Published online Jul 21, 2020. doi: 10.3748/wjg.v26.i27.3899
Peer-review started: February 27, 2020
First decision: April 30, 2020
Revised: May 12, 2020
Accepted: June 23, 2020
Article in press: June 23, 2020
Published online: July 21, 2020
Processing time: 144 Days and 22.4 Hours
The phenomenon of liver regeneration after partial hepatectomy (PH) is still a subject of considerable interest due to the increasing frequency of half liver transplantation on the one hand, and on the other hand, new surgical approaches which allow removal of massive space-occupying hepatic tumors, which earlier was considered as inoperable. Interestingly, the mechanisms of liver regeneration are extensively studied after PH but less attention is paid to the architectonics of the regenerated organ. Because of this, the question “How does the structure of regenerated liver differ from normal, regular liver?” has not been fully answered yet. Furthermore, almost without any attention is left the liver's structural transformation after repeated hepatectomy (of the re-regenereted liver).
To compare the architectonics of the lobules and circulatory bed of normal, re-generated and re-regenerated livers.
The livers of 40 adult, male, albino Wistar rats were studied. 14 rats were subjected to PH - the 1st study group (SG1); 10 rats underwent repeated PH – the 2nd study group (SG2); 16 rats were subjected to sham operation - control group (CG); The livers were studied after 9 months from PH, and after 6 months from repeated PH. Cytological (Schiff reaction for the determination of DNA concen-tration), histological (H&E, Masson trichrome, CK8 Immunohistochemical marker, transparent slides after Indian Ink injection, ), morphometrical (hepatocytes areas, perimeters and ploidy) and Electron Microscopical (Scanning Electron Microscopy of corrosion casts) methods were used.
In the SG1 and SG2, the area of hepatocytes and their perimeter are increased compared to the CG (P < 0.05). However, the areas and perimeters of the hepatocytes of the SG1 and SG2 groups reveal a lesser difference. In regenerated (SG1) and re-regenerated (SG2) livers, the hepatocytes form the remodeled lobules, which size (300-1200 µm) exceeds the sizes of the lobules from CG (300-600 µm). The remodeled lobules (especially the “mega-lobules” with the sizes 1000-1200 µm) contain the transformed meshworks of the sinusoids, the part of which is dilated asymmetrically. This meshwork might have originated from the several portal venules (interlobular and/or inlet). The boundaries between the adjacent lobules (including mega-lobules) are widened and filled by connective tissue fibers, which gives the liver parenchyma a nodular look. In SG2 the unevenness of sinusoid diameters, as well as the boundaries between the lobules (including the mega-lobules) are more vividly expressed in comparison with SG1. The liver tissue of both SG1 and SG2 is featured by the slightly expressed ductular reaction.
Regenerated and re-regenerated livers in comparison with normal liver contain hypertrophied hepatocytes with increased ploidy which together with transformed sinusoidal and biliary meshworks form the remodeled lobulli.
Core tip: Liver regeneration after partial hepatectomy (PH) is based on both proliferation and hypertrophy of hepatocytes. These cells unite into remodeled lobules, the sizes of which vary widely. The microcirculation of the liver is also re-modeled. At the same time, there is a suspicion that hyperplasia-hypertrophy and remodeling do not apply to all hepatocytes and all lobules; it is also possible that, in parallel with remodeling, new lobules are formed. After repeated PH, liver regeneration is based on the same type of transformations, although their intensity is less. To fully evaluate the lobular and microcirculatory architecture of regenerated liver after both, PH and repeated PH, it is necessary to investigate the architectonics of the initials of the biliary system.
- Citation: Tsomaia K, Patarashvili L, Karumidze N, Bebiashvili I, Azmaipharashvili E, Modebadze I, Dzidziguri D, Sareli M, Gusev S, Kordzaia D. Liver structural transformation after partial hepatectomy and repeated partial hepatectomy in rats: A renewed view on liver regeneration. World J Gastroenterol 2020; 26(27): 3899-3916
- URL: https://www.wjgnet.com/1007-9327/full/v26/i27/3899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i27.3899
The need for hepatectomy has increased over the last two decades due to increased space-occupying hepatic abnormalities[1,2] on the one hand, and on the other hand due to the increased frequency of the half liver transplantation from living[3-5]. The successful functioning of both transplanted, as well as the remnant liver of the donor, is largely determined by their unique regeneration ability. Liver regeneration, following the hepatectomy, is one of the most studied processes[6,7]. This is contributed to by the fact that various modifications of hepatectomy are easily modeled in experimental studies, particularly in small laboratory animals (rodents)[8-11]. Moreover, it is believed that the post-resection liver regeneration in rodents (rats) is the classic pattern of “regeneration”[12,13]. It was experimentally proved that following the liver resection (Partial hepatectomy - PH), the liver fully restores its size and mass on the 7th-8th (or upon 2 wk according to some authors) day from the surgery[13-15].
While studying the phenomenon of post-resection liver regeneration, researching the stimuli (triggers) inducing liver regeneration[6,7,16] on the one hand, and studying its termination mechanisms after restoring the liver mass is of key importance[12,16-19].
As of today, it has been confirmed that the process of post-resection regeneration of the liver and its termination upon restoring the mass, is based on a complex, interconnected and regulatory set of molecular, biological and biochemical mechanisms, trigger mechanisms of which are severe portal hypertension caused by sudden loss of significant functioning hepatic mass, and its leveling ability after the increase in the liver size[20].
Considering a plethora of papers dedicated to the study of liver regeneration, the fact that the structural side of the liver regeneration, following PH, is not studied is a paradox. In our previous paper reviewing the problems of liver regeneration we have mentioned that the issues like "What is the triger?" and "What is the mechanism?" are being actively investigated, while the issue "What kind of structural transformation is achieved?" remains out-of-focus[21].
A literature review suggests that most of the authors confirm the proliferation of hepatocytes following the PH[6,7,14,22-24]. Some authors highlight the hypertrophy of the hepatocytes[25-27] and/or the simultaneous presence of both processes[13,28].
The issue whether the size of the liver lobule is being increased (which would happen in the case of increasing the number of existing hepatocytes as well as their hypertrophy)[25,26,29] or there are developed new lobules, even with unusual architectonics[27], or both processes are occurring, remains unsolved.
The following also has to be specified: How are other tubular structures of the liver regenerating? How do they “follow” the growth of the body volume: By emerging “new branches” or by “extending” old branches? What transformations are the architectonics of the liver lobule and its microcirculatory module undergoing?
The question as to what happens after repeated liver resection following the partial hepatectomy, when the liver continues to “grow” and returns to its initial size, remains unanswered. Is the transformation of architectonics different, or is it performed in the same manner as in the case of the first regeneration?
Besides, it is important to study the liver tissue not only in the regeneration period immediately post-resection but also after passing a significant amount of time following the surgery, when the structural transformations are complete. Such a study should answer the question: “What is the difference between the architectonics of normal and regenerated liver?”.
The present study aims to describe the transformation of liver lobules and circulatory beds in remote periods from surgery after partial hepatectomy and repeated partial hepatectomy for the comparative analysis of architectonics of normal and regenerated liver.
The experiments were conducted on 40 adult, male, albino Wistar rats weighing 180-220 g. Our experimental study involved the control group (CG) and two study groups (SG1 and SG2). The control group was presented with sham-operated rats (surgery included laparotomy and abdominal cavity revision). The rodents of SG1 underwent 70% hepatectomy. The SG2 was presented with rats that underwent the repeated 70% hepatectomy 9 months after primary intervention. The liver tissues were studied after 9 months from sham-operation (CG) and primary 70% hepatectomy (SG1), also after six months from repeated 70% hepatectomy (SG2).
The livers were studied histologically [hematoxylin and eosin (H&E), Masson trichrome, CK8 Immunohistochemical marker, transparent slides after Indian Ink injection], morphometrically (hepatocytes areas, perimeters, and ploidy) and by scanning electron microscopy (SEM) of corrosion casts. The distribution of the experimental animals in accordance with experimental models and research methods is presented in the Table 1.
| Methods | Histology | SEM of corrosion cast | Ploidy of hepatocytes | Number of animals | ||||
| H&E | Histochemistry(Masson’s Trichrome) | IHC (CK8) | Morphometry(perimeters and areas of the hepatocytes and their nuclei) | Transparent slides after Indian Ink injection | ||||
| Control | 6* | 6* | 6* | 6* | 4 | 6 | 3* | 16 |
| Study group 1 (9 mo after PH) | 6** | 6** | 6** | 6** | 4 | 4 | 3** | 14 |
| Study group 2 (6 mo after repeated PH conducted after 9 mo from primary intervention) | 4*** | 4*** | 4*** | 4*** | 2 | 4 | 3*** | 10 |
| Total 40 | ||||||||
The surgical operations were performed on animals in the morning, in the fasting state, under diethyl ether general anesthesia. The animal protocol was designed to minimize pain or discomfort to the animals during the operation as well as in post-operative periods. The animals were acclimatized to laboratory conditions (22 °C, 12 h/12 h light/dark, 60% humidity, ad libitum access to food and water) prior to experimentation and after surgery (limitation was in place on the days before the operation and before the intervention).
Primary partial hepatectomy: PH was performed according to the Claudia Mitchell & Holger Willenbring protocol with the application of double knot surgery[10]. After opening the abdominal cavity of the rat, the liver was mobilized by sectioning the liver ligaments. The first ligature was followed by the excision of the left lateral lobe (about 26% of the liver mass), while the second ligature by the excision of the medial lobe of the liver (about 40% of the liver mass).
The resected liver tissue was examined macro- and microscopically to find out any pathology.
Repeated partial hepatectomy: Repeated PH was performed 9 months after the first surgery. The laparotomy and abdominal cavity revision were carried out. The remnant liver was represented by the regenerated upper and lower segments of the right lateral lobes and the anterior and posterior caudal lobes. The blood vessels of both segments of the right lateral lobe were ligated by applying the "single-knot method" so as not to hinder the blood flow in the lower vena cava. The resected liver tissue corresponded to about 70% of the remnant liver[11].
H&E staining: Liver tissue sections of 3-μm were stained by the standard H&E method and studied microscopically with different magnification.
Histology after Indian-ink/gelatin injection: Histological “transparent” slices of liver tissue were prepared after injection of the Indian-ink and gelatin (1:3) mixture into the portal vein. The mixture was prepared in accordance with the recommendations of Vellimana et al[30] and Aum et al[31]. The injection technique was the same as for the injection of a solidifying mass for SEM investigation (see below).
Histochemistry: Liver tissue sections of 3-5 μm were stained using Masson’s Trichrome kit (Sigma Aldrich Catalog Number: C970D37) according to the recommendation of the manufacturer.
Immunohistochemistry: Rabbit antibody keratin-8 (KRT8) produced by MyBiosourse (Catalog #MBS8510691) was used for the marking of hepatocyte’s cell membrane and cholangiocytes of formaldehyde-fixed liver tissue. The antibody was diluted 1:200 in 0.01 mol/L phosphate buffered saline pH 7.4 (Sigma Aldrich).
A heat mediated antigen retrieval step was performed in citrate buffer. The tissue was then blocked and incubated with the antibody for 2 h at 22 °C. An HRP conjugated goat anti-rabbit antibody was used as the secondary.
All light microscopy was conducted by Primo star ZEISS, Jena, Germany equipped with a digital video camera - ZEN 2.3 SP1.
Morphometry: The histological slides stained with CK8 marker were used for morphometric analysis. CK8 allowed good visualization of the hepatocyte membrane and ensured a high degree of accuracy of marking the measuring space.
The morphometrical analysis was conducted for:
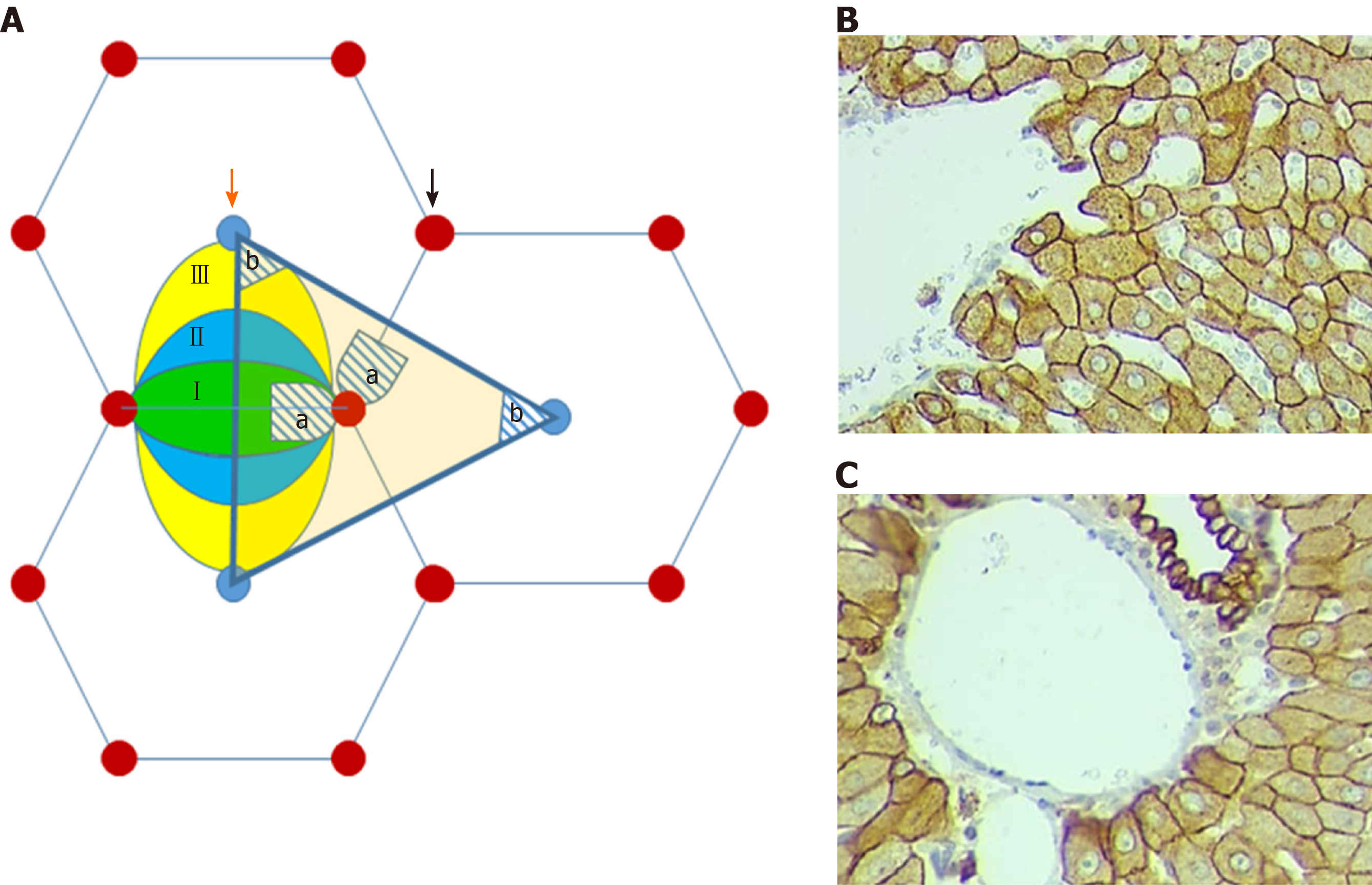
(1) Hepatocytes of the first zone of the liver acinus, located periportally on both sides of the line connecting neighboring portal triads (this corresponds to the area, adjoining the perpendicular line descending from the portal zone to the connective axis of the central veins of the adjacent classical lobules (hatched area “a” on Figure 1A and C).
And (2) Hepatocytes of the third zone of the liver acinus, which are located around the central vein of the classic liver lobule and correspond to the area near the top of the portal lobule (hatched area “b” on Figure 1A and B).
The hepatocytes of the second zone of liver acinus were not measured due to difficulty in the exact identification of the zone's borders.
The analysis of the hepatocyte area and perimeter was performed on caudal lobes of the livers of the animals from the control as well as from both study groups, taking into account that this lobe would be maintained even after repeated hepatectomy. Histological slides were scanned on a Motic Digital Slide Scanner and analyzed using motic digital scanner assistant software, Motic VM 3.0. The work area was enlarged 40 times and cell membranes were outlined manually because the shape of the hepatocytes does not fit any geometric figure as a rule. For morphometric analysis, there were chosen the cells with a fully visualized membrane and nucleus (Figure 1B and C).
Differences between control and study groups were calculated using t-test and Mann-Whitney U test and the value used for statistical significance was P < 0.05. All statistical analyses were performed with SAS v 9.4 software.
Determination of the DNA concentration of the hepatocytes: The liver tissue of rats of CG, SG1, and SG2 was dissociated into the cells using the phosphate buffers. From the obtained suspensions smears were prepared. The Feulgen method (Schiff reaction) was used to determine the concentration of DNA in the nuclei of dissociated cells[32,33]. The capture of the specimen stained with the Schiff's reagent was performed by using a light microscope (ZEN 2.3 SP1; 100 × 10). The intensity of the staining of the nuclei was evaluated using the computer program Image J.
The solidifying mass obtained by the mixture of 0.25 g MAYCRYL C. C. powder (4.7% of whole mass) + 0.08 g benzoyl peroxide (1.5% of whole mass) + 5.0 mL protacryl-M liquid component was injected into the liver blood circulatory network through the portal vein of the Wistar rats[34]. The injection was carried out under diethyl ether deep general anesthesia, after washing-out of the blood vessels with 0.1 mL. Heparin (5000 un/mL) mixed in 0.9% NaCl solution (washing-out speed – 20 mL/min). The details of the injection were described earlier[35] ISM was injected by using a manometer-syringe of the own construction[34]. The injection pressure varied between 20-30 mmHg.
The outflow of the injected mass occurred through the defect made in the right atrium. The manipulations were performed using microsurgical microscopy (Bino Scientific, Manufacturer: Nature of Business, India ISO 9001-2008).
Polymerization of the injected mass happened in the animal body placed in a water-bath at 37 0C for 2 h. The livers were excised and cut into small fragments which did not exceed 20 × 10 × 10 mm3. The fragments were corroded in 20% KOH solution, at room temperature. After 2 h fragments were removed from the KOH solution and washed 3 times in distilled water for 10 min. The resulting preparations were dried in air. The obtained specimens were mounted with the special (electro-conductive) glue on the appropriate tables for SEM examination with JEOL-JSM-6510LV allowing the visualization of the sample by both backscattered and secondary streams of the electrons, under high and low vacuum conditions. For this investigation in high vacuum conditions, the corrosion casts were preliminarily coated with gold in equipment JEC-3000FC (Tokyo BOEKI Group, Japan) (vacuum = 3.2 Pa; coating time = 180 s).
This research was adopted by the ethical committee of Aleksandre Natishvili Institute of Morphology, TSU in accordance with Directive 2010/63/ OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on the “protection of animals used for scientific purposes” (22 September 2010).
The results of the morphometric study of liver tissue reveal that the area of the CG hepatocytes, located in the first zone of the liver acinus is significantly less than the area of the hepatocytes in the third zone of the acinus (P < 0.05) while we could not find significant difference between the perimeters of the corresponding cells (P > 0.05). The cells of the first and third zones of the acini for the SG1 and SG2 do not differ significantly either by area or by the length of the perimeter (P > 0.05 in both cases). In the SG1 and SG2, the area of hepatocytes and their perimeter of both, the first zone and the third zone of the acini are increased significantly compared to the CG (P < 0.05 in all groups), The hepatocytes of the SG1 and SG2, located in the first zone of the acinus do not differ significantly from each other by areas or the length of the perimeter (P > 0.05 in both cases), whereas the area of the hepatocytes of the SG2, located in the third zone of the acinus significantly exceeds the area of the corresponding hepatocytes of the SG1 (P < 0.05) (Table 2).
| Study groups | III zone (mean ± SD) | I zone (mean ± SD) | ||
| Area | Perimeter | Area | Perimeter | |
| Control group (CG) | 283 ± 88 | 64 ± 10 | 255 ± 66 | 62 ± 8 |
| Study group 1 (SG1) | 331 ± 95 | 71 ± 11 | 348 ± 90 | 71 ± 10 |
| Study group 2 (SG2) | 390 ± 128 | 75 ± 11 | 372 ± 107 | 73 ± 11 |
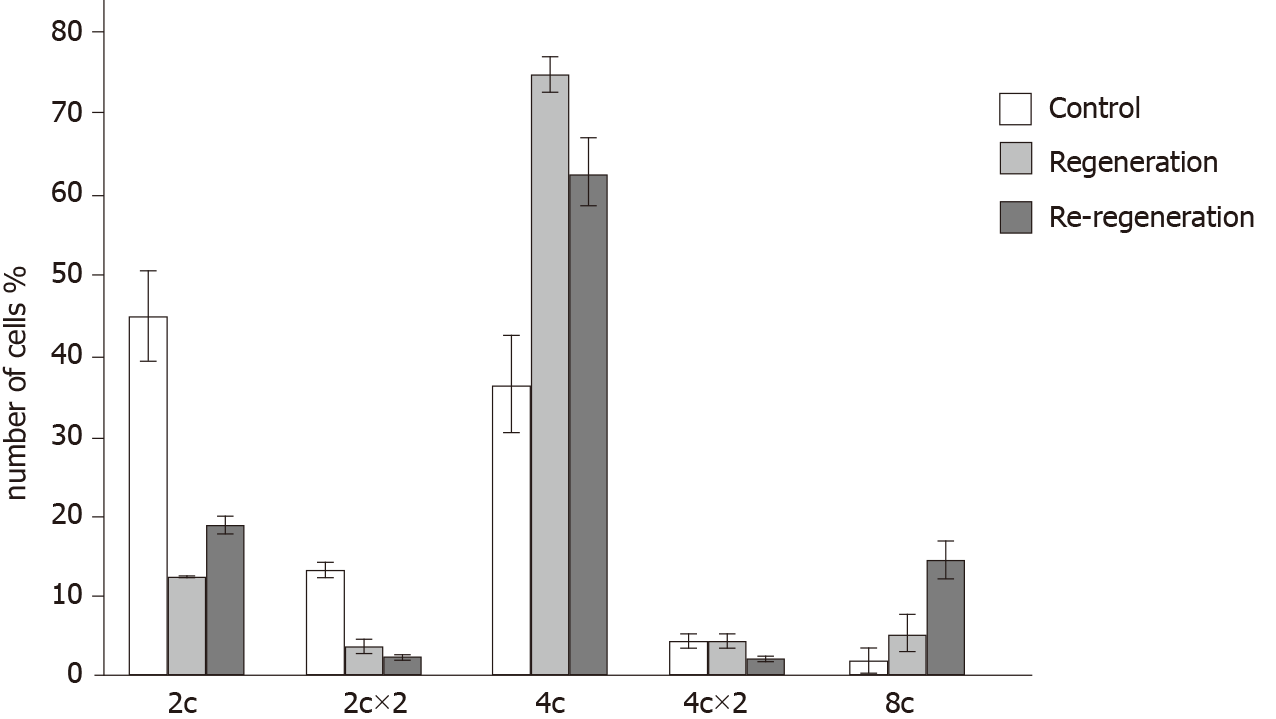
The number of diploid (2c) and binucleated cells (2c × 2) significantly decreased in the SG1 and SG2 groups compared to the CG group (P < 0.05), while the number of polyploidy (4c and 8c) cells is significantly increased (P < 0.05). At the same time, the number of high ploidy (8c) cells in SG2 increased in comparison with both CG and SG1 groups (the data of the measurements are presented in Figure 2).
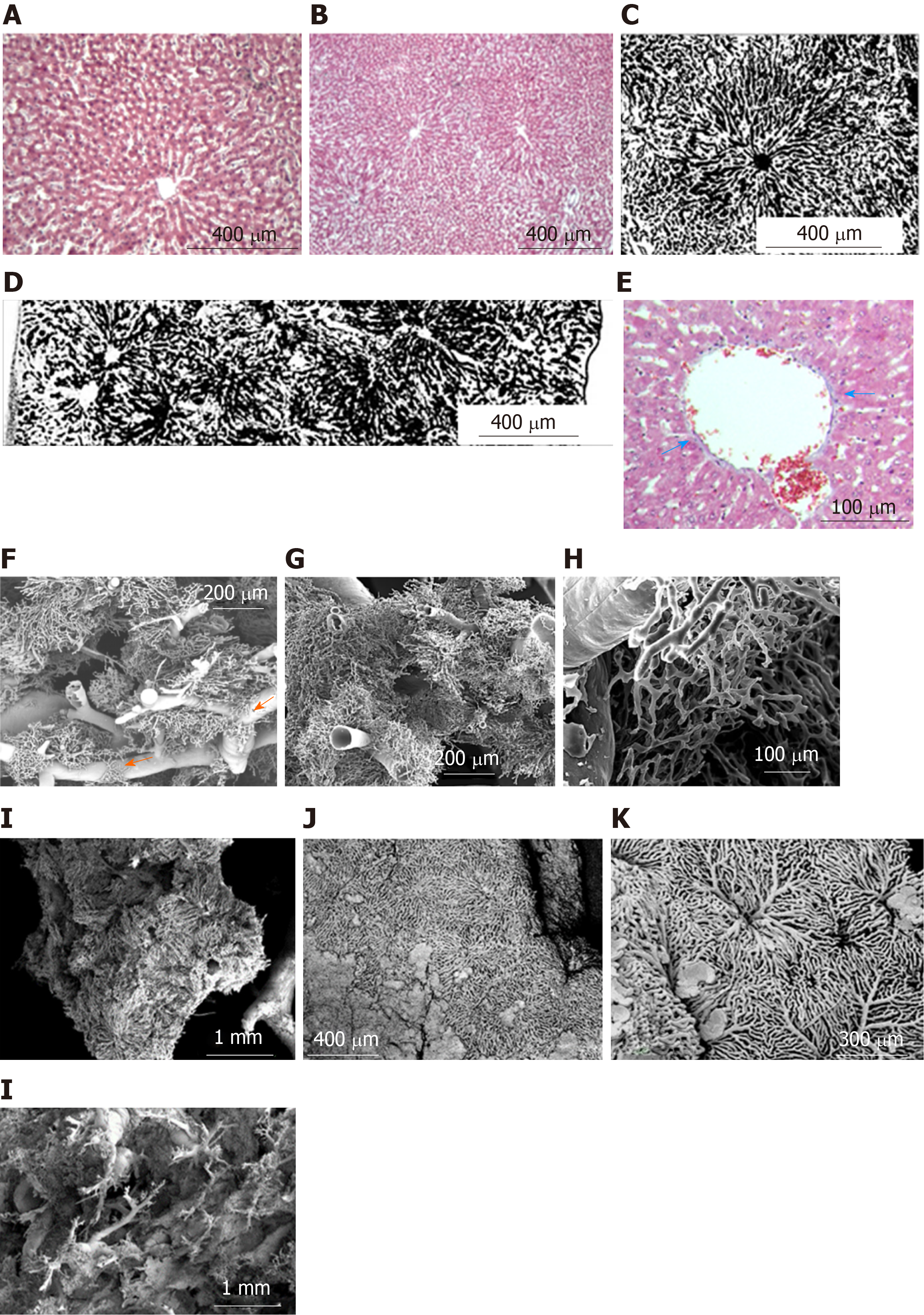
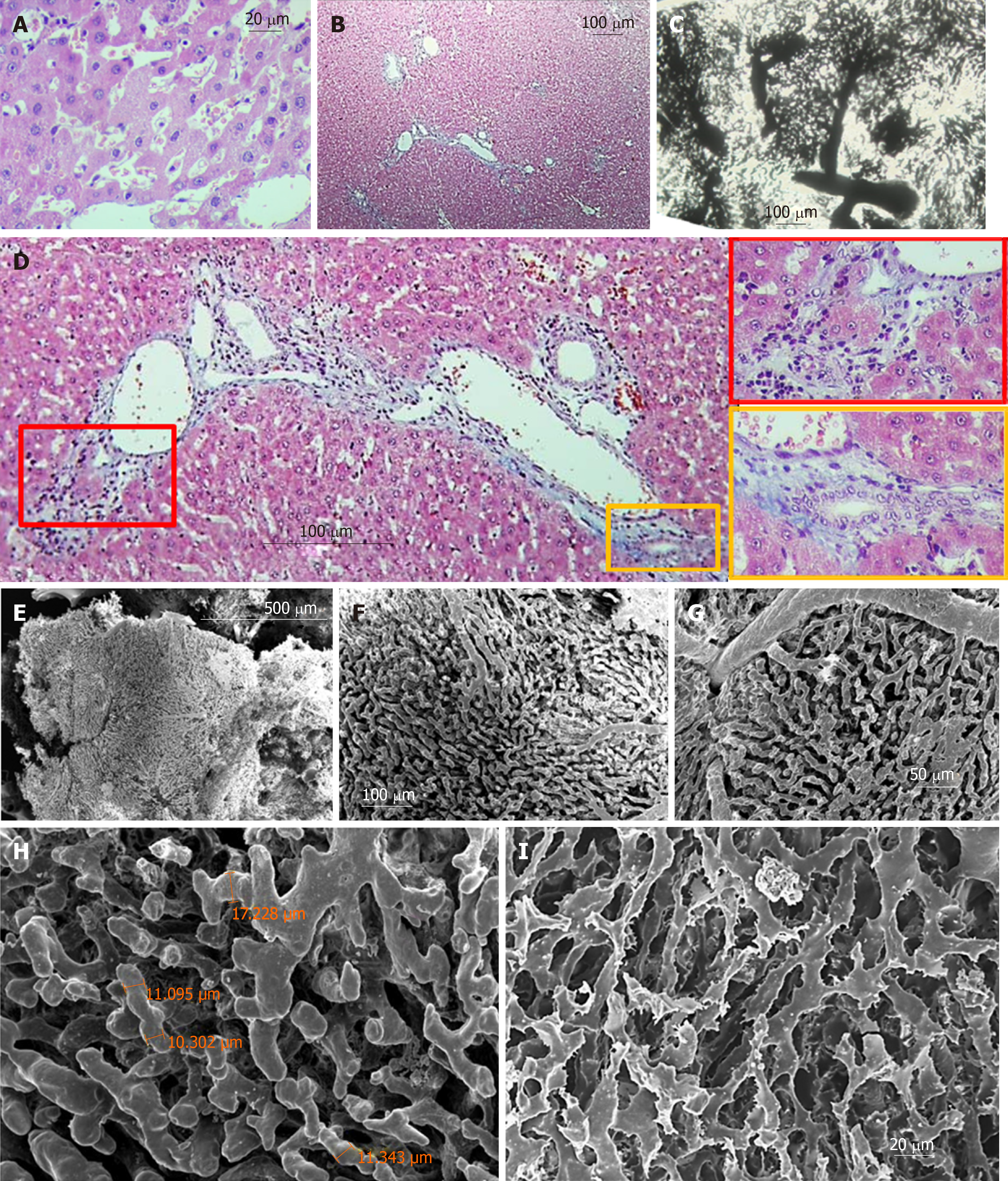
Regular shaped liver lobules of the histological slices (H&E) from the CG are represented by a circular or multi-dimensional geometrical structure close to the circle in the center of which the central vein can be identified (Figure 3A and B). The identification of such lobules is relatively easy on transparent histological slices, prepared after Indian Ink-Gelatin injection (Figure 3C). It should be noted that the diameter of such "classical" lobules varies significantly and ranges from 300 µm to 600 µm. The slices clearly indicate several adjacent lobules, the circulatory bed of which is interconnected. These intercommunicated lobules are filling the distance of ~ 2.6 millimeters between the diaphragmatic and visceral surfaces of the caudal lobe of the liver (Figure 3D).
The collagenous and elastic connective tissue fibers form sheaths for the portal tubular structures and tributaries of hepatic veins. The thickness of this capsule increases with the increasing diameter of the tubular structures. In addition, the connective tissue is more weakly expressed around the branches of the hepatic veins and is not visible in Disse's spaces (Figure 3E).
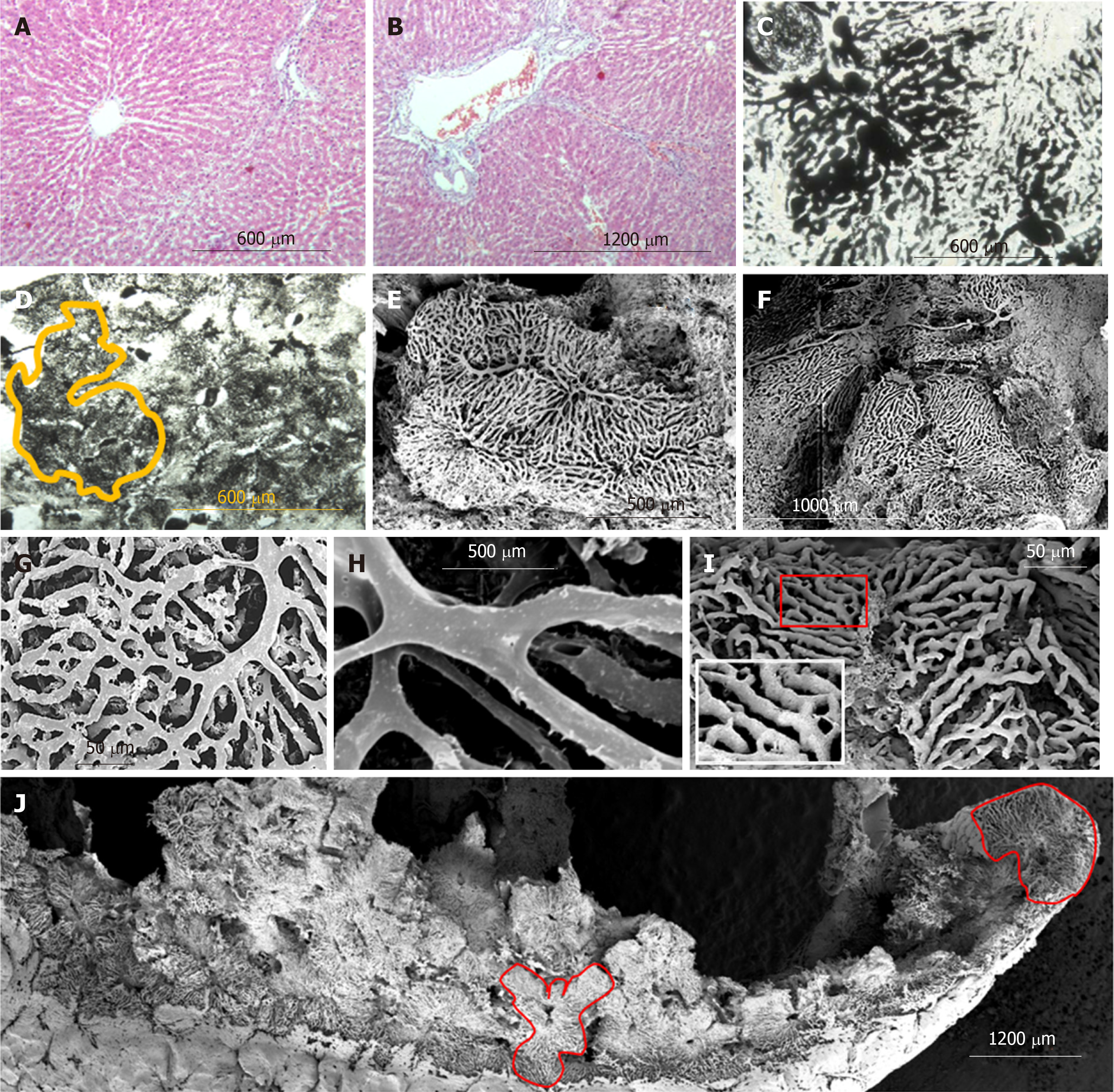
Nine months after PH (SG1) the sizes of those lobules of the histological slices (H&E), contours which can be identified, are higher than normal. Part of the sinusoids is dilated asymmetrically giving, to the liver tissue, a nonhomogenous view (Figure 4A and B). The sinusoidal non-homogeneity is better seen on transparent histological slices prepared after Indian Ink - Gelatin injection: the "mega-lobules" formed by the aggregation of merging several adjacent lobules, a unified sinusoidal network of which originates from several portal venules, can be clearly seen in different areas (Figure 4C and D). The boundaries between the adjacent lobules (including mega-lobules) are widened and filled by connective tissue fibers. The number of collagen and elastic connective tissue fibers around the portal triads and branches of the hepatic vein are increased. Moreover, single fibers are also observed in Disse's spaces (Figure 4B).
Six months later the repeated PH (SG2) the unevenness of sinusoid diameters (Figure 5A and C), as well as the boundaries between the lobules (including the mega-lobules) are more vividly expressed. The fibrotic changes in re-regenerated liver tissue are increased. The connective-tissue membranes around tubular structures are thickened; such membrane accompanies perilobular blood vessels, surrounding the atypical lobules (mega-lobules), which gives the liver parenchyma a nodular look. The number of connective tissue fibers is also increased in the Disse's spaces (Figure 5B and D).
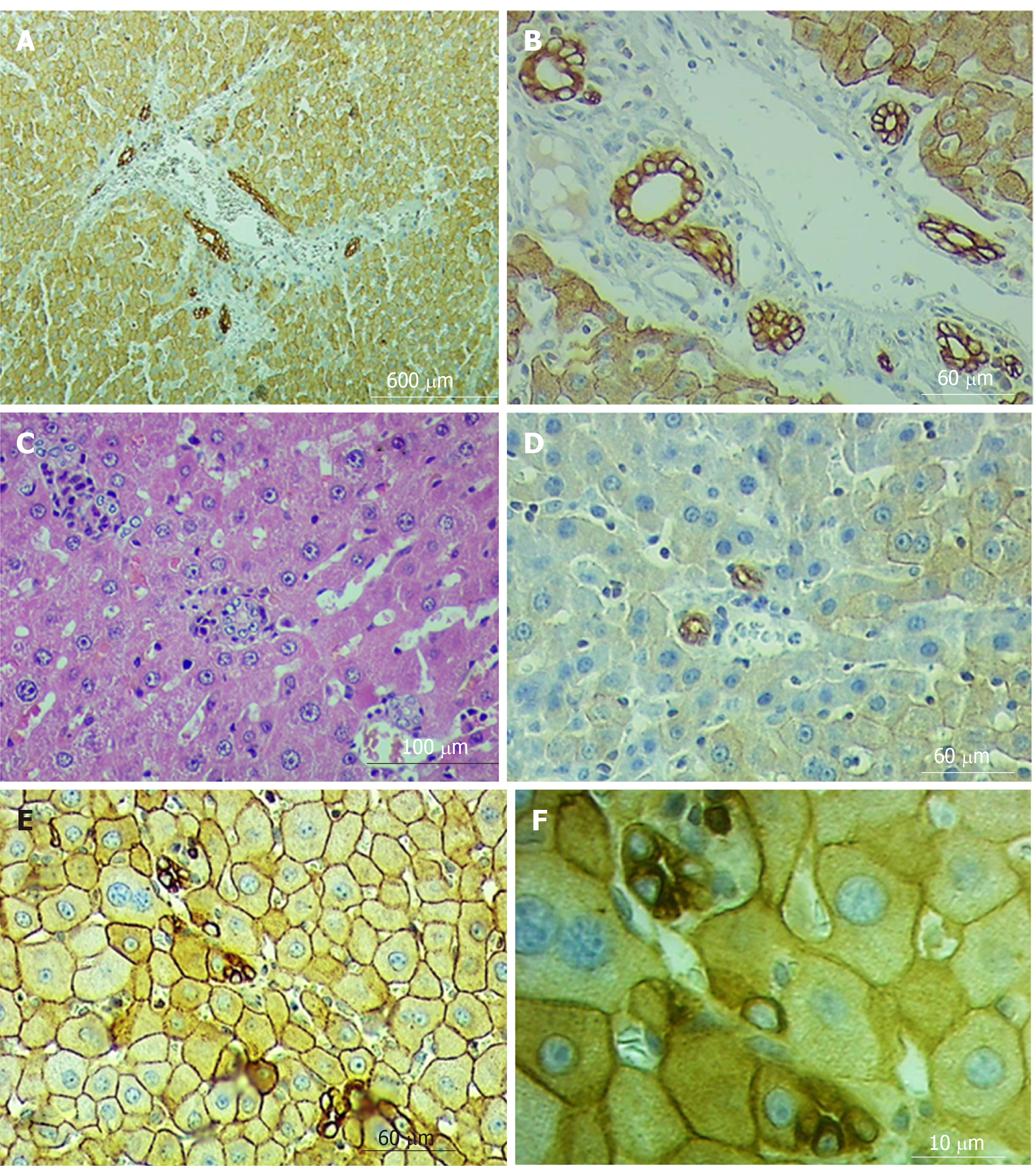
Biliary structures appear on histological slices, both stained with H&E and marked with CK8. In addition, the number of ductular profiles in the SG1 and SG2 was greater than in control animals; The ductular profiles are observed not only in the portal tracts or in the periportal zones of the liver parenchyma, but also within the liver lobules including thos adjacent to the various caliber branches of the liver veins (involving central and sublobular veins) (Figure 6A-F).
The results provided below are based on SEM investigation of the vascular casts of the liver containing blood vessels less than 300 µm in diameter. The sinusoidal vessels form both - the three-dimensional meshwork of more or less uniform regular form as well as the mesh with difficultly described geometry.
Three types of lobules can be distinguished in the liver tissue: Superficial lobules, the parietal surfaces of which are represented as an anastomosing meshwork with a circular or multi-facet shape. The casts of superficial sinusoids are associated with the casts of central/sublobular veins, rarely with the veins of bigger diameter. The diameters of the parietal surfaces of the superficial lobules range from 300 µm to 600 µm (Figure 3J and K). Deeply localized lobules have forms which are different from the superficial ones. Here the sinusoidal meshworks form spheral or ellipsoidal, multi-faceted constructs which are connected to the casts of the thinnest branches of the portal blood vessels (interlobular branches) and/or liver veins (central and sublobular veins) (Figure 3F, G, and I). The sizes of these lobules (conventionally, "diameters") range from 300 µm to 1000 µm. Some lobules reproduce the structure of either the classical lobule or the liver acinus. The later is commonly represented by a complex of the portal vein and so-called partially casted sinusoids “not reaching" the central veins (Figure 3F-H). There also may be distinguished the regions, where it is difficult or impossible to identify the lobule-like structures in the sinusoidal meshwork; Such regions are commonly found in the areas of bifurcation of the branches of the portal veins with diameters of 200-400 µm (Figure 3H).
The sinusoids may represent not only the meshwork connected to the terminal (interlobular) branches of the portal vein but also the meshwork, connected to the so-called “inlet” portal venules. These inlet venules are separated from the larger-caliber blood vessels at certain intervals (every 300-700 µm) on two opposite sides (Figure 4L). Rarely, there are observed the regions where several adjacent lobules are merged in such a way that they create an aggregation - "mega-lobule", the common sinusoidal meshwork of which originates from the various portal venules and is drained by several central (sublobular) veins.
Sinusoidal casts are characterized by a narrowing in the peripheries of the lobules. At such places, they are more twisted and interconnected and form a polygonal meshwork. The casts are somehow thicker and more “straightened” in the proximity of central (sublobular) veins. Occasionally, between the adjacent casts of the sinusoids, there is observed some kind of parallelism before adjoining the branches of the liver veins. The intermediate vessels localized between the periportal and pericentral zones have a transitional characteristic.
The diameters of the sinusoidal replicas vary in a wide range (from 6 µm up to 20 µm). The length of the sinusoidal vessels before the branching also varies (from 6 µm up to 50 µm).
On some regions of the corrosion specimen, we have observed small capillary meshworks, connected to the sinusoidal casts – a so-called “periportal meshwork”, which sometimes connects the sinusoids of the lobules, located on the opposite sites of the portal vein branch (Figure 3F, arrow).
After the 9 months from the partial hepatectomy, the so-called "megalobules" - formed by the merging of the adjacent lobules - are distinguished on the corrosion casts specimen, which differs from normal architectonics of the lobules both in form and in the construction of the sinusoidal network. The size of the longest diameter of such lobules (in the case of an ellipsoidal form) or the diagonal (in the case of a cuboid shape) exceeds 1 mm (Figure 4E and F). Sometimes it becomes possible to identify the small constructions (200-400 µm in diameter), forming the megalobule, though sometimes it is not possible (Figure 5E-J).
The diameter of sinusoidal casts of the regenerated liver lobules often exceeds the diameter of sinusoidal casts in the control group. The sinusoidal casts, similar to the casts in the control group, create two types of meshwork – with parallel, rarely interconnected fragments and twisted, often "anastomosing" fragments. However, the topography of the meshworks of these two types is not featured with the same topography as it was described in normal liver (parallel casts - pericentrally and twisted casts - periportally). For example, in the regenerated liver, both types of casts meshwork might be observed near the central vein (Figure 4G and I). Sinusoidal casts, in addition to loops with an inner diameter of 30-50 µm (which are commonly found normally as well), often form loops with an inner diameter of 2-4 µm. Sometimes these small loops are created not only by more or less even division and repeated merging of the whole casts but by the means of branched small wing-form outgrowths of the casts (Figure 4I) as well. A large scale imaging of the sinusoidal casts shows that except for the imprints of the endotheliocytes and/or their nuclear-containing zones (which are also found in the control group samples), in SG1 the villous outgrowths are observed on the casts. The presence of such villi gives the sinusoidal casts a “scratchy” look (Figure 4H).
SEM of the corrosion casts of the re-regenerated liver (SG2) indicates that the number and size of mega-lobules are increased (often exceeding 1 mm) (Figure 5E). The architectonics of these mega-lobules is quite different from the architectonics of the lobules from CG. At the same time, it is difficult to identify the boundaries between the smaller structures constituting mega-lobules while the boundaries between adjacent megalobules are vividly expressed. The diameters of the sinusoids and the associated blood vessels are greater than those of the corresponding blood vessels in the livers of CG and SG1 (Figure 5F-H).
Within the meshwork of the sinusoidal casts, there are frequently observed blind-end fragments (Figure 5H). At the same time, there are found the local dilations created by merging 3-4 sinusoids (similar to those which were observed either in CG or in SG1) (Figure 5G-I). The sinusoidal casts form loops of different inner diameters (5-30 µm). On part of the casts, there are imprints of the adjacent cells and/or their nuclear-containing zones. The villous outgrowths, like those observed on the sinusoidal casts of the regenerated liver (SG1), were found on the part of the sinusoidal casts, however, the size and frequency of these outgrowths in the re-regenerated liver (SG2) is much higher (Figure 5I).
As we have mentioned above, the mechanisms of liver regeneration after PH are extensively studied but less attention is paid to the architectonics of the regenerated organ/tissue. Because of this, the question “How does the structure of regenerated liver differ from normal, regular liver?” has not been fully answered yet. Furthermore, almost no attention has been paid to the liver's structural transformation after repeated hepatectomy (of the re-regenareted liver). Our research supports clarifying some issues of the above-mentioned statement.
The same length and varied area of the periportal (the 1st zone of the acinus) and pericentral (the 3rd zone of the acinus) hepatocytes in the livers of CG animals indicate the difference in the shape of these cell sections. Periportally located hepatocytes are often formed as polygonal configurations, drawn into the square, whereas pericentrally located hepatocytes have a more oblong shape (resembling polygonal figures, drawn in a rectangle). Liver regeneration is associated with hypertrophy of both hepatocytes, located periportally and pericentrally. At the same time, hepatocyte sections are more uniformed.
It is confirmed that the liver almost fully restores its size and mass on the 7th-8th day after PH (in 2 wk according to some authors)[13,14]. The rapid activation of hepatocyte proliferation, observed within a few days after liver resection, is associated with a complex of mechanisms involving signaling pathways controlled by mitogenic growth factors and their receptors, as well as multiple cytokines and other signaling molecules that trigger DNA synthesis in hepatocytes[32,36]. It is thought that the appearance of these mitogenic factors and signaling molecules (endothelial growth factor, transforming growth factor, tumor necrosis factor, NO, etc.) is associated with both mechanical damage of the liver tissue during PH and/or the realization by the blood vessels of residual liver[16,33,37,38]. The acute portal hypertension acts as a triggering mechanism in post-resection liver regeneration. It is determined by a sharp decrease in the volume of the intrahepatic portal bed due to the excision of 2/3 of the liver. The increased portal pressure causes the development of the so-called shear stress in the sinusoids and releasing the endothelial factors, including NO, by them that is initiating the hepatic regeneration[20,37-40]. In addition, the pressure increases not only in the sinusoids where lumens are covered by fenestrated endothelial cells[41,42], but also in the spaces of Disse, where there are situated the various cells (Ito cells, Kupffer cells, etc.), which also get involved in the production of the signaling molecules and factors and in the regulation of liver regeneration[43,44]. In the current study, the structure of liver tissue is investigated when the processes of regeneration and re-regeneration are completed. Considering the established opinion, when the normal liver mass/body mass ratio of 2.5% has been restored, liver regeneration would be terminated[16]. Based on our data, it may be assumed that liver mass growth is provided by the hypertrophy of repopulated hepatocytes appearing after post-PH mitosis, on the one hand, and by the dilation of the sinusoidal capillaries, increase of the blood-vessel sizes and number of connective tissue fibers, on the other hand.
According to Miyaoka et al[45] the number of hepatocytes in the remnant liver lobes after the 70% hepatectomy is increased 1.6 times, while the weight of these lobes is increased 2.4 times. This proves that hepatocytes are subject not only to hyperplasia but also to hypertrophy as well. These data confirm the results of our morphometric investigation.
The increase in hepatocyte areas in SG1 and SC2 might be conditioned by two simultaneous processes - cell hypertrophy and polyploidy[45]. Our data show that both the primary and repeated PH results in an increase in the number of polyploid cells. This supports the assumption that along with hypertrophy and proliferation the genome content of hepatocytes is multiplied. The relatively high content of 8с cells in the SG2 might be stipulated by the increase of the functional load of the re-regenerated liver tissue.
Thus, it might be thought that after PH the regenerated liver (regenerated lobes) should contain an increased number of hypertrophied hepatocytes (the part of which has the increased polyploidy). But in such a case, the size of all the lobes of the regenerated liver should exceed the size of the lobules from the control group, as mentioned by Wagenaar et al[26] showing that porto-central distance is increased 1.2-1.5 times after PH.
However, the results of our study of histological and corrosion specimens (H&E, Masson's trichrome, transparent slices prepared after injection of Indian-Ink/Gelatin) verify that together with the hypertrophied, to various degrees, lobules (including so-called Mega-lobules), the lobules with the same size and shape as the lobules from the control group are found in both - regenerated and re-regenerated livers. The existence of normal size and shape lobules indicates that the process of hyperplasia-hypertrophy did not affect all hepatocytes, and therefore all lobules. This assumption agrees with the observation of Miyaoka et al[13].
At the same time, the hepatocytes subjected to proliferation and hypertrophy have undergone remodeling and an increased size (hypertrophied) lobules including the non-standard shaped- and “Mega-lobules”. This coincides with the opinion of Wagenaar et al[30].
The results of our study do not allow estimation of whether the number of “supplying” (interlobular and inlet) and draining (central and sublobular) venules are increased or not. Therefore, no conclusions can be drawn regarding the proliferation of lobules, despite some researchers pointing out that the new lobules should be formed within 56-122 d from the PH[46]. Studying the corrosion casts and Indian-ink injected slices prepared after 9 months from the partial hepatectomy we registered a remarkable increase of the number of blood vessels in mega-lobules, but we still prefer to use the more accurate term “lobule re-modeling” rather than “lobule proliferation”. However, while discussing the fact of increasing the number of blood vessels, the data indicating the splitting ability of the “Mega-lobules” after the primary as well as repeated PH have to be considered[26].
Some researchers confirm that not only after PH, but also at those stages of ontogenesis, when the body and organs continue to grow, only the size of the liver lobules increases, and not their number[25,47]. However, these authors do not indicate whether such growth of the lobule size is caused by the increase in the number or the size of hepatocytes.
However considering the results of Iatropoulos[46] and Kandilis et al[27], it should be assumed that the possibility of creating new lobules (lobule proliferation) cannot be completely excluded. Doubt is reinforced by the fact that on the corrosion cast of the regenerated liver we have observed the lobules, which are smaller than the sizes of the liver lobules of the CG animals. Thus, confirmation or rejection of the hypothesis about the creation of new lobules after PH requires additional studies.
Remodeling of the liver cells and lobules, following the PH, is accompanied by increasing the number of connective tissue fibers, not only around the large blood vessels but also of their small ramifications including perilobular branches and even in the Disse's spaces. This indicates that both remodeled lobules and their hepatocytes are more firmly embedded in the connective tissue framework in comparison with the control group. Presumably, because of such embedding, the new wave of remodeling following the repeated PH should be less intensive. This assumption is confirmed by examination of both, histological as well as corrosion specimens.
In addition, after repeated PH, Masson's trichrome-stained specimen revealed remarkable increase in connective tissue fibers compared to the state after primary resection. The fibers accompany the perilobular blood vessels surrounding the remodeled lobules (including mega-lobules). Allegedly because of this, following the next hepatectomy (the third), the lobules would have even less remodeling ability. This assumption is partially supported by studies by Wagenaar et al[26], who noted that hepatocytes do not increase after a third hepatectomy.
The fact that the number of ductular profiles gradually increases after 9 months from PH and after 6 months from the repeated PH (following 9-month regeneration) indicates that biliary structures are also subjected to structural changes. At the same time, this ductular reaction (DR) should not be associated with either stem cell proliferation or hepatocyte transdifferentiation (types II and III (DR)[48,49]. Ductular profiles revealed on histological slices of regenerated and re-regenerated livers resemble the ductular profiles found in the early stages of common bile duct occlusion in our previous studies[50,51]. But bile duct occlusion does not occur in the case of PH and/or repeated PH. It may be assumed that the appearance of ductular profiles in regenerated and re-regenerated hepatic parenchyma may be associated with such remodeling-related structural transformations (including the package of the connective tissue fibers) that result in obstruction of the normal flow of bile through the intraportal, intralobular and Herring ductules[52]. This again confirms that the transformation of the liver microcirculatory network including 4-сompartments instead of 3 (likely other organs provided by the excretory duct system), cannot be discussed fully without assessing the state of the bile ductules/canaliculi[53-55].
SEM of the corrosion casts confirms that in CG the liver is made up of lobules of different shapes and sizes. At the same time, due to the absence of remarkable connective tissue borders, which for example are observed in the pig liver, where each lobule is bounded by perilobular blood vessels and the connective-tissue fibers, associated with them[56,57], it is almost impossible to identify the classic form of the lobule in the rat liver. This is especially true for the lobules, located deeply in the parenchyma. In contrast to them, surface lobules retain more or less typical circular or polygonal shape, although they are also characterized by wide variations in size.
The presence of abundant anastomoses between the sinusoidal meshworks of adjacent lobules enables one to imagine the sinusoidal meshwork of the liver in the form of a single united mesh that is supplied and drained by many blood vessels. The supplying blood vessels are represented by the intralobular and inlet venules. The last ones separate from the larger blood vessels in every 300-600 µm on two opposite sides. The distance between the inlet venules corresponds to the lobule sizes. As for the distance of the sinusoids opening in the central/sublobular venules, they correspond to the sizes of the pericentral hepatocytes, given that between two adjacent sinusoids one or two hepatocytes may be located. The sinusoidal mesh has additional specific inlets in the form of periportal and peribiliary vascular plexuses, which in turn originate from the branches of the portal vein and hepatic artery. All described features correspond to normal liver vascularization described by SEM investigation of normal vascular corrosion casts provided by Yamamoto et al[58], and Gaudio et al[59].
After PH, as well as repeated PH, sinusoidal capillaries participate in the lobules remodeling by dilatation, proliferation, and formation of new construction of the spatial meshwork. However, this remodeling does not apply equally to all segments of the sinusoidal meshwork similarly to the hepatocytes (as described above). In regenerated and re-regenerated livers similar to the livers of CG animals, two types of the sinusoids are distinguished: The parallel with rare intercommunication and twisted, with frequent anastomoses. However, the intralobular topography of the mentioned different form fragments does not reveal the similarity to that described in normal conditions. The dilatation and lengthening of the sinusoidal casts, creation of the “blind-ended” rami and the increase in the number of loops confirm the existence of a proliferative process, that might be based on both proliferation[60], as well as hypertrophy of the endothelial cells covering the lumens of the sinusoids. The latter is supposedly due to some sinusoidal casts provided with the imprints of endothelial cells and their nucleus-containing zones, the size of which is similar to the size of imprints of nucleus-containing zones of hepatocytes described earlier on the casts of bile canaliculi[61].
The appearance of the blind-ended ramifications on the sinusoidal casts should be considered as the confirmation of the proliferation of the sinusoidal capillaries by sprouting[62]. It is believed that exactly by merging such “blind” ramifications are produced sinusoidal loops. The small inner diameter (e.g., 2-4 µm) of some loops suggests that hepatocytes should not always be located here. It may be assumed that such sinusoidal loops surround small intralobular biliary ductules, described above.
Those loops, with an even smaller inner diameter, which are created by interconnecting small winged processes of the sinusoidal cast, deserve a separate notice. Supposedly, the presence of such processes and loops should indicate that the mechanism of intussusceptive angiogenesis (capillary “splitting” by creating an “endothelial curtain” [“septum”] in their lumen and dividing it into two, often uneven, parts) is involved in sinusoidal proliferation[63].
The further remodeling of the sinusoids after the repeated PH (SG2) in comparison with SG1 is verified by SEM of the corrosion casts. In addition to the increasing number of blind-ended ramifications, the local dilations resulted from the merging of 3-4 sinusoids (sinusoidal lakes) were observed. Quite often the areas containing such patterns are difficult to distinguish from the central veins on the histological slices.
The presence of small villose processes of the sinusoidal casts of both regenerated (SG1) and re-regenerated (SG2) livers should indicate the presence of dilations of the endothelial fenestras making available the “penetration” of the injectable solidifying mass through them. Increases of the size and number of these processes in SG2 in comparison with SG1 might be related to a greater dilation of the endothelial cell fenestras. Increased fenestration, in its turn, has to be associated with endothelial cell remodeling, which develops after resection[64] and supposedly becomes even stronger after repeated resection.
Thus, if we summarize all the above, it can be concluded that liver regeneration after PH is based on both, proliferation and hypertrophy of hepatocytes. These cells unite into remodeled lobules, the sizes of which vary widely. The microcirculation of the liver is also remodeled. At the same time, there is a suspicion that hyperplasia-hypertrophy and remodeling do not apply to all hepatocytes and all lobules. It is also possible that in parallel with remodeling, the new lobules are formed[31]. After repeated PH, liver regeneration is based on the same type of transformations, although their intensity is less. However, the obtained results of the evaluation of hepatocytes’ ploidy confirm that even after repeated PH the liver tissue retains the ability for further recovery.
To fully evaluate the lobular and microcirculatory architecture of regenerated liver after both PH and repeated PH it is necessary to investigate the architectonics of the initials of the biliary system.
The structural changes of the regenerated liver are left beyond the significant investigation, whereas architectural changes after re-regeneration are almost unknown.
The research motivation is to provide the possibility for detection of new lobules in regenerated liver.
This study aims to compare architectonics of the lobules and circulatory bed of normal, regenerated and re-regenerated livers.
Liver regeneration after partial hepatectomy (PH) as well as its re-regeneration after repeated PH is based on both - increase in size of hepatocytes and remodeling of liver lobules. The last is accompanied with transformation of meshwork of the dilated sinusoids. Besides the space between lobules are widened and filled with connective tissue. Regenerated and re-regenerated livers are featured by the ductular reaction.
The lobulli of the regenerated and re-regenerated livers in comparison with normal liver are remodeled. The remodeling is based on the hypertrophy of the hepatocytes with increased ploidy and transformation of sinusoidal and biliary meshworks.
Further studies are planned to investigate the involvement of stem cells in liver re-regeneration processes, which currently represent a gap in knowledge; It is also planned to compare the morphological features of the liver regeneration following the PH with the regeneration of the transplanted liver of the same size, given that the transplanted liver has impaired innervation and lymphatic drainage.
We would like to thank Professor Nikoloz Jalabadze and the young scientist Mrs. Lili Nadaraia (Institute of Physical Materials Science and Materials Technologies, Georgian Technical University) for providing the study with SEM investigation, Mr. Nikoloz Chkhartishvili for statistical analysis, and the laboratory assistant, of the Alexandre Natishvili Institute of Morphology, TSU.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Georgia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu J S-Editor: Gong ZM L-Editor: A E-Editor: Ma YJ
| 1. | Wen PH, Lin KH, Chen YL, Hsieh CE, Ko CJ, Kuo SJ. Extracorporeal hepatic resection and autotransplantation using temporary portocaval shunt provides an improved solution for conventionally unresectable HCC. Dig Dis Sci. 2013;58:3637-3640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Zhang GQ, Zhang ZW, Lau WY, Chen XP. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new strategy to increase resectability in liver surgery. Int J Surg. 2014;12:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Sharma A, Ashworth A, Behnke M, Cotterell A, Posner M, Fisher RA. Donor selection for adult-to-adult living donor liver transplantation: well begun is half done. Transplantation. 2013;95:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Irie R, Nakazawa A, Sakamoto S, Takeda M, Yanagi Y, Shimizu S, Uchida H, Fukuda A, Miyazaki O, Nosaka S, Kasahara M. Living donor liver transplantation for congenital hepatic fibrosis in children. Pathol Int. 2020;70:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Goldaracena N, Barbas AS. Living donor liver transplantation. Curr Opin Organ Transplant. 2019;24:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 7. | Abu Rmilah A, Zhou W, Nelson E, Lin L, Amiot B, Nyberg SL. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol. 2019;8:e340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Almau Trenard HM, Moulin LE, Padín JM, Stringa P, Gondolesi GE, Barros Schelotto P. Development of an experimental model of portal vein ligation associated with parenchymal transection (ALPPS) in rats. Cir Esp. 2014;92:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Wei W, Zhang T, Zafarnia S, Schenk A, Xie C, Kan C, Dirsch O, Settmacher U, Dahmen U. Establishment of a rat model: Associating liver partition with portal vein ligation for staged hepatectomy. Surgery. 2016;159:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 449] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 11. | Saito S, Togo S, Morioka D, Matsuo K, Yoshimoto N, Nagano Y, Tanaka K, Kubota T, Nagashima Y, Shimada H. A rat model of a repeat 70% major hepatectomy. J Surg Res. 2006;134:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Palmes D, Spiegel HU. Animal models of liver regeneration. Biomaterials. 2004;25:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Miyaoka Y, Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell Div. 2013;8:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 15. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 16. | Tao Y, Wang M, Chen E, Tang H. Liver Regeneration: Analysis of the Main Relevant Signaling Molecules. Mediators Inflamm. 2017;2017:4256352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Kang LI, Mars WM, Michalopoulos GK. Signals and cells involved in regulating liver regeneration. Cells. 2012;1:1261-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Köhler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Huck I, Gunewardena S, Espanol-Suner R, Willenbring H, Apte U. Hepatocyte Nuclear Factor 4 Alpha Activation Is Essential for Termination of Liver Regeneration in Mice. Hepatology. 2019;70:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Nishii K, Brodin E, Renshaw T, Weesner R, Moran E, Soker S, Sparks JL. Shear stress upregulates regeneration-related immediate early genes in liver progenitors in 3D ECM-like microenvironments. J Cell Physiol. 2018;233:4272-4281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Tsomaia K, Inauri N, Patarashvili L, Karumidze N, Azmaipharashvili E, Bebiashvili I, Kordzaia M, Kakabadze M, Dzidziguri D, Kordzaia D. To fill the missing fragments of a complex mosaic of liver regeneration. Transl Clin Med - Georg Med J. 2018;3:32-37. [DOI] [Full Text] |
| 22. | Bangru S, Arif W, Seimetz J, Bhate A, Chen J, Rashan EH, Carstens RP, Anakk S, Kalsotra A. Alternative splicing rewires Hippo signaling pathway in hepatocytes to promote liver regeneration. Nat Struct Mol Biol. 2018;25:928-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Tarlá MR, Ramalho FS, Ramalho LN, Silva Tde C, Brandão DF, Ferreira J, Silva Ode C, Zucoloto S. A molecular view of liver regeneration. Acta Cir Bras. 2006;21 Suppl 1:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1204] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 25. | Dezső K, Papp V, Bugyik E, Hegyesi H, Sáfrány G, Bödör C, Nagy P, Paku S. Structural analysis of oval-cell-mediated liver regeneration in rats. Hepatology. 2012;56:1457-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Wagenaar GT, Chamuleau RA, Pool CW, de Haan JG, Maas MA, Korfage HA, Lamers WH. Distribution and activity of glutamine synthase and carbamoylphosphate synthase upon enlargement of the liver lobule by repeated partial hepatectomies. J Hepatol. 1993;17:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Kandilis AN, Koskinas J, Vlachos I, Skaltsas S, Karandrea D, Karakitsos P, Pantopoulou A, Palaiologou M, Nikiteas N, Tiniakos DG, Perrea DN. Liver regeneration: immunohistochemical study of intrinsic hepatic innervation after partial hepatectomy in rats. BMC Gastroenterol. 2014;14:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Nagy P, Teramoto T, Factor VM, Sanchez A, Schnur J, Paku S, Thorgeirsson SS. Reconstitution of liver mass via cellular hypertrophy in the rat. Hepatology. 2001;33:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Dezso K, Bugyik E, Papp V, László V, Döme B, Tóvári J, Tímár J, Nagy P, Paku S. Development of arterial blood supply in experimental liver metastases. Am J Pathol. 2009;175:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Vellimana AK, Milner E, Azad TD, Harries MD, Zhou ML, Gidday JM, Han BH, Zipfel GJ. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2011;42:776-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Aum DJ, Vellimana AK, Singh I, Milner E, Nelson JW, Han BH, Zipfel GJ. A novel fluorescent imaging technique for assessment of cerebral vasospasm after experimental subarachnoid hemorrhage. Sci Rep. 2017;7:9126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Liu M, Chen P. Proliferation-inhibiting pathways in liver regeneration (Review). Mol Med Rep. 2017;16:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Hoffmann K, Nagel AJ, Tanabe K, Fuchs J, Dehlke K, Ghamarnejad O, Lemekhova A, Mehrabi A. Markers of liver regeneration-the role of growth factors and cytokines: a systematic review. BMC Surg. 2020;20:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Tsomaia K, Patarashvili L, Bebiashvili I, Azmaiparashvili E, Kakabadze M, Jalabadze N, Sareli M, Gusev S, Kordzaia D. New corrosion cast media and its ability for SEM and light microscope investigation. Microsc Res Tech. 2020;83:778-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Kordzaya DJ, Goderdzishvili VT. Bacterial translocation in obstructive jaundice in rats: role of mucosal lacteals. Eur J Surg. 2000;166:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2467] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 37. | Schoen Smith JM, Lautt WW. Nitric oxide and prostaglandins potentiate the liver regeneration cascade. Can J Gastroenterol. 2006;20:329-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997;27:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today. 1999;29:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 42. | Anthony P. Biopathology of the Liver. An Ultrastructural Approach. J Clin Pathol. 1989;42:445-446. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 43. | Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology. 2001;33:363-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 45. | Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 46. | Iatropoulos MJ. Cytoarchitecture of rat liver during compensatory growth. Anat Rec. 1971;169:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Papp V, Dezsö K, László V, Nagy P, Paku S. Architectural changes during regenerative and ontogenic liver growth in the rat. Liver Transpl. 2009;15:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. III. Implications for liver pathology. Virchows Arch. 2011;458:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 49. | Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995;191:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Azmaiparashvili E, Berishvili E, Kakabadze Z, Pilishvili O, Mikautadze E, Solomonia R, Jangavadze M, Kordzaia D. Ductular reaction at the early terms of common bile duct ligation in the rats. Acta Biol Hung. 2012;63:321-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Azmaiparashvili E, Bebiashvili I, Karumidze N, Tsomaia K, Kordzaia D. Ductular reaction at the early and late stages of biliary obstruction: is the mechanism the same? Georgian Med News. 2019;100-106. [PubMed] |
| 52. | Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 521] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 53. | Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis. 2004;24:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Kordzaia D, Jangavadze M. Unknown bile ductuli accompanying hepatic vein tributaries (experimental study). Georgian Med News. 2014;121-129. [PubMed] |
| 55. | Saxena R, Hytiroglou P, Thung SN, Theise ND. Destruction of canals of Hering in primary biliary cirrhosis. Hum Pathol. 2002;33:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Eberlova L, Liska V, Mirka H, Gregor T, Tonar Z, Palek R, Skala M, Bruha J, Vycital O, Kalusova K, Haviar S, Kralickova M, Lametschwandtner A. Porcine liver vascular bed in Biodur E20 corrosion casts. Folia Morphol (Warsz). 2016;75:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Eberlova L, Liska V, Mirka H, Tonar Z, Haviar S, Svoboda M, Benes J, Palek R, Emingr M, Rosendorf J, Mik P, Leupen S, Lametschwandtner A. The use of porcine corrosion casts for teaching human anatomy. Ann Anat. 2017;213:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Yamamoto K, Itoshima T, Tsuji T, Murakami T, Motta P. Motta P. Three-Dimensional Architecture of Blood, Lymphatic, and Biliary Pathways in the Liver by SEM of Corrosion Casts. In: Motta P.M., Murakami T. FH. Scanning Electron Microscopy of Vascular Casts: Methods and Applications. Boston, MA: Springer US; 1992: 71-82. [DOI] [Full Text] |
| 59. | Gaudio E, Pannarale L, Onori P, Riggio O. A scanning electron microscopic study of liver microcirculation disarrangement in experimental rat cirrhosis. Hepatology. 1993;17:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Widmann JJ, Fahimi HD. Proliferation of mononuclear phagocytes (Kupffer cells) and endothelial cells in regenerating rat liver. A light and electron microscopic cytochemical study. Am J Pathol. 1975;80:349-366. [PubMed] |
| 61. | Kordzaia DD. [Structure of the excretory ducts, intraorganic lymphatic vessels, tissue canals and intercellular space of various organs (data of scanning electron microscopy injection replicas)]. Arkh Anat Gistol Embriol. 1989;97:26-31. [PubMed] |
| 62. | Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 416] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 63. | Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 64. | Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, Ito H, Nakagawa K, Yoshidome H, Kataoka M, Nakajima N. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001;34:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |