Published online Jan 21, 2020. doi: 10.3748/wjg.v26.i3.324
Peer-review started: October 7, 2019
First decision: November 10, 2019
Revised: December 13, 2019
Accepted: December 21, 2019
Article in press: December 21, 2019
Published online: January 21, 2020
Processing time: 100 Days and 22.2 Hours
Liver cancer is the fifth most common cancer and the second cause of cancer-related deaths worldwide. Transarterial chemoembolization (TACE) is the best treatment of intermediate hepatocellular carcinoma (HCC). Doxorubicin is the most commonly used drug despite a low level of evidence.
To compare the objective response rate of idarubicin-based TACE (Ida-TACE) against doxorubicin-based TACE (Dox-TACE) in intermediate stage HCC.
Between January 2012 and December 2014, all patients treated with TACE at our academic hospital were screened. Inclusion criteria were patients with Child-Pugh score A or B, a performance status below or equal to 1, and no prior TACE. Either lipiodol TACE or drug-eluting beads TACE could be performed with 10 mg of idarubicin or 50 mg of doxorubicin. Each patient treated with idarubicin was matched with two doxorubicin-treated patients. The TACE response was assessed by independent radiologists according to the mRECIST criteria.
Sixty patients were treated with doxorubicin and thirty with idarubicin. There were 93% and 87% of cirrhotic patients and 87% and 70% of Child-Pugh A in the doxorubicin and idarubicin groups, respectively. The median number of HCC per patient was two in both groups with 31% and 26% of single nodules in doxorubicin and idarubicin groups, respectively. Objective response rate after first TACE was 76.7% and 73.3% (P = 0.797) with 41.7% and 40.0% complete response in doxorubicin and idarubicin groups, respectively. Progression-free survival was 7.7 mo in both groups, and liver transplant-free survival was 24.9 mo and 21.9 mo in doxorubicin and idarubicin groups, respectively. Safety profiles were similar in both groups, with grade 3-4 adverse events in 35% of Dox-TACE and 43% of Ida-TACEs.
Ida-TACE and Dox-TACE showed comparable results in terms of efficacy and safety. Ida-TACE may represent an interesting alternative to Dox-TACE in the management of patients with intermediate stage HCC.
Core tip: Transarterial chemoembolization in the treatment of choice for intermediate stage hepatocellular carcinoma. Doxorubicin is the most used drug without any satisfying evidence of its superiority compared with other drugs. An increasing number of preclinical and early phase clinical studies suggest the superiority of idarubicin anti-tumor efficacy in transarterial chemoembolization. With the limits relative to retrospective analysis, this study shows that idarubicin represents an alternative to doxorubicin in the treatment of hepatocellular carcinoma with comparable efficacy and safety. It needs to be confirmed by randomized clinical trials.
- Citation: Roth GS, Teyssier Y, Abousalihac M, Seigneurin A, Ghelfi J, Sengel C, Decaens T. Idarubicin vs doxorubicin in transarterial chemoembolization of intermediate stage hepatocellular carcinoma. World J Gastroenterol 2020; 26(3): 324-334
- URL: https://www.wjgnet.com/1007-9327/full/v26/i3/324.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i3.324
Liver cancer is the fifth most common cancer and the second cause of cancer-related deaths worldwide[1]. Hepatocellular carcinoma (HCC) represents 90% of liver cancer. Only 30% of HCC patients can have access to surgical resection, percutaneous ablation, or liver transplantation (LT). Among the recommended treatments for intermediate HCC, classified as Barcelona Clinic of Liver Cancer (BCLC) B, transarterial chemoembolization (TACE) is the best treatment based upon randomized controlled trial and meta-analysis[2,3].
In these studies, local control is obtained in 5%-55% of patients but with a short time-to-progression (6 mo to 12 mo), due to a high rate of recurrence. A recent systematic review including 10108 patients, showed a tumor response rate of 52.5%, and a median overall survival (OS) of 19.4 mo[4]. Chemotherapeutic agents can be delivered either through lipiodol emulsion [the conventional method (cTACE)] or loaded on drug-eluting microbeads (DEB-TACE), even though the latter method did not show any superiority in anti-tumor efficacy as well as no clear benefit in terms of safety[5,6]. Several methods were explored to improve results of TACE, such as the combination of systemic chemotherapies, without success[7,8]. Surprisingly, the choice of the drug used in TACE is still debated. Doxorubicin is commonly used, but no solid data confirmed its superiority compared to other drugs such as epirubicin, cisplatin, or idarubicin. In vitro data have shown superiority of idarubicin to doxorubicin, especially in the SNU-449 cell line[9]. Besides, two phase 1 trials studying idarubicin in HCC showed promising results either by using drug-eluting beads or lipiodol emulsion[10,11]. An open label and single arm phase 2 trial is currently ongoing to assess the anti-tumor efficacy of idarubicin-loaded beads in HCC (NCT02185768).
This study aims to compare idarubicin-based TACE (Ida-TACE) with doxorubicin-based TACE (Dox-TACE) in the treatment of intermediate HCC.
We retrospectively reviewed the medical files and imaging examinations of all consecutive patients undergoing TACE for HCC at our University hospital from 2012 to 2014. During this period, due to local drug supply constraints, patients were treated either by Dox-TACE or Ida-TACE.
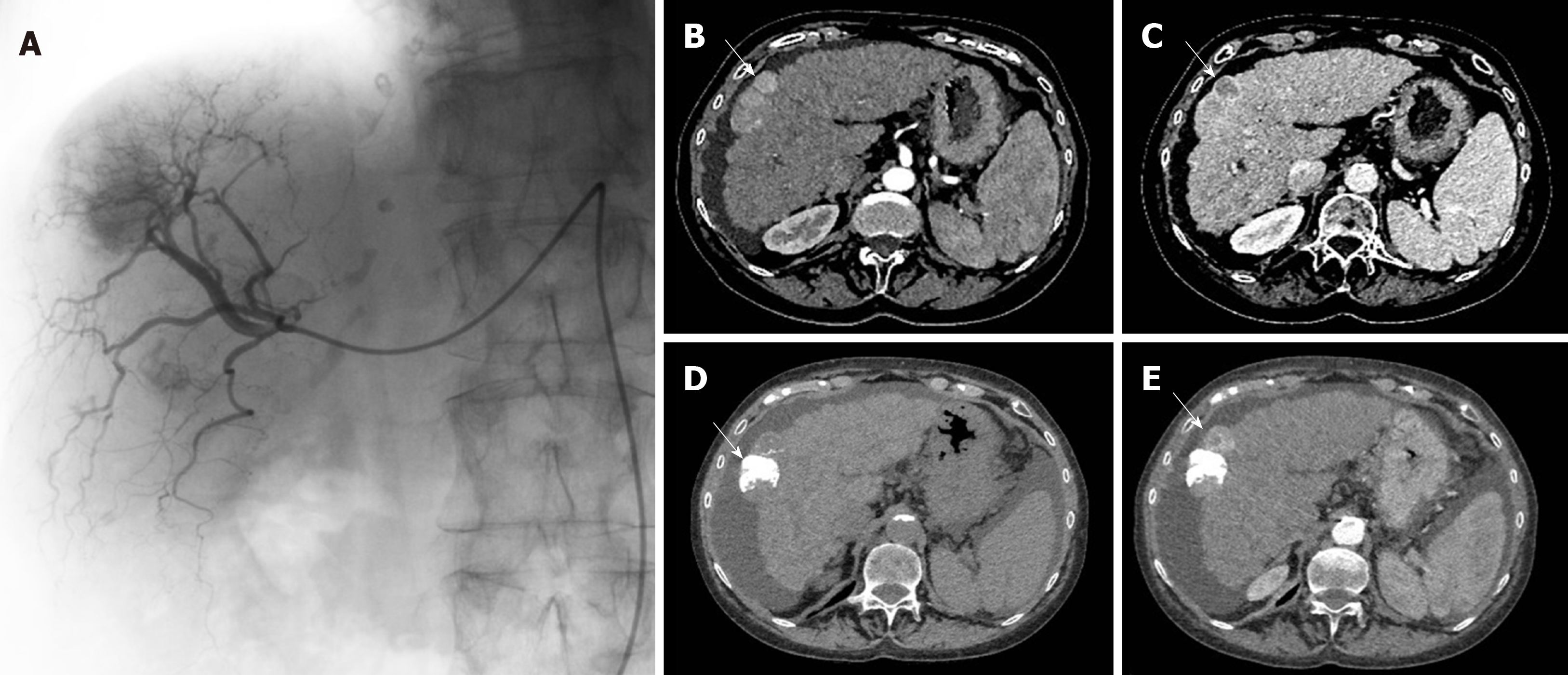
Patients were treated with TACE following standard local protocol. Each indication of TACE was validated during multidisciplinary tumor board including a hepatologist, an interventional radiologist, and a liver surgeon. Procedures were realized in an interventional radiology suite (Allura Integris, Philips Medical Systems, Eindhoven, Netherlands). The contrast media used was Xenetix 350 (Xenetix®, Guerbet, Roissy, France). First, diagnostic arteriography was performed under local anesthesia, through the right femoral artery, using a 4-French introducer sheath. Portal vein patency and arterial vascular anatomy were appreciated due to the catheterization of the superior mesenteric artery and the coeliac trunk (Figure 1). Chemoembolization was as selective as possible according to tumor localization and number. The use of a microcatheter was left to the radiologist’s discretion. cTACE or DEB-TACE could be performed. During cTACE, 50 mg of injectable lyophilized doxorubicin (Adriblastina®, Pfizer Pharma, United States) or 10 mg of injectable lyophilized idarubicin (Zavedos®, Pfizer Pharma, United States) were manually emulsified with 5-10 mL of iodized oil (Lipiodol® Ultra Fluide, Guerbet, France) before infusion. Drug-eluting beads (100 µm; Embozene Tandem® microspheres, Celonova Biosciences, Germany) were used for DEB-TACE procedures. Lipiodol emulsion or DEB were injected until saturation of tumor feeding arteries. In the case of cTACE, drug administration was immediately followed by embolization using absorbable gelatine sponge (Curaspon®; Curamedical, Netherlands) to obtain an arterial flow stop during 10 min. As recommended by European guidelines and as routinely done in our department, TACE could be repeated 2 mo after the first treatment in case of partial response (PR) on postoperative scan[12].
Inclusion criteria were patients treated with TACE for HCC, with Child-Pugh score A or B, with a performance status below or equal to 1, and with no prior TACE. TACE as a bridge to transplantation was not an exclusion criterion.
Every patient gave his written consent before TACE, and study ethics were approved by an independent institutional review board (CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, IRB 5891).
The primary aim of this study was to compare the objective response rate (ORR) after first TACE between doxorubicin and idarubicin treated patients. Secondary objectives were to compare disease control rate (DCR), progression-free survival (PFS), OS, liver transplant-free survival (LTFS), and adverse events (AEs) between doxorubicin and idarubicin groups. A safety analysis was also performed with collection of early AEs occurring within the 1st month. Grading of AEs was done based on Common Terminology Criteria for Adverse Events V5.0. Chronic liver toxicity considered as liver function deterioration between 30 d and 90 d after TACE was also analyzed.
All imaging examinations were archived in a picture archiving and communication system and were reviewed. Medical parameters were extracted from the patient’s electronic medical records by one of the authors who independently reviewed the entire medical record. Thus, radiological analyses were realized blindly to treatment type and clinical data.
The following patient characteristics were collected: Sex, age, World Health Organization (WHO) performance status, body mass index, etiology of chronic liver disease, and cirrhosis status.
Every patient had pre-operative imaging and post-operative imaging within 3 mo after TACE as recommended[12], to assess tumor response and determine the need to perform an additional TACE or not. Radiological examination was performed with a computed tomography scan (Figure 1) or magnetic resonance scan. Imaging data collected were tumor number and largest nodules measurements on preoperative imaging. Tumor response was assessed according to the mRECIST criteria[12] with complete response, PR, stable disease, and progressive disease.
During the post-embolization period, AEs were monitored on clinical examinations, systematic biological follow-up (renal and hepatic functions), and classical imaging follow-up.
Patients were matched as follows: Doxorubicin:idarubicin (2:1) based on tumor number, sum of the two largest tumors, Child-Pugh score, platelet count, WHO performance status level, cTACE, or DEB-TACE. Matching was performed blindly to tumor response data of TACE.
The ORR and DCR were compared using exact Fischer tests. The PFS and LTFS were computed by clinical characteristics using the Kaplan-Meier method. In LTFS, events corresponded to liver transplant or death. Log-rank tests were used to compare survival rates. Liver transplant events were censured in OS analyses. Analyses were performed using Stata version 13.0 (Stata Corporation, College Station, TX, United States).
Between 2012 and 2014, 90 patients who benefited from TACE for HCC were included in this study. Thirty patients treated with Ida-TACE were matched with sixty patients treated with Dox-TACE. Both groups were comparable concerning demographics data (Table 1).
| Characteristics | Doxorubicin, n = 60 | Idarubicin, n = 30 |
| Clinical and biological data | ||
| Sex, male/female | 54 (90.0)/6 (10.0) | 28 (93.3)/2 (6.7) |
| Age in yr, mean ± SD | 62.9 ± 9.0 | 61.9 ± 9.8 |
| WHO performance status 0/1 | 51 (85.0)/9 (15.0) | 25 (83.3)/5 (16.6) |
| BMI, median (IQR) | 27.6 (24.7-32.1) | 25.2 (22.9-27.2) |
| < 20 | 2 (3.3) | 2 (6.9) |
| 20-24.9 | 15 (25.0) | 12 (41.4) |
| ≥ 25 | 43 (71.7) | 15 (51.7) |
| Tumor characteristics | ||
| Tumor number, median (IQR) | 2 (1-3) | 2 (2-3.75) |
| 1 nodule | 17 (28.3) | 7 (23.3) |
| 2-3 nodules | 31 (51.7) | 15 (50.0) |
| > 3 nodules | 12 (20.0) | 8 (26.7) |
| 2 largest nodules sum in mm, median (IQR) | 49.5 (32.8-62.0) | 40.5 (30.5-58.8) |
| Partial portal vein thrombosis | 8 (13) | 3 (10) |
| Underlying cirrhosis | ||
| Yes/no | 56 (93.3)/4 (6.7) | 26 (86.7)/4 (13.3) |
| Child-Pugh class A/B | 52 (86.7)/8 (13.3) | 21 (70.0)/9 (30.0) |
| MELD, median (IQR) | 9.22 (8.13-10.57) | 8.19 (7.34-9.86) |
| Etiology | ||
| Alcohol | 18 (30.0) | 6 (20.0) |
| HBV/HCV infection | 11 (18.3) | 4 (13.3) |
| Metabolic | 7 (11.7) | 1 (3.3) |
| Alcohol + HBV/HCV | 3 (5.0) | 8 (26.7) |
| Alcohol + Metabolic | 16 (26.7) | 5 (16.7) |
| Hemochromatosis | 3 (5.0) | 2 (6.7) |
| Biological parameters | ||
| Platelet count in G/L, median (IQR) | 112.5 (82.8-171.5) | 106.5 (86.3-135.3) |
| Albumin in g/dL, median (IQR) | 3.7 (3.4-3.9) | 3.5 (3.1-3.9) |
| Total bilirubin in mg/dL, median (IQR) | 0.94 (0.58-1.18) | 0.73 (0.48-1.04) |
| AST in IU/L, median (IQR) | 46 (33-65) | 55 (34-68) |
| ALT in IU/L, median (IQR) | 44 (34-70) | 40 (29-60 |
| GGT in IU/L, median (IQR) | 152 (92-261) | 185 (141-332) |
| ALP in IU/L, median (IQR) | 105 (88-133) | 115 (88-139) |
| PT as %, median (IQR) | 79.5 (67.0-85.8) | 79.5 (63.3-89.3) |
| AFP in ng/mL, median (IQR) | 12.7 (4.6-109.3) | 16.0 (5.2-35.0) |
| Drug administration method | ||
| Conventional TACE/beads | 48 (80)/12 (20.0) | 24 (80)/6 (20.0) |
| Drug dose in mg, median (IQR) | 50 (50-50) | 10 (10-10) |
| TACE territory: Global/lobar/segmental | 34 (56.7)/20 (33.3)/6 (10.0) | 15 (50)/14(46.7)/1(3.3) |
| Portal hypertension evidence | ||
| Ascites on preoperative scan: No/yes | 49 (76.6)/15 (23.4) | 21 (65.6)/11 (34.4) |
| Splenomegaly on preoperative scan: No/yes | 27 (45.0)/33 (55.0) | 7 (23.3)/23 (76.7) |
| Esophageal varices | ||
| None | 28 (46.7) | 17 (56.7) |
| Grade 1/2/3 | 14 (23.3)/17 (28.3)/1 (1.7) | 2 (6.7)/9 (30.0)/2 (6.7) |
| Low platelet count as < 100 000/mm3: No/yes | 37 (61.6)/23 (38.3) | 18 (60.0)/12 (40.0) |
Considering tumor characteristics, median tumor number was two in both groups with a single nodule in 28.3% and 23.3% of cases, and a median tumor size (sum of the two largest tumors) of 49.5 mm [interquartile range (IQR): 32.8-62.0] and 40.5 mm (IQR: 30.5-58.8) in Dox-TACE and Ida-TACE patients, respectively.
Cirrhosis was present in 93.3% and 86.7% of patients with 86.7% and 70.0% of Child-Pugh A and a median Model for End-stage Liver Disease of 9.22 and 8.19 in Dox-TACE and Ida-TACE patients, respectively.
Considering portal hypertension, Dox-TACE and Ida-TACE patients respectively presented a splenomegaly in 55% and 76.7% of cases, a platelets count under 100000/mm3 in 38.3% and 40.0 % of cases, ascites on preoperative radiological exam in 23.4% and 34.4% of cases, and grade 2-3 esophageal varices of 30.0% and 36.7% of cases (Table 1).
Considering treatment characteristics, doxorubicin and idarubicin were administered with cTACE in 80% of the cases and DEB-TACE in 20% of cases in both groups. TACE was global in 56.7% and 50% of doxorubicin and idarubicin-treated patients, respectively. Median number of TACE per patient was two (IQR: 1-3) in the doxorubicin group and two (IQR: 2-3) in the idarubicin group. Sixteen patients (27%) were transplanted in the doxorubicin group and ten (33%) in the idarubicin group.
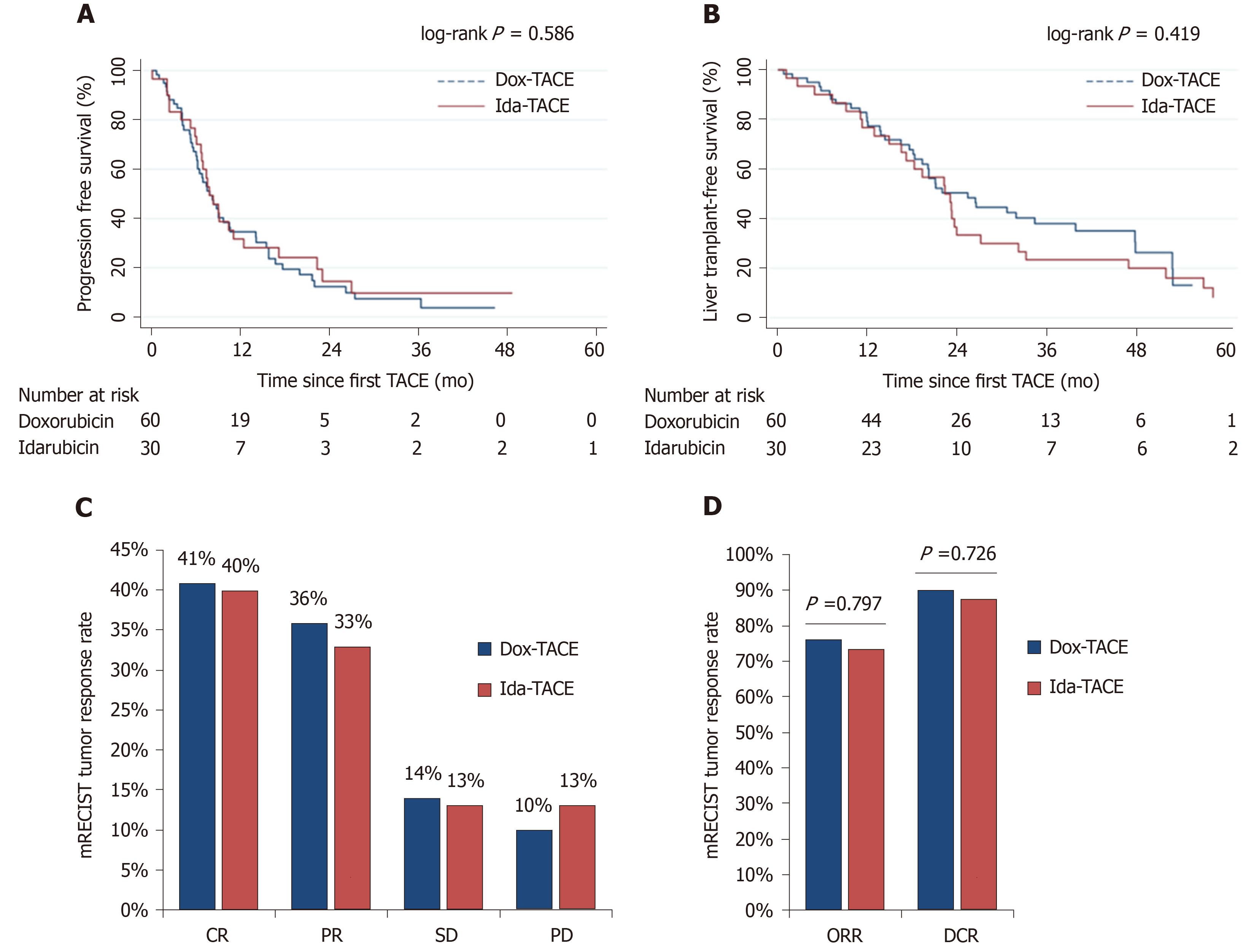
Tumor response evaluation within 3 mo post-TACE, according to mRECIST criteria, showed an ORR of 76% and 73% (P = 0.797) with 41% and 40% of complete response, and 36% and 33% of PR in Dox-TACE and Ida-TACE groups, respectively. DCR was 90% and 87%, respectively (P = 0.726) (Figure 2).
The median follow-up was 19.8 vs 21.8 mo in doxorubicin vs idarubicin patients with a PFS of 7.71 vs 7.68 mo (P = 0.586), and LTFS was 24.9 vs 21.9 mo in doxorubicin- vs idarubicin-treated patients, respectively (P = 0.434) (Figure 2).
Safety data is detailed in Table 2. Tolerance profiles were similar with AEs of any grade in 97% of patients in both arms. The most frequent AEs were post-embolization syndrome (Dox-TACE: 45%; Ida-TACE: 63%), pain, elevated transaminases, cholestasis, hyperbilirubinemia, and fatigue. At least one grade 3-4 AEs was found in 21 patients (35%) treated with doxorubicin and 13 patients (43%) treated with idarubicin. The most frequent grade 3-4 AEs were elevated aspartate aminotransferase (Dox-TACE: 33%; Ida-TACE: 43%), elevated alanine aminotransferase (23% in both arms), pain (Dox-TACE: 12%; Ida-TACE: 13%), fatigue (Dox-TACE: 11%; Ida-TACE: 10%), and liver failure (7% in both groups). One Dox-TACE patient died 23 d after TACE due to cardiac rhythm disturbances. The median duration of hospitalization was 3 d in both groups.
| Adverse events | Doxorubicin, n = 60 | Idarubicin, n = 30 | ||
| Any grade | Grade 3-4 | Any grade | Grade 3-4 | |
| Any adverse event | 58 (97) | 21 (35) | 29 (97) | 13 (43) |
| Treatment-related death | 1 (2) | 1 (2) | 0 | 0 |
| Post embolization syndrome | 27 (45) | NA | 19 (63) | NA |
| Fatigue | 27 (45) | 7 (11) | 19 (63) | 3 (10) |
| Pain | 9 (15) | 7 (12) | 5 (17) | 4 (13) |
| Gastrointestinal disorders | ||||
| Biliary complications | 5 (8) | 4 (7) | 1 (3) | 0 |
| Liver failure | 6 (10) | 4 (7) | 4 (13) | 2 (7) |
| Paralytic ileus | 1 (2) | 0 | 0 | 0 |
| Portal thrombosis | 0 | 0 | 1 (2) | 1 (2) |
| Liver biological parameters | ||||
| Elevated AST | 55 (92) | 20 (33) | 26 (87) | 13 (43) |
| Elevated ALT | 51 (85) | 14 (23) | 24 (80) | 7 (23) |
| Elevated GGT | 21 (35) | 0 | 12 (40) | 0 |
| Elevated ALP | 16 (27) | 0 | 5 (17) | 0 |
| Hyperbilirubinemia | 34 (57) | 0 | 22 (73) | 0 |
| Decreased prothrombin time | 28 (48) | 0 | 9 (32) | 0 |
| Neurological disorders | ||||
| Stroke | 1 (2) | 0 | 0 | 0 |
| Respiratory disorders | ||||
| Lung failure | 1 (2) | 0 | 1 (3) | 1 (3) |
| Lung infection | 0 | 0 | 2 (7) | 2 (7) |
| Urinary tract disorders | ||||
| Acute kidney failure | 1 (2) | 0 | 0 | 0 |
| Acute urine retention | 3 (5) | 0 | 1 (3) | 0 |
| Urinary tract infection | 1 (2) | 1 (2) | 0 | 0 |
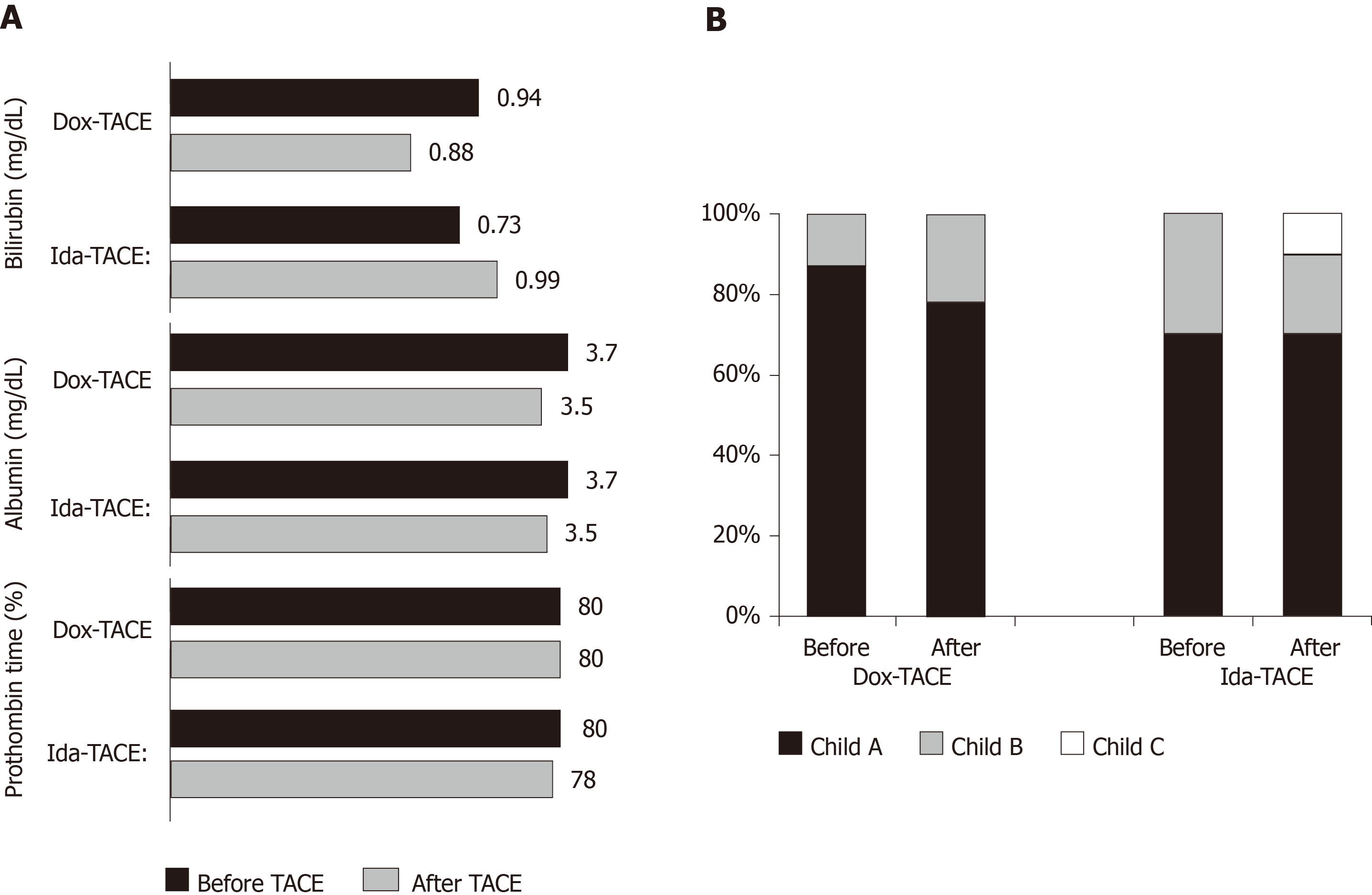
Considering chronic toxicity, mild changes of bilirubin, albumin, and prothrombin time were observed in both arms (Figure 3A), and median Child-Pugh score was 6 in both arms after TACE, compared with 5 and 6 before TACE in Dox-TACE and Ida-TACE patients, respectively. Consequences on changes in repartition of Child-Pugh grade A, B, or C are illustrated in Figure 3B.
The aim of this study was to report our single-center experience comparing TACE with idarubicin vs TACE with doxorubicin. We did not report a significant difference concerning our primary objective that was tumor response within 3 mo after TACE (P = 0.797). There was no differences between groups in terms of DCR, PFS, and LTFS.
Safety analyses revealed that idarubicin did not increase the occurrence of early AEs and chronic deterioration of liver function compared with doxorubicin. Safety profiles of both arms were comparable with TACE trials in the literature[2,13]. Interestingly, more severe biliary AEs were reported in the Dox-TACE group (n = 4) compared to Ida-TACE group (n = 0), but the difference was not statistically significant (P = 0.65).
To our knowledge, this study represents the largest cohort comparing TACE using idarubicin to TACE with doxorubicin. To avoid major biases, analysis of the primary objective was blindly performed, and Dox-TACE and Ida-TACE patients were matched with stratification on essential clinical characteristics such as tumor number, tumor size, liver function level, platelet count, WHO performance status, and TACE technique.
Nonetheless, this study presents several limitations. First, it is a retrospective and monocentric study. TACE techniques are very heterogeneous from one center to another. For example, ORR and DCR obtained in both groups were superior to tumor response available in the literature, which confirms that TACE results are strongly influenced by center experience[2,14]. A larger prospective multicenter trial is needed to confirm these results.
Both patients receiving TACE in a palliative setting or as a bridge to liver transplant were included. However, the proportions of patients on LT waiting list was well balanced between the groups with approximately 30% of patients in each group. To avoid impact on OS analysis of LT, LTFS was analyzed rather than OS to consider both death and liver transplant as events. This offers a better illustration of survival due to TACE.
Even though TACE is largely used in the treatment of intermediate HCC, several essential issues are still debated. Four meta-analyses[14-17] and a recent randomized trial including 101 patients[18] suggested that transarterial embolization, also called bland embolization, offers comparable outcomes to TACE. These studies suffer from methodological issues, and TACE is still the standard of care in BCLC B HCC.
The choice of the administered drug, its administration, and its dosage are also debated[14]. Different studies tried to optimize TACE efficacy through technical improvements, notably by using drug-eluting beads, but without success[5,19]. The use of doxorubicin is the most widespread, but it is only based on a low level of evidence[14]. In vitro studies showed that idarubicin has a toxicity index on HCC cell-lines 57-fold higher than doxorubicin[9]. While doxorubicin has a low lipophilicity binding, idarubicin is highly hydrophobic suggesting better cell penetrance[20] and allows more stable lipiodol–drug emulsion[21]. The 10 mg dose was chosen based on preliminary data of the IDASPHERE phase 1 trial that included 31 patients between 2010 and 2012 treated with beads loaded with idarubicin[10]. This dose was confirmed in two prospective single-arm trials including IDASPHERE II phase 2 trials published in 2019[22,23]. However, as 80% of patients were treated with cTACE in our study, the optimal dose may differ from DEB-TACE. Indeed, a phase 1 trial published in 2018 evaluating idarubicin dose in cTACE showed interesting results in terms of efficacy and safety with a maximum tolerated dose of idarubicin at 20 mg[11], suggesting that further studies at a higher dose such as 15 mg or 20 mg are required. Another explanation, is that the impact of drug type is probably mild in TACE as illustrated by the controversy around chemoembolization vs bland embolization with conflicting results in literature[14-18,24]. Thus, a higher number of patients is probably needed to prove superiority of idarubicin.
Finally, idarubicin was randomly assigned to patients in the setting of routine care due to the temporary shortage of doxorubicin, but as it is retrospective, we cannot control this aspect.
During the last decade, realization of Dox-TACE in BCLC B patients has become complex due to discontinuation of production of lyophilized doxorubicin by many pharmaceutical groups. For technical reasons, use of the lyophilized form is essential to obtain a mix with sufficient viscosity. This will induce tumor ischemia and increase the exposure time of the tumor to the drug, leading to a higher cytotoxicity, whereas liquid forms seem to be rapidly washed out in the blood flow. Nowadays, only Pfizer continues to produce lyophilized doxorubicin, with recurring shortage periods. Thus, finding alternatives to doxorubicin becomes a challenge to allow clinicians to perform TACE in optimal conditions.
Idarubicin showed comparable efficacy and safety to doxorubicin in this indication and may represent a new option in the management of patients with BCLC B HCC.
Transarterial chemoembolization (TACE) is the treatment of choice in intermediate hepatocellular carcinoma (HCC). Doxorubicin is largely used but without solid evidence in literature.
Growing research suggests that idarubicin is a serious candidate for use in TACE with one phase 1 clinical trial using lipiodol emulsion and a phase 1 as well as two phase 2 trials using drug-eluting beads. Idarubicin was never compared to doxorubicin in this setting. Because of multiple worldwide doxorubicin shortages, realization of TACE in patients with Barcelona Clinic Liver Cancer B becomes challenging and finding new alternatives to doxorubicin seems essential.
The objective of this study was to compare idarubicin-based TACE (Ida-TACE) with doxorubicin-based TACE (Dox-TACE) in the treatment of intermediate HCC in terms of anti-tumor efficacy and safety.
All patients undergoing TACE between January 2012 and December 2014 were screened and included with the following inclusion criteria: Child-Pugh score A or B, a performance status below or equal to 1, and no prior TACE. Lipiodol emulsion or drug-eluting beads TACE could be performed with 10 mg of idarubicin or 50 mg of doxorubicin. Each patient treated with idarubicin was matched with two doxorubicin-treated patients. Objective tumor response of TACE was assessed based on mRECIST criteria by independent radiologists. Progression-free survival and liver-transplant free survival were compared between groups using log-rank tests.
Both treatment group showed comparable characteristics. There were no differences in objective tumor response, progression-free survival, and liver-transplant free survival between Dox- and Ida-TACE. No additional toxicity was observed with idarubicin-TACE.
Idarubicin showed comparable efficacy and safety to doxorubicin in TACE and may represent a new option in the management of patients with Barcelona Clinic of Liver Cancer B HCC. In this study, due to the vast majority of patients treated by TACE using lipiodol, this constitutes the largest cohort of patients treated with idarubicin during TACE with delivery through lipiodol-emulsion.
Idarubicin represents a serious alternative to doxorubicin without complicating the procedure or increasing its toxicity. As lipiodol TACE is the most widespread technique to deliver chemotherapy, these results should easily help to improve HCC patient care worldwide. These results need to be confirmed by further clinical studies, and a phase II trial is scheduled.
Thank you to Marine Faure for her help in the safety analysis process.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qi XS, Yang L, Yao DF S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Li X
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25536] [Article Influence: 1824.0] [Reference Citation Analysis (7)] |
| 2. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2269] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 3. | Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 4. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 5. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1207] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 6. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, Bruix J. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 7. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 543] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 8. | Pinter M, Ulbrich G, Sieghart W, Kölblinger C, Reiberger T, Li S, Ferlitsch A, Müller C, Lammer J, Peck-Radosavljevic M. Hepatocellular Carcinoma: A Phase II Randomized Controlled Double-Blind Trial of Transarterial Chemoembolization in Combination with Biweekly Intravenous Administration of Bevacizumab or a Placebo. Radiology. 2015;277:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Boulin M, Guiu S, Chauffert B, Aho S, Cercueil JP, Ghiringhelli F, Krause D, Fagnoni P, Hillon P, Bedenne L, Guiu B. Screening of anticancer drugs for chemoembolization of hepatocellular carcinoma. Anticancer Drugs. 2011;22:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Boulin M, Hillon P, Cercueil JP, Bonnetain F, Dabakuyo S, Minello A, Jouve JL, Lepage C, Bardou M, Wendremaire M, Guerard P, Denys A, Grandvuillemin A, Chauffert B, Bedenne L, Guiu B. Idarubicin-loaded beads for chemoembolisation of hepatocellular carcinoma: results of the IDASPHERE phase I trial. Aliment Pharmacol Ther. 2014;39:1301-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Guiu B, Jouve JL, Schmitt A, Minello A, Bonnetain F, Cassinotto C, Piron L, Cercueil JP, Loffroy R, Latournerie M, Wendremaire M, Lepage C, Boulin M. Intra-arterial idarubicin_lipiodol without embolisation in hepatocellular carcinoma: The LIDA-B phase I trial. J Hepatol. 2018;68:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6020] [Article Influence: 860.0] [Reference Citation Analysis (3)] |
| 13. | Monier A, Guiu B, Duran R, Aho S, Bize P, Deltenre P, Dunet V, Denys A. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol. 2017;27:1431-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 617] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 15. | Xie ZB, Ma L, Wang XB, Bai T, Ye JZ, Zhong JH, Li LQ. Transarterial embolization with or without chemotherapy for advanced hepatocellular carcinoma: a systematic review. Tumour Biol. 2014;35:8451-8459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 17. | Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, Vendemiale G, Serviddio G. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United European Gastroenterol J. 2017;5:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, Jarnagin WR, D'Angelica MI, Allen PJ, Erinjeri JP, Brody LA, O'Neill GP, Johnson KN, Garcia AR, Beattie C, Zhao B, Solomon SB, Schwartz LH, DeMatteo R, Abou-Alfa GK. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol. 2016;34:2046-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 19. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F, PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 481] [Article Influence: 43.7] [Reference Citation Analysis (1)] |
| 20. | Gallois L, Fiallo M, Garnier-Suillerot A. Comparison of the interaction of doxorubicin, daunorubicin, idarubicin and idarubicinol with large unilamellar vesicles: Circular dichroism study. Biochim Biophys Acta. 1998;1370:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Boulin M, Schmitt A, Delhom E, Cercueil JP, Wendremaire M, Imbs DC, Fohlen A, Panaro F, Herrero A, Denys A, Guiu B. Improved stability of lipiodol-drug emulsion for transarterial chemoembolisation of hepatocellular carcinoma results in improved pharmacokinetic profile: Proof of concept using idarubicin. Eur Radiol. 2016;26:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Guiu B, Chevallier P, Assenat E, Barbier E, Merle P, Bouvier A, Dumortier J, Nguyen-Khac E, Gugenheim J, Rode A, Oberti F, Valette PJ, Yzet T, Chevallier O, Barbare JC, Latournerie M, Boulin M. Idarubicin-loaded Beads for Chemoembolization of Hepatocellular Carcinoma: The IDASPHERE II Single-Arm Phase II Trial. Radiology. 2019;291:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Guiu B, Colombat S, Piron L, Hermida M, Allimant C, Pierredon-Foulongne MA, Belgour A, Escal L, Cassinotto C, Boulin M. Transarterial Chemoembolization of Hepatocellular Carcinoma with Idarubicin-Loaded Tandem Drug-Eluting Embolics. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Qi X, Zhao Y, Li H, Guo X, Han G. Management of hepatocellular carcinoma: an overview of major findings from meta-analyses. Oncotarget. 2016;7:34703-34751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |