Published online Jan 21, 2020. doi: 10.3748/wjg.v26.i3.266
Peer-review started: November 25, 2019
First decision: December 4, 2019
Revised: December 17, 2019
Accepted: January 2, 2020
Article in press: January 2, 2020
Published online: January 21, 2020
Processing time: 51 Days and 19.3 Hours
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome are well recognized entities among surgical patients. Nevertheless, a number of prospective and retrospective observational studies have shown that IAH is prevalent in about half of the critically ill patients in the medical intensive care units (ICU) and has been widely recognized as an independent risk factor for mortality. It is alarming to note that many members of the critical care team in medical ICU are not aware of the consequences of untreated IAH and the delay in making the diagnosis leads to increased morbidity and mortality. Frequently it is underdiagnosed and undertreated in this patient population. Elevated intra-abdominal pressure decreases the blood flow to the kidneys and other abdominal viscera and also results in reduced cardiac output and difficulties in ventilating the patient because of increased intrathoracic pressure. When intraabdominal hypertension is not promptly recognized and treated, it leads to abdominal compartment syndrome, multiorgan dysfunction syndrome and death. Large volume fluid resuscitation is very common in medical ICU patients presenting with sepsis, shock and other inflammatory conditions like pancreatitis and it is one of the major risk factors for the development of intra-abdominal hypertension. This article presents an overview of the epidemiology, definitions, risk factors, pathophysiology and management of IAH and abdominal compartment syndrome in critically ill medical ICU patients.
Core tip: Intra-abdominal hypertension and abdominal compartment syndrome are very common in medical intensive care unit. Recognizing the risk factors for the development of intra-abdominal hypertension, timely measurement of intra-abdominal pressure and promptly implementing the resuscitation strategies can significantly reduce the morbidity and mortality associated with abdominal compartment syndrome.
- Citation: Rajasurya V, Surani S. Abdominal compartment syndrome: Often overlooked conditions in medical intensive care units. World J Gastroenterol 2020; 26(3): 266-278
- URL: https://www.wjgnet.com/1007-9327/full/v26/i3/266.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i3.266
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are frequently encountered in critically ill patients in the intensive care unit (ICU) and result in significant morbidity and mortality. The World Society of the Abdominal Compartment Syndrome has published evidence-based medicine consensus guidelines for diagnosing and managing elevated intra-abdominal pressure (IAP)[1]. IAH is a graded phenomenon or continuum of process which when not recognized leads to ACS. It is extremely important to recognize this entity in critically ill patients because of the impact of elevated intra-abdominal pressure on end organ function.
Overall IAH and ACS are widely under-recognized in medical ICU. An Australian study conducted a survey of critical care nurses knowledge of IAH and ACS and it was found that less than 20% were able to recognize the less apparent causes of IAH and lack of education related to IAP monitoring was identified by nearly half of respondents as the primary barrier to monitor IAP[2]. Results from a clinical survey indicate less than 30% of clinicians being aware of correct definition of IAH and ACS and only few of them applied the guidelines in managing patients with IAH[3]. General lack of clinical awareness and lack of clinical application of available knowledge resulted in not measuring the intra-abdominal pressures in a number of ICUs. A multidisciplinary survey on recognition and management of IAH and ACS was performed by Kimball et al[4]. The results demonstrated moderate recognition and understanding of IAH and ACS among surgical specialists and significant ignorance among medical specialists.
ACS is well described in surgical patients, but limited data is available regarding the prevalence of ACS in medical ICU patients. Study published by Malbrain et al[5] in 2004 showed that nearly 50% of all ICU patients were at risk for IAH and 8% were at risk for full-blown ACS and it also showed contrary to the popular belief, ACS was more prevalent in medical ICU patients than in surgical ICU patients. A prospective observational study that screened 491 ICU patients in 15 ICUs worldwide found IAH in 34% of patients on the day of admission and 48.9% of the patients developed IAH during the observational period. In this mixed ICU patient cohort, IAH occurred in almost half of the all ICU patients and was twice as prevalent in mechanically ventilated patients[6].
The World Society of the Abdominal Compartment Syndrome was founded in 2004 and has published consensus definitions on IAH and ACS as listed in the Table 1. IAH is defined as IAP equal to or greater than 12 mmHg and ACS is defined as sustained IAP above 20 mmHg with new onset of end organ dysfunction[7]. Normal IAP in healthy individuals ranges between 5 to 7 mmHg and obese patients tend to have higher baseline IAP values[8].
| 1 | IAP is the steady-state pressure concealed within the abdominal cavity |
| 2 | APP = MAP - IAP |
| 3 | FG = GFP-PTP = MAP - 2 × IAP |
| 4 | IAP should be expressed in mmHg and measured at end-expiration in the complete supine position after ensuring that abdominal muscle contractions are absent and with the transducer zeroed at the level of the mid-axillary line |
| 5 | The reference standard for intermittent IAP measurement is via the bladder with a maximal instillation volume of 25 mL of sterile saline |
| 6 | Normal IAP is approximately 5-7 mm Hg in critically ill adults |
| 7 | IAH is defined by a sustained or repeated pathologic elevation of IAP ≥ 12 mmHg |
| 8 | IAH is graded as follows: |
| Grade I: IAP 12-15 mmHg | |
| Grade II: IAP 16-20 mmHg | |
| Grade III: IAP 21-25 mmHg | |
| Grade IV: IAP > 25 mmHg | |
| 9 | ACS is defined as a sustained IAP > 20 mmHg (with or without an APP < 60 mmHg) that is associated with new organ dysfunction/failure |
| 10 | Primary ACS is a condition associated with injury or disease in the abdomino-pelvic region that frequently requires early surgical or interventional radiological intervention |
| 11 | Secondary ACS refers to conditions that do not originate from the abdomino-pelvic region |
| 12 | Recurrent ACS refers to the condition in which ACS redevelops following previous surgical or medical treatment of primary or secondary ACS |
The abdomen is a closed anatomic space and increase in intraabdominal volume results in proportional increase in IAP. The abdominal compliance is largely determined by the elastic recoil of the abdominal wall and the diaphragm. It is important to recognize the risk factors and conditions that predispose to development of IAH and ACS in a timely fashion to prevent the end organ damage. The presence of these risk factors should suggest clinicians to start monitoring the IAP. The risk factors can be classified into 4 categories as mentioned in Table 2. Decreased abdominal wall compliance, increased intraluminal contents, collection of contents in the abdominal cavity, capillary leak and fluid resuscitation are the broad categories that lead to the development of IAH and ACS[9].
| Reduced abdominal wall compliance |
| Obesity |
| Abdominal surgery |
| Prone positioning |
| Rectus sheath hematoma |
| Burns with abdominal eschars |
| Mechanical ventilation with high positive end-expiratory pressure |
| Ventilator dyssynchrony |
| Increased intra-luminal contents |
| Gastric distention |
| Gastroparesis |
| Colonic pseudo-obstruction |
| Volvulus |
| Abdominal tumor |
| Intra-abdominal or retroperitoneal tumor |
| Damage control laparotomy |
| Enteral feeding |
| Abdominal cavity collections |
| Ascites |
| Hemoperitoneum |
| Pneumoperitoneum |
| Major trauma |
| Laparoscopy with excessive inflation pressures |
| Peritoneal dialysis |
| Abdominal inflammation-peritonitis, pancreatitis |
| Abdominal abscess |
| Capillary leak and fluid resuscitation |
| Acidosis |
| Hypothermia |
| Coagulopathy |
| Massive transfusion |
| Trauma |
| Sepsis |
| Large volume fluid resuscitation |
| Major burns |
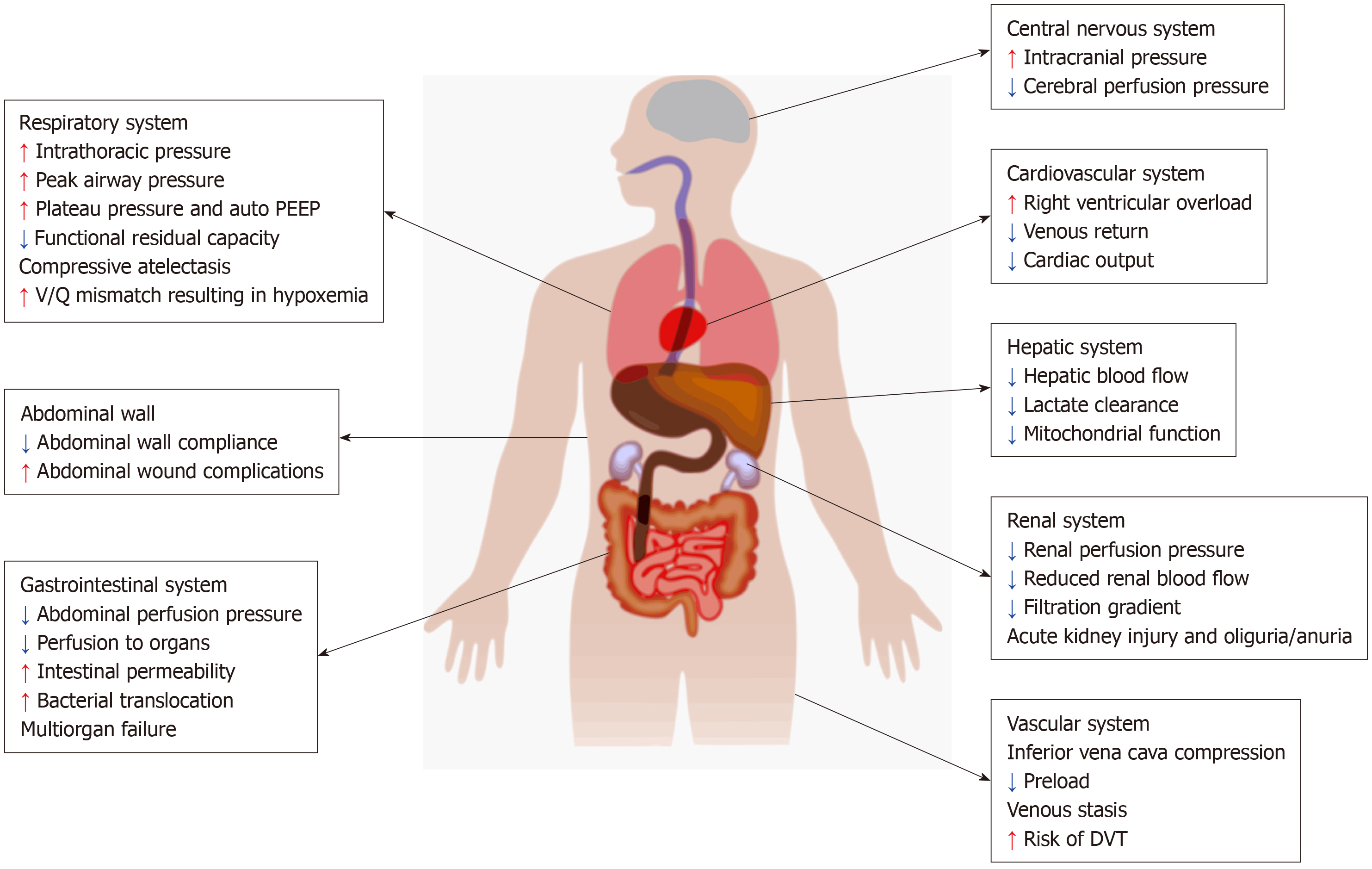
Increased IAP has significant impact on various organ systems and leads to physiologic derangements as shown in Figure 1. In addition, certain pre-existing comorbidities such as chronic renal failure, pulmonary disease, cardiomyopathy, morbid obesity which are frequently encountered in the medical ICU play an important role in aggravating the effects of elevated IAP.
When the IAP increases, the diaphragm moves cephalad and this in turn increases the intrathoracic pressure. Elevation in intrathoracic pressure reduces cardiac output by reducing the venous return. The cephalad movement of the diaphragm results in direct cardiac compression leading to reduced ventricular compliance and contractility[10]. Increased IAP compresses the aorta and pulmonary parenchyma leading to increased systemic vascular resistance and pulmonary vascular resistance respectively. IAH reduces venous return leading to peripheral edema and increased risk of development of deep venous thrombosis[11].
By increasing the intrathoracic pressure, IAH leads to increased peak airway pressure, reduced pulmonary compliance leading to alveolar volutrauma and ventilation perfusion mismatch respectively. The cephalad movement of diaphragm leads to atelectasis that results in intrapulmonary shunting and pneumonia[12].
IAH results in significant reduction in urinary output by reducing the renal blood flow and function. The resultant reduction in renal artery blood flow, increase in the renal vein pressure and the renal vascular resistance leads to impaired glomerular and tubular function[13]. Studies have shown that oliguria can develop at an IAP of 15 mmHg and anuria at an IAP of 30 mmHg[14]. The renal filtration gradient (FG) is the difference between glomerular filtration pressure (GFP) and proximal tubular pressure (PTP). When IAH occurs PTP is same as IAP[9].
FG = GFP - PTP
FG = (MAP - IAP) - IAP
FG = MAP - 2 × IAP
Hence doubling of IAP results in fourfold increase in filtration gradient, which significantly affects the net urine output.
Gut is very sensitive to elevations in IAP. Reduction in mesenteric flow can occur at IAP of only 10 mmHg. IAP of 40 mmHg can reduce the celiac artery blood flow by 43% and superior mesenteric artery flow by 69%[15]. This is further augmented by hypovolemia. IAH also compresses mesenteric veins resulting in intestinal edema which further increases IAP initiating a vicious cycle that results in worsening perfusion, bowel ischemia, decreased intraluminal pH, feeding intolerance, systemic metabolic acidosis and significantly increased mortality. IAH impedes the lymphatic flow by direct compression as well as by increasing the intrathoracic pressure and exacerbates intestinal edema and ascites[16]. ACS in turn leads to multiorgan dysfunction syndrome[17].
Increased IAP reduces abdominal wall blood flow resulting in ischemia and edema. This reduces abdominal wall compliance and exacerbates IAH[18].
Elevation of IAP impairs hepatic cell function and liver perfusion. Decreased hepatic arterial and portal venous flow results in decreased lactate clearance and altered mitochondrial function[19].
IAH results in elevation of intracranial pressure by decreased lumbar venous plexus blood flow, decreased cerebral venous outflow and increased cerebral blood flow secondary to increased PaCO2. This in turn significantly affects cerebral perfusion and function[20].
IAH is seen in at least 50% of patients with severe pancreatitis[21]. IAH is being increasingly recognized as a point of specific intervention in patients with acute pancreatitis and timely management has shown to improve mortality in this group of patients. IAH and ACS occurs early during the first 3 to 5 days of acute pancreatitis. Significant local and visceral edema from pancreatic and peripancreatic inflammation, ascites, ileus, aggressive volume resuscitation are some of the contributing factors[22]. IAP surveillance should be routinely done in patients with acute pancreatitis receiving aggressive fluid resuscitation, high severity, renal and respiratory complications and fluid accumulation in multiple areas as observed by the CT scan.
Large volume resuscitation is very common in the ICU setting and is the most common cause of IAH in patients without primary abdominal pathology. During the initial period of resuscitation large volume of fluids is frequently administered in critically ill patients with septic shock, hemorrhagic shock, burns, acute pancreatitis and other conditions associated with systemic inflammation. Although fluid resuscitation remains the cornerstone in managing critically ill patients, there is enough data to suggest that, administration of more than 5 L of fluids during the initial resuscitative phase can be harmful and leads to increased mortality.
IAH is very common in major burn patients and ACS was frequently seen in patients with more than 70% body surface area burns in the study published by Ivy et al[23] in 2000. This study also showed a linear correlation between volume of fluid infused and development of IAH. IAH is also frequently seen in cardiac surgery patients. Out of 69 patients undergoing cardiac surgery, 31% developed IAH and positive fluid balance was a strong independent risk factor for IAH[24].
Volume resuscitation and net fluid balance were also important risk factors for development of IAH in liver transplant recipients and major trauma patients. A prospective clinical study done in Italy found IAH in 32% of the liver transplant cases[25]. Adequate intravascular volume resuscitation is vital in management of patients with IAH and ACS as elevated intrathoracic pressure significantly reduces cardiac output by reducing the preload. Adequate fluid resuscitation has shown to improve survival by restoring end-organ perfusion and function. Excessive fluid resuscitation doubles the risk of developing IAH and ACS and organ failure compared to a more conservative fluid resuscitation strategy.
A prospective randomized study showed plasma resuscitated patients developed IAH less frequently than patients receiving crystalloids. Colloid and plasma resuscitation reduced fluid requirements and edema, improved cardiac parameters. Also, studies have shown that hypertonic saline during shock resuscitation in burn and trauma patients reduces the incidence of IAH. Oda et al[26] reported reduced risk for ACS when using hypertonic lactated saline for resuscitating burn patients and O’Mara et al[27] reported lower fluid requirements and lower IAP using colloids.
A prospective study screened medical ICU patients with a minimum net fluid balance of 5 L within the preceding 24 h and excluded patients with abdominal surgery, obesity. Out of the 468 medical ICU admissions screened, 40 patients met the 24-h fluid balance criteria and out of them 85% had IAH and 33% developed intra-abdominal pressure of more than 20 mmHg and 25% met the criteria for abdominal compartment syndrome. This study confirms the high incidence of IAH and ACS in medical ICU patients receiving large volume resuscitation[28].
IAP monitoring is a safe and cost-effective tool for identifying patients at risk for developing IAH and ACS. It helps in guiding resuscitative therapy and reducing mortality and morbidity associated with IAH and ACS.
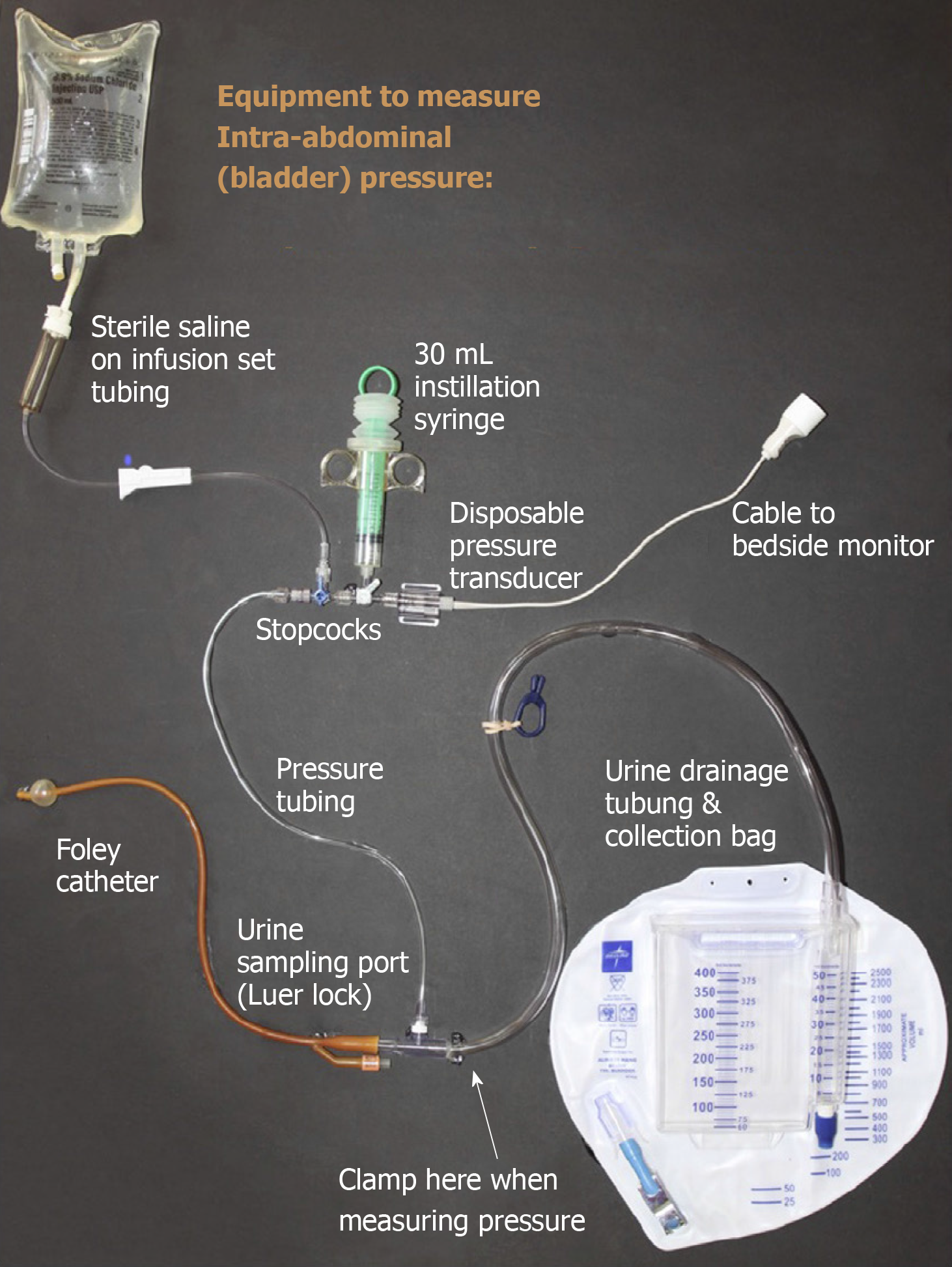
Intra vesicular saline instillation is the most common technique to monitor intra-abdominal pressure. A simple closed system that can be used to measure bladder pressure in ICU is shown in Figure 2. Clinical examination by palpation has shown to have a poor sensitivity and specificity in diagnosing IAH and ACS[29]. Other less commonly used techniques to measure intra-abdominal pressures are manometry from abdominal drain, intragastric pressure measurement through a nasogastric tube, measuring pressure from the central venous catheter placed through the femoral vein into inferior vena cava etc. Newer modalities include abdominal wall thickness measurement using point-of-care ultrasound, wireless motility capsule, continuous IAP measurement devices and noninvasive IAP estimation using near-infrared spectroscopy.
IAP should be measured at end expiration and patient should be in a complete supine position with the transducer zeroed in mid axillary line at the level of iliac crest. Maximal instillation volume of 25 mL of sterile saline should be used. IAP varies with BMI and obese patients tend to have a higher baseline IAP. Temperature of isotonic saline instilled can also affect IAP. Isotonic saline at room temperature significantly raises IAP due to contraction of the bladder muscle and hence it is important to wait for at least 30 to 60 s after isotonic saline instillation to allow warming of the fluid and relaxation of the detrusor muscle[30].
Although the prevalence of IAH and ACS is high in medical ICU patients, routine monitoring in all ICU patients is not feasible and not recommended. According to WSACS guidelines, in patients with 2 or more risk factors baseline IAP must be measured and if it is elevated frequent 4 to 6-h monitoring should be done[30].
The term poly-compartment syndrome refers to a condition where 2 or more anatomical compartments have elevated pressures. Within the poly-compartment syndrome, abdomen plays a central role and elevated IAP that results in ACS can interact with other compartments and result in thoracic compartment syndrome, intracranial compartment syndrome and extremity compartment syndrome. Initial management of poly-compartment syndrome is focusing on primary compartment and lowering compartment pressure, supporting organ perfusion and preventing specific adverse effects[31].
Acute bowel injury refers to a complex bowel injury caused by a first hit, either directly such as in abdominal sepsis, burns, pancreatitis, trauma or indirectly from ischemia due to shock followed by a second hit in the form of capillary leak, bowel edema and local ischemia resulting in IAH. This vicious cycle results in acute intestinal distress syndrome and abdominal compartment syndrome[9].
Pelvic compartment syndrome is a condition resulting from increased pressure within the true pelvis and typically follows an expanding hematoma secondary to a traumatic fracture of the pelvis[32]. Pelvic hypertension with organ dysfunction which is characterized by reduced pelvic venous return and ureteral dilatation results in pelvic compartment syndrome. It is diagnosed by elevated bladder pressure and should be differentiated from IAH/ACS. It is important to promptly recognize this syndrome and evacuate the hematoma or fluid collection through laparotomy or percutaneous drainage to decompress the pelvis[33].
Abdominal perfusion pressure (APP) is the difference between mean arterial pressure and intra-abdominal pressure. Targeting APP of more than 60 mmHg has been proposed as a better predictor of outcome in IAH than measuring IAP[34]. But the primary focus should be on reducing IAP rather than driving up the mean arterial pressure (MAP). The main strategies of conservative management are to improve abdominal wall compliance, evacuate intra and extraluminal contents and correct fluid balance to improve worsening IAH[35]. But when there is rapid progression to ACS, prompt surgical decompression must be undertaken.
Deep sedation, analgesia and neuromuscular blockade can improve thoracoabdominal muscle tone and abdominal wall compliance by reducing pain, agitation, ventilator dyssynchrony and accessory muscle use[36]. Ileus is very common among patients with pancreatitis, peritonitis, major trauma, abdominal surgery and after large volume fluid resuscitation. Nasogastric tube and rectal tube decompression help in reducing IAP in patients with IAH. The administration of bowel enemas and prokinetic agents also help in evacuating intraluminal contents and decrease visceral volume. Other techniques including supine positioning of patient in reducing IAP, large volume paracentesis in patients with ascites are also frequently done to reduce the risks of developing IAH. Intensivist performed percutaneous catheter decompression is an effective and less invasive modality for treating patients with IAH/ACS. Improved patient outcomes have been found in patients undergoing percutaneous drainage placement probably from early decompression when compared to open abdominal decompression[37].
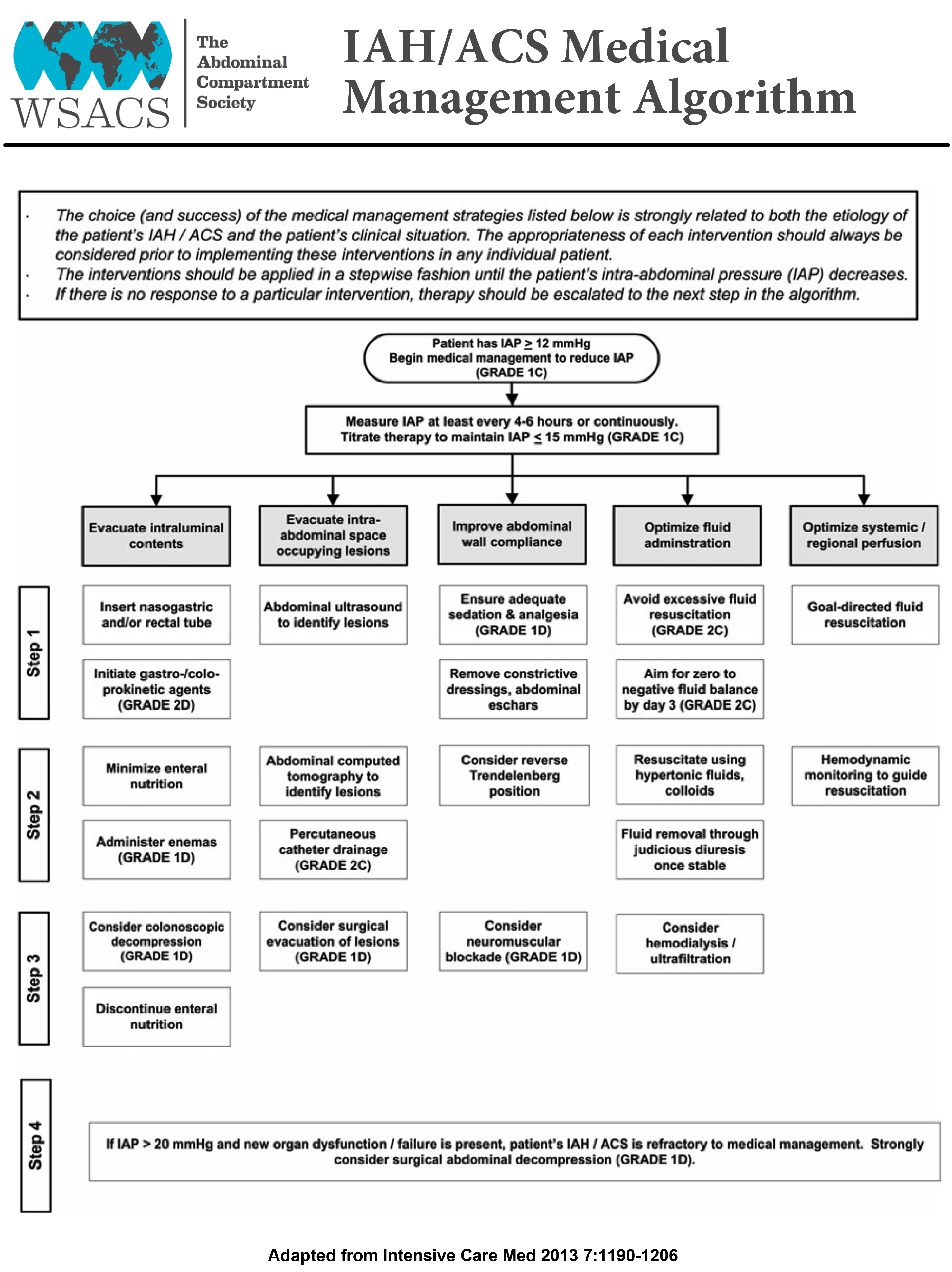
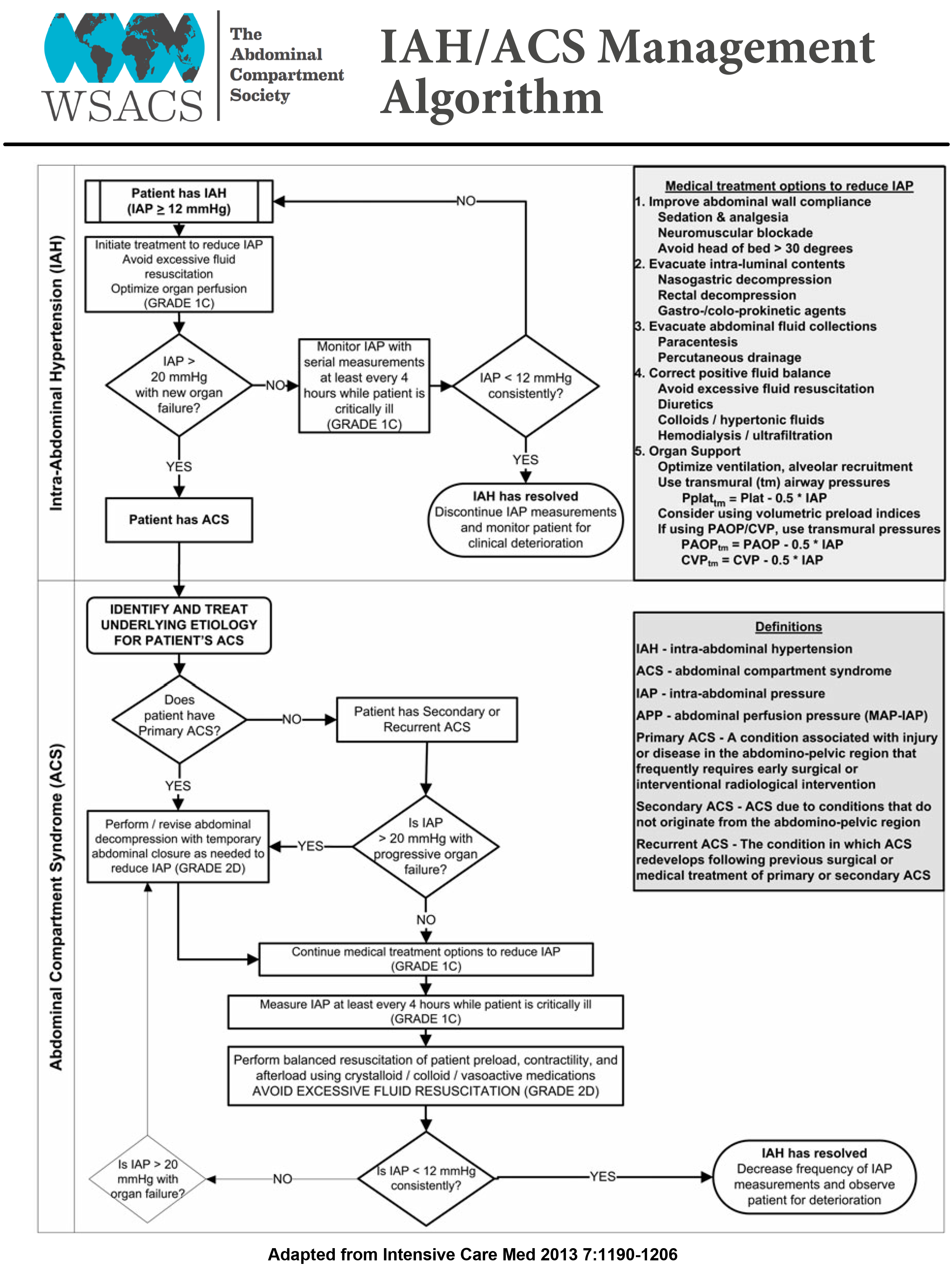
Aggressive fluid removal with diuretics or renal replacement therapy may help in reducing IAP. A review of 13 published case series suggested that an average total body fluid removal of 4.9 L resulted in reduction of IAP from 19.3 ± 9.1 mmHg to 11.5 ± 3.9 mmHg[38]. Because of the paucity of data on fluid removal, the focus should be more on avoiding excessive fluid administration during resuscitation phase rather than active fluid removal with diuresis in critically ill patients. The IAH/ACS management guidelines proposed by WSACS is mentioned in the Figures 3 and 4.
When the nonsurgical techniques fail to reduce the IAP, surgical abdominal decompression must be promptly initiated as it is the definitive management to reduce the risk of mortality from ACS. Decompressive laparotomy when combined with negative pressure peritoneal therapy reduces the IAP, improves visceral perfusion and reduces the transmission of inflammatory mediators into the bloodstream, thereby reducing the risk of developing multiorgan dysfunction from sepsis[39]. The term open abdomen refers to surgical management strategy whereby the abdominal wall incision is temporarily left unrepaired at the end of the surgery to relieve the intra-abdominal pressure. The open abdomen technique with temporary closure using vacuum-assisted closure, patch technique, silo technique (Bagota bag) and skin-only technique using towel clips are some of the methods that reduces the risk of development of IAH and ACS. In the recent years there has been increased awareness of the deleterious effects of IAH and this has led to more open abdomen management in ICU, which also reduces the risk of recurrent ACS. A collective review of 250 patients undergoing midline laparotomy found that decompression had an overall positive effect on cardiovascular, respiratory and renal function[40]. Although surgical decompression and open abdomen can be life-saving, it can also lead to certain complications like stimulation of a hypercatabolic state and protein loss through removal of peritoneal fluid, development of fistula, large ventral hernia or life threatening hemorrhagic complications including reperfusion syndrome[41,42]. Bacterial colonization of wounds and the risk of fistulas and ventral hernias increases, the longer the abdominal cavity is left open[43].
It is important to understand that most of the ICUs in United States have a combined model where medically trained intensivists manage both medical and surgical patients in the same ICU. As previously mentioned, the incidence of IAH/ACS is higher in the medical intensive care unit patients. In 2001, Kimball et al[4] conducted a survey to assess the understanding and clinical management of IAH/ACS of 4538 physician members of Society of Critical Care Medicine with a response rate of 35.7%. Of those surveyed 35% had a primary training in surgery and 31.5% in medicine. Nearly half (46.8%) of those surveyed worked in a combined medical/surgical ICU setting. It is interesting to note that surgeons and anesthesiologists chose intra-abdominal trauma/bleeding with large volume resuscitation as the most common cause of IAH/ACS and medical intensivists rated “third spacing of fluids” as the leading cause of IAH/ACS. Worsening oliguria, increasing ventilator pressure, decreasing cardiac output were some of the conditions that prompted both the medical and surgical intensivists to consider decompression laparotomy. Surgery trained intensivists had the most experience managing patients with IAH/ACS. The higher recognition rates of IAH/ACS among the surgical intensivists can partly be explained by the fact that the definitive treatment for this condition involves surgery. This however does lead to a component of bias and overdiagnosis. Of the medical intensivists 23% were not aware of the bladder pressure measurement procedure and 20% of them stated that they would never use decompression laparotomy to treat ACS compared with 3.6% of intensivists with surgical training. When it comes to management, it was interesting to note that 63% of surgery trained intensivists and 42% of the anesthesia trained intensivists frequently used decompression laparotomy as a management option for IAH/ACS compared to only 16% of medically trained intensivists. This survey also showed that the medical intensivists frequently considered percutaneous catheter drainage and paracentesis in managing patients with IAH/ACS, but this treatment was seldom used by the surgical intensivists[4].
Zhou et al[44] did a survey of 141 intensivists with majority of them (80%) having a background in internal medicine and found that diuresis, dialysis and paracentesis were commonly chosen over decompression laparotomy and 11% of the respondents stated that their surgeons would ‘never decompress patients with ACS’.
IAH is a continuum of pathophysiologic changes that results in ACS, which is a life-threatening condition caused by sustained acute elevation of IAP more than 20 mmHg with end organ failure. Observational studies have shown a high incidence of IAH and ACS in medical ICU patients. Large volume fluid resuscitation and inflammatory intra-abdominal conditions like acute pancreatitis are some of the frequent causes for IAH and ACS in medical ICU. Studies have shown less awareness and knowledge about IAH and ACS among clinicians and nurses working in medical ICU settings. It is very important to promptly recognize and diagnose IAH and ACS in the ICU setting because of its associated morbidity and mortality. Routine measurement of IAP in patients at risk is vital for early diagnosis and management of IAH and ACS. The gold standard, easy and cost-effective technique to measure IAP is measuring bladder pressure using intra -vesicular saline instillation. The current guidelines by WSACS on diagnosis and management of IAH and ACS should offer a roadmap for practicing intensivists in managing patients at risk. Once IAH is diagnosed, nonsurgical steps including gastric and bowel decompression, evacuation of intraluminal contents, paracentesis, diuresis, use of sedation should be promptly initiated to reduce IAP. Surgical abdominal decompression remains the mainstay of management in patients who don’t respond to the medical treatment.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Fellow, American College of Physician; Fellow, American College of Chest Physician; Fellow, American Academy of Sleep Medicine; Member, Society of Critical Care Medicine.
Specialty type: Gastroenterology and Hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee JG, Manenti A, Yeh YC, Zhang LY S-Editor: Ma L L-Editor: A E-Editor: Qi LL
| 1. | Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D'Amours S, Wendon J, Hillman K, Wilmer A. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med. 2007;33:951-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 538] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 2. | Hunt L, Frost SA, Newton PJ, Salamonson Y, Davidson PM. A survey of critical care nurses' knowledge of intra-abdominal hypertension and abdominal compartment syndrome. Aust Crit Care. 2017;30:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Wise R, Roberts DJ, Vandervelden S, Debergh D, De Waele JJ, De Laet I, Kirkpatrick AW, De Keulenaer BL, Malbrain ML. Awareness and knowledge of intra-abdominal hypertension and abdominal compartment syndrome: results of an international survey. Anaesthesiol Intensive Ther. 2015;47:14-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Kimball EJ, Rollins MD, Mone MC, Hansen HJ, Baraghoshi GK, Johnston C, Day ES, Jackson PR, Payne M, Barton RG. Survey of intensive care physicians on the recognition and management of intra-abdominal hypertension and abdominal compartment syndrome. Crit Care Med. 2006;34:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 6. | Reintam Blaser A, Regli A, De Keulenaer B, Kimball EJ, Starkopf L, Davis WA, Greiffenstein P, Starkopf J; Incidence, Risk Factors, and Outcomes of Intra-Abdominal (IROI) Study Investigators. Incidence, Risk Factors, and Outcomes of Intra-Abdominal Hypertension in Critically Ill Patients-A Prospective Multicenter Study (IROI Study). Crit Care Med. 2019;47:535-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 7. | Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D'Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 863] [Article Influence: 45.4] [Reference Citation Analysis (2)] |
| 8. | Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS. What is normal intra-abdominal pressure? Am Surg. 2001;67:243-248. [PubMed] |
| 9. | Malbrain ML, De laet IE. Intra-abdominal hypertension: evolving concepts. Clin Chest Med. 2009;30:45-70, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Cullen DJ, Coyle JP, Teplick R, Long MC. Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med. 1989;17:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 281] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Barnes GE, Laine GA, Giam PY, Smith EE, Granger HJ. Cardiovascular responses to elevation of intra-abdominal hydrostatic pressure. Am J Physiol. 1985;248:R208-R213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Pelosi P, Quintel M, Malbrain ML. Effect of intra-abdominal pressure on respiratory mechanics. Acta Clin Belg. 2007;62 Suppl 1:78-88. [PubMed] |
| 13. | Kirkpatrick AW, Colistro R, Laupland KB, Fox DL, Konkin DE, Kock V, Mayo JR, Nicolaou S. Renal arterial resistive index response to intraabdominal hypertension in a porcine model. Crit Care Med. 2007;35:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Richards WO, Scovill W, Shin B, Reed W. Acute renal failure associated with increased intra-abdominal pressure. Ann Surg. 1983;197:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 221] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Friedlander MH, Simon RJ, Ivatury R, DiRaimo R, Machiedo GW. Effect of hemorrhage on superior mesenteric artery flow during increased intra-abdominal pressures. J Trauma. 1998;45:433-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Malbrain ML, Pelosi P, De laet I, Lattuada M, Hedenstierna G. Lymphatic drainage between thorax and abdomen: please take good care of this well-performing machinery. Acta Clin Belg. 2007;62 Suppl 1:152-161. [PubMed] |
| 17. | Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Diebel L, Saxe J, Dulchavsky S. Effect of intra-abdominal pressure on abdominal wall blood flow. Am Surg. 1992;58:573-575; discussion 575-576. [PubMed] |
| 19. | Luca A, Cirera I, García-Pagán JC, Feu F, Pizcueta P, Bosch J, Rodés J. Hemodynamic effects of acute changes in intra-abdominal pressure in patients with cirrhosis. Gastroenterology. 1993;104:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Citerio G, Vascotto E, Villa F, Celotti S, Pesenti A. Induced abdominal compartment syndrome increases intracranial pressure in neurotrauma patients: a prospective study. Crit Care Med. 2001;29:1466-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Jaipuria J, Bhandari V, Chawla AS, Singh M. Intra-abdominal pressure: Time ripe to revise management guidelines of acute pancreatitis? World J Gastrointest Pathophysiol. 2016;7:186-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | De Waele JJ, Leppäniemi AK. Intra-abdominal hypertension in acute pancreatitis. World J Surg. 2009;33:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Ivy ME, Atweh NA, Palmer J, Possenti PP, Pineau M, D'Aiuto M. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 224] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Dalfino L, Sicolo A, Paparella D, Mongelli M, Rubino G, Brienza N. Intra-abdominal hypertension in cardiac surgery. Interact Cardiovasc Thorac Surg. 2013;17:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Biancofiore G, Bindi ML, Romanelli AM, Boldrini A, Consani G, Bisà M, Filipponi F, Vagelli A, Mosca F. Intra-abdominal pressure monitoring in liver transplant recipients: a prospective study. Intensive Care Med. 2003;29:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Oda J, Ueyama M, Yamashita K, Inoue T, Noborio M, Ode Y, Aoki Y, Sugimoto H. Hypertonic lactated saline resuscitation reduces the risk of abdominal compartment syndrome in severely burned patients. J Trauma. 2006;60:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | O'Mara MS, Slater H, Goldfarb IW, Caushaj PF. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005;58:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Daugherty EL, Hongyan Liang, Taichman D, Hansen-Flaschen J, Fuchs BD. Abdominal compartment syndrome is common in medical intensive care unit patients receiving large-volume resuscitation. J Intensive Care Med. 2007;22:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Sugrue M, Bauman A, Jones F, Bishop G, Flabouris A, Parr M, Stewart A, Hillman K, Deane SA. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg. 2002;26:1428-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 30. | Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, Sugrue M, Cheatham M, Ivatury R, Ball CG, Reintam Blaser A, Regli A, Balogh ZJ, D'Amours S, Debergh D, Kaplan M, Kimball E, Olvera C; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 857] [Article Influence: 71.4] [Reference Citation Analysis (2)] |
| 31. | Malbrain ML, Wilmer A. The polycompartment syndrome: towards an understanding of the interactions between different compartments! Intensive Care Med. 2007;33:1869-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Hessmann M, Rommens P. Does the intrapelvic compartment syndrome exist? Acta Chir Belg. 1998;98:18-22. [PubMed] |
| 33. | Manenti A, Giuliani E. The pelvic compartment syndrome. J Am Coll Surg. 2013;217:374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Cheatham ML, White MW, Sagraves SG, Johnson JL, Block EF. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621-626; discussion 626-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 218] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Cheatham ML. Nonoperative management of intraabdominal hypertension and abdominal compartment syndrome. World J Surg. 2009;33:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | De Laet I, Hoste E, Verholen E, De Waele JJ. The effect of neuromuscular blockers in patients with intra-abdominal hypertension. Intensive Care Med. 2007;33:1811-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Cheatham ML, Safcsak K. Percutaneous catheter decompression in the treatment of elevated intraabdominal pressure. Chest. 2011;140:1428-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46:361-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 39. | Kubiak BD, Albert SP, Gatto LA, Snyder KP, Maier KG, Vieau CJ, Roy S, Nieman GF. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock. 2010;34:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | De Waele JJ, Hoste EA, Malbrain ML. Decompressive laparotomy for abdominal compartment syndrome--a critical analysis. Crit Care. 2006;10:R51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Sugrue M. Abdominal compartment syndrome and the open abdomen: any unresolved issues? Curr Opin Crit Care. 2017;23:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 42. | Rogers WK, Garcia L. Intraabdominal Hypertension, Abdominal Compartment Syndrome, and the Open Abdomen. Chest. 2018;153:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 43. | Miller PR, Meredith JW, Johnson JC, Chang MC. Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann Surg. 2004;239:608-614; discussion 614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Zhou JC, Zhao HC, Pan KH, Xu QP. Current recognition and management of intra-abdominal hypertension and abdominal compartment syndrome among tertiary Chinese intensive care physicians. J Zhejiang Univ Sci B. 2011;12:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |