Published online Oct 14, 2019. doi: 10.3748/wjg.v25.i38.5800
Peer-review started: July 16, 2019
First decision: August 2, 2019
Revised: September 11, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: October 14, 2019
Processing time: 89 Days and 18.6 Hours
Sirtuin 1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase that is involved in various diseases, including cancers, metabolic diseases, and inflammation-associated diseases. However, the role of SIRT1 in ulcerative colitis (UC) is still confusing.
To investigate the role of SIRT1 in intestinal epithelial cells (IECs) in UC and further explore the underlying mechanisms.
We developed a coculture model using macrophages and Caco-2 cells. After treatment with the SIRT1 activator SRT1720 or inhibitor nicotinamide (NAM), the expression of occludin and zona occludens 1 (ZO-1) was assessed by Western blot analysis. Annexin V-APC/7-AAD assays were performed to evaluate Caco-2 apoptosis. Dextran sodium sulfate (DSS)-induced colitis mice were exposed to SRT1720 or NAM for 7 d. Transferase-mediated dUTP nick-end labeling (TUNEL) assays were conducted to assess apoptosis in colon tissues. The expression levels of glucose-regulated protein 78 (GRP78), CCAAT/enhancer-binding protein homologous protein (CHOP), caspase-12, caspase-9, and caspase-3 in Caco-2 cells and the colon tissues of treated mice were examined by quantitative real-time PCR and Western blot.
SRT1720 treatment increased the protein levels of occludin and ZO-1 and inhibited Caco-2 apoptosis, whereas NAM administration caused the opposite effects. DSS-induced colitis mice treated with SRT1720 had a lower disease activity index (P < 0.01), histological score (P < 0.001), inflammatory cytokine levels (P < 0.01), and apoptotic cell rate (P < 0.01), while exposure to NAM caused the opposite effects. Moreover, SIRT1 activation reduced the expression levels of GRP78, CHOP, cleaved caspase-12, cleaved caspase-9, and cleaved caspase-3 in Caco-2 cells and the colon tissues of treated mice.
SIRT1 activation reduces apoptosis of IECs via the suppression of endoplasmic reticulum stress-mediated apoptosis-associated molecules CHOP and caspase-12. SIRT1 activation may be a potential therapeutic strategy for UC.
Core tip: The purpose of this article was to investigate the role of sirtuin 1 (SIRT1) in intestinal epithelial cells (IECs) in ulcerative colitis (UC) in a UC coculture model and in mice with dextran sodium sulfate (DSS)-induced colitis. It was found that SIRT1 activation contributes to enhanced intestinal barrier and reduced apoptosis of IECs via the suppression of endoplasmic reticulum stress-mediated apoptosis-associated molecules CCAAT/enhancer-binding protein homologous protein and caspase-12. SIRT1 activation may be a potential therapeutic strategy for UC.
- Citation: Ren MT, Gu ML, Zhou XX, Yu MS, Pan HH, Ji F, Ding CY. Sirtuin 1 alleviates endoplasmic reticulum stress-mediated apoptosis of intestinal epithelial cells in ulcerative colitis. World J Gastroenterol 2019; 25(38): 5800-5813
- URL: https://www.wjgnet.com/1007-9327/full/v25/i38/5800.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i38.5800
Ulcerative colitis (UC), the main subtype of inflammatory bowel disease (IBD), is a chronic relapsing inflammatory disorder of the large intestine. The incidence and prevalence of UC have increased in recent years[1]. The etiology of UC remains obscure and involves a combination of genetics, environment, microbiota, and the immune system[2,3]. It is widely believed that dysregulation of cytokines (e.g., tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-10, and IL-21), oxidative stress, and abnormal immune responses are key players in the progression of UC[4]. Recent data demonstrate that the intestinal epithelium, which plays a crucial role in the occurrence and persistence of UC, is a highly dynamic tissue rather than a simple physical barrier[5]. Although a large number of therapeutic agents, including 5-ASA drugs, immunosuppressants, steroids, and emerging biological agents, have appeared in the past few years, most patients still experience severe complications or recurrence of the disease, which greatly reduces their quality of life[6]. Therefore, it is imperative to develop effective treatments for UC.
The endoplasmic reticulum (ER) is a principal compartment in eukaryotic cells for protein folding and trafficking. Cellular stresses such as perturbations of Ca2+ homeostasis and oxidative stress disrupt ER homeostasis, resulting in the accumulation of unfolded and misfolded proteins in the ER lumen, which initiates the unfolded protein response (UPR)[7]. The UPR reduces protein synthesis, accelerates protein folding, and activates ER-associated degradation to orchestrate the recovery of ER function. However, if ER stress is too severe or persistent, intrinsic apoptotic pathways are eventually triggered, leading to cell death[8,9]. Recently, increasing evidence suggests that ER stress and UPR are involved in the pathogenesis of UC by regulating apoptosis, autophagy, and inflammatory responses[6,10].
Sirtuin 1 (SIRT1), a member of the mammalian sirtuin family of proteins, is a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase and plays an essential role in caloric restriction, life span modulation, and cell fate de-termination[11,12]. Recently, a number of studies have demonstrated that SIRT1 plays a protective role in colitis[13-16]. In particular, a report by Melhem et al[14] illustrated that SIRT1 relieves experimental colitis by modulating ER stress and reducing the UPR. However, the mechanism underlying the regulatory effect of SIRT1 on ER stress-mediated apoptosis in colitis is still unclear.
In the present study, we aimed to investigate the role of SIRT1 in the intestinal barrier in a UC coculture model and in mice with dextran sodium sulfate (DSS)-induced colitis. The mechanisms underlying the effect of SIRT1 on ER stress-mediated apoptotic pathways within intestinal epithelial cells (IECs) were further explored.
The SIRT1 activator SRT1720 and inhibitor nicotinamide (NAM) were obtained from Selleck Chemicals (Houston, TX, United States). DSS was purchased from MP Biomedical (Santa Ana, CA, United States). Anti-occludin, anti-zona occludens 1 (ZO-1), and anti-caspase-3 primary antibodies were purchased from Proteintech (Wuhan, China), anti-caspase-12 and anti-caspase-9 antibodies were obtained from LSBio (Seattle, WA, United States), and anti-SIRT1, anti-glucose-regulated protein 78 (GRP78), anti-CCAAT/enhancer-binding protein homologous protein (CHOP), and anti-β-actin antibodies were obtained from Abcam (Cambridge, UK).
We established an in vitro coculture model of Caco-2 and THP-1 cells based on previous studies[17-19].The human colon carcinoma Caco-2 and monocyte THP-1 cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA, United States), cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, United States) and RPMI-1640 cell culture medium (Gibco), respectively, supplemented with 10% fetal bovine serum (FBS; Gibco), and incubated at 37 °C in a 5% CO2 atmosphere. To establish the coculture model, Caco-2 cells were cultured in 6-well culture inserts (Transwell inserts; Corning Costar, NY, United States) at a density of 2 × 105 cells/insert for 17-20 d to obtain an integrated monolayer. THP-1 cells were cultured in 6-well plates at a density of 1.5 × 106 cells/well and treated with serum-free RPMI-1640 medium containing 100 ng/mL phorbol-12-myristate-13-acetate (PMA; Sigma-Aldrich, St. Louis, MO, United States) and 0.3% bovine serum albumin (BSA; Sigma-Aldrich) for 48 h. After confirming that THP-1 cells had differentiated into macrophages, the Transwell insert on which Caco-2 cells had been cultured for 17-20 d was placed in the culture well in which human macrophage-like THP-1 cells were cultivated, then lipopolysaccharide (LPS; Sigma-Aldrich) was added to the lower chamber at a final concentration of 10 ng/ml. Ultimately, the two cell lines were cocultured for 24 h. Once the coculture model was established, the SIRT1 activator SRT1720 or inhibitor NAM was added to the upper chamber medium at a final concentration of 10 µM and 5 mM, respectively.
The levels of secreted inflammatory cytokines IL-1β and TNF-α in the coculture model as well as in the colon tissues of treated mice were assayed using ELISA kits (Boster, Wuhan, China) according to the manufacturer’s instructions. Cell-free supernatants from the upper chamber after coculture for 24 h and colon homogenate supernatants of mice were collected. The absorbance at 450 nm was detected with a microplate reader (Thermo Fisher Scientific, Waltham, MA, United States).
Caco-2 apoptosis was evaluated with an Annexin V-APC/7-AAD Apoptosis Detection kit (Keygen Biotech, Nanjing, China). After SRT1720 or NAM treatment for 48 h, Caco-2 cells were harvested with EDTA-free trypsin, washed twice with cold phosphate-buffered saline, and resuspended in 500 µL of 1 × binding buffer. The resuspended cells were incubated with 5 µL of Annexin V-APC and 5 µL of 7-AAD for 5 min in the dark prior to being analyzed with a CytoFLEX flow cytometer (Beckman Coulter, CA, United States).
Twenty-four female C57BL/6 mice (6-8 wk old, weighing 18-22 g) were obtained from SIPPR-BK Lab Animal Co. Ltd. (Shanghai, China) and kept at room temperature (22-23 °C), with a light/dark cycle of 12/12 h, and free access to food and water. All experimental protocols were designed to minimize pain or discomfort to the animals and were approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
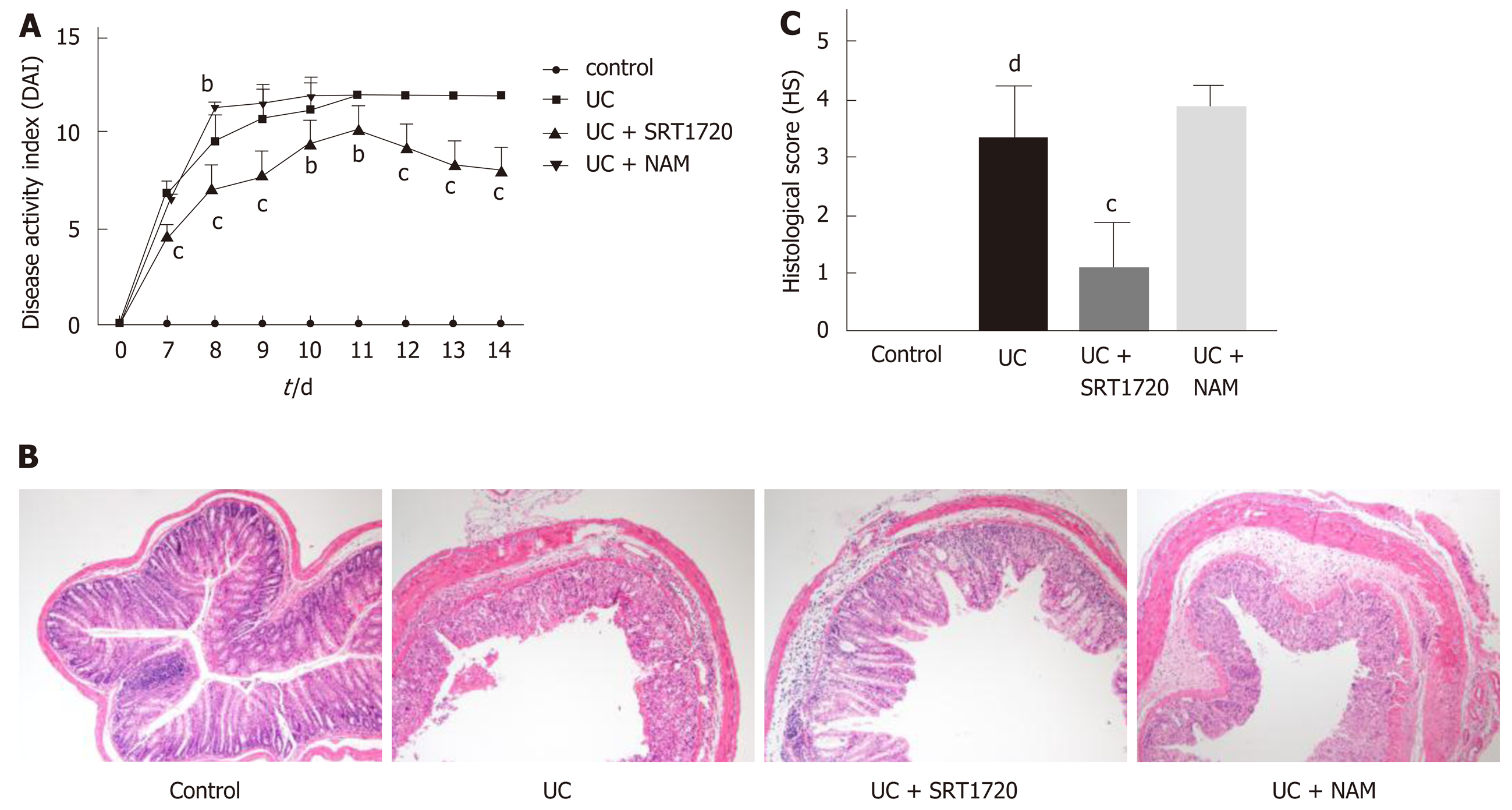
Female mice were randomly divided into four groups of six per group: The control group had free access to drinking water; the UC group was fed 3% DSS (w/v) for 7 consecutive days; and the UC + SRT1720 and UC + NAM groups received 3% DSS (w/v) for 7 consecutive days, followed by treatment with SRT1720 (100 mg/kg·d, intraperitoneal injection) or NAM (500 mg/kg·d, intraperitoneal injection) for another 7 d, respectively. The disease activity index (DAI) was measured daily after successful induction of acute colitis, as previously described[20].
All mice were sacrificed by decapitation. Distal colon samples were harvested for subsequent studies. The histological score (HS) of colon sections stained with hematoxylin and eosin was evaluated as described previously[20].
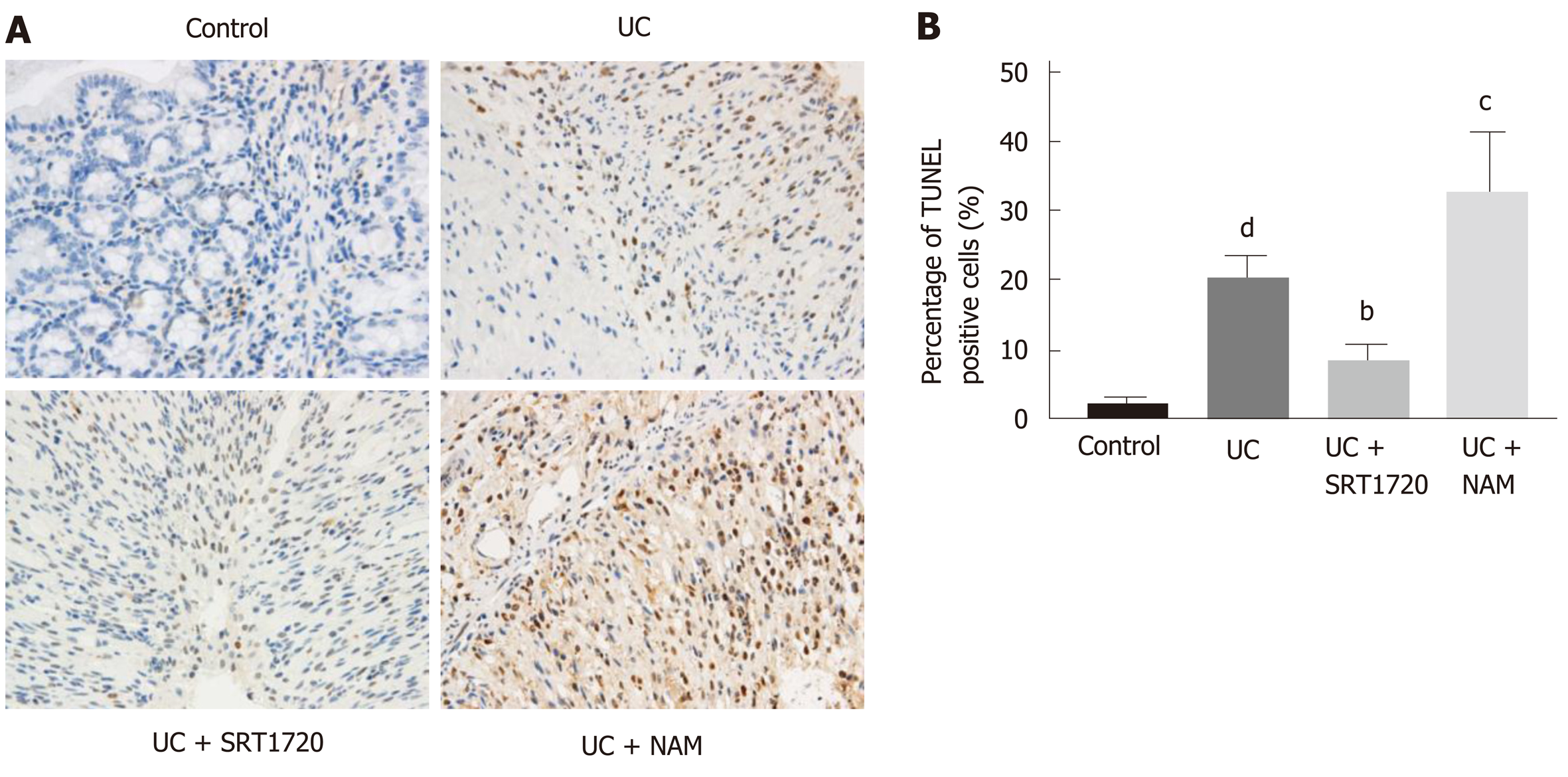
Apoptosis of cells in the colon tissue was assessed using a commercially available TUNEL assay kit (In Situ Cell Death Detection kit; Roche Applied Science, Basel, Switzerland) according to the manufacturer's instructions. In brief, tissue sections were incubated with proteinase K solution at 37 °C for 15 min. Afterwards, the enzyme solution and label solution were mixed (1:9) and added to the samples. The addition of 50 µL of converter-POD for 30 min was performed sequentially. Ten fields per section were assayed randomly in each experiment, and the percentage of positive cells was calculated.
Total RNA was extracted from human macrophage-like THP-1 and Caco-2 cells using TRIzol reagent (TaKaRa, Shiga, Japan) and reverse transcribed using a PrimeScriptTM RT reagent kit with gDNA Eraser (TaKaRa). qRT-PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR System using the SYBER Green Premix Ex Taq kit (TaKaRa) according to the manufacturer’s protocol. The primer sequences are listed in Table 1. The relative mRNA expression was analyzed by the 2-△△Ct method.
| Gene name | Primer sequence | |
| IL-1β | Forward | 5‘-ATGGCTTATTACAGTGGCA-3’ |
| Reverse | 5‘-TGTAGTGGTGGTCGGAGA-3’ | |
| TNF-α | Forward | 5‘-TCAGAGGGCCTGTACCTCAT-3’ |
| Reverse | 5‘-GGAAGACCCCTCCCAGATAG-3’ | |
| GRP78 | Forward | 5‘-GGAACCATCCCGTGGCATAA-3’ |
| Reverse | 5‘-CTTGGTAGGCACCACTGTGT-3’ | |
| CHOP | Forward | 5‘-CACCACTCTTGACCCTGCTTCTC-3’ |
| Reverse | 5‘-TGACCACTCTGTTTCCGTTTCC-3’ | |
| β-actin | Forward | 5’-AGCGAGCATCCCCCAAAGTT-3’ |
| Reverse | 5‘-GGGCACGAAGGCTCATCATT-3’ |
Total protein from Caco-2 cells and colon segments of mice was isolated with RIPA buffer (Beyotime Biotechnology, Shanghai, China). Protein was quantified by BCA assay, separated by 10% SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, United States). The membranes were blocked with 5% BSA diluted in TBS containing 5% Tween-20 for 2 h at room temperature, and incubated with primary antibodies at 4 °C overnight. Finally, the membranes were treated with ECL reagent and exposed to X-ray film. β-actin was used as an internal control.
Data are shown as the mean ± standard deviation (SD). All statistical analyses were performed with GraphPad Prism 7.0 (GraphPad Software, San Diego, United States) using unpaired Student’s t-test or one-way analysis of variance followed by Tukey’s test for multiple comparisons. P < 0.05 indicated a statistically significant difference.
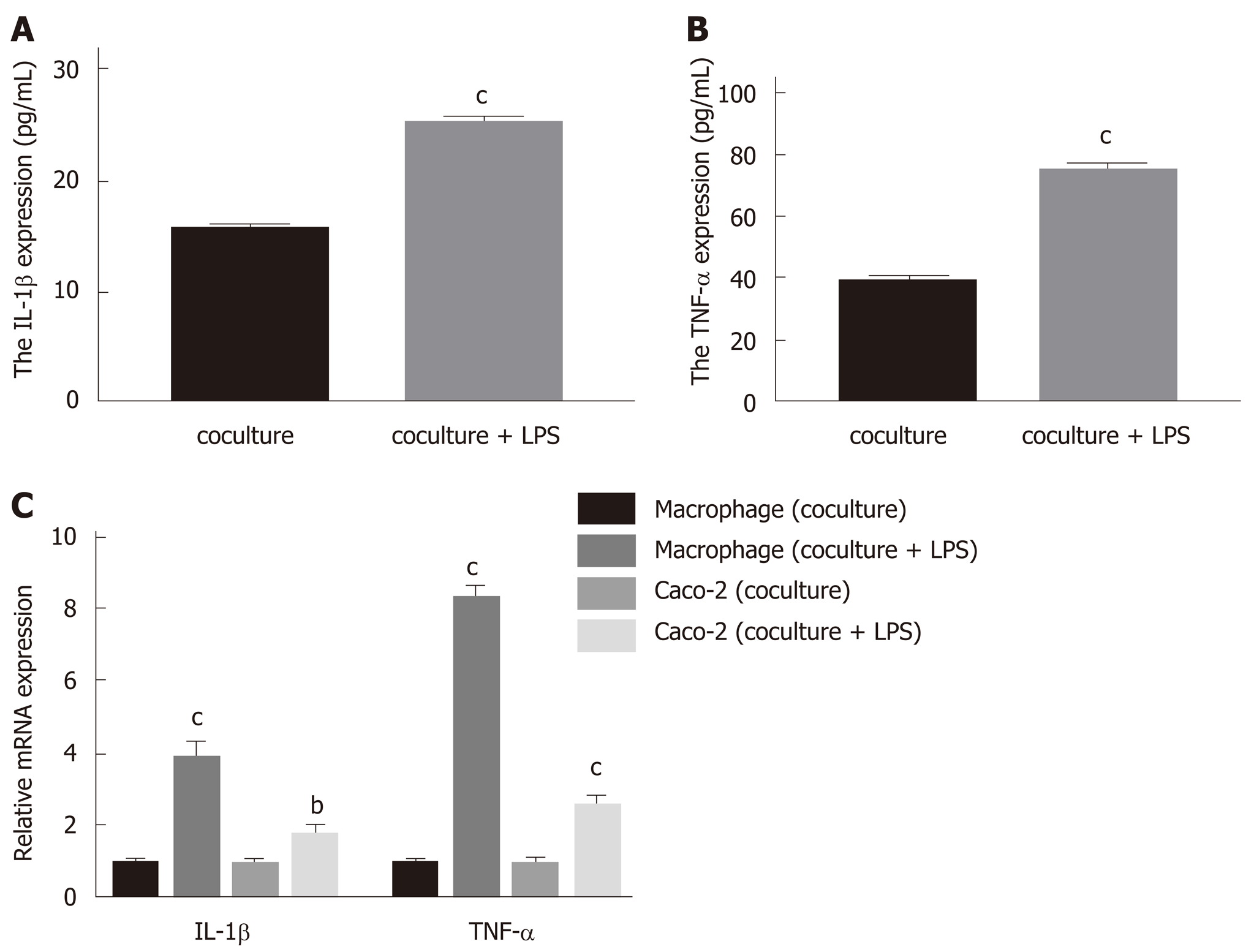
Cell-free supernatants from the upper chamber, human macrophage-like THP-1 cells, and Caco-2 cells were collected for the evaluation of IL-1β and TNF-α levels by ELISA or qRT-PCR. In the present study, the levels of secreted IL-1β and TNF-α in the upper chamber supernatants were dramatically increased upon LPS stimulation (Figure 1A and B; P < 0.001 vs coculture). In addition, the mRNA expression levels of inflammatory cytokines in macrophages and Caco-2 cells were significantly increased compared with the cells cocultured without added LPS (Figure 1C; P < 0.01 for IL-1β in Caco-2 vs coculture, P < 0.001 for IL-1β and TNF-α in macrophages and TNF-α in Caco-2 vs coculture). Herein, LPS administration for 24 h significantly increased IL-1β and TNF-α levels in the coculture model, which is in accordance with precious studies[17-19], suggesting that it is a suitable model to mimic acute colitis in vitro.
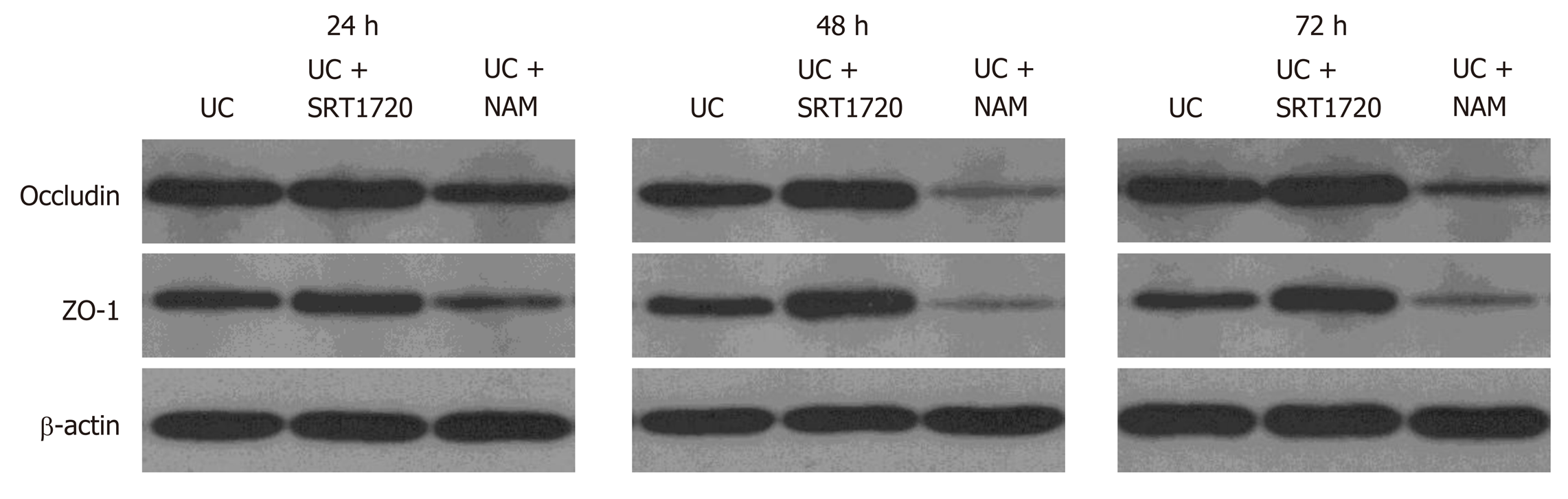
To assess the integrity of the intestinal barrier, we detected the expression levels of tight junction (TJ) proteins occludin and ZO-1. After coculturing with SRT1720 (10 µM) or NAM (5 mM), Caco-2 cells were collected, and the protein was extracted for Western blot analysis. As expected, compared with the UC group, SRT1720 administration significantly upregulated the expression of the TJ proteins occludin and ZO-1, while NAM treatment suppressed the expression of occludin and ZO-1 (Figure 2). Clearly, our data indicate that drug treatment for 48 h results in the strongest protective and damaging effect on Caco-2 monolayers, respectively. Therefore, we chose 48 h as the time point for drug treatment in subsequent experiments.
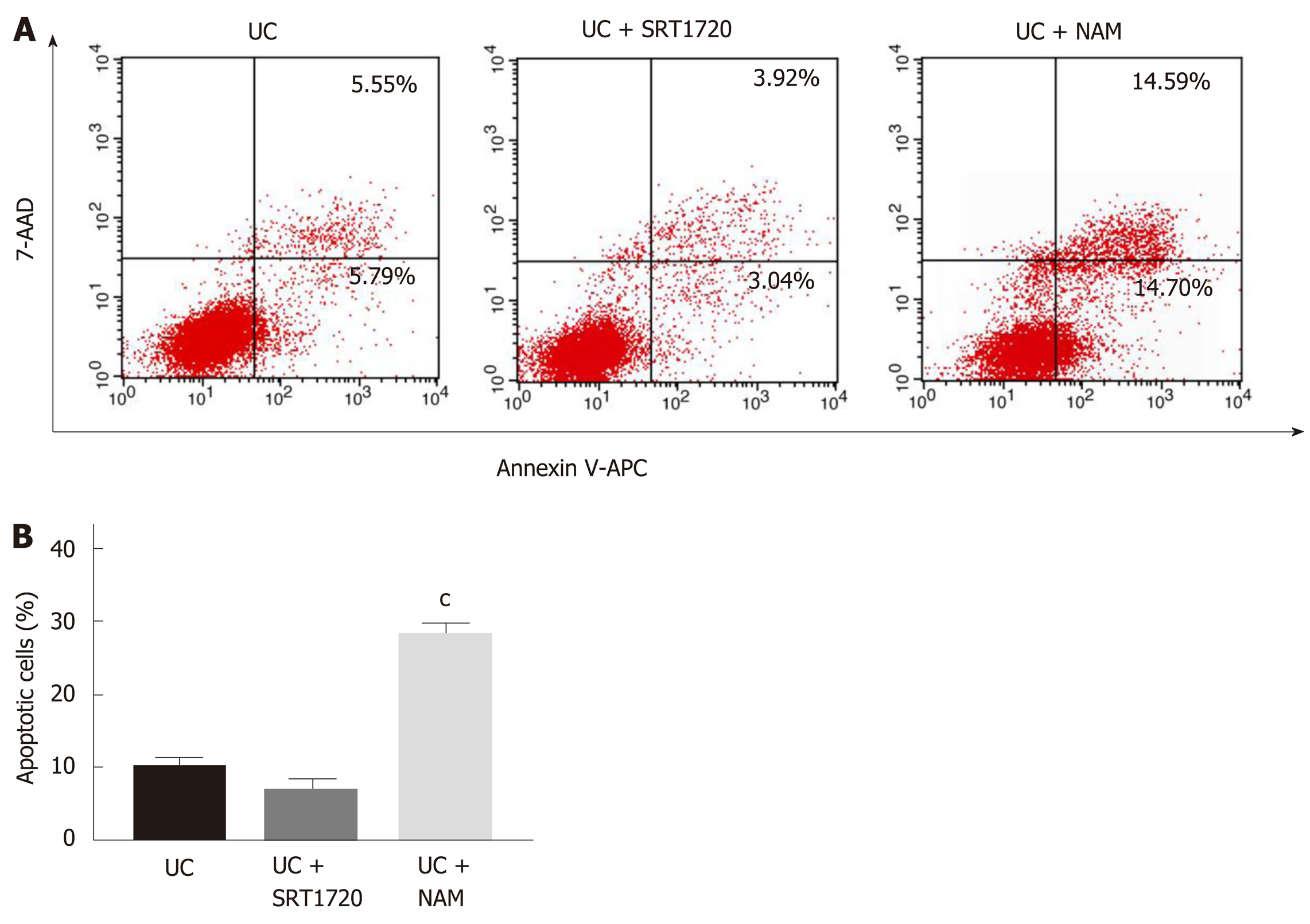
Annexin V-APC/7-AAD staining assays were applied to assess the apoptosis of Caco-2 cells treated with SRT1720 or NAM for 48 h. As shown in Figure 3A, the induction of colitis caused significant damage to the Caco-2 monolayers, with the apoptosis rate (Annexin V-APC+/7-AAD+ quadrant and Annexin V-APC+/7-AAD- quadrant) reaching 10.21%. Though administration of SRT1720 reduced the apoptosis rate of Caco-2 cells to some extent, there were no significant differences between the UC + SRT1720 group and the UC group (Figure 3B). However, NAM treatment led to a significant increase in the rate of apoptosis (Figure 3B; P < 0.001 vs UC).
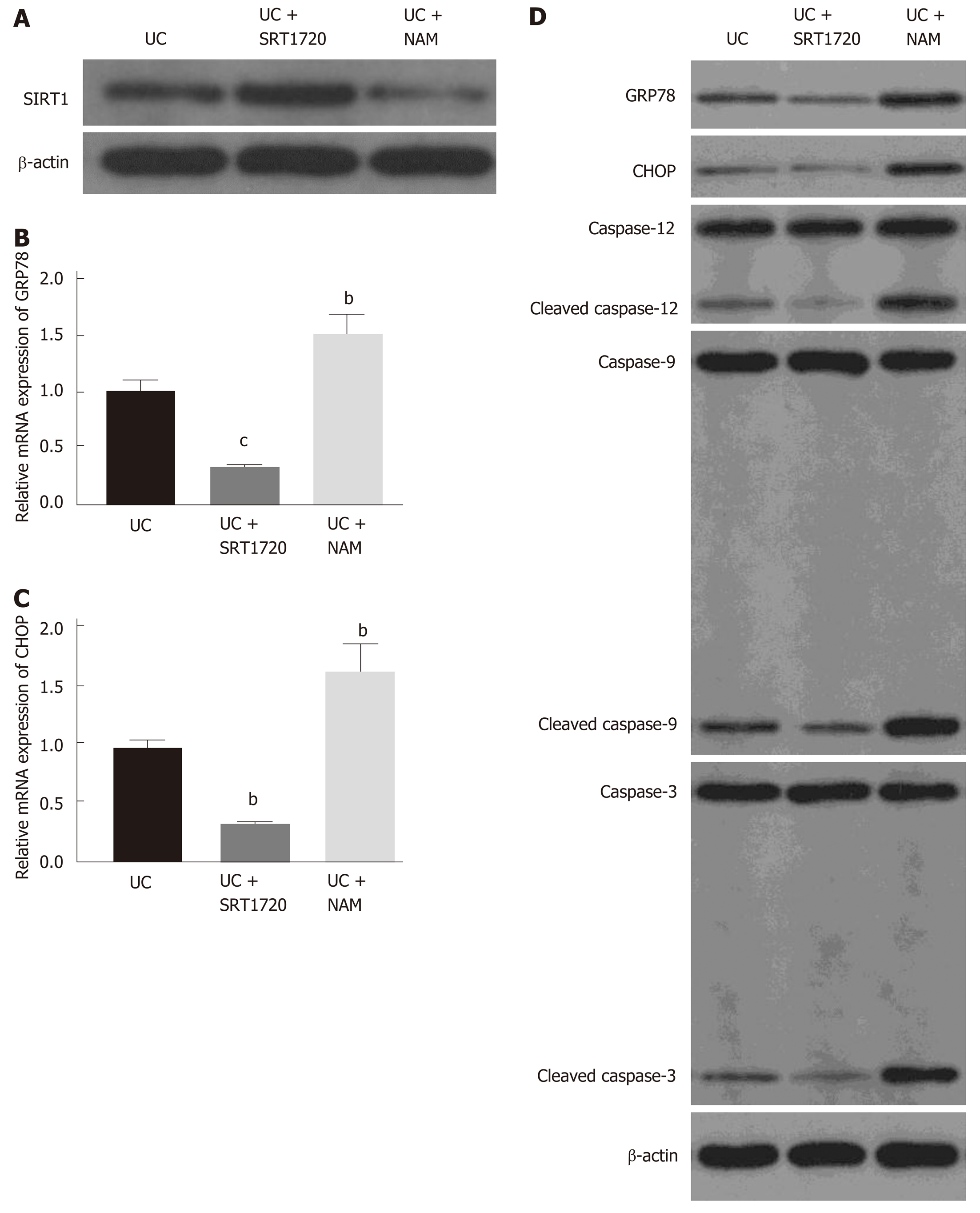
As shown in Figure 4A, SRT1720 administration increased the protein level of SIRT1, while NAM treatment downregulated its expression. To explore the molecular mechanisms underlying the protective role of SIRT1, we detected the mRNA levels of the ER stress chaperone GRP78 and the ER stress-induced apoptosis marker CHOP in Caco-2 cells. Exposure to SRT1720 for 48 h resulted in significantly decreased mRNA expression levels of GRP78 (Figure 4B; P < 0.001 vs UC) and CHOP (Figure 4C; P < 0.01 vs UC), whereas NAM treatment increased the expression of GRP78 and CHOP (Figure 4B and C; P < 0.01 vs UC). Western blot was also performed to verify the protein levels of ER stress- and apoptosis-related molecules. Consistent with the mRNA levels, we found that the level of GRP78 was significantly decreased in the SRT1720-treated group compared with the UC group (Figure 4D). In addition, the levels of CHOP and cleaved caspase-12, which play important roles in ER stress-induced apoptosis, were also decreased after SRT1720 treatment (Figure 4D). Moreover, the expression of downstream molecules, such as caspase-9 and caspase-3, was also suppressed (Figure 4D). In contrast, NAM treatment increased the expression of GRP78 and CHOP and upregulated the levels of the activated forms of caspase-12, caspase-9, and caspase-3 (Figure 4D).
Symptoms of acute colitis, including weight loss, diarrhea, and rectal bleeding, were observed daily after 7 d of DSS exposure. As expected, the administration of DSS successfully induced colitis, as the DAI dramatically increased in the UC group compared with the control group (Figure 5A; P < 0.001 vs control). The DAI score was higher in the UC + NAM group than in the UC group (Figure 5A; P < 0.01 vs UC), and SRT1720 treatment markedly reduced the DAI score (Figure 5A; P < 0.01 vs UC, P < 0.001 vs UC). Histologically, integrity loss, goblet cell damage, and inflammatory cell infiltration were observed in the DSS group compared with the control group (Figure 5B and C; P < 0.001 vs control). Compared with those in the UC group, the above changes were ameliorated in the SRT1720-treated group and aggravated in the NAM-treated group (Figure 5B). The HS of the UC + SRT1720 group was higher than that of the UC group (Figure 5C; P < 0.001 vs UC), while the UC + NAM group did not show significantly aggravated colitis (Figure 5C). Taken together, these data show that SIRT1 activation reduces susceptibility to DSS-induced acute colitis both clinically and histologically.
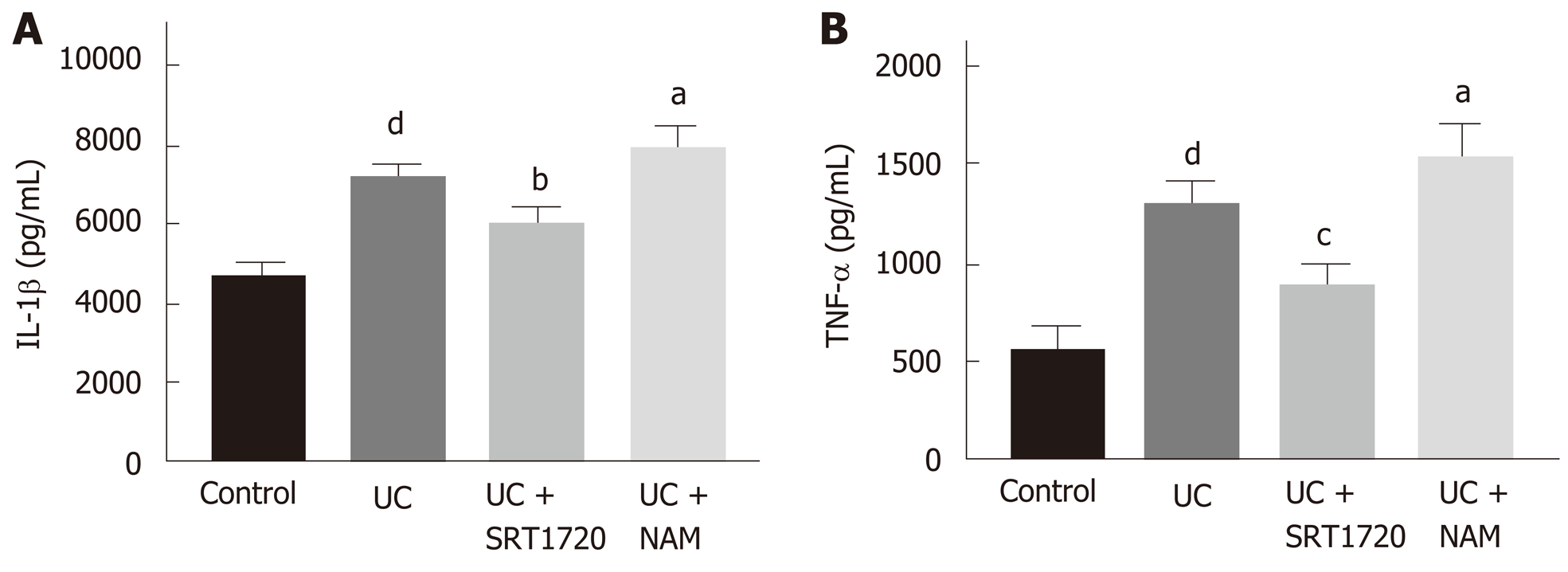
The expression levels of IL-1β and TNF-α in colon tissues were detected by ELISA to assess the inflammatory response. The data indicated that DSS treatment increased the levels of IL-1β and TNF-α significantly (Figure 6A and B; P < 0.001 vs control). Reduced expression levels of inflammatory cytokines were observed in the UC + SRT1720 group compared with the UC group (Figure 6A and B; P < 0.01 for IL-1β and P < 0.001 for TNF-α vs UC). In addition, the UC + NAM group showed increased expression levels of IL-1β and TNF-α (Figure 6A and B; P < 0.05 vs UC).
To estimate apoptosis in the colon tissue, TUNEL assays were performed, and positively stained cells were counted. The DSS group presented a dramatically larger number of apoptotic cells than the control group (Figure 7A and B; P < 0.001 vs control). There were fewer apoptotic cells in the UC + SRT1720 group than in the UC group (Figure 7A and B; P < 0.01 vs UC). Moreover, NAM administration decreased the percentage of apoptotic cells (Figure 7A and B; P < 0.001 vs UC).
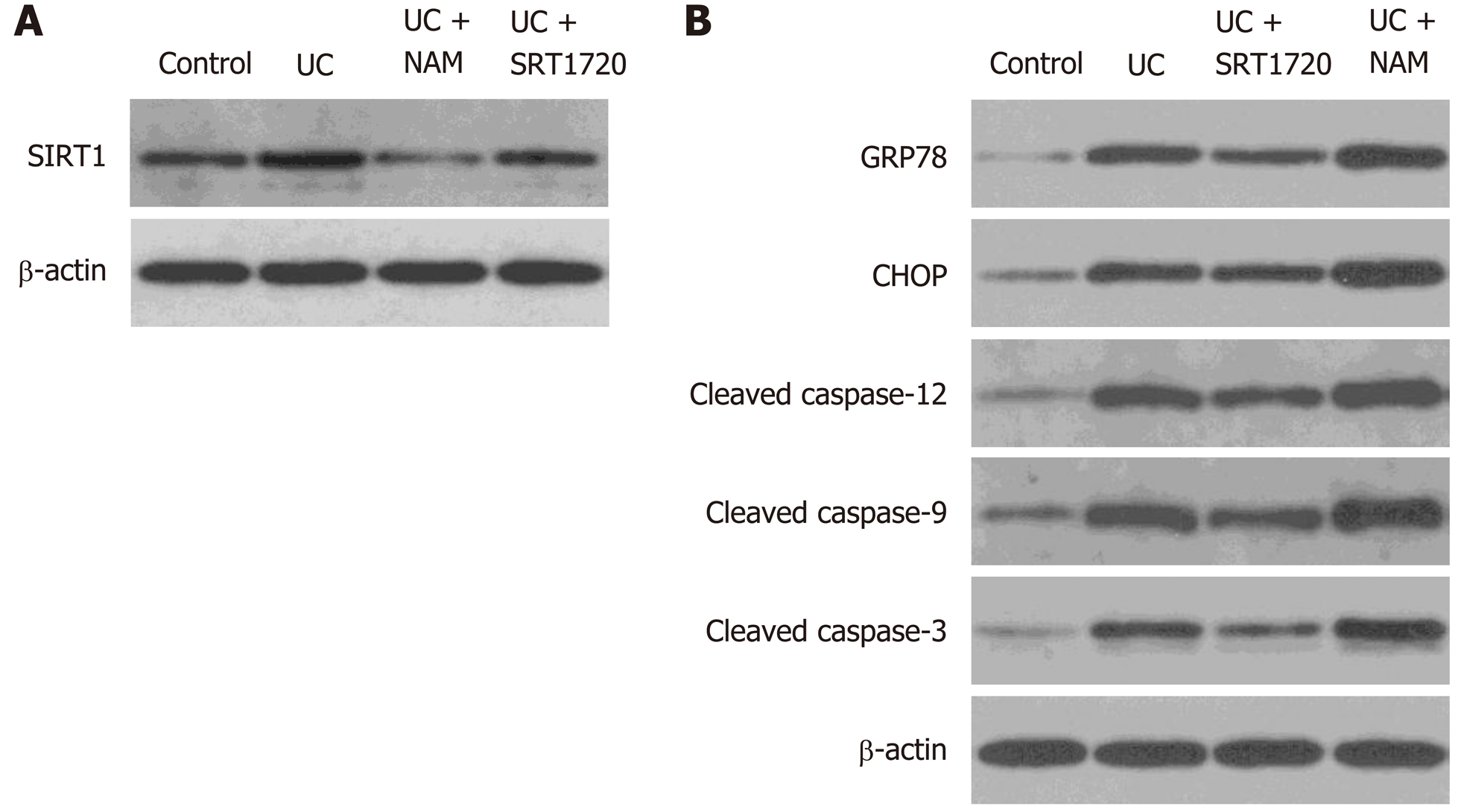
Protein levels in colon tissues of treated mice were assessed by Western blot. DSS administration caused a lower protein level of SIRT1. Besides, SRT1720 and NAM treatment upregulated and downregulated SIRT1 expression levels, respectively (Figure 8A). As shown in Figure 8B, compared with the control group, the DSS-treated group showed significantly elevated protein levels of GRP78, CHOP, and cleaved caspase-12, suggesting that ER stress and the UPR were activated. Consistent with the in vitro results, SRT1720 administration downregulated the protein levels of GRP78, CHOP, cleaved caspase-12, cleaved caspase-9, and cleaved caspase-3 (Figure 8B). Additionally, NAM administration caused the opposite effect (Figure 8B). Altogether, these results show that SIRT1 activation inhibits ER stress-mediated apoptotic pathways in DSS-induced colitis mice.
UC is characterized by chronic colonic mucosal inflammation and is a known risk for colorectal cancer. Although novel pharmacological therapies have been emerging recently, the existing treatments for UC are still not satisfactory[21]. Thus, developing effective drugs is essential. As a well-known modulator of lifespan, SIRT1 is involved in various diseases, including cancers, metabolic diseases, and inflammation-associated diseases[22-25]. However, the role of SIRT1 in the intestinal barrier and intestinal inflammation is still obscure. Here, we applied the SIRT1 activator SRT1720 and inhibitor NAM to investigate the potential effects of SIRT1 on the intestinal barrier in a UC coculture model and in mice with DSS-induced colitis. Our results demonstrate that the pharmacological activation of SIRT1 enhances TJ integrity of the intestinal barrier and reduces apoptosis of IECs by downregulating the expression of CHOP and suppressing the activation of caspase-12, which are key molecules in ER stress-mediated apoptotic pathways.
SIRT1 was found to be downregulated in colonic epithelium and lamina propria mononuclear cells (LPMCs) of IBD patients and elevated after successful infliximab treatment, suggesting that SIRT1 is involved in the development of IBD[13,14]. Furthermore, previous studies have identified the protective role of SIRT1 in intestinal inflammation, the molecular mechanisms of which include intestinal microbiota alteration and nuclear factor kappa B (NF-κB) pathway suppression[13,16,26]. In our study, SIRT1 activation significantly alleviated DSS-induced experimental colitis both clinically and histologically; this alleviation of colitis was accompanied by the downregulation of the levels of the inflammatory cytokines IL-1β and TNF-α in colon tissues, which is consistent with previous studies. Nevertheless, further studies are required to illuminate the underlying mechanisms.
The intestinal epithelial barrier isolates the internal milieu from the external environment and plays a crucial role in intestinal homeostasis. Intestinal inflammation is associated with increased permeability of the intestinal mucosa caused by intestinal barrier damage[27]. Occludin and ZO-1 are important TJ proteins and are essential for the maintenance of intestinal mucosal barrier integrity[28]. Occludin interacts directly with claudins and actin and takes part in the regulation of the intestinal barrier[29]. ZO-1 is a peripheral membrane protein that is essential for TJ assembly and maintenance[29]. A recent study revealed that occludin and ZO-1 expression in UC patients was not only significantly decreased compared with that of healthy controls but also positively related to intestinal mucosal healing[30]. Moreover, previous studies have demonstrated that SIRT1 enhances TJs in other physical barriers[31,32]. Herein, we found that SIRT1 activation significantly increased the expression of occludin and ZO-1 in Caco-2 monolayers, suggesting that SIRT1 may exert protective effects on colitis by promoting intestinal barrier integrity.
IECs, which comprise enterocytes, goblet cells, and Paneth cells, have well-developed ER structures for the biosynthesis of large amounts of proteins. Increasing evidence suggests that ER stress and the UPR in IECs are involved in the pathogenesis of UC[10,33,34]. Unresolved ER stress is a common character of the UC epithelium and results in the activation of the UPR to restore ER homeostasis or the induction of cell apoptosis if ER stress is too severe to be rescued[35]. Additionally, ER stress in IECs is related to intestinal dysbiosis and dysregulated immune response, leading to cell dysfunction, mucosal barrier damage, and intestinal inflammation[36]. In the present study, exposure to DSS increased the expression of the ER stress marker GRP78, which was accompanied by an increased rate of apoptosis in the colon and the activation of apoptosis-related proteases caspase-9 and caspase-3. GRP78 is an ER chaperone that binds to transmembrane ER stress sensors (inositol-requiring enzyme 1α (IRE1α), double-stranded RNA-activated protein kinase-like ER kinase (PERK), and activating transcription factor 6α (ATF6α)) in non-stressed cells[37]. It is released and contributes to the apoptosis of IECs upon ER stress[6]. Moreover, we found that SIRT1 activation reduced apoptosis in Caco-2 monolayers and colon tissues of DSS-induced colitis mice, indicating that SIRT1 may play a protective role in ER stress-induced injury.
To further confirm the protective effect of SIRT1, we detected the protein levels of CHOP and caspase-12. As a downstream transcriptional factor of PERK/eukaryotic translation initiation factor 2α (eIF2α)/activating transcription factor 4 (ATF4), CHOP plays a critical role in ER stress-induced apoptosis through suppression of the antiapoptotic protein Bcl-2 and induction of the proapoptotic molecules Bim, death receptor 5 (DR5), and telomere repeat binding factor 3 (TRB3)[38]. The intestinal epithelium of IBD patients shows higher expression of CHOP compared with normal people[39]. Furthermore, CHOP overexpression increases susceptibility to intestinal inflammation and mucosal tissue injury in mice, whereas knockdown of CHOP alleviates IEC apoptosis[40,41]. Previous studies have revealed that SIRT1 alleviates ER stress-mediated cell apoptosis through the downregulation of the PERK-eIF2α-CHOP axis in the UPR pathway in cardiac cells and chondrocytes[42,43]. Caspase-12 is another marker of ER stress-mediated apoptosis, which is separated from the ER membrane and cleaved into active fragments upon ER stress, resulting in caspase-3 cleavage and apoptosis[44]. Guo et al[45] reported that SIRT1 may alleviate ER stress-mediated apoptosis of cardiomyocytes via reduced expression levels of CHOP and cleaved caspase-12. Here, we show that SIRT1 activation decreases the expression of CHOP and suppresses the activation of caspase-12 in Caco-2 cells as well as in DSS-induced colitis mice, while treatment with the SIRT1 inhibitor NAM induced the opposite effect, which indicates that SIRT1 protects IECs from ER stress-induced apoptosis by suppressing CHOP, caspase-12, and their downstream signaling cascades.
In conclusion, we discovered that SIRT1 activation contributes to enhanced intestinal barrier integrity and reduced IEC apoptosis via the suppression of ER stress-mediated apoptotic proteins such as CHOP and caspase-12. SIRT1 may serve as a novel drug target, and SIRT1 activation is a promising therapeutic strategy for UC.
Ulcerative colitis (UC), the main subtype of inflammatory bowel disease (IBD), is a chronic relapsing inflammatory disorder of the large intestine. The incidence and prevalence of UC have increased in recent years. Sirtuin 1 (SIRT1), a member of the mammalian sirtuin family of proteins, is a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase and plays an essential role in caloric restriction, life span modulation, and cell fate determination. Recently, a number of studies have demonstrated that SIRT1 plays a protective role in colitis.
Although a large number of therapeutic agents, including 5-ASA drugs, immunosuppressants, steroids, and emerging biological agents, have appeared in the past few years, most patients still experience severe complications or recurrence of the disease, which greatly reduces their quality of life.
To investigate the role of SIRT1 in intestinal epithelial cells in UC and further explore the underlying mechanisms.
We developed a coculture model using macrophages and Caco-2 cells. After treatment with the SIRT1 activator SRT1720 or inhibitor nicotinamide (NAM), the expression of occludin and zona occludens 1 (ZO-1) was assessed by Western blot. Annexin V-APC/7-AAD assays were performed to evaluate Caco-2 apoptosis. DSS-induced colitis mice was exposed to SRT1720 or NAM for 7 d. Transferase-mediated dUTP nick-end labeling (TUNEL) assays were conducted to assess apoptosis in colon tissues. The expression levels of glucose-regulated protein 78 (GRP78), CCAAT/enhancer-binding protein homologous protein (CHOP), caspase-12, caspase-9, and caspase-3 in Caco-2 cells and the colon tissues of treated mice were examined by quantitative real-time PCR and Western blot.
SRT1720 treatment increased the protein levels of occludin and ZO-1 and inhibited Caco-2 apoptosis, whereas NAM administration caused the opposite effects. DSS-induced colitis mice treated with SRT1720 had a lower disease activity index (P < 0.01), histological score (P < 0.001), inflammatory cytokine levels (P < 0.01), and apoptotic cell rates (P < 0.01), while exposure to NAM caused the opposite effects. Moreover, SIRT1 activation reduced the expression levels of GRP78, CHOP, cleaved caspase-12, cleaved caspase-9, and cleaved caspase-3 in Caco-2 cells and the colon tissues of treated mice.
SIRT1 activation contributes to enhanced intestinal barrier integrity and reduced apoptosis of intestinal epithelial cells via the suppression of endoplasmic reticulum (ER) stress-mediated apoptosis-associated molecules CHOP and caspase-12.
SIRT1 may serve as a novel drug target, and SIRT1 activation is a promising therapeutic strategy for UC.
We are grateful to Dr. Wei-Xiang Zhong for excellent assistance in pathology.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chuang SM, Chen YK, Touil-Boukoffa C, Tanabe S S-Editor: Wang J L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3353] [Article Influence: 186.3] [Reference Citation Analysis (11)] |
| 3. | Sorrentino D. Microbial dysbiosis in spouses of ulcerative colitis patients: Any clues to disease pathogenesis? World J Gastroenterol. 2017;23:6747-6749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Chen ML, Sundrud MS. Cytokine Networks and T-Cell Subsets in Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 461] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 6. | Cao SS. Epithelial ER Stress in Crohn's Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Song S, Tan J, Miao Y, Zhang Q. Crosstalk of ER stress-mediated autophagy and ER-phagy: Involvement of UPR and the core autophagy machinery. J Cell Physiol. 2018;233:3867-3874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 8. | Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 3055] [Article Influence: 235.0] [Reference Citation Analysis (0)] |
| 9. | Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1840] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 10. | Zhang HS, Chen Y, Fan L, Xi QL, Wu GH, Li XX, Yuan TL, He SQ, Yu Y, Shao ML, Liu Y, Bai CG, Ling ZQ, Li M, Liu Y, Fang J. The Endoplasmic Reticulum Stress Sensor IRE1α in Intestinal Epithelial Cells Is Essential for Protecting against Colitis. J Biol Chem. 2015;290:15327-15336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 12. | Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, Liu H, Lu Y, Liao J, Chen X, Chu Y. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1α-dependent glycolysis. Cancer Res. 2014;74:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Caruso R, Marafini I, Franzè E, Stolfi C, Zorzi F, Monteleone I, Caprioli F, Colantoni A, Sarra M, Sedda S, Biancone L, Sileri P, Sica GS, MacDonald TT, Pallone F, Monteleone G. Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol. 2014;7:1467-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Melhem H, Hansmannel F, Bressenot A, Battaglia-Hsu SF, Billioud V, Alberto JM, Gueant JL, Peyrin-Biroulet L. Methyl-deficient diet promotes colitis and SIRT1-mediated endoplasmic reticulum stress. Gut. 2016;65:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4'-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NSX, Czopik A, Shanahan MT, Kang A, Chen W, Azcarate-Peril MA, Gulati AS, Fargo DC, Guarente L, Li X. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology. 2017;153:772-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Watanabe F, Satsu H, Mochizuki T, Nakano T, Shimizu M. Development of the method for evaluating protective effect of food factors on THP-1-induced damage to human intestinal Caco-2 monolayers. Biofactors. 2004;21:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Satsu H, Ishimoto Y, Nakano T, Mochizuki T, Iwanaga T, Shimizu M. Induction by activated macrophage-like THP-1 cells of apoptotic and necrotic cell death in intestinal epithelial Caco-2 monolayers via tumor necrosis factor-alpha. Exp Cell Res. 2006;312:3909-3919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Kämpfer AAM, Urbán P, Gioria S, Kanase N, Stone V, Kinsner-Ovaskainen A. Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol In Vitro. 2017;45:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 21. | Pagnini C, Pizarro TT, Cominelli F. Novel Pharmacological Therapy in Inflammatory Bowel Diseases: Beyond Anti-Tumor Necrosis Factor. Front Pharmacol. 2019;10:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 1445] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 23. | Wei Z, Jia J, Heng G, Xu H, Shan J, Wang G, Liu C, Xia J, Zhou H, Wu M, Yang Z, Wang M, Xiong Z, Huang H, Liu L, Qian C. Sirtuin-1/Mitochondrial Ribosomal Protein S5 Axis Enhances the Metabolic Flexibility of Liver Cancer Stem Cells. Hepatology. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Nassir F, Ibdah JA. Sirtuins and nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:10084-10092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J. Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L847-L853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Wang K, Li YF, Lv Q, Li XM, Dai Y, Wei ZF. Bergenin, Acting as an Agonist of PPARγ, Ameliorates Experimental Colitis in Mice through Improving Expression of SIRT1, and Therefore Inhibiting NF-κB-Mediated Macrophage Activation. Front Pharmacol. 2018;8:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Guo K, Ren J, Gu G, Wang G, Gong W, Wu X, Ren H, Hong Z, Li J. Hesperidin Protects Against Intestinal Inflammation by Restoring Intestinal Barrier Function and Up-Regulating Treg Cells. Mol Nutr Food Res. 2019;63:e1800975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467-G475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 320] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2709] [Article Influence: 169.3] [Reference Citation Analysis (0)] |
| 30. | Tan Y, Guan Y, Sun Y, Zheng C. Correlation of Intestinal Mucosal Healing and Tight Junction Protein Expression in Ulcerative Colitis Patients. Am J Med Sci. 2019;357:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Fu C, Hao S, Xu X, Zhou J, Liu Z, Lu H, Wang L, Jin W, Li S. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta Pharmacol Sin. 2019;40:630-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 32. | Stamatovic SM, Martinez-Revollar G, Hu A, Choi J, Keep RF, Andjelkovic AV. Decline in Sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol Dis. 2019;126:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Hooper KM, Barlow PG, Henderson P, Stevens C. Interactions Between Autophagy and the Unfolded Protein Response: Implications for Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:661-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, Flak MB, Cusick JL, Kohno K, Iwawaki T, Billmann-Born S, Raine T, Bharti R, Lucius R, Kweon MN, Marciniak SJ, Choi A, Hagen SJ, Schreiber S, Rosenstiel P, Kaser A, Blumberg RS. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 584] [Cited by in RCA: 576] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 35. | Tréton X, Pédruzzi E, Cazals-Hatem D, Grodet A, Panis Y, Groyer A, Moreau R, Bouhnik Y, Daniel F, Ogier-Denis E. Altered endoplasmic reticulum stress affects translation in inactive colon tissue from patients with ulcerative colitis. Gastroenterology. 2011;141:1024-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Ma X, Dai Z, Sun K, Zhang Y, Chen J, Yang Y, Tso P, Wu G, Wu Z. Intestinal Epithelial Cell Endoplasmic Reticulum Stress and Inflammatory Bowel Disease Pathogenesis: An Update Review. Front Immunol. 2017;8:1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 38. | Cao SS. Endoplasmic reticulum stress and unfolded protein response in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1177] [Cited by in RCA: 1141] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 40. | Waldschmitt N, Berger E, Rath E, Sartor RB, Weigmann B, Heikenwalder M, Gerhard M, Janssen KP, Haller D. C/EBP homologous protein inhibits tissue repair in response to gut injury and is inversely regulated with chronic inflammation. Mucosal Immunol. 2014;7:1452-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Lu X, Li C, Li C, Li P, Fu E, Xie Y, Jin F. Heat-Labile Enterotoxin-Induced PERK-CHOP Pathway Activation Causes Intestinal Epithelial Cell Apoptosis. Front Cell Infect Microbiol. 2017;7:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Prola A, Pires Da Silva J, Guilbert A, Lecru L, Piquereau J, Ribeiro M, Mateo P, Gressette M, Fortin D, Boursier C, Gallerne C, Caillard A, Samuel JL, François H, Sinclair DA, Eid P, Ventura-Clapier R, Garnier A, Lemaire C. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017;24:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 43. | Kang X, Yang W, Wang R, Xie T, Li H, Feng D, Jin X, Sun H, Wu S. Sirtuin-1 (SIRT1) stimulates growth-plate chondrogenesis by attenuating the PERK-eIF-2α-CHOP pathway in the unfolded protein response. J Biol Chem. 2018;293:8614-8625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Aoyama K, Burns DM, Suh SW, Garnier P, Matsumori Y, Shiina H, Swanson RA. Acidosis causes endoplasmic reticulum stress and caspase-12-mediated astrocyte death. J Cereb Blood Flow Metab. 2005;25:358-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Guo R, Liu W, Liu B, Zhang B, Li W, Xu Y. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism. Int J Cardiol. 2015;191:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |