Published online Jun 14, 2020. doi: 10.3748/wjg.v26.i22.3110
Peer-review started: March 15, 2020
First decision: April 25, 2020
Revised: April 29, 2020
Accepted: May 29, 2020
Article in press: May 29, 2020
Published online: June 14, 2020
Processing time: 91 Days and 1.9 Hours
Splenic artery aneurysm (SAA) and pseudoaneurysm are rare vessel’s lesions. Pseudoaneurysm is often symptomatic and secondary to pancreatitis or trauma. True SAA is the most common aneurysm of visceral vessels. In contrast to pseudoaneurysm, SAA is usually asymptomatic until the rupture, with high mortality rate. The clinical onset of SSA’s rupture is a massive life-threatening bleeding with hemodynamic instability, usually into the free peritoneal space and more rarely into the gastrointestinal tract.
We describe the case of a 35-year-old male patient, with negative past medical history, who presented to the emergency department for massive upper gastrointestinal bleeding, severe anemia and hypotension. An esophagogastroduodenoscopy performed in emergency showed a gastric bulging in the greater curvature/posterior wall with a small erosion on its surface, with a visible vessel, but no active bleeding. Endoscopic injection therapy with cyanoacrylate glue was performed. Urgent contrast-enhanced computed tomography was carried out due to the clinical scenario and the unclear endoscopic aspect: The radiological examination showed a giant SAA which was adherent to posterior stomach wall, and some smaller aneurysms of the left gastric and ileocolic artery. Because of the high risk of a two-stage rupture of the giant SAA with dramatic outcome, the patient underwent immediate open surgery with aneurysmectomy, splenectomy and distal pancreatectomy with a good postoperative outcome.
The management of a ruptured giant SAA into the stomach can be successful with surgical approach.
Core tip: Splenic artery aneurysm (SAA) is a rare vessel’s lesion, despite is the most common aneurysm of visceral vessels. We present herein, a rare case of spontaneous rupture of a giant SAA into the stomach, in a previously healthy male patient. This case highlights the importance of the contrast enhanced computed tomography in case of unclear endoscopic aspect and the urgent surgical treatment in order to prevent a two-stage rupture.
- Citation: Panzera F, Inchingolo R, Rizzi M, Biscaglia A, Schievenin MG, Tallarico E, Pacifico G, Di Venere B. Giant splenic artery aneurysm presenting with massive upper gastrointestinal bleeding: A case report and review of literature. World J Gastroenterol 2020; 26(22): 3110-3117
- URL: https://www.wjgnet.com/1007-9327/full/v26/i22/3110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i22.3110
An aneurysm is a dilation of the lumen of the artery, usually due to pathological changes taking place in its wall. Usually, the pathology concerns elastic fibers and smooth muscle cells of the middle part of the vessel (e.g., fibromuscular dysplasia, collagenopathy and atherosclerosis)[1]. There are true and false aneurysms. In a true aneurysm, the vessel wall thins and bulges as a consequence of the damage of elastic and muscle elements, which are replaced by non-elastic connective tissue bulging under the pressure of blood. In most of the case, a false aneurysm is a lesion of the vessel secondary to a trauma or to adhering inflammatory processes of the surrounding tissue. Splenic artery aneurysm (SAA) is quite rare, although it is the most frequent among the visceral vessel aneurysms with an incidence of 0.2% to 2%, and it is the third most frequent intra-abdominal aneurysm[2]. The annual risk of rupture of a SAA is among 2%-10%. These lesions have a higher incidence in females (4:1), presenting as clinical emergencies in 22% of the cases, with an overall mortality rate of 8.5%[3].
Small SAAs (up to 2 cm) are usually asymptomatic and considered incidental findings[4]. Conversely, larger SAAs (5 cm) are symptomatic and can leads to complications[5]. A giant SAA measure more than 10 cm in size and is rather uncommon[6]. The risk of rupture in giant SAAs is up to 28%[7], with a mortality rate of 40%[8]. Usually SAAs can break into the peritoneal cavity, less than 30% of them perforate into the lumen of intra-abdominal visceral organs[9]. Rupture of a SAA with erosion into the stomach is a rare cause of massive upper gastrointestinal bleeding (UGIB). The gastric fistula of a true giant SAA is even more rare and usually leads to death.
We report the case of a patient presented with massive UGIB who was found to have a true giant SAA. He was successfully treated using both endoscopic and surgical approach. This case report is, to our knowledge, the first case with a good outcome after sequential endoscopic and surgical approach of a giant true SAA presenting with massive UGIB, following its rupture into the stomach.
A previously healthy 35-year old male was referred from the emergency department (ED) of a primary care hospital to the ED of a level-1 hospital for one episode of massive UGIB.
On admission, he presented signs of hypovolemic shock with paleness, sweating, low blood pressure (BP of 100/50 mmHg), tachycardia (105 bpm) and acute anemia (hemoglobin level of 9.9 d/dL on first check, and of 8 d/dL on second one). A previously placed nasogastric tube showed drainage of 300 cc of blood.
No significant past medical history was recorded as well as drugs intake or alcohol abuse.
Intensive treatment including fluids challenge, blood transfusion of one unite of red blood cell, broad spectrum antibiotic and proton pump inhibitor intravenous (IV) therapy was started.
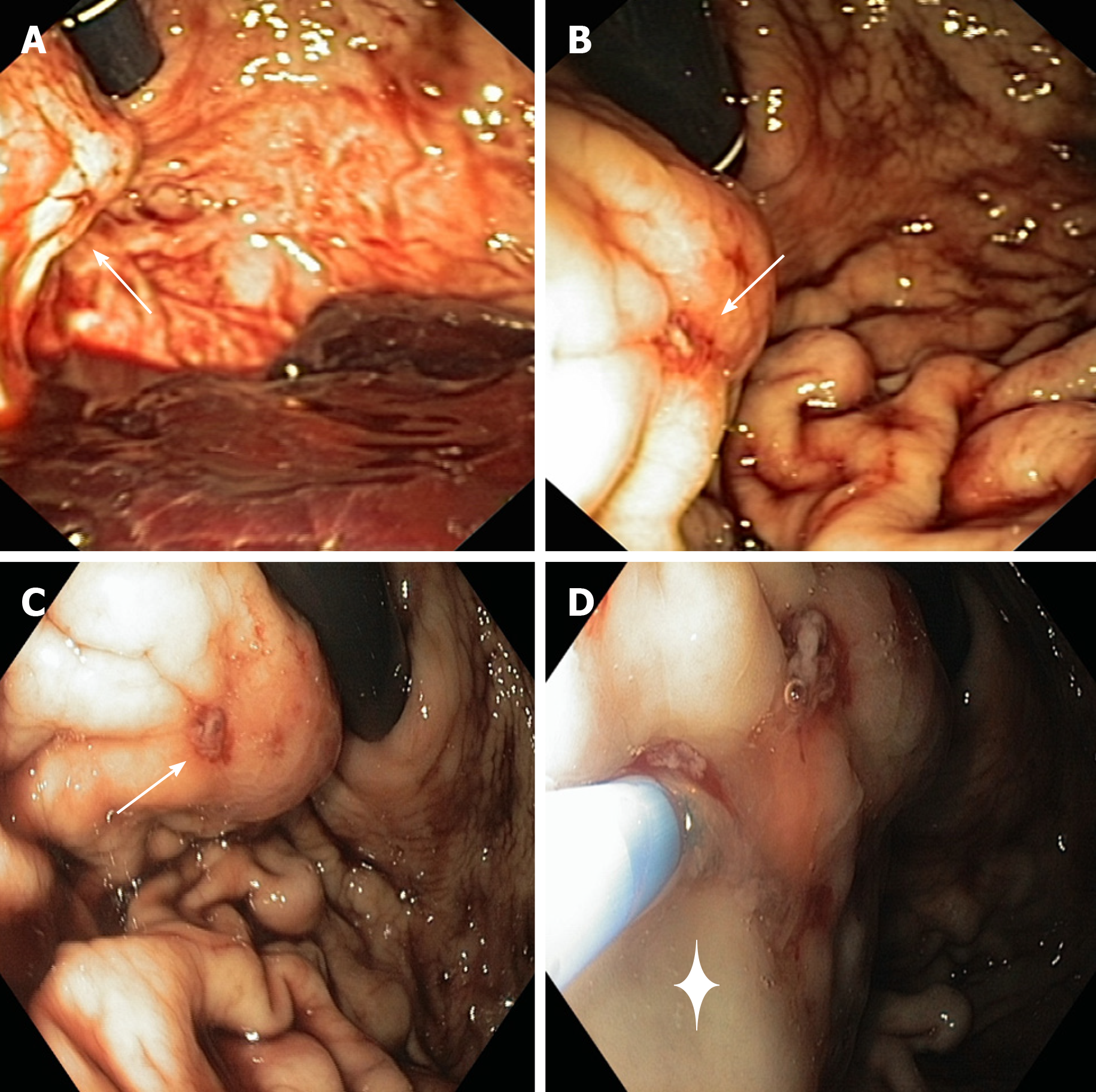
An esophagogastroduodenoscopy (EGD) was performed in emergency setting, with a standard gastroscope (Olympus Medical System® GIF-Q160). Endoscopy showed the presence of a non-pulsatile bulging at the proximal third of greater curvature/ posterior wall of the corpus of the stomach (Figure 1). The gastric bulging measured among 3 cm in diameter and presented a small erosion on its surface with a visible vessel, but no active bleeding at the moment of the examination, despite the gastric cavity was quite full of fresh blood. An endoscopic cyanoacrylate glue injection was performed (n-Butyl 2-cyanoacrylate, Glubran®2, GEM, Italy): The glue was delivered into the lesion through a 23-gauge injection needle catheter (Cook-Medical Inc.), followed by injection of 1.2 cc of sterile water. No other lesions of the upper GI tract were detected during the EGD.
The clinical scenario and the finding of such uncommon gastric lesion in a healthy young man with negative past medical history led us to perform an urgent contrast-enhanced computed tomography (CT) after the EGD.
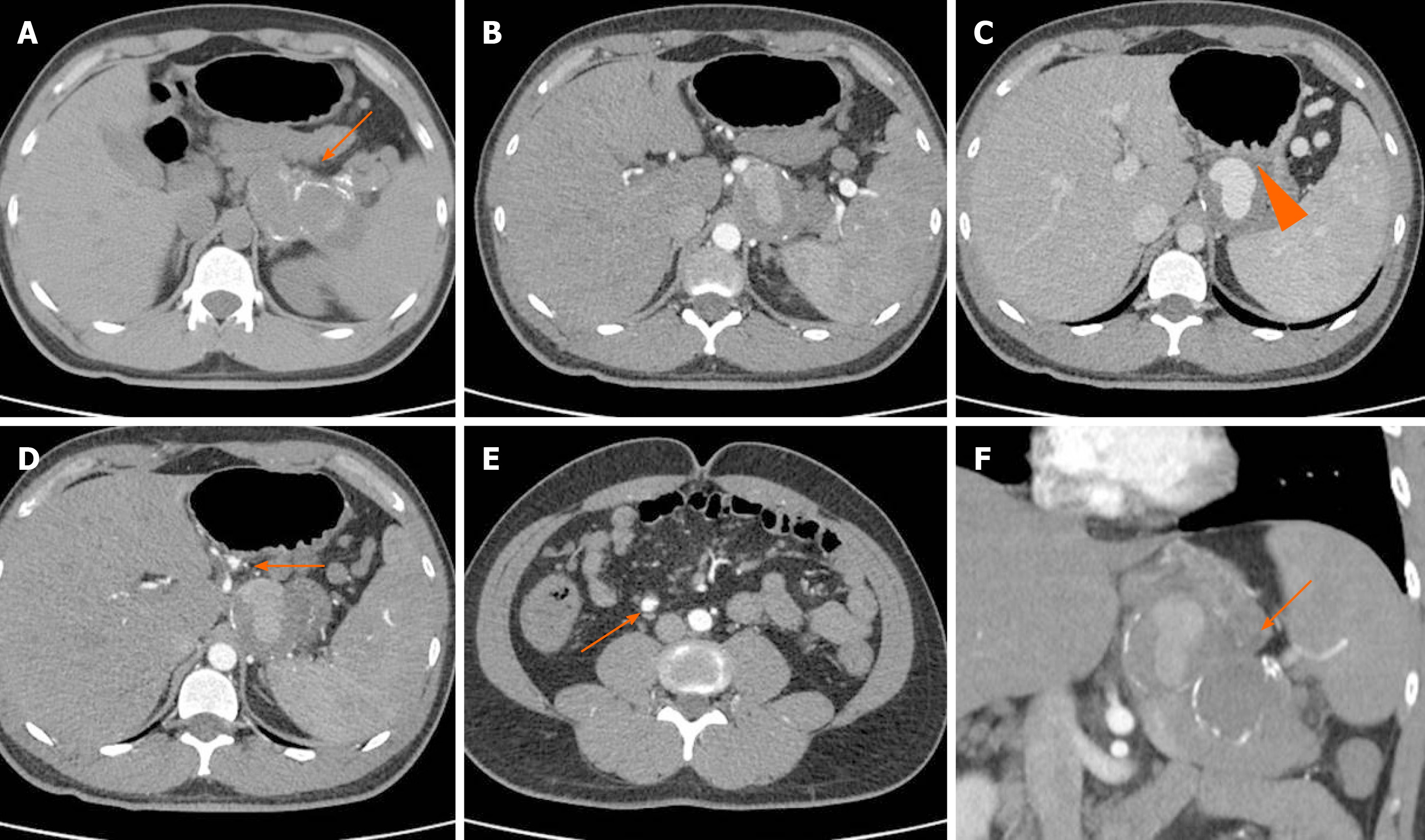
At the CT angiography (Figure 2), the middle and distal third of the splenic artery were fully replaced by a giant aneurysm (10.5 cm in maximum diameter); it was packed to the posterior wall of the stomach, with a plugged fistula. No active bleeding was noted, confirming that EGD treatment was successuful. The opacification of the aneurysm was partial, because of its large volume and low pressure. Two small aneurysms (< 2 cm in size) of the left gastric and ileocolic artery were also detected.
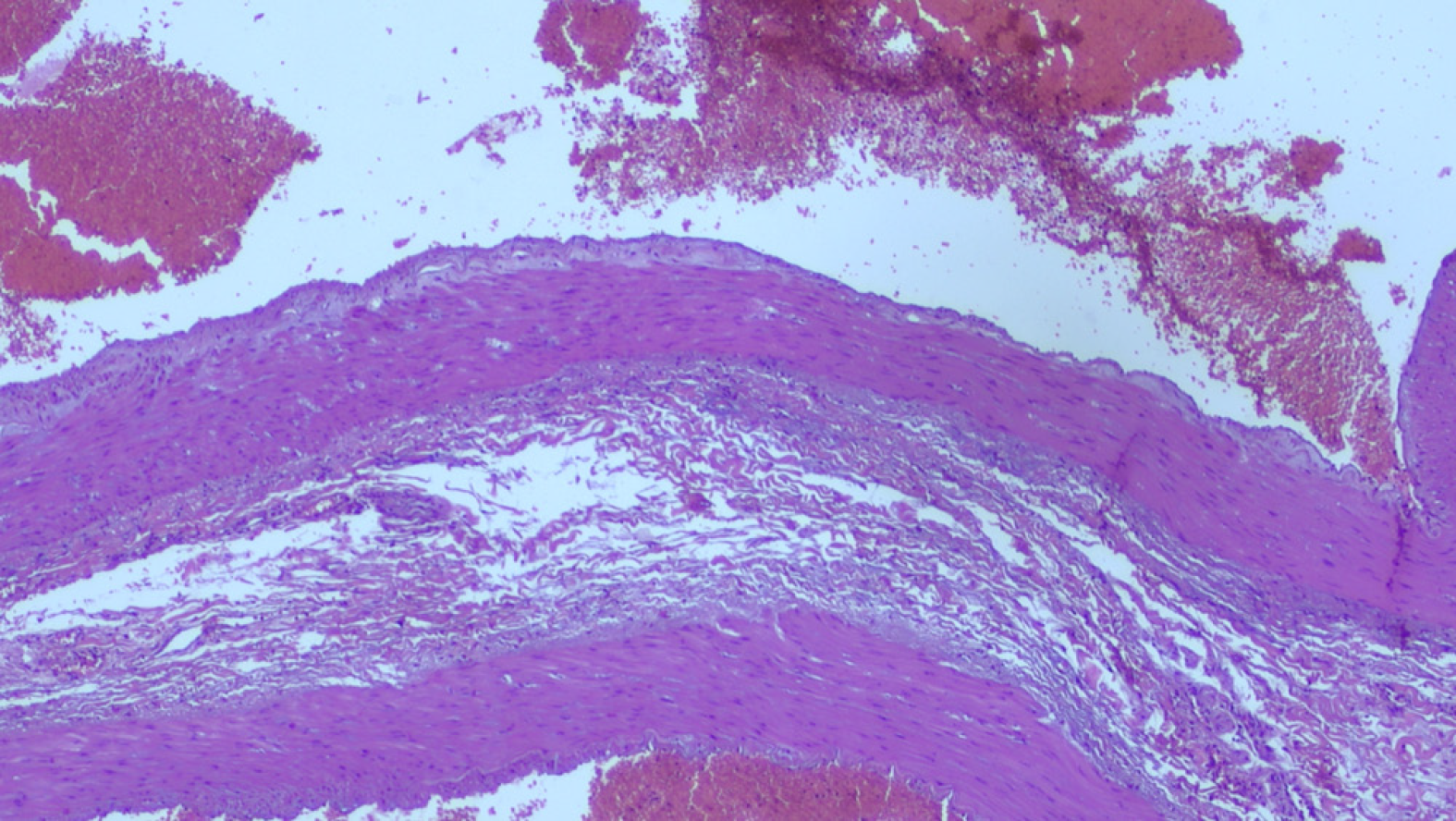
Histology of the resected specimen showed a spleen with features of congested and dilated vessels of the surface and a normal sample of distal pancreas. An aneurysmal dilatation of the middle-distal part of splenic artery was measuring 11 cm × 7 cm and it could be considered true since it was fusiform, involving all the wall layers and with no surrounding inflammation. Distorted elastin configuration, high collagen content, reduced number of smooth muscle cells, and a high number of medial microvessels and inflammatory cells were observed (Figure 3).
According to the available literature, coil embolization of the aneurysm was considered unfit for its size. Therefore, given the life-threatening related to an impending two-stage rupture of the giant SAA, after informed consent statement, the patient underwent immediate open surgery.
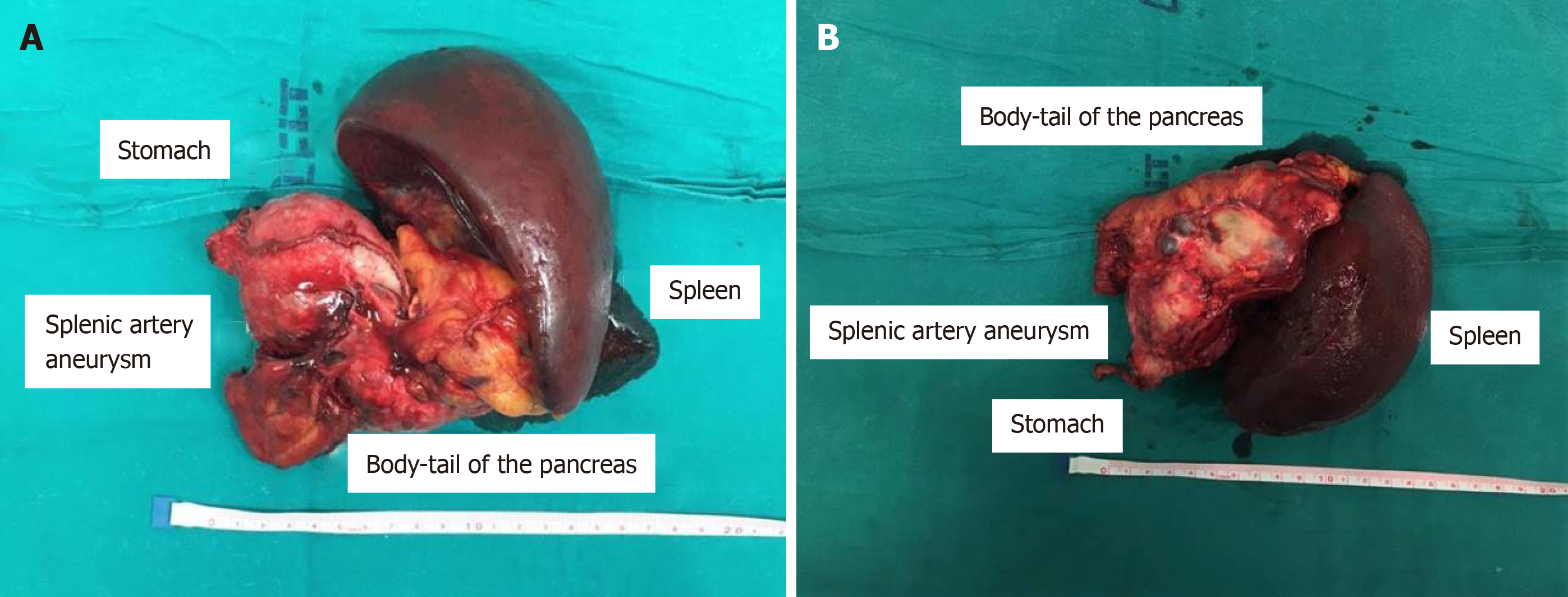
At laparotomy, no free fluid or emoperitoneum were evident. The aneurysm was located in the distal part of the splenic artery and strictly aderent to the pancreatic tail and the posterior wall of the stomach and fissureted at this level; it was undissectable from the other structures and with high risk of uncontrolled rupture. Due to those characteristics, after isolation of the origine of the splenic artery to obtain a good vascular control, in case of unintended rupture of the giant aneurysm during surgical manipulation, a standard distal splenopancreasectomy was performed including the aneurysm; in order to obtain the en-bloc excision of the aneurysm, also a wedge resection of the posterior gastric wall including the ulcer was carried out (Figure 4).
The length of postoperative care was two weeks, without complications. During the hospital stay the patient underwent radiological and clinical work-up. Total body multi-slice CT scan with IV contrast was performed in order to exclude any other site of visceral aneurysms (chest and brain). Disease such as arterial and portal hypertension, atherosclerosis, diabetes, alpha-1-antitrypsin deficiency, liver cirrhosis and collagenopathy were ruled out. Full antibody screening test was negative. Thrombophilia molecular screening evidenced only a heterozygous mutation in the MTHFR gene, that is not related, as reported in literature, to thrombophilia[10].
SAAs are mostly detected incidentally during various imaging studies. Those are also occasionally founded at emergency exploratory laparotomies performed for hemoperitoneum or during autopsy. Upper abdominal pain and severe dysphepsia are the most commonly reported symptoms for ruptured SAAs[11]. Our patient had a rare onset of rupture with massive UGIB, without previous symptoms of alarm.
Risk of rupture for true aneurysms is very low (2% to 3%), but it becomes seriously high for pseudoaneurysms (37% to 47%) with 90% mortality rate[12]. Spontaneous ruptures of true SAAs are more frequent in case of aneurysms larger than 2 cm in diameter and during the third trimester of pregnancy[12-15].
SAA rupture into stomach following fistula formation is less common than into the peritoneal cavity and it is rarely secondary to a true SAA[16-18]. A few cases of suspected true SAAs with intragastric rupture were reported, but the final histology did not confirm them to be true aneurysms[16,19]. In contrast, intragastric bleeding is a common feature of pseudoaneurysms of the splenic artery[20,21].
In case of intraperitoneal rupture of a SAA, the patient presents with acute abdomen and hypovolemic shock[22,23]. In those case SAA rupture may be sudden, or can take place in two stages, which occurs in 20 to 25% of cases[24]. “Double rupture” is a well described manifestation for intraperitoneal bleeding of true SAA, with a first, short, plugged bleeding into the lesser sac followed by more conspicuous bleeding into the peritoneal cavity.
Until now, only one case of recurrent intragastric bleeding from a true but small (< 3 cm) SSA was reported[25]. In their experience, De Silva et al[25] described the case of a young patient who presented with a first massive episode of UGIB and abdominal pain, followed by recurrent intragastric bleeding with initial negative EGD. The patient had a prolonged intermediate stable period, with a certain delay in making diagnosis followed by sudden circulatory collapse and savage laparotomy.
Giant true SAAs with penetrating fistula to stomach are extremely rare and fatal events. Our patient presented a sudden massive UGIB. The emergency endoscopy detected recent signs of bleeding and a gastric lesion with uncommon features, in relation to negative past medical history of the patient. Endoscopic haemostatis with glue injection was performed to guard against any trouble re-bleeding and an urgent CT angiography was carried out for further investigation, leading to the diagnosis of a giant SSA.
A few similar cases of prompt diagnose of SAA were reported: A case of double rupture of a splenic artery pseudoaneurysm, with negative EGD and ultrasono-graphy[21]. Another case of a SAA was suspected by Tannoury et al[26] after seeing during endoscopy a submucosal non-pulsatile gastric lesion. Boschmann et al[27] reported a case in which an abdominal ultrasound scan was crucial to suspect a SAA in a patient with recurrent GI bleeding.
Nowadays, no common guidelines are available for the management of SAA. Level 1 evidences are not available since the disease is rare, so the majority of studies are retrospective and with few patients. Small (< 2.0 cm) and asymptomatic SAAs can be followed up with radiological imaging[28]. Asymptomatic true aneurysms exceeding 2 cm in size are at a high risk of rupture, and so treatment is recommended[29-34]. Pseudoaneurysms should be treated because their risk of rupture is not related to their size[35]. Treatment is necessary for SAA that are growing rapidly, symptomatic, or ruptured[36].
Treatment options include open surgical, laparoscopic, endovascular or percutaneous transabdominal repair. The endovascular techniques most used include embolization with or without stent, thrombin or Gelfoam injection, administration of glue, plug placement, particle injection. Usually, in giant SAA, this can be a pre surgical treatment, in order to reduce the intra operative risk of bleeding.
Surgical intervention is the most commonly described treatment modality for giant SAA in literature, with an overall success rate (98%) comparable to that of recent series[11,30]. This approach is particularly indicated in treating aneurysms that cause mass effect, hemodynamic instability, in case of artero-venous fistula presence or concomitant other complications[11]. The size and local anatomy of giant SAA, however, create possible difficulties in surgical treatment. First, vascular control of the proximal splenic artery is difficult to obtain and necessitates additional maneuvers, to gain retroperitoneal access to the celiac trunk[37]. Second, giant SAAs can be usually associated with fistula formation or tight adherence to adjacent organs, which request further visceral resection as in our reported case.
The laparoscopic approach has recently been reported to be a safe and feasible treatment in SAA management; it gives the advantages of lower morbidity, shorter procedure and hospital stay when compared with open surgery. Nonetheless, it has rarely been used for management of giant SAA in emergency setting[38,39].
Although rare, true SAAs can result in intragastric rupture with catastrophic GI bleeding. The diagnosis of SAA should be excluded in case of recurrent UGIB with no other pathological evidence or when a subspected gastric lesion is detected after a massive haematemesis in an otherwise healty-patient. Perhaps, in this setting, the avilability of the ecoendoscopy could be helpful to promptly diagnose the SAA. The possibility of double or multiple ruptures should be considered when managing patients with SAA.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qayed E S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Kinoshita H, Kubota A, Kasuda S, Nishiguchi M, Takahashi M, Ouchi H, Minami T, Otsu N, Yoshida S, Adachi N, Matsui K, Yamamura T, Motomura H, Hishida S. An autopsy case of rupture of an aneurysm of the splenic artery. Soud Lek. 2008;53:44-45. [PubMed] |
| 2. | Illuminati G, LaMuraglia G, Nigri G, Vietri F. Surgical repair of an aberrant splenic artery aneurysm: report of a case. Ann Vasc Surg. 2007;21:216-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Rao S, Sivina M, Willis I, Sher T, Habibnejad S. Massive lower gastrointestinal tract bleeding due to splenic artery aneurysm: a case report. Ann Vasc Surg. 2007;21:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Tsokos M, Nolting RO, Lockemann U. Sudden, unexpected death due to splenic artery aneurysm rupture. Am J Forensic Med Pathol. 2005;26:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Pinarbaşi B, Poturoğlu S, Yanar H, Güven K, Akyüz F, Dizdaroğlu F, Güllüoğlu M, Taviloğlu K, Kaymakoğlu S, Mungan Z. A rare cause of hemosuccus pancreaticus: primary splenic artery aneurysm ruptured into pancreatic serous cystadenoma. Turk J Gastroenterol. 2008;19:57-63. [PubMed] |
| 6. | Huang IH, Zuckerman DA, Matthews JB. Occlusion of a giant splenic artery pseudoaneurysm with percutaneous thrombin-collagen injection. J Vasc Surg. 2004;40:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Jamsheer NS, Malik N. Ruptured splenic artery aneurysm. Ann Saudi Med. 2001;21:340-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Karaman K, Onat L, Sirvanci M, Olga R. Endovascular stent graft treatment in a patient with splenic artery aneurysm. Diagn Interv Radiol. 2005;11:119-121. [PubMed] |
| 9. | Liu CF, Kung CT, Liu BM, Ng SH, Huang CC, Ko SF. Splenic artery aneurysms encountered in the ED: 10 years' experience. Am J Emerg Med. 2007;25:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Dell'edera D, Tinelli A, Milazzo GN, Malvasi A, Domenico C, Pacella E, Pierluigi C, Giuseppe T, Marcello G, Francesco L, Epifania AA. Effect of multivitamins on plasma homocysteine in patients with the 5,10 methylenetetrahydrofolate reductase C677T homozygous state. Mol Med Rep. 2013;8:609-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Abbas MA, Stone WM, Fowl RJ, Gloviczki P, Oldenburg WA, Pairolero PC, Hallett JW, Bower TC, Panneton JM, Cherry KJ. Splenic artery aneurysms: two decades experience at Mayo clinic. Ann Vasc Surg. 2002;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Trastek VF, Pairolero PC, Joyce JW, Hollier LH, Bernatz PE. Splenic artery aneurysms. Surgery. 1982;91:694-699. [PubMed] |
| 13. | Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery - a review. Surgeon. 2010;8:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Jacobson J, Gorbatkin C, Good S, Sullivan S. Splenic artery aneurysm rupture in pregnancy. Am J Emerg Med. 2017;35:935.e5-935.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Monti JD. A rare cause of abdominal pain and hypotension in pregnancy. JAAPA. 2016;29:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Miao YD, Ye B. Intragastric rupture of splenic artery aneurysms: Three case reports and literature review. Pak J Med Sci. 2013;29:656-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Barnes J, Bouras G, Cooper L, Lam F, Shearman J, Menon V. Splenic artery aneurysm presenting with clinical features of a bleeding gastric gastrointestinal stromal tumour. J Surg Case Rep. 2011;2011:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Sadhu S, Sarkar S, Verma R, Dubey S, Roy M. Haemosuccus pancreaticus due to true splenic artery aneurysm: a rare cause of massive upper gastrointestinal bleeding. J Surg Case Rep. 2010;2010:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Akküçük S, Aydoğan A, Bayaroğulları H, Yetim I. Massive upper gastrointestinal bleeding due to giant splenic artery aneurysm with gastric fistula. Sakarya Med J. 2013;3:150-153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Schatz RA, Schabel S, Rockey DC. Idiopathic Splenic Artery Pseudoaneurysm Rupture as an Uncommon Cause of Hemorrhagic Shock. J Investig Med High Impact Case Rep. 2015;3:2324709615577816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Sawicki M, Marlicz W, Czapla N, Łokaj M, Skoczylas MM, Donotek M, Kołaczyk K. Massive Upper Gastrointestinal Bleeding from a Splenic Artery Pseudoaneurysm Caused by a Penetrating Gastric Ulcer: Case Report and Review of Literature. Pol J Radiol. 2015;80:384-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Aydın MT, Fersahoğlu MM, Tezer S, Okuducu M, Ağca B, Memişoğlu K. Spontaneous rupture of the splenic artery aneurysm: a rare clinical presentation of acute abdomen. Ulus Travma Acil Cerrahi Derg. 2016;22:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Papadomichelakis A, Anyfantakis D, Kastanakis M, Karona P, Bobolakis E. Rupture of a splenic artery aneurysm in a previously healthy 53-year-old male. J Med Life. 2014;7 Spec No. 2:69-70. [PubMed] |
| 24. | Summerour, VA, Bramhall, SR. Splenic artery aneurysms. Adv Emerg Med. 2018;8:1-2. |
| 25. | De Silva WSL, Gamlaksha DS, Jayasekara DP, Rajamanthri SD. A splenic artery aneurysm presenting with multiple episodes of upper gastrointestinal bleeding: a case report. J Med Case Rep. 2017;11:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Tannoury J, Honein K, Abboud B. Splenic artery aneurysm presenting as a submucosal gastric lesion: A case report. World J Gastrointest Endosc. 2016;8:496-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Boschmann H, Zimmermann HB, Wiechmann T, Wenisch HJ, Weinke T. [Ruptured splenic artery aneurysm--a rare cause of recurrent gastrointestinal hemorrhages]. Med Klin (Munich). 2001;96:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Tessier DJ, Stone WM, Fowl RJ, Abbas MA, Andrews JC, Bower TC, Gloviczki P. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg. 2003;38:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Hogendoorn W, Lavida A, Hunink MG, Moll FL, Geroulakos G, Muhs BE, Sumpio BE. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg. 2014;60:1667-76.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Pulli R, Dorigo W, Troisi N, Pratesi G, Innocenti AA, Pratesi C. Surgical treatment of visceral artery aneurysms: A 25-year experience. J Vasc Surg. 2008;48:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Martin D, Teixeira Farinha H, Dattner N, Rotman S, Demartines N, Sauvain MO. Spontaneous non-traumatic splenic artery aneurysm rupture: a case report and review of the literature. Eur Rev Med Pharmacol Sci. 2018;22:3147-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Patel A, Weintraub JL, Nowakowski FS, Kim E, Fischman AM, Ellozy SH, Faries PL, Vouyouka AG, Marin ML, Lookstein RA. Single-center experience with elective transcatheter coil embolization of splenic artery aneurysms: technique and midterm follow-up. J Vasc Interv Radiol. 2012;23:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Toukouki A, Verbeeck N, Weber J, Lens V. Intragastric Rupture of a Splenic Artery Aneurysm Associated with a Pancreatic Cancer. J Belg Soc Radiol. 2016;100:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Scheiermann P, Meimarakis G, Bamberg F, Weis F. Ruptured splenic artery aneurysm masquerading as a gastric hemorrhage. J Vasc Surg. 2012;56:509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Akbulut S, Otan E. Management of Giant Splenic Artery Aneurysm: Comprehensive Literature Review. Medicine (Baltimore). 2015;94:e1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Lakin RO, Bena JF, Sarac TP, Shah S, Krajewski LP, Srivastava SD, Clair DG, Kashyap VS. The contemporary management of splenic artery aneurysms. J Vasc Surg. 2011;53:958-64; discussion 965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Pescarus R, Montreuil B, Bendavid Y. Giant splenic artery aneurysms: case report and review of the literature. J Vasc Surg. 2005;42:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Tiberio GA, Bonardelli S, Gheza F, Arru L, Cervi E, Giulini SM. Prospective randomized comparison of open versus laparoscopic management of splenic artery aneurysms: a 10-year study. Surg Endosc. 2012;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Pietrabissa A, Ferrari M, Berchiolli R, Morelli L, Pugliese L, Ferrari V, Mosca F. Laparoscopic treatment of splenic artery aneurysms. J Vasc Surg. 2009;50:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |