Published online Aug 7, 2020. doi: 10.3748/wjg.v26.i29.4302
Peer-review started: April 3, 2020
First decision: May 26, 2020
Revised: June 9, 2020
Accepted: July 23, 2020
Article in press: July 23, 2020
Published online: August 7, 2020
Processing time: 126 Days and 2.1 Hours
The main endemic areas of alveolar echinococcosis (AE) are in Central Europe and Western China. Both the infiltration of intrahepatic vascular and bile duct structures as well as extrahepatic disease can lead to further complications and may increase morbidity in patients with AE.
To evaluate vascular/biliary involvement in hepatic AE and its distant extrahepatic disease manifestations in an international collective was the aim.
Consecutively, five experienced examiners evaluated contrast-enhanced abdominal computed tomography (CT) scans for 200 patients with hepatic AE of each of four locations (n = 50) in Germany, France and China. Therefore, we retrospectively included the 50 most recent abdominal contrast-enhanced CT examinations at each center, performed because of hepatic AE from September 21, 2007 to March 21, 2018. AE liver lesions were classified according to the echinococcosis multilocularis Ulm classification for CT (EMUC-CT). Distant extrahepatic manifestations were documented either by whole body positron emission tomography–CT or with the addition of thoracic CT and cranial magnetic resonance imaging. Vascular/biliary involvement of the hepatic disease as well as the presence of distant extrahepatic manifestations were correlated with the EMUC-CT types of liver lesion. Statistical analysis was performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, United States).
Distant extrahepatic AE manifestations were significantly more frequent in China than in Europe (P = 0.0091). A significant relationship was found between the presence of distant extrahepatic disease and AE liver lesion size (P = 0.0075). Vascular/biliary structures were involved by the liver lesions significantly more frequently in China than in Europe (P < 0.0001), and vascular/biliary involvement depended on lesion size. Different morphological types of AE liver lesions led to varying frequencies of vascular/biliary involvement and were associated with different frequencies of distant extrahepatic manifestations: Vascular/biliary involvement as a function of lesions primary morphology ranged from 5.88% of type IV liver lesions to 100% among type III lesions. Type IV differed significantly in these associations from types I, II, and III (P < 0.0001). With respect to extrahepatic disease, the primary morphology types IV and V of liver lesions were not associated with any case of distant extrahepatic disease. In contrast, distant extrahepatic manifestations in types I–III were found to varying degrees, with a maximum of 22% for type III.
Different CT morphological patterns of hepatic AE lesions influence vascular/biliary involvement and the occurrence of distant extrahepatic manifestations. There are intercontinental differences regarding the characteristics of AE manifestation.
Core tip: This study demonstrates for the first time how different computed tomography morphological types of liver lesions in alveolar echinococcosis (AE) affect the intrahepatic involvement pattern as well as distant extrahepatic disease manifestations. The disease shows different characteristics in an intercontinental comparison between Europe and China. These results may provide information about the behavior of this disease during its initial manifestation and its progression. A morphological classification of AE liver lesions seems therefore not only useful in order to facilitate the initial differential diagnosis but also indicates a direct clinical impact.
- Citation: Graeter T, Bao HH, Shi R, Liu WY, Li WX, Jiang Y, Schmidberger J, Brumpt E, Delabrousse E, Kratzer W, the XUUB Consortium. Evaluation of intrahepatic manifestation and distant extrahepatic disease in alveolar echinococcosis. World J Gastroenterol 2020; 26(29): 4302-4315
- URL: https://www.wjgnet.com/1007-9327/full/v26/i29/4302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i29.4302
Human alveolar echinococcosis (AE) is a rare malignant parasitic disease that results from infection with the larval stage of echinococcus multilocularis[1]. Because of the frequently exogenous tumor-like proliferation and destructive growth, AE resembles a malignant tumor in its behavior and appearance and can lead to infiltration of the affected organs and even to severe disease and death[2]. AE has become a serious global problem, occurring in moderate to cold climate zones in the northern hemispheres and being prevalent particularly across central Europe, a large part of north and central Asia, and some parts of North America[3].
Imaging tools such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and 18F fluorodeoxyglucose (FDG)-positron emission tomography (PET) are used to diagnose AE lesions, combined with results of immunodiagnosis (specific serology) and epidemiological findings[4-8]. CT scans can reveal the shape, number, size, and location of lesions more accurately than can ultrasound and also demonstrate the typical calcifications most clearly[9]. MRI best captures the structural alveolar characteristics in AE. Combined with CT or MRI, FDG-PET can be used to evaluate the local inflammatory activity induced by the lesion. Absence of metabolic activity, however, does not necessarily mean that the parasite is nonviable and may indicate suppressed immune defenses[10]. Despite abundant clinical resources and technical advances, the diagnosis of AE in infected individuals remains challenging, especially among inexperienced clinicians. With delayed diagnosis, therapy often begins in a late stage of the disease, which significantly limits treatment options[11].
The liver usually is the first organ affected by larval infestation. A manifestation outside the liver without liver involvement is rare[12]. Hepatic AE (HAE) can affect intrahepatic blood vessels and bile ducts. Especially with involvement of such structures in the hilum, a radical resection is difficult or impossible. In the literature (cases and small series), hepatobiliary complications in AE are reported with an incidence of 10%–30%[13-17]. Vascular complications include Budd–Chiari and vena cava syndromes, which are caused by occlusions of the hepatic veins or inferior vena cava, respectively[18-21]. Hepatobiliary complications represent a turning point in HAE and are crucial for the further clinical course of the disease[11,13].
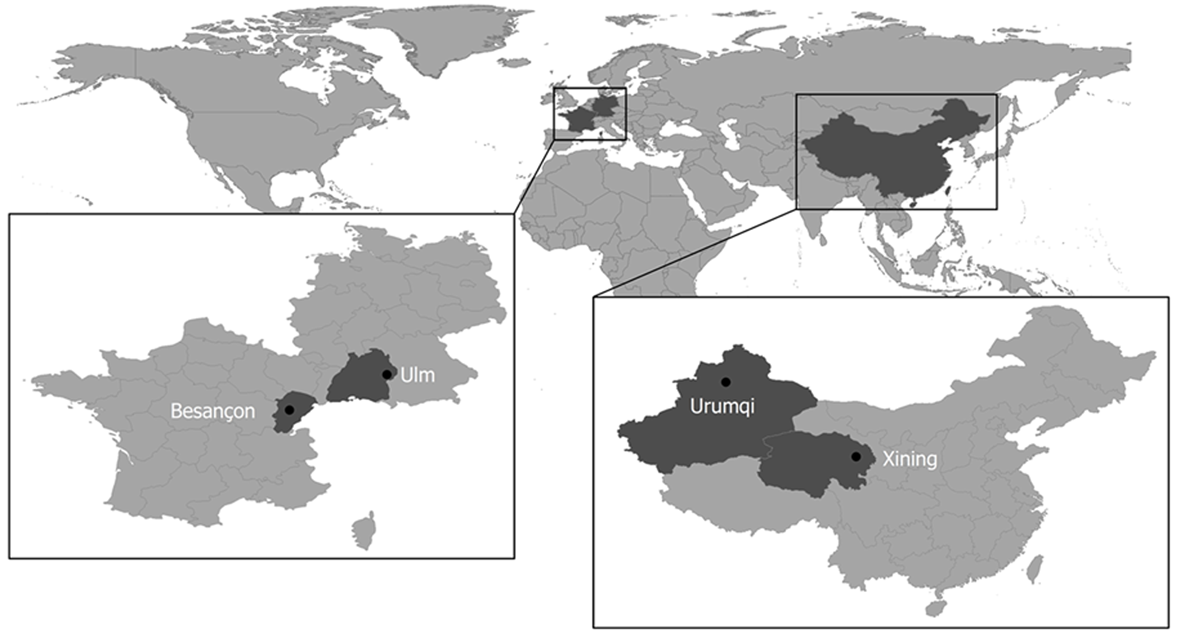
This multicenter study was based in two Chinese and two European university clinics that are international leaders in research and in treatment of AE. These centers are located in AE-endemic areas and established a research cooperation in 2016 to carry out the Xining-Urumqi-Ulm-Besançon (XUUB) imaging project. Xining is the regional capital of Qinghai province in central China, and Urumqi is the capital of the Uyghur autonomous region of Xinjiang in northwest China. Ulm is situated in the state of Baden-Württemberg in southwest Germany. Besançon is a city in the Franche-Comté region of eastern France (Figure 1).
The aim of the study was to assess the vascular/biliary involvement and the distant extrahepatic disease manifestations of the different cases in a collective of 200 German, French, and Chinese patients with HAE.
For German patients, the study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki (ref. No. 409/15). Because of its retrospective design and pseudonymized evaluation of imaging, no ethics approval was necessary for France and China. All data were analyzed anonymously.
The following inclusion criteria were defined. Retrospectively, we included the 50 most recent abdominal contrast-enhanced CT examinations at each center (n = 200), performed because of hepatic AE from 09/21/07 to 03/21/18. The number of cases was estimated after consultation with the respective countries involved and the number of CT examinations in recent months. The previous clinical and imaging morphological findings had to have been classified as confirmed AE according to Brunetti et al.’s case definition[2]. Antibody status, possible subsequent therapeutic strategies, and socioeconomic factors were not considered in the inclusion criteria.
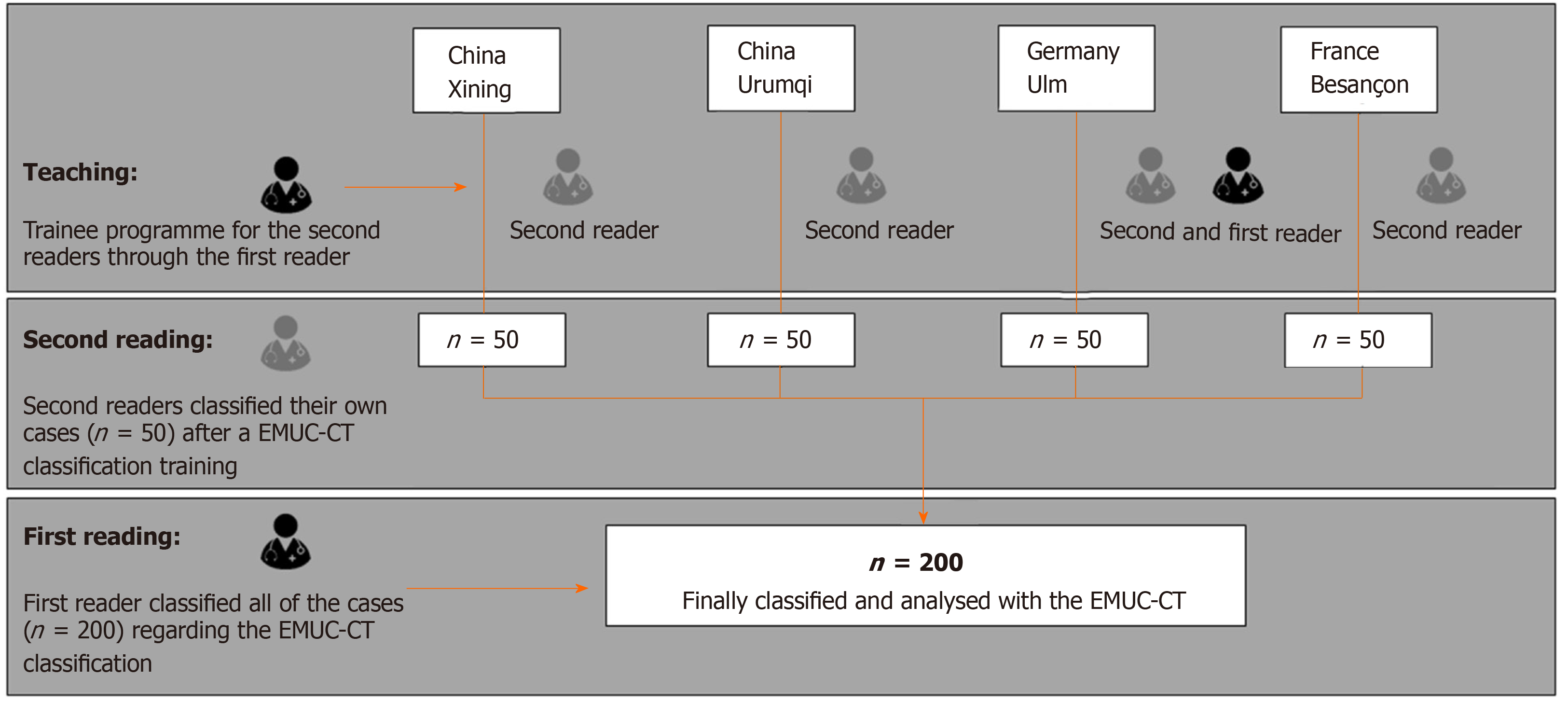
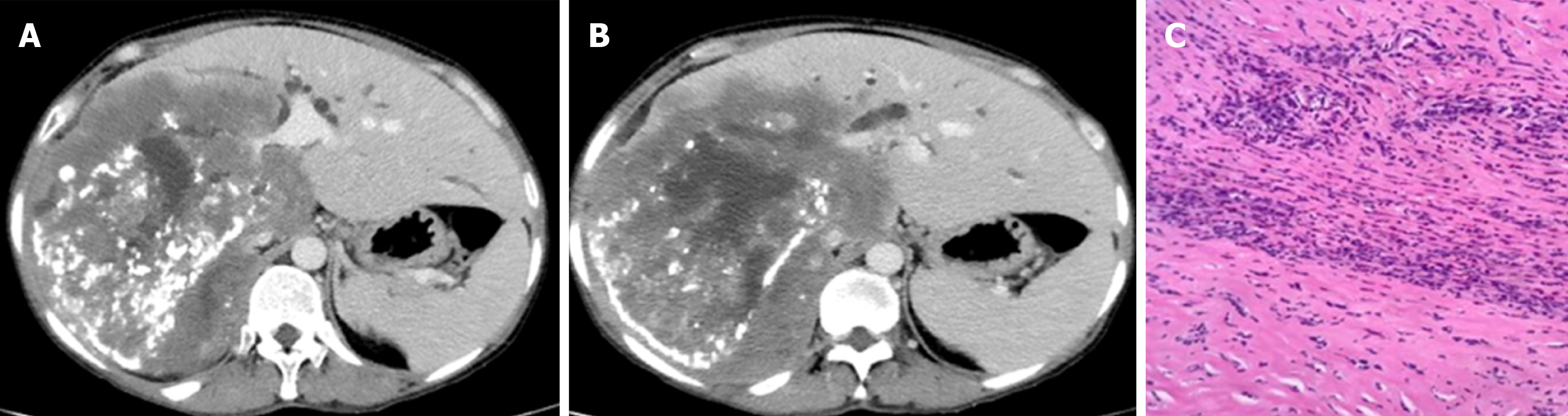
The echinococcus multilocularis Ulm classification for CT (EMUC-CT) provides a scheme for classifying the very different morphological appearances of HAE lesions (Table 1)[5]. The classification of all HAE cases according to the EMUC-CT was carried out by the first reader from April 09, 2018 to April 14, 2018. Only venous-phase CT scans were used to evaluate the lesions. The largest lesion within a liver was used to determine the primary morphological type, and all further evaluations in this study reference these. A local experienced radiologist at each of the four centers became the second reader for their own 50 cases and independently re-classified the local cases (Figure 2). Criteria concerning the classification of the lesions, as well as further technical and disease-related information, were collected on a detailed report form. In addition to the essential patient data (sex and age), technical information included the basic technical modality of the CT scan. The following CT scanners were used in the different centers: Philips ICT, United UCT (Xining); CT-Discovery CT 750 HD, GE Healthcare (Urumqi); Biograph mCT-S (40), Siemens Healthcare (Ulm) and Biograph; Siemens; CTI; Knoxville, TN (Besançon).
| Primary morphology (Subcriteria) | Pattern of calcification |
| Type I, Diffuse infiltrating (with cystoid portion / without cystoid portion) | Without calcifications |
| Type II, Primarily circumscribed tumor-like (with cystoid portion / without cystoid portion) | With feathery calcifications |
| Type III, Primarily cystoid - intermediate (IIIa), widespread (IIIb) - (with more solid portions at the edge / without more solid portions at the edge) | With focal calcifications |
| With diffuse calcifications | |
| Type IV, Small-cystoid/metastasis-like | With calcifications primarily at the edge |
| Type V, Mainly calcified | With a central calcification |
Disease-related information included the affected hepatic lobes and a detailed listing of the liver segments involved, as well as the number of lesions, any vascular/biliary involvement and the overall dimension of the biggest lesion. The evaluation regarding vascular/biliary involvement of the liver lesions was based on a detailed joint case discussion and the consensus of all five readers, all experienced radiologists. The involvement of large central or medium-sized peripheral portal venous, venous, or arterial vessel sections and a central or peripheral cholestasis (peripheral bile ducts more than 2 mm) caused by lesions was evaluated using CT. From an anatomical point of view, this association points to an involvement of the jointly running portal biliary and vascular structures. The criteria for vascular and biliary involvement were therefore considered to be common criteria.
Whether a distant extrahepatic disease manifestation was present was determined retrospectively based on respective whole-body staging examinations. The different centers occasionally handled these differently, depending on local conditions and practices. In Ulm and Besançon, full body imaging was performed during the present PET-CT examination. In the two Chinese centers, where no PET-CT examinations were performed, the chest was also examined using CT, and the cranium was examined by using complementary MRI, assessing the corresponding clinical symptoms. All distant extrahepatic manifestations were histologically confirmed as AE.
Solely accentuated but well-circumscribed lymph nodes without infiltrating aspect were not evaluated as extrahepatic manifestations. Furthermore, the direct infiltration of organs adjacent to the liver or an infiltration of parahepatic connective tissue or diaphragm, respectively, through the liver lesion was also not evaluated as a separate (metastasis-like) extrahepatic manifestation. For further calculations concerning extrahepatic disease manifestations, these cases were not included unless distant extrahepatic manifestations were simultaneously recorded, but they were documented separately. The presence of vascular/biliary involvement by the AE liver lesion as well as of distant extrahepatic disease manifestations was finally associated with the presented EMUC-CT type of liver lesion.
We performed statistical analyses using SAS Version 9.4 (SAS Institute Inc., Cary, NC, United States). Descriptive analysis of the data was performed to obtain absolute and relative frequencies, as well as measures of central tendency and dispersion. Pearson's χ2 and exact fisher tests were used to determine possible relationships and differences in the frequency distribution between dichotomous variables. Differences in metric variables from the four study centers were evaluated by post hoc Tukey and Kruskal–Wallis tests based on an analysis of variance. Inter-rater reliability between the reader was determined by kappa coefficients. The level of significance was set at α = 0.05, and a P value < 0.05 was considered to be statistically significant with a five percent probability of error.
The statistical methods of this study were reviewed by Dr. Julian Schmidberger, MPH, PhD, from the Department of Internal Medicine I, University Hospital Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany.
In the overall collective (n = 200), 55% were women. The complete demographic data can be found in Table 2. The fleiss kappa (k) inter-rater reliability for reporting the findings using the EMUC-CT was 76% (95%CI: 69%-83%; P < 0.0001). The distribution of the primary morphologies of the different centers is shown in Table 3.
| XUUB overall (n = 200) | Xining (n = 50) | Urumqi (n = 50) | Ulm (n = 50) | Besançon (n = 50) | |
| Sex, n (%) | |||||
| Male | 90 (45.0) | 22 (44.0) | 23 (46.0) | 21 (42.0) | 24 (48.0) |
| Female | 110 (55.0) | 28 (56.0) | 27 (54.0) | 29 (58.0) | 26 (52.0) |
| Age, yr, n (%) | |||||
| < 18 | 8 (4.0) | 6 (12.0) | 2 (4.0) | 0 (0.0) | 0 (0.0) |
| 18–40 | 59 (29.5) | 20 (40.0) | 26 (52.0) | 8 (16.0) | 5 (10.0) |
| 41–60 | 66 (33.0) | 24 (48.0) | 18 (36.0) | 12 (24.0) | 12 (24.0) |
| 61–80 | 50 (25.0) | 0 (0.0) | 4 (8.0) | 26 (52.0) | 20 (40.0) |
| > 81 | 17 (8.5) | 0 (0.0) | 0 (0.0) | 4 (8.0) | 13 (26.0) |
| Lesion size (mm) | 95.6 ± 51.8 | 108.0 ± 53.0 | 132.7 ± 46.0 | 71.4 ± 46.39 | 70.4 ± 32.3 |
| (11–261) | (21–261) | (36–253) | (11–202) | (13–173) | |
| Number of lesions | 3.2 ± 4.5 | 3.3 ± 5.3 | 1.8 ± 1.2 | 5.0 ± 6.5 | 2.7 ± 2.4 |
| (1–29) | (1–27) | (1–6) | (1–29) | (1–12) | |
| Age (yr) | 50.1 ± 20.5 | 35.5 ± 12.6 | 38.0 ± 13.9 | 61.3 ± 16.8 | 65.7 ± 18.3 |
| (11–91) | (11–55) | (16–77) | (18–85) | (18–91) |
| XUUB total (n = 200) | Xining (n = 50) | Urumqi (n = 50) | Ulm (n = 50) | Besançon (n = 50) | |
| Type I | 85 (42.5) | 18 (36.0) | 17 (34.0) | 22 (44.0) | 28 (56.0) |
| With cystoid portion | 55 (64.7) | 13 (72.2) | 13 (76.5) | 11 (50.0) | 18 (64.3) |
| Without cystoid portion | 30 (35.3) | 5 (27.8) | 4 (23.5) | 11 (50.0) | 10 (35.7) |
| Type II | 67 (33.5) | 17 (34.0) | 26 (52.0) | 12 (24.0) | 12 (24.0) |
| With cystoid portion | 55 (82.09) | 13 (76.5) | 22 (84.6) | 10 (83.3) | 10 (83.3) |
| Without cystoid portion | 12 (17.9) | 4 (23.5) | 4 (15.4) | 2 (16.7) | 2 (16.7) |
| Type III | 27 (13.5) | 13 (26.0) | 7 (14.0) | 4 (8.0) | 3 (6.0) |
| With more solid portions at the edge | 23 (85.19) | 11 (84.6) | 6 (85.7) | 4 (100.0) | 2 (66.7) |
| Without more solid portions at the edge | 4 (14.8) | 2 (15.4) | 1 (14.3) | 0 (0.0) | 1 (33.3) |
| Type iiia | 8 (4.0) | 2 (4.0) | 1 (2.0) | 2 (4.0) | 3 (6.0) |
| With more solid portions at the edge | 7 (87.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) |
| Without more solid portions at the edge | 1 (12.5) | 2 (100.0) | 1 (100.0) | 2 (100.0) | 1 (33.3) |
| Type iiib | 19 (9.5) | 11 (22.0) | 6 (12.0) | 2 (4.0) | 0 (0.0) |
| With more solid portions at the edge | 16 (84.21) | 9 (81.8) | 5 (83.3) | 0 (0.0) | 0 (0.0) |
| Without more solid portions at the edge | 3 (15.8) | 2 (18.2) | 1 (16.7) | 2 (100.0) | 0 (0.0) |
| Type IV | 17 (8.5) | 2 (4.0) | 0 (0.0) | 9 (18.0) | 6 (12.0) |
| Type V | 4 (2.0) | 0 (0.0) | 0 (0.0) | 3 (6.0) | 1 (2.0) |
Involvement of the right hepatic lobe was present in 80%, which is also reflected in similar values in the intercontinental comparison of 78% for Europe and 82% for China. For each of the four centers, segment VIII, which is centrally located on the right hepatic side, was most frequently involved, occurring in 57%.
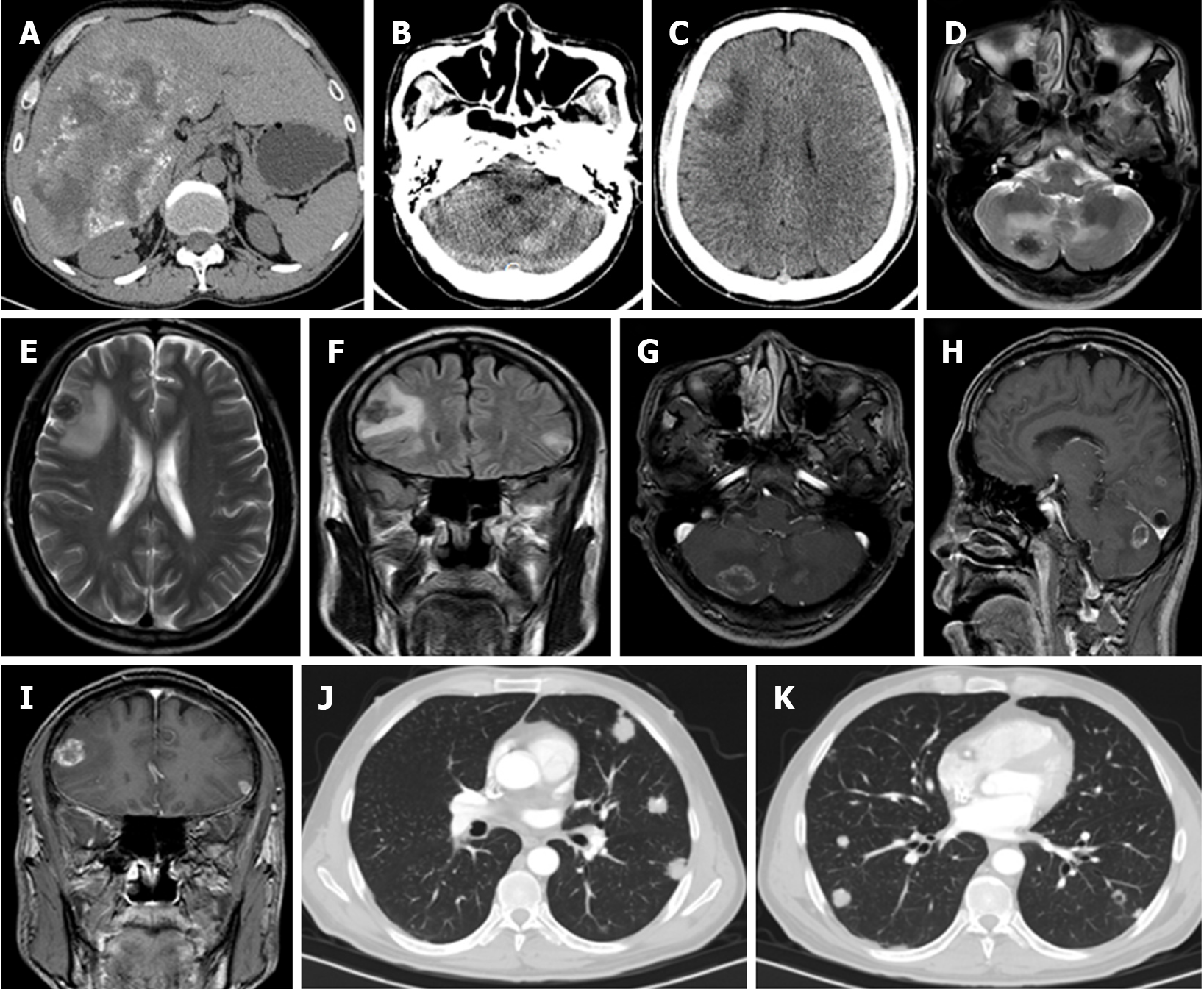
Distant extrahepatic manifestations were rather rare in the total collective, occurring in 16 of 200 (8%). Table 4 provides an overview of the localization of the respective distant extrahepatic manifestations. However, Europe and China differed significantly in rates of these features, with only three cases in Europe 3/100 (3%) compared to thirteen in China 13/100 (13%) (χ² = 6.7935; P = 0.0091). In the Chinese group, the presence of a distant extrahepatic manifestation was approximately balanced, with six cases in Urumqi and seven for Xining. The three European cases were all from the French data, with no cases recorded in the German cases.
| Patient | Center | Age | Sex | Involved organs |
| No. 1 | Besançon | 91 | Female | Spleen |
| No. 2 | Besançon | 86 | Male | Cranial calotte |
| No. 3 | Besançon | 89 | Female | Lung |
| No. 4 | Urumqi | 56 | Male | Lung |
| No. 5 | Urumqi | 49 | Male | Lung |
| No. 6 | Urumqi | 53 | Male | Retroperitoneal (distant from the liver) |
| No. 7 | Urumqi | 37 | Female | Retroperitoneal (distant from the liver) |
| No. 8 | Urumqi | 30 | Male | Lung |
| No. 9 | Urumqi | 45 | Female | Lung |
| No. 10 | Xining | 32 | Female | Lung |
| No. 11 | Xining | 51 | Female | Lung |
| No. 12 | Xining | 30 | Female | Brain, lung |
| No. 13 | Xining | 17 | Male | Lung |
| No. 14 | Xining | 29 | Male | Brain, lung |
| No. 15 | Xining | 50 | Male | Brain, lung |
| No. 16 | Xining | 49 | Male | Lung |
With respect to the primary morphology types of liver lesions, types IV and V were not associated with any case of distant extrahepatic disease. In contrast, distant extrahepatic manifestations in types I–III were found to varying degrees, with a maximum of 22% for type III.
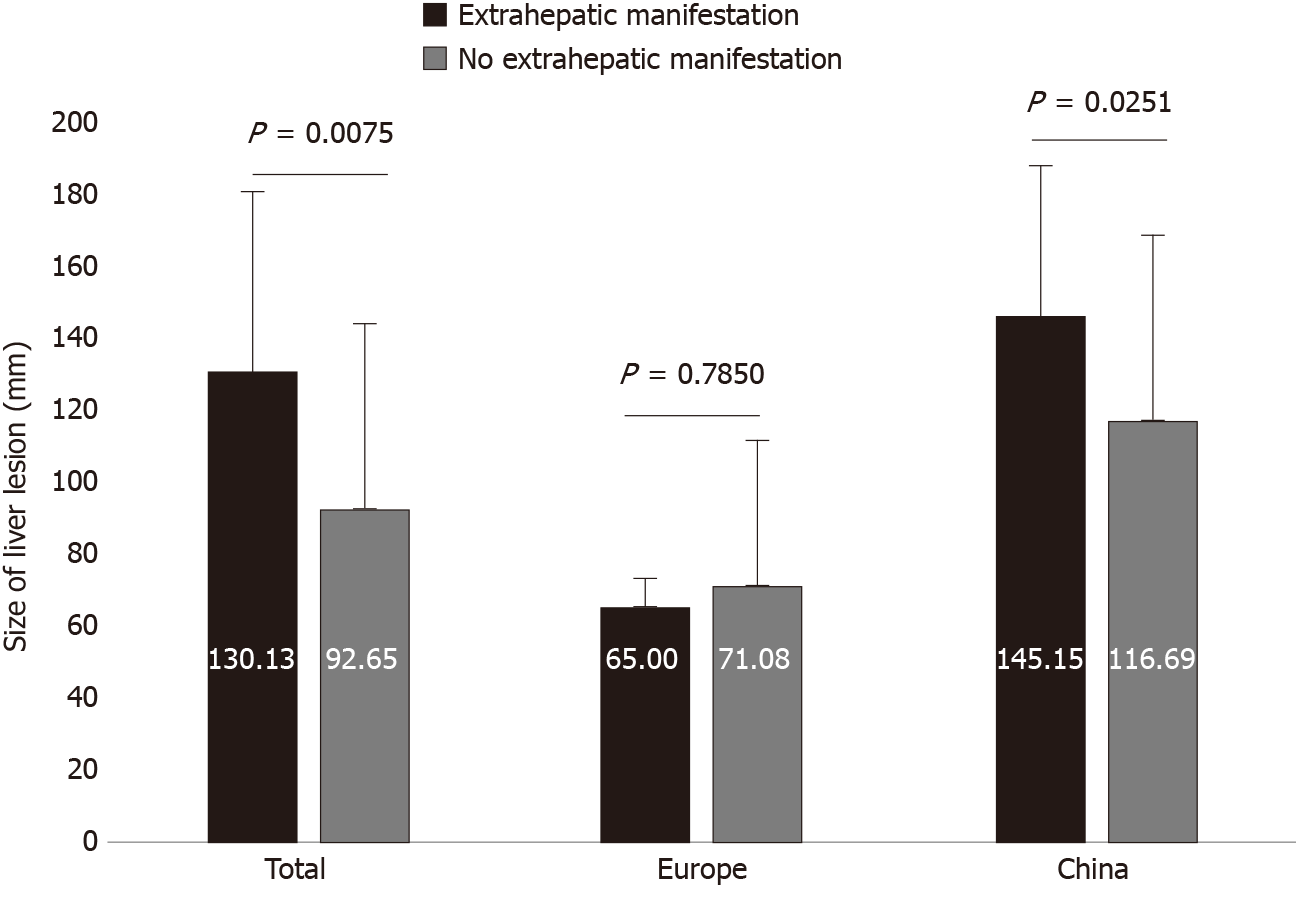
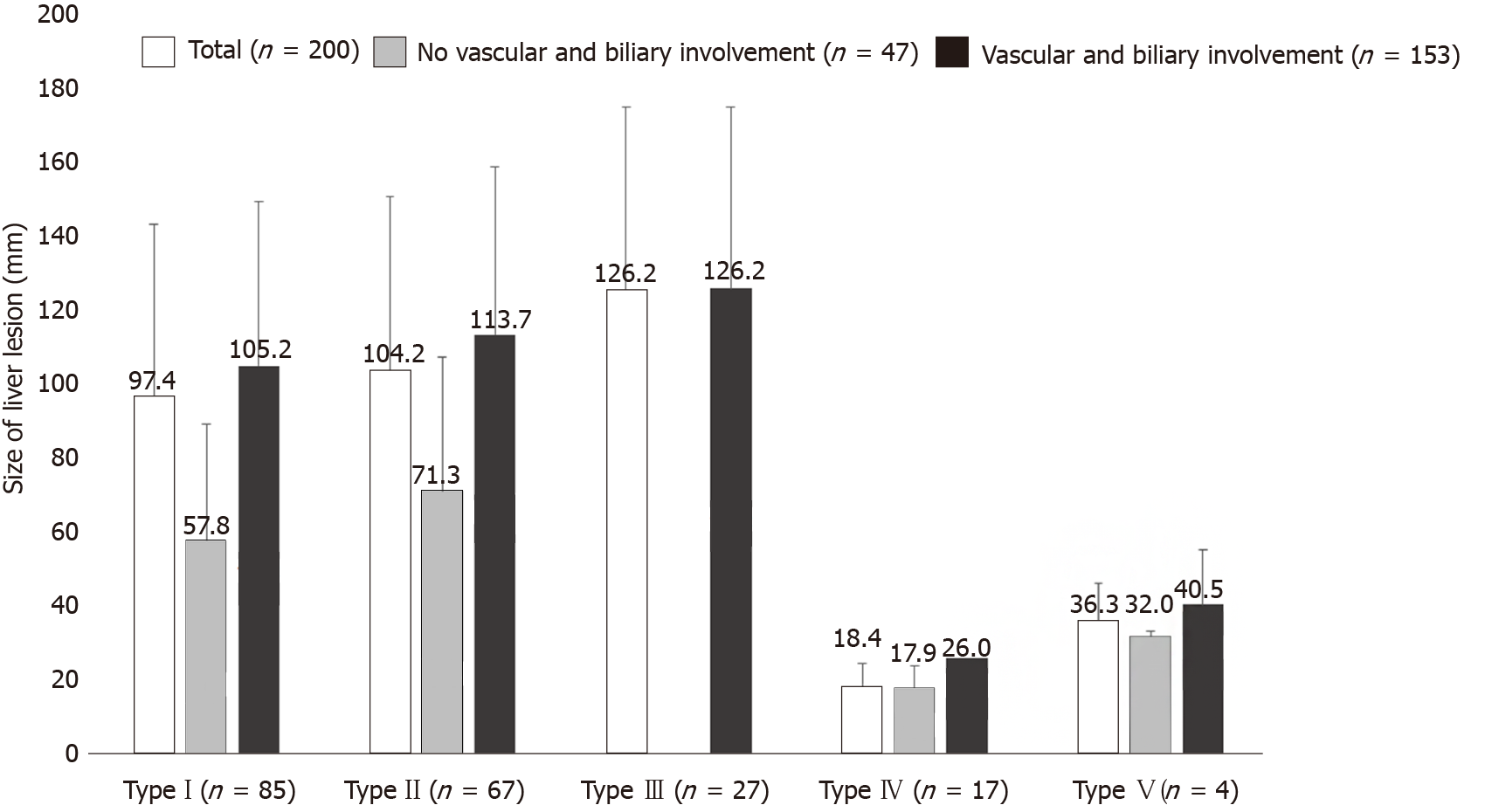
We identified a significant differences between the presence and non-presence of distant extrahepatic disease and the size of the liver lesion, for the total dataset (130.13 ± 49.81 vs 92.65 ± 51.05; P = 0.0075) and for the Chinese cases (145.15 ± 42.25 vs 116.69 ± 51.16; P = 0.0251). Overall, distant extrahepatic manifestations were significantly more common in larger lesions of the liver (Figures 3 and 4).
We did not adjudicate the following as distant extrahepatic manifestations: solely accentuated, well-circumscribed lymph nodes, and a continuous infiltration of neighboring organs or liver-adjacent connective tissue (including diaphragm) through the liver lesion. These features had the following distribution in this group of 200 cases: lymph nodes, n = 6; diaphragm, n = 3; retroperitoneum close to the liver, n = 3; right adrenal gland, n = 3; mediastinum/pericardium/right atrium, n = 1; and gallbladder, n = 2. Within those cases, simultaneous distant extrahepatic manifestations were recorded for two of six with accentuated lymph nodes, one of three with infiltration of the diaphragm, and one of three with local retroperitoneal infiltration.
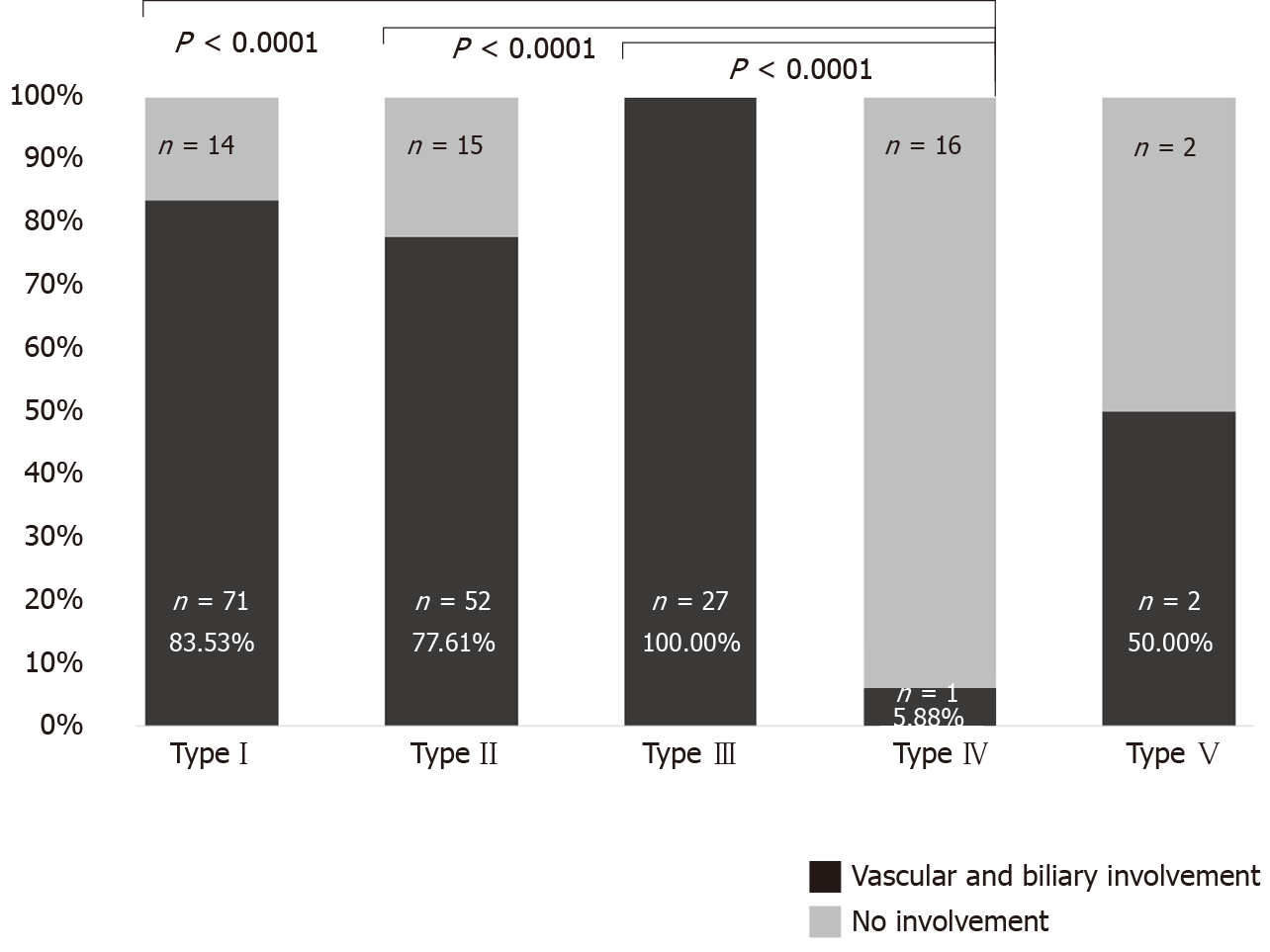
Vascular/biliary involvement of the largest liver lesion was found in 153 of the total 200 cases (76.50%). In the Chinese data, 92/100 (92%) showed this involvement, which was significantly higher than in the European data, with 61/100 (61%) (χ² = 26.7279, P < 0.0001, Figure 5).
The presence of vascular/biliary involvement highly significantly correlated with liver lesion size for the total group (110.45 ± 46.99 vs 47.44 ± 35.03; P < 0.0001) as well as for the European (88.04 ± 32.54 vs 44.07 ± 35.20; P < 0.0001) and Chinese datasets separately (125.30 ± 49.30 vs 63.87 ± 31.02; P = 0.0009).
Among the 200 cases, vascular/biliary involvement as a function of primary lesion morphology ranged from 5.88% of type IV liver lesions to 100% among type III lesions. Other primary morphological types occasionally were associated with very high rates of vascular/biliary involvement. Type IV differed significantly in these associations from types I, II, and III (fisher exact test; P < 0.0001) (Figure 6). In Europe, type I was most commonly associated with involvement of the corresponding structures, whereas in China it was type II, (P = 0.0056 for comparison between the two geographic regions). Figure 7 shows the distribution of primary morphology types depending on vascular and biliary involvement and non-involvement considering the lesion size.
In this study, a large international collective (n = 200) of German, French, and Chinese patients with HAE was evaluated after prior classification of the liver lesions based on EMUC-CT[5] concerning findings of vascular/biliary involvement and distant extrahepatic disease. The aim was to obtain information about the different manifestations of this parasitosis at the respective sites and yield useful information about the development and dynamics of this disease. Information on criteria such as extrahepatic manifestations and vascular/biliary involvement have never been studied in relation to different morphological types of AE liver lesions. It may be possible that such information can provide an approximation to a suspected stadium-like development of AE.
AE lesions appear almost exclusively in the liver. In addition to lesion size, this infiltration into vascular and biliary structures is clinically important[11,13-17]. Extrahepatic localizations of primary AE lesions are rare, but there may be invasion into neighboring organs or distant disease manifestations[1,12]. The exact prognostic relevance of distant extrahepatic manifestations in AE as well as the mechanism driving these so-called “distant metastases” of AE are debated[22-26]. Both the infiltration of intrahepatic vascular and bile duct structures as well as extrahepatic disease manifestations can lead to further complications and may increase morbidity in patients with AE.
Here, with regard to intrahepatic disease, the involvement of the right hepatic lobe was most common, occurring in 80% of the total cases. The same held for comparisons among regions (Europe vs China). The fact that segment VIII was most frequently involved for all four centers is certainly because of its central location and size, taking up a larger volume compared to the left lobe. Azizi et al[7] and Becce et al[27] also described a predominately right hepatic distribution of AE lesions.
In this study, if only a continuous infiltration of the liver lesion was present locoregionally with some extrahepatic tendency, these cases were not adjudicated as extrahepatic disease. Similarly, solely accentuated, well-circumscribed lymph nodes were not evaluated as extrahepatic manifestations. These nodes can exhibit so-called "small particles of echinococcus multilocularis,” which can lead to corresponding inflammatory reactivity without representing a confirmed parasitic disease manifestation[28].
True distant extrahepatic manifestations, which were quite rare overall, showed a significant difference in comparison between the European and Chinese centers, with more identified in the Chinese data. This finding could be viewed as an indication of more advanced cases in China at the time of diagnosis. Distant extrahepatic manifestations in the lung were most common in the overall group, followed by lesions in the brain. Distant extrahepatic AE manifestations are also described more frequently in these two organs in other studies[29-31].
With regard to the five primary morphology types of liver lesions after EMUC-CT, type IV and V were not associated with any cases of distant extrahepatic disease, and type III was most frequently associated with it (22.22%). This result may indicate different stages of development through the lesion types, with incrementally different degrees of progression of the overall disease. In agreement, in a recent comparative study of CT morphology and histology, type IV was described as the initial lesion and type III as the most advanced[32].
We found a significant relationship between the presence of distant extrahepatic disease and the size of the liver lesion in the overall group and in the Chinese data, with significantly more frequent extrahepatic involvement and simultaneously larger lesions of the liver in China. These findings imply that detection of distant extrahepatic manifestations is related to disease progression.
The vast majority of cases in the overall dataset (76.50%) showed vascular/biliary involvement of the largest liver lesion, and the patient population in China had significantly more frequent involvement compared to the European patients. Involvement of vascular and biliary structures also significantly correlated with lesion size. These observations add further support to the earlier evidence that Chinese cases are more advanced at detection, based on lesion size and type distribution[33]. In addition, type IV had the least vascular/biliary involvement at 5.88%, differing significantly from types I–III, and type III had the most, at 100%. These results also support the idea of disease progression through type IV to types I, II, and III successively[33-35].
A limitation of this retrospective study is the assessment of vascular/biliary involvement of HAE lesions, which was based purely on image morphological criteria, although all experienced reviewers agreed on the conclusions. Since no standardized examination protocol was used in the preparation of the routine CT scans, this may in principle have led to method-related limitations in the assessment of individual criteria. The study design precluded a histopathological evaluation of these criteria. Furthermore, the presence of distant extrahepatic disease was determined retrospectively based on whole-body staging examinations. As described above, these exams were occasionally handled differently for the different centers, depending on local conditions and practices.
In summary, the current findings show differences between Chinese and European AE cases in terms of vascular/biliary involvement, distant extrahepatic disease manifestations, and EMUC-CT types of liver lesions associated with these features.
The results may give indications about the behavior of this disease in the context of initial manifestation and progression. A morphological classification of AE liver lesions seems therefore not only useful in order to facilitate the initial differential diagnosis but also indicates a direct clinical impact.
The designation and evaluation of distant extrahepatic AE manifestations as "metastases," as in the PNM (P = parasitic mass in the liver, N = involvement of neighbouring organs, and M = metastasis) classification[36], may be re-evaluated in future studies. Such a re-evaluation is warranted as it remains unclear whether these distant manifestations are “metastases” originating from the liver lesion or if they also represent possibly more slowly growing primary manifestations, which we think is more likely.
Many important scientific questions concerning AE could so far only be answered through networks and cooperation at international, European and national level[1,37-39]. There is a need for future prospective studies combining standardized international radiological expertise and terminology in an Echino network[40].
In future internationally standardized examination protocols will be necessary to generate valid imaging data in order to improve their comparability.
Human alveolar echinococcosis (AE) is a zoonosis caused by the larval stage of the fox tapeworm echinococcus multilocularis. Untreated, the disease is fatal. The main endemic areas of alveolar echinococcosis (AE) are in Central Europe and Western China.
Early diagnosis is of crucial importance. Imaging techniques play the greatest role here. International comparative studies on imaging are not yet available.
Aim of this study was to evaluate the vascular/biliary involvement of hepatic alveolar echinococcosis and the extrahepatic disease manifestations in a collective of German, French, and Chinese cases.
Consecutively, five experienced examiners evaluated contrast-enhanced abdominal computed tomography (CT) scans for 200 patients with hepatic AE of each of four locations (n = 50) in Germany, France and China according to the echinococcosis multilocularis Ulm classification for CT (EMUC-CT). Vascular/biliary involvement of the hepatic disease as well as the presence of distant extrahepatic manifestations were correlated with the EMUC-CT types of liver lesion.
Distant extrahepatic AE manifestations were significantly more frequent in China than in Europe (P = 0.0091). A significant relationship was found between the presence of distant extrahepatic disease and AE liver lesion size (P = 0.0075). Vascular/biliary structures were involved by the liver lesions significantly more frequently in China than in Europe (P < 0.0001), and vascular/biliary involvement depended on lesion size. Different morphological types of AE liver lesions led to varying frequencies of vascular/biliary involvement and were associated with different frequencies of distant extrahepatic manifestations. Type IV differed significantly in these associations from types I, II, and III (P < 0.0001). With respect to extrahepatic disease, the primary morphology types IV and V of liver lesions were not associated with any case of distant extrahepatic disease. In contrast, distant extrahepatic manifestations in types I–III were found to varying degrees, with a maximum of 22% for type III.
Different CT morphological patterns of hepatic AE lesions influence vascular/biliary involvement and the occurrence of distant extrahepatic manifestations. There are intercontinental differences regarding the characteristics of AE manifestation.
The results may give indications about the behavior of this disease in the context of initial manifestation and progression. A morphological classification of AE liver lesions seems therefore not only useful in order to facilitate the initial differential diagnosis but also indicates a direct clinical impact.
Many thanks to the World Health Organization Informal Working Group on Echinococcosis, especially to Dominique A Vuitton, for initiating the XUUB cooperation.
Besançon, France: Anne-Pauline Bellanger, Oleg Blagosklonov, Solange Bresson-Hadni, Eleonore Brumpt, Eric Delabrousse, Florent Demonmerot, Frederic Grenouillet, Bruno Heyd, Jenny Knapp, Stephane Koch, Laurence Millon, Damien Montange, Josephine Moreau, Carine Richou, Celia Turco, Claire Vanlemmens, Dominique A Vuitton, Lucine Vuitton.
Ulm, Germany: Thomas FE Barth, Sven Baumann, Ambros J Beer, Meinrad Beer, Hartmut Döhner, Iris Fischer, Tilmann Graeter, Hans-Jürgen Groß, Beate Gruener, Doris Henne-Bruns, Andreas Hillenbrand, Silke Kapp-Schwörer, Katharina Klein, Wolfgang Kratzer, Patrycja Schlingeloff, Julian Schmidberger, Rong Shi, Steffen Stenger, Frauke Theis.
Urumqi, China: Yi Jiang, Renyong Lin, Wenya Liu, Yingmei Shao, Aji Tuerganaili, Jian Wang, Hao Wen, Wenbao Zhang.
Xining, China: Yanling Bai, Haihua Bao, Jiayuan Cao, Haining Fan, Yingli Kang, Weixia Li, Ren Li, Haijiu Wang, Xiaoping Wang, Shengbao Wen, Yousen Wu, Guixiu Yin, Zhixin Wang.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xing BC, Verran DJ S-Editor: Ma YJ L-Editor: A E-Editor: Ma YJ
| 1. | Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, Vuitton DA. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv Parasitol. 2017;96:259-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 2. | Brunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1333] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 3. | Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, Li W; interdisciplinary Echinococcosis Working Group Ulm. Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. 2019;47:703-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Kratzer W, Gruener B, Kaltenbach TE, Ansari-Bitzenberger S, Kern P, Fuchs M, Mason RA, Barth TF, Haenle MM, Hillenbrand A, Oeztuerk S, Graeter T. Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: Echinococcosis multilocularis Ulm classification-ultrasound. World J Gastroenterol. 2015;21:12392-12402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 5. | Graeter T, Kratzer W, Oeztuerk S, Haenle MM, Mason RA, Hillenbrand A, Kull T, Barth TF, Kern P, Gruener B. Proposal of a computed tomography classification for hepatic alveolar echinococcosis. World J Gastroenterol. 2016;22:3621-3631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Kodama Y, Fujita N, Shimizu T, Endo H, Nambu T, Sato N, Todo S, Miyasaka K. Alveolar echinococcosis: MR findings in the liver. Radiology. 2003;228:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Azizi A, Blagosklonov O, Lounis A, Berthet L, Vuitton DA, Bresson-Hadni S, Delabrousse E. Alveolar echinococcosis: correlation between hepatic MRI findings and FDG-PET/CT metabolic activity. Abdom Imaging. 2015;40:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Schweiger A, Grimm F, Tanner I, Müllhaupt B, Bertogg K, Müller N, Deplazes P. Serological diagnosis of echinococcosis: the diagnostic potential of native antigens. Infection. 2012;40:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Reuter S, Nüssle K, Kolokythas O, Haug U, Rieber A, Kern P, Kratzer W. Alveolar liver echinococcosis: a comparative study of three imaging techniques. Infection. 2001;29:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Reuter S, Buck A, Manfras B, Kratzer W, Seitz HM, Darge K, Reske SN, Kern P. Structured treatment interruption in patients with alveolar echinococcosis. Hepatology. 2004;39:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Graeter T, Ehing F, Oeztuerk S, Mason RA, Haenle MM, Kratzer W, Seufferlein T, Gruener B. Hepatobiliary complications of alveolar echinococcosis: A long-term follow-up study. World J Gastroenterol. 2015;21:4925-4932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Reuter S, Seitz HM, Kern P, Junghanss T. Extrahepatic alveolar echinococcosis without liver involvement: a rare manifestation. Infection. 2000;28:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Ambregna S, Koch S, Sulz MC, Grüner B, Öztürk S, Chevaux JB, Sulima M, de Gottardi A, Napoléon B, Abergel A, Bichard P, Boytchev I, Deprez P, Dumortier J, Frossard JL, Kull E, Meny B, Moradpour D, Prat F, Vanbiervliet G, Thevenot T, Vuitton DA, Bresson-Hadni S, Vuitton L. A European survey of perendoscopic treatment of biliary complications in patients with alveolar echinococcosis. Expert Rev Anti Infect Ther. 2017;15:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Stojkovic M, Junghanss T, Veeser M, Weber TF, Sauer P. Endoscopic Treatment of Biliary Stenosis in Patients with Alveolar Echinococcosis--Report of 7 Consecutive Patients with Serial ERC Approach. PLoS Negl Trop Dis. 2016;10:e0004278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Frei P, Misselwitz B, Prakash MK, Schoepfer AM, Prinz Vavricka BM, Müllhaupt B, Fried M, Lehmann K, Ammann RW, Vavricka SR. Late biliary complications in human alveolar echinococcosis are associated with high mortality. World J Gastroenterol. 2014;20:5881-5888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ozturk G, Polat KY, Yildirgan MI, Aydinli B, Atamanalp SS, Aydin U. Endoscopic retrograde cholangiopancreatography in hepatic alveolar echinococcosis. J Gastroenterol Hepatol. 2009;24:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Sezgin O, Altintaş E, Saritaş U, Sahin B. Hepatic alveolar echinococcosis: clinical and radiologic features and endoscopic management. J Clin Gastroenterol. 2005;39:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Çakmak E, Alagozlu H, Gumus C, Alí C. A case of Budd-Chiari syndrome associated with alveolar echinococcosis. Korean J Parasitol. 2013;51:475-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Vogel J, Görich J, Kramme E, Merkle E, Sokiranski R, Kern P, Brambs HJ. Alveolar echinococcosis of the liver: percutaneous stent therapy in Budd-Chiari syndrome. Gut. 1996;39:762-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Fleiner-Hoffmann AF, Pfammatter T, Leu AJ, Ammann RW, Hoffmann U. Alveolar echinococcosis of the liver: sequelae of chronic inferior vena cava obstructions in the hepatic segment. Arch Intern Med. 1998;158:2503-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Rossi IA, Delay D, Qanadli SD, Jaussi A. Inferior vena cava syndrome due to Echinococcus multilocularis. Echocardiography. 2009;26:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Wang H, Lu C, Liu X, Zhang W. Metastatic and prognostic factors in patients with alveolar echinococcosis. Int J Clin Exp Pathol. 2015;8:11192-11198. [PubMed] |
| 23. | Matsuhisa T. [The mechanism of distant metastases of alveolar hydatid disease]. Hokkaido Igaku Zasshi. 1996;71:369-376. [PubMed] |
| 24. | Kunze V, Layer G, Brüning R, Nägele M. ["Metastasizing" Echinococcus alveolar of the liver]. Radiologe. 1992;32:444-447. [PubMed] |
| 25. | Eckert J, Thompson RC, Mehlhorn H. Proliferation and metastases formation of larval Echinococcus multilocularis. I. Animal model, macroscopical and histological findings. Z Parasitenkd. 1983;69:737-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Mehlhorn H, Eckert J, Thompson RC. Proliferation and metastases formation of larval Echinococcus multilocularis. II. Ultrastructural investigations. Z Parasitenkd. 1983;69:749-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Becce F, Pomoni A, Uldry E, Halkic N, Yan P, Meuli R, Schmidt S. Alveolar echinococcosis of the liver: diffusion-weighted MRI findings and potential role in lesion characterisation. Eur J Radiol. 2014;83:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Hillenbrand A, Beck A, Kratzer W, Graeter T, Barth TFE, Schmidberger J, Möller P, Henne-Bruns D, Gruener B. Impact of affected lymph nodes on long-term outcome after surgical therapy of alveolar echinococcosis. Langenbecks Arch Surg. 2018;403:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Jiang C. Alveolar echinococcosis in China. Chin Med J (Engl). 1998;111:470-475. [PubMed] |
| 30. | Kvascevicius R, Lapteva O, Awar OA, Audronyte E, Neverauskiene L, Kvasceviciene E, Sokolovas V, Strupas K, Marcinkute A, Deplazes P, Müllhaupt B. Fatal Liver and Lung Alveolar Echinococcosis with Newly Developed Neurologic Symptoms due to the Brain Involvement. Surg J (N Y). 2016;2:e83-e88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 31. | Ozdol C, Yildirim AE, Daglioglu E, Divanlioglu D, Erdem E, Belen D. Alveolar hydatid cyst mimicking cerebellar metastatic tumor. Surg Neurol Int. 2011;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Grimm J, Beck A, Nell J, Schmidberger J, Hillenbrand A, Beer AJ, Dezsényi B, Shi R, Beer M, Kern P, Henne-Bruns D, Kratzer W, Moller P, Barth TFE, Gruener B, Graeter T. Combining Computed Tomography and Histology Leads to an Evolutionary Concept of Hepatic Alveolar Echinococcosis. Pathogens. 2020;9:634. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Graeter T, Bao H, Delabrousse E, Brumpt E, Shi R, Li W, Jiang Y, Schmidberger J, Kratzer W, Liu W; XUUB consortium. Hepatic alveolar echinococcosis: Comparative computed tomography study between two Chinese and two European centres. Food Waterborne Parasitol. 2020;19:e00082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Engler A, Shi R, Beer M, Schmidberger J, Kratzer W, Barth TFE, Grimm J, Hillenbrand A, Henne-Bruns D, Gruener B, Beer AJ, Graeter T. Simple liver cysts and cystoid lesions in hepatic alveolar echinococcosis: a retrospective cohort study with Hounsfield analysis. Parasite. 2019;26:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Aoki T, Hagiwara M, Yabuki H, Ito A. Unique MRI findings for differentiation of an early stage of hepatic alveolar echinococcosis. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283-S287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 37. | Said-Ali Z, Grenouillet F, Knapp J, Bresson-Hadni S, Vuitton DA, Raoul F, Richou C, Millon L, Giraudoux P; Francechino Network. Detecting nested clusters of human alveolar echinococcosis. Parasitology. 2013;140:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Piarroux M, Piarroux R, Knapp J, Bardonnet K, Dumortier J, Watelet J, Gerard A, Beytout J, Abergel A, Bresson-Hadni S, Gaudart J; FrancEchino Surveillance Network. Populations at risk for alveolar echinococcosis, France. Emerg Infect Dis. 2013;19:721-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Charbonnier A, Knapp J, Demonmerot F, Bresson-Hadni S, Raoul F, Grenouillet F, Millon L, Vuitton DA, Damy S. A new data management system for the French National Registry of human alveolar echinococcosis cases. Parasite. 2014;21:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Vuitton DA, McManus DP, Rogan MT, Romig T, Gottstein B, Naidich A, Tuxun T, Wen H, Menezes da Silva A; World Association of Echinococcosis. International consensus on terminology to be used in the field of echinococcoses. Parasite. 2020;27:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |