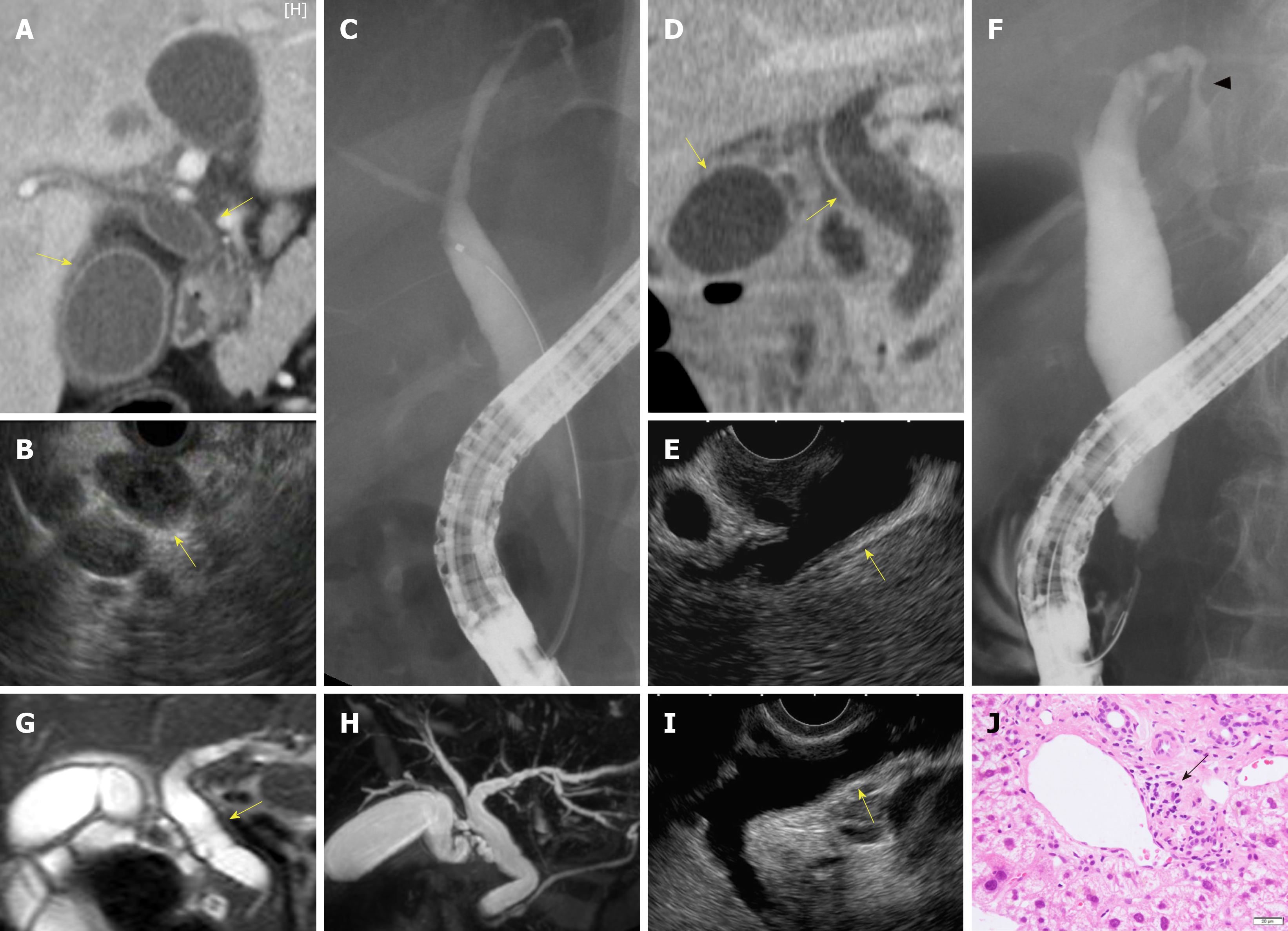
Biliary stenosis occurred in 8 patients (34.8%, 8/23); 7 had stenosis in the intrahepatic biliary tract, which included 4 patients with multiple strictures in the biliary tract. Biliary dilation was observed in 20 patients (76.9%, 20/26). Twenty patients had hypertrophy of the biliary tract (95.2%, 20/21), of which 19 cases were diffuse (Figure 2).
Peroral cholangioscopy was performed in only 5 cases[26,33,38]. Multiple scarred lesions and band-like narrowing were found in 4 patients, and 2 patients with PD-1 inhibitor-related SC showed diverticulum-like outpouching[33,38]. Ulcerative lesions with many black spots (e.g., “burned-out” epithelium) and yellow plaque were found in 1 patient[26].
In total, 23 patients underwent pathological evaluation; of these, 15 patients underwent liver biopsy. Fourteen patients had inflammatory changes in the bile duct and/or peribiliary tract, and CD8+ T cells were dominant in the inflammatory cells in 8 of these patients (53.3%, 8/15). Lobular hepatitis was found in 2 patients (13.3%, 2/15).
Transpapillary biopsy of the biliary tract with biopsy forceps was performed for 8 patients, and these pathological findings revealed Inflammatory cells infiltration in the bile duct. In 2 patients, CD8+ T cells were the dominant inflammatory cells in the bile duct.
Of the 6 patients that underwent biliary drainage, 5 did not respond.
Corticosteroids were the main treatment for PD inhibitor-related SC (83.8%, 26/31). Only 3 patients who the levels of liver and biliary enzymes were improved to normal level with steroid therapy, so that the response rate to corticosteroids was 11.5% (3/26). Eight patients with PD-1 inhibitor-related SC had poor response, no improvement of liver and biliary enzymes, to steroid therapy. In 15 patients who received steroid therapy, the levels of liver and biliary enzymes were improved, although normalization of enzyme activities was not achieved (i.e. only a moderate response occurred).
PD-1/PD-L1 inhibitors are used widely for the treatment of many types of malignancies. However, irAEs, including cardiac, respiratory, endocrine, gastrointestinal, musculoskeletal, skin, and, importantly, hepatobiliary disorders, were also reported[15-18]. However, the reasons for the occurrence of irAEs, including cholangitis, are unclear, although it may involve the T cell, antibody, and cytokine responses[39].
Gelsomino et al[20,21] reported the first case of PD-1 inhibitor-related SC; subsequently, this group and Kawakami et al[19] suggested the clinical features of PD-1 inhibitor-related SC. However, PD-1 inhibitor-related SC is still not well known. Kawakami et al[19] reported SC related to the PD-1 inhibitor nivolumab was characterized by: (1) Localized extrahepatic bile duct dilation without obstruction; (2) Diffuse hypertrophy of the extrahepatic bile duct wall; (3) A dominant increase in the biliary tract enzymes alkaline phosphatase and gamma-glutamyl transpeptidase relative to hepatic enzymes aspartate aminotransferase and alanine aminotransferase; (4) Normal or reduced levels of the serum immunological markers, such as antinuclear antibody, antimitochondrial antibody, smooth muscle antibody, and IgG4; (5) The pathological finding of biliary tract CD8+ T cell infiltration from liver biopsy; and (6) A moderate to poor response to steroid therapy. In our study, some clinical features, such as biliary dilation without obstruction, diffuse hypertrophy of the extrahepatic biliary tract, liver dysfunction with a dominant increase in the biliary tract enzymes relative to hepatic enzymes, normal level of serum IgG4, and a moderate to poor response to steroid therapy, were similar to those reported by Kawakami et al[19]. In contrast, Gelsomino et al[20] suggested that there were different types of PD-1 inhibitor-related SC, such as large duct cholangitis and small ducts cholangitis, and that those types have different clinical presentation and biochemical evolution and were associated with various outcomes. Indeed, in our case review, 15 patients had diffuse extrahepatic biliary hypertrophy without biliary stenosis (extrahepatic type). Three patients had multiple stenoses, especially in the intrahepatic bile duct, without extrahepatic biliary hypertrophy (intrahepatic type). Moreover, four patients had diffuse biliary tract hypertrophy with multiple stenoses of the intrahepatic and extrahepatic bile ducts (diffuse type). The clinical implications of these types of PD-1 inhibitor-SC is uncertain, but may be clarified by more cases in the future.
Zen et al[40] reported that CD8+ T lymphocytes were the predominant infiltrates in the bile duct of patients with PD-1 inhibitor-related SC, similar to hepatic irAEs. Moreover, they reported the clinical features and detailed pathological findings of 10 cases of hepatobiliary disorders caused by PD-1 inhibitors. In that study, the ratio of CD8+ to CD4+ cells was significantly higher than that in autoimmune hepatitis or idiosyncratic drug-induced liver injury[37]. Although CD8+ T cell infiltration is one of the clinical features of irAEs, in the pathological findings of PD-1 inhibitor-related SC, CD8+ T cells were not necessarily dominant, especially in the bile duct biopsy. Other inflammatory cells, such as eosinophils, neutrophils, plasma cells, and macrophages, were also observed in the biliary tract. Although this finding may be used for auxiliary diagnosis for PD-1 inhibitor-SC, it may not always be observable.
In general, steroid therapy was recommended for the treatment of irAEs[41,42], however, corticosteroids were not useful for the treatment of PD-1 inhibitor-related SC. Although four patients received high-dose steroid therapy (methylprednisolone, 500–1000 mg/d), a good response was not shown. Therefore, at least in our study, steroid therapy was not recommended for the treatment of PD-1 inhibitor-related SC. However, the response to steroid therapy may be dependent on the type of PD-1 inhibitor-related SC, as described above. Although 15 patients with extrahepatic and diffuse type PD-1 inhibitor-related SC received steroid therapy in our case review, a good response occurred only in one case (6.7%, 1/15). Meanwhile, only two patients with intrahepatic type PD-1 inhibitor-related SC received steroid therapy: One patient’s liver function was improved and the other had a moderate response, with a response ratio of 1:1. This finding is still uncertain in a few cases.
Ursodeoxycholic acid (UDCA) was used for treatment of PD-1 inhibitor-related SC in 13 patients. Two patients received only UDCA, with discontinuation of nivolumab, and displayed a moderate response. In contrast, no response was found in the single patient who received UDCA with bezafibrate. Seven patients received combination therapy of steroids and UDCA. The response rate to that therapy was 28.6% (2/7). Three patients received steroid therapy first; when no improvement was observed, UDCA was added and a moderate response was observed in these patients. Although the efficacy was insufficient, UDCA was considered a treatment for PD-1 inhibitor-related SC owing to the low rate of adverse events[43].
Other anti-inflammatory agents, including immunomodulators or infliximab were sometimes considered to using for treatment of irAEs[44]. Tacrolimus, an immunomodulator, was also used for one case of PD-1 inhibitor-related SC; however, the response was insufficient. Infliximab was used for some irAEs, such as colitis and pneumonitis. In our case review, infliximab was not used for the treatment of PD-1 inhibitor-related SC. More cases may be needed to evaluate the usefulness of these drugs for PD-1 inhibitor-related SC.
This study had some limitations. First, there are no current diagnostic criteria for PD-1 inhibitor-related SC. Second, some clinical cases, for which blood test data, image findings, and pathological evaluation were not presented, were included in this study. Therefore, our study may include different diseases that cause sclerosing cholangitis.
In conclusion, some clinical features of PD-1 inhibitor-related SC, such as biliary dilation without obstruction, diffuse hypertrophy of the extrahepatic biliary tract and/or multiple strictures of intrahepatic biliary tract, liver dysfunction with a dominant increase in biliary tract enzymes relative to hepatic enzymes, normal level of serum IgG4, and a moderate-to-poor response to steroid therapy, were revealed, although there were many unsolved questions in our study. To establish the diagnostic criteria for PD-1 inhibitor-related SC, more cases, for which clinical data including hepatobiliary enzymes, immunological marker, image findings, and pathological evaluation were presented clearly, need to be evaluated. Although CD8+ T cell infiltration is one of the pathological features of PD-1 inhibitor-related SC, it is not enough to exclude different diseases that cause sclerosing cholangitis. We will have to find more specific features of PD-1 inhibitor-related SC.
Programmed cell death-1 (PD-1) inhibitor has been indicated for many types of malignancies. On the other hands, these inhibitors cause immune-related adverse events (irAEs). Hepatobiliary disorder is a phenotype of irAEs that affect 0%–4.5% of patients treated with PD-1 inhibitors.
Recently, PD-1 inhibitor-related sclerosing cholangitis (SC), one of the irAEs, have been reported. However, the clinical and pathological features of PD-1 inhibitor-related SC are uncertain.
The objective of this study to evaluate the clinical and pathological features of PD-1 inhibitor-related SC through a systematic review of the literature.
We conducted an electronic search through databases of PubMed. The review was restricted to the period from January 2014 to September 2019 and focused on case reports/series on PD-1 inhibitor-related SC published in English. The reference lists of the identified papers were also scanned to find out further relevant studies. Six cases previously studied by us, including three that have not yet been published, were included in this review.
Thirty-one PD-1 inhibitor-related SC cases were evaluated. The median number of cycles until PD-1 inhibitor-related SC onset was 5.5 (range, 1–27). Abdominal pain or discomfort (35.5%, 11/31) was the most frequent symptom. Liver dysfunction with a notable increase in biliary tract enzymes relative to hepatic enzymes, and a normal level of serum IgG4 were shown in blood serum test. Biliary dilation without obstruction (76.9%, 20/26), diffuse hypertrophy of the extrahepatic biliary tract (90.5%, 19/21), and multiple strictures of the intrahepatic biliary tract (30.4%, 7/23) were noted. CD8+ T cells were the dominant inflammatory cells in the bile duct or peribiliary tract in 11/23 (47.8%) cases. The response rate of corticosteroids for PD inhibitor-related SC was 11.5% (3/26).
Some clinical features of PD-1 inhibitor-related SC, such as biliary dilation without obstruction, diffuse hypertrophy of the extrahepatic biliary tract and/or multiple strictures of intrahepatic biliary tract, liver dysfunction with a dominant increase in biliary tract enzymes relative to hepatic enzymes, normal level of serum IgG4, and a moderate-to-poor response to steroid therapy, were revealed.
To establish the diagnostic criteria for PD-1 inhibitor-related SC, more cases, for which clinical data including hepatobiliary enzymes, immunological marker, image findings, and pathological evaluation were presented clearly, need to be evaluated. We will have to find more specific features of PD-1 inhibitor-related SC.
We wish to thank to our colleagues in the Departments of Gastroenterology and Hepatology, and Pathology at Tottori University Faculty of Medicine (Tottori, Japan).
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Uhlmann D, Yang L S-Editor: Zhang L L-Editor: A E-Editor: Xing YX
| 1. | Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1835] [Cited by in RCA: 1776] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 2. | Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3192] [Cited by in RCA: 3321] [Article Influence: 276.8] [Reference Citation Analysis (0)] |
| 3. | Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2210] [Cited by in RCA: 2221] [Article Influence: 222.1] [Reference Citation Analysis (0)] |
| 4. | Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3964] [Cited by in RCA: 4350] [Article Influence: 435.0] [Reference Citation Analysis (0)] |
| 5. | Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5686] [Cited by in RCA: 6742] [Article Influence: 674.2] [Reference Citation Analysis (0)] |
| 6. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7537] [Article Influence: 753.7] [Reference Citation Analysis (0)] |
| 7. | Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2598] [Cited by in RCA: 2805] [Article Influence: 280.5] [Reference Citation Analysis (0)] |
| 8. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4605] [Article Influence: 460.5] [Reference Citation Analysis (0)] |
| 9. | Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1915] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 10. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1716] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 11. | Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 799] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 12. | Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A; KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4026] [Cited by in RCA: 4488] [Article Influence: 448.8] [Reference Citation Analysis (1)] |
| 13. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4953] [Article Influence: 619.1] [Reference Citation Analysis (0)] |
| 14. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7247] [Article Influence: 724.7] [Reference Citation Analysis (0)] |
| 15. | Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 614] [Article Influence: 76.8] [Reference Citation Analysis (1)] |
| 16. | Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 169] [Reference Citation Analysis (0)] |
| 17. | Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 638] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 18. | Raschi E, Mazzarella A, Antonazzo IC, Bendinelli N, Forcesi E, Tuccori M, Moretti U, Poluzzi E, De Ponti F. Toxicities with Immune Checkpoint Inhibitors: Emerging Priorities from Disproportionality Analysis of the FDA Adverse Event Reporting System. Target Oncol. 2019;14:205-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, Kamata K, Takenaka M, Kimura M, Chikugo T, Sato T, Kudo M, Ito A, Nakagawa K. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs. 2017;35:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Gelsomino F, Vitale G, Ardizzoni A. A case of nivolumab-related cholangitis and literature review: how to look for the right tools for a correct diagnosis of this rare immune-related adverse event. Invest New Drugs. 2018;36:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Gelsomino F, Vitale G, D'Errico A, Bertuzzi C, Andreone P, Ardizzoni A. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol. 2017;28:671-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Kashima J, Okuma Y, Shimizuguchi R, Chiba K. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: a case report. Cancer Immunol Immunother. 2018;67:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 23. | Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, Parkinson CA, Corrie PG. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open. 2017;2:e000268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Cho JH, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Late-Onset Cholecystitis with Cholangitis after Avelumab Treatment in Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13:e34-e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Hamoir C, de Vos M, Clinckart F, Nicaise G, Komuta M, Lanthier N. Hepatobiliary and Pancreatic: Nivolumab-related cholangiopathy. J Gastroenterol Hepatol. 2018;33:1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Kuraoka N, Hara K, Terai S, Yatabe Y, Horio Y. Peroral cholangioscopy of nivolumab-related (induced) ulcerative cholangitis in a patient with non-small cell lung cancer. Endoscopy. 2018;50:E259-E261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Ogawa K, Kamimura K, Terai S. Antiprogrammed Cell Death-1 Immunotherapy-Related Secondary Sclerosing Cholangitis. Hepatology. 2019;69:914-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Kono M, Sakurai T, Okamoto K, Masaki S, Nagai T, Komeda Y, Kamata K, Minaga K, Yamao K, Takenaka M, Watanabe T, Nishida N, Kudo M. Efficacy and Safety of Chemotherapy Following Anti-PD-1 Antibody Therapy for Gastric Cancer: A Case of Sclerosing Cholangitis. Intern Med. 2019;58:1263-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Noda-Narita S, Mizuno S, Noguchi S, Watanabe K, Nakai Y, Koike K, Kage H, Nagase T. Development of mild drug-induced sclerosing cholangitis after discontinuation of nivolumab. Eur J Cancer. 2019;107:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Sawada K, Shonaka T, Nishikawa Y, Hasegawa K, Hayashi H, Hasebe T, Nakajima S, Ikuta K, Fujiya M, Furukawa H, Okumura T. Successful Treatment of Nivolumab-related Cholangitis with Prednisolone: A Case Report and Review of the Literature. Intern Med. 2019;58:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Le Tallec E, Ricordel C, Triquet L, Deniel A, Marcorelles P, Lena H, Jego P, Belhomme N. An Original Case of an Association of Eosinophilic Fasciitis with Cholangitis Induced by Nivolumab. J Thorac Oncol. 2019;14:e13-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Oda H, Ishihara M, Miyahara Y, Nakamura J, Kozuka Y, Iwasa M, Tsunoda A, Yamashita Y, Saito K, Mizuno T, Shiku H, Katayama N. First Case of Cytokine Release Syndrome after Nivolumab for Gastric Cancer. Case Rep Oncol. 2019;12:147-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Koya Y, Shibata M, Shinohara N, Nebuya S, Oe S, Honma Y, Senju M, Sato N, Harada M. Secondary sclerosing cholangitis with hemobilia induced by pembrolizumab: Case report and review of published work. Hepatol Res. 2019;49:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Fouchard M, Jantzem H, Quere G, Descourt R, Robinet G, Poureau PG. Three cases of immune cholangitis related to anti-programmed cell death and programmed cell death ligand agents for the treatment of non-small cell lung cancer. Eur J Cancer. 2019;115:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Cǎlugǎreanu A, Rompteaux P, Bohelay G, Goldfarb L, Barrau V, Cucherousset N, Heidelberger V, Nault JC, Ziol M, Caux F, Maubec E. Late onset of nivolumab-induced severe gastroduodenitis and cholangitis in a patient with stage IV melanoma. Immunotherapy. 2019;11:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Anderson B, Dawe DE. Nivolumab-Induced Secondary Sclerosing Cholangitis with Deterioration Despite Immunosuppression. J Thorac Oncol. 2019;14:e205-e206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Zen Y, Chen YY, Jeng YM, Tsai HW, Yeh MM. Immune-related adverse reactions in the hepatobiliary system: second-generation check-point inhibitors highlight diverse histological changes. Histopathology. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Onoyama T, Takeda Y, Kato M, Edano M, Tarumoto R, Matsumoto K, Isomoto H. Peroral cholangioscopy of programmed cell death-1 inhibitor-related sclerosing cholangitis: three case reports. Endoscopy. 2019;51:E402-E403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3160] [Article Influence: 451.4] [Reference Citation Analysis (0)] |
| 40. | Zen Y, Yeh MM. Checkpoint inhibitor-induced liver injury: A novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol. 2019;36:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119-iv142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 1504] [Article Influence: 188.0] [Reference Citation Analysis (1)] |
| 42. | Brahmer JR, Lacchetti C, Thompson JA. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2018;14:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 43. | Zhu GQ, Shi KQ, Huang GQ, Wang LR, Lin YQ, Braddock M, Chen YP, Zhou MT, Zheng MH. A network meta-analysis of the efficacy and side effects of UDCA-based therapies for primary sclerosing cholangitis. Oncotarget. 2015;6:26757-26769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1423] [Article Influence: 177.9] [Reference Citation Analysis (0)] |