Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2276
Peer-review started: December 24, 2019
First decision: March 27, 2020
Revised: April 1, 2020
Accepted: April 29, 2020
Article in press: April 29, 2020
Published online: May 21, 2020
Processing time: 149 Days and 7.8 Hours
In recent years, the serrated neoplasia pathway where serrated polyps arise as a colorectal cancer has gained considerable attention as a new carcinogenic pathway. Colorectal serrated polyps are histopathologically classified into hyperplastic polyps (HPs), sessile serrated lesions, and traditional serrated adenomas; in the serrated neoplasia pathway, the latter two are considered to be premalignant. In western countries, all colorectal polyps, including serrated polyps, apart from diminutive rectosigmoid HPs are removed. However, in Asian countries, the treatment strategy for colorectal serrated polyps has remained unestablished. Therefore, in this review, we described the clinicopathological features of colorectal serrated polyps and proposed to remove HPs and sessile serrated lesions ≥ 6 mm in size, and traditional serrated adenomas of any size.
Core tip: In western countries, generally all colorectal polyps are removed, including serrated polyps, except rectosigmoid hyperplastic polyps ≤ 5 mm in size. However, no treatment strategy has been developed for colorectal serrated polyps in Asian countries. Hence, in this review, we described the clinicopathological features of colorectal serrated polyps and proposed to remove hyperplastic polyps and sessile serrated lesions ≥ 6 mm in size, and traditional serrated adenomas of any size by narrow-band imaging observation.
- Citation: Sano W, Hirata D, Teramoto A, Iwatate M, Hattori S, Fujita M, Sano Y. Serrated polyps of the colon and rectum: Remove or not? World J Gastroenterol 2020; 26(19): 2276-2285
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2276.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2276
Apart from adenoma-carcinoma sequence and de novo carcinogenic pathways[1,2], the serrated neoplasia pathway where serrated polyps emerge as a colorectal cancer has been recently gaining considerable research interest as a new carcinogenic pathway. Hyperplastic polyps (HPs), sessile serrated lesions (SSLs), and traditional serrated adenomas (TSAs) are the histopathological classifications of colorectal serrated polyps[3]. Of these, SSLs and TSAs are regarded as premalignant lesions in the serrated neoplasia pathway.
SSLs had been previously described as sessile serrated adenoma/polyps[4]. They possess molecular features, such as hypermethylation of the CpG islands in the promoter regions of tumor suppressor genes (i.e., the CpG island methylator phenotype-high: CIMP-high) and BRAF mutations, and they progress to either of the following colorectal cancer types: (1) Microsatellite instable colorectal cancers with MLH1 inactivation; or (2) Microsatellite stable colorectal cancers without MLH1 inactivation. Meanwhile, TSAs progress to microsatellite stable colorectal cancers through the following molecular features: (1) CIMP-high and BRAF mutations without MLH1 inactivation; or (2) KRAS mutations without any of the CIMP-high, BRAF mutations, or MLH1 inactivation[1-3,5-7].
Generally, western countries remove all colorectal polyps, including serrated polyps, apart from rectosigmoid HPs ≤ 5 mm in size. Unfortunately, Asian countries still have no treatment strategy for colorectal serrated polyps. Hence, in this review, we described the clinicopathological features of colorectal serrated polyps and proposed a treatment strategy for them.
To clarify the prevalence of colorectal serrated polyps, particularly SSLs, we conducted a prospective study[8]. Between June 2013 and May 2014, patients aged ≥ 40 years undergoing colonoscopy for standard clinical indications at our center were prospectively enrolled. During colonoscopy, 0.05% indigo carmine dye was sprayed throughout the colorectum by using waterjet function to highlight the lesions. All detected lesions were diagnosed by high-definition magnifying narrow-band imaging (NBI) and were removed endoscopically or surgically, aside from rectosigmoid NICE (NBI international colorectal endoscopic)[9,10] or JNET (Japan NBI Expert Team)[11] type 1 lesions ≤ 5 mm in size. Regarding rectosigmoid NICE or JNET type 1 lesions measuring ≤ 5 mm, we recorded their total number by using tally counter and then removed some of them. In 315 out of 343 eligible patients, 1301 colorectal lesions were removed endoscopically or surgically. Of them, 165 HPs in 97 patients, 21 SSLs in 17 patients, and 13 TSAs in 12 patients were identified histopathologically. Furthermore, the prevalence of HPs, SSLs, and TSAs, defined as the proportion of patients with ≥ 1 HP, SSL, and TSA lesions, was 28.3%, 5.0%, and 3.5%, respectively.
In the study of Hazewinkel et al[12] of 1426 patients with a median age of 60 years who underwent screening colonoscopy, the prevalence of HPs, SSLs, and TSAs was 23.8%, 4.8%, and 0.1%, respectively. In another study of 1479 patients by Carr et al[13], the prevalence of HPs, SSLs, and TSAs was 30%, 3.9%, and 0.7%, respectively. Abdeljawad et al[14] reported that the prevalence of SSLs was 8.1% in their study of 1910 patients aged ≥ 50 years who underwent screening colonoscopy. The previously reported prevalence of colorectal serrated polyps varies due to the study design and population, colonoscopy equipment and observation method, experience of the endoscopists, and the histopathological diagnostic criteria. However, SSLs, which have recently attracted attention as precursors of microsatellite instable colorectal cancers, seem not to be rare in clinical practice[14,15].
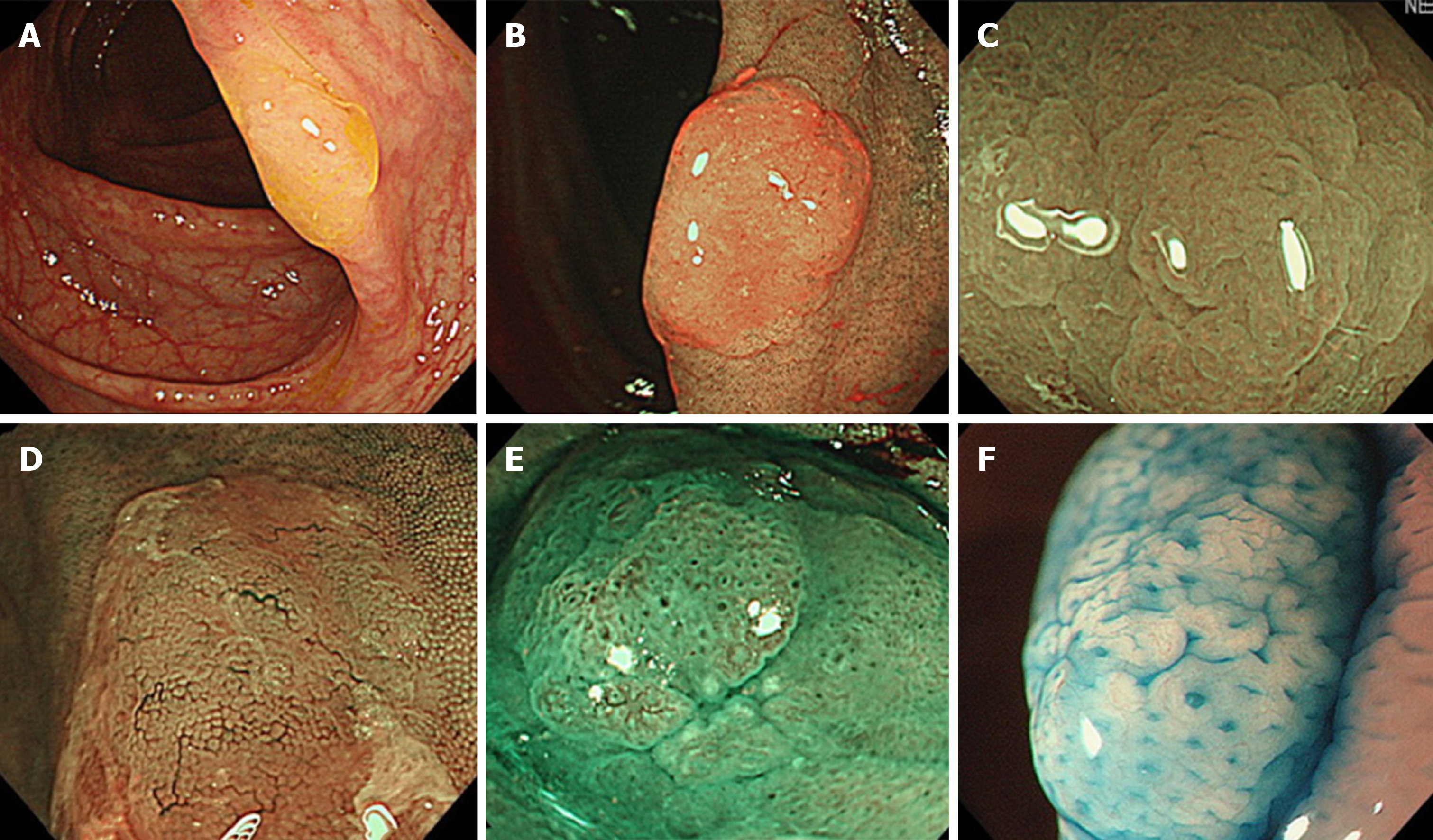
HPs commonly exist in the left colorectum, particularly rectosigmoid colon, and are often ≤ 5 mm in size. In white light endoscopy, they appear as discolored, flat elevated lesions (Figure 1A). In NBI endoscopy, they appear as whitish lesions (NICE or JNET type 1) without expanded, brown meshed capillary vessels (MC vessels)[16], which are seen in conventional adenomas (Figure 1B). In chromoendoscopy, Kudo type II asteroid pits are observed on the lesion surface (Figure 1C).
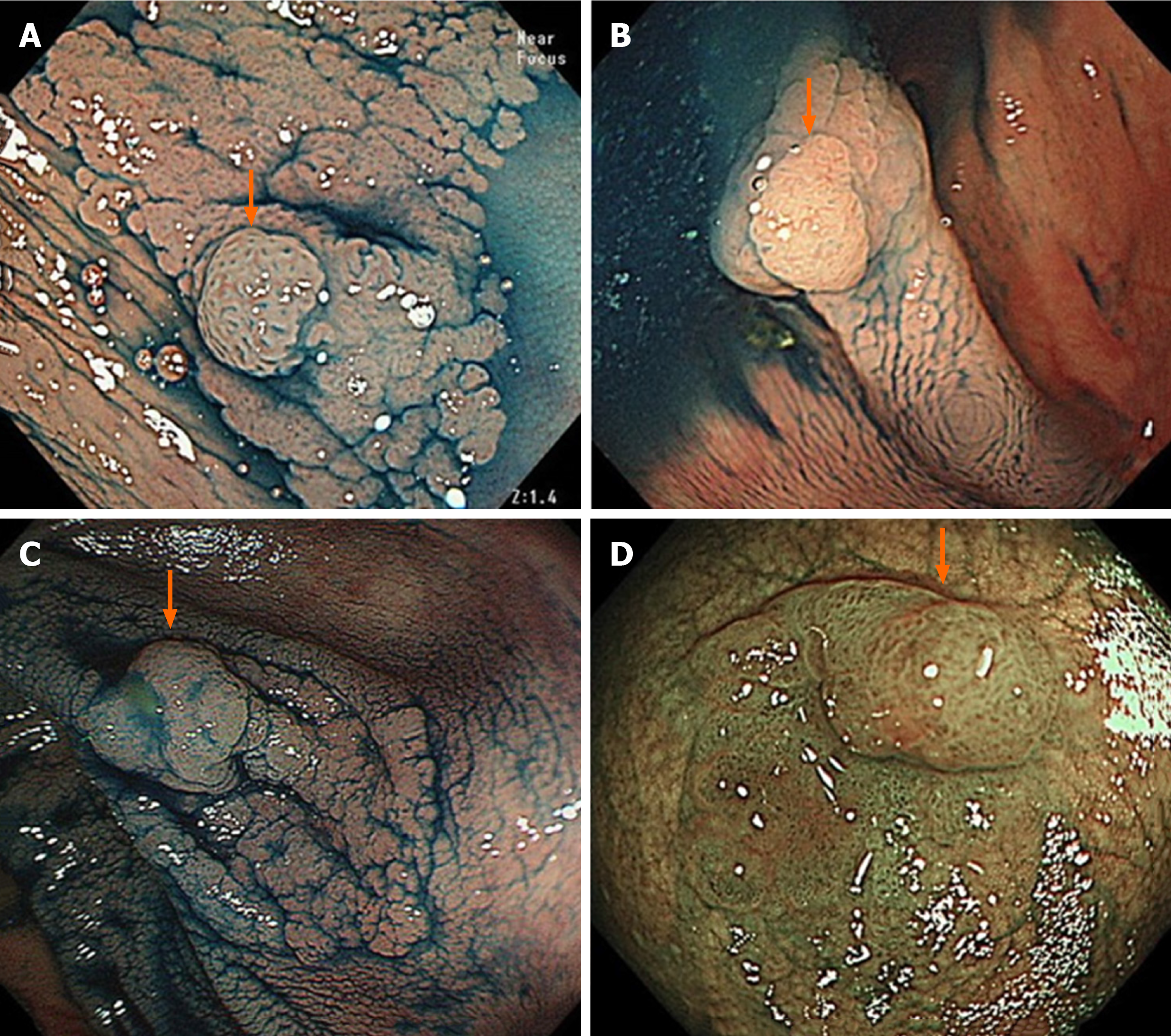
SSLs typically occur in the right colon and are slightly larger than HPs. The characteristic findings of SSLs include various endoscopic findings, such as a mucous cap in white light endoscopy[17], a red cap sign in NBI endoscopy[18], a cloud-like surface in white light or NBI endoscopy[19], dilated and branching vessels in NBI endoscopy[20], expanded crypt openings in NBI endoscopy[21], and type II open-shape (type II-O) pits in chromoendoscopy[22] (Figure 2). However, SSLs resemble HPs endoscopically, and most of them are detected as discolored, flat elevated lesions in white light endoscopy and whitish lesions (NICE or JNET type 1) in NBI endoscopy; therefore, differentiating between HPs and SSLs endoscopically in clinical practice remains often challenging.
SSL is generally accompanied with dysplasia during carcinogenesis[1-4,6]. Endoscopic morphological findings, such as large or small nodules on the surface and partial protrusion of SSLs, were useful indicators of dysplasia within SSLs, with an accuracy of 93.3% on the analysis of 326 SSLs (Figure 3)[23]. Burgess et al[24] also reported that the presence of any 0-Is or nodular components within SSLs was associated with dysplasia within SSLs, with 71.0% accuracy on the analysis of 207 SSLs ≥ 20 mm in size.
Regarding the pit pattern analysis of SSLs with dysplasia (SSLDs) using magnifying chromoendoscopy, Burgess et al[24] stated that SSLDs exhibited an adenomatous pit pattern (Kudo type III, IV, or V), with 70.6% accuracy on the analysis of 201 SSLs ≥ 20 mm in size. Moreover, Murakami et al[25] reported that SSLDs exhibited Kudo type III, IV, VI, or VN pit patterns in addition to a type II pit pattern, with 99.4% accuracy on the analysis of 314 SSLs. Tanaka et al[26] also described that SSLDs exhibited an adenomatous pit pattern (Kudo type III or IV) in addition to a type II or II-O pit pattern, with 89.4% accuracy on the analysis of 123 SSLs.
Utilizing nonmagnifying NBI observation, Tate et al[27] reported that a demarcated area with a neoplastic pit pattern (Kudo type III/IV or NICE type 2) was a useful indicator of dysplasia within SSLs, with 95.0% accuracy on the analysis of 141 SSLs ≥ 8 mm in size.
Thus, high-accuracy endoscopic diagnosis of SSLDs is becoming possible; however, some SSLDs do not exhibit the abovementioned characteristic endoscopic findings.
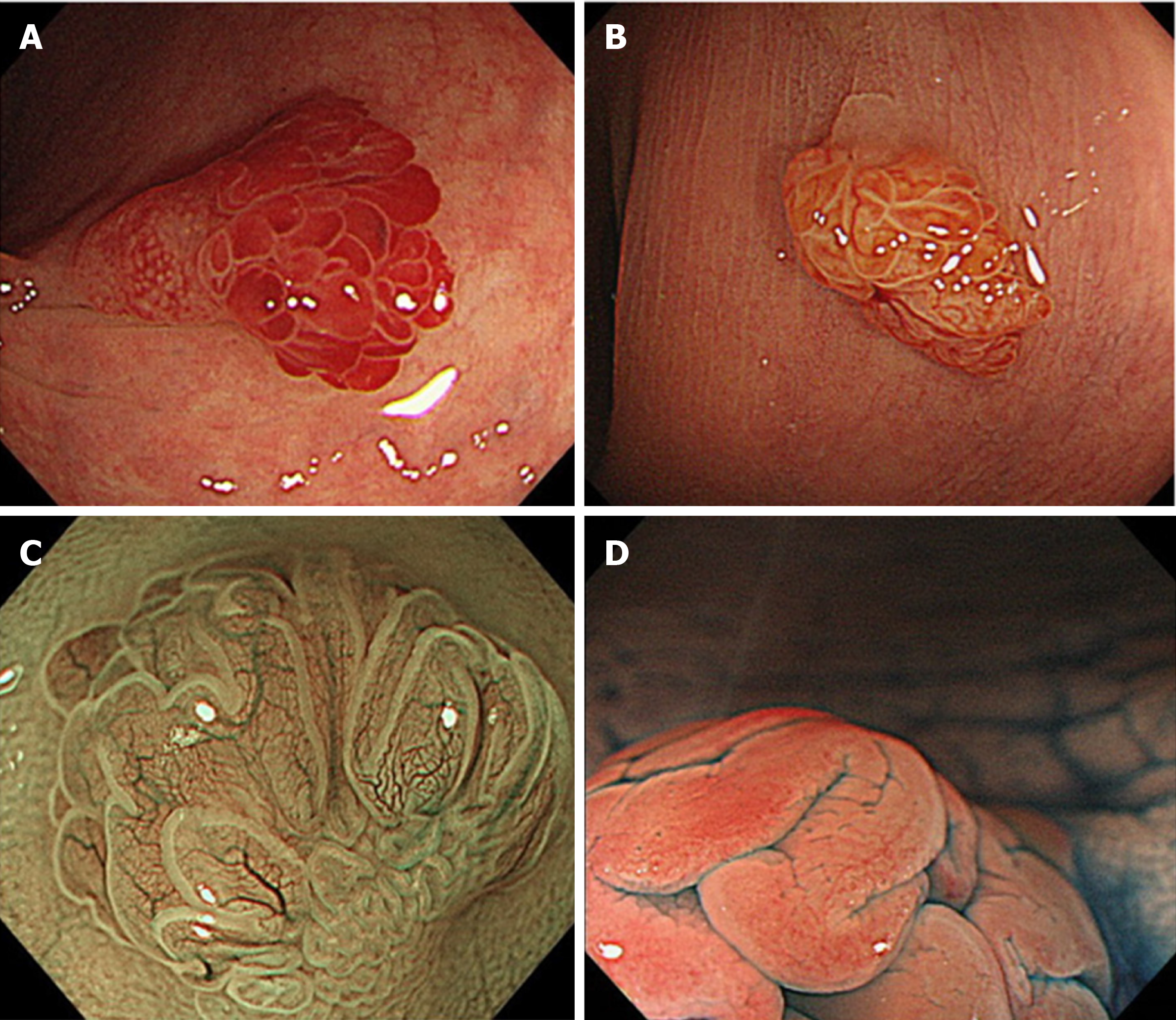
TSAs commonly appear in the left colorectum, similar to HPs. They appear as reddish, protruded or pedunculated lesions in white light observation and “pine cone-like” or “branch coral-like” lesions in macroscopic observation (Figure 4A and B). In NBI endoscopy, “leaf vein-like” expanded, brown capillary vessels, which differ from MC vessels seen in conventional adenomas, are identified in the large stromal area around the crypts, and TSAs appear as brownish resembling conventional adenomas (NICE or JNET type 2) (Figure 4C). In chromoendoscopy, they are characterized by type IIIH pits, which are Kudo type IIIL-like tubular pits accompanied with serration in the crypt margin, or type IVH pits, which are Kudo type IV-like villous pits accompanied with serration in the crypt margin (Figure 4D)[28].
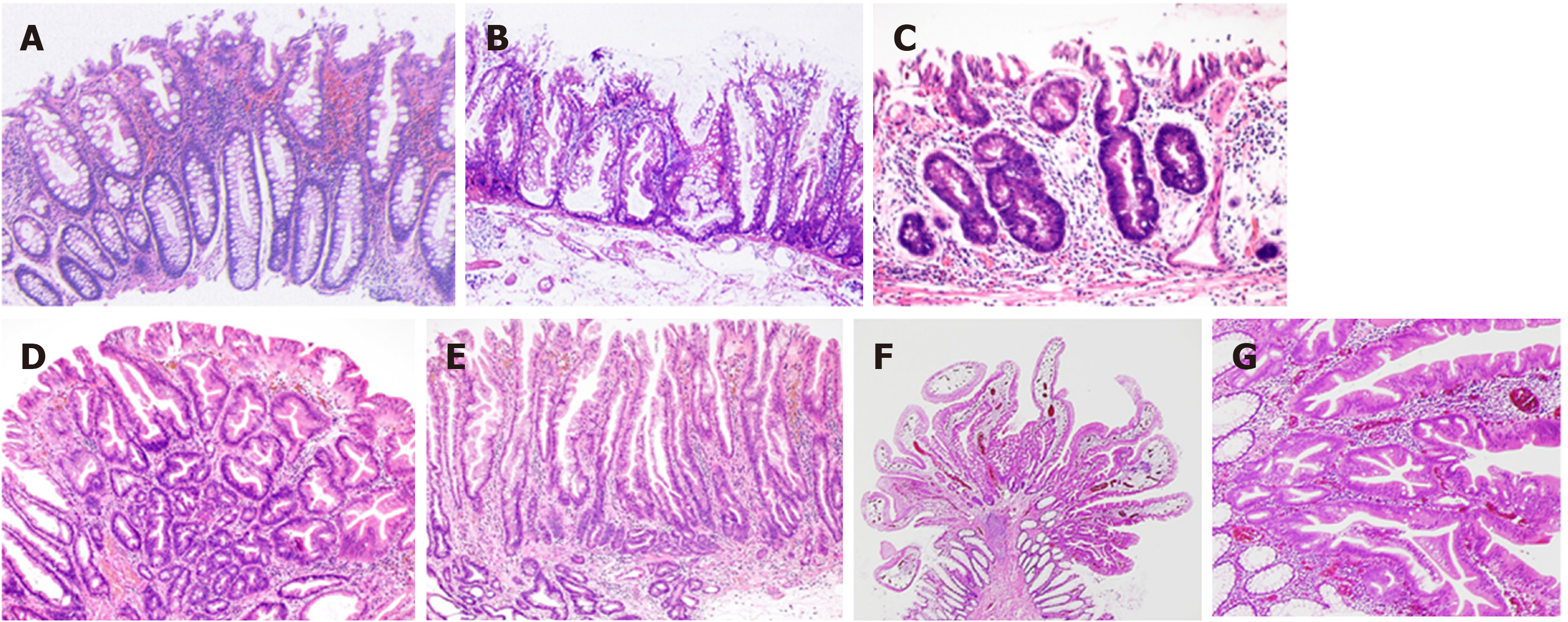
On the basis of mucin type, HPs are subclassified into three types, namely, microvesicular HPs (MVHPs), goblet-cell-rich HPs, and mucin-poor HPs[2]. MVHPs and goblet-cell-rich HPs account for up to 60% and 30%, respectively, whereas mucin-poor HPs remain rare. All these HPs are histologically characterized by the elongation of the intestinal crypts, with serration of the upper part of the crypts (Figure 5A). Conversely, the lower part of the crypts is narrow, without any serration. The basal part of the crypts exhibits uniform proliferation. Meanwhile, cytological dysplasia is not seen in any HP types.
SSLs are histopathologically characterized by (1) crypt dilation; (2) irregularly branching crypts; and (3) horizontally arranged basal crypts (inverted T- and/or L-shaped crypts) (Figure 5B)[29,30]. The proliferation zone of the crypts often shifts from the basal part to the side of the crypts[2].
According to the Japanese Society for Cancer of the Colon and Rectum criteria established in 2011, when at least two of these three histopathological findings were found in ≥ 10% of the lesion area, the serrated polyps are diagnosed as SSLs[29,30]. However, those that do not meet the abovementioned criteria are diagnosed as HPs.
Generally, cytological dysplasia arising in SSLs is divided into two types, namely, conventional adenoma-like dysplasia and serrated dysplasia (Figure 5C and D)[3,4,31,32].
Conventional adenoma-like dysplasia is histopathologically characterized by the presence of elongated cells with penicillate and hyperchromatic pseudostratified nuclei, basophilic cytoplasm, and increased mitoses, and it resembles a conventional tubular or tubulovillous adenoma cytologically. Meanwhile, serrated dysplasia is histopathologically characterized by a proliferation of cuboidal atypical cells, eosinophilic cytoplasm, enlarged round nuclei with open vesicular prominent chromatin, prominent nucleoli, and increased mitoses[3,4,31,32].
After the analysis of 326 SSLs, we also found a previously unreported type of dysplasia, termed as “cryptal dysplasia,” which is localized in the crypt base but cannot be classified as either conventional adenoma-like or serrated dysplasia (Figure 5E)[23]. Although the clinical significance of “cryptal dysplasia” remains unclear, this type was common within SSLDs and mostly coexisted with conventional adenoma-like and/or serrated dysplasia to a varied extent. Further assessment of the clinical significance of “cryptal dysplasia” is necessary to become widely recognized and be applied by endoscopists and pathologists in their diagnostic examinations.
TSAs are histopathologically characterized by tubulovillous or villous architecture, tall columnar cells with intensely eosinophilic cytoplasm and pencillate nuclei, slit-like serrations due to narrow slits in the deeply eosinophilic epithelium resembling normal small intestinal mucosa, and ectopic crypt formations due to the abnormal crypt development with loss of orientation toward the muscularis mucosae (Figure 5F and G)[3,7].
NBI observation detects both HPs and SSLs as NICE or JNET type 1 lesions. In our study, the probability of NICE or JNET type 1 lesions being SSLs significantly increased with the lesion size, with 6 mm as the boundary (≤ 5 mm: 0.7%; 6-9 mm: 29.0%; ≥ 10 mm: 70%) (Table 1)[8]. In addition, 89.1% and 10.9% of HPs were ≤ 5 and ≥ 6 mm in size, respectively, showing a pyramid-type distribution; meanwhile, 76.2% and 23.8% of SSLs were ≥ 6 and ≤ 5 mm in size, respectively, indicating an inverted pyramid-type distribution. Furthermore, HPs, particularly MVHPs, harbor genetic abnormalities, such as CIMP-high and BRAF mutations, similar to SSLs[33-35]. Thus, HPs will transition to SSLs with an increase of the lesion size, with a boundary of approximately 6 mm.
If differentiating between HPs and SSLs is easy, SSLs can be removed selectively; however, it is often challenging in clinical practice. In addition, NICE or JNET type 1 lesions ≥ 6 mm in size, even if HPs, may eventually transition to SSLs if left unattended. Therefore, we propose to remove all NICE or JNET type 1 lesions ≥ 6 mm in size. In our study, most NICE or JNET type 1 lesions were ≤ 5 mm, and only 1.1% of them were ≥ 6 mm in size[8]. Additionally, the prevalence of NICE or JNET type 1 lesions ≥ 6 mm in size was not high at 8.5%, suggesting that the removal of all NICE or JNET type 1 lesions ≥ 6 mm in size seems feasible in clinical practice.
Meanwhile, in our study, the probability of NICE or JNET type 1 lesions ≤ 5 mm in size being SSLs was only 0.7% (Table 1)[8]. In our other study, the rate of dysplasia within SSLs increased as the lesion size increased (≤ 5 mm, 0%; 6-9 mm, 6.0%; ≥ 10 mm, 13.6%) (Table 2)[23], similar to a stepwise increase of the dysplasia degree or cancer rate within conventional adenomas that occurs along with an increase in lesion size[36-38]. Thus, the probability of NICE or JNET type 1 lesions ≤ 5 mm in size being SSLs was extremely low, and even if they are SSLs, the rate of dysplasia within SSLs ≤ 5 mm in size was also extremely low, suggesting that NICE or JNET type 1 lesions ≤ 5 mm in size can be left in situ.
Western countries remove all NICE or JNET type 1 lesions ≤ 5 mm in the cecum, ascending, transverse, and descending colon. However, in our study, despite the relatively high (25.1%) prevalence of these lesions, the probability of these being SSLs was quite low (1.6%)[8]. Therefore, removing all NICE / JNET type 1 lesions ≤ 5 mm in the whole colon apart from rectosigmoid colon appears to have more disadvantages such as the probable adverse events including post-polypectomy bleeding and perforation, the burden on the endoscopists, and the time-consuming examinations, than the advantages of removing SSLs ≤ 5 mm in size. However, these lesions include HPs, which have the potential to transition to SSLs, and some SSLs. Hence, we recommend following up these lesions at appropriate intervals (e.g., 5 years[39,40]) if left unattended.
As mentioned, SSLs are frequently accompanied with dysplasia during carcinogenesis[1-4,6], and they may rapidly progress to carcinomas after they start to develop dysplasia within them[41]. In our study, the rate of dysplasia within SSLs increased as the lesion size also increased, and all SSLDs were ≥ 6 mm in size (Table 2)[23]. Therefore, if all SSLs ≥ 6 mm in size are removed, we could possibly remove almost all SSLDs with a high sensitivity. Given that endoscopically diagnosing SSLDs with a high accuracy is gradually becoming possible[23-27], colorectal lesions suspected of SSLDs should be removed en bloc for accurate pathological assessment and prevention of recurrent cancers due to incomplete removal.
In NBI endoscopy, TSAs are detected as NICE or JNET type 2 brownish lesions similar to conventional adenomas. Considering the lower prevalence[8,12,13] and similar malignant potential[42,43] (e.g., cancer rate within the lesions) of TSAs compared with conventional adenomas, we propose to remove all TSAs of any size in the same fashion as conventional adenomas.
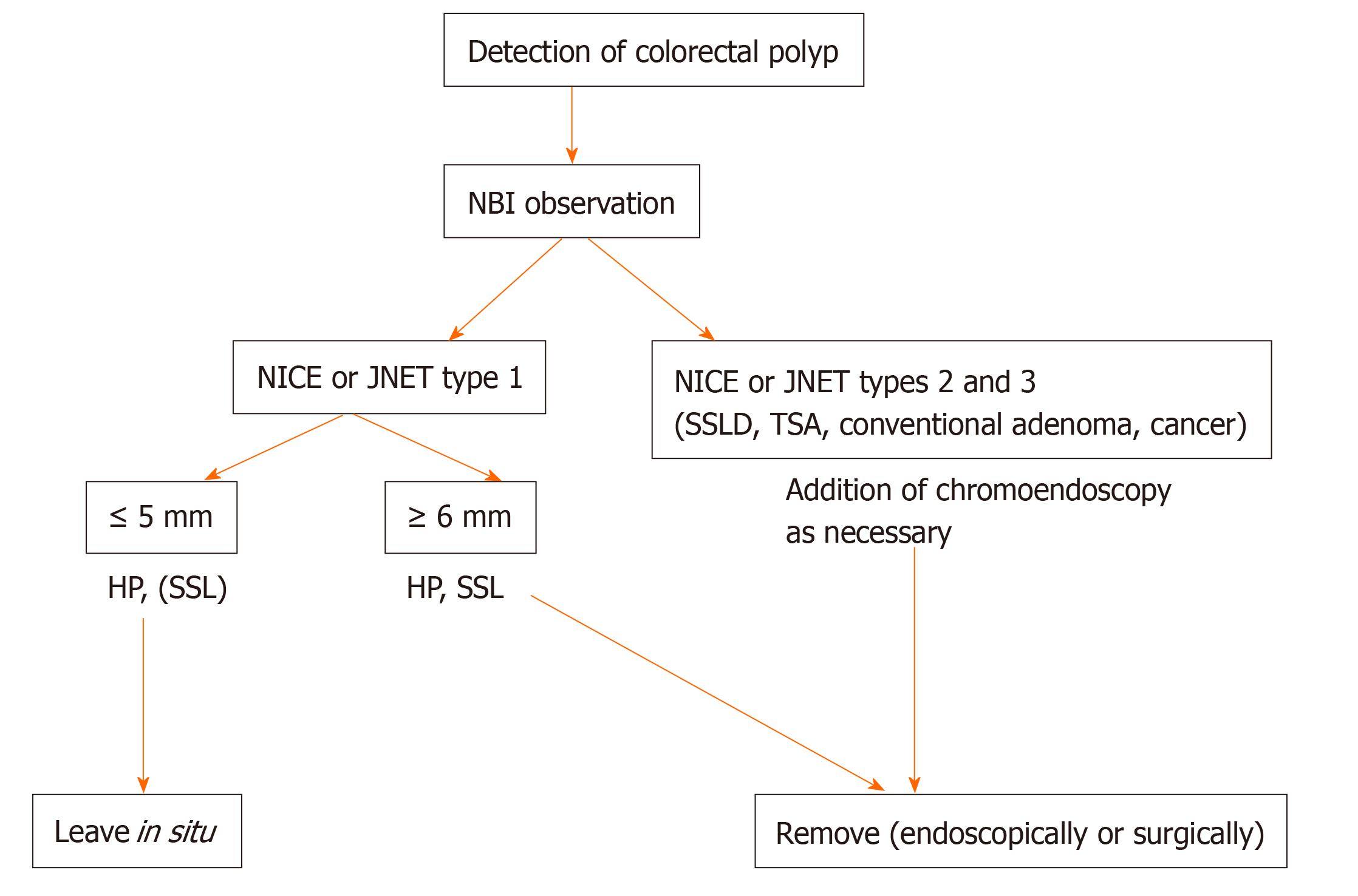
In this review, we described the clinicopathological features of colorectal serrated polyps and proposed a treatment strategy for them. When the removal of all conventional adenomas and cancers, which exhibit NICE or JNET types 2 and 3 on NBI observation, is added to our proposed treatment strategy, the treatment algorithm for colorectal polyps, including serrated polyps, using NBI observation is as follows (Figure 6): (1) Leave in situ NICE or JNET type 1 lesions, such as HPs and few SSLs, at ≤ 5 mm in size, (2) Remove NICE or JNET type 1 lesions, such as HPs and SSLs, at ≥ 6 mm in size, and (3) Remove NICE or JNET types 2 and 3 lesions, including SSLDs, TSAs, conventional adenomas, and cancers, of any size. This treatment algorithm for colorectal polyps is simple and seems feasible in clinical practice. We believe that this simple treatment strategy will result in the decline of colorectal cancer morbidity and mortality in Asian countries.
Our proposal of the treatment strategy for colorectal serrated polyps has the limitation that it is mainly based on studies of Japanese patients. Therefore, further studies are needed to evaluate the applicability of our treatment strategy in other Asian countries. We hope that in the future, treatment guidelines for colorectal serrated polyps will be established also in Asian countries, as well as surveillance guidelines based on these.
We would like to thank Dr. Kazuhito Ichikawa for capturing the histopathological pictures as well as Dr. Takahiro Fujimori for his useful advice regarding histopathological assessment.
Manuscript source: Invited Manuscript
Corresponding Author's Membership in Professional Societies: Japanese Society of Gastroenterology, No. 032993.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sun XT, Yamada A, Yu B S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ
| 1. | Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 725] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 2. | IJspeert JE, Vermeulen L, Meijer GA, Dekker E. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol. 2015;12:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Pai RK, Mäkinen MJ, Rosty C. Colorectal serrated lesions and polyps. In: Nagtegaal ID, Arends MJ, Odze RD, Lam AK. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press, 2019: 163-169. |
| 4. | Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis. In: Nagtegaal ID, Arends MJ, Odze RD, Lam AK. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press, 2010: 160-165. |
| 5. | Gaiser T, Meinhardt S, Hirsch D, Killian JK, Gaedcke J, Jo P, Ponsa I, Miró R, Rüschoff J, Seitz G, Hu Y, Camps J, Ried T. Molecular patterns in the evolution of serrated lesion of the colorectum. Int J Cancer. 2013;132:1800-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Murakami T, Akazawa Y, Yatagai N, Hiromoto T, Sasahara N, Saito T, Sakamoto N, Nagahara A, Yao T. Molecular characterization of sessile serrated adenoma/polyps with dysplasia/carcinoma based on immunohistochemistry, next-generation sequencing, and microsatellite instability testing: a case series study. Diagn Pathol. 2018;13:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | McCarthy AJ, Serra S, Chetty R. Traditional serrated adenoma: an overview of pathology and emphasis on molecular pathogenesis. BMJ Open Gastroenterol. 2019;6:e000317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Sano W, Sano Y, Iwatate M, Hasuike N, Hattori S, Kosaka H, Ikumoto T, Kotaka M, Fujimori T. Prospective evaluation of the proportion of sessile serrated adenoma/polyps in endoscopically diagnosed colorectal polyps with hyperplastic features. Endosc Int Open. 2015;3:E354-E358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 416] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 11. | Iwatate M, Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Ishikawa H, Murakami Y, Yoshida S, Saito Y; Japan NBI Expert Team (JNET). Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Hazewinkel Y, de Wijkerslooth TR, Stoop EM, Bossuyt PM, Biermann K, van de Vijver MJ, Fockens P, van Leerdam ME, Kuipers EJ, Dekker E. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Abdeljawad K, Vemulapalli KC, Kahi CJ, Cummings OW, Snover DC, Rex DK. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc. 2015;81:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW, Appleyard M, Hewett D, Togashi K, Jass JR, Leggett BA. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 16. | Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 17. | Tadepalli US, Feihel D, Miller KM, Itzkowitz SH, Freedman JS, Kornacki S, Cohen LB, Bamji ND, Bodian CA, Aisenberg J. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video). Gastrointest Endosc. 2011;74:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Nakao Y, Saito S, Ohya T, Aihara H, Arihiro S, Kato T, Ikegami M, Tajiri H. Endoscopic features of colorectal serrated lesions using image-enhanced endoscopy with pathological analysis. Eur J Gastroenterol Hepatol. 2013;25:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Hazewinkel Y, López-Cerón M, East JE, Rastogi A, Pellisé M, Nakajima T, van Eeden S, Tytgat KM, Fockens P, Dekker E. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Yamada M, Sakamoto T, Otake Y, Nakajima T, Kuchiba A, Taniguchi H, Sekine S, Kushima R, Ramberan H, Parra-Blanco A, Fujii T, Matsuda T, Saito Y. Investigating endoscopic features of sessile serrated adenomas/polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2015;82:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Yamashina T, Takeuchi Y, Uedo N, Aoi K, Matsuura N, Nagai K, Matsui F, Ito T, Fujii M, Yamamoto S, Hanaoka N, Higashino K, Ishihara R, Tomita Y, Iishi H. Diagnostic features of sessile serrated adenoma/polyps on magnifying narrow band imaging: a prospective study of diagnostic accuracy. J Gastroenterol Hepatol. 2015;30:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 22. | Kimura T, Yamamoto E, Yamano HO, Suzuki H, Kamimae S, Nojima M, Sawada T, Ashida M, Yoshikawa K, Takagi R, Kato R, Harada T, Suzuki R, Maruyama R, Kai M, Imai K, Shinomura Y, Sugai T, Toyota M. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Sano W, Fujimori T, Ichikawa K, Sunakawa H, Utsumi T, Iwatate M, Hasuike N, Hattori S, Kosaka H, Sano Y. Clinical and endoscopic evaluations of sessile serrated adenoma/polyps with cytological dysplasia. J Gastroenterol Hepatol. 2018;33:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Burgess NG, Pellise M, Nanda KS, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, P'Ng H, McLeod D, Bourke MJ. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut. 2016;65:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Murakami T, Sakamoto N, Ritsuno H, Shibuya T, Osada T, Mitomi H, Yao T, Watanabe S. Distinct endoscopic characteristics of sessile serrated adenoma/polyp with and without dysplasia/carcinoma. Gastrointest Endosc. 2017;85:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Tanaka Y, Yamano HO, Yamamoto E, Matushita HO, Aoki H, Yoshikawa K, Takagi R, Harada E, Nakaoka M, Yoshida Y, Eizuka M, Sugai T, Suzuki H, Nakase H. Endoscopic and molecular characterization of colorectal sessile serrated adenoma/polyps with cytologic dysplasia. Gastrointest Endosc. 2017;86:1131-1138.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Tate DJ, Jayanna M, Awadie H, Desomer L, Lee R, Heitman SJ, Sidhu M, Goodrick K, Burgess NG, Mahajan H, McLeod D, Bourke MJ. A standardized imaging protocol for the endoscopic prediction of dysplasia within sessile serrated polyps (with video). Gastrointest Endosc. 2018;87:222-231.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Sano Y, Saito Y, Fu KI, Matsuda T, Uraoka T, Kobayashi N, Ito H, Machida H, Iwasaki J, Emura F, Hanafusa M, Yoshino T, Kato S, Fujii T. Efficacy of magnifying chromoendoscopy for the differential diagnosis of colorectal lesions. Dig Endosc. 2005;17:105-116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Yao T, Sugai T, Iwashita A, Fujimori T, Kushima R, Nokubi M, Mitomi H, Ajioka Y, Konishi F. Histopathological characteristics and diagnostic criteria of SSA/P, from project research “potential of cancerization of colorectal serrated lesions” of the Japanese Society for Cancer of the Colon and Rectum. Stomach Intestine. 2011;46:442-448. |
| 30. | Fujimori Y, Fujimori T, Imura J, Sugai T, Yao T, Wada R, Ajioka Y, Ohkura Y. An assessment of the diagnostic criteria for sessile serrated adenoma/polyps: SSA/Ps using image processing software analysis for Ki67 immunohistochemistry. Diagn Pathol. 2012;7:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O'Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-29; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 829] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 32. | Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 33. | Yang S, Farraye FA, Mack C, Posnik O, O'Brien MJ. BRAF and KRAS Mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | O'Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, Amorosino M, Farraye FA. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 35. | Kim KM, Lee EJ, Ha S, Kang SY, Jang KT, Park CK, Kim JY, Kim YH, Chang DK, Odze RD. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol. 2011;35:1274-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | O'Brien MJ, Winawer SJ, Zauber AG, Gottlieb LS, Sternberg SS, Diaz B, Dickersin GR, Ewing S, Geller S, Kasimian D. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98:371-379. [PubMed] |
| 37. | Gschwantler M, Kriwanek S, Langner E, Göritzer B, Schrutka-Kölbl C, Brownstone E, Feichtinger H, Weiss W. High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol. 2002;14:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Sakamoto T, Matsuda T, Nakajima T, Saito Y. Clinicopathological features of colorectal polyps: evaluation of the 'predict, resect and discard' strategies. Colorectal Dis. 2013;15:e295-e300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A, Hazewinkel Y, Jover R, Kalager M, Loberg M, Pox C, Rembacken B, Lieberman D; European Society of Gastrointestinal Endoscopy. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 40. | Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, Robertson DJ, Shaukat A, Syngal S, Rex DK. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 41. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, McKeone D, Pearson SA, Leggett B, Whitehall V. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 42. | Huang CS, O'brien MJ, Yang S, Farraye FA. Hyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathway. Am J Gastroenterol. 2004;99:2242-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Kim KM, Lee EJ, Kim YH, Chang DK, Odze RD. KRAS mutations in traditional serrated adenomas from Korea herald an aggressive phenotype. Am J Surg Pathol. 2010;34:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |