Published online Dec 7, 2019. doi: 10.3748/wjg.v25.i45.6607
Peer-review started: October 16, 2019
First decision: November 4, 2019
Revised: November 10, 2019
Accepted: November 23, 2019
Article in press: November 24, 2019
Published online: December 7, 2019
Processing time: 50 Days and 23.2 Hours
It is well known that nonalcoholic fatty liver disease (NAFLD) is associated with insulin resistance (IR). LB100, a serine/threonine protein phosphatase 2A (PP2A) inhibitor, is closely related to IR. However, there is little data regarding its direct influence on NAFLD.
To elucidate the effect and underlying mechanism of LB100 in NAFLD.
After 10 wk of high fat diet (HFD) feeding, male C57BL/6 mice were injected intraperitoneally with vehicle or LB100 for an additional 6 wk (three times a week). The L02 cell line was treated with LB100 and free fatty acids (FFAs) for 24 h. Hematoxylin and eosin and oil red O staining were performed for histological examination. Western blot analysis was used to detect the protein expression of Sirtuin 1 (Sirt1), total and phosphorylated AMP-activated protein kinase α (AMPKα), and the proteins involved in lipogenesis and fatty acid oxidation. The mRNA levels were determined by qPCR. Pharmacological inhibition of AMPK was performed to further examine the exact mechanism of LB100 in NAFLD.
LB100 significantly ameliorated HFD-induced obesity, hepatic lipid accumulation and hepatic injury in mice. In addition, LB100 significantly downregulated the protein levels of acetyl-CoA carboxylase, sterol regulatory element-binding protein 1 and its lipogenesis target genes, including stearoyl-CoA desaturase-1 and fatty acid synthase, and upregulated the levels of proteins involved in fatty acid β-oxidation, such as peroxisome proliferator-activated receptor α (PPARα), peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), carnitine palmitoyltransferase 1α, acyl-CoA oxidase 1 and uncoupling protein 2, as well as the upstream mediators Sirt1 and AMPKα in the livers of HFD-fed mice. In vitro, LB100 alleviated FFA-induced lipid accumulation in L02 cells through the AMPK/Sirt1 signaling pathway. Further studies showed that the curative effect of LB100 on lipid accumulation was abolished by inhibiting AMPKα in L02 cells.
PP2A inhibition by LB100 significantly ameliorates hepatic steatosis by regulating hepatic lipogenesis and fatty acid oxidation via the AMPK/Sirt1 pathway. LB100 may be a potential therapeutic agent for NAFLD.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases worldwide, which greatly increases the medical and economic burden. We aimed to investigate the effect and underlying mechanism of LB100 in lipid accumulation during NAFLD development in mice fed a high fat diet and L02 cells treated with free fatty acids. Our study provided, for the first time, in vivo and in vitro evidence that LB100 can effectively inhibit hepatic lipogenesis via the AMPK/Sirt1 pathway and could be a therapeutic strategy for NAFLD.
- Citation: Chen XY, Cai CZ, Yu ML, Feng ZM, Zhang YW, Liu PH, Zeng H, Yu CH. LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway. World J Gastroenterol 2019; 25(45): 6607-6618
- URL: https://www.wjgnet.com/1007-9327/full/v25/i45/6607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i45.6607
Nonalcoholic fatty liver disease (NAFLD) includes a series of hepatic metabolic disorders characterized by excessive hepatic fat accumulation without a history of significant alcohol consumption[1]. It ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), irreversible fibrosis, cirrhosis, and eventually hepatocellular carcinoma (HCC)[2]. NAFLD is one of the most common chronic liver diseases worldwide, with a global prevalence of 25.2% and a prevalence of 29.2% in China[3,4], which greatly increases the medical and economic burden. However, its exact pathogenesis remains poorly understood. Lifestyle modification is advocated for treating patients with NAFLD, but its efficacy is limited. Optional medications include vitamin E, pioglitazone and pentoxifylline, but there is a lack of Food and Drug Administration-approved treatments[5]. Therefore, there is an urgent need to develop effective drug therapies for NAFLD.
AMP-activated protein kinase (AMPK) is a monitor of cellular energy status; once activated, it inhibits various anabolic pathways, stimulates catabolic pathways, suppresses ATP consumption, and increases ATP production to restore energy homeostasis[6,7]. AMPK enhances Sirtuin 1 (Sirt1) activity by increasing cellular NAD+ levels, further leading to the deacetylation and activity regulation of downstream Sirt1 targets such as Peroxisome proliferator-activated receptor gamma coactivator-1α (PGC1α)[8]. Numerous studies have found that AMPK and Sirt1 are closely related to lipid metabolism and activate each other in a finely tuned network[8,9]. Phosphorylated AMPK can directly target Acetyl-CoA carboxylase (ACC), phosphorylating and inactivating it[10]. AMPK activation also reduces the transcriptional activation of sterol regulatory element-binding protein 1c (SREBP-1c), which is a critical transcription factor in the regulation of lipogenic genes, including ACC, fatty acid synthase (FAS) and stearoyl-CoA desaturase-1 (SCD1)[11,12]. In addition, AMPK has been shown to be closely related to fatty acid β oxidation[13,14]. In summary, the AMPK-Sirt1 axis plays an important role in lipid metabolism.
LB100 (Supplementary Figure 1A) is a first-line serine/threonine protein phosphatase 2A (PP2A) small molecule inhibitor that is water-soluble and was initially designed to increase the sensitivity of solid tumors to chemotherapy[15]. Emerging evidence suggests that overactivation of PP2A is closely related to elevated insulin resistance (IR) in the liver[16,17]. PP2A regulates the phosphorylation of AMPK by dephosphorylating Thr-172, a residue that increases kinase activity when phosphorylated[18,19]. The direct interaction between PP2A and AMPK was confirmed by co-immunoprecipitation and co-localization experiments[19]. Therefore, we hypothesized that the effect of LB100 on lipid metabolism was at least partly mediated through the AMPK/Sirt1 pathway.
In this study, we investigated the effect of LB100 on ameliorating hepatic lipid accumulation in both in vivo and in vitro NAFLD models and its potential interaction with the AMPK/Sirt1 pathway, which may provide a new approach for the effective treatment of NAFLD.
Male C57BL/6 mice (6 wk, 18-22 g), purchased from B&K Laboratory Animal Corp., Ltd. (Shanghai, China) were randomly distributed into four treatment groups: Standard chow diet (SCD) + vehicle, SCD + LB100 1.5 mg/kg, HFD + vehicle, HFD + LB100 1.5 mg/kg. The mice were housed in a specific pathogen-free environment (24-26°C, relative humidity: 50%-60%) with a 12-h light/dark cycle and free access to food and water. For 10 wk, the mice were fed either a SCD or a HFD (60% of kilocalories as fat; Product D12492, Research Diets, New Brunswick, NJ, United States). The animals were then injected intraperitoneally with the vehicle or LB100 (three times a week) dissolved in normal saline for the next 6 wk. Blood and tissue samples were stored at -80°C. All animal experiments were performed according to the guidelines approved by the Animal Care and Use Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Permit number: 2018-842).
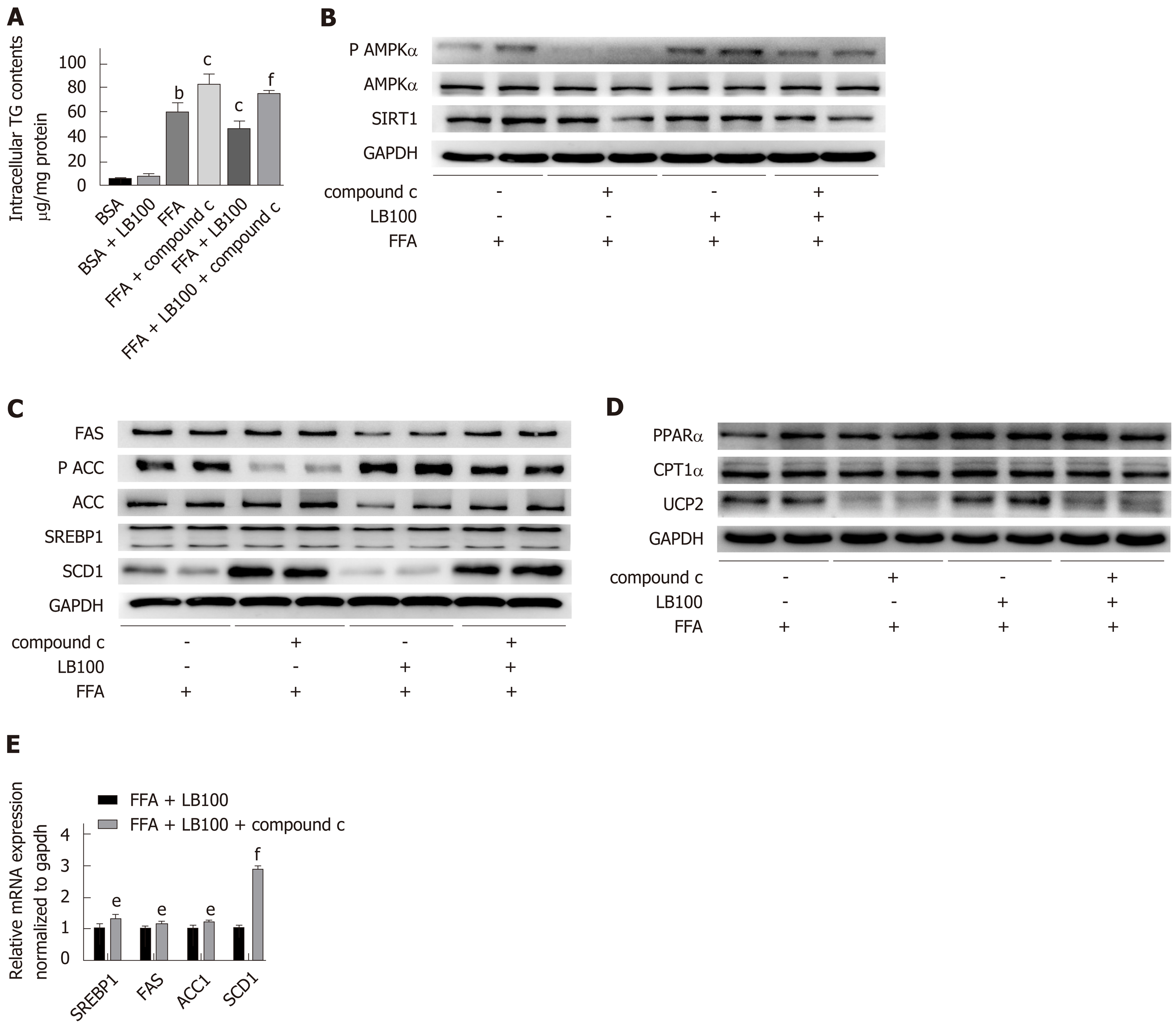
The normal human hepatic cell line (L02), obtained from the Chinese Academy of Science (Shanghai, China), was maintained in Dulbecco’s modified Eagle’s medium (high glucose) supplemented with 10% fetal bovine serum and 1% antibiotics at 37°C with 95% humidified air/5% CO2. L02 cells were exposed to a mixture of free fatty acids (FFAs; oleate acid and palmitate acid, final ratio 2:1; Sigma-Aldrich, St. Louis, MO, United States) at a final concentration of 1 mmol/L with or without LB100 (Selleckchem; 6 μmol/L) for 24 h. To investigate the effect of the AMPK signaling pathway on LB100-regulated lipid metabolism, the cells were pretreated with the AMPK inhibitor compound C (10 μmol/L) for 2 h and treated with LB100 supplemented with FFAs for 24 h.
L02 cells were seeded in 96-well plates at a density of 5 × 103 cells/well and cultured overnight. Subsequently, the cells were pretreated with various concentrations of LB100 (100 nmol/L–100 µmol/L) for 24 h at 37 °C. Cell viability was estimated using the cell counting kit-8 colorimetric assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
Total proteins were extracted from cells and liver tissues using RIPA buffer (Applygen Technologies Inc., Beijing, China) supplemented with protease and phosphatase inhibitors (Sigma). Equal amounts of protein were separated by 8%-10% SDS-PAGE followed by transfer to PVDF membranes (Millipore, Inc., Darmstadt, Germany). The membranes were blocked with 5% nonfat dry milk in TBST, followed by incubation overnight with the following primary antibodies: anti-PP2A subunit C (2259), anti-p-AMPKα (2535), anti-AMPKα (2603), anti-Sirt1 (8469), anti-GAPDH (2118), anti-SCD1 (2438), anti-PGC1α (2178), anti-carnitine palmitoyltransferase 1α (CPT1α) (12252), anti-uncoupling protein 2 (UCP2) (89326) (Cell Signaling Technology, Beverly, MA, USA), anti-PP2A α+β (methyl L309) (ab66597), anti-p-ACC (ab31931), anti-ACC (ab45174), anti-FAS (ab128856), anti-SREBP1 (ab3259), and anti-PPARα (ab8934) (Abcam, Cambridge, UK). Proteins were visualized with an enhanced ECL kit (Fudebio, Hangzhou, China).
Total RNA was extracted from cells or livers with RNA plus (Takara, Dalian, China), and cDNA synthesis was performed with a One Step PrimeScript® RT-PCR kit (Takara, Japan) using 2.5 μg total RNA. Real-time PCR analysis was carried out using the SYBR Premix-Ex Tag Kit (Takara) on an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA, United States). The primer sequences are listed in Supplementary Table 1.
Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed after feeding the mice for 14 or 15 wk with the HFD, respectively. For GTTs, the mice were injected intraperitoneally with glucose solution (1 mg/g body weight) after a 16-h fast. For ITTs, the mice were injected intraperitoneally with insulin solution (0.75 mU/g body weight) after a 6-h fast. Blood glucose levels were measured from the tail tip using a Freestyle brand glucometer (LifeScan, Shanghai, China).
After the designated LB100 treatment, L02 cells or mouse livers were lysed in the previously described RIPA lysis buffer. Supernatants containing 50 μg of total cellular proteins were assayed with the PP2A assay kit (Millipore) according to the manufacturer's instructions. The PP2A activity data are presented as the percentage of relative PP2A activity compared with that of the control.
Liver tissue homogenate and cells were collected for intrahepatic and intracellular triglyceride (TG) determination using a commercial kit (Applygen Technologies Inc.) according the manufacturer’s instructions. TG values were normalized to total protein content.
Liver sections were fixed in 10% formalin overnight, embedded in paraffin, sectioned and then stained with hematoxylin and eosin (H and E) for histological examination. For the detection of lipid accumulation, frozen liver sections and cell slides were stained with oil red O according to a standard protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Finally, liver sections and cell slides were imaged at 200 × magnification (Olympus, Tokyo, Japan). The data shown are from one representative experiment of three independent repeats.
The statistical methods used in this study were reviewed by Yi Zhang from the Institute of Statistics, School of Mathematical Sciences, Zhejiang University of China. Student’s t-test or two-way ANOVA was used in the statistical analysis. All statistical analyses were performed with SPSS 23 (IBM, Chicago, IL, United States). P < 0.05 was considered statistically significant. Data are presented as the mean ± standard deviation. All experiments were repeated at least three times independently.
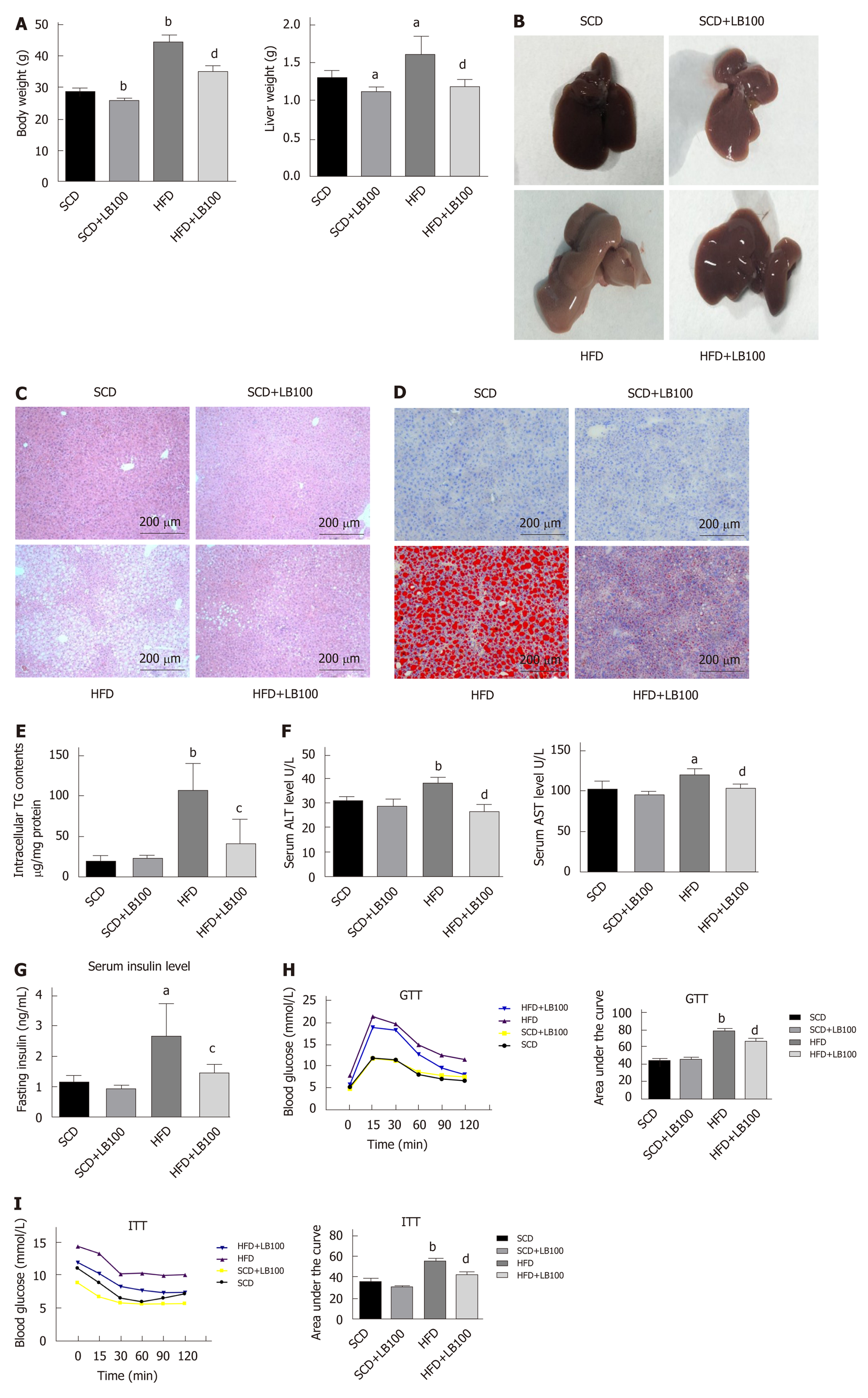
Mice supplemented with LB100 for 6 wk (HFD+LB100 mice) showed significant reductions in body and liver weights compared with those of mice fed the HFD (HFD mice) (Figure 1A). With regard to liver appearance, HFD mice had markedly larger and paler livers than those of HFD+LB100 mice (Figure 1B). In accordance with H and E staining, oil red O staining and hepatic TG analysis showed a dramatic decrease in lipid levels in HFD+LB100 mice compared with those in HFD mice, whereas no difference was observed in LB100-treated SCD-fed mice (SCD+LB100 mice) and SCD-fed mice (SCD mice) (Figure 1C-E). LB100 also attenuated HFD-induced liver injury with a marked decrease in serum alanine aminotransferase and aspartate aminotransferase levels (Figure 1F). However, LB100 did not significantly decrease serum total cholesterol and TG levels in HFD mice (Supplementary Figure 2A and B). HFD mice showed elevated fasting insulin levels compared with those of HFD+LB100 mice (Figure 1G). In addition, we performed GTTs and ITTs to determine if glucose homeostasis was regulated by LB100 and found that glucose homeostasis and insulin sensitivity were improved in HFD+LB100 mice (Figure 1H-I). In addition, we evaluated liver fibrosis by staining with both Sirius red and Masson's trichrome, and found there were almost no signs of fibrosis (Supplementary Figure 3A and B). These findings demonstrate that LB100 enhances insulin sensitivity and ameliorates body weight, hepatic steatosis and liver injury in HFD mice.
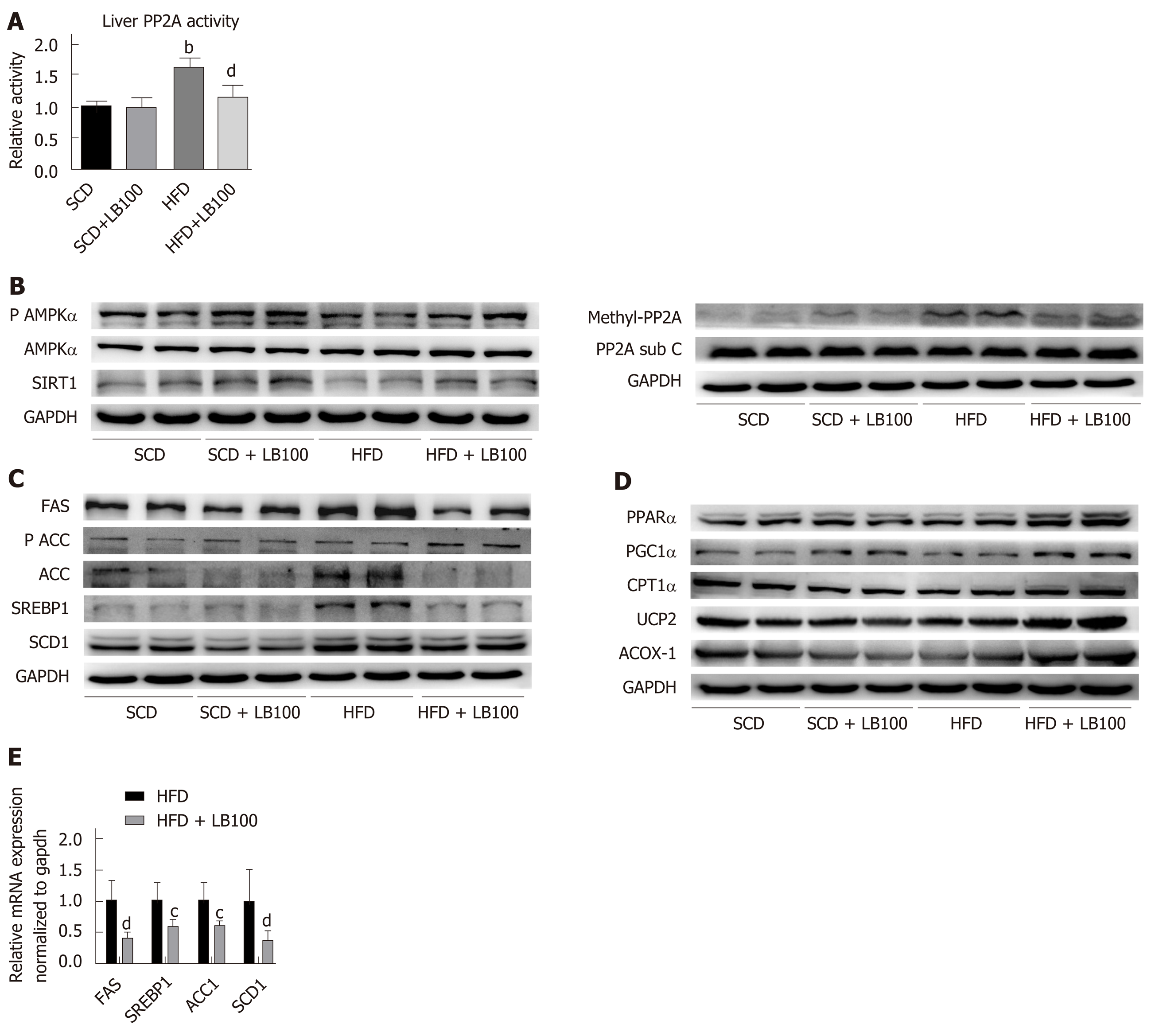
As expected, fatty acids increased liver PP2A activity, while LB100 reduced liver PP2A activity but did not affect protein levels of the PP2A catalytic subunit C (Figure 2A). In addition, the level of PP2A methylation in the livers of HFD mice was increased, while LB100 downregulated PP2A methylation levels (Figure 2A). As shown in Figure 2B, protein expression of Sirt1 and the ratio of P-AMPK (Thr172)/AMPK were markedly increased in HFD+LB100 mice. LB100 treatment significantly downregulated the levels of proteins involved in fatty acid synthesis (Figure 2C). In contrast, LB100 increased the expression of proteins involved in fatty acid β-oxidation, such as PPARα, PGC-1α, CPT1α, ACOX-1 and UCP-2, in the livers of HFD mice (Figure 2D). Moreover, we found that LB100 treatment significantly upregulated the mRNA levels of SREBP1, FAS, SCD1 and CPT1α (Figure 2E). These results indicate that LB100 regulates lipid metabolism by inhibiting PP2A activity and activating the AMPK/Sirt1 pathway in vivo.
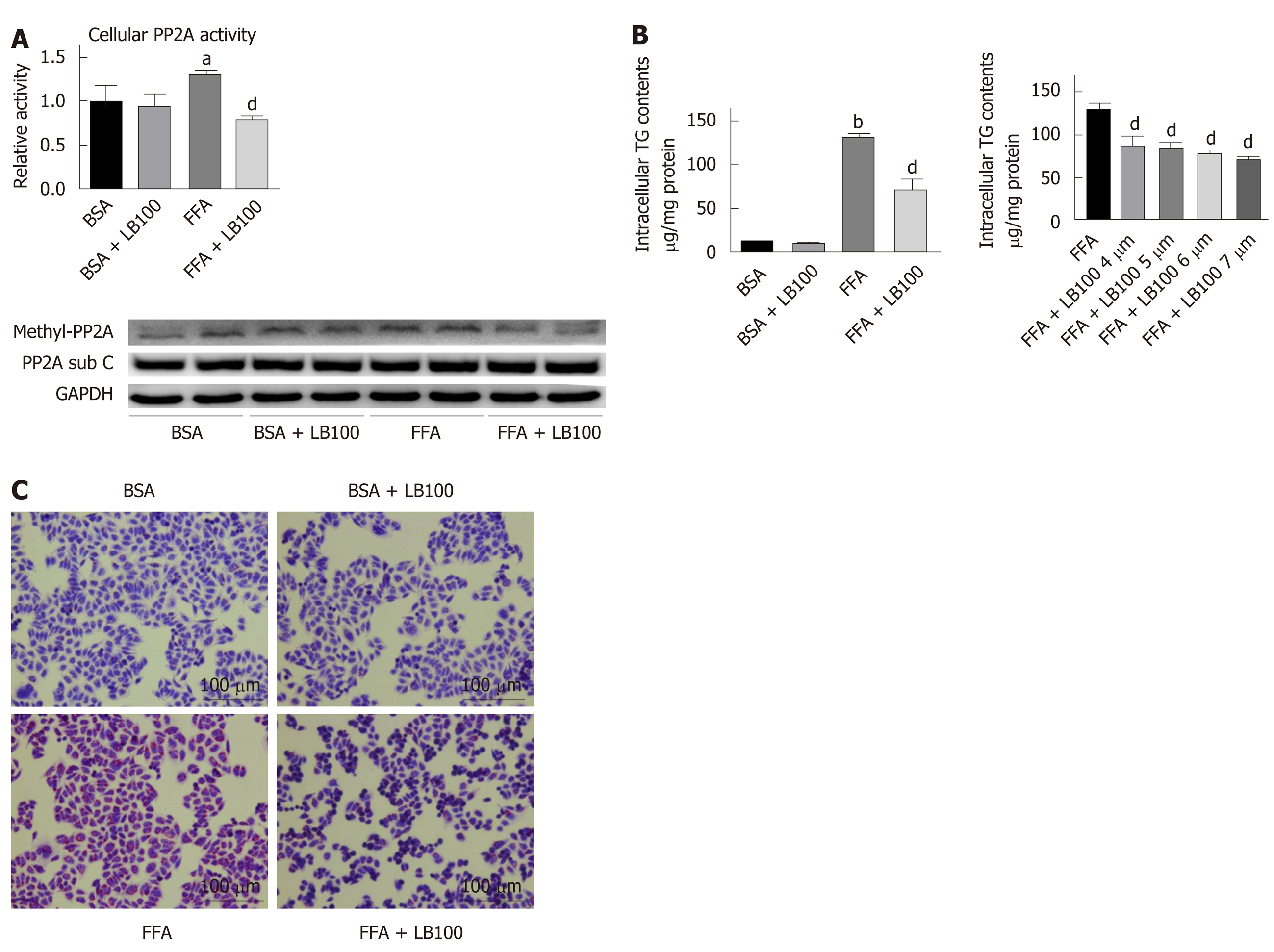
We also investigated the effect of LB100 on FFA-induced lipid deposition in vitro. The optimal concentration of LB100 treatment was 6 μmol/L, as evaluated by CCK-8 assays (Supplementary Figure 1B). Consistent with the in vivo results, fatty acids induced overactivation of PP2A, while LB100 impaired PP2A activity (Figure 3A). LB100 attenuated the FFA-induced intracellular TG accumulation in a dose-dependent manner and attenuated lipid accumulation, as shown by oil red O staining (Figure 3B and C). These findings reveal that LB100 alleviates FFA-induced lipid deposition in hepatocytes.
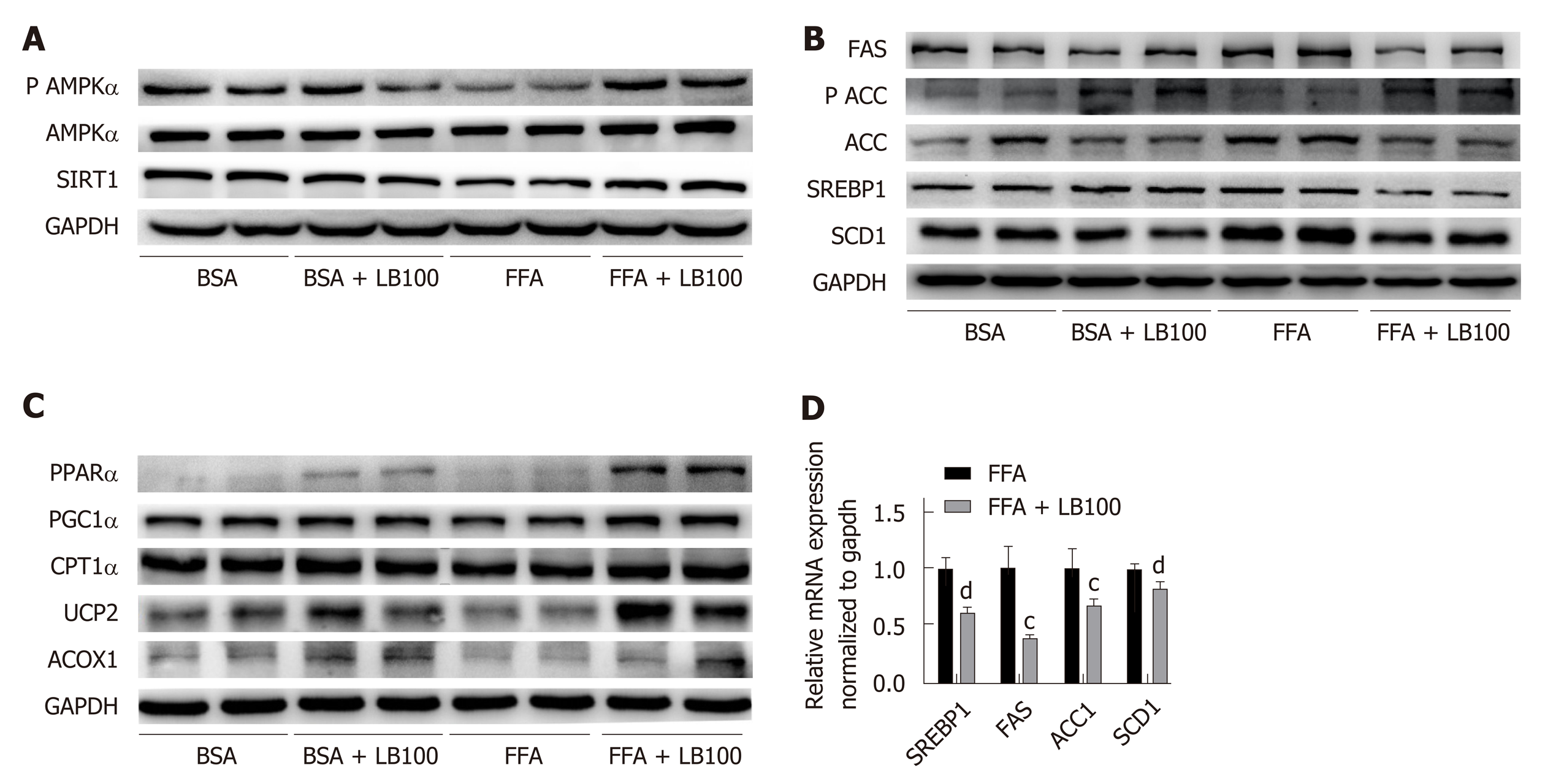
Consistent with the in vivo findings, LB100 upregulated the expression of Sirt1 and p-AMPKα and significantly decreased the protein levels of SREBP1 and its target genes, including SCD1, FAS and ACC, in FFA-treated L02 cells (Figure 4A and B). LB100 also induced PPARα, PGC-1α, CPT1α, ACOX-1 and UCP-2 expression in vitro (Figure 4C). As expected, LB100 significantly downregulated the mRNA levels of SREBP1, FAS, ACC1 and SCD1 (Figure 4D). These results indicate that LB100 ameliorates hepatic lipid accumulation in vitro by activating the AMPK/Sirt1 signaling pathway.
We further examined whether AMPK plays a key role in LB100-mediated alleviation of hepatic steatosis. As predicted, the inhibitory effect of LB100 on hepatic lipid accumulation was eliminated by preincubation with the AMPK inhibitor compound C. Cellular TG analysis showed a dramatic increase in lipid level with compound C use (Figure 5A). Compound C treatment counteracted the activation of the AMPK/Sirt1 signaling pathway by LB100 (Figure 5B) and significantly upregulated the protein levels of ACC and some other proteins involved in fatty acid synthesis (Figure 5C). Inhibition of AMPK also counteracted the LB100-induced increase in lipid oxidation protein levels, such as PPARα, CPT1α and UCP2 (Figure 5D). However, there was no significant difference in the protein levels of PGC1α and ACOX-1. (Supplementary Figure 4A). In addition, compound C abolished the LB100-mediated reduction in the mRNA levels of SREBP1, FAS, ACC1 and SCD1 (Figure 5E). Taken together, these data suggest that AMPK is vital for the role of LB100 in lipid metabolism.
In this study, we demonstrated that LB100 can play an important role in liver lipid metabolism to prevent HFD-induced obesity, hepatic steatosis and IR in a NAFLD mouse model. LB100 reduced hepatic lipogenesis and promoted fatty acid β-oxidation via the AMPK/Sirt1 pathway in HFD-fed mice, although no similar changes were observed in SCD-fed mice. These findings provide molecular evidence supporting LB100 as a promising therapeutic strategy for NAFLD.
NAFLD is characterized by fat accumulation without excessive alcohol consumption and has complex potential mechanisms. At present, a widely recognized pathogenesis of NAFLD is the double-hit theory: The first “hit” is IR, which induces an increase in peripheral fat decomposition, abnormal liver lipid metabolism and hyperinsulinemia, leading to liver steatosis; then the second "hit" of endoplasmic reticulum stress occurs, leading to the development of hepatocyte inflammation, necrosis and fibrosis, further promoting the progression of NAFLD[20,21]. Therefore, inhibition of de novo lipogenesis, enhancement of fatty liver β-oxidation and improvement in IR are promising strategies for the prevention and treatment of NAFLD.
A series of studies have demonstrated that FFAs induce overactivation of PP2A both in mice and in cultured cell lines, and PP2A is a negative regulator of insulin signaling pathways[16,22]. Moreover, inhibition of PP2A protects against HFD-induced obesity and IR in mice[16,17]. LB100 is a derivative of the natural product cantharidin, which shows no obvious systemic toxicity and could be used as a pharmacologic inhibitor of PP2A[15]. However, the underlying mechanism by which LB100 inhibits hepatic steatosis remains unclear. In this study, we first tested the activity of PP2A by measuring the methylation status of the catalytic subunit of PP2A and using immunoprecipitation activity assays, and then we confirmed FFA-induced PP2A overactivation both in vivo and in vitro. After treatment with LB100, the activity of PP2A decreased with amelioration of lipid accumulation both in vivo and in vitro. In addition, LB100 improved glucose metabolism and insulin sensitivity in HFD-fed mice. These results indicate that LB100 plays an important role in the development of IR and NAFLD.
Then, we further examined the potential mechanism of LB100 in lipid metabolism. Liangpunsakul et al[23] demonstrated, for the first time, that AMPK and PP2A can interact directly by immunoprecipitation. PP2A negatively regulates the phosphorylation state of AMPK by dephosphorylating Thr-172, as reported by Joseph et al[19]. We also found that LB100 increased the protein expression of P-AMPK (Thr172)/AMPK and Sirt1 both in vivo and in vitro. The results of the present study show that PP2A has essential roles in the regulation of autophagy. Marta Varela-Rey’s study showed that blocking PP2A activity restored autophagy flux in hepatocytes and ameliorated liver steatosis[24]. Some studies have described that inhibition of PP2A by okadaic acid suppresses autophagy in hepatocytes[25,26]. Our study, in which LB100 works through the activation of the AMPK/Sirt1 pathway, adds an exciting new mechanism through which LB100 exerts its beneficial effects. Undoubtedly, it is interesting to perform further studies to establish whether LB100 regulates lipid metabolism through the regulation of autophagy.
Recently, it was found that lipid accumulation in the liver inhibits AMPK activation and affects ACC expression, accelerating fatty acid synthesis and the development of NAFLD[27,28]. AMPK and Sirt1 are closely related to lipid metabolism and activate each other in a finely tuned network[8,9]. Treatments that enhance AMPK/Sirt1 expression to inhibit ACC activity and to increase lipolysis and β-oxidation can improve NAFLD in HFD-fed mice[29,30]. Sirt1 regulates lipid homeostasis through multiple nutrient sensors such as SREBP1, PGC-1α and PPARα[31,32]. In this study, as expected, LB100 reduced the expression of adipogenic genes such as SREBP1, FAS, ACC and SCD1 both at the protein and mRNA levels and increased the protein expression of fatty acid β-oxidation genes, including PPARα, PGC1α, CPT1α, ACOX1 and UCP2. Finally, the use of the AMPK inhibitor compound C largely counteracted the effect of LB100 on the attenuation of fatty accumulation in vitro. Compound C significantly upregulated SREBP1, FAS, ACC and SCD1, at both the protein and mRNA levels, and downregulated the protein expression of PPARα, CPT1α and UCP2. However, there was no significant difference in the protein levels of PGC1α and ACOX1 after compound C treatment, which we consider to be a negative feedback response; the regulation of these two proteins may also be through another pathway. All of these findings indicate that AMPK signaling is a critical metabolic cue involved in the process by which LB100 alleviates lipid deposition both in vivo and in vitro.
However, there are some limitations to this study. First, the function of PP2A is extensive and complex, and AMPK may not be the only target gene regulated by LB100; therefore, further research is required to clarify the detailed downstream mechanism. Second, compound C treatment failed to neutralize the increased expression of PGC1α and ACOX1, and the regulatory mechanisms require further investigation.
In summary, our study provided, for the first time, in vivo and in vitro evidence that LB100 can effectively inhibit hepatic lipogenesis via the AMPK/Sirt1 pathway and could be a therapeutic strategy for NAFLD.
Nowadays, no pharmacological therapy is approved for nonalcoholic fatty liver disease (NAFLD). Recent studies have shown that serine/threonine protein phosphatase 2A (PP2A) is closely related to obesity and insulin resistance. LB100 is a water-soluble PP2A small molecule inhibitor. Thus, we hypothesize that LB100 can ameliorate hepatic lipid accumulation in fatty liver.
Increasing evidence indicates that PP2A regulates the AMPK, which is a monitor of cellular energy status. AMPK and Sirtuin 1 (Sirt1) are closely related to lipid metabolism and activate each other in a finely tuned network. However, as a first-line PP2A inhibitor, there is little data regarding the influence of LB100 on NAFLD and its underlying mechanism. Thus, studies on the potential effect of LB100 on NAFLD are urgently required.
To elucidate the effect and underlying mechanism of LB100 in NAFLD.
This research was performed using C57BL/6 mice fed a high fat diet (HFD) for 16 wk and L02 cells stimulated with free fatty acids (FFAs) for 24 h to establish in vivo and in vitro models of hepatic steatosis. Mice were injected intraperitoneally with vehicle or LB100 (1.5 mg/kg, three times a week) and L02 cells were treated with LB100 (6 μmol/L) to determine the effect of LB100 on NAFLD.
LB100 significantly ameliorated HFD-induced obesity, hepatic lipid accumulation and hepatic injury in mice accompanied by activation of the AMPK/Sirt1 signaling pathway. Similar results were observed in L02 cells stimulated with FFAs. Further studies showed that the curative effect of LB100 on lipid deposition was abolished by pharmacological inhibition of AMPK in L02 cells.
PP2A inhibition by LB100 significantly ameliorates hepatic steatosis by regulating hepatic lipogenesis and fatty acid oxidation via the AMPK/Sirt1 pathway.
LB100 is a promising therapeutic strategy for NAFLD. Further clinical application should be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Leo A, Jamali R, Tarantino G, Treeprasertsuk S S-Editor: Tang JZ L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 2. | Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7544] [Article Influence: 838.2] [Reference Citation Analysis (0)] |
| 4. | Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology. 2019;70:1119-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 421] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 5. | Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 984] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 7. | Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond). 2008;32 Suppl 4:S7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 522] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 8. | Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2659] [Cited by in RCA: 2563] [Article Influence: 160.2] [Reference Citation Analysis (0)] |
| 9. | Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015-20026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 650] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 10. | Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3802] [Cited by in RCA: 4212] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 11. | Shimano H. Sterol regulatory element-binding protein-1 as a dominant transcription factor for gene regulation of lipogenic enzymes in the liver. Trends Cardiovasc Med. 2000;10:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Latasa MJ, Moon YS, Kim KH, Sul HS. Nutritional regulation of the fatty acid synthase promoter in vivo: sterol regulatory element binding protein functions through an upstream region containing a sterol regulatory element. Proc Natl Acad Sci USA. 2000;97:10619-10624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Osler ME, Zierath JR. Adenosine 5'-monophosphate-activated protein kinase regulation of fatty acid oxidation in skeletal muscle. Endocrinology. 2008;149:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1554] [Cited by in RCA: 1462] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 15. | Lu J, Kovach JS, Johnson F, Chiang J, Hodes R, Lonser R, Zhuang Z. Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms. Proc Natl Acad Sci USA. 2009;106:11697-11702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Galbo T, Olsen GS, Quistorff B, Nishimura E. Free fatty acid-induced PP2A hyperactivity selectively impairs hepatic insulin action on glucose metabolism. PLoS One. 2011;6:e27424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Xian L, Hou S, Huang Z, Tang A, Shi P, Wang Q, Song A, Jiang S, Lin Z, Guo S, Gao X. Liver-specific deletion of Ppp2cα enhances glucose metabolism and insulin sensitivity. Aging (Albany NY). 2015;7:223-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Park S, Scheffler TL, Rossie SS, Gerrard DE. AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. Cell Calcium. 2013;53:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Joseph BK, Liu HY, Francisco J, Pandya D, Donigan M, Gallo-Ebert C, Giordano C, Bata A, Nickels JT. Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J Biol Chem. 2015;290:10588-10598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3130] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 21. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1660] [Article Influence: 118.6] [Reference Citation Analysis (2)] |
| 22. | Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol. 2004;24:8778-8789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Liangpunsakul S, Wou SE, Zeng Y, Ross RA, Jayaram HN, Crabb DW. Effect of ethanol on hydrogen peroxide-induced AMPK phosphorylation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1173-G1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Zubiete-Franco I, García-Rodríguez JL, Martínez-Uña M, Martínez-Lopez N, Woodhoo A, Juan VG, Beraza N, Lage-Medina S, Andrade F, Fernandez ML, Aldámiz-Echevarría L, Fernández-Ramos D, Falcon-Perez JM, Lopitz-Otsoa F, Fernandez-Tussy P, Barbier-Torres L, Luka Z, Wagner C, García-Monzón C, Lu SC, Aspichueta P, Mato JM, Martínez-Chantar ML, Varela-Rey M. Methionine and S-adenosylmethionine levels are critical regulators of PP2A activity modulating lipophagy during steatosis. J Hepatol. 2016;64:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Samari HR, Møller MT, Holden L, Asmyhr T, Seglen PO. Stimulation of hepatocytic AMP-activated protein kinase by okadaic acid and other autophagy-suppressive toxins. Biochem J. 2005;386:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Blankson H, Holen I, Seglen PO. Disruption of the cytokeratin cytoskeleton and inhibition of hepatocytic autophagy by okadaic acid. Exp Cell Res. 1995;218:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Davies SP, Carling D, Munday MR, Hardie DG. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992;203:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | López M. Hypothalamic AMPK and energy balance. Eur J Clin Invest. 2018;48:e12996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Liou CJ, Dai YW, Wang CL, Fang LW, Huang WC. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019;33:11791-11803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Salomone F, Barbagallo I, Godos J, Lembo V, Currenti W, Cinà D, Avola R, D'Orazio N, Morisco F, Galvano F, Li Volti G. Silibinin Restores NAD⁺ Levels and Induces the SIRT1/AMPK Pathway in Non-Alcoholic Fatty Liver. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 912] [Cited by in RCA: 883] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 32. | Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959-33970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 438] [Article Influence: 29.2] [Reference Citation Analysis (0)] |