Published online Aug 21, 2019. doi: 10.3748/wjg.v25.i31.4512
Peer-review started: April 15, 2019
First decision: May 16, 2019
Revised: July 5, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 21, 2019
Processing time: 128 Days and 14.4 Hours
Esophageal cancer (EC) is associated with a poor prognosis, particularly so in Africa where an alarmingly high mortality to incidence ratio prevails for this disease.
To provide further understanding of EC in the context of the unique cultural and genetic diversity, and socio-economic challenges faced on the African continent.
We performed a systematic review of studies from Africa to obtain data on epidemiology, risk factors, management and outcomes of EC. A non-systematic review was used to obtain incidence data from the International Agency for Research on Cancer, and the Cancer in Sub-Saharan reports. We searched EMBASE, PubMed, Web of Science, and Cochrane Central from inception to March 2019 and reviewed the list of articles retrieved. Random effects meta-analyses were used to assess heterogeneity between studies and to obtain odds ratio (OR) of the associations between EC and risk factors; and incidence rate ratios for EC between sexes with their respective 95% confidence intervals (CI).
The incidence of EC is higher in males than females, except in North Africa where it is similar for both sexes. The highest age-standardized rate is from Malawi (30.3 and 19.4 cases/year/100000 population for males and females, respectively) followed by Kenya (28.7 cases/year/100000 population for both sexes). The incidence of EC rises sharply after the age of 40 years and reaches a peak at 75 years old. Meta-analysis shows a strong association with tobacco (OR 3.15, 95%CI: 2.83-3.50). There was significant heterogeneity between studies on alcohol consumption (OR 2.28, 95%CI: 1.94-2.65) and on low socioeconomic status (OR 139, 95%CI: 1.25-1.54) as risk factors, but these could also contribute to increasing the incidence of EC. The best treatment outcomes were with esophagectomy with survival rates of 76.6% at 3 years, and chemo-radiotherapy with an overall combined survival time of 267.50 d.
Africa has high incidence and mortality rates of EC, with preventable and non-modifiable risk factors. Men in this setting are at increased risk due to their higher prevalence of tobacco and alcohol consumption. Management requires a multidisciplinary approach, and survival is significantly improved in the setting of esophagectomy and chemoradiation therapy.
Core tip: Esophageal cancer is an important cause of cancer related deaths world-wide, but particularly in Africa where mortality rates due to this condition have been found to be higher. We systematically reviewed the literature on esophageal cancer in Africa and present our findings on the incidence, risk factors, management strategies, resultant outcomes and overall survival.
- Citation: Asombang AW, Chishinga N, Nkhoma A, Chipaila J, Nsokolo B, Manda-Mapalo M, Montiero JFG, Banda L, Dua KS. Systematic review and meta-analysis of esophageal cancer in Africa: Epidemiology, risk factors, management and outcomes. World J Gastroenterol 2019; 25(31): 4512-4533
- URL: https://www.wjgnet.com/1007-9327/full/v25/i31/4512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i31.4512
Esophageal cancer (EC) is the sixth most common cause of mortality among all cancers worldwide, and the seventh most common cancer worldwide, with an estimated 572034 new cases (3.2% of all cancers) and 508585 cancer deaths (5.3% of all cancer deaths) in 2018[1]. The high mortality to incidence ratio of 88.9% (= 508585/572034) worldwide shows that EC has a poor prognosis. Of these 572034 new cases worldwide, about 28494 (5.0%) occurred in African countries. The mortality to incidence ratio is even higher in Africa at 97.2% (= 27703/28494). Clinically, patients can be asymptomatic, however as the disease progresses, patients present with dysphagia which may be associated with weight loss. Histologically, EC can be squamous cell carcinoma (ESCC) or adenocarcinoma (EAC). Both types are common in males more than females[1]. Squamous cell carcinomas are more commonly encountered in individuals from low-resource regions, whereas adenocarcinomas tend to arise in high-resource populations. Globally, squamous cell carcinoma is more common. In Africa, squamous cell carcinoma is predominant, while adenocarcinoma is more common in the United States[2].
Risk factors for developing ESCC include tobacco consumption (smoking)[3-5], heavy alcohol consumption[6,7], drinking hot tea[8,9], consuming red meat[10], poor oral health[11], low intake of fresh fruit and vegetables[10,12], and low socioeconomic status[6,13]. Esophageal mucosa dysplasia from acid reflux and Barrett’s esophagus is a risk factor for developing EAC[14]. Management of EC includes surgery, radiation, chemotherapy and laser therapy. Various systematic reviews of studies on EC in Africa have been conducted[15,16] and there are significant new data to update these reviews. The aim of this article is to systematically review published studies of EC in Africa. The specific objectives of the review are to: (1) Estimate the incidence and geographic variation of EC in Africa; (2) Determine the risk factors associated with EC in this region; (3) Describe the management and outcomes of EC in Africa; and (4) Assess the quality of research papers included in the quantitative analyses of this review.
Systematic review and non-systematic review methods were used. The systematic review was used to obtain data on epidemiology, risk factors, management and outcomes of EC, while the non-systematic review was used to obtain incidence data.
Age standardized incidence data for EC in Africa were obtained from the current International Agency for Research on Cancer (IARC) report[17], and from the Cancer in Sub-Saharan Africa report[18]. The Cancer in Sub-Saharan Africa report collates population-based cancer incidence data from 25 cancer registries in 20 sub-Saharan African countries that are part of the African Cancer Registry Network. The IARC report includes countries in North Africa. The target group for both reports is to use registries that report the incidence of cancers in the general population in contrast to other types of registries, such as those based on patients who are treated at specific hospitals or who belong to a specific insurance plan. The reports use ICD-10 codes to show the age-standardised incidence per 100000 population stratified by sex and type of cancer for each country. We extracted data from code C.15 where EC is reported.
Search strategy: Research studies were eligible if they were published in English and conducted on humans and in Africa. We searched EMBASE, PubMed, Web of Science, and Cochrane Central from inception to March 2019 for primary publications. The following search terms were used in the four databases: (Esophageal can* OR oesophageal can* OR esophageal tumor OR oesophageal tumor OR esophagus can* OR oesophagus can*) AND (epidemiology OR incidence OR prevalence OR risk factor* OR treatment OR management OR outcome*) AND (Africa OR East* Africa OR West Africa OR Southern Africa OR North* Africa OR Algeria* OR Angola* OR Benin OR Botswana OR “Burkina Faso” OR Burundi OR Cameroon* OR "Cabo Verde" OR “Cape Verde” OR “Central African Republic” OR Chad OR Comoros OR Congo OR “Cote d`Ivoire” OR Djibouti OR Egypt* OR “Democratic Republic of Congo” OR “Equatorial Guinea” OR Eritrea* OR “Eswatini” OR Swaziland OR Ethiopia* OR Gabon* OR Gambia* OR Ghana* OR Guinea OR “Guinea Bissau” OR Kenya* OR Lesotho OR Liberia* OR Libya* OR Madagascar OR Malawi* OR Mali OR Mauritania* OR Mauritius OR Morocc* OR Mozambique OR Namibia* OR Niger OR Nigeria* OR Rwanda* OR “Sao Tome and Principe” OR Senegal* OR Seychelle* OR “Sierra Leone” OR Somali* OR “South African” OR “South Africa” OR “South African” OR Sudan* OR “North Sudan” OR “South Sudan” OR Tanzania* OR Togo* OR Tunisia* OR Uganda* OR Zambia* OR Zimbabwe*).
Study selection: Epidemiological studies that provided a quantitative measure of disease occurrence (prevalence, incidence) or mortality (survival, mortality rate), and studies that had a quantitative association between risk factors (explanatory variables) and EC were eligible. In vitro studies were excluded because they do not provide an estimate of disease occurrence. Titles and abstracts of all articles identified were screened independently by several authors (AA, NC, AN, JC, BN, MM). Full texts of the remaining articles were reviewed by the authors, and consensus reached on potential eligibility.
Data extraction: We used a data extraction form to collect the following information from each eligible article identified from the database search: (1) First author and year of publication; (2) Year the study was conducted; (3) Country; (4) Study population; (5) Sample size; (6) Incidence or prevalence of EC; (7) Study design; (8) Factors associated with EC; (9) Management of EC; (10) Outcomes; and (11) Measure of association if available.
Quality assessment: A modified quality assessment of the final papers included in this systematic review was adapted from the quality assessment tool for systematic reviews of observational studies (QATSO)[19]. Items included in QATSO tool are: (1) External validity (representativeness of sampling procedures used in each study); (2) Response rate, which we modified to include three categories (> 80%, 60%-80%, < 60% or not reported); (3) Validity of measurement methods; (4) Bias in the measurement of the outcomes; and (5) Control for important confounders. The final score is the mean across the items. Studies achieving a final score of 60% or above in at least 4 of these items were considered adequate in this review[19].
We considered the following outcomes from the incidence data in the non-systematic review: (1) Age-standardized incidence rate (= cases/year/100000 population); and (2) Mortality-incidence ratio for countries with available information. For studies included in the systematic review, the following were the outcomes: (1) Prevalence of EC (= cases/study sample size) for studies on epidemiology; (2) Odds ratio (OR) of developing EC for studies on risk factors associated with EC; and (3) Median survival in days after management of EC for studies on management and outcomes of EC.
We analyzed the incidence data from the non-systematic review and the findings from the systematic review separately. Given the low incidence of EC, we used the rare disease assumption to assume that the OR approaches relative risk or risk ratio (RR) in studies that presented their measures of effects as RRs. For results with available or calculable ORs or incidence rate ratio estimates (IRRs), we conducted a meta-analysis from a fixed effects model. The ORs or IRRs and their 95% confidence intervals (CIs) were graphically represented on Forest plots. We assessed the heterogeneity between studies using the I2 statistic, which estimates the extent (percentage) the ORs or IRRs vary between studies[20]. Meta-analyses were conducted using the Stata version 14.2 (Stata Corporation, College Station, TX, United States).
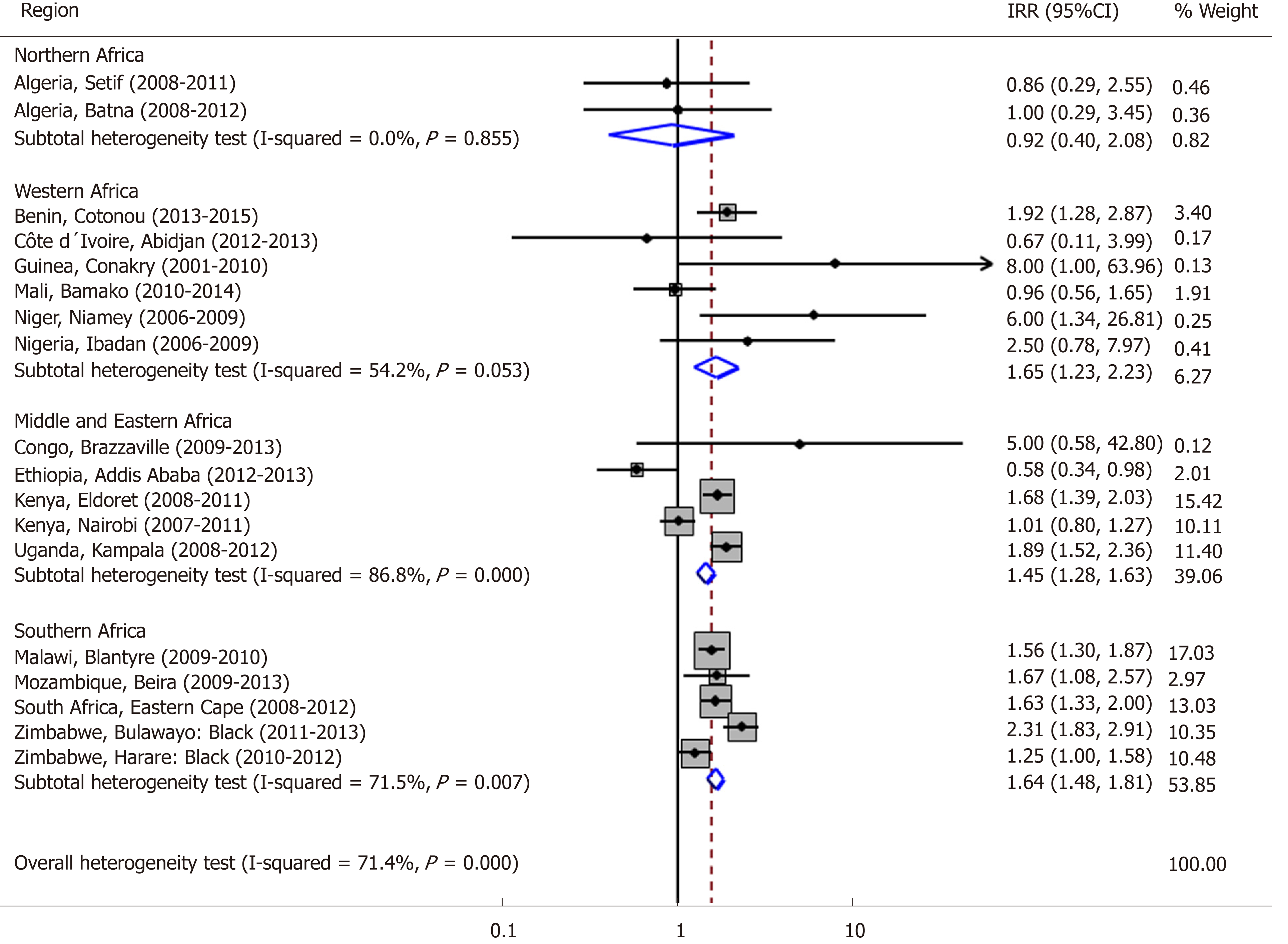
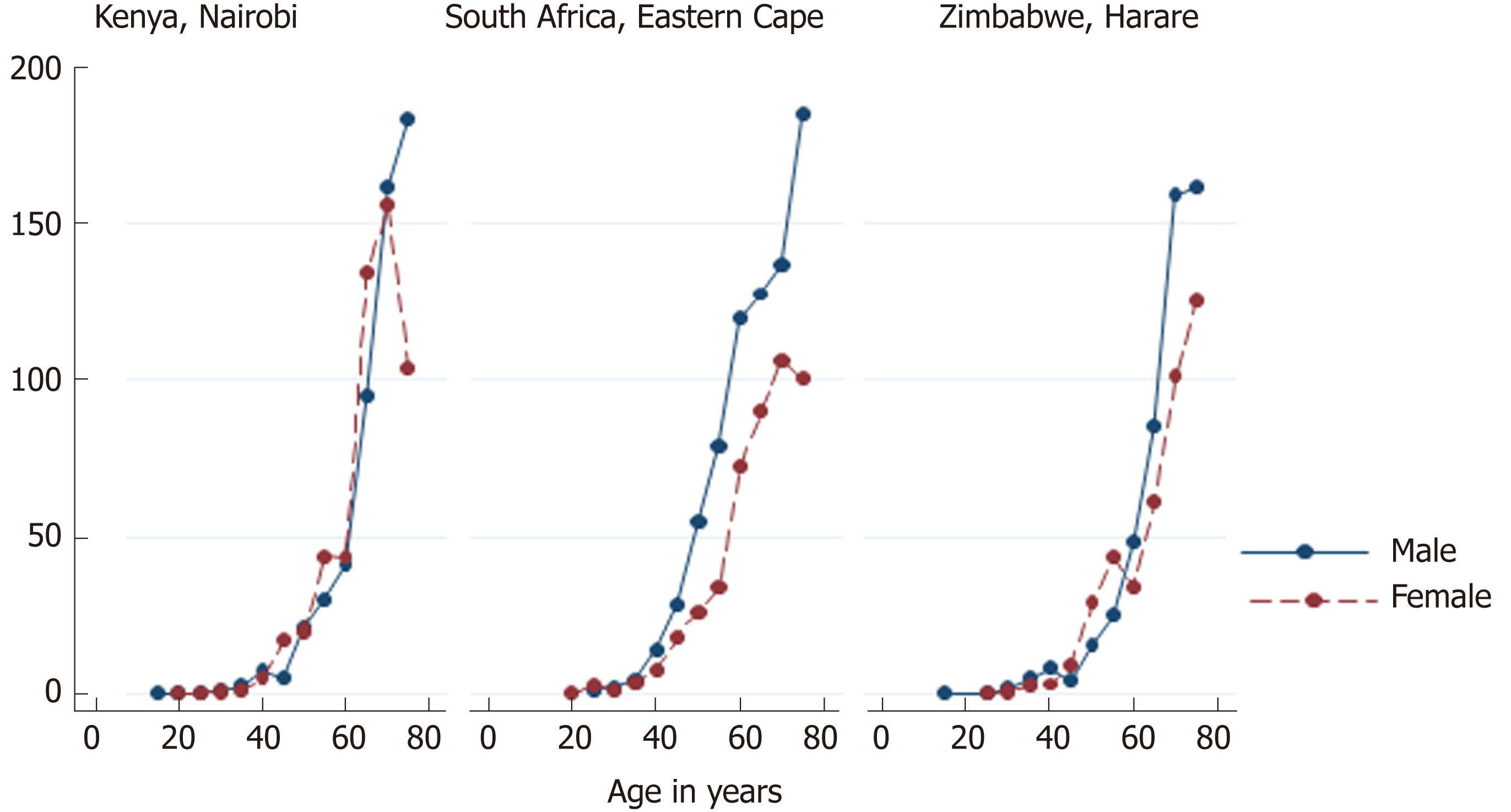
Age-standardized incidence rates of EC in Africa are generally higher in men than in women (Table 1). An analysis of the subgroups (regions) suggests that the incidence of EC is similar in males and females in Northern Africa, but higher in men in Western, Middle and Eastern, and Southern Africa. For Northern and Western African urban populations, there was no or little evidence of heterogeneity in the IRRs for males versus females (I2 statistics 0.0% and 54.2%, respectively). Therefore, males in Western African countries were 65% more likely to develop EC than females. However, there was significant heterogeneity based on I2 statistics in the Middle and Eastern African, and in Southern African urban populations, and therefore, we do not report the pooled IRRs for these regions (Figure 1). The incidence of EC is not constant across age groups. It is rare in persons under 15 years of age. After age 40, the incidence of EC rises sharply, reaching a peak among persons aged 75 years and above (Figure 2).
| Age-standardized incidence rate (cases/year/100000 pop) | ||
| Country (period covered) | Males | Females |
| Algeria, Setif (2008-2011) | 0.6 | 0.7 |
| Algeria, Batna (2008-2012) | 0.5 | 0.5 |
| Benin, Cotonou (2013-2015) | 6.9 | 3.6 |
| Botswana (2005-2008) | 11.4 | 3.9 |
| Congo, Brazzaville (2009-2013) | 0.5 | 0.1 |
| Côte d´Ivoire, Abidjan (2012-2013) | 0.2 | 0.3 |
| Ethiopia, Addis Ababa (2012-2013) | 2.2 | 3.8 |
| The Gambia (2007-2011) | 1.0 | 0.5 |
| Guinea, Conakry (2001-2010) | 0.8 | 0.1 |
| Kenya, Eldoret (2008-2011) | 28.7 | 17.1 |
| Kenya, Nairobi (2007-2011) | 14.1 | 14.0 |
| Malawi, Blantyre (2009-2010) | 30.3 | 19.4 |
| Mali, Bamako (2010-2014) | 2.6 | 2.7 |
| Mauritius (2010-2012) | 3.2 | 1.0 |
| Mozambique, Beira (2009-2013) | 5.5 | 3.3 |
| Niger, Niamey (2006-2009) | 1.2 | 0.2 |
| Nigeria, Abuja (2013) | 0.7 | 0.0 |
| Nigeria, Calabar (2009-2013) | 0.0 | 0.0 |
| Nigeria, Ibadan (2006-2009) | 1.0 | 0.4 |
| Namibia (2009) | 2.4 | 0.1 |
| Seychelles (2009-2012) | 7.5 | 1.9 |
| South Africa, Eastern Cape (2008-2012) | 23.8 | 14.6 |
| South Africa: National Cancer Registry of South Africa (2007) | 6.2 | 3.1 |
| Uganda, Kampala (2008-2012) | 22.9 | 12.1 |
| Zimbabwe, Bulawayo: Black population (2011-2013) | 23.8 | 10.3 |
| Zimbabwe, Harare: Black population (2010-2012) | 16.4 | 13.1 |
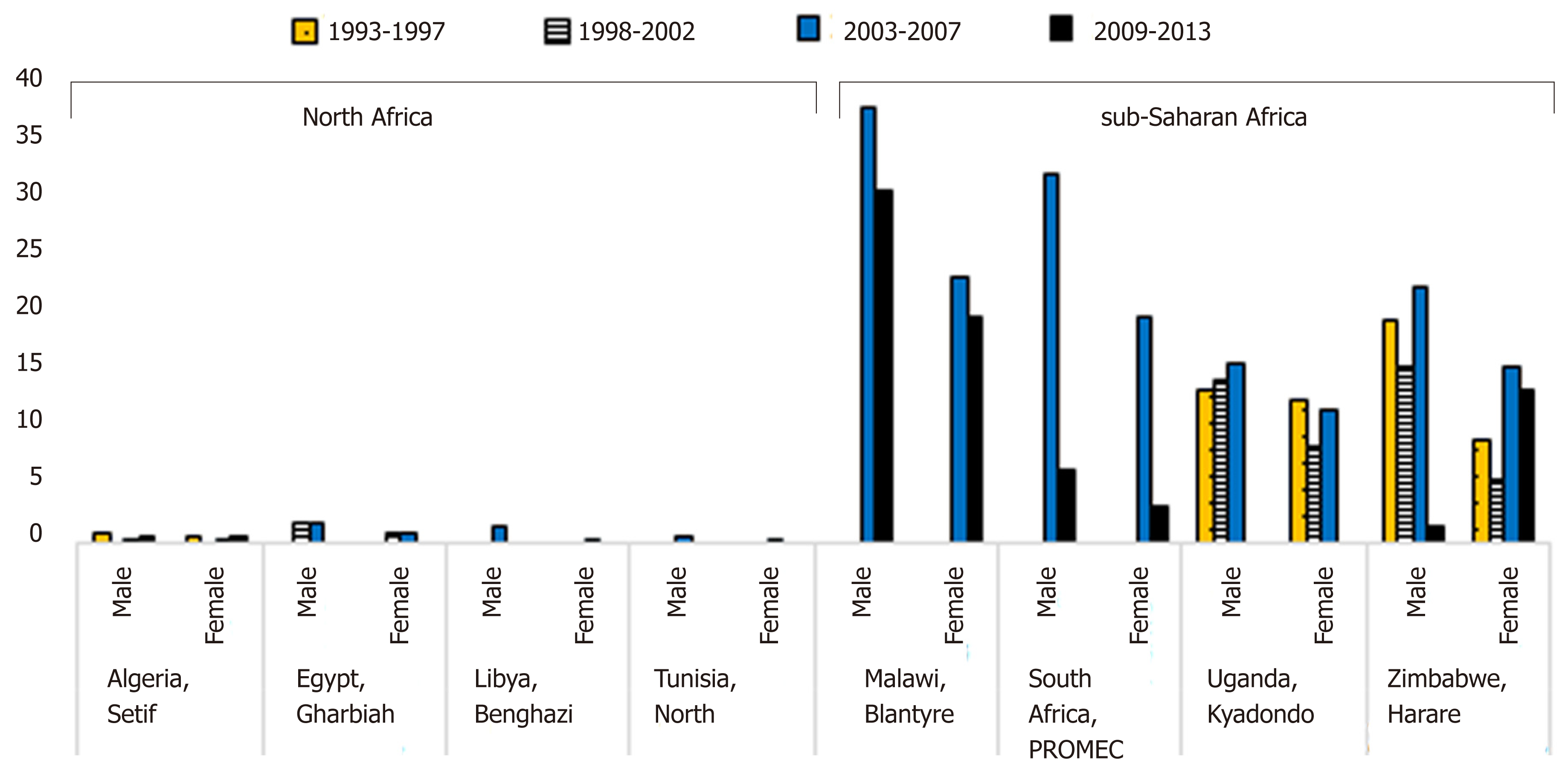
The age-standardized incidence rates of EC for males and females for the calendar years 1993 through 2013 show a variation in the trends for new EC cases (Figure 3). In Northern African populations (Algeria, Egypt, and Libya), the incidence rates of EC have been low in both males and females. In Malawi, the percentage decrease was greater for males (10.8%) than for females (8.7%) over the 2003-2007 to 2009-2013 period, while in South Africa over this same period, the percentage decrease was greater for females (72.7%) than for males (67.5%). In Uganda, the increase in incidence rates in males was continuous and progressive from the 1993-1997 to the 2003-2007 period. For females, however, there was a decrease between 1998 and 2002, then a slight increase between 2003 and 2007. In Zimbabwe, there was a decrease in incidence rates between 1998 and 2002 for males, then an increase between 2003 and 2007, followed by a substantial percentage decrease in incidence rates (86.4%) in the 2009-2013 period from the 2003-2007 period. For females, however, there was a decrease in incidence rates between 1998 and 2002, followed by a generally increase thereafter.
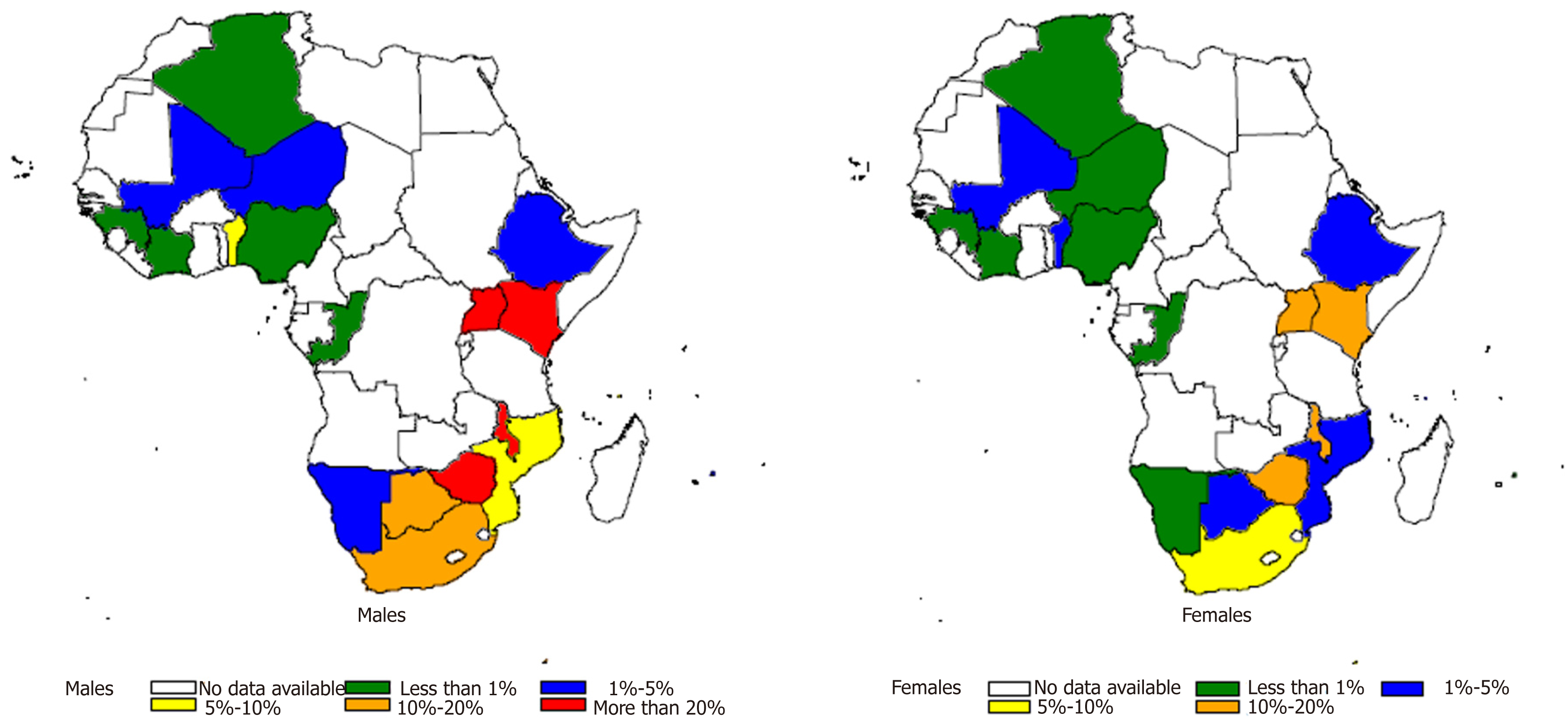
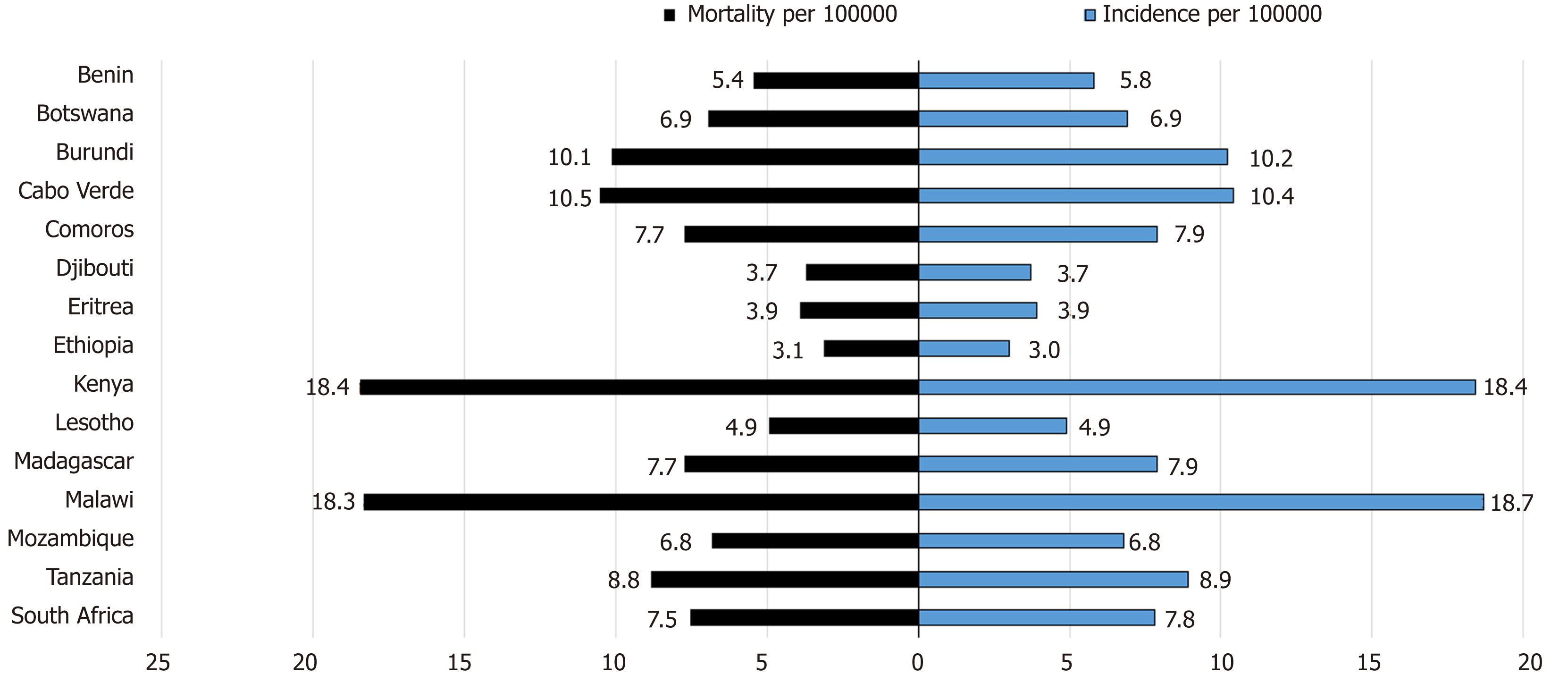
In addition to variation by gender and age, the incidence of EC also varies by geographic location (Figure 4). The highest incidence rate between 2009 and 2013 was 30.3 cases/year/100000 among males and 19.4 cases/year/100000 among females in Malawi, followed by 28.7 cases/year/100000 for males and 28.7 cases/year/100000 for females in Kenya, Eldoret in this same period[18,21]. The mortality to incidence ratio of EC is high in African countries. Kenya exhibits the highest mortality rate for both men and women, while Malawi has the highest incidence rate for both men and women (Figure 5).
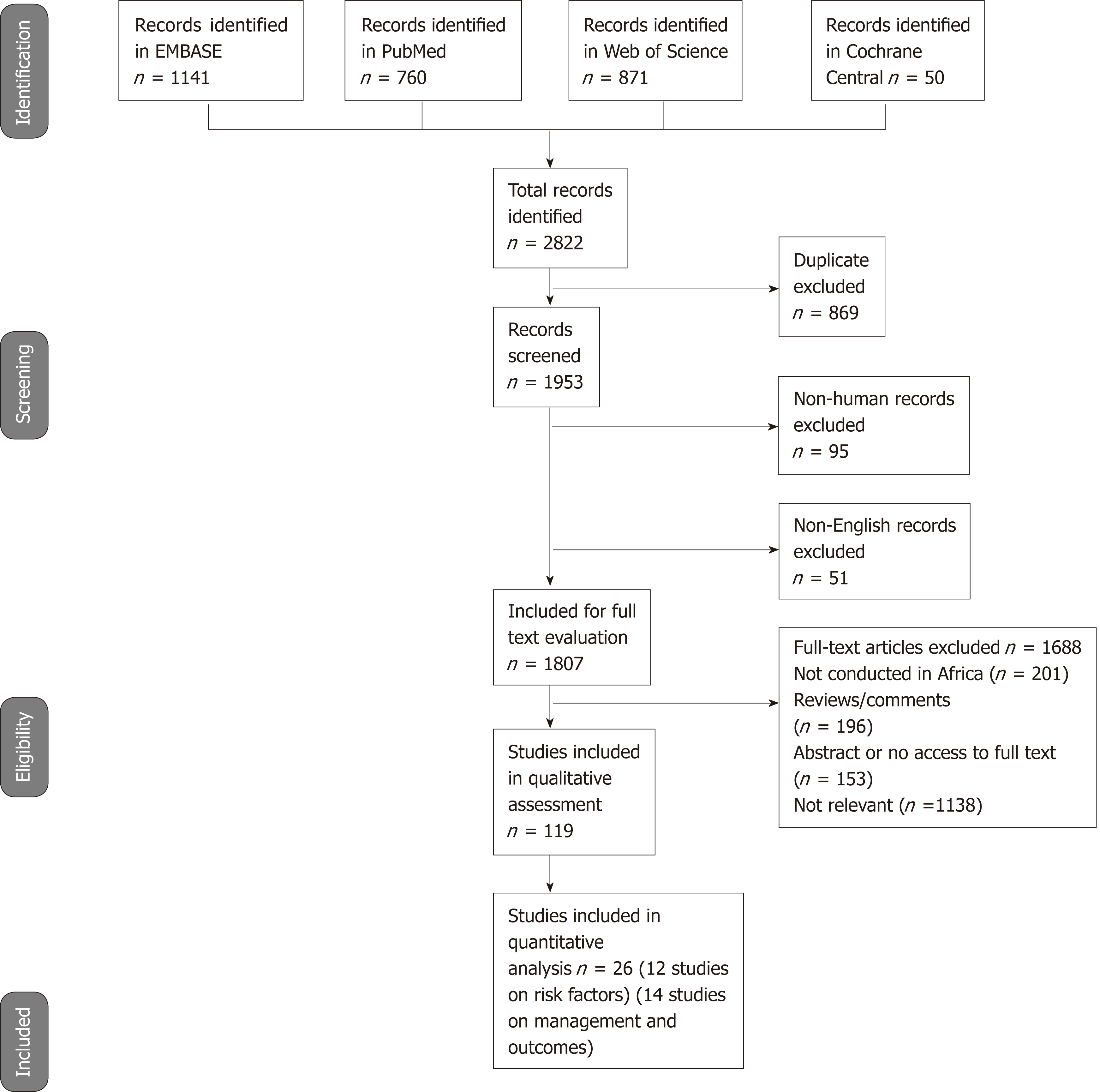
Results of search strategy: We identified 2822 published study citations from four databases, of which 869 were duplicates. There were 1953 titles that were therefore screened for initial eligibility, of which 146 were excluded (51 were not in English, and 95 were not human studies). We conducted further screening for studies reporting on epidemiology or risk factors or management and outcomes of EC and identified 1807 studies for full article review. Of these 1807 studies, 201 were not conducted in Africa, 196 were review articles, we could not access 153 full articles, and 1138 were not relevant. We thus identified 119 eligible for inclusion in the review (Figure 6).
Description of studies: Of the 119 eligible studies, 48 reported on the epidemiology, 36 studies on risk factors and 34 studies on management and outcomes of EC. All studies were conducted in both males and females, except for studies on epidemiology by Harington et al[22] and Robertson et al[23] that involved males only (Table 2).
| SA | Ken | Uga | Zim | Mal | Sud | Nig | Tan | Zam | Egy | Moz | Sen | Bot | Gha | Eth | Multi | |
| Studies on epidemiology (n = 48) | ||||||||||||||||
| Total number of studies per country | 13 | 8 | 4 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | – | – | 1 |
| Number of studies in cities/provinces | 8 | 5 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| Studies on risk factors (n = 37) | ||||||||||||||||
| Total number of studies per country | 18 | 5 | 3 | 2 | 1 | – | 1 | 1 | 2 | 1 | – | – | – | 2 | 1 | – |
| Number of studies on tobacco consumption | 10 | 3 | 2 | 2 | 2 | – | – | 1 | 2 | – | – | – | – | 1 | 1 | – |
| Number of studies on alcohol consumption | 5 | 1 | 1 | 1 | – | – | – | – | – | – | – | – | – | – | 1 | – |
| Studies on management and outcomes (n = 34) | ||||||||||||||||
| Total number of studies per country | 18 | 3 | – | – | 1 | 2 | 1 | – | – | 7 | – | – | – | – | 1 | 1 |
| Number of studies that report on chemotherapy | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Number of studies that report on radiotherapy | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Number of studies that report on chemoradiotherapy | 4 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – |
Of the 48 studies on epidemiology of EC, 13 (27.1%) were conducted in South Africa[23-35], 8 (16.7%) in Kenya[36-43], 4 (8.3%) in Uganda[44-47], 3 each in Zimbabwe (6.3%)[48-50], Malawi (6.3%)[51-53], Sudan (6.3%)[54-56] and Nigeria (6.3%)[57-59], 2 each in Tanzania (4.2%)[60,61] and Zambia (4.2%)[62,63], 1 each in Egypt (2.1%)[64], Mozambique (2.1%)[65], Senegal (2.1%)[66] and Botswana (2.1%)[67], and 3 (6.3%) studies were multi-country[22,68,69].
Of the 37 studies on risk factors for EC, 18 (48.6%) were conducted in South Africa[10,70-86], 5 (13.5%) in Kenya[11,13,87-89], 3 (8.1%) in Uganda[4,5,90], 2 each in Ghana (5.4%)[7,91], Zambia (5.4%)[3,92] and Zimbabwe (5.4%)[93,94], and 1 each in Malawi (2.7%)[95], Ethiopia (2.7%)[12], Tanzania (2.7%)[6], Egypt (2.7%)[96] and Nigeria (2.7%)[97].
Of the 34 studies on management and outcomes of EC, 18 (52.9%) were conducted in South Africa[98-114], 7 (20.6%) were conducted in Egypt[115-121], and 3 in Kenya (8.8%)[122-124] , 2 in Sudan (5.9%)[125,126], 1 each in Ethiopia (2.9%)[127], Malawi (2.9%)[128] and Nigeria (2.9%)[129], and 1 (2.9%) multi-country[130] (Table 2).
All 48 studies on the epidemiology of EC involved hospital-based patients (unlike the non-systematic review methods results above that report on population-based incidence data). Most of these studies were conducted in South Africa with most studies concentrated in cities or provinces (Table 2). The earliest studies on EC were conducted in Botswana between 1963 and 1968[67] and in South Africa between 1963 and 1964[24]. Over time there has been significant geographic variations in the prevalence of EC within and between countries. In Uganda, 7.5% of gastroscopy patients seen between 2009 and 2011 had EC[44]. In Zimbabwe, 6% of all cancers seen between 1991 and 2010 had EC[48]. In South Africa, 17% of all tumors seen in 1998 were EC[26]. In Zambia, 2.3% of total number of endoscopy patients seen between 1999 and 2005 had EC[63]. In Kenya, 19% total neoplasms seen between 1989 and 1998 were EC[42].
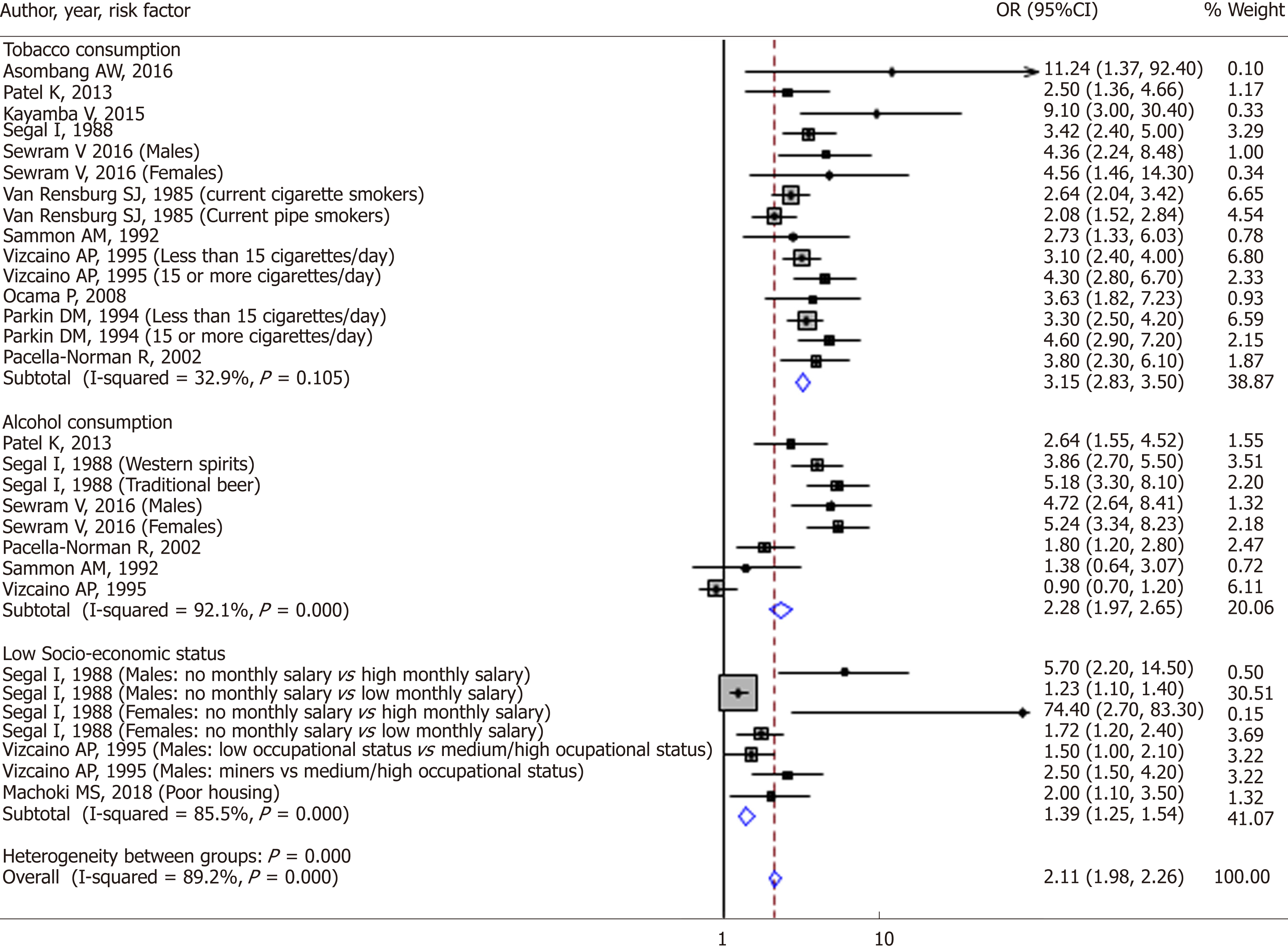
There were 12 studies included in the quantitative analysis that assessed the association between risk factors and EC. A qualitative assessment of these 12 studies achieved a final score of 91.7% and thus had adequate quality (Table 3). Some studies assessed more than one risk factor and thus there was an overlap in the number of studies. Of these 12 studies, 11 case control studies assessed tobacco consumption as a risk factor for EC, 6 case control studies assessed alcohol consumption as a risk factor, and 3 case control studies that assessed socio-economic status as a risk factor for EC (Figure 7).
| Quality variable | Quality variable categories | Number of studies | Proportion (column %) |
| Representativeness of sampling procedures used in each study | Non-probability | 12 | 100.0 |
| Probability | 0 | ||
| Response rate | Above 80% | 10 | 83.3 |
| Between 60% and 80% | 2 | ||
| Not reported | 0 | ||
| Validity of measurement methods for risk factors | Face to face interview | 12 | 100.0 |
| Self-administered questionnaire | |||
| Bias in the measurement of the esophageal cancer | Histology | 10 | 83.3 |
| Endoscopy | 1 | ||
| Not reported | 1 | ||
| Control for important confounders | Yes | 11 | 91.7 |
| Not reported | 1 | ||
| Final score (mean across the items) | 91.7 |
Tobacco: Tobacco smoking and chewing have been found to be major risk factors for ESCC across many African countries, with OR of approximately 2.5-11.24; 95%CI: 1.36-92.4, P = 0.0001 to 0.04[3,5,88,92].
Several case control studies also found a high relative risk of developing ESCC in smokers of approximately 1.29 to 5.7[72,79,80,94]. Among cases of ESCC, higher proportions (74% to 82.2%) of smokers have also been found among ESCC cases in cross sectional studies across Africa[6,7]. In a Zimbabwean study, Parkin et al found that male smokers had a relatively higher risk of developing ESCC compared to female smokers OR 2.7, 95%CI: 1.8-4.2 vs OR 1.3, 95%CI: 0.8-2.1[93]. In a similar study in Zimbabwe, tobacco smoking in males was significantly associated with risk of EC with RR rising to 5.7 among smokers of 15 or more g/day; this effect was independent of alcohol drinking[94]. Among women who had ever smoked tobacco the RR was 4.0 compared to those who had never smoked. However, another study in South Africa found a relatively higher risk of developing ESCC in black African female who smoked cigarettes, OR 1.3, 95%CI: 0.8-2.1[71]. As elsewhere in the world, the prevalence of tobacco consumption is higher in males than females in Africa. In a study among 159 hospital patients with ESCC in South Africa by Loots et al[35], men were found more likely to use tobacco than females (OR 7.8, P < 0.001).
Alcoholic beverages: Alcohol consumption was found to be significantly associated with EC, OR 2.64, 95%CI: 1.55-4.52[88]. Segal et al[80] found a high relative risk of developing EC in different groups of men and women who drunk large amounts of traditional beer. In men the relative risk was 1.25-3.9; 95%CI: 1.1-6.9, P = 0.0001, while in females it was 1.72-25.70, 95%CI: 1.2-238.4, P = 0.0043. The risk was even higher in the groups of men who drunk large amounts of commercial spirits, OR 1.93-7.20, 95%CI: 1.2-33.2. EC was also found to be associated with alcohol consumption in another case control study in South Africa in both males and females who had taken alcohol before (men: OR 3.48; 95%CI: 2.00-6.09, females: OR 2.23; 95%CI: 1.60-3.11)[81]. They went further to categorise the participants into groups with varying numbers of days they drunk maize beer per week and also by the amount they drunk per week. Those who drunk for 5-7 d a week had the highest odds of developing oesophageal as did the ones who drunk higher quantities of the beer; (males: OR 4.68, 95%CI: 2.32-9.44; females: OR 2.65, 95%CI: 1.49-4.71) and (males: OR 4.26, 95%CI: 2.39-7.62; females: OR 5.05, 95%CI: 3.26-7.82) respectively. The same was done for sorghum beer, commercial spirits, home-made spirits and wine. The results for all the types of alcohol were similar in both sexes except for home-made spirits which had a relatively higher OR in females of 15; 95%CI: 3.31-71.18. There is increased exposure to alcohol in a male in Africa like in other parts of the world. In a study conducted among hospital patients with EC in South Africa, Loots et al[35] found that men were more likely to consume alcohol than females, OR 7.7, P < 0.001. In a study done in Nigeria, the ASR attributable to alcohol in males was 0.3/100000 while in females it was 0.1/100000[97]. The risk of oesophageal in black South African males and females who consumed alcohol was similar, OR 1.8, 95%CI: 1.2-2.8 and OR 1.7, 95%CI: 1.0-2.9 respectively[71]. No association was found between alcohol consumption and oesophageal cancer in a study done in Transkei region of South Africa[79].
Diet: Solanum nigrum vegetable consumption had a relative risk of developing oesophageal cancer of 3.62, 95%CI: 0.92-4.66[79]. The same study found no association of various other types of food with oesophageal cancer. There was an association between purchased maize meal and EC in Zulu men of South Africa, with a relative risk of 5.7[72]. In a study done in Eastern Cape Province of South Africa, Sewram et al[10] found associations between different types of diet and EC. Individually, maize or sorghum consumption versus never or rare consumption were not associated with EC (P > 0.1). Males and females consuming green leafy vegetables 5-7 day/week had 38% (P = 0.04) and 50% (P = 0.007) reduced odds of developing EC, respectively, compared with consumption ≤ 1 d/wk. A similar reduction in odds was observed with fruit consumption. Principal component factor analysis revealed 3 distinct dietary patterns. In females, high vs low consumption of Pattern 1 (sorghum, green leafy vegetables, green legumes, fruits, meat) was inversely associated with EC development (OR = 0.54, 95%CI: 0.34-0.89), whereas for Pattern 2 (maize, wild greens-imifino, dry beans) the odds were elevated (OR = 1.67, 95%CI: 1.04-2.67). Compared with low adherence, high adherence to Pattern 3 (wheat-based products) reduced the odds by 35% for both sexes[10].
Social economic status: Not having a monthly salary was associated with EC in men; the relative risk of developing EC was high in those that had no monthly salary compared to those that received a high salary (RR 5.7, 95%CI: 2.2-14.5). The relative risk of developing EC was still significantly high in those that had no monthly income compared to those that received a low salary (RR 1.23, 95%CI: 1.1-1.4), P = 0.0003. A similar association was observed in females who had no monthly income compared to those who received a high monthly income (RR 74.4, 95%CI: 2.7-83.3); also compared to those that received a low monthly income (RR 1.72, 95%CI: 1.2-2.4), P = 0.0043[80]. Low occupational status (OR 1.5, 95%CI: 1.0-2.1) and working as a miner (OR 2.5, 95%CI: 1.5-4.2) were associated with increased risks of EC compared to men of medium or high occupational status[94]. Poor housing was found to be an independent predictor of EC in a study done in Kenya, (OR 2.0, 95%CI: 1.1-3.5)[13]. No association was found between socio-economic status and EC in a study done in Transkei region of South Africa[79].
Infections: HIV infection on its own conferred increased risk of developing ESCC (OR 2.3, 95%CI: 1.0-5.1, P = 0.03). The OR was stronger when only people under 60 years were included (OR 4.3, 95%CI: 1.5-13.2, P = 0.003)[92]. An association between Helicobactor pylori and EC was found in South-Western Uganda, OR 7.2, 95%CI: 1.68-31.04, P = 0.003[90]. However, in another study, Helicobactor Pylori prevalence was found to be similar between cases of oesophageal cancer and general population (51%)[75].
Micronutrients: Within a high EC rate area occupant of households with previously recorded EC cases had significantly lower levels of Selenium (Se, P < 0.0025), but not of other mineral elements, than subjects from households without known cases of EC[74]. Mean Se levels of subjects with premalignant or malignant esophageal cytological changes were significantly lower than those of subjects without such lesions, P < 0.0025. An inverse relationship was found between Se status and the degree of cytologic abnormality. These findings suggest that Se deficiency may play a role in the development of EC[74]. In a cross-sectional study in Western Kenya, Pritchet et al[89] found a high serum Se associated with prevalence of esophageal squamous dysplasia. (Q4 vs Q1: OR 3.03, 95%CI: 1.05-8.74), after adjusting for potential confounders (Q4 vs Q1: OR: 3.87, 95%CI: 1.06-14.19).
A direct comparison between subjects with carcinoma of the oesophagus and the 1976 group showed that the geometric mean hepatic iron concentrations in the carcinoma patients were significantly higher in the 40-49, 50-59 and over-60 age groups[73]. While geometric mean hepatic iron concentrations in the various age groups were also higher than those obtained in the 1959/1960 study, the differences only reached statistical significance in subjects over the age of 60 years. These results suggest that the excessive consumption of home-brewed alcoholic drinks contaminated with iron may be directly or indirectly associated with the development of carcinoma of the oesophagus in urban black adults[73].
Genetic factors: Females were twice more likely to report a family history of cancer, P = 0.017, OR 2.6[35]. Genome wide association studies have shown an association of genetic regions and EC in an African population when compared to a non-African population with increase EC incidence. However, these variations are noted within the same African population and require further analysis[70]. In a retrospective analysis of patients with esophageal biopsies, Simpong et al[91] evaluated HER-2 protein expression in tissue. Of 91 esophageal biopsies, they found 8 cases (8.79%) of esophageal adenocarcinoma, of which 3 (37%) had HER-2 overexpression. The implications of this are developing targeted treatment protocols[91]. Mutation in ras gene is thought to promote carcinogenesis[82]. Victor et al[82] evaluated 27 EC samples from a high incidence are in South Africa, and found no evidence of ras mutation, however the authours conclude that point mutation may not be directly involved but possibly involvement of ras via other mechanisms.
Another studied gene is the MSH3, involved with DNA mismatch repair. A mutation in MSH3 has been shown to promote carcinogenesis of the colon. When investigating the methylation status of MSH3 gene in tumors and matching adjacent normal-looking tissues from EC patients in a high-risk South African population in order to further elucidate possible role of MSH3 in esophageal tumorigenesis, overall, promoter methylation was detected in 91.9% of tumors, which was significantly higher compared to 76.0% in adjacent normal-looking esophageal tissues (P = 0.008)[83]. When samples were grouped according to different demographics (including age, gender and ethnicity) and smoking status of patients, methylation frequencies were found to be significantly higher in tumor tissues of Black subjects (P = 0.024), patients of 55-65 years of age (P = 0.032), males (P = 0.037) and tobacco smokers (P = 0.015). Furthermore, methylation of the MSH3 promoter was significantly more frequent in tumor samples from smokers compared to tumor samples from non-smokers (OR = 31.9, P = 0.031)[83].
The mismatch repair system (MMR) is involved repairing errors that occur in DNA replication. Some of the proteins in the MMR system include MSH3, PMS1 and MLH3. A mutation in these can result in carcinogenesis. Vogelsang et al[83], assessed the cumulative effects of polymorphisms in the DNA mismatch repair genes and tobacco smoking in EC risk in a South African population[83,84]. In a mixed ancestry group, they found the MSH3 rs26279 G/G vs A/A or A/G genotype was positively associated with cancer (OR = 2.71, 95%CI: 1.34-5.50). Similar associations were observed for PMS1 rs5742938 (GG vs AA or AG: OR = 1.73, 95%CI: 1.07-2.79) and MLH3 rs28756991 (AA or GA vs GG: OR = 2.07; 95%CI: 1.04-4.12)[84]. In Black individuals, however, no association between MMR polymorphisms and cancer risk was observed in individual single nucleotide polymorphisms analysis)[84]. In a similar study of South Africans with Black ancestry (368 cases and 583 controls) and mixed ancestry (197 cases and 420 controls), Wang et al[85] sought to investigate the associations of 3 individual microRNA single nucleotide polymorphisms with the risk of developing esophageal SCC. In Blacks, rs6505162 A>C was associated with ESCC under dominant, additive and recessive models with ORs 1.353, 1.404, and 2.858, respectively[85]. This locus showed very strong interactions with smoke inhalation from burning wood or charcoal used for heating and cooking in very poorly ventilated areas The AArs213210-CTrs7372209 genotype had a protective effect on OSCC risk (in the Black, OR = 0.229, P = 0.012; and the Mixed Ancestry groups, OR = 0.230, P = 0.00014)[85]. In a Kenyan study, first degree family history of ESCC was found to be an independent predictor of ESCC, (OR 3.5, 95%CI: 1.3-9.5)[13].. In another study in Egypt, family history of EC was found to be a risk for EC, (OR 6.21, 95%CI: 3.12-11.81)[96].
Qat chewing: In a study done in Ethiopia, qat use was more frequent in EC cases (36%) than in controls (26%). A 2-fold elevation in EC risk was observed in ever qat chewers compared with never users in unadjusted conditional logistic regression (OR = 2.12, 95%CI = 0.94-4.74), an association that disappeared after adjusting for differences in tobacco use, consumption of alcohol and green vegetables, education level, and religion (OR = 0.95, 95%CI: 0.22-4.22)[12]. Among never tobacco users, however, a non-significant increase in EC risk was suggested in ever qat users also after adjustment[12].
Age and gender: With a median age of 60 years for cases vs 45 years for controls, an association with oesophageal cancer was observed in the older age with an OR of 1.63, 95%CI: 1.34-1.98, P < 0.001, while male gender also showed an association, OR 2.17, 95%CI: 1.07-4.41, P = 0.032[5]. Males and the elderly were found to be more susceptible to EC than females and younger ones respectively in a study done in Egypt (OR 3.12, 95%CI: 2.41-4.29) and (OR 1.32, 95%CI: 1.21-1.4) respectively[96].
Ethnicity and residence: Kalenjin ethnicity was found to be associated with EC in western Kenya, OR 5.7, 95%CI: 2.1-15.1[87]. Long-term residence in the Transkei region of South Africa was found to be associated with EC in females, OR 14.7, 95%CI: 4.7-46.0[71].
Urine isoprostane: High level of Urine isoprostane was found to be associated with ESCC in Zambia, OR = 2.35, 95%CI: 1.19-4.65, P = 0.014[3].
Non-acid gastro-oesophageal reflux: Non-acid gastro-oesophageal reflux was associated with development of ESCC, OR 8.8, 95%CI: 3.2-24.5, P < 0.0001[76]. Alcohol and smoking had no effect on these results.
Caustic ingestion: Caustic ingestion was found to be an independent predictor of EC in a study done in Kenya (OR 11.3,95%CI: 3.0-42.5)[13].
Tooth loss, cooking, hot beverage consumption, dental fluorosis and using mursik: Tooth loss, cooking, hot beverage consumption and using mursik were found to be associated with EC in the Rift Valley of Kenya, OR 5.28, 95%CI: 2.98-9.41; OR 2.32, 95%CI: 1.41-3.84; OR 12.78, 95%CI: 6.98-23.6; and OR 3.72, 95%CI: 1.96-7.14 respectively[88]. Menya et al[11] conducted a case control study in Kenya evaluating the association of dental fluorosis and EC. They assessed association of EC with tobacco use, alcohol consumption, education, oral hygiene and missing/decayed teeth. After adjusting for the other factors, they found 40% of EC cases had moderate/sever fluorosis, OR 9.4, 95%CI: 4.6-19.1 and 43 % cases with mild fluorosis, OR 2.3, 95%CI: 1.3-4[11].
Traditional Xhosa medications: There was an association between taking traditional Xhosa medications and EC with a relative risk of 2.08, 95%CI: 0.92-4.66[79].
Baker’s yeast: There was an association between consumption of bakers yeast and EC in men, RR 2.17; 95%CI: 1.1-4.2, P = 0.0234[80].
Endoscopic pulsion intubation: This was only reported in three studies, and of the three studies, only two reported on the number of patients with a combined total of 200 patients[122,131]. This is a palliative endoscopic procedure to relieve patients from malignant dysphagia, in which the oesophagus is endoscopically intubated, then an endoprosthesis place at site of obstruction[122,131,132]. Jani et al[122] reported it to be affordable and effective method of palliative treatment.
Stenting: Esophageal self-expandable metallic stent (SEMS), are used for palliative treatment of malignant dysphagia or esophago-respiratory fistula. We had 10 different studies with a combined total study population of 1699 patients that reported a marked improvement in symptoms of malignant dysphagia and/or esophago-respiratory fistula[103,107,108,119,120,123,124,128,133,134]. The type of stents used includes Ultraflex (Boston Scientific Corporation, MA, United States), AT&M SEMS (Advanced Technology and Materials, Beijing, China), Wallstent (Boston Scientific Corporation, MA, United States) among others[108,120,124].
Chemotherapy: Chemotherapy is a systemic therapy with the potential to eradicate micrometastatic disease by decreasing the number of cancer-cell dissemination. Five studies with a combined study population of 214 patients were reported in which chemotherapy (as single agent or combination) was used in the treatment of oesophageal with varying outcomes[99,100,106,131,135].
Radiotherapy: Different modalities of radiotherapy were employed as a sole modality to treat malignant dysphagia with improved outcomes. The different types of radiotherapy used included; brachytherapy such as high dose rate brachytherapy (HDRBT) in a study with a study population of 139 patients[130], high dose rate intraluminal brachytherapy (HDRILBT) with a study population of 172 patients[111], microselectron high dose rate brachytherapy(MHDRBT) with a study population of 232 patients[112], intraluminal brachytherapy, telebrachytherapy[110], and external beam radiotherapy(EBRT)[136]. A combined radiotherapy technique was used in some studies such as HDRBT/EBRT and EBRT/Intraluminal brachytherapy[136].
Chemo radiotherapy: Radiotherapy and chemotherapy were simultaneously used in some studies to enhance survival and improve the patients’ quality of life. Both therapies are known to improve the control of both the systemic disease and local disease by down staging the cancer thus increasing the surgical resectability. Four studies[98,109,114,117] reported on chemo radiotherapy (CRT) in which different regimes were used such as Cisplatin/fluorouracil/radiotherapy[117], Bleomycin or Vimblastine/radiotherapy[98], Cisplatin and 5-Fluorouracil/radiotherapy[109,135].
Esophagectomy: Esophagectomy remains the primary treatment modality of early oesophageal cancer, however in our study most studies attempted to find out its role in palliation for patients with advanced oesophageal cancer. Different approaches were employed that include; transhiatal esophagectomies[104,129], transthoracic approach esophagectomies such as left transthoracic and Ivor Lewis appro-aches[125-127,137] and McKeon approach[126].
Only 2 studies on esophageal stenting studies reported on mortality. The two studies reported stent application associated mortality of 3.5% in a study population of 58 patients[107] and of 0.5% in a study of 951 patients[124]. Three studies reported on the morbidity associated with this same intervention[119,120,124]. The early morbidities reported include oesophageal perforation, bleeding, chest pains and death while the late morbidities include migration, Tracheo-Oesophageal Fistula (TOF) and tumour overgrowth[119,120,124]. No study reported on any mortality associated with use of chemotherapy only. However, three studies reported on complications of the chemotherapy only. The common complications include haematological toxicity of different spectrums, stomatitis, cheilitis, xeroderma, nausea, anorexia and vomiting[99,100,106]. Only one study reported on mortality associated with use of radiotherapy (HDRILBT) alone[111]. A mortality of 12.8% was reported in this study with a study population of 172 patients[111]. The same study also reports on oesophageal strictures as the common complication occurring in 25% of patients on HDRILBT. Patients treated with telebrachytherapy at 20GY and 15GY dosages reported a complication free survival rate of 55% and 83% respectively[110].
No study reported on any mortality associated with use of chemo-radiotherapy. Only one study with a study population of 25 patients reported on complications such as haematological toxicity, mucositis, oesophagitis, nausea, vomiting and diarrhoea[117]. Esophagectomy associated mortality was reported in seven studies. The highest 30 d perioperative mortality was 50% in a study with a population of 10 patients, however, in the follow-up study with 21 patients the morbidity decreased to 14.3%[129]. The lowest mortality reported was 10% in a study with a study population of 100 patients[126]. The 30 d perioperative mortality was even lower (6.5%) in a study with combined treatment of Esophagectomy and CRT[138]. Other studies reported a mortality 11.8% in a study population of 127 patients[104], mortality 27.8% in a resected population of 61 patients[125], mortality 28% in a resected population of 34 patients and mortality 30% in a resected population of 25 patients[137]. Four studies reported on morbidity and the common complications includes; hemorrhage, anaesthetic complication, pneumonia, respiratory failure, pleural enteritis, chest wall infection, anastomotic leakage and heart failure[125,127,129,137].
The mean dysphagia score was measured using the Mellow and Pinka’s dysphagia score and was reported to improve after stent application in 10 studies[107,108,115,119,120,123,124,128,133,134]. A study with a population of 40 patients had the best mean dysphagia score improvement which was from a score of 3 to a score of 0[108]. The other mean dysphagia score improvements reported include; score 3.3 to 1.0 in a study population of 951 patients[124], score 3.5 to score 0.5 in a study population of 70 patients[134], score 3.5 to score 1.2 in a study population of 124 patients[119], score 2.98 to score 1.08 in a study population of 58 patients[107] and score 3.0 to score 1.0 in a study population of 350 patients[115].
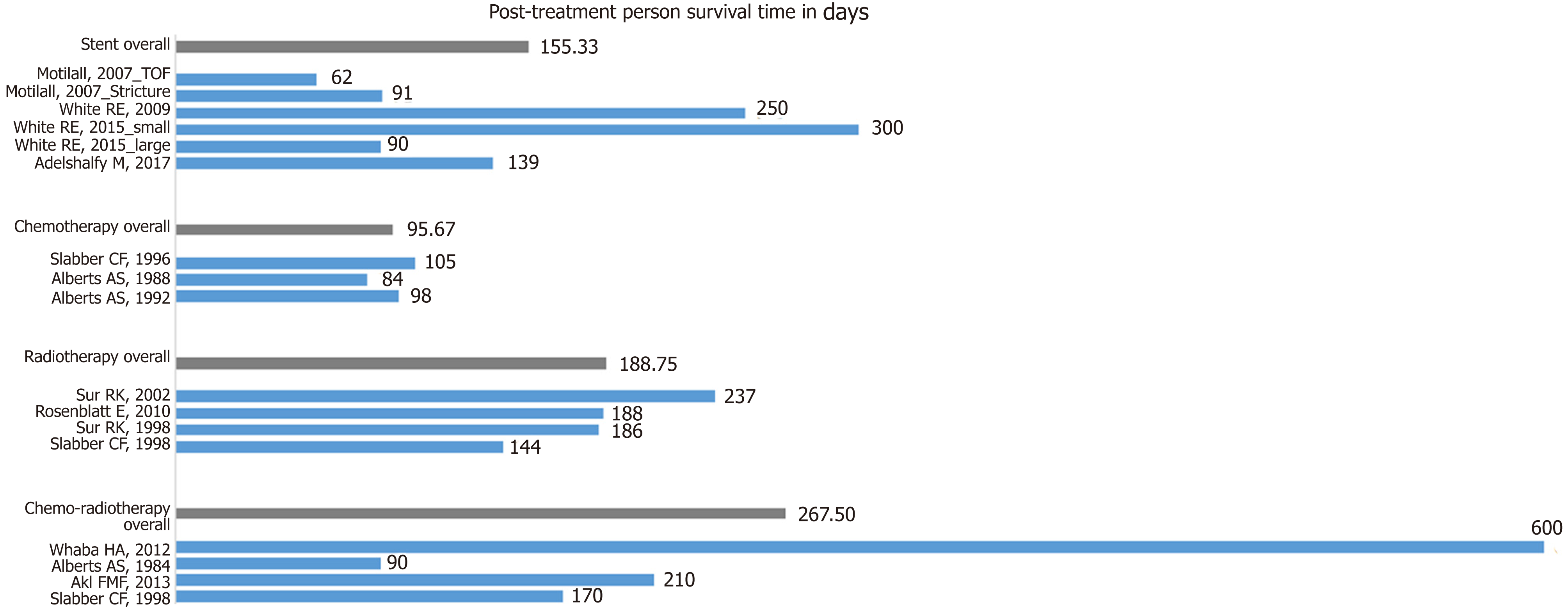
There were 14 studies included in the quantitative analysis that assessed post-treatment person survival. Some studies assessed survival from more than one treatment modality and thus there was an overlap in the number of studies. Of these 14 studies, 6 studies that assessed survival after stent placement, 3 studies assessed survival after chemotherapy, 4 on the effects of radiotherapy and 4 studies on the effects of chemotherapy on survival. The overall means for each treatment modality were calculated and chemo-radiotherapy had the longest post-treatment patient survival of 267.50 d. Radiotherapy had an overall mean survival time of 188.75 d, Stents 155.33 days, and chemotherapy 95.67 d (Figure 8).
Five studies reported on esophagectomy for the treatment of EC. Resection margins are classified as: RO - no microscopic evidence of remnant tissue, R1 - microscopically positive margins, and R2 - macroscopically positive margins. The goal of resection is to achieve RO margins. Only one study reported on R1 esophagectomy, of which it was only achieved in 2.2% of the resected patients[138]. The overall survival rates reported in the esophagectomy patients includes; 76.6% in 3 years[138], 30% in 3 years[101], 64% in 1 year and 40% in 2 years[125], 13.2% in 5 years[104], 52% in 2 years and 70.6% in 1 year[126].
Five studies reported on the use of stent placement in the treatment of EC. Of these, two studies found the median patient survival time to be less in patients who had TOFs as a complication EC, (62 d and 142 d), compared to those without TOF (91 d and 250 d)[107,124]. The best median patient survival time reported in stent application was 300 d (5.9 mo) in a study with a population of 100 patients[133]. The median patient survival times reported in other studies include 139 d in a study population of 350 patients[115], 216 d for males and 304 females in a study population of 151 patients[124].
Four studies reported on median patient survival and six studies on improvement of the patient’s mean dysphagia score after radiotherapy as single treatment for EC[109-112,130,136]. The best median patient survival time among these studies was 237 d (7.9 mo)[112]. The other studies reported a median patient survival time of 188 d with an overall survival rate of 18% in 1 year[130], 186 d with an overall survival rate of 19.4% in 1 year (20). The lowest median patient survival time reported was 144 d[109].
Four studies reported on the use of CRT for the treatment of EC. CRT reported the highest median patient survival time of 600 d (20 mo) compared to any other treatment modality[121]. The lowest reported median patient survival time of patients on CRT was 90 d (3 mo) in a study with a population of 271 patients[98]. The other studies on CRT reported a median overall survival time of 210 d (7 mo)[117] and 170 d (5.6 mo)[109]. Two studies reported on the improvement of the patient’s mean dysphagia score by 75%[117] and 67%[98]. Only one study reported on the use of endoscopic Nd-YAG laser for palliation in patients with oesophageal cancer. It reported an improvement of the mean dysphagia score from 3.3 to 1.2[116].
To our knowledge, this is the first comprehensive systematic review reporting the epidemiology, risk factors, management and outcomes of EC in Africa. The current incidence of 9.7 to 17.9/100000 is likely to be an underestimation. The predominant histopathological abnormality is squamous cell carcinoma in sub-Saharan Africa. We found that EC occurs at a younger age compared to other cancers, with a rise in incidence from age 40, peaking at persons aged 75. The incidence cannot be accurately recorded as some countries in Africa have not reported any studies on EC, and in those countries where studies have been carried out, there tends to be regional variation.
Within Sub-Saharan Africa there is a lot of geographic variability with the highest incidence of squamous cell carcinoma in Malawi of 30.3 cases /year/100000 among males and 19.4 cases /year/100000 among females between 2009 and 2013. The incidence is higher in males in sub-Saharan Africa but is equal between the sexes in North Africa. The reason for the high burden EC in several parts of Eastern and Southern Africa, especially among males could be explained in part by the strong association between tobacco consumption and EC as demonstrated in our meta-analysis (I-squared = 32.9%, OR 3.15, 95%CI: 2.83-3.50). There was significant heterogeneity between studies that examined the association between alcohol consumption and EC (I-squared = 92.1%, OR 2.28, 95%CI: 1.94-2.65); also between studies that examined the association between low socioeconomic status and EC (I-squared = 85.5%, OR 139, 95%CI: 1.25-1.54) as risk factors, but these could also contribute to increasing the incidence of EC. The geographic variation of EC within Africa can be explained by several environmental risk factors, and some host factors related to metabolic pathways of handling of oxidative stress and toxins. There may be a synergy to these risk factors. Fungal and mycotoxin contamination of staple cereals including white maize, wheat and Kocho (Qocho) in Ethiopia. Consumption of Mursik a fermented goat/sheep milk product has been identified as a risk in Kenya. The toxin associated with this risk is not very clear. Consumption of hot beverages including tea and porridge, is a risk as is poor oral health and fluorosis in some parts of Kenya. A low consumption of fruits and vegetables and deficiency in Se are considered as risks.
Host factors including mutation in genes coding for enzymes responsible for Alcohol and Acetaldehyde metabolism, and handling of oxidative stress in alcohol and contaminated food were found to be associated with EC and are important. Human papilloma virus infection has been observed to be a risk in some studies, but this risk is not consistent in other studies. Exogenous or endogenous acetaldehyde as a product of alcohol and oral fermentation (poor dental hygiene) is carcinogenic. Acetaldehyde dehydrogenase detoxifies both alcohol and Acetaldehyde. Formation of Acetaldehyde DNA adducts causes mutagenesis. ALDH2 polymorphism has been implicated in the development of oesophageal cancer. Genetic polymorphisms associated with modulation of oxidative stress, including N-acetyl transferases NAT1 and NAT2 mutations, Glutathione S transferase GSTT2B have been implicated in development of ESCC. Somatic mutations including p53 and p16/CDKN2 genes and those associated with genetic polymorphisms associated with DNA repair like RUNX1 rs2014300 have been observed as an independent risk in some small studies.
EC in Africa is associated with high mortality. The mortality relates to advanced disease at presentation, lack of availability of treatment modalities. There is morbidity and mortality associated with the treatment modalities. The high mortality is related to late presentation of disease. Radiotherapy delivered as brachytherapy or external beam improved, symptoms and a combination of these radiotherapy modalities was more effective. Chemotherapy has been used with palliative intent and was more effective in symptom control with additional radiotherapy (Chemoradiotherapy). Palliative care with self expanding mental stents improves symptoms of dysphagia.
Preventive strategies include public health interventions can modulate these risks, including discouraging smoking and alcohol consumption, changing practices of alcohol brewing and access to dental care. Agricultural and food storage practices to encourage dry storage of staple cereals could minimise fungal infection and thus limit mycotoxin contamination. Consumption of fruits and vegetables can be encouraged. Food with high antioxidant properties can also be identified and incorporated into local diets.
There is no obvious “at risk” population for squamous cell dysplasia or other risk factors to guide in formulation of screening programs for ESCC surveillance. Dysphagia is a late symptom in oesophageal cancer, as such it is difficult to formulate screening program in these high-risk populations, other than those with underlying Barrett’s oesophagus, a risk for oesophageal adenocarcinoma in North Africa.
The high mortality is related to late presentation with advanced stage disease as symptomatic EC is often advanced. As some studies show high perioperative mortality, this may be related to surgery on advanced and unstaged disease, perioperative malnutrition due to lack of nutritional assessments and optimisation and general postsurgical care and critical care facility availability. Optimisation of nutrition, local staging including endoscopic ultrasound and staging with computed tomography (CT) and positron emission tomography-CT scans for disseminated would inform treatment choices, and thus prognosis. The aim for surgical resection should be R0 resection. This required surgical expertise, but such characterisation also requires a skilled pathologist. Adjuvant chemotherapy would improve the prognosis in those with excision specimens more than R0. Evidence based outcomes in management of these patients would therefore require multi-disciplinary team assessment of these patients to choose the most appropriate treatment. Early detection of cancers, endoscopic resection of such cancers and palliative stenting require skilled endoscopists. Investment in such skills and these facilities would improve mortality and morbidity in Africa. There is also a need for investment in oncological services including chemotherapy and radiotherapy. Patients with advanced oesophageal cancer would require high quality palliative care, to manage stricture, aspiration due to tracheoesophageal fistulae with stenting, and chemo-radiotherapy to manage strictures. As with the other services, skills in endoscopy, nutrition and oncology are required.
There are some limitations to this systematic review. There is a variation in sample size and study design, with most having a small sample size, retrospective, less prospective and low number of randomized controlled trials. Measures of association such as relative risk or OR were not included, rather percentage of subjects with an identified risk factor. Some of the published data are from the 1970s, 80s and 90s with limitations to the full manuscript access and applicability to the current era given the socioeconomic and environmental changes. There is a lack of data from African countries, particularly mortality data. The mortality outcome studies are not clearly linked to preoperative or resection staging, treatment options are not clearly based on staging and the genetic polymorphism/associated mutation studies have not been replicated in larger studies hence the need for multicenter studies to address these issues. Further research is required to understand, the other risk factors can direct future targeted drug treatments that account for geographic variation, epidemiology, better treatments.
Esophageal cancer (EC) is associated with a poor prognosis, particularly so in Africa where an alarmingly high mortality to incidence ratio prevails for this disease. This paper aims to provide further understanding of EC in the context of the unique cultural and genetic diversity, and socio-economic challenges faced on the African continent.
The incidence rates of EC in Africa have been uptrending in contrast to those seen in other parts of the world, with some of the highest incidence rates being found in East Africa. In addition, the histological pattern of disease shows a geographic variation. There is a need for further study in order to understand the various predisposing factors leading to these trends; as well as the treatment strategies and survival outcomes.
By conducting a comprehensive review of published studies of EC in Africa, this article aims to estimate the incidence and geographic variation of EC in Africa; to determine the risk factors associated with EC in this region, as well as describing the management and treatment outcomes.
Both systematic and non-systematic systematic review methods were used. The systematic review was used to obtain data on epidemiology, risk factors, management and outcomes of EC, while the non-systematic review was to obtain incidence data from the International Agency for Research on Cancer, and the Cancer in Sub-Saharan reports. Databases including EMBASE, PubMed, Web of Science, and Cochrane Central were searched from inception to March 2019 and the list of articles retrieved was reviewed. Random effects meta-analyses models were used to assess heterogeneity between studies and to obtain odds ratio and 95% confidence intervals of the associations between EC and risk factors, incidence rate ratios for EC between sexes, and survival following treatment.
Apart from confirming well-established risk factors such as tobacco use and alcohol consumption, other associations were established - including dietary and genetic factors, socio-economic status and infections such as HIV. The age-standardized incidence rates of EC were noted to be higher in men than women, except in parts of North Africa were the rates were similar. The highest incidence rates were found to be in Malawi (30.3 and 19.4 cases /year/100000 population for males and females, respectively) followed by Kenya (28.7 cases/year/100000 population for both sexes). The best treatment outcomes were seen in patients who underwent esophagectomies and achieved overall survival rates of 76.6% at 3 years, as well as in those who underwent chemoradiotherapy and had a median patient survival time of 20 mo.
Africa has high incidence and mortality rates of EC, with preventable as well as non-modifiable risk factors, and the highest rates occurring in Malawi and East Africa. The management of this disease requires a multidisciplinary approach, and survival is significantly improved in the setting of esophagectomy and chemoradiation therapy.
Although advanced EC has a poor prognosis, a better understanding of the associated risk factors and challenges faced in managing this condition in the African setting can better inform the development of strategies and policies to improve earlier detection, optimize management and prolong survival.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kato K, Okamoto H, Otowa Y S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | GLOBOCAN 2018: Estimated cancer incidence, mortality and prevalence worldwide in 2018. Available from: http://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-sheet.pdf. |
| 2. | Parkin DM. Ferlay J, Hamdi-Cheri M, Sitas F, Thomas JO, Wahunga H, Whelan SL. Cancer in Africa: Epidemiology and Prevention. IARC Scientific Publication, 2003. . |
| 3. | Asombang AW, Kayamba V, Lisulo MM, Trinkaus K, Mudenda V, Sinkala E, Mwanamakondo S, Banda T, Soko R, Kelly P. Esophageal squamous cell cancer in a highly endemic region. World J Gastroenterol. 2016;22:2811-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Okello S, Churchill C, Owori R, Nasasira B, Tumuhimbise C, Abonga CL, Mutiibwa D, Christiani DC, Corey KE. Population attributable fraction of Esophageal squamous cell carcinoma due to smoking and alcohol in Uganda. BMC Cancer. 2016;16:446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Ocama P, Kagimu MM, Odida M, Wabinga H, Opio CK, Colebunders B, van Ierssel S, Colebunders R. Factors associated with carcinoma of the oesophagus at Mulago Hospital, Uganda. Afr Health Sci. 2008;8:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | McHembe MD, Rambau PF, Chalya PL, Jaka H, Koy M, Mahalu W. Endoscopic and clinicopathological patterns of esophageal cancer in Tanzania: Experiences from two tertiary health institutions. World J Surg Oncol. 2013;11:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Tettey M, Edwin F, Aniteye E, Sereboe L, Tamatey M, Ofosu-Appiah E, Adzamli I. The changing epidemiology of esophageal cancer in sub-Saharan Africa - the case of Ghana. Pan Afr Med J. 2012;13:6. [PubMed] |
| 8. | Middleton DR, Menya D, Kigen N, Oduor M, Maina SK, Some F, Chumba D, Ayuo P, Osano O, Schüz J, McCormack V. Hot beverages and oesophageal cancer risk in western Kenya: Findings from the ESCCAPE case-control study. Int J Cancer. 2019;144:2669-2676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Munishi MO, Hanisch R, Mapunda O, Ndyetabura T, Ndaro A, Schüz J, Kibiki G, McCormack V. Africa's oesophageal cancer corridor: Do hot beverages contribute? Cancer Causes Control. 2015;26:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Sewram V, Sitas F, O'Connell D, Myers J. Diet and esophageal cancer risk in the Eastern Cape Province of South Africa. Nutr Cancer. 2014;66:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Menya D, Maina SK, Kibosia C, Kigen N, Oduor M, Some F, Chumba D, Ayuo P, Middleton DRS, Osano O, Abedi-Ardekani B, Schüz J, McCormack VA. Dental fluorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case-control study and a pan-African perspective. Int J Cancer. 2019;145:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Leon ME, Assefa M, Kassa E, Bane A, Gemechu T, Tilahun Y, Endalafer N, Ferro G, Straif K, Ward E, Aseffa A, Schüz J, Jemal A. Qat use and esophageal cancer in Ethiopia: A pilot case-control study. PLoS One. 2017;12:e0178911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Machoki MS, Saidi H, Raja A, Ndonga A, Njue A, Biomdo I, Kimani S, Arudo J, Mushtaq A. Risk factors for esophageal squamous cell carcinoma in a Kenyan population. Ann Afr Surg. 2018;15:38. |
| 14. | Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett's Esophagus and Esophageal Adenocarcinoma. Gastroenterol Clin North Am. 2015;44:203-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Middleton DRS, Bouaoun L, Hanisch R, Bray F, Dzamalala C, Chasimpha S, Menya D, Mbalawa CG, N'Da G, Woldegeorgis MA, Njie R, Koulibaly M, Buziba N, Ferro J, Nouhou H, Ogunbiyi F, Wabinga HR, Chokunonga E, Borok MZ, Korir AR, Mwasamwaja AO, Mmbaga BT, Schüz J, McCormack VA. Esophageal cancer male to female incidence ratios in Africa: A systematic review and meta-analysis of geographic, time and age trends. Cancer Epidemiol. 2018;53:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Kachala R. Systematic review: Epidemiology of oesophageal cancer in Sub-Saharan Africa. Malawi Med J. 2010;22:65-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R and Ferlay J, editors. Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer 2017; . |
| 18. | Parkin DM, Ferlay J, Jemal A, Borok M, Manraj SS, N'da GG, Ogunbiyi JO, Liu B, Bray F. Cancer in Sub-Saharan Africa. IARC Scientific Publications. 2018;167. |
| 19. | Wong WC, Cheung CS, Hart GJ. Development of a quality assessment tool for systematic reviews of observational studies (QATSO) of HIV prevalence in men having sex with men and associated risk behaviours. Emerg Themes Epidemiol. 2008;5:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25814] [Article Influence: 1122.3] [Reference Citation Analysis (0)] |
| 21. | Available from: https://gco.iarc.fr/today/. |
| 22. | Harington JS, McGlashan ND, Bradshaw E, Geddes EW, Purves LR. A spatial and temporal analysis of four cancers in African gold miners from Southern Africa. Br J Cancer. 1975;31:665-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Robertson MA, Harington JS, Bradshaw E. The cancer pattern in African gold miners. Br J Cancer. 1971;25:395-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Bradshaw E, Schonland M. Smoking, drinking and oesophageal cancer in African males of Johannesburg, South Africa. Br J Cancer. 1974;30:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Dlamini Z, Bhoola K. Esophageal cancer in African blacks of Kwazulu Natal, South Africa: An epidemiological brief. Ethn Dis. 2005;15:786-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Gamieldien W, Victor TC, Mugwanya D, Stepien A, Gelderblom WC, Marasas WF, Geiger DH, van Helden PD. p53 and p16/CDKN2 gene mutations in esophageal tumors from a high-incidence area in South Africa. Int J Cancer. 1998;78:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Bizos D, Morgan H, Motha N, Makda M, Domingo A, Tiedt S, Wing J, Munanga M, Tembo J, Hale M, Gould A. Comparison of the incidence of oesophageal cancer in two 6-year periods from selected hospitals in and around Gauteng Province, South Africa. S Afr J Surg. 2015;53:55. |
| 28. | Jaskiewicz K, Marasas WF, van der Walt FE. Oesophageal and other main cancer patterns in four districts of Transkei, 1981-1984. S Afr Med J. 1987;72:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 29. | Kgomo M, Elnagar AA, Nagel J, Mokoena T. Prevalence of Squamous Cell Carcinoma of the Esophagus in a Single Tertiary Center of South Africa: A Cross Sectional Analytic Study. J Public Health Afr. 2017;8:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Mannell A, Murray W. Oesophageal cancer in South Africa. A review of 1926 cases. Cancer. 1989;64:2604-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | McGlashan ND, Harington JS, Chelkowska E. Changes in the geographical and temporal patterns of cancer incidence among black gold miners working in South Africa, 1964-1996. Br J Cancer. 2003;88:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Rose EF, McGlashan ND. The spatial distribution of oesophageal carcinoma in the Transkei, South Africa. Br J Cancer. 1975;31:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Somdyala NI, Parkin DM, Sithole N, Bradshaw D. Trends in cancer incidence in rural Eastern Cape Province; South Africa, 1998-2012. Int J Cancer. 2015;136:E470-E474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Somdyala NI, Bradshaw D, Gelderblom WC, Parkin DM. Cancer incidence in a rural population of South Africa, 1998-2002. Int J Cancer. 2010;127:2420-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Loots E, Sartorius B, Madiba TE, Mulder CJJ, Clarke DL. Oesophageal squamous cell cancer in a South African tertiary hospital: A risk factor and presentation analysis. S Afr J Surg. 2017;55:42-46. [PubMed] |
| 36. | Ahmed N, Cook P. The incidence of cancer of the oesophagus in West Kenya. Br J Cancer. 1969;23:302-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Gatei DG, Odhiambo PA, Orinda DA, Muruka FJ, Wasunna A. Retrospective study of carcinoma of the esophagus in Kenya. Cancer Res. 1978;38:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Korir A, Okerosi N, Ronoh V, Mutuma G, Parkin M. Incidence of cancer in Nairobi, Kenya (2004-2008). Int J Cancer. 2015;137:2053-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Ojuka D, Dindi K, Awori M. Prevalence of esophageal adenocarcinoma. Ann Afr Surg. 2018;15:82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Tenge CN, Kuremu RT, Buziba NG, Patel K, Were PA. Burden and pattern of cancer in Western Kenya. East Afr Med J. 2009;86:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Wakhisi J, Patel K, Buziba N, Rotich J. Esophageal cancer in north rift valley of Western Kenya. Afr Health Sci. 2005;5:157-163. [PubMed] |
| 42. | White RE, Abnet CC, Mungatana CK, Dawsey SM. Oesophageal cancer: A common malignancy in young people of Bomet District, Kenya. Lancet. 2002;360:462-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | White RE, Mungatana C, Mutuma G, Robert ME, Daniel RW, Topazian MD, Shah KV. Absence of human papillomavirus in esophageal carcinomas from southwestern Kenya. Dis Esophagus. 2005;18:28-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Alema ON, Iva B. Cancer of the esophagus: Histopathological sub-types in northern Uganda. Afr Health Sci. 2014;14:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Gondos A, Brenner H, Wabinga H, Parkin DM. Cancer survival in Kampala, Uganda. Br J Cancer. 2005;92:1808-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Parkin DM, Nambooze S, Wabwire-Mangen F, Wabinga HR. Changing cancer incidence in Kampala, Uganda, 1991-2006. Int J Cancer. 2010;126:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda 1991-2010. Int J Cancer. 2014;135:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 48. | Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991-2010. Int J Cancer. 2013;133:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Chokunonga E, Windridge P, Sasieni P, Borok M, Parkin DM. Black-white differences in cancer risk in Harare, Zimbabwe, during 1991-2010. Int J Cancer. 2016;138:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Watts TE. Cancer of the oesophagus in Zimbabwe. Cent Afr J Med. 1992;38:185-187. [PubMed] |
| 51. | Chasimpha SJD, Parkin DM, Masamba L, Dzamalala CP. Three-year cancer incidence in Blantyre, Malawi (2008-2010). Int J Cancer. 2017;141:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Chetwood JD, Finch PJ, Kankwatira A, Mallewa J, Gordon MA, Masamba L. Five-year single-centre experience of carcinoma of the oesophagus from Blantyre, Malawi. BMJ Open Gastroenterol. 2018;5:e000232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Msyamboza KP, Dzamalala C, Mdokwe C, Kamiza S, Lemerani M, Dzowela T, Kathyola D. Burden of cancer in Malawi; common types, incidence and trends: National population-based cancer registry. BMC Res Notes. 2012;5:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Babekir AR, el Fahal AH, Alla AH. Oesophageal cancer in Sudan. Trop Doct. 1989;19:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Gasmelseed N, Abudris D, Elhaj A, Eltayeb EA, Elmadani A, Elhassan MM, Mohammed K, Elgaili EM, Elbalal M, Schuz J, Leon ME. Patterns of Esophageal Cancer in the National Cancer Institute at the University of Gezira, in Gezira State, Sudan, in 1999-2012. Asian Pac J Cancer Prev. 2015;16:6481-6490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Mohammed ME, Abuidris DO, Elgaili EM, Gasmelseed N. Predominance of females with oesophageal cancer in Gezira, Central Sudan. Arab J Gastroenterol. 2012;13:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Abdulkareem FB, Onyekwere CA, Awolola NA, Banjo AA. A clinicopathologic review of oesophageal carcinoma in Lagos. Nig Q J Hosp Med. 2008;18:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Ajao OG, Solanke TF. Carcinoma of the esophagus. J Natl Med Assoc. 1979;71:703-705. [PubMed] |
| 59. | Ihekwaba FN, Solanke TF. Carcinoma of the oesophagus. Br J Surg. 1984;71:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Gabel JV, Chamberlain RM, Ngoma T, Mwaiselage J, Schmid KK, Kahesa C, Soliman AS. Clinical and epidemiologic variations of esophageal cancer in Tanzania. World J Gastrointest Oncol. 2016;8:314-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Mmbaga EJ, Deardorff KV, Mushi B, Mgisha W, Merritt M, Hiatt RA, Mwaiselage J, Zhang L, Van Loon K. Characteristics of Esophageal Cancer Cases in Tanzania. J Glob Oncol. 2018;4:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Kayamba V, Sinkala E, Mwanamakondo S, Soko R, Kawimbe B, Amadi B, Zulu I, Nzaisenga JB, Banda T, Mumbwe C, Phiri E, Munkonge P, Kelly P. Trends in upper gastrointestinal diagnosis over four decades in Lusaka, Zambia: A retrospective analysis of endoscopic findings. BMC Gastroenterol. 2015;15:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Kelly P, Katema M, Amadi B, Zimba L, Aparicio S, Mudenda V, Baboo KS, Zulu I. Gastrointestinal pathology in the University Teaching Hospital, Lusaka, Zambia: Review of endoscopic and pathology records. Trans R Soc Trop Med Hyg. 2008;102:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Khalil KA, Salama OE, El Zeiny NA, El din Khalil S, Esmail NF. A study of pattern of gastrointestinal malignant neoplasms in the last decade (1987-1996) in Alexandria. J Egypt Public Health Assoc. 1999;74:503-527. [PubMed] |
| 65. | Come J, Castro C, Morais A, Cossa M, Modcoicar P, Tulsidâs S, Cunha L, Lobo V, Morais AG, Cotton S, Lunet N, Carrilho C, Santos LL. Clinical and Pathologic Profiles of Esophageal Cancer in Mozambique: A Study of Consecutive Patients Admitted to Maputo Central Hospital. J Glob Oncol. 2018;4:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Dia D, Bassene ML, Ndiaye-Bâ N, Halim A, Diallo S, Fall S, Diouf MF, Mbengue M, Diouf ML. [Endoscopic features of esophageal cancer in Dakar, Senegal: Study of 76 observations]. Med Trop (Mars). 2011;71:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Macrae SM, Cook BV. A retrospective study of the cancer patterns among hospital in-patients in Botswana 1960-72. Br J Cancer. 1975;32:121-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 68. | Cheng ML, Zhang L, Borok M, Chokunonga E, Dzamamala C, Korir A, Wabinga HR, Hiatt RA, Parkin DM, Van Loon K. The incidence of oesophageal cancer in Eastern Africa: Identification of a new geographic hot spot? Cancer Epidemiol. 2015;39:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | Pilleron S, Soerjomataram I, Charvat H, Chokunonga E, Somdyala NIM, Wabinga H, Korir A, Bray F, Jemal A, Maxwell Parkin D. Cancer incidence in older adults in selected regions of sub-Saharan Africa, 2008-2012. Int J Cancer. 2019;144:1824-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Bye H. Prescott NJ, Lewis CM, Matejcic M, Moodley L, Robertson B, Rensburg Cv, Parker MI, Mathew CG. Distinct genetic association at the PLCE1 locus with oesophageal squamous cell carcinoma in the South African population. Carcinogenesis. 2012;33:2155-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Pacella-Norman R, Urban MI, Sitas F, Carrara H, Sur R, Hale M, Ruff P, Patel M, Newton R, Bull D, Beral V. Risk factors for oesophageal, lung, oral and laryngeal cancers in black South Africans. Br J Cancer. 2002;86:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Van Rensburg SJ, Bradshaw ES, Bradshaw D, Rose EF. Oesophageal cancer in Zulu men, South Africa: A case-control study. Br J Cancer. 1985;51:399-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Isaacson C, Bothwell TH, MacPhail AP, Simon M. The iron status of urban black subjects with carcinoma of the oesophagus. S Afr Med J. 1985;67:591-593. [PubMed] |
| 74. | Jaskiewicz K, Marasas WF, Rossouw JE, Van Niekerk FE, Heine Tech EW. Selenium and other mineral elements in populations at risk for esophageal cancer. Cancer. 1988;62:2635-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Kgomo M, Elnagar AA, Mokoena T, Jeske C, Nagel GJ. Prevalence of Helicobacter pylori Infection in Patients with Squamous Cell Carcinoma of the Oesophagus. A Descriptive Case Series Study. J Gastrointest Cancer. 2016;47:396-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Kgomo M, Mokoena TR, Ker JA. Non-acid gastro-oesophageal reflux is associated with squamous cell carcinoma of the oesophagus. BMJ Open Gastroenterol. 2017;4:e000180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Li D, Dandara C, Parker MI. The 341C/T polymorphism in the GSTP1 gene is associated with increased risk of oesophageal cancer. BMC Genet. 2010;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Li DP, Dandara C, Walther G, Parker MI. Genetic polymorphisms of alcohol metabolising enzymes: Their role in susceptibility to oesophageal cancer. Clin Chem Lab Med. 2008;46:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Sammon AM. A case-control study of diet and social factors in cancer of the esophagus in Transkei. Cancer. 1992;69:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Segal I, Reinach SG, de Beer M. Factors associated with oesophageal cancer in Soweto, South Africa. Br J Cancer. 1988;58:681-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Sewram V, Sitas F, O'Connell D, Myers J. Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa. Cancer Epidemiol. 2016;41:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Victor T, Du Toit R, Jordaan AM, Bester AJ, van Helden PD. No evidence for point mutations in codons 12, 13, and 61 of the ras gene in a high-incidence area for esophageal and gastric cancers. Cancer Res. 1990;50:4911-4914. [PubMed] |
| 83. | Vogelsang M, Paccez JD, Schäfer G, Dzobo K, Zerbini LF, Parker MI. Aberrant methylation of the MSH3 promoter and distal enhancer in esophageal cancer patients exposed to first-hand tobacco smoke. J Cancer Res Clin Oncol. 2014;140:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Vogelsang M, Wang Y, Veber N, Mwapagha LM, Parker MI. The cumulative effects of polymorphisms in the DNA mismatch repair genes and tobacco smoking in oesophageal cancer risk. PLoS One. 2012;7:e36962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 85. | Wang Y, Vogelsang M, Schäfer G, Matejcic M, Parker MI. MicroRNA polymorphisms and environmental smoke exposure as risk factors for oesophageal squamous cell carcinoma. PLoS One. 2013;8:e78520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 86. | Zaahl MG, Warnich L, Victor TC, Kotze MJ. Association of functional polymorphisms of SLC11A1 with risk of esophageal cancer in the South African Colored population. Cancer Genet Cytogenet. 2005;159:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Parker RK, Dawsey SM, Abnet CC, White RE. Frequent occurrence of esophageal cancer in young people in western Kenya. Dis Esophagus. 2010;23:128-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Patel K, Wakhisi J, Mining S, Mwangi A, Patel R. Esophageal Cancer, the Topmost Cancer at MTRH in the Rift Valley, Kenya, and Its Potential Risk Factors. ISRN Oncol. 2013;2013:503249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Pritchett NR, Burgert SL, Murphy GA, Brockman JD, White RE, Lando J, Chepkwony R, Topazian MD, Abnet CC, Dawsey SM, Mwachiro MM. Cross sectional study of serum selenium concentration and esophageal squamous dysplasia in western Kenya. BMC Cancer. 2017;17:835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Obayo S, Muzoora C, Ocama P, Cooney MM, Wilson T, Probert CS. Upper gastrointestinal diseases in patients for endoscopy in South-Western Uganda. Afr Health Sci. 2015;15:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 91. | Simpong DL, Asmah RH, Krampah C, Akakpo PK, Adu P, Asante DB, Naporo S, Adjei AA, Gyasi RK. HER-2 Protein Overexpression in Patients with Gastric and Oesophageal Adenocarcinoma at a Tertiary Care Facility in Ghana. ScientificWorldJournal. 2018;2018:1564150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Kayamba V, Bateman AC, Asombang AW, Shibemba A, Zyambo K, Banda T, Soko R, Kelly P. HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: A case-control study. Cancer Med. 2015;4:588-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Parkin DM, Vizcaino AP, Skinner ME, Ndhlovu A. Cancer patterns and risk factors in the African population of southwestern Zimbabwe, 1963-1977. Cancer Epidemiol Biomarkers Prev. 1994;3:537-547. [PubMed] |
| 94. | Vizcaino AP, Parkin DM, Skinner ME. Risk factors associated with oesophageal cancer in Bulawayo, Zimbabwe. Br J Cancer. 1995;72:769-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Geßner AL, Borkowetz A, Baier M, Göhlert A, Wilhelm TJ, Thumbs A, Borgstein E, Jansen L, Beer K, Mothes H, Dürst M. Detection of HPV16 in Esophageal Cancer in a High-Incidence Region of Malawi. Int J Mol Sci. 2018;19:pii: E557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Mansour AH, Mohammed MA, Anwar R, Elzafrany ME, Omar NM. ABO blood group and risk of malignancies in Egyptians. Int J Cancer Res. 2014;10:81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 97. | Odutola MK, Jedy-Agba EE, Dareng EO, Adebamowo SN, Oga EA, Igbinoba F, Otu T, Ezeome E, Hassan R, Adebamowo CA. Cancers Attributable to Alcohol Consumption in Nigeria: 2012-2014. Front Oncol. 2017;7:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Alberts AS, Anderson JD, Goedhals L, Cronje JD, Doman MJ. [Palliative radiation schedules with and without chemotherapy in advanced carcinoma of the esophagus]. S Afr Med J. 1984;66:289-291. [PubMed] |
| 99. | Alberts AS, Falkson G, Badata M, Terblanche AP, Schmid EU. Trimetrexate in advanced carcinoma of the esophagus. Invest New Drugs. 1988;6:319-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Alberts AS, Schoeman L, Burger W, Greef F, Falkson G. A phase II study of 5-fluorouracil and leucovorin in advanced carcinoma of the esophagus. Am J Clin Oncol. 1992;15:35-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 101. | Kneebone RL, Mannell A. Cancer of the oesophagus in Soweto. S Afr Med J. 1985;67:839-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 102. | Liakos D, Dower DWR, Florizoone M, Bizos DB. Is oesophageal stenting for cancer the answer? A report from a secondary hospital in the developing world. S Afr J Surg. 2010;48:43. |
| 103. | Loots E, Anderson F, Clarke DL, Mulder CJ, Madiba TE. Self-Expandable Metal Stents in Esophageal Cancer in a High HIV Prevalence Area: A Survival Analysis and Evaluation of Prediction Scores. Surg Laparosc Endosc Percutan Tech. 2016;26:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Mannell A, Becker PJ. Evaluation of the results of oesophagectomy for oesophageal cancer. Br J Surg. 1991;78:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Mannell A, Becker PJ, Nissenbaum M. Bypass surgery for unresectable oesophageal cancer: Early and late results in 124 cases. Br J Surg. 1988;75:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Mannell A, Winters Z. Carboplatin in the treatment of oesophageal cancer. S Afr Med J. 1989;76:213-214. [PubMed] |
| 107. | Motilall SR, Modiba MC, Tsatsi LD, Becker PJ. Trial of self-expandable metallic stents in the palliation of tracheo-oesophageal fistula in carcinoma of the oesophagus. S Afr J Surg. 2007;45:24-27. [PubMed] |
| 108. | Sanyika C, Corr P, Haffejee A. Palliative treatment of oesophageal carcinoma--efficacy of plastic versus self-expandable stents. S Afr Med J. 1999;89:640-643. [PubMed] |
| 109. | Slabber CF, Nel JS, Schoeman L, Burger W, Falkson G, Falkson CI. A randomized study of radiotherapy alone versus radiotherapy plus 5-fluorouracil and platinum in patients with inoperable, locally advanced squamous cancer of the esophagus. Am J Clin Oncol. 1998;21:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Sur RK, Levin VC, Donde B, Uijs RR, Luhana F. Adverse effects of teletherapy dose fractionation on complications in telebrachytherapy treatment of esophageal cancer: Final report. Endocurietherapy/Hyperthermia Oncol. 1996;12:171. |
| 111. | Sur RK, Donde B, Levin VC, Mannell A. Fractionated high dose rate intraluminal brachytherapy in palliation of advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;40:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Sur RK, Levin CV, Donde B, Sharma V, Miszczyk L, Nag S. Prospective randomized trial of HDR brachytherapy as a sole modality in palliation of advanced esophageal carcinoma--an International Atomic Energy Agency study. Int J Radiat Oncol Biol Phys. 2002;53:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Walker AR, Walker BF, Isaacson C, Segal I, Pryor S. Short duration of survival among South African blacks with oesophageal cancer. S Afr Med J. 1984;66:877-878. [PubMed] |
| 114. | Werner ID, Silber W, Madden PC, Birkenstock WE, Perkin H, Stein D. Carcinoma of the thoraco-abdominal oesophagus. S Afr Med J. 1975;49:653-656. [PubMed] |
| 115. | Abdelshafy M, Omar MA, Abdel Bary M. Wahaman MM, Bakheet RAE. Self-expandable metal stent for palliation of malignant dysphagia quality of life improvement in advanced cancer esophagus: Upper Egypt experience. J Egypt Soc Cardio-Thoracic Surg. 2017;25:262. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 116. | Abdel-Wahab M, Gad-Elhak N, Denewer A, El-Ebidy G, Sultan A, Abou-Elenin A, Fathy O, Abou-Zid M, El-Ghawalby N, Ezzat F. Endoscopic laser treatment of progressive dysphagia in patients with advanced esophageal carcinoma. Hepatogastroenterology. 1998;45:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 117. | Akl FM, Elsayed-Abd-Alkhalek S, Salah T. Palliative concurrent chemoradiotherapy in locally advanced and metastatic esophageal cancer patients with dysphagia. Ann Palliat Med. 2013;2:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 118. | Aslan M, Elbastawisy A. Clinic-pathologic study of gastric and esophageal malignancies at the National Cancer Institute, Cairo University, Egypt, 2008-2012. Eur J Cancer. 2017;72:S80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 119. | Elsharkawy AA, El-Geidie AA. Self-expanding metal stents in palliation of malignant dysphagia: Outcome of 124 Egyptian patients. Eur Arch Otorhinolaryngol. 2010;267:1123-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 120. | Shaker M, Deif A, Abdelaal A. Role of fluoroscopic guided self expandable metallic stents in the management of malignant esophageal strictures. Egypt J Radiol Nucl Med. 2016;47:811. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 121. | Wahba HA, El-Hadaad HA, Abd-Ellatif EA. Neoadjuvant concurrent chemoradiotherapy with capecitabine and oxaliplatin in patients with locally advanced esophegeal cancer. Med Oncol. 2012;29:1693-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 122. | Jani PG. Out-patient palliative therapy for advanced cancer of the oesophagus. East Afr Med J. 2000;77:456-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 123. | Parker RK, White RE, Topazian M, Chepkwony R, Dawsey S, Enders F. Stents for proximal esophageal cancer: A case-control study. Gastrointest Endosc. 2011;73:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | White RE, Parker RK, Fitzwater JW, Kasepoi Z, Topazian M. Stents as sole therapy for oesophageal cancer: A prospective analysis of outcomes after placement. Lancet Oncol. 2009;10:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Ahmed ME. The surgical management and outcome of oesophageal cancer in Khartoum. J R Coll Surg Edinb. 1993;38:16-18. [PubMed] |
| 126. | Ahmed ME, Mahadi SI, Ali BM. The surgical treatment of esophageal cancer in Sudan: A 100 consecutive cases. Int J Surg. 2016;29:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Ahmed AA. The surgical management and outcome of oesophageal cancer in Addis Ababa. Ethiop Med J. 2000;38:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 128. | Thumbs A, Borgstein E, Vigna L, Kingham TP, Kushner AL, Hellberg K, Bates J, Wilhelm TJ. Self-expanding metal stents (SEMS) for patients with advanced esophageal cancer in Malawi: An effective palliative treatment. J Surg Oncol. 2012;105:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 129. | Adegboye VO, Obajimi MO, Ogunseyinde AO, Brimmo IA, Adebo AO. Trans-hiatal oesophagectomy as palliative treatment for carcinoma of the oesophagus. East Afr Med J. 2002;79:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 130. | Rosenblatt E, Jones G, Sur RK, Donde B, Salvajoli JV, Ghosh-Laskar S, Frobe A, Suleiman A, Xiao Z, Nag S. Adding external beam to intra-luminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: A prospective multi-centre randomized trial of the International Atomic Energy Agency. Radiother Oncol. 2010;97:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 131. | Schmid EU, Alberts AS, Greeff F, Terblanche AP, Schoeman L, Burger W, Shiels RA, Friediger D, Van der Hoven A, Falkson G. The value of radiotherapy or chemotherapy after intubation for advanced esophageal carcinoma--a prospective randomized trial. Radiother Oncol. 1993;28:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 132. | Ogendo SW. Follow up of oesophageal cancer therapy at the Kenyatta National Hospital, Nairobi. East Afr Med J. 2001;78:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 133. | White RE, Chepkwony R, Mwachiro M, Burgert SL, Enders FT, Topazian M. Randomized Trial of Small-diameter Versus Large-diameter Esophageal Stents for Palliation of Malignant Esophageal Obstruction. J Clin Gastroenterol. 2015;49:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | White RE, Mungatana C, Topazian M. Esophageal stent placement without fluoroscopy. Gastrointest Endosc. 2001;53:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 135. | Slabber CF, Falkson G, Burger W, Schoeman L. 13-Cis-retinoic acid and interferon alpha-2a in patients with advanced esophageal cancer: A phase II trial. Invest New Drugs. 1996;14:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 136. | Sur RK, Levin V, Malas S, Donde B, Ahmed N, Retter E. Initial experience with telebrachytherapy treatment in palliation of advanced oesophageal cancer. S Afr J Surg. 1995;33:67-69. [PubMed] |
| 137. | Sinzobahamvya N. Oesophagectomy for carcinoma of the oesophagus--early results. Cent Afr J Med. 1990;36:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 138. | Rahouma M, Elkassem FA, Loay I, Yehia M, Abdelrahman A. P2.04-031 Predictors of pathological complete response (TRG=1) among esophageal cancer cases; NCI Pooled Data. J Thoracic Oncol. 2017;12:S1015. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |