Published online Jun 28, 2020. doi: 10.3748/wjg.v26.i24.3344
Peer-review started: February 26, 2020
First decision: May 1, 2020
Revised: May 11, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: June 28, 2020
Processing time: 122 Days and 19.5 Hours
Ca2+ has an important role in the maintenance of the skeleton and is involved in the main physiological processes. Its homeostasis is controlled by the intestine, kidney, bone and parathyroid glands. The intestinal Ca2+ absorption occurs mainly via the paracellular and the transcellular pathways. The proteins involved in both ways are regulated by calcitriol and other hormones as well as dietary factors. Fibroblast growth factor 23 (FGF-23) is a strong antagonist of vitamin D action. Part of the intestinal Ca2+ movement seems to be vitamin D independent. Intestinal Ca2+ absorption changes according to different physiological conditions. It is promoted under high Ca2+ demands such as growth, pregnancy, lactation, dietary Ca2+ deficiency and high physical activity. In contrast, the intestinal Ca2+ transport decreases with aging. Oxidative stress inhibits the intestinal Ca2+ absorption whereas the antioxidants counteract the effects of prooxidants leading to the normalization of this physiological process. Several pathologies such as celiac disease, inflammatory bowel diseases, Turner syndrome and others occur with inhibition of intestinal Ca2+ absorption, some hypercalciurias show Ca2+ hyperabsorption, most of these alterations are related to the vitamin D endocrine system. Further research work should be accomplished in order not only to know more molecular details but also to detect possible therapeutic targets to ameliorate or avoid the consequences of altered intestinal Ca2+ absorption.
Core tip: The intestinal Ca2+ absorption occurs mainly via the paracellular and the transcellular pathways. Both ways are regulated by calcitriol and other hormones as well as dietary factors. Fibroblast growth factor 23 (FGF-23) is a strong antagonist of vitamin D action. Part of the intestinal Ca2+ movement seems to be vitamin D independent. Intestinal Ca2+ absorption changes according to different physiological conditions. Oxidative stress inhibits the intestinal Ca2+ absorption whereas the antioxidants counteract the prooxidant effects. Most diseases that occur with altered intestinal Ca2+ absorption is related to changes in the vitamin D endocrine system. Further research could clarify many unknown points in this subject.
- Citation: Areco VA, Kohan R, Talamoni G, Tolosa de Talamoni NG, Peralta López ME. Intestinal Ca2+ absorption revisited: A molecular and clinical approach. World J Gastroenterol 2020; 26(24): 3344-3364
- URL: https://www.wjgnet.com/1007-9327/full/v26/i24/3344.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i24.3344
Ca2+ plays a relevant role in the skeleton, being the bones the storage site of 99% of total body Ca2+, mainly in the form of hydroxyapatite crystals[1]. Ca2+ is essential for the acquisition of an optimal peak bone mass within the first two decades, as well as for the maintenance of bone mineral density (BMD) in adulthood[2]. Apart from skeletal mineralization, this divalent cation is an important intracellular messenger and it actively participates in multiple physiological functions such as nerve excitation and transmission, muscle and cardiac contraction, blood coagulation, gland secretion and enzyme activation, among others. It is also implicated in cell differentiation and apoptosis as well as in immune response, thus having a significant role in tumorigenesis and cancer development[3]. This vast scope of functions raises the need for a homeostatic regulatory system. Extracellular and metabolic Ca2+ homeostasis is achieved by the concerted action of several Ca2+ regulating hormones: Parathyroid hormone (PTH), 1,25(OH)2D3 or calcitriol and calcitonin (CT). Estrogen, insulin-like growth factor (IGF-1), prolactin (PRL) and fibroblast growth factor (FGF-23) also participate in Ca2+ regulation[4].
Intestinal absorption is the unique way for Ca2+ to enter the organism from dietary nutrients. It occurs via two different mechanisms. When luminal Ca2+ is higher than that in the plasma, Ca2+ predominantly enters the intestine via the paracellular pathway through tight junctions between neighbouring enterocytes[5]. This is a passive non saturable transport, which takes place all along the small intestine without significant variations. In contrast, when luminal Ca2+ is lower than plasmatic Ca2+, the cation is actively absorbed via the transcellular pathway, which is an active saturable system that prevails in the duodenum[5,6]. Both mechanisms are regulated by different endocrine and non-endocrine factors.
Intestinal Ca2+ absorption in humans reaches approximately 35% of dietary load[7]. The amount of Ca2+ absorbed mainly depends on the quantity of Ca2+ consumed, the transit time in different parts of the gut and the solubility of Ca2+, which is inversely related to luminal pH[8]. Even though the absorption is more efficient in the duodenum and jejunum, where pH is lower, the amount of Ca2+ absorbed is greater in the ileum, since intestinal content remains ten times longer in this portion, which is finally responsible for almost 65% of Ca2+ absorption[9].
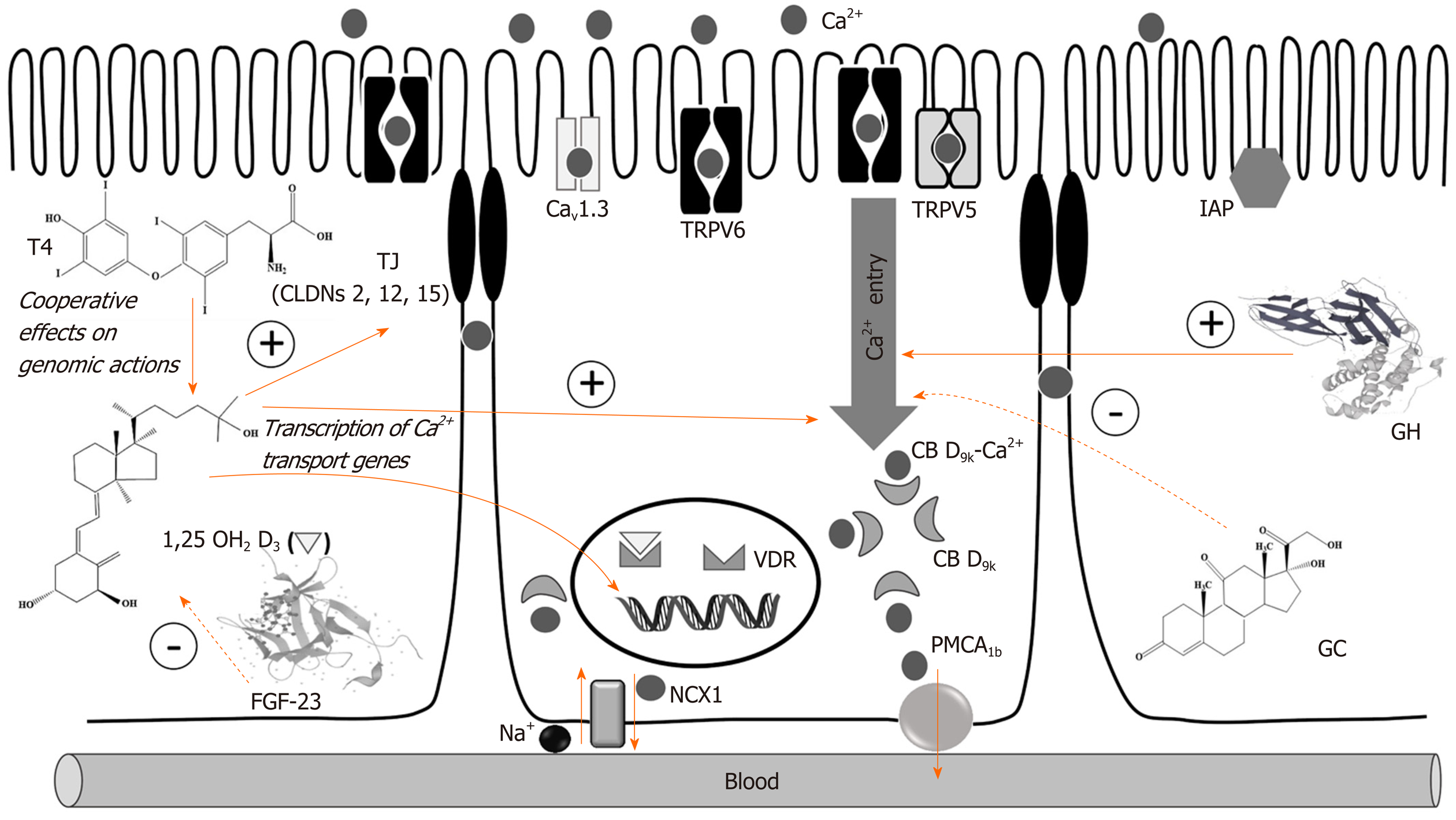
This absorptive route comprises the entrance of Ca2+ through electrically-charged watery space delimited by two neighbouring enterocytes. As it is the result of passive diffusion, this movement of the cation depends on Ca2+ concentration and voltage across intestinal epithelium. Rats fed a Ca2+-replete diet and humans show a chemical gradient which is favorable to the inward movement of the cation, since free Ca2+ is present in the duodenal lumen in a concentration of 2-6 mmol/L, compared to the much lower concentration in the interstitial fluid or plasma (1.25 mmol/L)[9,10]. As to electrical potential, the plasma is positive compared to the lumen, which would determine a secretion movement and exit of Ca2+. However, since this voltage gradient is very low, (2.5 mV), there is no outward flow of the cation due to electrodiffusion[11]. Apart from chemical gradient, Ca2+ together with other ions and small hydrophilic molecules enter through paracellular space along with the stream of water. This solvent drag-induced mechanism depends on the activation of sodium-glucose co-transporter 1 (SGLT-1) and Na+/K+-ATPase. Sodium, which enters through SGLT-1 and other sodium-coupled apical transporters, is pumped into the paracellular space by Na+/K+-ATPase, thus increasing sodium paracellular concentration and consequently augmenting osmotic water flow through this space. For this reason luminal glucose and galactose, which are substrates of SGLT-1, increase duodenal Ca2+ absorption[12]. Finally, duodenal epithelium has a preferential selectivity for small mono or divalent cations, such as Na+ or Ca2+, over larger or negative ions. This selectivity depends on some proteins such as occludin and claudins (CLDNs) in the tight junctions, which are specialized membrane domains in the apical region of enterocytes. CLDNs 2, 12, and 15 are associated with intestinal Ca2+ entering[13,14]. CLDNs 1 and 5 have sealing functions that could also diminish Ca2+ transport[15,16]. The expression of four candidate genes of the paracellular pathway, CLDN2 and tight junction proteins 1, 2, and 3, has been shown to be enhanced in the small intestine of laying hens after sexual maturity[6].
This active transport is carried out in three main steps; firstly, Ca2+ enters the enterocytes passively across the apical membranes. Afterwards, it binds to intracytoplasmatic proteins, which transfer the cation to the basolateral membrane (BLM). Finally, plasma membrane Ca2+-ATPase (PMCA1b) is the main Ca2+ transporter that extrudes the cation by primary active transport, at the expense of stored ATP.
Brush border membrane (BBM) is the first barrier that Ca2+ has to overcome in its way from intestinal lumen into the blood. Since Ca2+ is not able to move freely across the lipid bilayer of the plasma membrane, intestinal absorptive cells express some Ca2+ transporters. There are two epithelial Ca2+ channels that favor the passive transport of the cation across the apical membrane into enterocyte cytoplasm: Transient receptor potential vanilloid 5 (TRPV5) also known as ECaC1 or CaT2, and transient receptor potential vanilloid 6 (TRPV6); also known as ECaC2 or CaT1[2]. TRPVs are very important for maintaining blood Ca2+ levels in higher organisms, humans included. They are localized in apical membrane of Ca2+-transporting epithelial tissues and respond to 1,25(OH)2D3. They are structurally very similar since they share 75% of aminoacid identity. However, they differ in their distribution: TRPV5 expression is almost restricted to the kidney[17] and determines the level of urinary excretion of Ca2+ whereas TRPV6 shows intense expression in the intestine, thus being particularly important in intestinal Ca2+ absorption. More ubiquitously expressed, TRPV6 is also present in the kidney, placenta, epididymis and exocrine glands among other tissues[17,18]. Apical entrance of Ca2+ through TRPV6 is warranted by the favourable inwardly oriented electrochemical gradient for Ca2+, usually in low intracellular concentrations (100 nmol/L)[19].
Although TRPV6 is central for active Ca2+ absorption, studies in TRPV6 KO-mice have demonstrated that some Ca2+ is still absorbed when TRPV6 is absent[20]. Even more, the stimulating effect of 1,25(OH)2D3 on intestinal Ca2+ absorption is partially preserved in these animals[21]. In the same line, Woudenberg-Vrenken et al[22] obtained similar results when evaluating transepithelial Ca2+ absorption in a mouse-model carrying a nonfunctional TRPV6. They found that mice experimentally modified in one critic aminoacid residing in the pore site of the channel had lower intestinal Ca2+ uptake than wild-type controls and interestingly, showed a compensatory upregulation of TRPV5; however, insufficient to completely correct the diminished Ca2+ absorption. These findings strongly suggest that some other molecules may participate in the apical crossing of the electrolyte.
Another type of Ca2+ channel is present in the surface of enterocytes, Cav1.3, an apical L-type voltage-dependent channel present from the duodenum to the ileum, which might have a complementary role in Ca2+ permeation through TRPV6. The latter channel predominates in the duodenum, is activated by hyperpolarization, mainly overnight and between meals, and depends on vitamin D. In contrast, Ca(v) 1.3, predominates in mid-ileum, is activated when membrane is depolarized, mainly during postprandial active digestion, and is not modulated by vitamin D[23].
Once Ca2+ has passively entered the enterocyte, it binds to calbindins (CBs) and is subsequently transferred to BLM. All these proteins have a high α-helical content and share EF-hand structures with a helix-loop-helix sites that constitute the Ca2+-binding domains. These EF-hand motifs are held together in a single globular fold via hydrophobic interactions[24]. CB9k is present in the intestine of mammals, being highly abundant in the duodenum and gradually decreasing downwardly to become undetectable in distal ileum and colon[25]. CBs belong to a superfamily of Ca2+-binding proteins that also includes calmodulin and troponin C[26]. It has been shown that CB28K undergoes structural changes upon Ca2+ binding, which is indicative of a Ca2+-sensing protein[27].
Even though Ca2+ can cross the aqueous cytoplasmic environment at a higher rate than when associated with proteins[28], its binding to CBs prevents the free flow of Ca2+, helping to maintain intracellular Ca2+ lower than 10-7 mol/L. This Ca2+ buffering is extremely important since it prevents the potential deleterious pro-apoptotic effect of the cation, which has been confirmed in different tissues[29].
Since excessive free-ionized Ca2+ next to the BBM could deactivate TRPV6 restricting Ca2+ entry, the Ca2+ transferring function of these proteins may also contribute to warranting a persistent Ca2+ entry through the apical membrane[30]. In this direction, Song et al[31] have found that both TRPV6 and CB9k are similarly regulated, both being induced at weaning or under low Ca2+diets.
The expression of CB9k in duodenal enterocytes strongly depends on 1,25(OH)2D3[32], as also does the expression of other Ca2+-binding proteins such as CB28k, calmodulin, parvalbumin and sorcin[33], although the significance of their contribution to Ca2+ transferring remains to be specified. However, similarly to what we have referred for TRPV6, CB9k-KO mice partially preserve Ca2+ translocation capacity[20], which reinforces the potential role in cytoplasmatic Ca2+ translocation of several proteins other than CB9k. In this sense, Teerapornpuntakit et al[34] have found an upregulation of CB28k and parvalbumin expression in pregnancy and lactation, as a complementary mechanism to supply for the high demand of Ca2+ in these physiological conditions. Hwang et al[35] have observed an increase in the expression of most tight junction genes in the duodenum of normally fed CB9k-KO mice compared to wild-type controls. These findings suggest that the transcellular Ca2+-binding proteins may also exert some regulatory effect on paracellular Ca2+ absorption, suggesting that active and passive Ca2+ transport pathways may function cooperatively.
The active Ca2+ transport is required to overcome the unfavourable electrochemical gradient for Ca2+ across the BLM. PMCA1b and Na+/Ca2+ exchanger (NCX1) are the two proteins in charge of extruding Ca2+ out of the enterocyte, thus completing the transcellular Ca2+absorption.
PMCA1b, responsible for almost 80% of Ca2+ extrusion[36], is highly active in the duodenum, as detected in rats and it is a primary active transporter able to hydrolyze directly ATP to transport the cation[37]. The expression and activity of this pump is higher in enterocytes from the tip of the villi than in those from the crypt. This difference goes in line with the concept that mature enterocytes show a greater efficiency in transcellular Ca2+ movement[38]. NCX1, in contrast, is a secondary active transporter, coupled with Na+/Ca2+-ATPase, which creates a sodium gradient for NCX1-mediated Ca2+ efflux. It has a Na+/Ca2+ stoichiometry of 3:1 and it can either extrude or intrude Ca2+depending on cation gradients and the potential across the plasma membrane[39]. It appears to be responsible for approximately 20% of basolateral uphill Ca2+ extrusion from the enterocyte into blood stream[37]. Despite the importance of NCX1 in several tissues such as cardiac muscle, vascular smooth muscle and the nerves[40-42], the lower implication of this transporter in Ca2+ absorption as compared to PMCA1b activity caused NCX1 to be neglected in many studies concerning the intestine.
Basolateral Ca2+ absorption is closely related to the uptake of the cation in the apical membrane and its intracellular translocation. In this sense, a linear relationship between apical uptake and PMCA activity has been found in the duodenum of male rats[43]. In addition, CB9k and CB28k, calmodulin and parvalbumin activate PMCA[44,45]. This concerted mechanism contributes to avoiding intracellular accumulation of Ca2+, which would block Ca2+ entry from the lumen and could lead to apoptosis.
Vitamin D: 1,25(OH)2D3, the active metabolite of vitamin D, is the main regulating hormone of intestinal Ca2+ absorption. It induces structural and functional modifications in enterocytes and helps to enhance both transcellular and paracellular pathways, either by genomic or nongenomic actions[46,47]. 1,25(OH)2D3 can reach the intestinal target coming from two different sources: Either from the plasma, once its synthesis has been completed by 25(OH)D3 1α-hydroxylase (CYP27B1) in renal proximal tube (endocrine source)[48] or from de novo synthesis in the cytoplasm of the enterocyte, performed by a duodenal 1α-hydroxylase (intracrine source)[49].
This calciotropic lipophilic vitamin passes through the plasma membrane and binds to vitamin D receptor (VDR), its nuclear receptor. Once bound to the ligand, VDR forms a heterodimer with retinoid X receptor (RXR) and the new 1,25(OH)2D3-VDR- RXR complex functions as a transcription factor which binds to different vitamin D response elements in various target genes[50]. This determines a significant increase in the expression of all Ca2+ transporting proteins in the enterocyte: TRPV 6, CB9K, PMCA1b and NCX1, as has been demonstrated in animal models and humans[2,4,49,51-54]. Vitamin D-mediated Ca2+ absorption has mainly been studied in the proximal intestine, where Ca2+ is more efficiently absorbed. However, Christakos et al[30] have recently studied this process in mice with transgenic expression of VDR exclusively in the ileum, cecum and colon of VDR KO mice. Interestingly, these animals did not present the abnormalities in Ca2+ homeostasis and bone mineralization usually seen in VDR KO mice. These findings emphasize the importance of 1,25(OH)2D3-mediated Ca2+ absorption in the distal intestine[55].
Apart from these Ca2+-transporting proteins, 1,25(OH)2D3 can regulate other important genes in Ca2+ metabolism, such as the one of 24-hydroxylase (CYP24A1) which converts 1,25(OH)2D3 into 1,24,25(OH)3D3 and 25(OH)D3 into 24,25(OH)2D3, and CYP27B1, involved in the renal synthesis of 1,25(OH)2D3[56], but also expressed in the intestine[57] and parathyroid gland[58].
In addition to the genomic action described, there is some evidence that 1,25(OH)2D3 also binds to a plasma membrane receptor (MARRS: Membrane-associated, rapid response steroid-binding protein), which, in turn, activates other second messenger systems such as phospholipase A2 and protein kinase C[59-61]. Details of the underlying molecular mechanism of 1,25(OH)2D3-MARRS and its rapid minute-to-minute regulatory capacity remain to be elucidated.
Even though the transcellular pathway has been the focus of most studies concerning the effect of calcitriol on intestinal Ca2+ absorption, vitamin D has proved to exert a positive effect on the paracellular absorptive route as well. 1,25(OH)2D3 is able to change the permeability and selectivity of the tight junctions by altering certain crucial proteins such as CLDNs 2 and 12. This would help to enhance passive diffusion of Ca2+[62].
Rexhepaj et al[63] have observed that 1,25(OH)2D3 could also stimulate Na+/Ca2+-ATPase and SGLT, and consequently increase water-movement through the junction, thus carrying more Ca2+ inwardly with the flow. Tudpor et al[64] have demonstrated a dose dependent increase in solvent drag-induced Ca2+ movement one hour after direct exposure of rats to 10-100 nmol/L 1,25(OH)2D3. This rapid effect, abolished by inhibitors of phosphatidylinositol 3-kinase, protein kinase C, and MEK, would be mediated by nongenomic mechanisms involving 1,25(OH)2D3-MARRS.
It has also been reported that 1,25(OH)2D3 downregulates intestinal cadherin-17 (involved in cell-to-cell contact) and aquaporin-8 (associated with epithelial selectivity towards cations), which might also affect the Ca2+ absorption[20,65].
PTH: PTH, a hypercalcemic hormone secreted by parathyroid glands, is the other classical hormone known to exert a positive regulatory effect on intestinal Ca2+ absorption. However, this stimulatory effect is achieved indirectly after increasing CYP27B1 transcription for 1α-hydroxylase, the renal enzyme that completes the synthesis of 1,25(OH)2D3 in the kidney. As a result, 1,25(OH)2D3 production augments. In addition, PTH also suppresses the transcription of CYP24A1 that codifies for 24-hydroxylase, a renal enzyme which degrades 1,25(OH)2D3 by converting it into 1,24,25(OH)3D3[66]. Both actions lead to an increase in plasmatic 1,25(OH)2D3, which in turn enhances Ca2+ absorption as we have already revised.
Thyroid hormones: Thyroxine (T4) and triiodothyronine (T3) are known to regulate metabolism in general. Overproduction of T4 or T3 in the context of hyperthyroidism can lead to hypercalcemia due to an excessive bone turnover and consequently lead to bone demineralization[67,68]. However, there is some evidence that thyroid hormones would cooperate with vitamin D by increasing the genomic actions of 1,25(OH)2D3 in the intestine[69]. Cross et al[70] have shown that calcitriol added to cultures of 20-day-old embryonic chick small intestine, stimulated Na+ uptake. The calcitriol-mediated increase in Na+ uptake appeared to be related to increased tight-junctional or paracellular permeability[70]. It can be speculated that this effect could favor the paracelullar entry of Ca2+ as well. More recently, Kumar et al[71] have shown that Ca2+ influx in BBM vesicles was higher in enterocytes from hyperthyroid rats as compared to those of hypothyroid ones. The authors have proposed that this could be related to a change in membrane fluidity induced by thyroid hormones. Similarly, they have also observed that efflux of calcium across BLM was also higher in hyperthyroid rats. This difference was associated with a higher NCX1 activity triggered by thyroid hormones, possibly through the cAMP-mediated pathway. cAMP is a potent activator of Na+/Ca2+ exchanger and it was significantly higher in intestinal mucosa of hyperthyroid rats as compared to euthyroid animals. In addition to these actions, thyroid treatment increases serum PTH and 1,25(OH)2D3 levels, which contributes to enhancing Ca2+ absorption indirectly through vitamin D[7].
Growth hormone: Growth hormone (GH) has a central role in longitudinal bone growth and mineralization during childhood and adolescence. However, this metabolic hormone has receptors in most tissues and exerts various actions apart from skeletal growth. There is evidence that GH has proliferative effects on intestinal mucosa[72]. GH has been used to treat inflammatory bowel disease in pediatric[73] and adult patients[74]. Interestingly, FDA has approved the use of recombinant human GH to treat short bowel syndrome, where it improves absorption of carbohydrates, amino acids and fats[75,76]. GH can also stimulate intestinal Ca2+ absorption, which would occur indirectly by activating renal CYP27B1 and consequently increasing serum 1,25(OH)2D3 levels[77]. It has been demonstrated that GH can prevent the loss of intestinal VDR in ovariectomized (OVX) rats[78], which would suggest that it could increase intestinal sensitivity to 1,25(OH)2D3 by regulating tissue VDR levels[79]. However, the positive effect of GH on Ca2+ absorption would not be exclusively dependent on vitamin D. Fleet et al[80] have shown that GH increases intestinal Ca2+ absorption and duodenal CB9k levels in aged rats without increasing serum 1,25(OH)2D3 levels. Analogous results have been found in humans. In adult men, Ca2+ absorption has a positively correlation with IGF-1 and age-related decline in IGF-1 has a negative impact on Ca2+ absorption that could not be justified by a decrease in serum 1,25(OH)2D3[81].
Estrogens: There is evidence that post-menopausal women experiment an increase in bone resorption together with a reduction in Ca2+ absorption and an increase in urinary Ca2+ excretion as a consequence of estrogen loss[82,83]. Post-menopausal low estrogen levels have been associated with reduced serum 1,25(OH)2D3[84]. However, OVX rats have no reduction in serum 1,25(OH)2D3 levels[85], which would suggest the implication of vitamin D independent mechanisms. Thus, O’Loughlin et al[86] observed that estradiol replacement in OVX rats increases intestinal Ca2+ absorption without stimulation of circulating 1,25(OH)2D3 levels. In the same line, van Abel et al[87] found increased duodenal gene expression of TRPV5, TRPV6, CB9k, and PMCA1b in OVX rats treated with estradiol. In order to determine whether this stimulatory effect on Ca2+ transporting proteins was calcitriol-dependent, they used CYP27B1 KO mice and found that estradiol treatment increased mRNA levels of duodenal TRPV6. In contrast, Gennari et al[88] found that oophorectomy in young women reduces the intestinal Ca2+absorption induced by vitamin D, which was reversed by estrogen repletion. Other studies suggest the possibility of a deficient intestinal responsiveness to vitamin D due to a reduction of VDR levels[78,89,90]. However, the loss of intestinal VDR levels following estrogen reduction could not be confirmed in all studies[91].
Cell-culture experiments suggest that estrogen is able to reverse the decline in the efficiency of intestinal Ca2+ absorption at menopause onset[92], but the mechanisms that underlie this effect remain to be elucidated. Estrogen receptor alpha (ERα) KO mice showed a decrease in duodenal TRPV6 mRNA expression, without modification in CB9k, PMCA1b and VDR levels. Therefore, it seems that the genomic effects of estrogen on mice intestinal mucosa are mainly mediated by ERα[93]. However, Nie et al[94] have recently reported that estrogen regulates duodenal Ca2+ absorption through differential effects of ERα and ERβ on TRPV6 and PMCA1b expressions in duodenal epithelial cells, respectively.
PRL: The main lactogenic hormone, PRL, is elevated during pregnancy and lactation. Apparently, this pituitary hormone is able to enhance Ca2+ absorption in order to supply calcium for milk production. It has been shown that PRL enhances CYP27B1 protein expression and increases levels of 1,25(OH)2D3 during lactation, a moment when there is an increased Ca2+ requirement for the neonate[95]. However, its calciotropic action is not only achieved via vitamin D. It has been shown that PRL stimulates active intestinal Ca2+ transport in vitamin D-deficient rats[96]. Charoenphandhu et al[11] demonstrated that PRL directly stimulates active duodenal Ca2+ transport. Wongdee et al[97] observed that lactating rats exhibit some adaptive changes in their intestinal mucosa tending to increase the absorptive surface area. These rats have larger duodenal, jejunal and ileal villous as well as deeper cecal crypts than age-matched nulliparous rats. These histological modifications were diminished by bromocriptine, an inhibitor of pituitary PRL release. PRL also upregulated TRPV6 and PMCA1b in the duodenum of lactating rats. These changes were associated with a compensatory increase in FGF-23 expression, a local negative regulator of Ca2+ absorption, presumably to prevent Ca2+ hyperabsorption. Bromocriptine also manages to abolish FGF-23 increment, confirming it was induced by PRL. In addition, it has been suggested that PRL has also a stimulating effect on paracellular pathway by upregulating CLDN 15 in the tight junctions[98].
FGF-23: It is a glycoprotein secreted by osteocytes and osteoclasts and regulated by plasma levels of 1,25(OH)2D3 and Pi. The enhancement of these regulators leads to the serum increase in FGF-23, which in turn reduces the concentration of 1,25(OH)2D3 by inhibiting 1α-hydroxylase and stimulating 24 α-hydroxylase. As for Pi, FGF-23 increases its renal excretion[99].
FGF-23 has been indicated as a vitamin D antagonist in intestinal absorption of Ca2+. Khuituan et al[32] have demonstrated that intravenous administration of FGF-23 to male rats abolished the increase in intestinal absorption of Ca2+ caused by the injection of 1,25(OH)2D3. However, the inhibitory effect of FGF-23 could not be observed in the absence of the previous supply of 1,25(OH)2D3. The mechanisms underlying the effect of FGF-23 would be related to the decrease in the gene expression of TRPV5, TRPV6 and CB9k caused by this phosphaturic hormone. In this same work, the presence of FGFR1-4 in the BLM of rat enterocytes was confirmed. However, their functions are unclear since the direct exposure of the intestinal epithelium to FGF-23 did not cause alterations.
FGF-23 also blocks the stimulatory effect of 1,25(OH)2D3 on the paracellular pathway of intestinal Ca2+ absorption[100]. Since vitamin D favors this process by increasing water flow across paracellular space and consequently dragging solutes as Ca2+, it has been proposed that FGF-23 could decrease the water flow and the dragging of this cation.
The activation of the mechanisms mediated by FGF-23 would be crucial to avoid the Ca2+ hyperabsorption. Therefore, it was thinkable that a molecule that senses extracellular Ca2+ as the calcium sensing receptor (CaSR) would play an important role. In fact, Rodrat et al[101] have demonstrated that CaSR was involved in the inhibition of intestinal Ca2+ absorption mediated by FGF-23. According to their findings in a cell monolayer, the use of allosteric inhibitors of CaSR could reverse the inhibitory effect of FGF-23 on the stimulation of Ca2+ transport triggered by 1,25(OH)2D3.
Glucocorticoids: The negative side effects of glucocorticoid (GC) treatment on bone health are well known. Impaired function and number of osteoblasts and osteoclasts, high resorption rate, deficiency in mineralization are some of the effects of chronic treatment that lead to the development of GC-induced osteoporosis (GIO)[102]. GIO is also partially due to the alterations that GC produces in intestinal Ca2+ absorption. Van Cromphaut et al[103] evaluated the effect of dexamethasone treatment on the gene expression of proteins involved in the intestinal absorption of the cation. They did not find alterations in gene expression or Ca2+ absorption in the treated mice, justifying the absence of effects with the short treatment duration. Kim et al[104] determined that a single dose of dexamethasone increased the gene expression of TRPV6 and CB9k, while when it was given for 5 days, it led to a reduction in the expression of both genes. In concordance with these results, mRNA levels for duodenal VDR increased on day one, while they were reduced after 5 days of treatment. Zhang et al[105] observed reduced protein expression of TRPV6 and CB9k in the intestine of male mice injected with dexamethasone 3 times a week, for 12 weeks, effect that was accompanied by hypercalciuria and reduction in serum Ca2+ levels. Although the role of GC in intestinal Ca2+ absorption is not clear, the results presented would allow infer a certain negative effect of GC on cation transfer from the lumen to the interstitium.
CT: The role of CT on intestinal Ca2+absorption is controversial. Some studies have suggested that CT inhibits the process; in contrast, others indicate that has a stimulatory effect. Swaminathan et al[106] have demonstrated that CT may produce an inhibitory effect at low doses, whereas high doses increase the intestinal Ca2+ absorption. CT effect could be mediated by the vitamin D endocrine system, since it has been demonstrated in diabetic rats that CT increases 1,25(OH)2D3 synthesis at renal level[107]. Use of CT has been suggested to treat patients with β-thalassemia because they usually have low plasma levels of this hormone. CT chronic use has benefited osteoporosis associated with thalassemia, not only for its inhibitory effect on osteoclasts but also for the possible role in the 1,25(OH)2D3 synthesis[108].
The main dietary factor that can modify intestinal Ca2+ absorption is calcium itself. Low-calcium uptake could eventually produce hypocalcemia, which would augment PTH secretion leading to stimulate vitamin D endocrine system and demineralize bone[109]. On the other hand, high calcium diets and calcium hyperabsorption could increase cardiovascular risk associated with vascular calcification, nephrolithiasis and dementia, among other conditions[110,111]. Since the gut is the only gate for Ca2+ uptake, it is subjected to both local and systemic regulations, which protect against either insufficient or excessive Ca2+ absorption[112]. Low calcium diets enhance serum levels of vitamin D and, consequently, activate the endocrine actions of this vitamin. Thus, a chronic dietary Ca2+ deficiency increases all transcellular pathway genes and proteins[109,113,114], and increases the activity of the intestinal PMCA1b and NCX1 all along the villus, independently of cell maturation degree[38]. Benn et al[20] have gone further to demonstrate that this adaptive increase in Ca2+ absorption is present even in TRPV6 KO and CB9k KO mice, suggesting that TRPV6, which has been postulated as the rate-limiting factor in transcellular pathway, may not be so or it may be successfully replaced by other factors able to partially compensate its function. In our laboratory, we have observed in animals under low Ca2+ diets that the increment in Ca2+ transport is accompanied by a concomitant increase in the activity of intestinal alkaline phosphatase (IAP), a marker enzyme of enterocytic differentiation that may have a role in intestinal Ca2+ absorption[38]. Brun et al[115] have reported that luminal Ca2+ concentration increases the activity of IAP and simultaneously decreases the percentage of Ca2+ absorption, functioning as a minute-to-minute local regulatory mechanism of Ca2+ entry, independent of vitamin D. This would limit an excessive Ca2+ intake secondary to dietary calcium restriction, thus preventing possible acute toxic effects. This regulatory mechanism may probably be one of the reasons why high Ca2+ intake (1500 mg/d) was not followed by a significant increase in Ca2+ absorption in a clinical trial[116,117], as it would have been expected from the positive effect of stimulated vitamin D endocrine system. Interestingly, L-Phenylalanine, an inhibitor of IAP, prevented this regulatory effect and Ca2+ uptake remained increased. A more recent study showed that IAP activity induced by luminal calcium concentration provoked changes in luminal pH that could modulate intestinal Ca2+ absorption[118] In addition, a recent study revealed that IAP KO mice have higher intestinal Ca2+ uptake, which correlates with better biomechanical properties of trabecular bone[119].
It has also been suggested that CaSR, abundantly expressed in apical and basolateral membranes of enterocytes in humans, rats and mice[120-122] may also participate in the local regulation of intestinal Ca2+ absorption. Intestinal CaSR -specific KO mice showed an altered intestinal integrity, disbalanced gut microbiota and a pro-inflammatory status[123-125]. Rodrat et al[101] have recently observed that high-dose of 1,25(OH)2D3 or high concentration of luminal calcium reduced Ca2+ transport across a Caco-2 monolayer. The authors proposed that CaSR would sense luminal calcium triggering a local inhibitory feedback mechanism to restrict excessive Ca2+ uptake[101]. This inhibitory loop could possibly involve locally produced FGF-23, which has been observed to counteract the enhanced duodenal Ca2+ transport in mice exposed to 1,25(OH)2D3 for a long term[32,100].
Intestinal Ca2+ absorption changes according to the different physiological conditions. It is promoted under high Ca2+ demands such as growth, pregnancy, lactation, dietary Ca2+ deficiency and high physical activity. In contrast, the intestinal Ca2+ transport decreases with aging.
In small and premature infants, who need higher dietary calcium to have a positive balance, the intestinal Ca2+ absorption occurs through a passive paracellular pathway. These infants are unable to upregulate the transcellular pathway[126]. A progressive declination in the predominance of the paracellular pathway has been observed from childhood to adulthood[127]. Similarly, in rodents the intestinal Ca2+ absorption changes with age in order to reach the requirements for bone mineralization during growth. In suckling rat, the intestinal Ca2+ transport occurs predominantly through paracellular pathway; at weaning a transition to saturable absorption occurs indicating a larger contribution by the transcellular pathway during development, and then the absorption goes back to the paracellular pathway in adulthood[128]. The molecular changes associated with these alterations in the intestinal Ca2+ absorption is not well elucidated. With respect to the transcellular movement, the mRNA expressions of Trpv6, Cabp9K, and Pmca1 in the duodenum have been first noted at 14 d and peaked at 21 days in rodents[129]. Akhter et al[130] have demonstrated at 6 weeks of age that Cabp9K was highly expressed in the duodenum with a small amount in the jejunum and cecum, and at 44 wk it was no longer detected in jejunum and cecum, but remained in the duodenum. With regard to paracellular pathway, Holmes et al[131] have demonstrated in the mice jejunum a decrease in Cldn2 and increases in Cldn12 and Cldn15 with age. These studies highlight that the levels of molecules involved in the intestinal Ca2+ absorption are not constant throughout growth. The potential modulators of changes in the intestinal Ca2+ absorption during postnatal development are calcitriol, PRL and milk lactose[132].
One of the physiological changes in pregnancy involves alterations in Ca2+metabolism. The adequate growth and development of the fetus is associated with increased intestinal Ca2+ absorption and renal reabsorption in the mother. In the first trimester of human pregnancy, the intestinal Ca2+ absorption increases twofold being maintained this increment to term. This doubling in the intestinal Ca2+ absorption seems to be the major maternal adaptation to reach the fetal requirements for Ca2+either in humans or in rodents[133]. The classical calciotropic hormones as calcitriol, PTH and CT seem not to be main responsible for the Ca2+ demand during pregnancy and lactation[134]. The intestinal Ca2+ absorption has been shown to be VDR-independent in pregnant VDR knockout mice[135]. Pregnancy up-regulates intestinal Ca2+ absorption and skeletal mineralization independently of the vitamin D receptor. It has been suggested that other hormones such as PRL, placental lactogen and GH or other factors could contribute to the doubling of intestinal Ca2+ absorption in normal pregnancy[133].
After birth, the maternal mammalian gland secretes an elevated amount of Ca2+, which could reach up to 1000 mg/day of milk Ca2+. In order to provide an extra Ca2+for milk production during lactating period, the osteoclast-mediated bone resorption and the intestinal Ca2+ absorption is increased. The hormone responsible for milk calcium secretion in the stage of lactation remains uncertain, but there is some evidence that the lactogenic hormone PRL regulates that process. Charoenphandhu et al[134] have proposed that PRL stimulates the intestinal Ca2+ absorption in a two-step manner. In step 1, PRL increases the baseline of intestinal Ca2+ absorption in lactating rats through an increment in the TRPV6 mRNA, whereas in step 2, the suckling-induced PRL could induce further increased intestinal Ca2+ absorption twofold over the newly increased baseline. High levels of PRL (400-800 mg/mL) are required to induce an acute enhancement in the intestinal Ca2+ absorption, which is not attained without suckling[136]. The suckling-increased Ca2+ transport in rats occurs either in the small intestine or the large intestine. This increment peaks after 30 min of suckling and lasts for 30-45 min post-suckling[12]. Wongdee et al[97] have demonstrated that PRL upregulated the expression of TRPV6 and PMCA1b in the duodenum of lactating rats. In addition, they have observed upregulation of FGF-23 protein expression in the duodenum and cecum of the same animals. They interpreted that PRL was responsible for the intestinal adaptation induced by lactation, which was compensated with an increase in FGF-23 to prevent excessive Ca2+ absorption that might be harmful to lactating rats.
The role of calcitriol in the hyperabsorption of Ca2+ during lactation is not clear. Kovacs[137] has reported that preterm and term babies absorb Ca2+ through a passive non saturable process, which is facilitated by the lactose content in the milk. As they mature, they begin to absorb via a saturable calcitriol-dependent mechanism. This explains why vitamin D-deficient rickets appears much later, six to 18 mo after birth[138]. The phenotype of poor weight gain and low BMD in mice with a nonfunctional VDR is only observed after weaning, which indicates that intestinal Ca2+ absorption is not calcitriol-dependent while suckling[132]. Recently, Zhang et al[139] have demonstrated the effects of maternal 25(OH) D3 administration during lactation on sows and piglets. The intestinal Ca2+absorption was higher in treated sows as compared to non-treated sows, which are attributed to increased mRNA expressions of renal CYP27B1 and duodenal VDR, TRPV6, and CaBP-D9k. The piglets suckling sows receiving 25(OH) D3 exhibited higher Ca2+ content in tibia and femur; these effects were associated with higher plasma levels of calcitriol, which increased the gene expression of proteins involved in the intestinal Ca2+ transport, e.g., VDR and Cldn-2 in ileum and VDR and CB9k in colon. In other words, 25(OH)D3 supplementation during lactation improved bone health of both sow and piglet.
The temporal loss of bone mass during lactation is recovered promptly by mechanisms not quite clear. The bone health of the mother could be slightly or severely compromised leading to fragility fractures in some women. Full recovery of calcium content and bone strength is not always achieved after weaning. Nevertheless, changes in calcium and bone metabolism during pregnancy and lactation in most women are normal, transient and without deleterious effects in the long-term[140].
As a consequence of aging, the intestinal Ca2+ absorption decreases either in humans or in rodents. In humans, malabsorption of Ca2+ begins approximately at between 65 and 70 years[141]. In postmenopausal women, this deterioration begins earlier, but is reversible with estrogen therapy[142]. Among the different reasons for this decrease related to vitamin D metabolism could be mentioned: (1) Decreased renal synthesis of calcitriol by aged kidney; (2) Intestinal resistance to circulating calcitriol; (3) Decreased intestinal VDR; (4) Decreased skin synthesis of vitamin D; and (5) Substrate deficiency of vitamin D[143]. Song et al[144] have demonstrated that low levels of VDR in mice heterozygous for the VDR gene KO cause resistance of intestinal Ca2+ absorption to calcitriol. This resistance appears to be generated by the low translation of CB9k, which occurs after binding VDR with its ligand. Ramsubeik et al[142] have reported that beyond the traditional focus on Ca2+ and vitamin D, some other factors also influence intestinal Ca2+ absorption in post-menopausal women such as dietary intake of kilocalories, carbohydrates, and potassium. See Figure 1 for details about the molecular mechanisms involved in the intestinal Ca2+ absorption and the regulation by hormones.
Reactive oxygen species (ROS) are by-products of normal cellular metabolism and there are enzymatic and non-enzymatic defense-systems in charge of maintaining a balance between ROS production and depletion. When this equilibrium fails, it is due to overproduction of ROS or to a deficiency in protective responses, oxidative stress arises, which can alter lipids, proteins and nucleic acids, thus provoking cell dysfunction and tissue damage. Gastrointestinal tract is an important source of ROS. Despite the protective barrier provided by intestinal mucosa and its adequately-distributed microbiota, digestion-endproducts and pathogens can trigger inflammatory response which favors oxidative stress. Consequently, various gastrointestinal pathologies such as gastroduodenal ulcers, cancer and inflammatory bowel disease are associated with oxidative stress[145,146].
It has been demonstrated that intestinal Ca2+ absorption is also affected by oxidative stress. A study carried out in our laboratory demonstrated that DL-buthionine-S, R-sulfoximine (BSO) reduced intestinal Ca2+ absorption in rachitic chicks treated with cholecalciferol. BSO is an aminoacid-analogue which inhibits the synthesis of glutathione (GSH), one of the most important non-enzymatic intestinal antioxidant. The gut redox status was restored after intraluminal addition of GSH and intestinal Ca2+ absorption returned to baseline[147]. BSO also reduced the activity of IAP, an enzyme presumed to play a role in Ca2+ absorption, which was affected by the overabundance of ROS triggered by BSO. This reinforces the idea about the potential inhibitory effect of oxidative stress on the intestinal absorption of the cation[148].
Posterior studies with different pro-oxidant drugs gave more information about the inhibition of Ca2+ absorption by ROS overproduction. Such is the case of menadione (MEN), a synthetic precursor of vitamin K used in anti-cancer therapy. MEN metabolism starts by one-electron reduction and originates unstable semiquinone-radicals which rapidly react with O2. As a result, the semiquinone-radical cycles back to MEN and .O2- is generated, which becomes H2O2 through spontaneous or enzymatic dismutation[149]. Since GSH acts as an electron donor, intestinal administration of MEN (2.5 μmol /kg b. w.) depletes this antioxidant tripeptide triggering oxidative stress and diminishing the enzymatic activity of IAP and PMCA1b[150]. In the same direction, Areco et al[151] have observed that intraperitoneal MEN was also able to reduce the expression of PMCA1b, CB28K and CLDN 2 in the intestinal mucosa of chicks treated with the quinone. This would contribute to explaining the transient reduction in Ca2+ absorption caused by MEN, which appeared 30 minutes after treatment and lasted for less than ten hours. It is worth noting that this pro-oxidant also provoked apoptosis of enterocytes, thus determining the loss of approximately 30% of absorptive epithelial cells. The apoptotic process involved both the intrinsic and extrinsic pathways. An initial mitochondrial GSH depletion produced a reduction in mitochondrial membrane potential followed by the release of cytochrome c into the cytoplasm and DNA fragmentation (intrinsic apoptotic pathway). Mitochondrial dysfunction induced by MEN affected Krebs-cycle only partially, since it reduced the activity of malate dehydrogenase in 18% and the one of α-ketoglutarate dehydrogenase in 30% [152]. Extrinsic apoptotic pathway was also favored by MEN, which was evidenced by the expression of FAS, FASL and caspase-3[153]. Interestingly, quercetin, an anti-inflammatory and anti-apoptotic flavonoid with important protective properties in the intestine[154], could reverse the inhibitory effect of MEN. This flavonoid blocked the alterations in the mitochondria membrane potential triggered by MEN, thus blocking the apoptotic route dependent on FAS/FASL-caspase 3. This anti-apoptotic effect, based on the capacity of quercetin to preserve GSH levels, contributed to maintaining the absorptive enterocytes functioning[153].
The inhibitory effect of MEN on Ca2+ movement can also be prevented or restored by some protective drugs such as glutamine[155,156], an anti-inflammatory and anti-apoptotic drug associated with diverse functions of intestinal mucosa such as growth and reparation[157,158]. Moine et al[155,156] have observed that glutamine normalized the content of different molecules involved in both calcium absorption pathways as well as the levels of GSH and the activity of antioxidant enzymes.
Similarly, a monodosis of 10 mg/kg b. w. of melatonin (MEL), a pineal hormone also secreted in the gastrointestinal tract, has been shown to restore Ca2+ absorption previously reduced by MEN. This effect was the result of the normalization of the activity of antioxidant enzymes superoxide dismutase and catalase and the restoration of .O2- levels to basal status. MEL also restored the expression of proteins involved in both Ca2+ absorptive pathways[151]. These protective properties of MEL were reinforced in various studies that revealed anti-inflammatory effects of MEL in the intestine[159], helping to maintain epithelial integrity and digestive function[160], and reducing the risk of cancer[161].
Sodium deoxycholate (NaDOC) is a bile salt that depletes GSH, exerting a similar effect to the one of MEN. Rivoira et al[162] have demonstrated that high physiological doses of this salt inhibit intestinal Ca2+ absorption in a time and dose-dependent fashion. NaDOC mainly affects the transcellular pathway since it inhibits the expression of PMCA1b, CB D28k and NCX1. In addition, this bile-salt generates ROS and mitochondrial changes which eventually lead to apoptosis[162]. However, there are some bile acids that are able to preserve Ca2+ absorption. Lithocholic (LCA) is a secondary bile acid that binds to VDR and acts as an endogenous agonist of vitamin D[163], which has been proposed as a potential antitumoral agent[164]. LCA has proved to normalize the expression of genes and proteins involved in the transcellular pathway of Ca2+ absorption affected by NaDOC and restore oxidative stress parameters such as .O2- and the levels of protein carbonyl groups. This acid also attenuates the increase in the permeability of mitochondrial membrane triggered by NaDOC, being able to block the apoptosis induced by NaDOC when co-administered intraluminally with this drug. As a result, LCA avoids the reduction in the transcellular Ca2+ absorption provoked by NaDOC[165].
There are also pathological conditions that can favor oxidative stress and consequently lead to a reduction in the intestinal Ca2+ absorption. One of these conditions is Type-1 diabetes mellitus (T1DM). Rivoira et al[166] have shown that diabetic Wistar rats induced by the injection of streptozotocin (STZ), absorbed less Ca2+ than the control group. This reduction was transitory and reversible by insulin treatment. Interestingly, STZ-induced diabetes produced an overexpression of the proteins involved in the transcellular pathway which returned to basal levels after 60 d. This initial increase in the expression of NCX1, PMCA1b and TRPV6 has been interpreted as a possible compensatory effect to counteract the reduction in Ca2+ absorption, probably associated with an imbalanced redox status.
Ca2+ transport across intestinal epithelium is also impaired in experimental metabolic syndrome. Rodriguez et al[167] have observed that animals with a fructose-rich diet presented alterations in intestinal redox status, which were evidenced by a marked increase in .O2-, lower activity of antioxidant enzymes and a reduction in GSH. These animals also had nitrosative stress with increased nitric oxide and higher nitrotyrosine content of proteins. This global redox disequilibrium determined a combined alteration of both trans and paracellular pathways of Ca2+ absorption that might have been aggravated by a pro-inflammatory state with increased IL-6 and NF-kB. It is noteworthy that a subcutaneous injection of naringin (40 mg/kg b. w. during 4 wk on a daily basis), an antioxidant flavonoid present in grape fruit and other citrics, duplicated GSH level and blocked both inflammation and redox unbalance triggered by fructose-rich diet, consequently protecting intestinal Ca2+ absorption[167]. These findings go in line with similar antioxidant effects of naringin in ischemia-reperfusion models[168]. The anti-inflammatory potential of naringin on intestinal mucosa has been confirmed by different studies in ulcerative colitis[169], sepsis-induced intestinal injury[170], and gastrointestinal tumorigenesis[171]. It has also been found that naringin contributes to maintaining an equilibrium between the different components of the microbiota[172], thus preventing dysbiotic processes that could lead to ROS overproduction and indirectly affect Ca2+ absorption[173].
Table 1 shows the influence of prooxidant conditions on the intestinal Ca2+ absorption and the reversal/protection by antioxidants.
| Pro-oxidant condition | Effects on genes and proteins involved in intestinal Ca2+ absorption | Effect on REDOX state | Effects of antioxidant/ protective molecules | Effects on apoptosis |
| BSO[147,148] | Inhibition of IAP activity | Decrease in GSH content | GSH administration normalized intestinal Ca2+ absorption | Not evaluated |
| MEN[149-156] | Decrease in PMCA1b gene-protein expression and activity. Decrease in CB D28k and CLDN 2 gene-protein expression | Depletion of GSH content; Increase in ROS and protein carbonyls; Enhancement in SOD and CAT activity | QT, MEL and GLT administration normalized intestinal Ca2+ absorption and associated parameters | Activation of intrinsic and extrinsic pathways |
| NaDOC[162,165] | Decrease in PMCA1b mRNA Inhibition of PMCA1b, CBD28k and NCX1 protein expression | Depletion of GSH content; Increase in ROS and activity of SOD, CAT and GPx; Increase in iNOS protein expression and NO• content | QT and UDCA administration avoided the inhibition of intestinal Ca2+ absorption caused by NaDOC | Activation of intrinsic and extrinsic pathway |
| Diabetes[166] | Enhancement in expression of NCX1, PMCA1b and TRPV6 proteins and CLDN 2 gene expression | Decrease in GSH content; Increase in SOD activity and ROS levels | Insulin treatment restored redox state and intestinal Ca2+ absorption | Not evaluated |
| Metabolic syndrome[167] | Decrease in TRPV6, PMCA1b, CB D9k, CLDN 2, CLDN 12 and VDR protein expression; Decrease in IAP activity | Enhancement in protein carbonyls, NO• levels and nitrotyrosine content in proteins; Decrease in SOD and CAT activity | Administration of NAR prevented the reduction of intestinal Ca2+ absorption caused by fructose-rich diet | Not evaluated |
Crohn's disease and ulcerative colitis, the main forms of inflammatory bowel disease, are characterized by chronic inflammation of the intestine that can deteriorate the intestinal Ca2+ absorption[174]. In patients with Crohn's disease, this alteration may be due to vitamin D deficiency, magnesium deficiency, excessive use of glucocorticoids and/or intestinal resection. Vitamin D treatment has been shown to improve the intestinal Ca2+ absorption in these patients[175].
Patients with celiac disease (CD) frequently present Ca2+ deficiency, low BMD and metabolic bone diseases. In children and adolescents with CD, the Ca2+ deficiency may produce growth alterations and difficulties in peak bone mass achievement[176]. In the elderly, Ca2+ deficiency leads to low BMD and increased fracture risk[177]. Bone alterations partially result from impaired intestinal Ca2+ absorption due principally to the loss of villous cells in the duodenum, where the active Ca2+ transport occurs[178]. Steatorrhea, vitamin D deficit and changes in the mechanisms of Ca2+ absorption are other factors that contribute to the Ca2+malabsorption[179]. Hipovitaminosis D in CD patients is also a consequence of its malabsorption and the intestinal mucosal lesion[180], which lead to reduce the plasma calcitriol levels, and therefore, the intestinal Ca2+ absorption.
Bariatric surgery (BS) is a valuable option to treat the morbid obesity. However, these procedures may produce a decrease in the BMD increasing the risk of bone fractures, particularly when the duodenum is bypassed, as occurs in Roux-en-Y Gastric Bypass (RYGB). Since the duodenum is the site where the active transport of Ca2+occurs[181], the RYGB contributes to decreasing not only the intestinal Ca2+ absorption but also the absorption of other mineral and nutrients. The RYGB prevents the active calcitriol-mediated Ca2+ transcellular pathway in the duodenum and proximal jejunum, which resembles in certain extension a proximal intestine-specific Vdr KO animal model[182]. Furthermore, hipovitaminosis D is common in patients exposed to BS, which seems to be multifactorial, some factors being related to obesity and others related to the type of the surgical procedure and its consequences[183,184]. Indeed, the vitamin D deficiency would contribute to inhibiting the intestinal Ca2+ absorption, leading to impaired Ca2+ homeostasis and bone density. The reduction in the intestinal Ca2+ absorption[185] produces secondary hyperparathyroidism, and ultimately triggers bone loss[186]. Vitamin D supplementation after RYGB has not always avoided a decrease in BMD, suggesting that other factors should be involved in the bone loss[132]. In the Sleeve Gastrectomy, another very common procedure of BS, the contact time between Ca2+ and intestinal cells is shortened and, hence, the intestinal Ca2+ absorption decreases.
The intestinal Ca2+ absorption in diabetes has been poorly addressed. Most studies have dealt with experimental diabetes provoked by alloxan or STZ. Schneider et al[187] have demonstrated that the intestinal Ca2+ absorption is decreased in rats made diabetic with alloxan, which is due to a decrease in the lumen-to-plasma Ca2+ flux in the duodenum and ileum. A reduction in intestinal Ca2+ absorption has been reported to be associated with low circulating levels of calcitriol, decreased VDR number and CB9k content in diabetic rats[188,189]. In our laboratory we have also observed that the intestinal Ca2+ absorption decreases by insulin deficiency in rats injected with STZ, a model of T1DM. However, the effect was relatively rapid and transient leading to a time dependent adaptation, returning the intestinal Ca2+ absorption to normal values. The inhibition was accompanied by redox changes that produce oxidative stress, which may lead to alterations in the duodenum permeability. Both the redox state of the intestine and the intestinal Ca2+ absorption were normalized after insulin administration, which was independent of vitamin D status[166]. In a clinical study with adolescent girls using a dual-stable isotope approach to evaluate Ca2+ absorption, Weber et al[190] did not find adverse effects of T1DM on gastrointestinal Ca2+ absorption. Since T1DM is characterized by bone loss, alteration in bone remodeling, low BMD and increased risk of fractures[191], further investigation should be done in order to clarify whether a reduction in intestinal Ca2+ absorption contributes to development of bone disease associated to insulin deficiency.
Hypercalciuria is very common in patients with kidney stones. Although the molecular mechanisms underlying hypercalciuria are not well elucidated, it is considered that increased intestinal Ca2+ absorption contributes to the pathogenesis[192]. The idiopatic hypercalciuria is the most common form, but it has a polygenic trait, which makes more difficult to understand the pathogenesis. In the Dent disease, a monogenic disorder associated with hypercalciuria, it has also been found Ca2+ hyperabsorption. Wu et al[193] have demonstrated that the disruption of PI(4,5)P2 5-phosphatase activity by Dent-causing mutations of OCRL gene may explain the increased intestinal Ca2+ absorption. The authors conclude that the TRPV6 activity is enhanced due to the increased transcription of TRPV6 gene provoked by increased calcitriol[194] and/or release of TRPV6 suppression under Dent conditions.
The hereditary hypophosphatemic rickets with hypercalciuria is a rare autosomal recessive disorder with a prevalence of 1:250000. The patients carry loss-of-function mutations in the sodium-phosphate co-transporter NaPi-2c, which cause an increase in the urinary Pi excretion, hypophosphatemia, bowing, short stature and elevated calcitriol levels. Consequently, the intestinal Ca2+ absorption increases, the PTH dependent Ca2+-reabsorption in the distal renal tubules decreases, resulting in hypercalcemia, which leads to nephrocalcinosis in half of patients[195].
Women with Turner syndrome have an increased risk of osteoporosis mainly due to inadequately treated primary ovarian insufficiency and intrinsic bone abnormalities. These patients usually present some comorbid conditions that may further increase the risk of osteoporosis, such as vitamin D deficiency, CD and inflammatory bowel disease[196]. All these conditions may be associated with deficient Ca2+ absorption via different mechanisms such as endocrine deregulation of Ca2+ metabolism or oxidative stress among others.
Osteoporosis and impairment of Ca2+ homeostasis are frequent complications of thalassemia. Studies in thalassemic patients and animal models suggested that a defective Ca2+ absorption might be a cause of thalassemic bone disorder. The possible mechanisms associated with intestinal Ca2+ malabsorption in thalassemia are alterations in the Ca2+ transporters and hormonal controls of the transcellular and paracellular intestinal transport systems[108].
There has been an important progress in molecular studies related to the effects of calcitriol on intestinal Ca2+ absorption in rodents and birds. However, information about the role of other hormones and dietary factors are scarce. The lack of information about the molecular alterations in the intestinal Ca2+ absorption that accompany human pathologies is even greater. Since oxidative stress has shown to produce a powerful influence on the intestinal Ca2+ absorption and the prevention or restoration by antioxidants in experimental animals have proved to be very successful, it would be worth investigating these aspects in humans carrying pathologies associated with altered intestinal Ca2+ absorption.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Corresponding Author's Membership in Professional Societies: Prof. Nori Tolosa de Talamoni and Dr. Vanessa Areco are Members of Investigator Career from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina.
P-Reviewer: Chen Y S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Vannucci L, Fossi C, Quattrini S, Guasti L, Pampaloni B, Gronchi G, Giusti F, Romagnoli C, Cianferotti L, Marcucci G, Brandi ML. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients. 2018;10:1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 2. | Diaz de Barboza G, Guizzardi S, Tolosa de Talamoni N. Molecular aspects of intestinal calcium absorption. World J Gastroenterol. 2015;21:7142-7154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (9)] |
| 3. | Liao QS, Du Q, Lou J, Xu JY, Xie R. Roles of Na+/Ca2+ exchanger 1 in digestive system physiology and pathophysiology. World J Gastroenterol. 2019;25:287-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 4. | Wongdee K, Charoenphandhu N, Litwack G. Vitamin D-Enhanced Duodenal Calcium Transport. Litwack G. In: Litwack G. Vitamins and hormones. New York: Elsevier, 2015: 407-440. |
| 5. | Bronner F. Calcium absorption--a paradigm for mineral absorption. J Nutr. 1998;128:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Gloux A, Le Roy N, Brionne A, Bonin E, Juanchich A, Benzoni G, Piketty ML, Prié D, Nys Y, Gautron J, Narcy A, Duclos MJ. Candidate genes of the transcellular and paracellular calcium absorption pathways in the small intestine of laying hens. Poult Sci. 2019;98:6005-6018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci. 2010;47:181-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | van der Velde RY, Brouwers JR, Geusens PP, Lems WF, van den Bergh JP. Calcium and vitamin D supplementation: state of the art for daily practice. Food Nutr Res. 2014;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Duflos C, Bellaton C, Pansu D, Bronner F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J Nutr. 1995;125:2348-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Wasserman RH. Vitamin D and the dual processes of intestinal calcium absorption. J Nutr. 2004;134:3137-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Charoenphandhu N, Limlomwongse L, Krishnamra N. Prolactin directly stimulates transcellular active calcium transport in the duodenum of female rats. Can J Physiol Pharmacol. 2001;79:430-438. [PubMed] |
| 12. | Suntornsaratoon P, Kraidith K, Teerapornpuntakit J, Dorkkam N, Wongdee K, Krishnamra N, Charoenphandhu N. Pre-suckling calcium supplementation effectively prevents lactation-induced osteopenia in rats. Am J Physiol Endocrinol Metab. 2014;306:E177-E188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem. 2006;54:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Inai T, Sengoku A, Guan X, Hirose E, Iida H, Shibata Y. Heterogeneity in expression and subcellular localization of tight junction proteins, claudin-10 and -15, examined by RT-PCR and immunofluorescence microscopy. Arch Histol Cytol. 2005;68:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. 1999;78:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, Gitter AH, Schulzke JD, Fromm M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | van Goor MKC, Hoenderop JGJ, van der Wijst J. TRP channels in calcium homeostasis: from hormonal control to structure-function relationship of TRPV5 and TRPV6. Biochim Biophys Acta Mol Cell Res. 2017;1864:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Peng JB, Suzuki Y, Gyimesi G, Hediger MA, Kozak J, Putney JJ. TRPV5 and TRPV6 Calcium-Selective Channels [Internet]. Kozak J, Putney JJ. In: Kozak J, Putney JJ. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018: 241-274. |
| 19. | Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 452] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196-3205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Kutuzova GD, Sundersingh F, Vaughan J, Tadi BP, Ansay SE, Christakos S, Deluca HF. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci USA. 2008;105:19655-19659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Woudenberg-Vrenken TE, Lameris AL, Weißgerber P, Olausson J, Flockerzi V, Bindels RJ, Freichel M, Hoenderop JG. Functional TRPV6 channels are crucial for transepithelial Ca2+ absorption. Am J Physiol Gastrointest Liver Physiol. 2012;303:G879-G885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Kellett GL. Alternative perspective on intestinal calcium absorption: proposed complementary actions of Ca(v)1.3 and TRPV6. Nutr Rev. 2011;69:347-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Kojetin DJ, Venters RA, Kordys DR, Thompson RJ, Kumar R, Cavanagh J. Structure, binding interface and hydrophobic transitions of Ca2+-loaded calbindin-D(28K). Nat Struct Mol Biol. 2006;13:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Walters JR, Howard A, Lowery LJ, Mawer EB, Legon S. Expression of genes involved in calcium absorption in human duodenum. Eur J Clin Invest. 1999;29:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Noble JW, Almalki R, Roe SM, Wagner A, Duman R, Atack JR. The X-ray structure of human calbindin-D28K: an improved model. Acta Crystallogr D Struct Biol. 2018;74:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Berggård T, Miron S, Onnerfjord P, Thulin E, Akerfeldt KS, Enghild JJ, Akke M, Linse S. Calbindin D28k exhibits properties characteristic of a Ca2+ sensor. J Biol Chem. 2002;277:16662-16672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 840] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 29. | Merico V, de Barboza GD, Vasco C, Ponce R, Rodriguez V, Garagna S, Tolosa de Talamoni N. A mitochondrial mechanism is involved in apoptosis of Robertsonian mouse male germ cells. Reproduction. 2008;135:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Christakos S, Lieben L, Masuyama R, Carmeliet G. Vitamin D endocrine system and the intestine. Bonekey Rep. 2014;3:496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885-3894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Khuituan P, Teerapornpuntakit J, Wongdee K, Suntornsaratoon P, Konthapakdee N, Sangsaksri J, Sripong C, Krishnamra N, Charoenphandhu N. Fibroblast growth factor-23 abolishes 1,25-dihydroxyvitamin D₃-enhanced duodenal calcium transport in male mice. Am J Physiol Endocrinol Metab. 2012;302:E903-E913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Khanal RC, Nemere I. Endocrine regulation of calcium transport in epithelia. Clin Exp Pharmacol Physiol. 2008;35:1277-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Teerapornpuntakit J, Klanchui A, Karoonuthaisiri N, Wongdee K, Charoenphandhu N. Expression of transcripts related to intestinal ion and nutrient absorption in pregnant and lactating rats as determined by custom-designed cDNA microarray. Mol Cell Biochem. 2014;391:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Hwang I, Yang H, Kang HS, Ahn C, Hong EJ, An BS, Jeung EB. Alteration of tight junction gene expression by calcium- and vitamin D-deficient diet in the duodenum of calbindin-null mice. Int J Mol Sci. 2013;14:22997-23010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Freeman TC, Howard A, Bentsen BS, Legon S, Walters JR. Cellular and regional expression of transcripts of the plasma membrane calcium pump PMCA1 in rabbit intestine. Am J Physiol. 1995;269:G126-G131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Ghijsen WE, De Jong MD, Van Os CH. Kinetic properties of Na+/Ca2+ exchange in basolateral plasma membranes of rat small intestine. Biochim Biophys Acta. 1983;730:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Centeno VA, Díaz de Barboza GE, Marchionatti AM, Alisio AE, Dallorso ME, Nasif R, Tolosa de Talamoni NG. Dietary calcium deficiency increases Ca2+ uptake and Ca2+ extrusion mechanisms in chick enterocytes. Comp Biochem Physiol A Mol Integr Physiol. 2004;139:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Nishiyama K, Azuma YT, Kita S, Azuma N, Hayashi S, Nakajima H, Iwamoto T, Takeuchi T. Na⁺/Ca²⁺ exchanger 1/2 double-heterozygote knockout mice display increased nitric oxide component and altered colonic motility. J Pharmacol Sci. 2013;123:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Kofuji P, Hadley RW, Kieval RS, Lederer WJ, Schulze DH. Expression of the Na-Ca exchanger in diverse tissues: a study using the cloned human cardiac Na-Ca exchanger. Am J Physiol. 1992;263:C1241-C1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Iwamoto T, Kita S, Katsuragi T. Salt-sensitive hypertension, Na+/Ca2+ exchanger, and vascular smooth muscle. Trends Cardiovasc Med. 2005;15:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Khananshvili D. Sodium-calcium exchangers (NCX): molecular hallmarks underlying the tissue-specific and systemic functions. Pflugers Arch. 2014;466:43-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Claassen N, Coetzer H, De Winter R, Haag M, Kruger M. Relationship between duodenal calcium uptake and Ca2+-Mg2+-ATPase activity. Med Sci Res. 1995;24:809-811. |
| 44. | Timmermans JA, Bindels RJ, Van Os CH. Stimulation of plasma membrane Ca2+ pump by calbindin-D28k and calmodulin is additive in EGTA-free solutions. J Nutr. 1995;125:1981S-1986S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Walters JR. Calbindin-D9k stimulates the calcium pump in rat enterocyte basolateral membranes. Am J Physiol. 1989;256:G124-G128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Tolosa de Talamoni N, Morero R, Cañas F. Vitamin D3 administration increases the membrane fluidity of intestinal mitochondria. Biochem Int. 1989;19:701-707. [PubMed] |
| 47. | Alisio A, Cañas F, de Bronia DH, Pereira R, Tolosa de Talamoni N. Effect of vitamin D deficiency on lipid composition and calcium transport in basolateral membrane vesicles from chick intestine. Biochem Mol Biol Int. 1997;42:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Kopic S, Geibel JP. Gastric acid, calcium absorption, and their impact on bone health. Physiol Rev. 2013;93:189-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 49. | Balesaria S, Sangha S, Walters JR. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1193-G1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 508] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 51. | Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 478] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 52. | Wasserman RH, Smith CA, Brindak ME, De Talamoni N, Fullmer CS, Penniston JT, Kumar R. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Centeno V, Picotto G, Pérez A, Alisio A, Tolosa de Talamoni N. Intestinal Na(+)/Ca(2+) exchanger protein and gene expression are regulated by 1,25(OH)(2)D(3) in vitamin D-deficient chicks. Arch Biochem Biophys. 2011;509:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D(3) regulation of intestinal calcium absorption. Arch Biochem Biophys. 2012;523:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Dhawan P, Veldurthy V, Yehia G, Hsaio C, Porta A, Kim KI, Patel N, Lieben L, Verlinden L, Carmeliet G, Christakos S. Transgenic Expression of the Vitamin D Receptor Restricted to the Ileum, Cecum, and Colon of Vitamin D Receptor Knockout Mice Rescues Vitamin D Receptor-Dependent Rickets. Endocrinology. 2017;158:3792-3804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Fahkri H, Zhang B, Fajol A, Hernando N, Elvira B, Mannheim JG, Pichler BJ, Daniel C, Amann K, Hirao A, Haight J, Mak TW, Lang F, Föller M. Checkpoint kinase Chk2 controls renal Cyp27b1 expression, calcitriol formation, and calcium-phosphate metabolism. Pflugers Arch. 2015;467:1871-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Cross HS, Nittke T, Kallay E. Colonic vitamin D metabolism: implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol Cell Endocrinol. 2011;347:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Ritter CS, Haughey BH, Armbrecht HJ, Brown AJ. Distribution and regulation of the 25-hydroxyvitamin D3 1α-hydroxylase in human parathyroid glands. J Steroid Biochem Mol Biol. 2012;130:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Nemere I, Garbi N, Hämmerling GJ, Khanal RC. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J Biol Chem. 2010;285:31859-31866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Doroudi M, Schwartz Z, Boyan BD. Membrane-mediated actions of 1,25-dihydroxy vitamin D3: a review of the roles of phospholipase A2 activating protein and Ca(2+)/calmodulin-dependent protein kinase II. J Steroid Biochem Mol Biol. 2015;147:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Schwartz Z, Graham EJ, Wang L, Lossdörfer S, Gay I, Johnson-Pais TL, Carnes DL, Sylvia VL, Boyan BD. Phospholipase A2 activating protein (PLAA) is required for 1alpha,25(OH)2D3 signaling in growth plate chondrocytes. J Cell Physiol. 2005;203:54-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 333] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 63. | Rexhepaj R, Alesutan I, Gu S, Pelzl L, Eichenmüller M, Pathare G, Föller M, Kuhl D, Lang F. SGK1-dependent stimulation of intestinal SGLT1 activity by vitamin D. Pflugers Arch. 2011;462:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Tudpor K, Teerapornpuntakit J, Jantarajit W, Krishnamra N, Charoenphandhu N. 1,25-dihydroxyvitamin D(3) rapidly stimulates the solvent drag-induced paracellular calcium transport in the duodenum of female rats. J Physiol Sci. 2008;58:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Kutuzova GD, Deluca HF. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys. 2004;432:152-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Zierold C, Mings JA, DeLuca HF. Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J Cell Biochem. 2003;88:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Pantazi H, Papapetrou PD. Changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Karunakaran P, Maharajan C, Chockalingam R, Asokumar P, Koramadai Karuppusamy K, Sadasivam V. The effect of total thyroidectomy on the recovery of bone mineral density in subjects with hyperthyroidism. Surgery. 2019;165:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Cross HS, Debiec H, Peterlik M. Thyroid hormone enhances the genomic action of calcitriol in the small intestine. Prog Clin Biol Res. 1990;332:163-180. [PubMed] |
| 70. | Cross HS, Peterlik M. Cooperative effect of thyroid hormones and vitamin D on intestinal calcium and phosphate transport. Prog Clin Biol Res. 1988;252:331-336. [PubMed] |
| 71. | Kumar V, Prasad R. Thyroid hormones stimulate calcium transport systems in rat intestine. Biochim Biophys Acta. 2003;1639:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Shulman DI. Gastrointestinal effects of growth hormone. Endocrine. 2000;12:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Denson LA, Kim MO, Bezold R, Carey R, Osuntokun B, Nylund C, Willson T, Bonkowski E, Li D, Ballard E, Collins M, Moyer MS, Klein DJ. A randomized controlled trial of growth hormone in active pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2010;51:130-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Slonim AE, Bulone L, Damore MB, Goldberg T, Wingertzahn MA, McKinley MJ. A preliminary study of growth hormone therapy for Crohn's disease. N Engl J Med. 2000;342:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Byrne TA, Wilmore DW, Iyer K, Dibaise J, Clancy K, Robinson MK, Chang P, Gertner JM, Lautz D. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg. 2005;242:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Seguy D, Vahedi K, Kapel N, Souberbielle JC, Messing B. Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: a positive study. Gastroenterology. 2003;124:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 77. | Zoidis E, Gosteli-Peter M, Ghirlanda-Keller C, Meinel L, Zapf J, Schmid C. IGF-I and GH stimulate Phex mRNA expression in lungs and bones and 1,25-dihydroxyvitamin D(3) production in hypophysectomized rats. Eur J Endocrinol. 2002;146:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Chen C, Noland KA, Kalu DN. Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech Ageing Dev. 1997;99:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Fleet JC. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol Cell Endocrinol. 2017;453:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 80. | Fleet JC, Bruns ME, Hock JM, Wood RJ. Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology. 1994;134:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Fatayerji D, Mawer EB, Eastell R. The role of insulin-like growth factor I in age-related changes in calcium homeostasis in men. J Clin Endocrinol Metab. 2000;85:4657-4662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Heaney RP, Recker RR, Saville PD. Menopausal changes in calcium balance performance. J Lab Clin Med. 1978;92:953-963. [PubMed] |
| 83. | Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 993] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 84. | Gallagher JC, Riggs BL, DeLuca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980;51:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 255] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 85. | Pavlovitch H, Clemens TL, Laouari D, O'Riordan JL, Balsan S. Lack of effect on ovariectomy on the metabolism of vitamin D and intestinal calcium-binding protein in female rats. J Endocrinol. 1980;86:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | O'Loughlin PD, Morris HA. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol. 1998;511:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ. Regulation of the epithelial Ca2+ channels in small intestine as studied by quantitative mRNA detection. Am J Physiol Gastrointest Liver Physiol. 2003;285:G78-G85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Gennari C, Agnusdei D, Nardi P, Civitelli R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab. 1990;71:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 122] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Arjmandi BH, Hollis BW, Kalu DN. In vivo effect of 17 beta-estradiol on intestinal calcium absorption in rats. Bone Miner. 1994;26:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Colin EM, Van Den Bemd GJ, Van Aken M, Christakos S, De Jonge HR, Deluca HF, Prahl JM, Birkenhäger JC, Buurman CJ, Pols HA, Van Leeuwen JP. Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the Rat. J Bone Miner Res. 1999;14:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Cotter AA, Cashman KD. Effect of 17beta-oestradiol on transepithelial calcium transport in human intestinal-like Caco-2 cells and its interactions with 1,25-dihydroxycholecalciferol and 9-cis retinoic acid. Eur J Nutr. 2006;45:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632-640. [PubMed] |
| 94. | Nie X, Jin H, Wen G, Xu J, An J, Liu X, Xie R, Tuo B. Estrogen Regulates Duodenal Calcium Absorption Through Differential Role of Estrogen Receptor on Calcium Transport Proteins. Dig Dis Sci. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Ajibade DV, Dhawan P, Fechner AJ, Meyer MB, Pike JW, Christakos S. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology. 2010;151:2974-2984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 96. | Pahuja DN, DeLuca HF. Stimulation of intestinal calcium transport and bone calcium mobilization by prolactin in vitamin D-deficient rats. Science. 1981;214:1038-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 83] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Wongdee K, Teerapornpuntakit J, Sripong C, Longkunan A, Chankamngoen W, Keadsai C, Kraidith K, Krishnamra N, Charoenphandhu N. Intestinal mucosal changes and upregulated calcium transporter and FGF-23 expression during lactation: Contribution of lactogenic hormone prolactin. Arch Biochem Biophys. 2016;590:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Charoenphandhu N, Nakkrasae LI, Kraidith K, Teerapornpuntakit J, Thongchote K, Thongon N, Krishnamra N. Two-step stimulation of intestinal Ca(2+) absorption during lactation by long-term prolactin exposure and suckling-induced prolactin surge. Am J Physiol Endocrinol Metab. 2009;297:E609-E619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 99. | Marcucci G, Masi L, Ferrarì S, Haffner D, Javaid MK, Kamenický P, Reginster JY, Rizzoli R, Brandi ML. Phosphate wasting disorders in adults. Osteoporos Int. 2018;29:2369-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Khuituan P, Wongdee K, Jantarajit W, Suntornsaratoon P, Krishnamra N, Charoenphandhu N. Fibroblast growth factor-23 negates 1,25(OH)2D3-induced intestinal calcium transport by reducing the transcellular and paracellular calcium fluxes. Arch Biochem Biophys. 2013;536:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Rodrat M, Wongdee K, Panupinthu N, Thongbunchoo J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Prolonged exposure to 1,25(OH)2D3 and high ionized calcium induces FGF-23 production in intestinal epithelium-like Caco-2 monolayer: A local negative feedback for preventing excessive calcium transport. Arch Biochem Biophys. 2018;640:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Sutter SA, Stein EM. The Skeletal Effects of Inhaled Glucocorticoids. Curr Osteoporos Rep. 2016;14:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Van Cromphaut SJ, Stockmans I, Torrekens S, Van Herck E, Carmeliet G, Bouillon R. Duodenal calcium absorption in dexamethasone-treated mice: functional and molecular aspects. Arch Biochem Biophys. 2007;460:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Kim MH, Lee GS, Jung EM, Choi KC, Jeung EB. The negative effect of dexamethasone on calcium-processing gene expressions is associated with a glucocorticoid-induced calcium-absorbing disorder. Life Sci. 2009;85:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Zhang Y, Shao J, Wang Z, Yang T, Liu S, Liu Y, Fan X, Ye W. Aqueous extract of pomegranate seed attenuates glucocorticoid-induced bone loss and hypercalciuria in mice: A comparative study with alendronate. Int J Mol Med. 2016;38:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Swaminathan R, Ker J, Care D. Calcitonin and intestinal calcium absorption. J Endocrinol. 1974;61:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 107. | Wongsurawat N, Armbrecht HJ. Calcitonin stimulates 1,25-dihydroxyvitamin D production in diabetic rat kidney. Metabolism. 1991;40:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 108. | Lertsuwan K, Wongdee K, Teerapornpuntakit J, Charoenphandhu N. Intestinal calcium transport and its regulation in thalassemia: interaction between calcium and iron metabolism. J Physiol Sci. 2018;68:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 109. | Centeno V, de Barboza GD, Marchionatti A, Rodríguez V, Tolosa de Talamoni N. Molecular mechanisms triggered by low-calcium diets. Nutr Res Rev. 2009;22:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Anderson JJ, Kruszka B, Delaney JA, He K, Burke GL, Alonso A, Bild DE, Budoff M, Michos ED. Calcium Intake From Diet and Supplements and the Risk of Coronary Artery Calcification and its Progression Among Older Adults: 10-Year Follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 111. | Rule AD, Roger VL, Melton LJ, Bergstralh EJ, Li X, Peyser PA, Krambeck AE, Lieske JC. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 112. | Wongdee K, Rodrat M, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Factors inhibiting intestinal calcium absorption: hormones and luminal factors that prevent excessive calcium uptake. J Physiol Sci. 2019;69:683-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 113. | Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 114. | Brown AJ, Krits I, Armbrecht HJ. Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Arch Biochem Biophys. 2005;437:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Brun LR, Brance ML, Rigalli A. Luminal calcium concentration controls intestinal calcium absorption by modification of intestinal alkaline phosphatase activity. Br J Nutr. 2012;108:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Bangkok T. Human Vitamin and Mineral Requirements. Rome, Italy: 2001. Available from: http://www.fao.org/3/a-y2809e.pdf. |
| 117. | Favus MJ, Goltzman D. Regulation of Calcium and Magnesium. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Ames: John Wiley & Sons, Inc.; 2013: 171-179. |
| 118. | Brun LR, Brance ML, Lombarte M, Lupo M, Di Loreto VE, Rigalli A. Regulation of intestinal calcium absorption by luminal calcium content: role of intestinal alkaline phosphatase. Mol Nutr Food Res. 2014;58:1546-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Brun LR, Lombarte M, Roma S, Perez F, Millán JL, Rigalli A. Increased calcium uptake and improved trabecular bone properties in intestinal alkaline phosphatase knockout mice. J Bone Miner Metab. 2018;36:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G240-G250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 121. | Rutten MJ, Bacon KD, Marlink KL, Stoney M, Meichsner CL, Lee FP, Hobson SA, Rodland KD, Sheppard BC, Trunkey DD, Deveney KE, Deveney CW. Identification of a functional Ca2+-sensing receptor in normal human gastric mucous epithelial cells. Am J Physiol. 1999;277:G662-G670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 122. | Xie R, Dong X, Wong C, Vallon V, Tang B, Sun J, Yang S, Dong H. Molecular mechanisms of calcium-sensing receptor-mediated calcium signaling in the modulation of epithelial ion transport and bicarbonate secretion. J Biol Chem. 2014;289:34642-34653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 123. | Cheng SX, Lightfoot YL, Yang T, Zadeh M, Tang L, Sahay B, Wang GP, Owen JL, Mohamadzadeh M. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014;588:4158-4166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 124. | Owen JL, Cheng SX, Ge Y, Sahay B, Mohamadzadeh M. The role of the calcium-sensing receptor in gastrointestinal inflammation. Semin Cell Dev Biol. 2016;49:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Iamartino L, Elajnaf T, Kallay E, Schepelmann M. Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives. World J Gastroenterol. 2018;24:4119-4131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 126. | Hillman LS, Johnson LS, Lee DZ, Vieira NE, Yergey AL. Measurement of true absorption, endogenous fecal excretion, urinary excretion, and retention of calcium in term infants by using a dual-tracer, stable-isotope method. J Pediatr. 1993;123:444-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Alevizaki CC, Ikkos DG, Singhelakis P. Progressive decrease of true intestinal calcium absorption with age in normal man. J Nucl Med. 1973;14:760-762. [PubMed] |
| 128. | Ghishan FK, Parker P, Nichols S, Hoyumpa A. Kinetics of intestinal calcium transport during maturation in rats. Pediatr Res. 1984;18:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 129. | Lee GS, Lee KY, Choi KC, Ryu YH, Paik SG, Oh GT, Jeung EB. Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J Bone Miner Res. 2007;22:1968-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 130. | Akhter S, Kutuzova GD, Christakos S, DeLuca HF. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch Biochem Biophys. 2007;460:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 131. | Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 132. | Beggs MR, Alexander RT. Intestinal absorption and renal reabsorption of calcium throughout postnatal development. Exp Biol Med (Maywood). 2017;242:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Kovacs CS. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol Rev. 2016;96:449-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 134. | Charoenphandhu N, Wongdee K, Krishnamra N. Is prolactin the cardinal calciotropic maternal hormone? Trends Endocrinol Metab. 2010;21:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 135. | Fudge NJ, Kovacs CS. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology. 2010;151:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 136. | Jantarajit W, Thongon N, Pandaranandaka J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Prolactin-stimulated transepithelial calcium transport in duodenum and Caco-2 monolayer are mediated by the phosphoinositide 3-kinase pathway. Am J Physiol Endocrinol Metab. 2007;293:E372-E384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 137. | Kovacs CS. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr. 2012;32:97-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 138. | Kovacs C. Fetal mineral homeostasis [Internet]. In: Glorieux F, Pettifor J, Juppner H. Pediatric Bone: Biology and Diseases. Endotext: Comprehensive free online endocrinoloy book. San Diego, CA: Elsevier/Academic; 2011: 247-275. |
| 139. | Zhang L, Hu J, Li M, Shang Q, Liu S, Piao X. Maternal 25-hydroxycholecalciferol during lactation improves intestinal calcium absorption and bone properties in sow-suckling piglet pairs. J Bone Miner Metab. 2019;37:1083-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Kovacs CS, Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP. Calcium and Phosphate Metabolism and Related Disorders During Pregnancy and Lactation 2000. [PubMed] |
| 141. | Bullamore JR, Wilkinson R, Gallagher JC, Nordin BE, Marshall DH. Effect of age on calcium absorption. Lancet. 1970;2:535-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 234] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 142. | Ramsubeik K, Keuler NS, Davis LA, Hansen KE. Factors associated with calcium absorption in postmenopausal women: a post hoc analysis of dual-isotope studies. J Acad Nutr Diet. 2014;114:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am. 2013;42:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 144. | Song Y, Fleet JC. Intestinal resistance to 1,25 dihydroxyvitamin D in mice heterozygous for the vitamin D receptor knockout allele. Endocrinology. 2007;148:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 145. | Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1566] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 146. | Clark A, Mach N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front Physiol. 2017;8:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 147. | Tolosa de Talamoni N, Marchionatti A, Baudino V, Alisio A. Glutathione plays a role in the chick intestinal calcium absorption. Comp Biochem Physiol A Physiol. 1996;115:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 148. | Marchionatti A, Alisio A, Díaz de Barboza G, Baudino V, Tolosa de Talamoni N. DL-Buthionine-S,R-sulfoximine affects intestinal alkaline phosphatase activity. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:85-91. [PubMed] |
| 149. | Diaz de Barboza G, Guizzardi S, Moine L, Tolosa de Talamoni N. Oxidative stress, antioxidants and intestinal calcium absorption. World J Gastroenterol. 2017;23:2841-2853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 150. | Marchionatti AM, Díaz de Barboza GE, Centeno VA, Alisio AE, Tolosa de Talamoni NG. Effects of a single dose of menadione on the intestinal calcium absorption and associated variables. J Nutr Biochem. 2003;14:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Areco V, Rodriguez V, Marchionatti A, Carpentieri A, Tolosa de Talamoni N. Melatonin not only restores but also prevents the inhibition of the intestinal Ca(2+) absorption caused by glutathione depleting drugs. Comp Biochem Physiol A Mol Integr Physiol. 2016;197:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 152. | Marchionatti AM, Perez AV, Diaz de Barboza GE, Pereira BM, Tolosa de Talamoni NG. Mitochondrial dysfunction is responsible for the intestinal calcium absorption inhibition induced by menadione. Biochim Biophys Acta. 2008;1780:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 153. | Marchionatti AM, Pacciaroni A, Tolosa de Talamoni NG. Effects of quercetin and menadione on intestinal calcium absorption and the underlying mechanisms. Comp Biochem Physiol A Mol Integr Physiol. 2013;164:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 154. | Hong Z, Piao M. Effect of Quercetin Monoglycosides on Oxidative Stress and Gut Microbiota Diversity in Mice with Dextran Sodium Sulphate-Induced Colitis. Biomed Res Int. 2018;2018:8343052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 155. | Moine L, Díaz de Barboza G, Pérez A, Benedetto M, Tolosa de Talamoni N. Glutamine protects intestinal calcium absorption against oxidative stress and apoptosis. Comp Biochem Physiol A Mol Integr Physiol. 2017;212:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 156. | Moine L, Pérez A, Maldonado C, Tolosa de Talamoni N, Díaz de Barboza G. Glutamine protects both transcellular and paracellular pathways of chick intestinal calcium absorption under oxidant conditions. Comp Biochem Physiol A Mol Integr Physiol. 2019;238:110553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 157. | Chen J, Zhang S, Wu J, Wu S, Xu G, Wei D. Essential Role of Nonessential Amino Acid Glutamine in Atherosclerotic Cardiovascular Disease. DNA Cell Biol. 2020;39:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 158. | Kim MH, Kim H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 159. | Carrascal L, Nunez-Abades P, Ayala A, Cano M. Role of Melatonin in the Inflammatory Process and its Therapeutic Potential. Curr Pharm Des. 2018;24:1563-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 160. | Li RX, Li J, Zhang SY, Mi YL, Zhang CQ. Attenuating effect of melatonin on lipopolysaccharide-induced chicken small intestine inflammation. Poult Sci. 2018;97:2295-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 161. | Shafabakhsh R, Reiter RJ, Davoodabadi A, Asemi Z. Melatonin as a potential inhibitor of colorectal cancer: Molecular mechanisms. J Cell Biochem. 2019;120:12216-12223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 162. | Rivoira MA, Marchionatti AM, Centeno VA, Díaz de Barboza GE, Peralta López ME, Tolosa de Talamoni NG. Sodium deoxycholate inhibits chick duodenal calcium absorption through oxidative stress and apoptosis. Comp Biochem Physiol A Mol Integr Physiol. 2012;162:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 163. | Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 862] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 164. | Zhao MX, Cai ZC, Zhu BJ, Zhang ZQ. The Apoptosis Effect on Liver Cancer Cells of Gold Nanoparticles Modified with Lithocholic Acid. Nanoscale Res Lett. 2018;13:304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Marchionatti AM, Pérez A, Rivoira MA, Rodríguez VA, Tolosa de Talamoni NG. Lithocholic acid: a new emergent protector of intestinal calcium absorption under oxidant conditions. Biochem Cell Biol. 2017;95:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 166. | Rivoira M, Rodríguez V, López MP, Tolosa de Talamoni N. Time dependent changes in the intestinal Ca²⁺ absorption in rats with type I diabetes mellitus are associated with alterations in the intestinal redox state. Biochim Biophys Acta. 2015;1852:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 167. | Rodríguez V, Rivoira M, Guizzardi S, Tolosa de Talamoni N. Naringin prevents the inhibition of intestinal Ca2+ absorption induced by a fructose rich diet. Arch Biochem Biophys. 2017;636:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 168. | Bakar E, Ulucam E, Cerkezkayabekir A, Sanal F, Inan M. Investigation of the effects of naringin on intestinal ischemia reperfusion model at the ultrastructural and biochemical level. Biomed Pharmacother. 2019;109:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 169. | Cao H, Liu J, Shen P, Cai J, Han Y, Zhu K, Fu Y, Zhang N, Zhang Z, Cao Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J Agric Food Chem. 2018;66:13133-13140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 170. | Li Z, Gao M, Yang B, Zhang H, Wang K, Liu Z, Xiao X, Yang M. Naringin attenuates MLC phosphorylation and NF-κB activation to protect sepsis-induced intestinal injury via RhoA/ROCK pathway. Biomed Pharmacother. 2018;103:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 171. | Zhang YS, Li Y, Wang Y, Sun SY, Jiang T, Li C, Cui SX, Qu XJ. Naringin, a natural dietary compound, prevents intestinal tumorigenesis in Apc (Min/+) mouse model. J Cancer Res Clin Oncol. 2016;142:913-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Lima ACD, Cecatti C, Fidélix MP, Adorno MAT, Sakamoto IK, Cesar TB, Sivieri K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J Med Food. 2019;22:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 173. | D'Amelio P, Sassi F. Gut Microbiota, Immune System, and Bone. Calcif Tissue Int. 2018;102:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 174. | Ardizzone S, Cassinotti A, Bevilacqua M, Clerici M, Porro GB. Vitamin D and inflammatory bowel disease. Vitam Horm. 2011;86:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 175. | Kumari M, Khazai NB, Ziegler TR, Nanes MS, Abrams SA, Tangpricha V. Vitamin D-mediated calcium absorption in patients with clinically stable Crohn's disease: a pilot study. Mol Nutr Food Res. 2010;54:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 176. | Motta ME, Faria ME, Silva GA. Prevalence of low bone mineral density in children and adolescents with celiac disease under treatment. Sao Paulo Med J. 2009;127:278-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 177. | Vasquez H, Mazure R, Gonzalez D, Flores D, Pedreira S, Niveloni S, Smecuol E, Mauriño E, Bai JC. Risk of fractures in celiac disease patients: a cross-sectional, case-control study. Am J Gastroenterol. 2000;95:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 178. | Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 179. | Krupa-Kozak U, Drabińska N. Calcium in Gluten-Free Life: Health-Related and Nutritional Implications. Foods. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 180. | Zanchi C, Di Leo G, Ronfani L, Martelossi S, Not T, Ventura A. Bone metabolism in celiac disease. J Pediatr. 2008;153:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 181. | Campanha-Versiani L, Pereira DAG, Ribeiro-Samora GA, Ramos AV, de Sander Diniz MFH, De Marco LA, Soares MMS. The Effect of a Muscle Weight-Bearing and Aerobic Exercise Program on the Body Composition, Muscular Strength, Biochemical Markers, and Bone Mass of Obese Patients Who Have Undergone Gastric Bypass Surgery. Obes Surg. 2017;27:2129-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 182. | Abegg K, Gehring N, Wagner CA, Liesegang A, Schiesser M, Bueter M, Lutz TA. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R999-R1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 183. | Chakhtoura MT, Nakhoul NN, Shawwa K, Mantzoros C, El Hajj Fuleihan GA. Hypovitaminosis D in bariatric surgery: A systematic review of observational studies. Metabolism. 2016;65:574-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 184. | Corbeels K, Verlinden L, Lannoo M, Simoens C, Matthys C, Verstuyf A, Meulemans A, Carmeliet G, Van der Schueren B. Thin bones: Vitamin D and calcium handling after bariatric surgery. Bone Rep. 2018;8:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 185. | Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring). 2006;14:1940-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 186. | Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735-3740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 187. | Schneider LE, Schedl HP. Diabetes and intestinal calcium absorption in the rat. Am J Physiol. 1972;223:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 188. | Seino Y, Sierra RI, Sonn YM, Jafari A, Birge SJ, Avioli LV. The duodenal 1 alpha,25-dihydroxyvitamin D3 receptor in rats with experimentally induced diabetes. Endocrinology. 1983;113:1721-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 189. | Nyomba BL, Verhaeghe J, Thomasset M, Lissens W, Bouillon R. Bone mineral homeostasis in spontaneously diabetic BB rats. I. Abnormal vitamin D metabolism and impaired active intestinal calcium absorption. Endocrinology. 1989;124:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 190. | Weber DR, O'Brien KO, Schwartz GJ. Evidence of disordered calcium metabolism in adolescent girls with type 1 diabetes: An observational study using a dual-stable calcium isotope technique. Bone. 2017;105:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 191. | Grieco GE, Cataldo D, Ceccarelli E, Nigi L, Catalano G, Brusco N, Mancarella F, Ventriglia G, Fondelli C, Guarino E, Crisci I, Sebastiani G, Dotta F. Serum Levels of miR-148a and miR-21-5p Are Increased in Type 1 Diabetic Patients and Correlated with Markers of Bone Strength and Metabolism. Noncoding RNA. 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 192. | Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol. 2003;14:1082-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 193. | Wu G, Zhang W, Na T, Jing H, Wu H, Peng JB. Suppression of intestinal calcium entry channel TRPV6 by OCRL, a lipid phosphatase associated with Lowe syndrome and Dent disease. Am J Physiol Cell Physiol. 2012;302:C1479-C1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 194. | Silva IV, Cebotaru V, Wang H, Wang XT, Wang SS, Guo G, Devuyst O, Thakker RV, Guggino WB, Guggino SE. The ClC-5 knockout mouse model of Dent's disease has renal hypercalciuria and increased bone turnover. J Bone Miner Res. 2003;18:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 195. | Bergwitz C, Miyamoto KI. Hereditary hypophosphatemic rickets with hypercalciuria: pathophysiology, clinical presentation, diagnosis and therapy. Pflugers Arch. 2019;471:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 196. | Augoulea A, Zachou G, Lambrinoudaki I. Turner syndrome and osteoporosis. Maturitas. 2019;130:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |