Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4172
Peer-review started: April 8, 2019
First decision: May 30, 2019
Revised: June 20, 2019
Accepted: July 2, 2019
Article in press: July 3, 2019
Published online: August 14, 2019
Processing time: 128 Days and 20.3 Hours
Lysosomal acid lipase (LAL) plays a key role in intracellular lipid metabolism. Reduced LAL activity promotes increased multi-organ lysosomal cholesterol ester storage, as observed in two recessive autosomal genetic diseases, Wolman disease and Cholesterol ester storage disease. Severe liver steatosis and accelerated liver fibrosis are common features in patients with genetic LAL deficiency. By contrast, few reliable data are available on the modulation of LAL activity in vivo and on the epigenetic and metabolic factors capable of regulating its activity in subjects without homozygous mutations of the Lipase A gene. In the last few years, a less severe and non-genetic reduction of LAL activity was reported in children and adults with non-alcoholic fatty liver disease (NAFLD), suggesting a possible role of LAL reduction in the pathogenesis and progression of the disease. Patients with NAFLD show a significant, progressive reduction of LAL activity from simple steatosis to non-alcoholic steatohepatitis and cryptogenic cirrhosis. Among cirrhosis of different etiologies, those with cryptogenic cirrhosis show the most significant reductions of LAL activity. These findings suggest that the modulation of LAL activity may become a possible new therapeutic target for patients with more advanced forms of NAFLD. Moreover, the measurement of LAL activity may represent a possible new marker of disease severity in this clinical setting.
Core tip: Reduced lysosomal acid lipase (LAL) activity promotes increased multi-organ lysosomal cholesterol ester storage, as observed in two recessive autosomal genetic diseases, Wolman disease and Cholesterol ester storage disease. A less severe and non-genetic reduction of LAL activity has been reported in children and adults with non-alcoholic fatty liver disease (NAFLD). Patients with NAFLD show a significant, progressive reduction of LAL activity from simple steatosis to non-alcoholic steatohepatitis and cryptogenic cirrhosis. In the future, modulation of LAL activity may become a possible new therapeutic target for patients with more advanced forms of NAFLD and represent a possible new marker of disease severity.
- Citation: Baratta F, Pastori D, Ferro D, Carluccio G, Tozzi G, Angelico F, Violi F, Del Ben M. Reduced lysosomal acid lipase activity: A new marker of liver disease severity across the clinical continuum of non-alcoholic fatty liver disease? World J Gastroenterol 2019; 25(30): 4172-4180
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4172
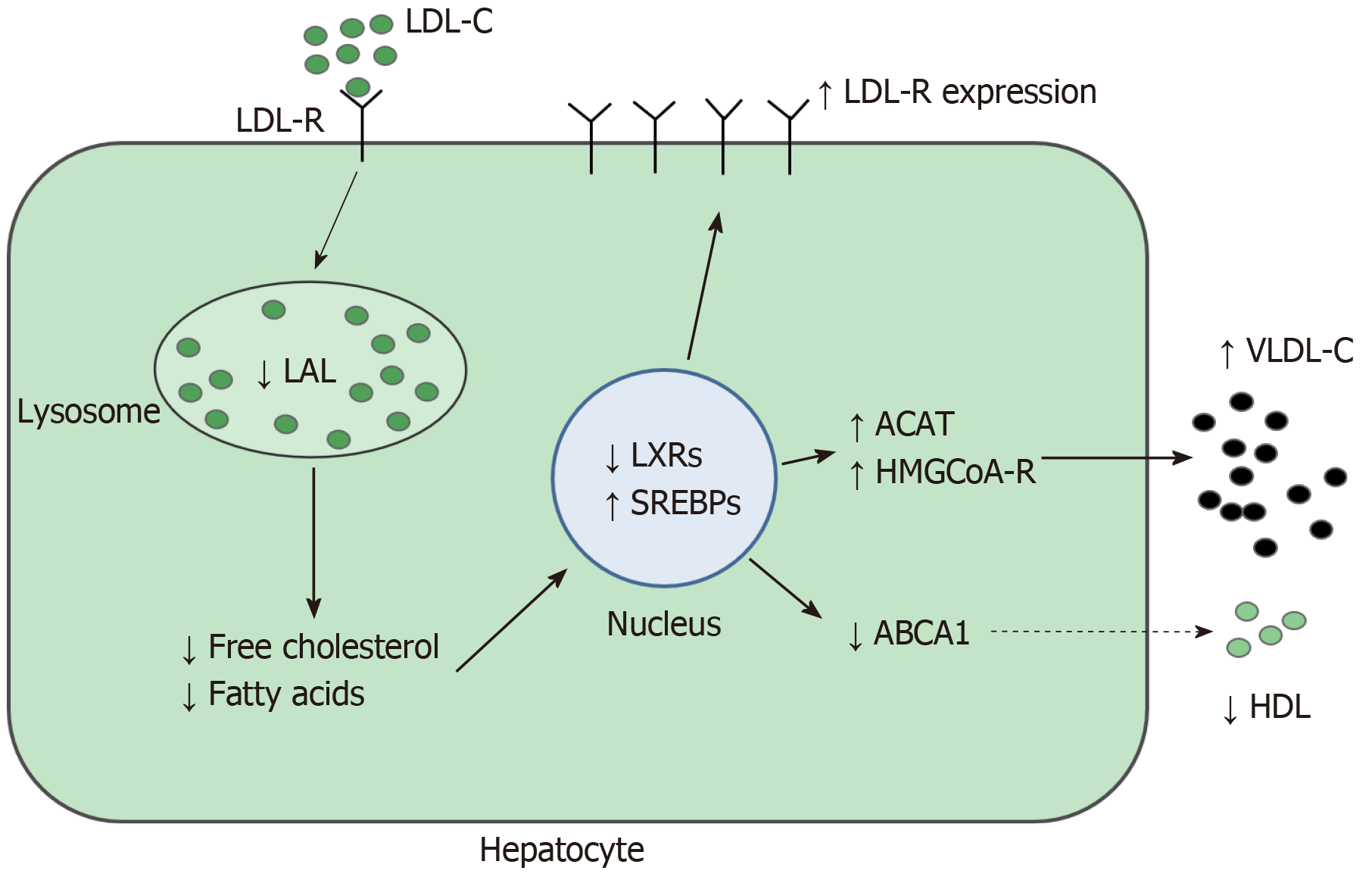
Lysosomal acid lipase (LAL) is a key enzyme for intracellular lipid metabolism, which regulates the intra-lysosomal hydrolysis of cholesterol esters (CE) and triglycerides (TG), producing free cholesterol and fatty acids[1]. The LAL activity reduction causes intra-lysosomal accumulation of CE and lowers free cholesterol in cytosol[2]. This reduction increases transcription factor sterol regulatory element binding protein activity, which promotes lipogenesis and synthesis of cholesterol and of very low-density lipoproteins. In addition, there is a reduction in liver X receptors expression resulting in impaired cholesterol efflux and high-density lipoprotein (HDL) production[3]. Moreover, low-density lipoprotein (LDL) receptor synthesis and the receptor-mediated LDL uptake are amplified.
In patients with both heterozygous or homozygous deletion of lipase A (LIPA) gene, a lipid phenotype similar to that observed in patients with familial hypercholesterolemia (FH) has been described[4,5]. Therefore, in presence of hypercholesterolemia with type IIa phenotype, it is very important, but not always easy, to make a differential diagnosis with heterozygous FH (Table 1). A family history for early cardiovascular disease and/or hypercholesterolemia supports a diagnosis of heterozygous FH. On the contrary, in the absence of diagnostic criteria for FH, a LAL defect could be suspected, especially in patients with hyper-cholesterolemia associated with low levels of HDL cholesterol. The Dutch Lipid Clinic Network score[6,7] or the Simon Broome criteria[8] for FH may be two useful tools for a differential diagnosis.
| Disease | TC | LDL | HDL | TG | Phenotype |
| LAL-related dyslipidemia | ↑↑↑ | ↑↑↑ | ↓ | ↑ | IIa, IIb, |
| Familial hypercholesterolemia | ↑↑↑ | ↑↑↑ | =/↓ | = | IIa, IIb |
| Familial combined hyperlipidemia | ↑↑ | ↑↑ | ↓ | ↑ | IIa, IIb, IV, V |
| Familial hypertriglyceridemia | N/ | ↓ | ↓↓ | ↑↑ | IV, V |
| Type III hyperlipidemia (dysbetalipoproteinemia) | ↑↑ | ↓ | = | ↑↑ | III, IV |
| Iperchylomicronemia | ↑ | ↓ | ↓↓↓ | ↑↑↑ | I, V |
| Hypoalfalipoproteinemia | N/↓ | N | ↓↓↓↓ | N | Low HDL |
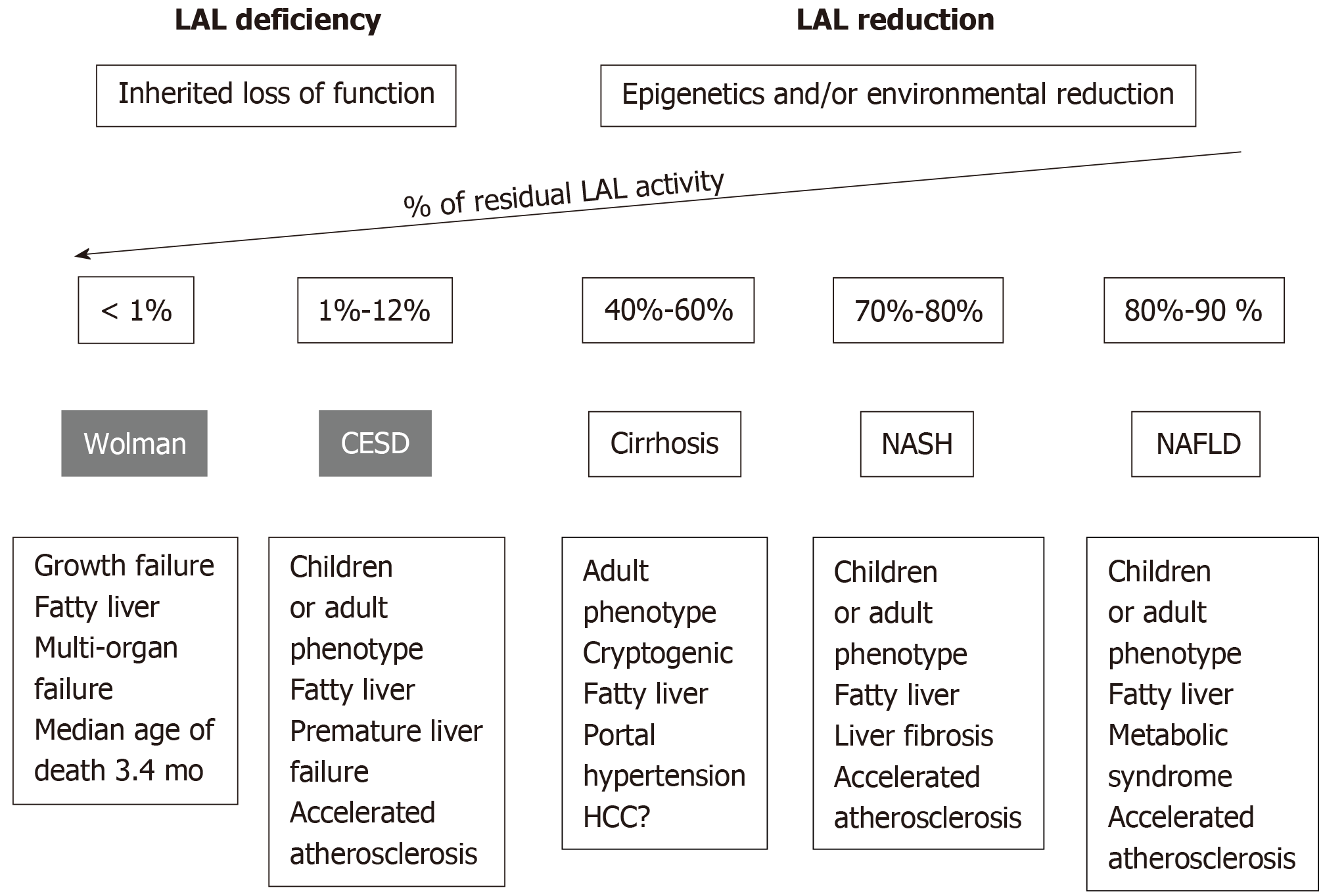
LAL deficiency (LAL-D) is a rare autosomal recessive genetic disease due to a mutation in the LIPA gene, characterized by the presence of CE and TG in numerous tissues. The most common mutation is the E8SJM variant, which has an estimated frequency of 0.00025 in the general population (i.e., 1 carrier per 200 individuals in Western countries). The LAL-D is a heterogeneous disorder that may present with two different phenotypes based on residual LAL activity levels (Figures 1): Wolman’s disease and cholesterol ester storage disease (CESD)[1,4].
Wolman’s disease starts prematurely during the 6th or 7th mo of life and quickly leads to death, with only a small proportion of patients surviving beyond the first year of age. Infants with LAL-D show delayed growth, associated with signs of malabsorption, hepatosplenomegaly, severe hepatic dysfunction, rapidly progressive anemia, and multi-organ failure; the adrenal calcifications are the pathognomonic sign of Wolman’s disease. In these patients, LAL activity is almost null[1].
CESD is the late onset phenotype, being manifested during childhood, adolescence, or in adulthood, with onset age ranging from 5 to 44 years or more. It presents with hepatic steatosis, high levels of aminotransferase, hepatomegaly, and dyslipidemia. As the clinical manifestations of CESD are not very characteristic, the diagnosis is often occasional. The clinical phenotype and disease severity are very variable and depend on the residual enzymatic activity, which is usually less than 12%. Therefore, the coexistence of hepatic steatosis and hypercholesterolemia, in particular in non-obese subjects, should lead to the differential diagnosis between LAL-D and other metabolic causes of non-alcoholic fatty liver disease (NAFLD), such as metabolic syndrome, type II diabetes, hypertriglyceridemia, and central obesity[9].
LAL-D leads to CE and TG accumulation in hepatocytes and liver-resident macrophages (Kupffer cells) with subsequent progression to fibrosis (Figures 2). The high prevalence of severe fibrosis in LAL-D and its rapid progression to cirrhosis suggest that lysosomal CE and TG accumulation is a potent driver of liver fibrosis[9-11]. A recent study showed increased transaminases in hepatocyte-specific LAL-deficient mice (Liv-Lipa -/-) as well as upregulation of hepatic cytokines and chemokines, known to drive inflammation and leading to Kupffer cell activation and liver damage[12]. In addition, lysosomal CE accumulation induces Kupffer cell activation, causing inflammation and liver damage in high fat/high cholesterol fed Liv-Lipa-/-mice. These findings indicated that hepatocytes’ LAL plays a critical role for liver homeostasis and function.
A recent study reported data on allograft recurrence, liver failure, and other clinical outcomes in 18 liver transplantation (LT) LAL-D patients. LT was necessary for treatment of LAL-D-associated liver failure but, interestingly, did not correct LAL activity, which remained deficient post-LT[13]. Therefore, LT does not correct deficient LAL enzyme in bone marrow derived histiocytes; moreover, LT does not even prevent multi-organ disease progression or liver disease recurrence, as observed in liver biopsies within the first year following LT.
In addition, Burton et al[14] have shown how, in patients with genetic LAL deficiency, 20 wk treatment with enzyme replacement therapy (Sebelipase-alpha) is able to reduce hepatic fat evaluated by magnetic resonance. In addition, treated patients showed serum liver enzymes and serum lipids improvement.
The term NAFLD indicates a set of diseases associated with the presence of excessive accumulation of hepatic fat in the absence of chronic viral infection and alcohol abuse. NAFLD is the most common hepatic disease. It is estimated that the prevalence in the general population is about 20%-30%, reaching up to 70%-90% in the obese or diabetic population[15].
NAFLD, in the initial phases, presents as simple steatosis, whose main histological finding is the presence of predominantly macrocytic steatosis in at least 5% of hepatocytes. In some cases, simple steatosis evolves into non-alcoholic steatohepatitis (NASH), in which the histological picture includes steatosis, ballooning, and inflammation with a progressive increase in fibrosis. In the past, NAFLD was considered a benign condition; however, recent evidence suggests a less favorable prognosis, due to the possible evolution in cirrhosis, hepatocellular carcinoma, and hepatic failure[16]. Today, NAFLD is considered the main cause of cryptogenic cirrhosis, the prevalence of which is increasing in recent years, especially in patients with history of obesity and type II diabetes. NAFLD is the second indication for liver transplantation in the US and is expected to exceed hepatitis C virus (HCV) in the next few years, becoming the first cause for liver transplant[17].
Numerous pathogenic factors contribute to the accumulation of lipids in the hepatocytes and, in a proportion of patients, the development of fibrotic processes[18]. Among them, insulin resistance, oxidative stress, and low-grade chronic inflammation supported by the production of cytokines deriving from visceral fat. However, the pathogenic mechanisms underlying the progression of simple steatosis to NASH and cirrhosis are not yet fully clarified nor are tools available to predict the evolution of NAFLD.
Prospective studies suggest that the first cause of death in patients diagnosed with NAFLD is cardiovascular disease[19,20]. Atherosclerosis is very common in these subjects, and many of them, especially before the onset of liver complications, develop coronary heart disease[21]. The relationship between NAFLD and cardiovascular risk has long been investigated[15,22-24].
According to the "multiple parallel hits hypothesis", many insults, including insulin resistance, the presence of gene variants, oxidative stress, and alteration of the intestinal microbiota, act simultaneously on the liver causing lipid infiltration and inflammation[18]. It remains to be clarified whether it is the metabolic syndrome that promotes steatosis through insulin resistance, or whether it is NAFLD that induces hyperinsulinemia through a defective mechanism of insulin degradation. The current opinion suggests a bidirectional link between NAFLD and the metabolic syndrome[25].
Few studies have so far assessed the activity of LAL in patients with NAFLD, and the possible role of LAL as one of the multiple hits in NAFLD pathogenesis is under debate[26].
Our group[27] has recently demonstrated reduced LAL activity in patients with NAFLD. LAL activity was significantly reduced in 240 patients with NAFLD compared to 100 heathy subjects (HS) [0.78 (0.61-1.01) nmol/spot per hour vs 1.15 (0.94-1.72) nmol/spot per hour, P < 0.001]. An even more marked reduction was observed in patients with histologically diagnosed NASH [0.67 (0.51-0.77) nmol/spot per hour, P < 0.001 vs HS; P < 0.001, between the groups]. In addition, patients with NAFLD who exhibited enzymatic activity below the median had higher serum total cholesterol (P < 0.05) and LDL cholesterol (P < 0.05) and higher levels of transaminases and gamma-glutamyltransferase (alanine aminotransferase, P < 0.001; aspartate aminotransferase, P < 0.01; gamma-glutamyltransferase, P < 0.01).
Therefore, based on our findings, it was possible to hypothesize that the reduction of LAL activity, as well as a predisposing factor for the development of NAFLD, could be considered as a further pathophysiological mechanism for progression to NASH and eventually to cryptogenic cirrhosis. Moreover, based on the above observations[28], we could also hypothesize that LAL activity can constitute a possible tool for the identification of the subjects with more advanced forms of NAFLD and possibly for the monitoring of the response to therapy.
Shortly thereafter[29], a significant reduction in LAL activity in a series of pediatric cases was also observed. In this study, children with significant fibrosis (stage 2-3, n = 64) had a significantly lower LAL activity compared to those with mild fibrosis (stage 0-1, n = 104), suggesting a potential role of reduced LAL activity in the pathogenesis of NAFLD-induced fibrosis.
In a further study[30], we found that NAFLD patients disclosed a relatively high prevalence of spleen enlargement and splenomegaly, which were significantly associated with a reduced LAL activity, suggesting that LAL may contribute to spleen enlargement in this setting. Although the degree of LAL activity reduction was less pronounced compared to genetic forms of LAL deficiency, which develop severe fat accumulation in the spleen, a similar mechanism may be hypothesized.
More recently, Tovoli et al[31] performed a study of LAL activity in 81 patients with a diagnosis of NAFLD and 78 matched controls with HCV-related liver disease. LAL activity was significantly reduced in NAFLD compared to that in HCV patients, suggesting that NAFLD is characterized by a specific deficit in LAL activity.
Finally, in a study by Gomaraschi M et al[32], 164 patients with biopsy-proven NAFLD were compared with 60 dyslipidemic patients with similar prevalence of metabolic syndrome and 30 controls. LAL activity on dried blood spot (DBS) was reduced in NAFLD patients compared to controls and those with dyslipidemia.
Therefore, we may conclude that the reduction of LAL activity, even in the absence of genetic diseases, seemed to be associated with the development of progressive hepatic steatosis (Table 2).
| NAFLD | ||||
| Year | Paper | Study populations | Results1 | Conclusions |
| 2015 | Baratta et al[27] | 100 HS; 240 NAFLD patients; (35 biopsy-proven NASH) | Median LAL activity was: 1.15 (0.95-1.72) in HS; 0.78 (0.61-1.01) in NAFLD; 0.67 (0.51-0.77) in NASH | A significant reduction of LAL activity in NAFLD patients compared to HS. In particular, in the subgroup of patients with biopsy proven NASH |
| 2016 | Selvakumar et al[29] | 168 children with biopsy-proven NAFLD; (80 NAFL and 88 NASH) | Mean LAL activity was: 1.3 ± 0.57 in NAFL patients; 1.2 ± 0.80 in NASH patients; 1.4 ± 0.80 in patients with F0-F1; 1.1 ± 0.45 in patients with F2-F3 | No significant difference in LAL activity between children with NASH compared to those without NASH; Reduced blood LAL activity correlates with severity of liver fibrosis |
| 2017 | Polimeni et al[30] | 315 NAFLD patients; with US spleen dimensions evaluation | Median LAL activity was: 0.9 (0.7-1.2) in patients with normal spleen; 0.7 (0.6-0.9) in patients with splenomegaly | Spleen enlargement and splenomegaly were significantly associated with a reduced LAL activity |
| 2017 | Tovoli et al[31] | 81 NAFLD patients; (53.1% with cirrhosis) | Median LAL activity was: 0.55 (0.41-0.81) in non-cirrhotic NAFLD patients; 0.84 (0.69-1.07) in non-cirrhotic HCV patients | LAL activity is significantly reduced in non-cirrhotic NAFLD, compared to that in non-cirrhotic HCV patients. |
| 78 HCV patients (59.0% with cirrhosis) | ||||
| Liver cirrhosis | ||||
| 2016 | Vespasiani-Gentilucci et al[34] | 63 CC patients 88 KAC patients 97 HS | Median LAL activity: 0.62 (0.44-0.86) in CC patients; 0.54 (0.42-0.79) in KAC patients; 0.96 (0.75-1.25) in HS | Liver cirrhosis is characterized by a severe acquired reduction of LAL-activity; The difference between the two groups of cirrhotics was not significant […]; LAL activity was not associated with liver function as determined with Child-Pugh class […] |
| 2016 | Shteyer et al[35] | 22 patients aged 1-75 years who underwent liver biopsy; 13 at high risk for LAL-D (microvesicular steatosis or with cryptogenic cirrhosis); 9 at low risk for LAL-D; (microvesicular steatosis in metabolic/NAFLD patients) | Mean LAL activity was 0.74 ± 0.28 and was similar in both risk groups; 37.5% had LAL < 0.5 | LAL < 0.5 was associated with markers of liver disease severity |
| 2017 | Tovoli et al[31] | 81 NAFLD patients; (53.1% with cirrhosis) | Median LAL activity was: 0.53 (0.29-0.69) in cirrhotic NAFLD patients; 0.67 (0.50-0.89) in cirrhotic HCV patients | LAL activity is significantly reduced in NAFLD-related cirrhosis compared to HCV-cirrhosis |
| 78 HCV patients; (59.0% with cirrhosis) | ||||
| 2017 | Angelico et al[33] | 133 CC patients; 141 KAC patients | Median LAL activity was: 0.49 (0.38-0.75) in CC patients; 0.65 (0.46-0.94) KAC patients | A strong association between LAL activity reduction and severity of liver disease was found. A marked reduction of LAL activity in patients with cryptogenic cirrhosis compared to the other known etiologies despite a more severe liver disease in the latter |
Based on follow-up studies, 25% of patients affected by NASH may develop cirrhosis and eventually hepatocellular carcinoma. In fact, current knowledge strongly indicates that cryptogenic cirrhosis is, in truth, the evolution of NASH. However, the mechanisms underlying disease progression remain poorly understood. Following evidence from NAFLD, we hypothesized that epigenetic and/or environmental modulation of LAL activity could be also an unrecognized contributing factor in the progression to cryptogenic cirrhosis.
To evaluate a possible role of LAL in cryptogenic cirrhosis, we carried out a cohort study including 274 patients with liver cirrhosis of different etiology from 19 centers in Italy[33]. Median LAL activity value was 0.58 nmol/spot per hour, 0.49 and 0.65 in the cryptogenic cirrhosis and alcoholic/viral cirrhosis groups, respectively (P < 0.002). Approximately 30% of patients with cryptogenic cirrhosis showed a severe reduction of LAL activity (i.e., < 0.40 nmol/spot per hour). The reduction was more evident in patients with cryptogenic cirrhosis, despite less severe liver disease. Furthermore, we observed a strong association between LAL activity reduction and severity of liver disease assessed by Child-Turcotte-Pugh and Model for End-Stage Liver Disease scores.
In a further single-center study carried out by Vespasiani-Gentilucci et al[34] , LAL-activity was severely reduced in patients with cryptogenic cirrhosis with respect to healthy subjects (HS) [0.62 (0.44-0.86) vs 0.96 (0.75-1.25) nmol/spot per hour, P < 0.001)], but it was also reduced in known-etiology cirrhotics [0.54 (0.42-0.79) nmol/spot per hour, P < 0.001 vs HS]. In this study, authors sequenced the LIPA gene and excluded genetic etiology for the observed LAL reduction.
Shteyer et al[35] performed a study on medical records of 22 patients with microvescicular steatosis and cryptogenic cirrhosis and 9 with NAFLD diagnosed with liver biopsies. LAL activity inversely predicted liver disease severity, and LAL level of 0.5 was the most sensitive for detecting both histologic and non-invasive markers for disease severity (Table 2).
LAL-D should be suspected in non-obese subjects with NAFLD and/or cryptogenic cirrhosis, unexplained persistently elevated levels of transaminases, and/or high levels of LDL cholesterol and low HDL cholesterol.
In these subjects, it would be appropriate to measure LAL activity using the DBS test, which is a simple and inexpensive test that determines the enzymatic activity on the blood spot, subtracting from the activity of the total lipase obtained after addition of a specific LAL inhibitor (Lalistat 2). All patients with a marked reduction in LAL activity (≤ 0.4 nmol/spot per hour) should be investigated for the presence of LIPA gene mutations. Patients homozygous for LAL deficiency have a residual activity equal to or close to 0. The lower limit of the range of normality in the validation tests of the method was 0.8 nmol/spot per hour.
A reduced LAL activity may contribute to liver damage in patients with NAFLD, but several issues need to be addressed to better understand the role of LAL in liver diseases. First, there are no reliable in vivo data on LAL activity modulation. In particular, epigenetic and environmental factors that are able to regulate its activity in subjects without homozygous mutations of the LIPA gene have not yet been identified. For example, it is not known whether an intervention on modifiable cardio-metabolic risk factors, typically associated with NAFLD, such as metabolic syndrome, diabetes, overweight, or oxidative stress, may affect the modulation of LAL activity in a favorable manner. Moreover, it is still unclear whether low LAL activity may predict liver disease progression and or cardio-metabolic events.
Besides, the specific contribution of circulating cells to the total activity of LAL on the DBS test is still unclear. In a study of a random sample of 300 subjects, LAL activity, measured with 4-methyl-umbelliferyl oleate as the substrate, was present in high concentration in lymphocytes and monocytes but not in polymorphonuclear leukocytes[36]. Recently, in a study performed in 172 HS aged ≥ 18 years, LAL activity in white blood cells was significantly higher than in platelets (458.9 ± 133.6 nmol/mg per hour vs 235.0 ± 88.3 nmol/mg per hour, P < 0.001). However, LAL activity in DBS correlated more strongly with that in platelets, suggesting that platelet count is recommended before interpreting LAL activity in DBS[37].
In addition, it remains to be clarified whether any improvement in the enzymatic activity could result in a reduction of hepatic fat in patients with NAFLD. In light of the results of a recent clinical trial with Sebelipase-alfa, it can be hypothesized that the modulation of LAL activity may become a possible new therapeutic target in the future, even in conditions of less severe reductions of LAL activity[14]. This could mainly concern patients with more advanced forms of NAFLD, such as those with NASH or cryptogenic cirrhosis for which, at present, there are no effective therapies[33,38].
In conclusion, the measurement of LAL activity in patients with NAFLD may be a new non-invasive marker of liver disease severity.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Jamali R S-Editor: Ma RY L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Fasano T, Pisciotta L, Bocchi L, Guardamagna O, Assandro P, Rabacchi C, Zanoni P, Filocamo M, Bertolini S, Calandra S. Lysosomal lipase deficiency: molecular characterization of eleven patients with Wolman or cholesteryl ester storage disease. Mol Genet Metab. 2012;105:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Dubland JA, Francis GA. Lysosomal acid lipase: at the crossroads of normal and atherogenic cholesterol metabolism. Front Cell Dev Biol. 2015;3:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Reiner Ž, Guardamagna O, Nair D, Soran H, Hovingh K, Bertolini S, Jones S, Ćorić M, Calandra S, Hamilton J, Eagleton T, Ros E. Lysosomal acid lipase deficiency--an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Bernstein DL, Hülkova H, Bialer MG, Desnick RJ. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J Hepatol. 2013;58:1230-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 5. | Valayannopoulos V, Malinova V, Honzík T, Balwani M, Breen C, Deegan PB, Enns GM, Jones SA, Kane JP, Stock EO, Tripuraneni R, Eckert S, Schneider E, Hamilton G, Middleton MS, Sirlin C, Kessler B, Bourdon C, Boyadjiev SA, Sharma R, Twelves C, Whitley CB, Quinn AG. Sebelipase alfa over 52 weeks reduces serum transaminases, liver volume and improves serum lipids in patients with lysosomal acid lipase deficiency. J Hepatol. 2014;61:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Casula M, Olmastroni E, Pirillo A, Catapano AL. MEMBERS OF THE LIPIGEN STEERING COMMETTEE, PRINCIPAL INVESTIGATORS: Coordinator center, Participant Centers, Participant Laboratories, COLLABORATORS, STUDY CENTRAL LABORATORY AND ANALYSIS GROUP. Evaluation of the performance of Dutch Lipid Clinic Network score in an Italian FH population: The LIPIGEN study. Atherosclerosis. 2018;277:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Borén J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg-Hansen A; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478-390a. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1632] [Cited by in RCA: 1972] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 8. | Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ. 1991;303:893-896. [PubMed] |
| 9. | Pant M, Oshima K. Cholesteryl Ester Storage Disease: An underdiagnosed cause of cirrhosis in adults. Ann Diagn Pathol. 2017;31:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Wolman M. Wolman disease and its treatment. Clin Pediatr (Phila). 1995;34:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Chatrath H, Keilin S, Attar BM. Cholesterol ester storage disease (CESD) diagnosed in an asymptomatic adult. Dig Dis Sci. 2009;54:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Leopold C, Duta-Mare M, Sachdev V, Goeritzer M, Maresch LK, Kolb D, Reicher H, Wagner B, Stojakovic T, Ruelicke T, Haemmerle G, Hoefler G, Sattler W, Kratky D. Hepatocyte-specific lysosomal acid lipase deficiency protects mice from diet-induced obesity but promotes hepatic inflammation. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:500-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Bernstein DL, Lobritto S, Iuga A, Remotti H, Schiano T, Fiel MI, Balwani M. Lysosomal acid lipase deficiency allograft recurrence and liver failure- clinical outcomes of 18 liver transplantation patients. Mol Genet Metab. 2018;124:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Burton BK, Balwani M, Feillet F, Barić I, Burrow TA, Camarena Grande C, Coker M, Consuelo-Sánchez A, Deegan P, Di Rocco M, Enns GM, Erbe R, Ezgu F, Ficicioglu C, Furuya KN, Kane J, Laukaitis C, Mengel E, Neilan EG, Nightingale S, Peters H, Scarpa M, Schwab KO, Smolka V, Valayannopoulos V, Wood M, Goodman Z, Yang Y, Eckert S, Rojas-Caro S, Quinn AG. A Phase 3 Trial of Sebelipase Alfa in Lysosomal Acid Lipase Deficiency. N Engl J Med. 2015;373:1010-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Del Ben M, Polimeni L, Baratta F, Pastori D, Loffredo L, Angelico F. Modern approach to the clinical management of non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:8341-8350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 919] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 17. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1384] [Article Influence: 138.4] [Reference Citation Analysis (1)] |
| 18. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1822] [Article Influence: 121.5] [Reference Citation Analysis (0)] |
| 19. | Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 20. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1007] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 21. | Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Targher G. Non-alcoholic fatty liver disease as driving force in coronary heart disease? Gut. 2017;66:213-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Pastori D, Baratta F, Novo M, Cocomello N, Violi F, Angelico F, Del Ben M. Remnant Lipoprotein Cholesterol and Cardiovascular and Cerebrovascular Events in Patients with Non-Alcoholic Fatty Liver Disease. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Pastori D, Loffredo L, Perri L, Baratta F, Scardella L, Polimeni L, Pani A, Brancorsini M, Albanese F, Catasca E, Del Ben M, Violi F, Angelico F. Relation of nonalcoholic fatty liver disease and Framingham Risk Score to flow-mediated dilation in patients with cardiometabolic risk factors. Am J Cardiol. 2015;115:1402-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Zhang T, Zhang C, Tang F, Zhong N, Li H, Song X, Lin H, Liu Y, Xue F. Identification of reciprocal causality between non-alcoholic fatty liver disease and metabolic syndrome by a simplified Bayesian network in a Chinese population. BMJ Open. 2015;5:e008204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Baratta F, Pastori D, Polimeni L, Tozzi G, Violi F, Angelico F, Del Ben M. Does Lysosomial Acid Lipase Reduction Play a Role in Adult Non-Alcoholic Fatty Liver Disease? Int J Mol Sci. 2015;16:28014-28021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Baratta F, Pastori D, Del Ben M, Polimeni L, Labbadia G, Di Santo S, Piemonte F, Tozzi G, Violi F, Angelico F. Reduced Lysosomal Acid Lipase Activity in Adult Patients With Non-alcoholic Fatty Liver Disease. EBioMedicine. 2015;2:750-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Ramirez CM, Lopez AM, Turley SD. Lysosomal Acid Lipase Activity: A Tool for the Detection and Management of Fatty Liver Disease? EBioMedicine. 2015;2:638-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Selvakumar PK, Kabbany MN, Lopez R, Tozzi G, Alisi A, Alkhouri N, Nobili V. Reduced lysosomal acid lipase activity - A potential role in the pathogenesis of non alcoholic fatty liver disease in pediatric patients. Dig Liver Dis. 2016;48:909-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Polimeni L, Pastori D, Baratta F, Tozzi G, Novo M, Vicinanza R, Troisi G, Pannitteri G, Ceci F, Scardella L, Violi F, Angelico F, Del Ben M. Spleen dimensions are inversely associated with lysosomal acid lipase activity in patients with non-alcoholic fatty liver disease. Intern Emerg Med. 2017;12:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Tovoli F, Napoli L, Negrini G, D'Addato S, Tozzi G, D'Amico J, Piscaglia F, Bolondi L. A Relative Deficiency of Lysosomal Acid Lypase Activity Characterizes Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Gomaraschi MFA, Pavanello C, Branchi A, Calabresi L, Fargion S. Lysosomal acid lipase activity is reduced in NAFLD: Mechanisms and rescue by PPAR-alpha agonists. Atherosclerosis. 2018;e24-e25. |
| 33. | Angelico F, Corradini SG, Pastori D, Fargion S, Fracanzani AL, Angelico M, Bolondi L, Tozzi G, Pujatti PL, Labbadia G, Corazza GR, Averna M, Perticone F, Croce G, Persico M, Bucci T, Baratta F, Polimeni L, Del Ben M, Violi F; LAL-Cirrhosis Collaborative Research Group. Severe reduction of blood lysosomal acid lipase activity in cryptogenic cirrhosis: A nationwide multicentre cohort study. Atherosclerosis. 2017;262:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Vespasiani-Gentilucci U, Gallo P, Piemonte F, Riva E, Porcari A, Vorini F, Tozzi G, Piccioni L, Galati G, De Vincentis A, Carotti S, Morini S, D'Amico J, Angeletti S, Pedone C, Picardi A. Lysosomal Acid Lipase Activity Is Reduced Both in Cryptogenic Cirrhosis and in Cirrhosis of Known Etiology. PLoS One. 2016;11:e0156113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Shteyer E, Villenchik R, Mahamid M, Nator N, Safadi R. Low Serum Lysosomal Acid Lipase Activity Correlates with Advanced Liver Disease. Int J Mol Sci. 2016;17:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Paul M, Coates JAC. Lysosomal acid lipase activity in human lymphocytes: population studies. Pediatric Research. 1977;454. |
| 37. | Vespasiani-Gentilucci U, D'Amico J, De Vincentis A, Tozzi G, Vorini F, Gallo P, Carotti S, Valentini F, Galati G, dell'Unto C, Piemonte F, Picardi A. Platelet count may impact on lysosomal acid lipase activity determination in dried blood spot. Clin Biochem. 2017;50:726-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Block RC, Razani B. Linking lysosomal acid lipase insufficiency to the development of cryptogenic cirrhosis. Atherosclerosis. 2017;262:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |