Published online Oct 21, 2019. doi: 10.3748/wjg.v25.i39.5953
Peer-review started: July 25, 2019
First decision: August 17, 2019
Revised: August 28, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 21, 2019
Processing time: 87 Days and 21.2 Hours
Portal hypertension (PHT) is primarily caused by an increase in resistance to portal outflow and secondarily by an increase in splanchnic blood flow. Vascular hyporeactivity both in systemic circulation and in the mesenteric artery plays a role in the hyperdynamic circulatory syndrome.
To explore gender differences and the role of endogenous sex hormones in PHT and vascular reactivity of mesenteric arterioles in rats.
Cirrhosis and PHT were established by subcutaneous injection of carbon tetrachloride (CCl4) in both male and female integral and castrated rats (ovariectomized [OVX] in female rats, orchiectomy [ORX] in male rats). The third-order branch of the mensenteric artery was divided and used to measure vascular reactivity to vasoconstrictors.
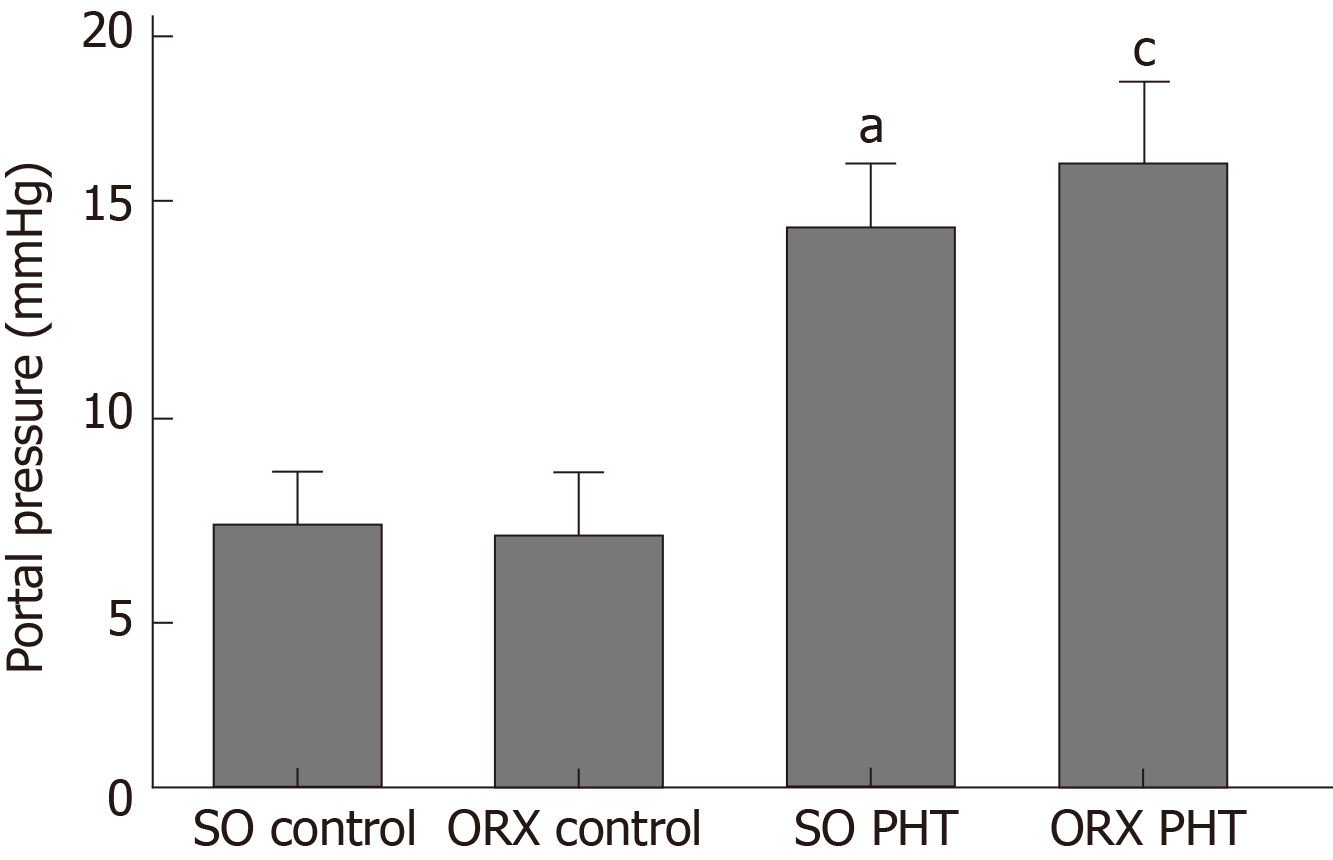
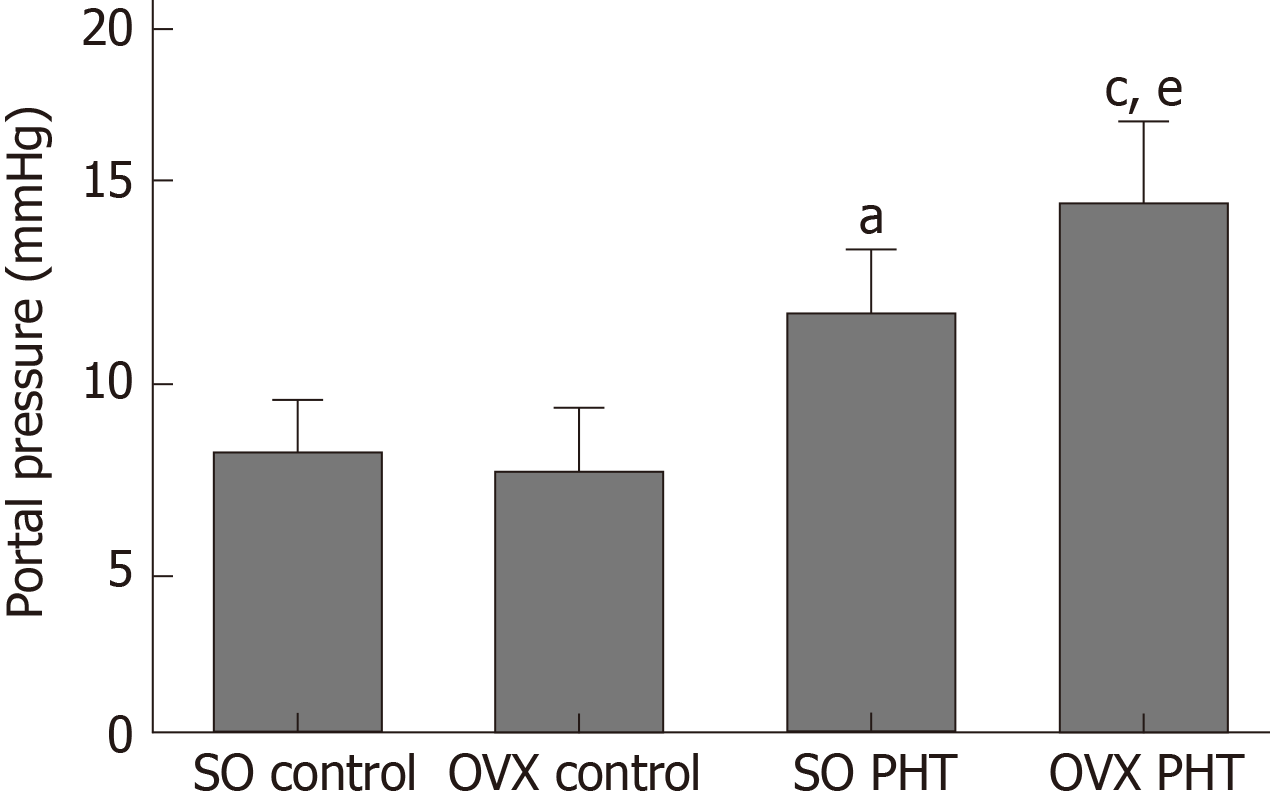
No significant difference in portal pressure was observed between integral and castrated male PHT rats (15.2 ± 2.1 mmHg vs 16.7 ± 2.7 mmHg, P > 0.05). The portal pressure in integral female PHT rats was lower than that in OVX female PHT rats (12.7 ± 2.7 mmHg vs 16.5 ± 2.4 mmHg, P < 0.05). In PHT rats, the concentration response curves of the mesenteric arterioles to norepinephrine were shifted to the right, and the maximal responses (Emax) values were decreased and effective concentrations causing half maximum responses (EC50) values were increased, compared to those of non-PHT rats, both in male and female rats. Compared to non-PHT integral male rats, the sensitivity of the mesenteric arterioles of non-PHT ORX male rats to norepinephrine was decreased (P > 0.05). However, there was no difference between integral and ORX male rats with PHT. In integral female PHT rats, the concentration response curves were shifted to the left (P < 0.05), and the Emax values were increased and EC50 values were decreased compared to OVX female PHT rats.
Clear gender differences were observed in mesenteric vascular reactivity in CCl4-induced cirrhotic and PHT rats. Conservation of estrogen can retain the sensitivity of the mesenteric arterioles to vasoconstrictors and has a protective effect on splanchnic vascular function in PHT.
Core tip: In cirrhosis, extrahepatic vascular hypocontractility leads to splanchnic vasodilation and decreased splanchnic vascular resistance. In this study, clear gender differences were observed in mesenteric vascular reactivity in carbon tetrachloride-induced cirrhotic and portal hypertensive rats. Conservation of estrogen can retain the sensitivity of mesenteric arterioles to vasoconstrictors and has a protective effect on splanchnic vascular function in portal hypertension.
- Citation: Zhang B, Ji LH, Zhang CG, Zhao G, Wu ZY. Gender differences in vascular reactivity of mesenteric arterioles in portal hypertensive and non-portal hypertensive rats. World J Gastroenterol 2019; 25(39): 5953-5960
- URL: https://www.wjgnet.com/1007-9327/full/v25/i39/5953.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i39.5953
Portal hypertension (PHT) is primarily caused by an increase in resistance to portal outflow and secondarily by an increase in splanchnic blood flow, which worsens and maintains the increased portal pressure[1,2]. Vascular hyporeactivity both in systemic circulation and in the mesenteric artery plays a role in the hyperdynamic circulatory syndrome[1,2].
Gender differences in the incidence of liver cirrhosis, PHT, and vascular responsiveness have been demonstrated by some epidemiological and experimental studies[3-6]. Cirrhotic rats treated with estradiol showed a significant decrease in portal pressure and a significant increase in hepatic blood flow, consistent with increased nitric oxide synthase in sinusoidal endothelial cells and inhibited activation of hepatic stellate cells. However, ICI-182.780 (an estrogen receptor antagonist) completely inhibits the reduction of portal pressure and elevation of hepatic blood flow[6,7]. Estradiol inhibits the activation of transcription factors by suppressing reactive oxygen species generation and mitogen-activated protein kinase pathways, and inactivates the downstream transcription processes involved in transforming growth factor-β1 expression and hepatic stellate cell activation. In contrast, progesterone acts in opposition to the favorable effects of estradiol and its effects are blocked by estradiol[8]. In male rats with PHT, the phenylephrine concentration–response curves of aortic rings with and without endothelium are lowered and shifted to the right. However, PHT does not induce vascular hyporesponsiveness in female rats[3].
The aim of this study was to investigate the influence of endogenous sex hormones on PHT and hyporeactivity of mesenteric arteries. Therefore, we investigated the gender difference in PHT and vascular reactivity of mesenteric arterioles by establishing a carbon tetrachloride (CCl4)-induced PHT model with both male and female integral and castrated rats.
Animal maintenance and experimental procedures were performed in accordance with the guidelines of the Laboratory Animal Care and Use Committee at Shanghai Jiao Tong University School of Medicine and were approved by the local Animal Ethics Committee of Renji Hospital (Shanghai, China).
Forty female (weighing 183 ± 12 g) and forty male (weighing 202 ± 18 g) Sprague–Dawley rats, obtained from SLAC (Shanghai, China), with an average age of approximately 8 wk, were housed in a temperature- and humidity-controlled environment with 12-h light/dark cycles and free access to food and water.
Half of the female rats underwent bilateral ovariectomized (OVX) and the other half underwent sham operation (SO). Meanwhile, half of the male rats underwent bilateral orchiectomy (ORX) and the other half underwent SO. At 2 wk after the primary surgery, the female rats were randomly divided as follows into four groups of 10 rats each: SO control, OVX control, SO PHT, and OVX PHT. The male rats were similarly divided into four groups: SO control, ORX control, SO PHT, and ORX PHT. The PHT groups were subcutaneously injected with 40% CCl4 in peanut oil at a dose of 0.4 mL/100 g body weight twice weekly, for 12 wk. The control groups were treated subcutaneously with the same volume of saline.
At the end of the 12-wk experimental period, the rats were anesthetized with 1% sodium pentobarbital (0.4 mL/100 g body weight). A 22 G catheter was introduced into the portal vein to measure portal pressure after making an incision at the midline of the abdomen. All parameters were recorded using the SP840 pressure transducer and a multichannel recorder (Philips, Irvine, CA, United States)[4].
Following the determination of portal pressure, the mesenteric arteries were removed, as previously described[4]. Briefly, the third-order arterioles of the mesentery were carefully dissected, and transferred to a vascular perfusion system[4]. Cumulative norepinephrine (NE) concentration response curves (10−8 mol/L-10−4 mol/L) were obtained by increasing the concentration in quarter-log increments[4].
Cumulative NE concentration response curves were fitted by a non-linear regression analysis (GraphPad Software Inc., San Diego, CA, United States). Maximal responses (Emax) and effective concentrations causing half maximum responses (EC50) were obtained from the curves. Values are expressed as the means ± standard deviations. Statistical comparisons were performed using one-way analysis of variance. P < 0.05 was considered significant. All statistical analyses were performed by GraphPad Software.
In male rats, administration of CCl4 induced significant PHT; however, no difference was found between SO PHT and ORX PHT rats (15.2 ± 2.1 mmHg vs 16.7 ± 2.7 mmHg, P > 0.05; Figure 1).
In female rats, administration of CCl4 also induced significant PHT; however, the portal pressure in SO PHT rats was lower than that in OVX PHT rats (12.7 ± 2.7 mmHg vs 16.5 ± 2.4 mmHg, P < 0.05; Figure 2).
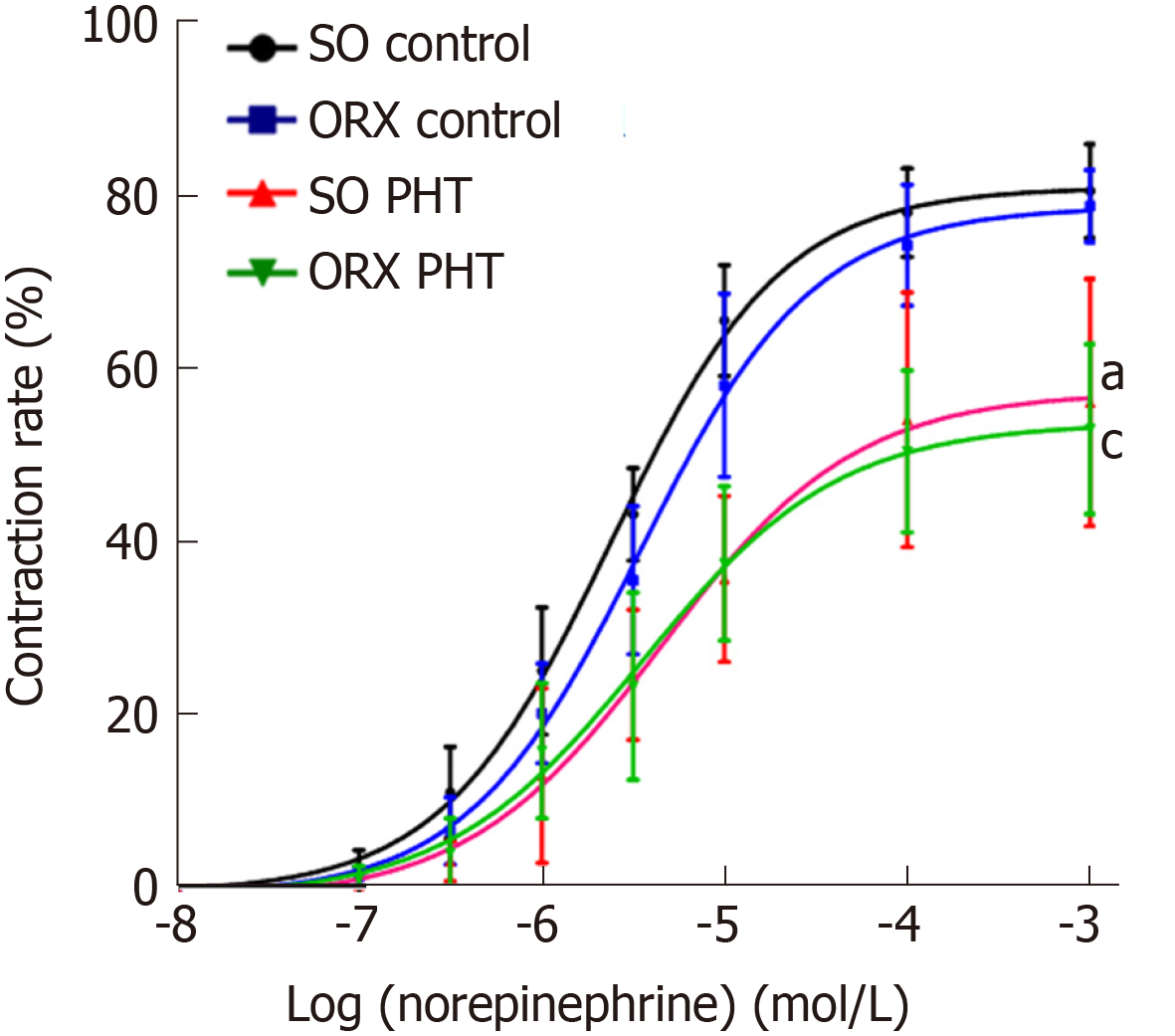
In non-PHT male rats, cumulative NE concentration response curves of mesenteric arterioles in ORX control rats was shifted to the right compared to that in SO control rats, with a similar Emax (78.71 ± 4.80% vs 80.95 ± 6.18%, P > 0.05), but a higher EC50 (4.17 ± 2.45 × 10-6 mol/L vs 2.51 ± 0.63 × 10−6 mol/L, P > 0.05), indicating that the sensitivity of mesenteric arterioles to NE might be slightly decreased because of castration (Figure 3, Table 1).
In the SO and ORX PHT rats, the concentration response curves were shifted to the right, with decreased Emax values (56.93 ± 15.33% and 52.76 ± 10.29% vs 78.71 ± 4.80%, P < 0.05) and increased EC50 values (4.77 ± 2.17 × 10-6 mol/L and 4.31 ± 2.89 × 10-6 mol/L vs 2.51 ± 0.63 × 10-6 mol/L, P > 0.05 and P < 0.05, respectively), compared to non-PHT integral male rats.
The concentration response curves between SO PHT and ORX PHT male rats coincided with each other, with similar Emax (56.93 ± 15.33% vs 52.76 ± 10.29%, P > 0.05) and similar EC50 (4.77 ± 2.17 × 10-6 mol/L vs 4.31 ± 2.89 × 10-6 mol/L, P > 0.05).
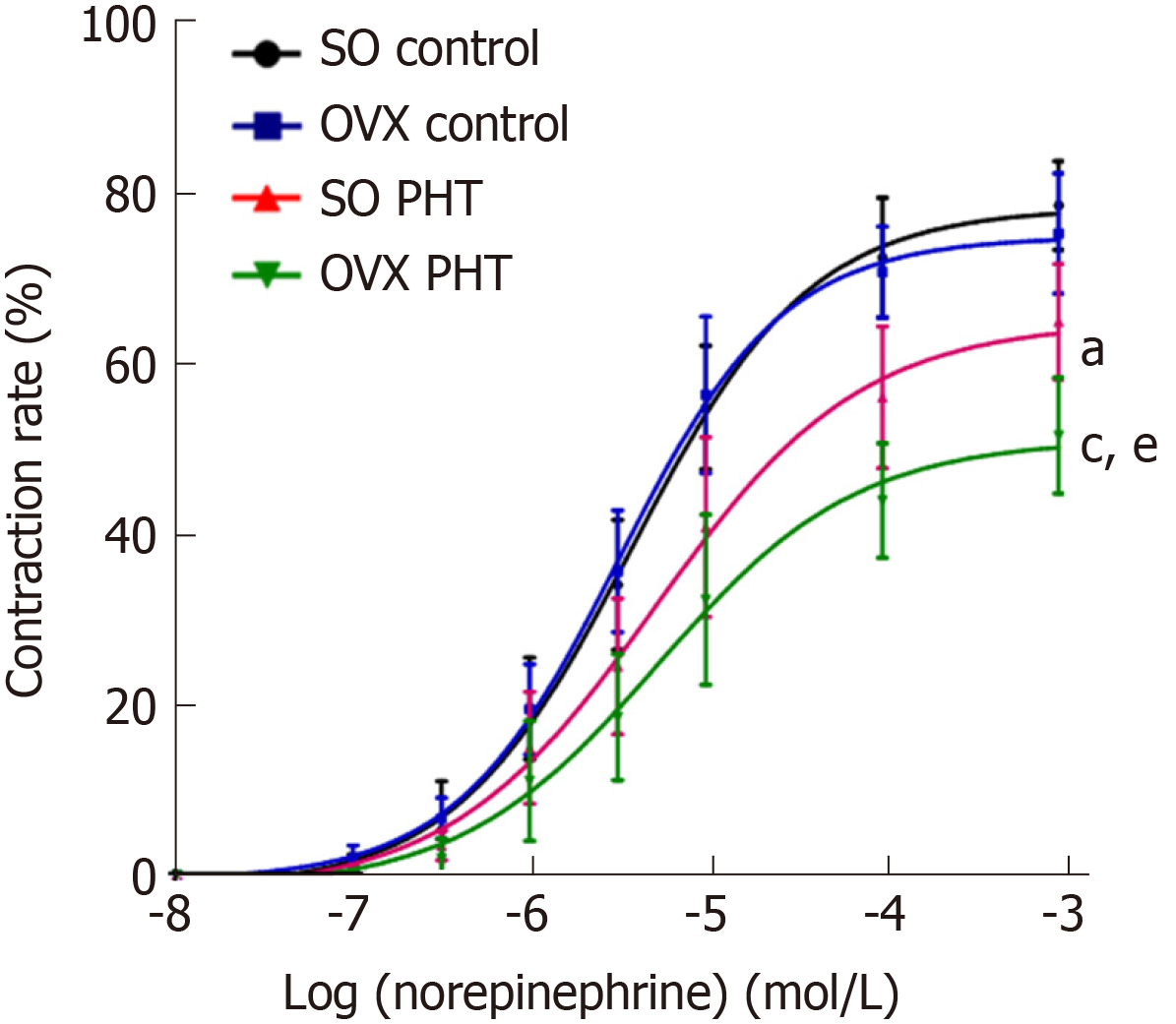
In non-PHT female rats, concentration response curves coincided with each other in SO control and OVX control rats, with similar Emax values (77.27 ± 6.37% vs 74.84 ± 5.91%, P > 0.05) and EC50 values (4.22 ± 1.97 × 10-6 mol/L vs 3.50 ± 1.48 × 10-6 mol/L, P > 0.05, Figure 4, Table 2).
In the SO PHT and OVX PHT rats, the concentration response curves were shifted to the right, with decreased Emax values (64.71 ± 7.53% and 53.70 ± 10.49% vs 77.27 ± 6.37%, P < 0.05) and increased EC50 values (7.14 ± 7.71 × 10−6 mol/L and 7.78 ± 9.28 × 10−6 mol/L vs 4.22 ± 1.97 × 10−6 mol/L, P > 0.05), compared to non-PHT integral female (SO control) rats.
However, the concentration response curve was lowered and shifted to the right in OVX PHT rats compared to SO PHT rats, with a lower Emax (53.70 ± 10.49% vs 64.71 ± 7.53%, P < 0.05) and higher EC50 (7.78 ± 9.28 × 10-6 mol/L vs 7.14 ± 7.71 × 10-6 mol/L, P > 0.05).
Splanchnic vasodilation is the pathophysiological hallmark in the development of hyperdynamic circulatory syndrome in liver cirrhosis and PTH[9,10]. This has been attributed mainly to marked vascular hyporeactivity to endogenous vasoconstrictors. In cirrhosis, extrahepatic vascular hypocontractility leads to vasodilation and contributes to PHT[9,10]. The increased portal tributary blood flow is attributable to decreased splanchnic vascular resistance and consecutive splanchnic vasodilation[11]. This splanchnic vasodilation is mediated by overproduction of vasodilators (such as nitric oxide [NO]) and by concomitant defects in contractile signaling pathways (such as RhoA/Rho-kinase signaling pathway)[11].
Previous studies on vascular reactivity mostly used isolated aorta, peripheral arteries, or mesenteric arteries. However, vascular resistance mainly depends on the arterioles rather than the aorta, and the physiological mechanisms of regulating vasoconstriction in arterioles and aortas are not entirely the same[12,13]. The resistance of the splanchnic arteries in PHT depends mainly on the mesenteric arteries, especially the pre-capillary resistance vessels (diameter within 260 μm)[14]. In this study of vascular reactivity, we investigated the change in inner diameter of the third branches of the mesenteric arteries (diameter~100 μm) under the microamplification system. By this technique, we evaluated small changes in the blood vessels by exogeneous vasoconstrictors, which showed good effects in our previous experiments[15].
Our study showed that ORX decreased the sensitivity to vasoconstrictors of the mesenteric arterioles of non-PHT male rats, which is consistent with the study of Rorbert et al[3], indicating that androgen affects vascular tone in physiological conditions[16,17]. However, in cirrhotic and PHT rats, androgens had little effect on the vascular reaction to vasoconstrictors.
In contrast to male rats, OVX had no effect on the vascular reaction to NE in non-PHT female rats. Compared to OVX female PHT rats, the sensitivity of the mesenteric arterioles to NE in integral female PHT rats was enhanced, indicating that conservation of estrogen can retain the sensitivity of the mesenteric arterioles to vasoconstrictors and have a protective effect in splanchnic vascular function in PHT.
Estrogen plays an important role in reducing the portal pressure in cirrhotic rats, mainly by the modulation of endothelial NO synthase and NO production, oxidative stress and RhoA/ROCK pathway, either in sinusoidal endothelial cells of cirrhotic liver or extrahepatic arteries, which could be blocked by ICI-182.780[7,18].
In summary, estrogen can improve hyporeactivity of the splanchnic arteries to vasoconstrictors, while androgens cannot. Further investigations are required to explain these differences.
Portal hypertension (PHT) is primarily caused by an increase in resistance to portal outflow and secondarily by an increase in splanchnic blood flow. Vascular hyporeactivity both in systemic circulation and in the mesenteric artery plays a role in the hyperdynamic circulatory syndrome. Gender differences in the incidence of liver cirrhosis, PHT and vascular responsiveness have been demonstrated by some epidemiological and experimental studies. Cirrhotic rats treated with estradiol showed a significant decrease in portal pressure and a significant increase in hepatic blood flow, consistent with increased nitric oxide synthase in sinusoidal endothelial cells and inhibited activation of hepatic stellate cells. Previous studies on vascular reactivity mostly used isolated aorta, peripheral arteries, or mesenteric arteries. In this study of vascular reactivity, we investigated the change in inner diameter of the third branches of the mesenteric arteries (diameter ~100 μm) under the microamplification system.
Despite the increased level of circulating endogenous vasoconstrictors in PHT, the sensitivity of blood vessels to them is significantly reduced. The pathogenetic mechanisms of this phenomenon have not been fully investigated.
The aim of this study was to investigate the influence of endogenous sex hormones on PHT and hyporeactivity of mesenteric arteries.
Cirrhosis and PHT were established by subcutaneous injection of CCl4 in both male and female integral and castrated rats (ovariectomized [OVX] in female rats, orchiectomy [ORX] in male rats). The third-order branch of the mensenteric artery was divided and used to measure vascular reactivity to vasoconstrictors. The third-order arterioles of the mesentery were carefully dissected and transferred to a vascular perfusion system. Two glass micropipettes (top diameter, 50 μm) were inserted into each end of the arteriole. Cumulative norepinephrine (NE) concentration response curves (10-8 mol/L-10-4 mol/L) were obtained by increasing the concentration in quarter-log increments.
ORX decreased the sensitivity to vasoconstrictors of the mesenteric arterioles of non-PHT male rats, indicating that androgen affects vascular tone in physiological conditions. However, in cirrhotic and PHT rats, conservation of androgens had little effect on the vascular reaction to vasoconstrictors. OVX had no effect on the vascular reaction to NE in non-PHT female rats. Compared to OVX female PHT rats, the sensitivity of mesenteric arterioles to NE in integral female PHT rats was enhanced, indicating that conservation of estrogen can retain the sensitivity of the mesenteric arterioles to vasoconstrictors and has a protective effect on splanchnic vascular function in PHT.
Clear gender differences were observed in mesenteric vascular reactivity in carbon tetrachloride-induced cirrhotic and PHT rats. Conservation of estrogen can retain the sensitivity of the mesenteric arterioles to vasoconstrictors and has a protective effect on splanchnic vascular function in PHT.
Estrogen can improve hyporeactivity of the splanchnic arteries to vasoconstrictors, while androgens cannot. Endothelial NO synthase and NO production, oxidative stress, and some signal pathways may participate in the underlying mechanism.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kreisel W, Garbuzenko DV S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Gatta A, Bolognesi M, Merkel C. Vasoactive factors and hemodynamic mechanisms in the pathophysiology of portal hypertension in cirrhosis. Mol Aspects Med. 2008;29:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Tsai MH, Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125:1452-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Robert R, Chagneau-Derrode C, Carretier M, Mauco G, Silvain C. Gender differences in vascular reactivity of aortas from rats with and without portal hypertension. J Gastroenterol Hepatol. 2005;20:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Zhang B, Zhang CG, Zhou QB, Chen W, Wu ZY. Estrogen improves the hyperdynamic circulation and hyporeactivity of mesenteric arteries by alleviating oxidative stress in partial portal vein ligated rats. World J Gastroenterol. 2013;19:6863-6868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Giard JM, Terrault NA. Women with Cirrhosis: Prevalence, Natural History, and Management. Gastroenterol Clin North Am. 2016;45:345-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Zhang B, Wu ZY. Estrogen derivatives: novel therapeutic agents for liver cirrhosis and portal hypertension. Eur J Gastroenterol Hepatol. 2013;25:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Sakamoto M, Ueno T, Nakamura T, Sakata R, Hasimoto O, Torimura T, Sata M. Improvement of portal hypertension and hepatic blood flow in cirrhotic rats by oestrogen. Eur J Clin Invest. 2005;35:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Itagaki T, Shimizu I, Cheng X, Yuan Y, Oshio A, Tamaki K, Fukuno H, Honda H, Okamura Y, Ito S. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 2005;54:1782-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 9. | Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl (4)-induced liver cirrhosis. Eur J Clin Invest. 2009;39:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Hennenberg M, Trebicka J, Biecker E, Schepke M, Sauerbruch T, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology. 2007;45:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Trebicka J, Leifeld L, Hennenberg M, Biecker E, Eckhardt A, Fischer N, Pröbsting AS, Clemens C, Lammert F, Sauerbruch T, Heller J. Hemodynamic effects of urotensin II and its specific receptor antagonist palosuran in cirrhotic rats. Hepatology. 2008;47:1264-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Limbu R, Cottrell GS, McNeish AJ. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS One. 2018;13:e0192484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Schmidt PM, Escobar AG, Torres JG, Martinez CS, Rizzetti DA, Kunz SN, Vassallo DV, Alonso MJ, Peçanha FM, Wiggers GA. Aluminum exposure for one hour decreases vascular reactivity in conductance and resistance arteries in rats. Toxicol Appl Pharmacol. 2016;313:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Jadeja RN, Thounaojam MC, Khurana S. Characterization of pressure-mediated vascular tone in resistance arteries from bile duct-ligated rats. Oncotarget. 2017;8:30706-30722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Chen W, Liu DJ, Huo YM, Wu ZY, Sun YW. Reactive oxygen species are involved in regulating hypocontractility of mesenteric artery to norepinephrine in cirrhotic rats with portal hypertension. Int J Biol Sci. 2014;10:386-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Pál É, Hadjadj L, Fontányi Z, Monori-Kiss A, Lippai N, Horváth EM, Magyar A, Horváth E, Monos E, Nádasy GL, Benyó Z, Várbíró S. Gender, hyperandrogenism and vitamin D deficiency related functional and morphological alterations of rat cerebral arteries. PLoS One. 2019;14:e0216951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, Böhm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |