Published online Feb 14, 2019. doi: 10.3748/wjg.v25.i6.683
Peer-review started: November 14, 2018
First decision: December 28, 2018
Revised: January 10, 2019
Accepted: January 20, 2019
Article in press: January 21, 2019
Published online: February 14, 2019
Obesity worsens inflammatory organ injury in acute pancreatitis (AP), but there is no effective preventive strategy. Sheng-jiang powder (SJP) has been shown to alleviate multiple-organ inflammatory injury in rats with high-fat diet-induced obesity. Hence, SJP is supposed to have an effect on multiple-organ inflammatory injury in AP in rats fed a high-fat diet.
To explore how obesity may contribute to aggravating inflammatory organ injury in AP in rats and observe the effect of SJP on multiple-organ inflammatory injury in AP in rats fed a high-fat diet.
Rats were randomly assigned to a control group (CG), an obese group (OG), and an SJP treatment group (SG), with eight rats per group. The rats in the OG and SG were fed a high-fat diet. From the third week, the rats in the SG were given oral doses of SJP (5 g/kg of body weight). After 12 wk, AP was induced in the three groups. Serum amylase level, body weight, Lee’s index, serum biochemistry parameters, and serum inflammatory cytokine and tissue cytokine levels were assessed, and the tissue histopathological scores were evaluated and compared.
Compared with the CG, serum triglyceride, total cholesterol, interleukin-6, and interleukin-10 levels were significantly higher in the OG, and serum high-density lipoprotein cholesterol level was significantly lower in the OG. Moreover, enhanced oxidative damage was observed in the pancreas, heart, spleen, lung, intestine, liver, and kidney. Evidence of an imbalanced antioxidant defense system, especially in the pancreas, spleen, and intestine, was observed in the obese AP rats. Compared with the OG, serum high-density lipoprotein cholesterol, interleukin-10, and superoxide dismutase expression levels in the pancreas, spleen, and intestine were increased in the SG. Additionally, SJP intervention led to a decrease in the following parameters: body weight; Lee’s index; serum triglyceride levels; serum total cholesterol levels; malondialdehyde expression levels in the pancreas, heart, spleen, lung, and liver; myeloperoxidase expression levels in the lung; and pathological scores in the liver.
Obesity may aggravate the inflammatory reaction and pathological multiple-organ injury in AP rats, and SJP may alleviate multiple-organ inflammatory injury in AP in rats fed a high-fat diet.
Core tip: Obesity worsens inflammatory organ injury in acute pancreatitis (AP), but there is no effective strategy. Sheng-jiang powder (SJP) has been shown to alleviate obesity-induced multiple-organ inflammatory injury. This study demonstrates that obesity may aggravate the inflammatory reaction and pathological injury in multiple organs in AP rats and that SJP may alleviate multiple-organ inflammatory injury in AP in rats fed a high-fat diet.
- Citation: Miao YF, Kang HX, Li J, Zhang YM, Ren HY, Zhu L, Chen H, Yuan L, Su H, Wan MH, Tang WF. Effect of Sheng-jiang powder on multiple-organ inflammatory injury in acute pancreatitis in rats fed a high-fat diet. World J Gastroenterol 2019; 25(6): 683-695
- URL: https://www.wjgnet.com/1007-9327/full/v25/i6/683.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i6.683
The prevalence and incidence of obesity have sharply increased worldwide over the past 40 years[1]. Several studies have shown that obesity has contributed to the increased incidence[2] and severity[3,4] of acute pancreatitis (AP), specifically by increasing the risk of multisystem organ failure[5]. Although increasing evidence has confirmed the adverse effects of obesity on the course and prognosis of AP, the mechanism by which obesity influences AP has not been elucidated to date. There is no effective strategy to prevent AP from causing organ deterioration in obesity; thus, the current management standard is supportive care and the management of complications as they occur[6].
The most common mechanisms underlying the obesity-related increase in AP severity include visceral fat-induced acute lipotoxicity and the inflammatory response[7,8]. An increase in obesity-associated intrapancreatic fat and peripancreatic fat enables the unregulated lipolysis of visceral fat enriched in triglycerides, resulting in systemic unsaturated fatty acid (UFA) release, pancreatic necrosis, and respiratory, cardiovascular, and renal failure[3]. In addition to visceral fat-induced acute lipotoxicity, an obesity-induced imbalance between pro- and anti-inflammatory reactions is another mechanism involved in the exacerbation of AP in obesity. Adipose tissue leads to abundant macrophage infiltration, followed by increased secretion of proinflammatory cytokines [e.g., interleukin-6 (IL-6) and tumor necrosis factor alpha] and decreased production of anti-inflammatory cytokines [e.g., interleukin-10 (IL-10) and adiponectin], promoting inflammation, impairing insulin sensitivity, and dysregulating lipid metabolism[9]. Moreover, in obese AP rats, oxidative stress occurs locally and systemically along with the upregulation of proinflammatory cytokines and exacerbation of lipid peroxidation[10]. Our previous studies have demonstrated that high-fat diet-induced obesity can cause extensive inflammatory damage, especially multiple-organ inflammatory injury, in rats[11-13]. More importantly, obese individuals have an increased risk of developing multisystem organ failure in AP[5]. However, the mechanism of this effect is still unknown. Therefore, in this study, we focused on inflammatory organ injury differences in AP between obese and lean rats.
According to traditional Chinese medicine (TCM) theory, obesity belongs to the category of “Turbidity”[14]. Sheng-Jiang powder (SJP), which is composed of Jiangchan (Bombyx batryticatus), Chantui (Periostracum cicada), Jianghuang (Curcuma longa L.), and Dahuang (Rheum palmatum L.)[15], has been widely used for the treatment of “Turbidity” for hundreds of years in China. SJP has been reported to exhibit diverse biological properties, including anti-inflammatory, lipid-lowering, and immune regulatory characteristics[16]. Our previous studies have demonstrated that SJP can ameliorate the inflammatory response and histopathological lesions in multiple organs in obese rats[11-13]. In addition, a previous study reported that SJP can reduce the inflammatory response and improve the clinical symptoms and prognosis of patients with AP[17]. Could SJP alleviate multiple-organ inflammatory injury in AP by preventing obesity in rats? To address this question, we aimed to explore how obesity may contribute to aggravating inflammatory organ injury in AP in rats and observe the effect of SJP on multiple-organ inflammatory injury in AP in rats fed a high-fat diet.
Dahuang (batch No. 16110150), Jianghuang (batch No. 16080008), Jiangchan (batch No. 16100147), and Chantui (batch No. 16080020) spray-dried drug powders were purchased from the Affiliated Hospital of Chengdu University of TCM (Chengdu, China) and authenticated by Professor Wang WM (Department of Herbal Pharmacy, West China Hospital, Sichuan University, China) according to the Chinese Pharmacopoeia (The Pharmacopoeia Commission of People's Republic of China, 2010). Voucher specimens were deposited in our laboratory. The spray-dried drug powders were mixed and reconstituted with sterile double-distilled water (concentration: 1 g/mL) according to the standard proportion of 4:3:2:1 based on Wan-Bing-Hui-Chun, which is a famous, classic TCM book[14]. This SJP solution was stored at 4 °C until use and administered orally to the rats at a dose of 5 mL/kg of body weight.
Male Sprague-Dawley rats (n = 24) weighing 60-80 g (3-4 wk of age) were purchased from Chengdu Dashuo Experimental Animal Co., Ltd. (Chengdu, China). The rats were acclimatized to the laboratory conditions (22 ± 2°C, 65% ± 10% relative humidity, 12-h light/dark cycle, and ad libitum access to water and food) for one week prior to the special feeding and fasted for 12 h prior to the induction of the AP model. The protocol was approved by the Institution Animal Care and Use Committee of Sichuan University (Chengdu, China) (protocol number, 2017052A).
The rats were randomly selected and assigned to three groups (eight rats per group) according to the type of diet and treatment. As shown in Table 1, a control group (CG) was fed a control diet (#LAD3001G; Trophic Animal Feed High-Tech Co., Ltd., Nantong, China) and treated with normal saline; an obese group (OG) was fed a high-fat diet (#TP23300; Trophic Animal Feed High-Tech Co., Ltd.) and treated with normal saline; and an SJP treatment group (SG) was fed a high-fat diet and treated with SJP (5 g/kg of body weight). AIN93G is a type of diet that has been extensively used worldwide and designed for growing rodents[18], and the composition of the control diet (Table 2) used in this study is similar to that of AIN93G. The high-fat diet, in which approximately 33% of the calories are derived from fat, primarily lard (Table 2), was appropriate for inducing an obesity rodent model[19]. All rats were acclimatized to the respective diets for 2 wk before the experiment started. Then, the rats were orally treated with SJP/normal saline once a day for 10 wk.
| Group | Rats (n) | Food | Treatment (oral administration for 10 wk) |
| CG | 8 | Control diet | Equal volumes of normal saline. |
| OG | 8 | High-fat diet | Equal volumes of normal saline. |
| SG | 8 | High-fat diet | Sheng-jiang powder (5 g/kg of body weight) |
| Ingredient | Control diet (LAD3001G) | High-fat diet (TP23300) | ||
| g | kcal | g | kcal | |
| Casein | 200 | 753.20 | 267 | 1335 |
| Corn starch | 397 | 1495.10 | 0 | 0 |
| Maltodextrin | 132 | 497.11 | 157 | 785 |
| Sucrose | 100 | 376.60 | 89 | 445 |
| Soybean oil | 70 | 263.62 | 33 | 165 |
| Lard | 0 | 0.00 | 301 | 1505 |
| Cellulose | 50 | 188.30 | 67 | 335 |
| Mineral mixture | 35 | 131.81 | 66 | 330 |
| Vitamin mixture | 10 | 37.66 | 13 | 65 |
| L-cystine | 3 | 11.30 | 4 | 20 |
| Choline bitartrate | 0 | 0.00 | 3 | 15 |
| Choline chloride | 3 | 11.30 | 0 | 0 |
| Total | 1000 | 3766 | 1000 | 5000 |
After 12 weeks, the rats were fasted for 12 h. After performing intraperitoneal anesthesia with 2% sodium pentobarbital at 40 mg/kg of body weight, blood from the tail vein was collected for amylase detection, and an AP model was induced by a retrograde injection of 3.5% sodium taurocholate (Sigma, St. Louis, MO, United States; 1 mL/kg of body weight) with a microinfusion pump at a rate of 0.2 mL/min into the biliopancreatic ducts of the rats in each group. Twenty-four hours after the AP induction, the rats were anesthetized (2% sodium pentobarbital, intraperitoneal injection, 40 mg/kg of body weight), and blood samples were collected from each rat into tubes using cardiac puncture to test the levels of serum biochemistry parameters and cytokines (IL-6 and IL-10). Lee’s index, which is a rapid means of determining obesity in rats, was calculated by using the following formula[20]:
Lee’s index = [body weight (g)]1/3 × 103/naso - anal length (cm).
Pancreas, liver, heart, spleen, lung, kidney, and intestinal tissue samples were collected after euthanizing the rats (2% sodium pentobarbital, intraperitoneal injection, 200 mg/kg of body weight) for the pathological and tissue cytokine analyses.
The blood samples were centrifuged at 2500 rpm for 5 min to collect the supernatants for analysis. The levels of triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and amylase were measured using a HITACHI automatic biochemical analyzer (7170A, HITACHI, Tokyo, Japan) at the Affiliated Hospital of Chengdu University of TCM (Chengdu, China).
The blood samples were centrifuged at 2500 rpm for 5 min to collect the supernatants for analysis. The levels of IL-6 and IL-10 were measured by enzyme-linked immunosorbent assay (ELISA) using a Rat IL-6 ELISA kit (EKT24498, Friendbio, Wuhan, China) and a Rat IL-10 ELISA kit (EKT25325, Friendbio, Wuhan, China), respectively. According to the manufacturer’s protocol, the absorbance was measured at 450 nm with a High-Throughput Universal Microplate Assay. Then, the sample values were read based on the standard curve, and the relative concentrations were calculated.
Fresh tissue samples used for the pathological analysis were fixed in 4% paraformaldehyde (AR1068, BOSTER, Wuhan, China), embedded in paraffin, sectioned into 5-μm sections, and stained with hematoxylin and eosin. All histopathological sections were observed and scored by two independent blinded pathologists. The total histopathology score represents the mean of the combined scores of each parameter assigned by the two investigators. The histopathological severity of pancreatitis was determined according to Kusske et al[21] (points 0-4, edema, inflammation, hemorrhage, and necrosis). The scoring system described by Mikawa et al[22] (points 0-4: alveolar congestion, hemorrhage, infiltration or aggregation of neutrophils in the airspace or the vessel wall, and thickness of the alveolar wall/hyaline membrane formation) was used to score acute lung injury. Inflammation-associated histological alterations in the intestinal mucosa were graded using a scoring system according to Wirtz et al[23]. The liver, heart, spleen, and kidney sections were examined for signs of edema, inflammatory infiltration, fat necrosis, parenchymal necrosis, and hemorrhage.
Tissue samples were homogenized, sonicated, and centrifuged at 10000 rpm for 10 min at 4 °C. The supernatants were collected for the cytokine analysis. Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), reactive oxygen species (ROS), and myeloperoxidase (MPO) levels were determined using the following reagent kits according to the manufacturer's protocols: rat MDA ELISA assay kit (EKT2065-75-0), SOD ELISA assay kit (EKT2486), GSH-Px ELISA assay kit (EKT2876), ROS ELISA assay kit (EKT25346), and MPO ELISA assay kit (EKT303413) (obtained from Friendbio Biotechnology Co., Ltd., Wuhan, China). Briefly, the absorbance was measured at 450 nm with a High-Throughput Universal Microplate Assay. Then, the sample values were read based on the standard curve, and the relative concentrations were calculated.
The statistical methods used in this study were reviewed by Dr. Hai Niu from the College of Mathematics, Sichuan University. Normality was assessed by the Shapiro-Wilk normality test, and homogeneity of variance was assessed by the Bartlett’s test. In this study, all data, which are expressed as the mean ± standard deviation, passed the normality test. If the variances of the three experimental groups were equal, two-sided one-way analysis of variance (followed by multiple pairwise comparisons using the Dunnett-t test) was used to discover the differences among the groups; if the variances were unequal, the Kruskal-Wallis test was used. In addition, Student’s t-test was used to compare the difference in the amylase levels between the CG and CG’ (CG before AP induction). Statistical analyses were performed using GraphPad Prism 6.01 software (GraphPad Software Inc., San Diego, CA, United States). Statistical significance is expressed as aP < 0.05, bP < 0.01 vs CG; cP < 0.05, dP < 0.01 vs OG; or eP < 0.01 vs CG’.
At the end of the experiment, compared with the CG, the body weight of the rats in the OG increased by 26.67% (P < 0.01; Table 3); furthermore, Lee's index also increased by 10.45% in the OG (P < 0.05; Table 3). The levels of serum triglyceride and total cholesterol in the OG were significantly higher than those in the CG (P < 0.01; Table 3). SJP treatment significantly reduced the above four parameters in the rats fed a high-fat diet (P < 0.01 or P < 0.05; Table 3). In addition, serum HDL-c level in the OG was significantly lower than that in the CG (P < 0.01; Table 3), and SJP treatment significantly improved the low levels of serum HDL-c in the rats fed a high-fat diet (P < 0.05; Table 3). However, serum LDL-c values did not significantly differ among the experimental groups.
| Parameter | CG | OG | SG |
| Body weight (g) | 480.10 ± 43.62 | 608.10 ± 36.96b | 536.60 ± 46.77d |
| Lee’s index | 301.40 ± 23.36 | 332.90 ± 19.52a | 307.30 ± 19.40b |
| TG (mmol/L) | 1.35 ± 0.51 | 2.69 ± 0.86b | 1.60 ± 0.60d |
| TC (mmol/L) | 1.46 ± 0.32 | 1.97 ± 0.21b | 1.68 ± 0.19b |
| HDL-c (mmol/L) | 0.68 ± 0.11 | 0.46 ± 0.07b | 0.59 ± 0.12b |
| LDL-c (mmol/L) | 0.54 ± 0.13 | 0.49 ± 0.11 | 0.48 ± 0.11 |
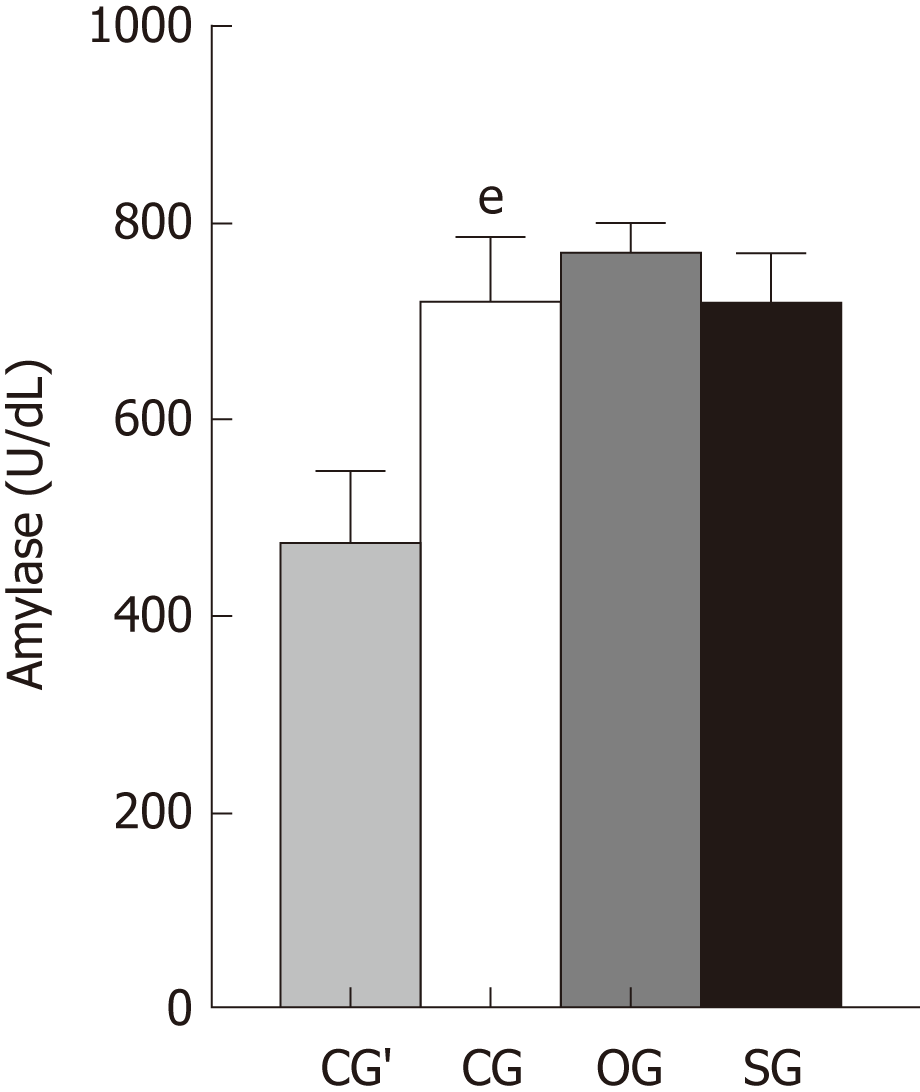
Sodium taurocholate treatment led to a significant increase in serum amylase levels in the rats in the CG’ (P < 0.01; Figure 1). However, serum amylase levels only slightly changed among the three groups after AP induction (Figure 1).
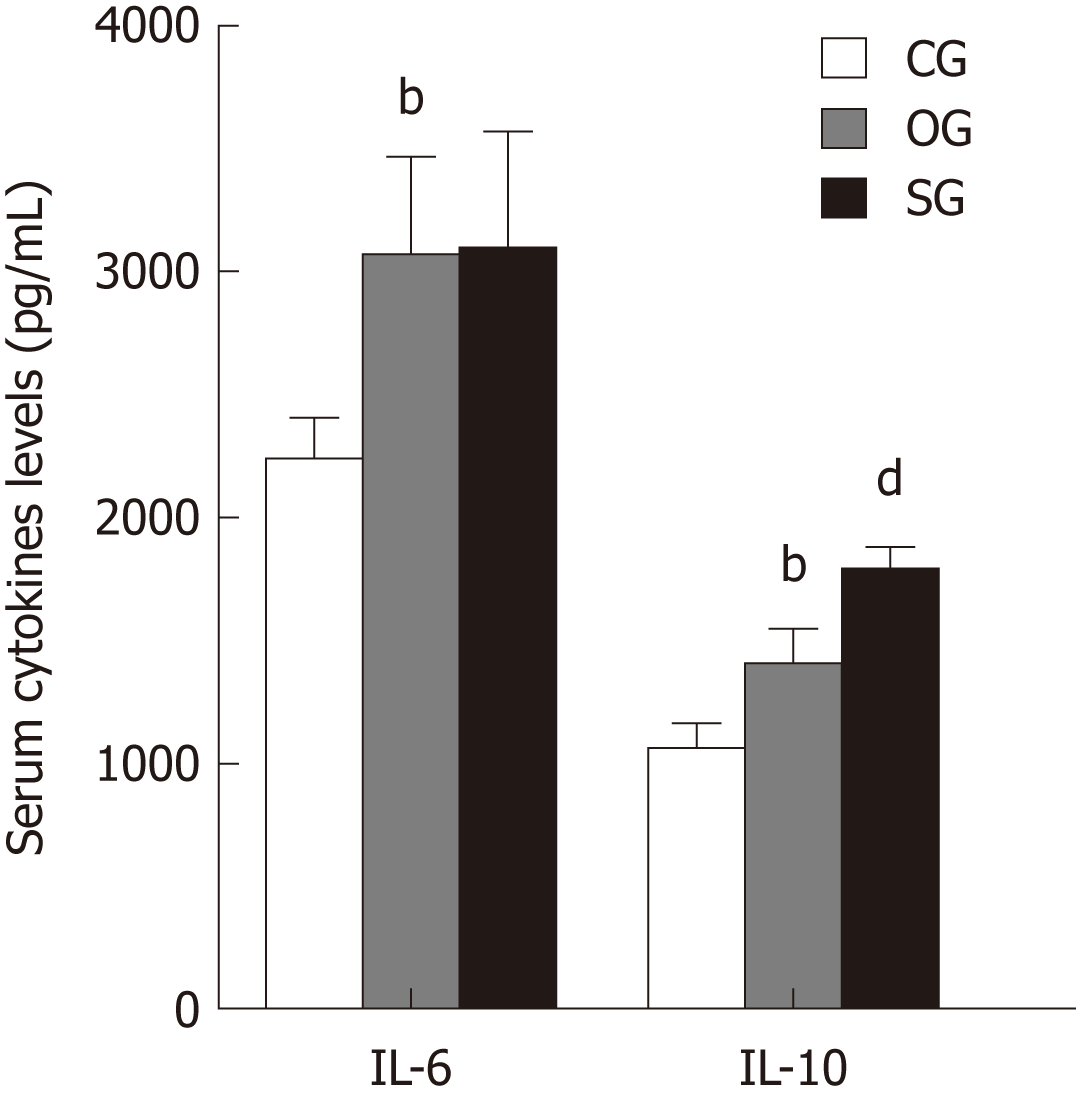
Compared with the CG group, serum levels of IL-6 and IL-10 in the OG group were increased (P < 0.01, Figure 2). Serum IL-10 level in the SG group was significantly higher than that in the OG group (P < 0.01, Figure 2).
MDA levels in the pancreas, heart, spleen, liver, lung, and kidney from the obese rats with AP were higher than those in the lean rats with AP (P < 0.05 for the lung, P < 0.01 for other tissues, Table 4). SOD levels in the pancreas, spleen, intestine, and liver from the obese rats with AP were lower than those in the lean rats with AP (P < 0.01, Table 4). In contrast, the SOD levels in the lung and kidney from the obese rats with AP were higher than those in the lean rats with AP (P < 0.01, Table 4). Similarly, the MPO level in the intestine from the obese rats with AP was also higher than that in the lean rats with AP (P < 0.01, Table 4). However, the ROS and GSH-Px levels in the liver and kidney from the rats showed minimal changes between the CG and OG. SJP markedly reduced the MDA levels in the pancreas, heart, spleen, lung, and liver (P < 0.05 for the spleen, P < 0.01 for other tissues, Table 4) and the MPO level in the lung (P < 0.05, Table 4). In contrast, SJP markedly increased the SOD levels in the pancreas, spleen, and intestine (P < 0.01, Table 4) and the GSH-Px level in the kidney (P < 0.05, Table 4).
| Organ | CG | OG | SG |
| Pancreas | |||
| MDA (pmol/mL) | 1048 ± 116 | 1584 ± 278b | 1066 ± 156d |
| SOD (U/mL) | 338 ± 31 | 244±21b | 285 ± 18d |
| Heart | |||
| MDA (pmol/mL) | 1358 ± 199 | 1685 ± 87b | 1297 ± 83d |
| SOD (U/mL) | 313 ± 24 | 321 ± 42 | 351 ± 14 |
| Spleen | |||
| MDA (pmol/mL) | 1247 ± 205 | 1748 ± 96b | 1569 ± 185b |
| SOD (U/mL) | 288 ± 17 | 214 ± 15b | 322 ± 44d |
| Lung | |||
| MDA (pmol/mL) | 1359 ± 283 | 1745 ± 86a | 1282 ± 140d |
| SOD (U/mL) | 153 ± 31 | 268 ± 21b | 241 ± 23 |
| MPO (ng/mL) | 77 ± 5 | 79 ± 12 | 56 ± 6b |
| Intestine | |||
| MDA (pmol/mL) | 1266 ± 141 | 1412 ± 325 | 1414 ± 235 |
| SOD (U/mL) | 347 ± 12 | 240 ± 19b | 341 ± 29d |
| MPO (ng/mL) | 46 ± 3 | 75 ± 9b | 64 ± 3 |
| Liver | |||
| MDA (pmol/mL) | 1285 ± 146 | 1549 ± 92b | 1235 ± 160d |
| SOD (U/mL) | 308 ± 47 | 229 ± 11b | 231 ± 12 |
| ROS (IU/mL) | 718 ± 146 | 819 ± 96 | 730 ± 85 |
| GSH-Px (mIU/mL) | 86 ± 11 | 71 ± 20 | 80 ± 6 |
| Kidney | |||
| MDA (pmol/mL) | 1077 ± 236 | 1519 ± 217b | 1513 ± 223 |
| SOD (U/mL) | 240 ± 22 | 350 ± 22b | 316 ± 55 |
| ROS (IU/mL) | 598 ± 85 | 677 ± 142 | 666 ± 49 |
| GSH-Px (mIU/mL) | 53 ± 7 | 66 ± 17 | 82 ± 13b |
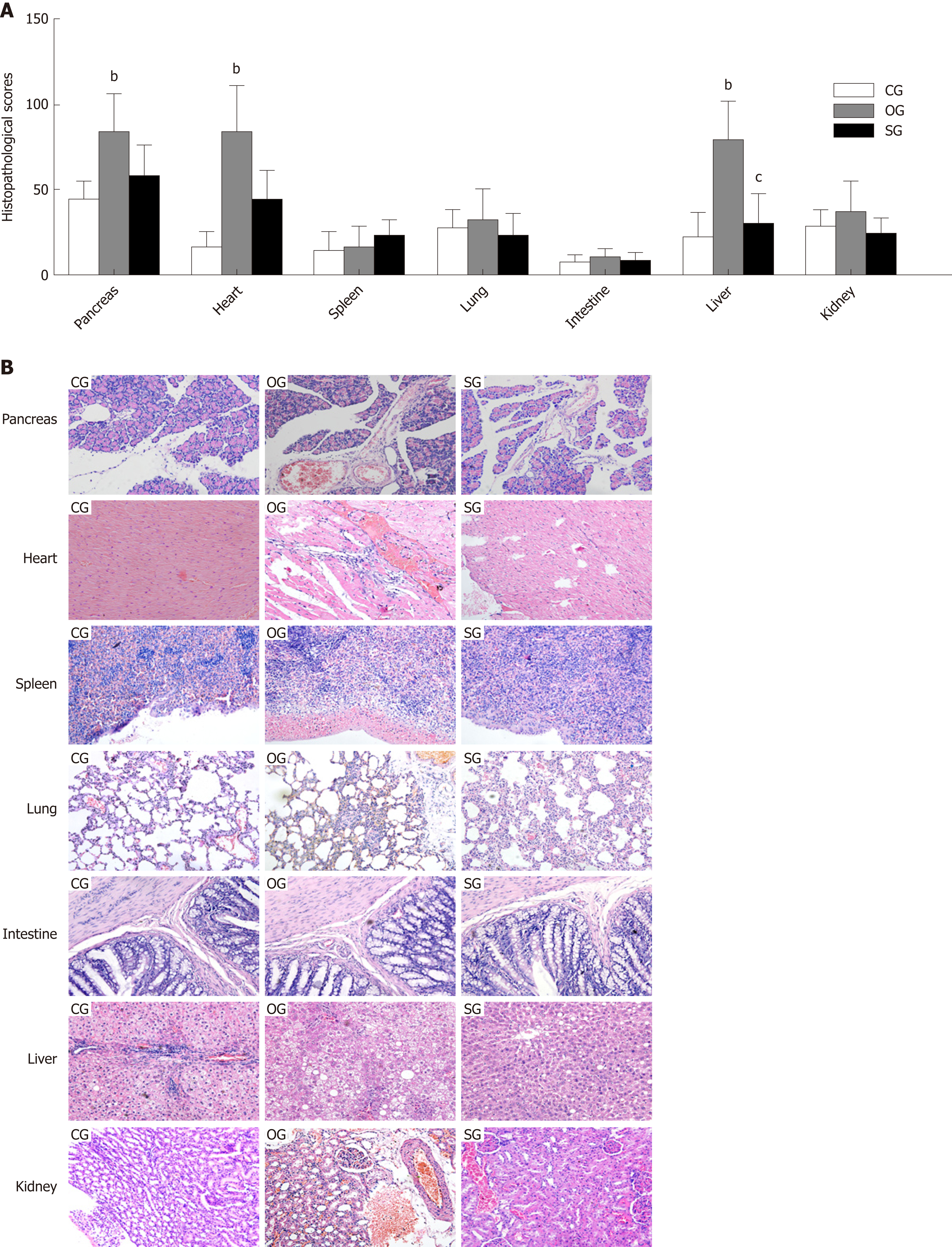
Compared with the organs from the CG group rats, obesity exacerbated the pathological damage to the pancreas, liver, and heart, and more inflammatory cell infiltration, a large degree of severe tissue edema, more hemorrhage, and more necrosis were observed (P < 0.01, Figure 3A and B). However, SJP mitigated the hepatic pathological damage, and less inflammatory cell infiltration, mild tissue edema, and less necrosis were observed (P < 0.05, Figure 3A and B).
In the present study, serum triglyceride, total cholesterol, IL-6, and IL-10 levels in the obese AP rats were extremely high, and serum HDL-c level was significantly low. Enhanced oxidative damage was observed in the pancreas, heart, spleen, lung, intestine, liver, and kidney. Additionally, an imbalance in the antioxidant defense system, especially in the pancreas, spleen, intestine, and liver, was observed in the obese AP rats. Importantly, SJP treatment significantly increased serum HDL-c and IL-10 levels, decreased serum triglyceride and total cholesterol levels, induced oxidative stress in multiple organs, and ultimately ameliorated the pathological damage to the liver.
As one becomes more obese, more fat accumulates in and around the viscera, including the pancreas[3]. Fat in adipocytes is known to be composed of triglycerides, which are three free fatty acids hinged to a glycerol backbone, forming > 80% of the adipocyte mass[4]. More significantly, increased free fatty acids, particularly unsaturated fatty acids (UFAs), have been documented in serum from patients with severe AP[24], and serum triglyceride levels in AP patients are independently and proportionally correlated with persistent organ failure regardless of the etiology[25]. Furthermore, sufficient evidence suggests that UFAs are directly toxic to pancreatic acinar cells due to cytosolic calcium, largely causing extracellular release, decreased ATP levels, the inhibition of mitochondrial complexes I and V, and, ultimately, necrosis[3]. In AP associated with obesity, the expression levels of cytokines are significantly elevated in serum or organs involved in AP (pancreas, liver, and lungs)[26,27]. One study demonstrated that obesity reduces IL-10 expression in the spleen and that spleen-derived IL-10 protects against obesity-induced inflammatory responses in the pancreas[28]. In addition, in obese mice, the levels of proinflammatory cytokines, including tumor necrosis factor alpha and IL-6, increased along with serum UFAs, but treatment with the triglyceride lipolysis inhibitor orlistat significantly reduced these cytokines and UFAs and prevented organ failure and mortality[29]. Hence, in addition to being directly responsible for necrosis, lipolysis is likely a contributor to the milieu of exaggerated inflammation characterizing AP. In this study, high-fat diet-induced obesity resulted in high serum levels of triglycerides, IL-6, and IL-10 in AP rats, but SJP prevented lipotoxicity and the systemic inflammatory response in obese AP rats. Moreover, previous studies have shown that SJP can reduce systemic inflammatory injury and downregulate the production of proinflammatory cytokines in mice and humans with sepsis[30].
Oxidative stress occurs when there is an imbalance between the production of reactive metabolites and the activity of antioxidant enzymes. Many studies have shown that oxidative stress is significantly increased in obese patients and animals and causes direct or indirect damage to various organs[31,32]. MDA is a toxic decomposition product of lipid peroxidation degradation, and the MDA content can reflect the degree of oxidative stress and cell damage[33]. During lipid peroxidation in pancreatitis, the MDA levels in plasma and ascites in obese animals are always higher than those in lean animals[34]. Hayam Ateyya et al[35] revealed that in rats with AP, the MDA levels are markedly increased when the SOD and glutathione levels are reduced in the pancreas and distant organs (i.e., lung, liver, and kidney). In our study, we found similar results in multiple organs (i.e., pancreas, liver, heart, spleen, lung, and kidney) from obese AP rats, suggesting that these organs might experience very serious conditions of oxidative stress and cell damage.
Various proinflammatory transcription factors can induce lipid peroxidation, causing the release of inflammatory cytokines and exacerbating oxidative stress, establishing a vicious circle[10]. Therefore, the synergistic effect of proinflammatory cytokines released during AP combined with the increased MDA levels in obese rats may aggravate the multiple-organ damage. ROS, which are chemical species containing oxygen, are byproducts of aerobic metabolism that play significant roles in cell signaling and homeostasis[36]. ROS levels can dramatically increase following oxidative stress in obesity, which may significantly damage the cell structure[36]. According to this study, ROS levels in the liver and kidney changed minimally between the obese and lean rats, but MDA levels in the obese rats were significantly increased. We speculate that this result may be due to the longer half-life of MDA compared with that of ROS, indicating that MDA is able to spread and reach distant intracellular and extracellular targets, thereby amplifying the effects of oxidative stress[37].
MPO is a characteristic enzyme of neutrophils, and its activity reflects the extent of neutrophil infiltration; sustained activation of MPO can cause tissue damage and aggravate inflammatory lesions[38]. Intestinal injury, which is considered a primary complication after AP onset, is well known to occur during early disease progression[39]. In addition, the intestine is considered an important target organ for obesity outcomes because of the effects of diet on this organ. An increase in intestinal ROS production and MPO activity was observed in rats fed a high-energy diet[40]. The increased MPO levels in the intestinal tissues in our study may suggest that obesity aggravates intestinal inflammatory injury in AP rats.
SOD, catalase, and GSH-Px are important antioxidant enzymes in organs and are involved in free-radical scavenging[41]. Erythrocyte SOD and GSH-Px activities are decreased in rats with AP[42]. Furthermore, low basal levels of pancreatic SOD activity are associated with increased mortality and tissue damage in rats with AP[43]. In the present work, SOD levels in the liver, pancreas, spleen, and intestinal tissues from the obese rats were lower than those from the lean animals, indicating that the antioxidant capacity in obese rats with AP may be lower than that in nonobese rats; this compromised antioxidant defense system may contribute to the increase in multiple-organ damage in obese rats with AP.
In China, SJP is widely used for the treatment of inflammatory diseases or syndromes, such as sepsis, pneumonia, and systemic inflammatory response syndrome; in addition, SJP has a positive effect on obesity-related glomerulopathy and obesity after contraception by subcutaneous Norplant implants (Supplementary material). Notably, our previous study showed that rhein and bisdemethoxycurcumin may be the potential active components of SJP for the treatment of AP[44]. Curcumin, an effective compound extracted from Curcuma longa L., exerts its ameliorative effects against AP by inhibiting oxidative stress and inflammation[45]. One study showed that curcumin administration reduces MDA levels in skeletal muscle to improve oxidative stress in high-fat diet-induced obese rats[46]. In contrast, curcumin is effective at countering the depletion of antioxidants. Kempaiah et al[47] reported that curcumin countered the depletion of GSH in erythrocytes and livers of high-fat diet-fed rats. Elevated hepatic SOD activity has also been found to be decreased by curcumin treatment. Moreover, emodin, which is derived from Rheum palmatum L., can prevent diet-induced obesity and associated metabolic syndrome by inhibiting the sterol regulatory element-binding protein pathway[48]. In this study, administration of SJP to obese rats reduced the activity of MPO in the lung; decreased the content of MDA in the pancreas, heart, spleen, lung, and liver; and increased the activity of SOD in the pancreas, spleen, and intestine. These results suggest that SJP can prevent AP from causing multiple-organ inflammatory injury-related deterioration in obese rats by reducing reactive oxygen free radicals, improving the activities of antioxidant enzymes, and alleviating the systemic inflammatory response.
However, there were some limitations to this study. First, SJP was not administered to obese rats, but it was administered at the time obesity was induced. According to the obesity and Lee’s index results, SJP appears to largely prevent the development of obesity. Hence, the preventive effect of SJP on AP in obesity needs further exploration. Second, this study provided partial information about multiple-organ injuries in obese AP rats; however, the more specific mechanism needs further study. Third, the specific effective monomer components and tissue pharmacokinetics of SJP should be considered. Finally, studies investigating the effectiveness of SJP in obesity are limited to case reports and small-sample single-center randomized controlled trials in which SJP is always combined with other complementary or alternative medicine therapy (acupuncture or ear acupressure); hence, large sample multicenter randomized controlled trials should be considered.
In conclusion, obesity may aggravate the inflammatory reaction and pathological injury to multiple organs in AP rats, but SJP may alleviate multiple-organ inflammatory injury in AP in rats fed a high-fat diet. The results of this study may provide an experimental basis for clinical discoveries. Future research should investigate the active components and mechanisms of action of SJP before it can be transformed into a potentially useful treatment in human trials (Supplementary Table 1).
Obesity has contributed to the increased incidence and severity of acute pancreatitis (AP), specifically by increasing the risk of multisystem organ failure. However, the mechanism by which obesity influences AP has not been elucidated to date. There is no effective strategy for preventing AP from causing organ deterioration in obesity; thus, the current management standard is supportive and symptomatic. Therefore, investigations of how obesity may contribute to aggravating inflammatory organ injury in AP and identification of potential treatments are urgently required.
Our previous studies have demonstrated that high-fat diet-induced obesity can cause extensive inflammatory damage, especially multiple-organ inflammatory injury, in rats. However, the effect of high-fat diet-induced obesity on AP is still unknown. Sheng-jiang powder (SJP) is considered able to ameliorate the inflammatory response and histopathological lesions in multiple organs in obese rats. Could SJP alleviate multiple-organ inflammatory injury in AP by preventing obesity in rats? Therefore, this study aimed to explore the mechanisms of the effect of high-fat diet-induced obesity on inflammatory organ injury in AP rats and observe the effect of SJP on multiple-organ inflammatory injury in AP in rats fed a high-fat diet to provide an experimental basis for its clinical application in the future.
To explore how high-fat diet-induced obesity may contribute to aggravating inflammatory organ injury in AP rats and observe the effect of SJP on multiple-organ inflammatory injury in AP in rats fed a high-fat diet.
In this study, an obese rat model was induced by high-fat diet feeding, which is widely accepted and used for the induction of obesity in rats. The AP rat model was induced by a retrograde injection of sodium taurocholate into the biliopancreatic ducts, which is widely used to induce AP in rats. The levels of serum biochemistry parameters [triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and amylase] were measured using an HITACHI automatic biochemical analyzer (7170A, HITACHI, Tokyo, Japan). The levels of serum inflammatory cytokines (IL-6 and IL-10) and tissue oxidative stress cytokines [malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), reactive oxygen species (ROS), and myeloperoxidase (MPO)] were measured by ELISA, which is a simple, rapid, accurate, and sensitive method. All histopathological sections were observed and scored by two independent blinded pathologists using different scoring systems specific to different tissues.
Statistical analyses were performed with GraphPad Prism 6.01 software. All data are expressed as the mean ± standard deviation and passed the normality test. Two-sided one-way analysis of variance (followed by multiple pairwise comparisons using the Dunnett-t test) or the Kruskal-Wallis test was used to discover the differences among the three groups. In addition, Student’s t-test was used to compare the difference in the amylase levels between the control group (CG) and CG’ (CG before AP induction).
In the present study, serum triglyceride, total cholesterol, interleukin (IL)-6, and IL-10 levels in the obese AP rats were extremely high, and serum HDL-c level was significantly low. Enhanced oxidative damage was observed in the pancreas, heart, spleen, lung, intestine, liver, and kidney. Additionally, an imbalance in the antioxidant defense system, especially in the pancreas, spleen, intestine, and liver, was observed in the obese AP rats. Interestingly, SJP significantly increased serum HDL-c and IL-10 levels, decreased serum triglyceride and total cholesterol levels, induced oxidative stress in multiple organs, and ultimately ameliorated the pathological damage to the liver.
The preventive effect of SJP on AP in obesity remains to be determined. Moreover, this study provides partial information about multiple-organ injuries in obese AP rats, but the more specific mechanism needs further study. Finally, the specific effective monomer components and tissue pharmacokinetics of SJP should be considered to provide more systematic and comprehensive evidence for the clinical application of this Chinese decoction.
This study demonstrates that high-fat diet-induced obesity may aggravate the inflammatory reaction and pathological injury to multiple organs, especially leading to a strong and extensive oxidative stress response in organs, in sodium taurocholate-induced AP rats. In addition, SJP may alleviate multiple-organ inflammatory injury in AP in rats fed a high-fat diet.
As we observed that SJP may ameliorate multiple-organ inflammatory injury in AP in rats fed a high-fat diet by regulating the oxidative stress response, further investigation of the underlying molecular mechanism is urgently required to provide experimental evidence for wider clinical usage.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Negoi I, Neri V, Tao R, Venu RP, Yago MD S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY
| 1. | World Health Organization. Obesity and overweight. Accessed November 2, 2018 Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. . [Cited in This Article: ] |
| 2. | Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, Jensen ET, Lund JL, Pasricha S, Runge T, Schmidt M, Shaheen NJ, Sandler RS. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149:1731-1741.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 639] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 3. | Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT, Furlan A, Behari J, Liu S, McHale T, Nichols L, Papachristou GI, Yadav D, Singh VP. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 275] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 4. | Acharya C, Navina S, Singh VP. Role of pancreatic fat in the outcomes of pancreatitis. Pancreatology. 2014;14:403-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1181] [Cited by in F6Publishing: 1092] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 7. | Sadr-Azodi O, Orsini N, Andrén-Sandberg Å, Wolk A. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol. 2013;108:133-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 8. | Yashima Y, Isayama H, Tsujino T, Nagano R, Yamamoto K, Mizuno S, Yagioka H, Kawakubo K, Sasaki T, Kogure H, Nakai Y, Hirano K, Sasahira N, Tada M, Kawabe T, Koike K, Omata M. A large volume of visceral adipose tissue leads to severe acute pancreatitis. J Gastroenterol. 2011;46:1213-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Zyromski NJ, Mathur A, Pitt HA, Lu D, Gripe JT, Walker JJ, Yancey K, Wade TE, Swartz-Basile DA. A murine model of obesity implicates the adipokine milieu in the pathogenesis of severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G552-G558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 722] [Cited by in F6Publishing: 753] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 11. | Li J, Zhang YM, Li JY, Zhu L, Kang HX, Ren HY, Chen H, Yuan L, Miao YF, Wan MH, Tang WF. Effect of Sheng-Jiang Powder on Obesity-Induced Multiple Organ Injuries in Rats. Evid Based Complement Alternat Med. 2017;2017:6575276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Miao YF, Li J, Zhang YM, Zhu L, Chen H, Yuan L, Hu J, Yi XL, Wu QT, Wan MH, Tang WF. Sheng-jiang powder ameliorates obesity-induced pancreatic inflammatory injury via stimulating activation of the AMPK signalling pathway in rats. World J Gastroenterol. 2018;24:4448-4461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Li J, Zhu L, Zhang YM, Chen H, Miao YF, Kang HX, Ren HY, Wan MH, Long D, Tang WF. Sheng-Jiang Powder Ameliorates High Fat Diet Induced Nonalcoholic Fatty Liver Disease via Inhibiting Activation of Akt/mTOR/S6 Pathway in Rats. Evid Based Complement Alternat Med. 2018;2018:6190254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Liu XM, Tong XL, Wang PQ. Discussion on the theory of turbidity. Shijie Zhongxiyi Jiehe Zazhi. 2009;4:839-842. [DOI] [Cited in This Article: ] |
| 15. | Tian SX, Li SM. Clinical application on the effect of Sheng-jiang powder. Hebei Zhongxiyi Xuebao. 1994;9:40-44. [DOI] [Cited in This Article: ] |
| 16. | Liu WJ, Xue YX, Hu DP. Research Progress on Modern Pharmacological Mechanism of Sheng-Jiang Powder. Beijing Zhongyiyao. 2012;31:939-943. [DOI] [Cited in This Article: ] |
| 17. | Gu WY, Zhao L, Qian FH, Wu Y, Cao DF. Treatment of acute pancreatitis with Sheng-Jiang Powder. Jilin Zhongyiyao. 2013;33:1232-1234. [DOI] [Cited in This Article: ] |
| 18. | Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939-1951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5738] [Cited by in F6Publishing: 5770] [Article Influence: 186.1] [Reference Citation Analysis (0)] |
| 19. | Wang H, Storlien LH, Huang XF. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Physiol Endocrinol Metab. 2002;282:E1352-E1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Li M, Ye T, Wang XX, Li X, Qiang O, Yu T, Tang CW, Liu R. Effect of Octreotide on Hepatic Steatosis in Diet-Induced Obesity in Rats. PLoS One. 2016;11:e0152085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284-8; discussion 289. [PubMed] [Cited in This Article: ] |
| 22. | Mikawa K, Maekawa N, Nishina K, Takao Y, Yaku H, Obara H. Effect of lidocaine pretreatment on endotoxin-induced lung injury in rabbits. Anesthesiology. 1994;81:689-699. [PubMed] [Cited in This Article: ] |
| 23. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1160] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 24. | Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Nawaz H, Koutroumpakis E, Easler J, Slivka A, Whitcomb DC, Singh VP, Yadav D, Papachristou GI. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110:1497-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Segersvärd R, Tsai JA, Herrington MK, Wang F. Obesity alters cytokine gene expression and promotes liver injury in rats with acute pancreatitis. Obesity (Silver Spring). 2008;16:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Park J, Chang JH, Park SH, Lee HJ, Lim YS, Kim TH, Kim CW, Han SW. Interleukin-6 is associated with obesity, central fat distribution, and disease severity in patients with acute pancreatitis. Pancreatology. 2015;15:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Gotoh K, Inoue M, Shiraishi K, Masaki T, Chiba S, Mitsutomi K, Shimasaki T, Ando H, Fujiwara K, Katsuragi I, Kakuma T, Seike M, Sakata T, Yoshimatsu H. Spleen-derived interleukin-10 downregulates the severity of high-fat diet-induced non-alcoholic fatty pancreas disease. PLoS One. 2012;7:e53154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Malecki EA, Castellanos KJ, Cabay RJ, Fantuzzi G. Therapeutic administration of orlistat, rosiglitazone, or the chemokine receptor antagonist RS102895 fails to improve the severity of acute pancreatitis in obese mice. Pancreas. 2014;43:903-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Zhu L, Zhao L, Qian FH, Qi LL, Xia YC, Qian YM Xi Y. Research on the Inhibition of Inflammatory Cytokines by Shengjiang San in Sepsis Mice. Zhongguo Zhongyi Jizheng. 2015;24:384-386. [DOI] [Cited in This Article: ] |
| 31. | Dimassi S, Chahed K, Boumiza S, Canault M, Tabka Z, Laurant P, Riva C. Role of eNOS- and NOX-containing microparticles in endothelial dysfunction in patients with obesity. Obesity (Silver Spring). 2016;24:1305-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Fu J, Zeng C, Zeng Z, Wang B, Gong D. Cinnamomum camphora Seed Kernel Oil Ameliorates Oxidative Stress and Inflammation in Diet-Induced Obese Rats. J Food Sci. 2016;81:H1295-H1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 937] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 34. | Pereda J, Pérez S, Escobar J, Arduini A, Asensi M, Serviddio G, Sabater L, Aparisi L, Sastre J. Obese rats exhibit high levels of fat necrosis and isoprostanes in taurocholate-induced acute pancreatitis. PLoS One. 2012;7:e44383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Ateyya H, Wagih HM, El-Sherbeeny NA. Effect of tiron on remote organ injury in rats with severe acute pancreatitis induced by L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:873-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | McMurray F, Patten DA, Harper ME. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity (Silver Spring). 2016;24:2301-2310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 37. | Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 38. | Odobasic D, Kitching AR, Yang Y, O'Sullivan KM, Muljadi RC, Edgtton KL, Tan DS, Summers SA, Morand EF, Holdsworth SR. Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood. 2013;121:4195-4204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2013;17:349-355. [PubMed] [Cited in This Article: ] |
| 40. | Gil-Cardoso K, Ginés I, Pinent M, Ardévol A, Terra X, Blay M. A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats. Br J Nutr. 2017;117:218-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Czakó L, Takács T, Varga IS, Tiszlavicz L, Hai DQ, Hegyi P, Matkovics B, Lonovics J. Oxidative stress in distant organs and the effects of allopurinol during experimental acute pancreatitis. Int J Pancreatol. 2000;27:209-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Tang QQ, Su SY, Fang MY. Zinc supplement modulates oxidative stress and antioxidant values in rats with severe acute pancreatitis. Biol Trace Elem Res. 2014;159:320-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Ren J, Luo Z, Tian F, Wang Q, Li K, Wang C. Hydrogen-rich saline reduces the oxidative stress and relieves the severity of trauma-induced acute pancreatitis in rats. J Trauma Acute Care Surg. 2012;72:1555-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Zhu L, Li JY, Zhang YM, Kang HX, Chen H, Su H, Li J, Tang WF. Pharmacokinetics and pharmacodynamics of Shengjiang decoction in rats with acute pancreatitis for protecting against multiple organ injury. World J Gastroenterol. 2017;23:8169-8181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Shafik NM, Abou-Fard GM. Ameliorative Effects of Curcumin on Fibrinogen-Like Protein-2 Gene Expression, Some Oxido-Inflammatory and Apoptotic Markers in a Rat Model of l-Arginine-Induced Acute Pancreatitis. J Biochem Mol Toxicol. 2016;30:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Sripradha R. Preventive effect of curcumin on inflammation, oxidative stress and insulin resistance in high-fat fed obese rats. J Complement Integr Med. 2016;13:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Kempaiah RK, Srinivasan K. Antioxidant status of red blood cells and liver in hypercholesterolemic rats fed hypolipidemic spices. Int J Vitam Nutr Res. 2004;74:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Li J, Ding L, Song B, Xiao X, Qi M, Yang Q, Yang Q, Tang X, Wang Z, Yang L. Emodin improves lipid and glucose metabolism in high fat diet-induced obese mice through regulating SREBP pathway. Eur J Pharmacol. 2016;770:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |