Published online Jan 7, 2020. doi: 10.3748/wjg.v26.i1.97
Peer-review started: October 31, 2019
First decision: November 22, 2019
Revised: December 4, 2019
Accepted: December 14, 2019
Article in press: December 14, 2019
Published online: January 7, 2020
Processing time: 67 Days and 21.5 Hours
Autotaxin (ATX) has been reported as a direct biomarker for estimating the evaluation of liver fibrosis. But available data on ATX as a useful biomarker for the complications of liver cirrhosis (LC) are scant.
To assess the clinical usefulness of ATX for assessing the complications of LC.
This multicenter, retrospective study was conducted at six locations in Japan. We include patients with LC, n = 400. The ATX level was evaluated separately in men and women because of its high level in female patients. To assess the clinical usefulness of ATX for the complications of LC, the area under the curve (AUC) of ATX assessing for the severe complications was analyzed in comparison with the model for end-stage liver disease score, albumin-bilirubin (ALBI) score, fibrosis-4 index, and aspartate aminotransferase-to-platelet ratio index.
The mean age was 68.4 ± 11.4 years, 240 patients (60.0%) were male. A total of 213 (53.3%) and 187 (46.8%) patients were compensated and decompensated, respectively. The numbers of patients with varix rupture, hepatic ascites, and hepatic encephalopathy were 35 (8.8%), 131 (32.8%), and 103 (25.8%), respectively. The AUCs of ATX in men for hepatic encephalopathy, hepatic ascites, and varix ruptures were 0.853, 0.816, and 0.706, respectively. The AUCs of ATX in women for hepatic encephalopathy, hepatic ascites, and varix rupture were 0.759, 0.717, and 0.697, respectively. The AUCs of ATX in men were higher than those in women, as were all the other biomarkers used to detect encephalopathy and varix ruptures. However, for detecting ascites, the AUC of ALBI in men was more effective than using ATX.
ATX in men was more effective than any other biomarkers for detecting hepatic encephalopathy and varix ruptures.
Core tip: Autotaxin (ATX) has been reported as a novel biomarker for estimating liver fibrosis stages, and researchers have reported that this fibrosis marker correlated closely with more advanced fibrosis. Especially, the assessment for the complications of liver cirrhosis (LC) is valuable in helping to make treatment decisions. However, data on the clinical usefulness of novel fibrosis markers in assessing the complications of LC are scant. This study showed that ATX is a useful biomarker for assessing the complications of LC. Especially, the use of ATX in men was more effective than any other biomarker for detecting hepatic encephalopathy and varix ruptures.
- Citation: Shao X, Uojima H, Setsu T, Okubo T, Atsukawa M, Furuichi Y, Arase Y, Hidaka H, Tanaka Y, Nakazawa T, Kako M, Kagawa T, Iwakiri K, Terai S, Koizumi W. Usefulness of autotaxin for the complications of liver cirrhosis. World J Gastroenterol 2020; 26(1): 97-108
- URL: https://www.wjgnet.com/1007-9327/full/v26/i1/97.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i1.97
Progression of liver disease to cirrhosis, liver failure, hepatic cellular cancer, varix, or ascites is correlated with the histological change in liver fibrosis[1]. Liver biopsy is the standard procedure to assess the stages of liver fibrosis and is well known to be diagnostically accurate. However, liver biopsy is not an ideal method for the diagnosis of liver fibrosis because the small sample volume with each biopsy leads to sampling errors[2]. Furthermore, liver biopsy is an invasive procedure, with a significantly high risk of life-threatening complications for patients with advanced liver cirrhosis (LC)[3,4]. These limitations have led to the development of serum biomarkers for the assessment of liver fibrosis in recent years. Serum biomarkers are categorized into direct and indirect biomarkers whether or not they reflect extracellular matrix (ECM) turnover. Direct biomarkers have clinical values involving both the evaluation of liver fibrosis and monitoring the behavior of fibrogenesis and ECM metabolism[5]. Autotaxin (ATX) has been reported as a direct biomarker for estimating the evaluation of liver fibrosis. This biomarker, ATX, also known as ectonucleotide pyrophosphatase/phosphodiesterase 2, is a secreted lysophospholipase D that hydrolyzes lysophosphatidylcholine to generate lysopho-sphatidic acid, a lipid mediator that activates G-protein-coupled receptors to evoke various cellular responses[6,7].
Because ATX is subsequently cleared from the circulation in liver sinusoidal endothelial cells, liver fibrosis reduces the capacity to metabolize ATX, resulting in deterioration of liver function, which in turn leads to an increase in the serum ATX level[7-9]. According to the progression of fibrosis, the degree of impairment of the uptake of various substances in the liver sinusoidal endothelium was exacerbated. Especially, for decompensated LC with ascites, hepatic encephalopathy, and varix rupture, impairment of the uptake of the phenotypic changes in liver sinusoidal endothelial cells leads to an extreme reduction[10].
Developments of serum biomarkers have focused on the diagnosis of cirrhosis, but more recent researches have emphasized the availability of these markers to assess patients with more advanced fibrosis[5]. The ATX level may be a useful biomarker to select treatment therapy for ascites, hepatic encephalopathy, and varix ruptures. And the assessment for the complications of LC is especially valuable in helping to make treatment decisions[11]. However, available data on ATX as a useful biomarker for the complications of LC are scant. The aim of this study was to assess the clinical usefulness of ATX for assessing the complications of LC.
This study was approved by the Institutional Review Board Ethics Committee of the Tokushukai Medical Group (No. TGE01178-024), and the protocol for this study conforms to the provisions of the Declaration of Helsinki. This study is registered in the UMIN Clinical Trials Registry as UMIN000036221. This study is a retrospective observational study. Informed consent was obtained from all individual participants included in the study by the opt-out method of our hospital website.
This multicenter, retrospective study was comprised of 400 patients with LC conducted at six locations in Japan. Cirrhosis in these patients was caused by hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic liver disease, nonalcoholic steatohepatitis, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, the Budd-Chiari syndrome, and chronic right-sided heart failure. Patients with poorly controlled heart failure, severe renal dysfunction, and malignancies other than HCC were excluded.
Diagnosis of LC was based on noninvasive imaging using computed tomography and/or magnetic resonance imaging revealing a hepatic cirrhotic appearance, portosystemic collaterals, an enlarged spleen, esophageal varices, and/or nonmalignant ascites. Ultrasound-guided liver biopsy was not conducted for histological assessment.
Serum concentrations of ATX were measured using a two-site enzyme immunoassay and an automated immunoassay analyzer (Tosoh Corp., Tokyo, Japan). The assay reagent was compatible with a commercial automated immunoassay analyzer AIA System (Tosho Corp., Tokyo Japan). This system included automated 10 μL of specimen dispensation, incubation of the reaction cup, bound-free washing, 4-methylumbelliferyl phosphate substrate dispensation and fluorometric detection[8-10].
Indirect biomarkers for the aspartate aminotransferase (AST)-to-platelet ratio index (APRI), the fibrosis-4 (Fib-4) index, the albumin-bilirubin (ALBI) score, and the model for end-stage liver disease (MELD) score were calculated as: APRI = [AST (35 IU/L)/platelets (103/μL)] × 100; Fib-4 index = [AST (IU/L) × age (years)]/[alanine aminotransferase (ALT; IU/L)1/2 × platelets (103/μL)]; ALBI score = −0.085 × [albumin (g/L)] + 0.66 × log10 [serum bilirubin (μmol/L)][12]; and MELD score = 3.78 × loge [serum bilirubin (mg/dL)] + 11.2 × loge [PT-international normalized ratio] + 9.57 × loge [serum creatinine (mg/dL)] + 6.43[13].
To assess the clinical usefulness of ATX for the complications of LC, we examined ATX levels in cirrhotic patients with severe complications and compared the area under the curve (AUC) of serum biomarkers assessing for the complications of LC. Severe complications were defined as those of a decompensated phase, such as hepatic ascites, hepatic encephalopathy, and varix ruptures according to the guidelines by the American Association for the Study of Liver Diseases[14]. We also determined if ATX was associated with other serum biomarkers or other laboratory data. Correlations between the ATX score in LC and the characteristics were determined using Pearson’s r coefficient. Laboratory tests were conducted to measure the white blood cells, hemoglobin, platelets, prothrombin time, serum albumin, blood urea nitrogen, serum creatinine, AST, ALT, total bilirubin, serum sodium, serum potassium, hemoglobin A1c, total cholesterol, the branched-chain amino acid and tyrosine ratio, ammonia, and alpha-fetoprotein levels. All laboratory data were obtained on the same day as the ATX measurements.
Data were analyzed using SPSS version 24.0 (IBM Corp., Armonk, NY, United States). All data were expressed as mean ± SD. Continuous variables in Child Pugh class (CPC) were compared using Tukey’s honestly significant difference (HSD) test, and unpaired groups were compared using the unpaired t-test. Receiver operating characteristic (ROC) plots were constructed to establish sensitivity-specificity relationships. All differences with a P value of < 0.05 were considered significant.
A total of 400 patients with LC were enrolled. The baseline clinical characteristics of the 400 patients are summarized in Table 1. The mean age was 68.4 ± 11.4 years (range 22–93 years), 240 patients (60.0%) were male, and 84 patients (21.0%) had liver cancer. The mean body weight was 62.8 ± 13.3 kg (range 31.5–97.0 kg), and the mean body mass index was 24.0 ± 4.11 (range 13.7–41.4). LC was caused by hepatic (n = 180) and nonhepatic viruses (n = 220). A total of 213 (53.3%) and 187 (46.8%) patients were compensated and decompensated, respectively. The numbers (and proportions) of decompensated LC in varix rupture, hepatic ascites, and hepatic encephalopathy were 35 (8.8%), 131 (32.8%), and 103 (25.8%), respectively.
| Characteristics | Value |
| Number of patients, n | 400 |
| Age, yr | 68.4 ± 11.4 |
| Gender: Male, n (%) | 240 (60.0) |
| Weight, kg | 62.8 ± 13.3 |
| Body mass index, kg/m2 | 24.0 ± 4.11 |
| Aetiology: HCV/HBV/Alcohol/NASH/AIH/PBC/etc., n | 130/50/108/60/18/9/25 |
| HCV: SVR/non-SVR, n | 84/46 |
| Liver cancer, n (%) | 84 (21) |
| Diabetes mellitus, n (%) | 124 (31.0) |
| Hypertension, n (%) | 120 (30.0) |
| Compensated/Decompensated, n | 213/187 |
| Hepatic ascites, n (%) | 131 (32.8) |
| Hepatic encephalopathy, n (%) | 103 (25.8) |
| Varicose vein rupture, n (%) | 35 (8.8) |
| Child-Pugh score | 6.62 ± 1.88 |
| Child-Pugh class A/B/C, n | 232/127/41 |
| MELD-score | 7.16 ± 4.48 |
| ALBI score | -2.29 ± 0.68 |
| White blood cells, /μL | 4845 ± 3609 |
| Hemoglobin, g/dL | 12.5 ± 2.29 |
| Platelets, × 104/μL | 12.1 ± 6.53 |
| Prothrombin time, % | 75.9 ± 20.2 |
| Serum albumin, g/dL | 3.66 ± 0.72 |
| Blood urea nitrogen, mg/dL | 17.4 ± 9.9 |
| Serum creatinine, mg/dL | 0.97 ± 0.68 |
| Aspartate aminotransferase, IU/L | 39.4 ± 23.5 |
| Alanine aminotransferase, IU/L | 28.1 ± 18.7 |
| Total bilirubin, mg/dL | 1.25 ± 0.97 |
| Serum sodium, mEq/L | 139 ± 2.75 |
| Hemoglobin A1c, % | 5.94 ± 1.18 |
| Total cholesterol, mg/dL | 176 ± 40.8 |
| Branched chain amino acid | 4.97 ± 2.24 |
| Ammonia, μg/dL | 52.3 ± 40.9 |
| Alpha-fetoprotein, ng/mL | 54.4 ± 564 |
The average ATX levels (mg/L) were 1.58 ± 0.68 in men and 1.99 ± 0.73 in women. A significantly higher ATX level was observed in women (P < 0.001).
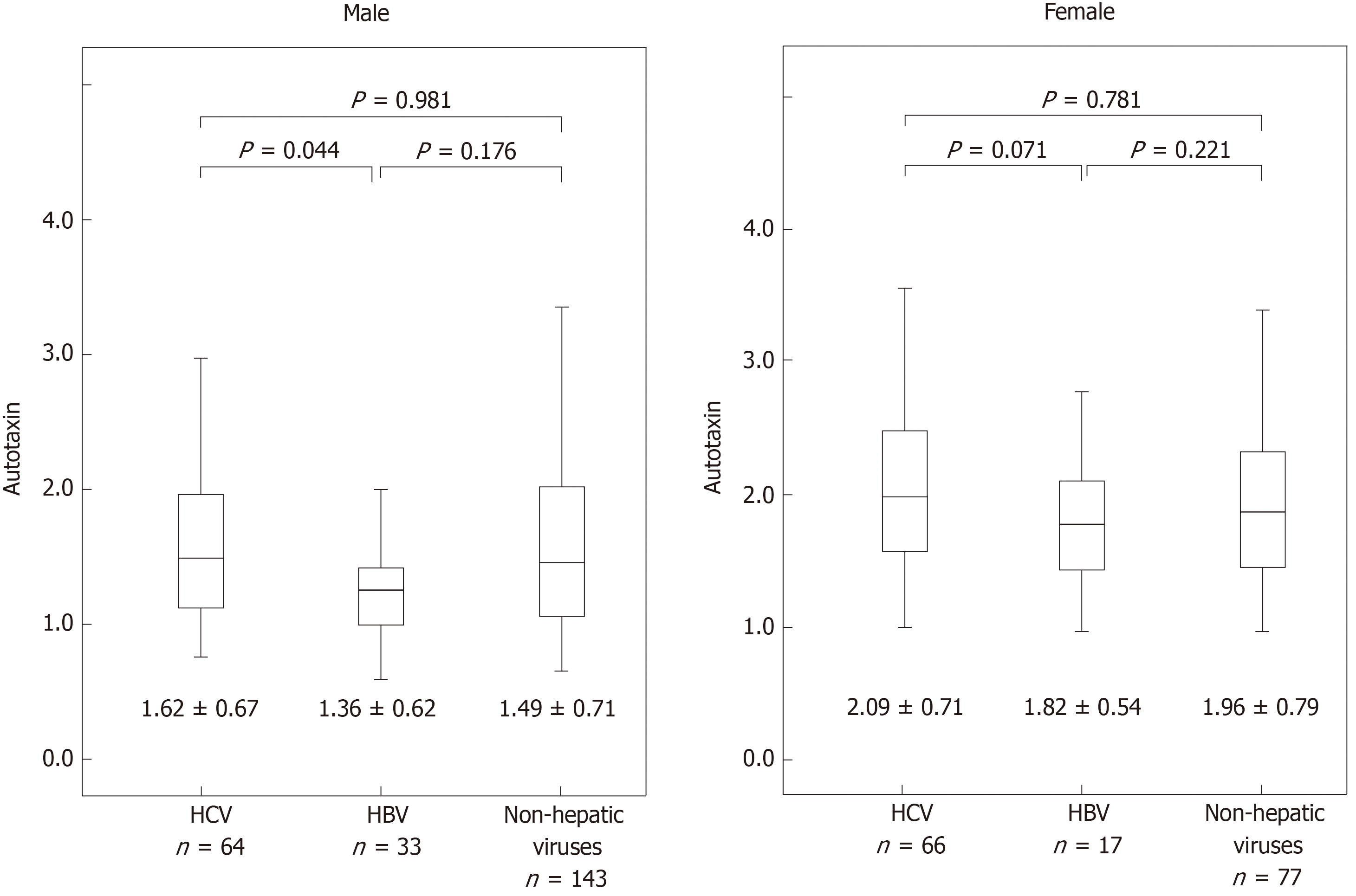
The average ATX levels in men and women were 1.62 ± 0.67 and 2.09 ± 0.71 mg/L for the HCV group, 1.36 ± 0.62 and 1.82 ± 0.54 mg/L for the HBV group, and 1.49 ± 0.71 and 1.96 ± 0.79 mg/L for the nonviral group, respectively. Tukey’s HSD test confirmed that the ATX levels in men were significantly different in the HCV and HBV groups (P = 0.044). However, the ATX levels were not significantly different in the other groups (Figure 1).
We analyzed the proportion of patients with different ATX levels stratified by CPC. The numbers (and proportions) of cirrhosis in CPCs A, B, and C were 232 (58.0%), 127 (31.7%), and 41 (10.3%), respectively. The average ATX levels in men and women were 1.23 ± 0.39 and 1.76 ± 0.67 mg/L, respectively, for CPC A, 1.88 ± 0.59 and 2.21 ± 0.66 mg/L, respectively, for CPC B, and 2.68 ± 0.81 and 2.74 ± 0.62 mg/L, respectively, for CPC C. Tukey’s HSD test confirmed that the ATX levels in both men and women increased significantly with increasing CPCs (P < 0.001).
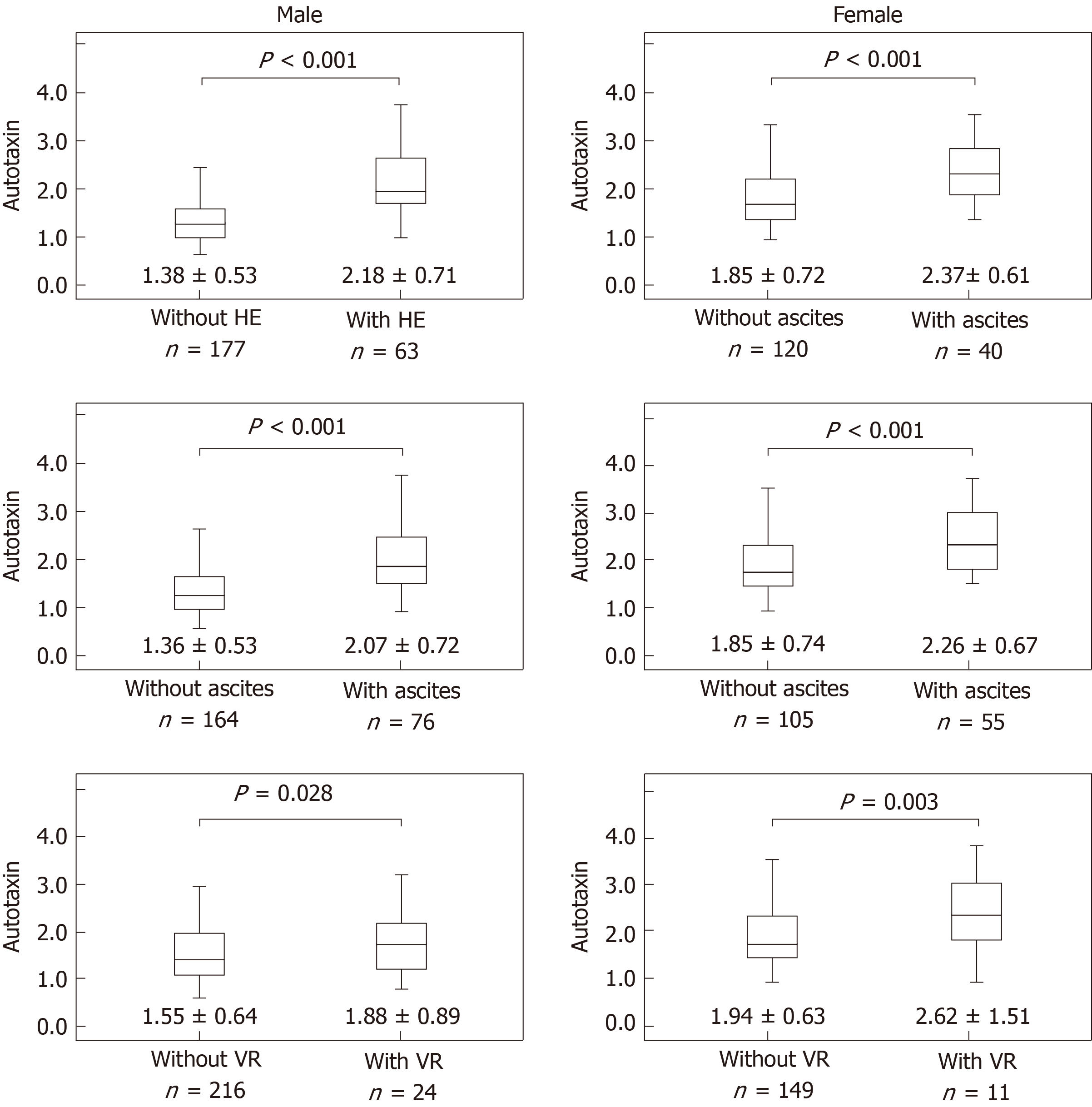
The average ATX levels (mg/L) in men and women were 1.21 ± 0.40 and 1.73 ± 0.57, respectively, for the compensated group and 1.98 ± 0.71 and 2.33 ± 0.80, respectively, for the decompensated group. A significantly higher ATX level in men and women was observed in the decompensated group (P < 0.001). The average ATX levels in men with varix rupture, hepatic ascites, and hepatic encephalopathy were 1.88 ± 0.89, 2.07 ± 0.72, and 2.18 ± 0.71, respectively. By contrast, the average ATX levels (mg/L) in men without varix rupture, hepatic ascites, and hepatic encephalopathy were 1.55 ± 0.64, 1.36 ± 0.53, and 1.38 ± 0.53, respectively (Figure 2). A significantly higher ATX levels in men were observed in patients with varix rupture, hepatic ascites, and hepatic encephalopathy (P = 0.028; P < 0.001; P < 0.001, respectively). The average ATX levels (mg/L) in women with varix ruptures, hepatic ascites, and hepatic encephalopathy were 2.62 ± 1.51, 2.26 ± 0.67, and 2.37 ± 0.61, respectively. By contrast, the average ATX levels in women without varix rupture, hepatic ascites, and hepatic encephalopathy were 1.94 ± 0.63, 1.85 ± 0.74, and 1.85 ± 0.72, respectively. Significantly higher ATX levels in women were observed in patients with varix rupture, hepatic ascites, and hepatic encephalopathy (P = 0.003; P < 0.001; P < 0.001, respectively).
The average ATX levels (mg/L) in men and women were 1.65 ± 0.40 and 2.02 ± 0.68, respectively, for patients with HCC and 1.56 ± 0.54 and 1.97 ± 0.76, respectively, for patients without HCC. However, the ATX levels were not significantly different among patients with and those without HCC in men and women (Men: P = 0.178, women P = 0.215).
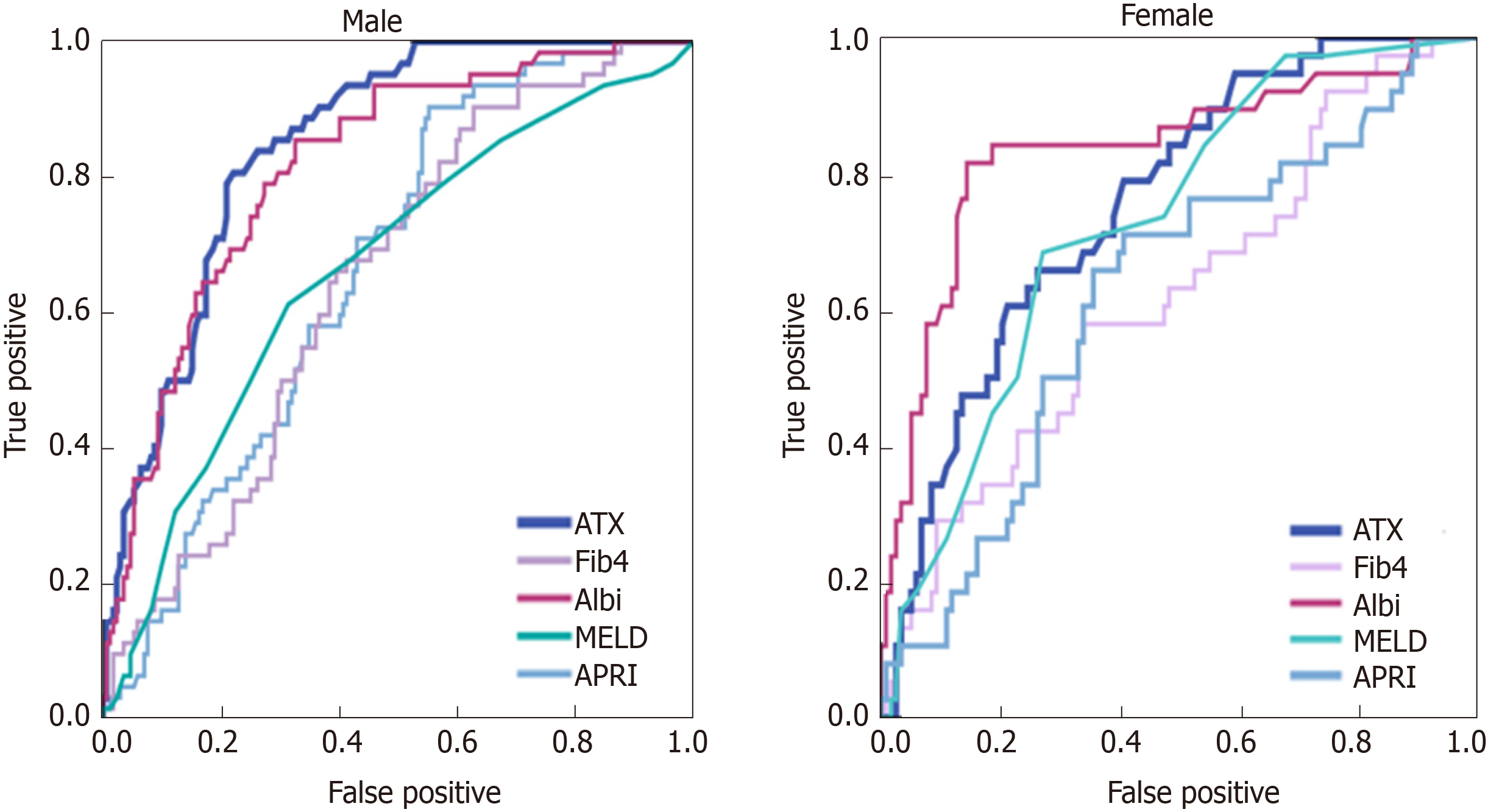
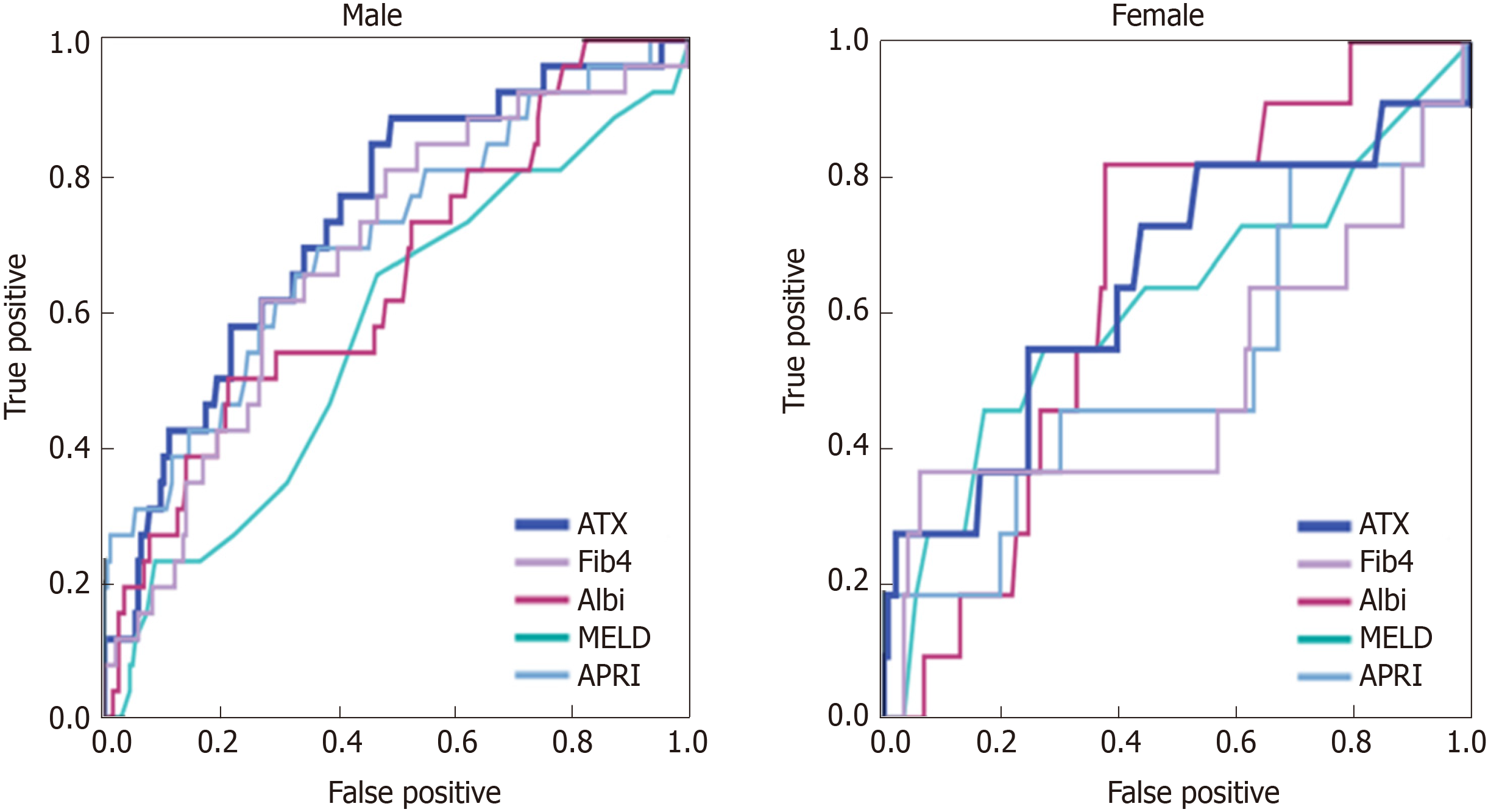
ATX for the assessment of complications of LC was analyzed in comparison with the MELD score, ALBI score, Fib-4 index, and APRI (Table 2). The AUCs of ATX for hepatic encephalopathy were 0.853 [95% confidence interval (CI): 0.795–0.911] in men and 0.759 (95%CI: 0.658–0.860) in women (Figure 3). The AUCs of ATX in men were higher than those in women and higher than those using other biomarkers for detecting encephalopathy. The AUCs of ATX for hepatic ascites were 0.816 (95%CI: 0.756–0.877) in men and 0.717 (95%CI: 0.616–0.819) in women. The AUCs of ATX in men were higher than those in women and higher than those using other serum biomarkers for detecting hepatic ascites. The AUCs of ALBI in men were higher than those using other biomarkers for detecting ascites. The AUCs of ATX for varix rupture were 0.706 (95%CI: 0.558–0.855) in men and 0.697 (95%CI: 0.512–0.882) in women. The AUCs of ATX in men were higher than those in women and higher than those using other serum biomarkers for detecting varix rupture (Figure 4).
| Item | Parameter | Male | Female |
| AUC (95%CI) | AUC (95%CI) | ||
| Hepatic encephalopathy | ATX | 0.853 (0.795-0.911) | 0.759 (0.658-0.860) |
| MELD | 0.644 (0.563-0.725) | 0.727 (0.640-0.814) | |
| Fib 4 index | 0.651 (0.568-0.734) | 0.674 (0.538-0.789) | |
| APRI | 0.670 (0.590-0.750) | 0.640 (0.516-0.778) | |
| ALBI | 0.825 (0.757-0.893) | 0.815 (0.685-0.811) | |
| Hepatic ascites | ATX | 0.816 (0.756-0.877) | 0.717 (0.616-0.819) |
| MELD | 0.640 (0.560-0.720) | 0.744 (0.665-0.823) | |
| Fib 4 index | 0.654 (0.569-0.738) | 0.653 (0.540-0.765) | |
| APRI | 0.663 (0.581-0.744) | 0.633 (0.520-0.746) | |
| ALBI | 0.863 (0.807-0.918) | 0.787 (0.690-0.878) | |
| Varix rupture | ATX | 0.706 (0.558-0.855) | 0.697 (0.512-0.882) |
| MELD | 0.514 (0.386-0.643) | 0.612 (0.413-0.810) | |
| Fib 4 index | 0.619 (0.462-0.775) | 0.566 (0.299-0.833) | |
| APRI | 0.653 (0.474-0.833) | 0.579 (0.333-0.826) | |
| ALBI | 0.608 (0.449-0.768) | 0.614 (0.524-0.803) |
Table 3 shows the correlation between ATX and clinical characteristics. The MELD score, Fib-4 index, APRI, and ALBI score were correlated with the ATX level in both men and women. The correlation coefficient between ALBI and ATX in men was strong. We also evaluated the correlation between ATX and clinical laboratory data. Platelets, prothrombin time, serum albumin, AST, ALT, total bilirubin, total cholesterol, branched-chain amino acid (BCAA), tyrosine (TYR), the BCAA-to-TYR ratio and ammonia were correlated with ATX levels in both men and women. The correlation coefficient between TYR and ATX in men was strong.
| Characteristics | Male | Female | ||
| r | P value | r | P value | |
| Serum biomarker | ||||
| MELD-score | 0.293 | 0.001 | 0.211 | 0.040 |
| Fibrosis 4 index | 0.470 | < 0.001 | 0.335 | < 0.001 |
| APRI | 0.438 | 0.001 | 0.414 | < 0.001 |
| ALBI | 0.614 | < 0.001 | 0.452 | < 0.001 |
| Clinical laboratory data | ||||
| White blood cells | -0.123 | 0.057 | -0.130 | 0.102 |
| Hemoglobin | -0.260 | 0.001 | -0.268 | 0.001 |
| Platelets | -0.289 | 0.001 | -0.308 | 0.001 |
| Prothrombin time | -0.443 | < 0.001 | -0.279 | 0.001 |
| Serum albumin | -0.539 | < 0.001 | -0.451 | < 0.001 |
| Blood urea nitrogen | -0.093 | 0.152 | -0.123 | 0.716 |
| Serum creatinine | -0.036 | 0.580 | -0.139 | 0.387 |
| Aspartate aminotransferase | 0.443 | 0.001 | 0.329 | 0.001 |
| Alanine aminotransferase | 0.225 | 0.022 | 0.249 | 0.001 |
| Total bilirubin | 0.538 | < 0.001 | 0.300 | 0.001 |
| Serum sodium | -0.282 | 0.001 | -0.192 | 0.015 |
| Hemoglobin A1c | -0.151 | 0.109 | -0.094 | 0.282 |
| Total cholesterol | -0.244 | 0.001 | -0.231 | 0.001 |
| BTR | -0.596 | 0.004 | -0.298 | 0.003 |
| Branched chain amino acid | -0.338 | 0.001 | -0.260 | 0.374 |
| Tyrosine | 0.585 | < 0.001 | 0.455 | < 0.001 |
| Ammonia | 0.369 | < 0.001 | 0.407 | < 0.001 |
| Alpha-fetoprotein | 0.074 | 0.269 | -0.051 | 0.549 |
To the best of our knowledge, this is the first report that directly compared the levels of serum liver fibrosis markers and ATX in patients with the complications of LC. Direct biomarkers reflect not only hepatic fibrosis but also hepatic function[5]. We observed that ATX is a useful biomarker for assessing the complications of LC. Especially, the performance of ATX in men was better than that in women and of the ALBI score, Fib-4 index, and APRI in detecting hepatic encephalopathy and varix ruptures. These results demonstrated that ATX levels may be helpful for detecting clinically evident decompensating events due to portal hypertension.
Portal hypertension is caused by increased portal venous flow and/or enhanced intrahepatic vascular resistance resulting from the activation of hepatic stellate cells and the dysfunction of endothelial cells[10]. Because endothelial dysfunction lead to decrease ATX clearance, ATX may indicate portal hypertension. Pleli et al[10] reported a possible causative link between the extent of portal hypertension and ATX levels. Especially, patients suffering from portal hypertensive gastropathy and hepatic encephalopathy showed significantly higher ATX serum concentrations. Increased portal venous flow or enhanced intrahepatic vascular resistance lead to diversion of blood away from the liver toward low-resistance portosystemic vessels[10,15]. Portal hypertensive collateral formation leads to “varices” that are dilated end-organ veins with a high risk of rupture, as well as “shunts” that may lead to recurrent and refractory hepatic encephalopathy[16]. By contrast, for detecting ascites, the AUC of ATX was lower than that using ALBI. The significance of albumin in ascites has been established since the 1940s[17]. Cirrhotic patients with hypoalbuminemia developed ascites while patients without hypoalbuminemia did not[18,19]. The ALBI grades, which are calculated from albumin and serum bilirubin, may be indicated better than those of any other biomarkers for detecting hepatic ascites.
Pleli et al[10] reported serum levels of ATX levels from subjects with LC were elevated compared to healthy control subjects, and serum ATX levels correlated with the Child-Pugh score in predicting the severity of the disease. That is, dysfunction of endothelial cells from the progression of fibrosis lead to reduced ATX clearance and increased serum ATX. However, the mechanism for the high levels of ATX could be complicated. A unique aspect of ATX is its high level in female patients, but the exact mechanism is unknown[20-22]. Sex-dependent differences in serum ATX levels need to be considered when using the ATX level as a marker of liver fibrosis. In the present study, ATX was more accurate in male patients than in female patients as a liver fibrosis marker for detecting liver disease severity. ATX levels in women may overestimate liver disease severity compared with any other serum biomarkers.
Furthermore, previous reports indicated that elevated ATX levels were associated with inflammatory liver damage[23-25]. In the present study, the ATX levels for men in the HBV and HCV groups differed slightly (P = 0.044). The reason for this may correlate with the different mechanisms for liver inflammation from HBV and HCV. Patients with chronic HCV infection exhibited persistent inflammatory responses and fibrogenesis throughout the clinical course even after progression to LC[26]. Meanwhile, chronic HBV infection was quiescent inflammation because of seroconversion from HBe antigen to HBe antibody in most cases[27]. Therefore, suppression of liver inflammation after the eradication of HCV may lead to a better clinical outcome in patients with HCV who have an elevated ATX fibrosis marker. In point of fact, the ATX levels of patients with eradicated HCV through direct-acting antiviral therapy were comparable to those of uninfected patients[28].
This study had some limitations. First, this was a retrospective study. Second, this study included 400 patients with chronic liver diseases whose etiologies were not uniform. We made ATX comparisons among HBV, HCV, and nonviral groups. However, the proportion of patients with complications of LC was different among these three etiologies. Third, the gold standard for assessment of the severity of portal hypertension is the hepatic venous pressure gradient (HVPG). Regrettably, however, the correlation between ATX and HVPG was unknown because these data were not evaluated for all the enrolled patients due to it being a retrospective study. Future studies on the HVPG may make it the first-choice biomarker for the assessment of portal hypertension. Fourth, this study was a multiple center study. Therefore, there may have been inconsistencies between the experimental equipment and standardization.
In conclusion, our study revealed that ATX is a useful clinical biomarker for assessing the complications of LC because it could reflect not only hepatic fibrosis but also hepatic function. Using ATX as a biomarker in men was more efficacious than that of any other biomarkers for hepatic encephalopathy and varix ruptures. To make treatment decisions, it is necessary to consider that patients with high ATX levels may have complications of LC.
Developments of serum biomarkers have focused on the diagnosis of cirrhosis, but more recent researches have emphasized the availability of these markers to assess patients with more advanced fibrosis.
The autotaxin (ATX) level may be a useful biomarker to select treatment therapy for ascites, hepatic encephalopathy, and varix ruptures. And the assessment for the complications of liver cirrhosis (LC) is especially valuable in helping to make treatment decisions.
The aim of this study was to assess the clinical usefulness of ATX for assessing the complications of LC.
This multicenter, retrospective study was conducted at six locations in Japan. We include patients with LC, n = 400. The ATX level was evaluated separately in men and women because of its high level in female patients. To assess the clinical usefulness of ATX for the complications of LC, the area under the curve (AUC) of ATX assessing for the severe complications was analyzed in comparison with the model for end-stage liver disease score, albumin-bilirubin (ALBI) score, fibrosis-4 index, and aspartate aminotransferase-to-platelet ratio index.
The AUCs of ATX in men for hepatic encephalopathy, hepatic ascites, and varix ruptures were 0.853, 0.816, and 0.706, respectively. The AUCs of ATX in women for hepatic encephalopathy, hepatic ascites, and varix rupture were 0.759, 0.717, and 0.697, respectively. The AUCs of ATX in men were higher than those in women, as were all the other biomarkers used to detect encephalopathy and varix ruptures. However, for detecting ascites, the AUC of ALBI in men was more effective than using ATX.
ATX is a useful biomarker for assessing the complications of LC. Especially, the use of ATX in men was more effective than any other biomarker for detecting hepatic encephalopathy and varix ruptures. The ATX level is especially valuable in helping to make treatment decisions for hepatic encephalopathy and varix ruptures. ATX in men was more effective than any other biomarkers for detecting hepatic encephalopathy and varix ruptures. Developments of serum biomarkers have focused on the diagnosis of cirrhosis and the assessment of advanced fibrosis. Using ATX as a biomarker in men was more efficacious than that of any other biomarkers for hepatic encephalopathy and varix ruptures. To make treatment decisions, it is necessary to consider that patients with high ATX levels may have complications of LC. ATX is a useful clinical biomarker for assessing the complications of LC because it could reflect not only hepatic fibrosis but also hepatic function. Direct biomarkers reflect not only hepatic fibrosis but also hepatic function. The gold standard for assessment of the severity of portal hypertension is the hepatic venous pressure gradient (HVPG). Future studies on the HVPG may make it the first-choice biomarker for the assessment of portal hypertension.
Direct biomarkers reflect not only hepatic fibrosis but also hepatic function. The gold standard for assessment of the severity of portal hypertension is the HVPG. Future studies on the HVPG may make it the first-choice biomarker for the assessment of portal hypertension. The best method is a direct comparison of the ATX and HVPG for assessing the complications of LC.
We thank Ayumu Sugitani, of the Institute of Biomedical Research, Sapporo Higashi Tokushukai Hospital, Hokkaido, Japan, for assistance with the statistical analyses, and Robert E Brandt, Founder, CEO, and CME of MedEd Japan, for editing and formatting the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bourgoin SG, Chen GX, Song B S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1576] [Article Influence: 98.5] [Reference Citation Analysis (1)] |
| 2. | Romanelli RG, Stasi C. Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr Drug Targets. 2016;17:1804-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Neuman MG, Cohen LB, Nanau RM. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem. 2016;49:302-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, Gotoh M, Narimatsu H, Korenaga M, Mizokami M, Nishie A, Aishima S, Maehara Y. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25:218-231. [PubMed] |
| 6. | Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524-2529. [PubMed] |
| 7. | Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 906] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 8. | Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 590] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Pleli T, Martin D, Kronenberger B, Brunner F, Köberle V, Grammatikos G, Farnik H, Martinez Y, Finkelmeier F, Labocha S, Ferreirós N, Zeuzem S, Piiper A, Waidmann O. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis--a prospective cohort study. PLoS One. 2014;9:e103532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 12. | Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Kawamura T, Yamago H, Suga Y, Miyamoto Y, Tomida H, Azemoto N, Mori K, Miyata H, Ninomiya T, Kawasaki H. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 13. | Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1431] [Article Influence: 178.9] [Reference Citation Analysis (3)] |
| 15. | Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Philips CA, Rajesh S, Augustine P, Padsalgi G, Ahamed R. Portosystemic shunts and refractory hepatic encephalopathy: patient selection and current options. Hepat Med. 2019;11:23-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Walayat S, Martin D, Patel J, Ahmed U, N Asghar M, Pai AU, Dhillon S. Role of albumin in cirrhosis: from a hospitalist's perspective. J Community Hosp Intern Med Perspect. 2017;7:8-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Wood LJ, Colman J, Dudley FJ. The relationship between portal pressure and plasma albumin in the development of cirrhotic ascites. J Gastroenterol Hepatol. 1987;2:525-531. [DOI] [Full Text] |
| 19. | Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, Torres M, Humbert P, Rimola A, Llach J. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 412] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Ando W, Yokomori H, Kaneko F, Kaneko M, Igarashi K, Suzuki H. Serum Autotaxin Concentrations Reflect Changes in Liver Stiffness and Fibrosis After Antiviral Therapy in Patients with Chronic Hepatitis C. Hepatol Commun. 2018;2:1111-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, Ohta H, Yamori T, Watanabe M, Chun J, Arai H. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279:17634-17639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Nakagawa H, Ikeda H, Nakamura K, Ohkawa R, Masuzaki R, Tateishi R, Yoshida H, Watanabe N, Tejima K, Kume Y, Iwai T, Suzuki A, Tomiya T, Inoue Y, Nishikawa T, Ohtomo N, Tanoue Y, Omata M, Igarashi K, Aoki J, Koike K, Yatomi Y. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta. 2011;412:1201-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Nakamura K, Ohkawa R, Okubo S, Tozuka M, Okada M, Aoki S, Aoki J, Arai H, Ikeda H, Yatomi Y. Measurement of lysophospholipase D/autotaxin activity in human serum samples. Clin Biochem. 2007;40:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436-39442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 602] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 25. | Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822-25830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 26. | van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradère JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015-5022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 441] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 27. | Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, Paik YH, Lee KS, Park YN, Han KH. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Shimamatsu K, Kage M, Nakashima O, Kojiro M. Pathomorphological study of HCV antibody-positive liver cirrhosis. J Gastroenterol Hepatol. 1994;9:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |