Published online Jun 21, 2019. doi: 10.3748/wjg.v25.i23.2898
Peer-review started: April 4, 2019
First decision: April 11, 2019
Revised: April 27, 2019
Accepted: May 18, 2019
Article in press: May 18, 2019
Published online: June 21, 2019
Processing time: 78 Days and 11.4 Hours
NIMA related kinase 2 (NEK2) is closely related to mitosis, and it is currently considered to be over-expressed frequently in many poorly prognostic cancers. However, the effect of the up-regulated NEK2 on cellular signaling in tumors, such as gastric cancer (GC), is con-fusing.
To determine the role of the up-regulation of NEK2 in GC.
To investigate the pathological significance of NEK2 in GC, the expression pattern of NEK2 in GC was investigated based on the “Oncomain” database and compared between 30 pairs of cancer samples and adjacent tissues. The co-expression of NEK2 and ERK in GC was analyzed using The Cancer Genome Atlas (TCGA) database and confirmed in clinical samples by quantitative real-time PCR (qRT-PCR), and the survival curve was also plotted. Western blot or qRT-PCR was used to analyze the effect of NEK2 on the phosphorylation levels of ERK and c-JUN in two GC cell lines (BGC823 and SGC7901) with NEK2 overexpression, and the expression of the downstream effector cyclin D1. Furthermore, CCK8, EdU incorporation assay, and flow cytometry were used to detect the proliferative ability of BGC823 and SGC7901 cells with stably silenced ERK.
NEK2 was significantly up-regulated in human GC tissues. ERK was significantly associated with NEK2 expression in human clinical specimens, and combined overexpression of NEK2 and ERK potentially forecasted a poor prognosis and survival in GC patients. NEK2 knockdown in GC cells inhibited ERK and c-JUN phosphory-lation and reduced the transcription of cyclin D1. More interestingly, NEK2 can rescue the inhibition of cellular viability, proliferation, and cell cycle progression due to ERK knockdown.
Our results indicate that NEK2 plays a carcinogenic role in the malignant proliferation of GC cells via the ERK/MAPK signaling, which may be important for treatment and improving patient survival.
Core tip: Expression of NIMA related kinase 2 (NEK2) is significantly up-regulated in human gastric cancer (GC) tissues and cells. Combined NEK2 and ERK expression may predict overall survival in patients with GC. NEK2 regulates the expression of cyclin D1 by affecting the phosphorylation of ERK and c-JUN in GC and mediates cell proliferation via the ERK/c-JUN/cyclin D1 axis.
- Citation: Fan WD, Chen T, Liu PJ. NIMA related kinase 2 promotes gastric cancer cell proliferation via ERK/MAPK signaling. World J Gastroenterol 2019; 25(23): 2898-2910
- URL: https://www.wjgnet.com/1007-9327/full/v25/i23/2898.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i23.2898
Gastric cancer (GC) is a type of malignant tumor in the digestive system with high incidence, and it is the third cause of human cancer-related death[1-3]. Although surgery, radiation therapy, and chemotherapy can improve patient survival to some extent, the 5-year survival rate of GC patients is still low (25%-30%), partly because the complexity of clinical heterogeneity[4-6]. Due to high cell proliferation is associated with poor survival in patients with GC[7], exploring the mechanisms of malignant proliferation of GC cells is important for the treatment and improvement of patient survival[8].
In mammals, NIMA related kinase 2 (NEK2), as an S/G2 phase kinase, usually shows a high expression level in various forms of cancer, mediating the cellular mitosis during cell division[9-11]. In vivo, silencing NEK2 can significantly slow tumor growth in mice and result in reduced tumor burden in tumor-forming organs[12-14]. Furthermore, some studies have shown that up-regulated NEK2 leads to malignant proliferation and drug resistance in vivo, and NEK2 siRNA can significantly reduce tumor formation ability in allogeneic tumor formation studies[15,16]. Although the exact molecular mechanism of NEK2 in the regulation of tumor formation and progression is largely unclear, the fact that NEK2 silencing induces the slower proliferation of cells suggests that NEK2 inhibition can resist tumor progression in vivo, and inhibition of NEK2 expression may become an effective treatment for GC[14,17-20].
The ERK/MAPK pathway communicates intracellular phosphorylation kinase signaling from a receptor on the surface of the cell to the DNA in the nucleus, and produces some changes in cell processes to respond to the extracellular stress[21,22]. Overall, once the extracellular mitogen binds to the receptor on the membrane, ERK will be activated and its downstream effector c-Jun will also be switched by transferring a phosphate group[23], participating in the ERK/MAPK signal tran-sduction[24,25]. Many studies show that activation of the ERK/MAPK pathway plays an important role in tumorigenesis, progression, survival in GC[26-28].
Here, we showed that NEK2 promotes gastric tumorigenesis by regulating the cell cycle. Based on the rise of NEK2 expression in GC and the application of bio-informatics and clinical sample analysis, our study focused on the combined expression of NEK2 and ERK in GC and demonstrated that up-regulation of NEK2 in GC cells by targeting ERK/c-JUN/cyclin D1 signaling effectively promoted the proliferation of GC cells and significantly affected the clinical prognosis of GC patients. Our study revealed the combined role of the NEK2 and ERK/MAPK pa-thways in the cell cycle, which is important for the GC treatment and improvement of patient survival.
GC and paired adjacent tissues were obtained from 30 patients treated at Zhang jiagang First People's Hospital from 2016 to 2018. All patients had no history of chemotherapy or immunotherapy, and all samples were classified as GC by two pathologists. The sample use was approved by the patients and the Hospital Ethics Committee, and all patients provided informed consent.
The AGS, MGC803, BGC823, and SGC7901 cell lines and human normal gastric epithelial cell line (GES1) used in the study were purchased from the Chinese Academy of Medical Sciences Cell Resource Platform (Beijing, China). All cell lines were cultured in DMEM (Gibco) containing 10% FBS (Gibco) and 1% antibiotic (Gibco).
We transfected siRNA oligos into cells using Lipofectamine 3000 (Invitrogen). SiRNAs against NEK2 (siNEK2-1, 5'- GUCAGAUAUUGAGAAAAAUUACCAA-3'; siNEK2-2, 5'- AAUGAGAAAUCAGAUAUCUGGUCAT-3') and control siRNA [siN.C, 5'-UUCUCCGAACGUGUCACGUd(TT)-3'] were obtained from GenePharma (Suzhou). After 48 h of transfection, cells were harvested for subsequent experiments.
The shRNA targeting the ERK gene (5'- AACCTTTAAAGACTGATATTCAAAT-3') was designed and cloned into the pGV307-RFP vector (Addgene). Lentiviral packaging, purification, and infection of the cells of interest were performed according to the protocol provided by the manufacturer. Cells with stable ERK knock-down were selected using 5 μg/mL puromycin (Sigma) at 3 d after infection.
RNA was extracted from cells or tissue samples using TRIzol Reagent (Sigma), and 2 μg of RNA was transcribed into cDNA with a Prim primer RT Master Mix Kit (Takara) using random primers, followed by qRT-PCR using GoTaq qRT-PCR Master Mix (Promega, cat). The ΔΔCT method was used to calculate the expression level of the target mRNA. Experiments were performed in triplicate. The primers used in our study are listed in Table 1.
| Gene | Primers (5’ to 3’, forward/reverse) |
| NEK2 | TGCTTCGTGAACTGAAACATCC |
| CCAGAGTCAACTGAGTCATCACT | |
| ERK | CGGGGCATCTTCGAGATCG |
| CAGAACAACGCCGTTTCAGTT | |
| c-JUN | GTCCTCCATAAATGCCTGTTCC |
| GATGCAACCCACTGACCAGAT | |
| Cyclin D1 | ACGAAGGTCTGCGCGTGTT |
| CCGCTGGCCATGAACTACCT | |
| GAPDH | GGAGCGAGATCCCTCCAAAAT |
| GGCTGTTGTCATACTTCTCATGG |
Paraffin-embedded tissue sections were deparaffinized and dehydrated, and im-munohistochemical staining for NEK2 and ERK in tissues was performed using a DAB substrate kit (Maxin). The staining intensity and staining range were respectively scored using the standard method, and according to the final score (staining intensity × staining range), a score of less than 6 was considered to be low staining, and > 6 high staining.
Total cellular protein was extracted using RIPA lysis containing protease and phosphatase inhibitors. Protein concentration was measured according to the instructions of the BCA kit (Thermo Scientific). SDS-PAGE separation and im-munoblotting were then performed according to standard methods. The primary antibodies used in our study are listed below: EK2 (Abcam), ERK1/2 (Cell Signaling Technology), c-JUN (Cell Signaling Technology), phospho-Erk1/2 (Thr202/Tyr204) (Cell Signaling Technology), phosphorylated c-JUN (Ser63) (Cell Signaling Technology), cyclin D1 (Abcam), and β-actin (Abcam).
Cells with stable ERK knockdown or NEK2 overexpression were seeded in 96-well plates at a density of 1000 cells per well. After incubation for 24, 48, and 72 h, CCK-8 reagent (Dojindo) was added at a concentration of 10% to each well, and after in-cubation for 1 h, the optical density (OD) was measured at 490 nm. Experiments were performed in triplicate.
EdU incorporation assay was performed with an EdU Apollo 488 in vitro imaging kit (RiboBio). One thousand cells were seeded into each well of a 96-well plate. After incubation for 24 h, the medium was changed to a medium containing 20 mmol/L EdU, and incubation was continued at 37 °C for 1 h. After fixing the cells with 4% paraformaldehyde, Apollo staining was performed according to the standard instructions. Cell proliferation rates are shown as the percentage of EdU positive cells. Experiments were performed in triplicate.
When the cells in the 6-well plate reached 80% confluence, they were harvested using trypsin and fixed overnight in pre-cooled 70% ethanol. After washing, cells were incubated in a dark environment with 500 μL of PI/RNase staining buffer (BD Pharmingen) for 15 min. Cell cycle changes were detected and analyzed using the FACSCalibur system (BD Biosciences). Experiments were performed in triplicate.
Data were analyzed using GraphPad Prism 5 (La Jolla, CA, United States). The chi-square test or Fisher's exact test was used to determine the significance of categorical data. The Student's t-test or one-way ANOVA was used to determine the significance of the mean. Differences were considered statistically significant at P < 0.05 (aP < 0.05, bP < 0.01, cP < 0.001).
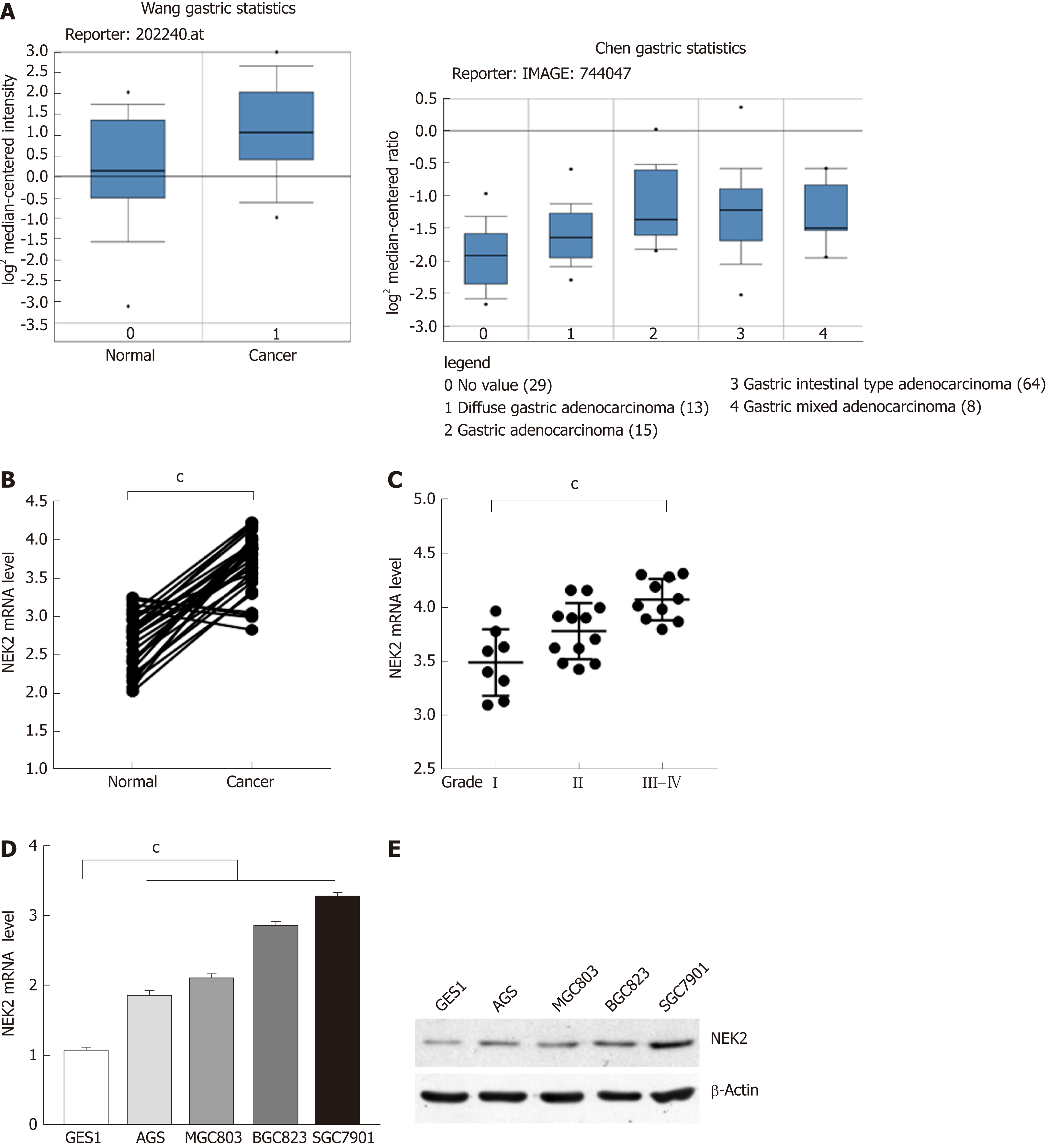
The amplification of NEK2 has an oncogene effect in various types of tumors, regulating the process of tumor proliferation and invasion. To clarify the expression pattern of NEK2 in human GC, we searched the "Oncomine" database for GC data published by different researchers. It was surprised to find that NEK2 expression in GC tissues was significantly higher compared to that in normal tissues (Figure 1A). To confirm the high level of NEK2 expression in GC, we detected the NEK2 expression level in 30 pairs of GC and adjacent tissues. The results showed that the NEK2 mRNA level in GC tissues was significantly higher than that in non-cancerous tissues (Figure 1B).
In addition, in order to understand whether the high expression of NEK2 has an effect on the progression of GC, we divided 30 human GC samples into three grades (I, II, and III-IV) according to the WHO criteria for clinical GC tumors to evaluate the expression pattern of NEK2 in various stages of GC. Interestingly, high-grade tumors (Grade III-IV) showed a higher NEK2 expression level than low-grade tumors (Figure 1C), giving a suggestion that NEK2 expression is gradually increasing in the progression of GC. These results indicated that the progressively up-regulated expression of NEK2 may be a tumor-promoting regulator in the progression of GC.
To further verify that NEK2 is up-regulated in GC, the mRNA and protein expression levels of NEK2 were detected in four gastric tumor cell lines (AGS, MGC803, BGC823, and SGC7901) and gastric epithelial cell line (GES1). In the four GC cell lines, both the mRNA and protein expression levels of NEK2 were higher than those in the normal gastric epithelial cell line (Figure 1D and E), which is consistent with the expression pattern of NEK2 in the tissues. Taken together, these data can confirm that NEK2 expression shows a dynamic up-regulation pattern in GC tissues, and NEK2 plays a potential tumor-promoting role in the pathological process of GC.
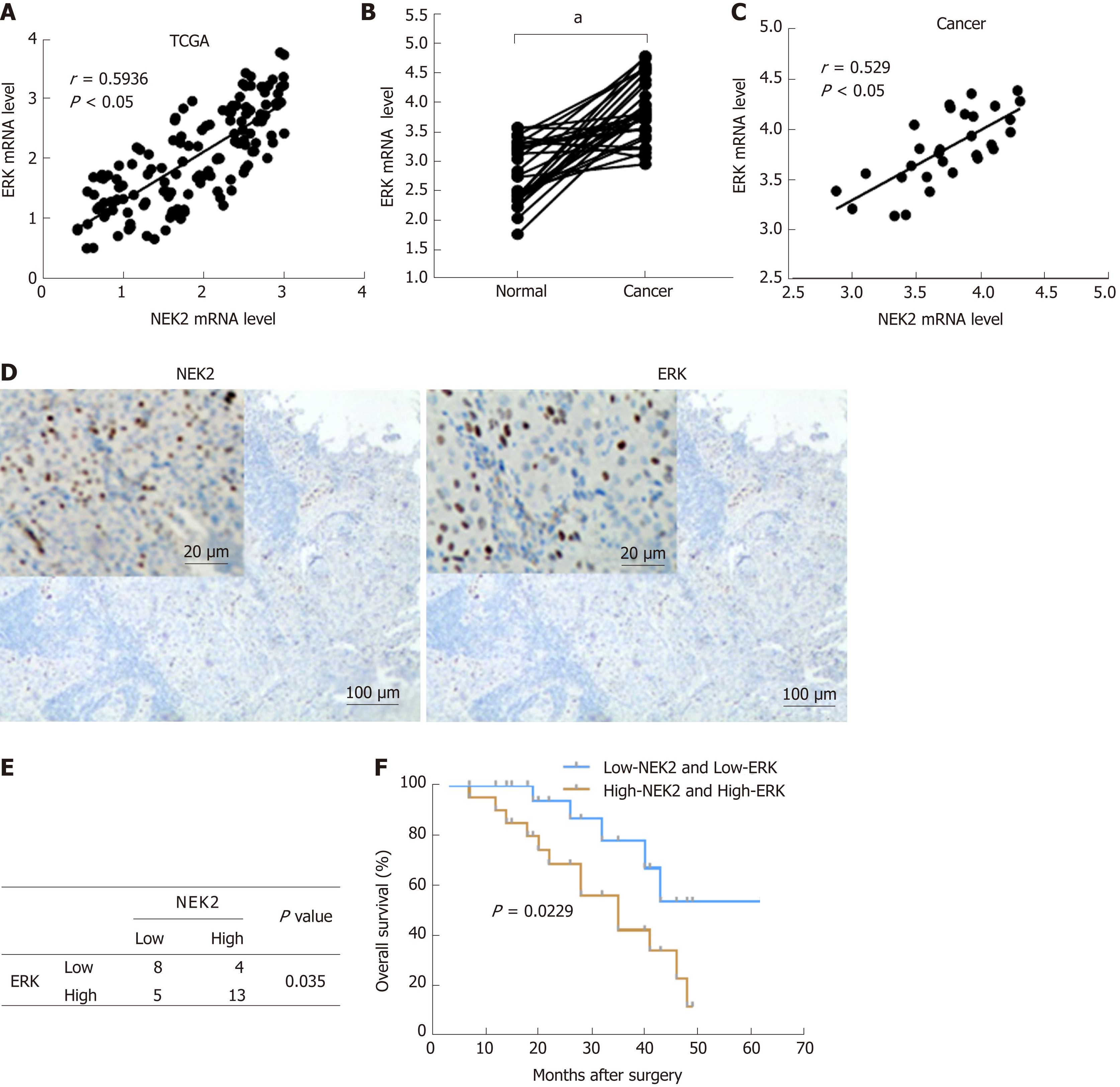
Research has shown that there is a correlation between the activation of ERK1/2 and NEK2 during mitosis[29]. In order to further elucidate the molecular mechanism of the correlation of ERK1/2 and NEK2, we tried to detect the correlation of ERK1/2 and NEK2 expression in GC. First, we found that NEK2 and ERK had strong co-expression relationships in GC based on the TCGA database (Figure 2A). We next examined the ERK expression level in GC tissues, and the result showed that ERK was significantly up-regulated in GC tissue relative to normal tissue (Figure 2B). In addition, in order to confirm the expression relationship between NEK2 and ERK in GC, we further examined the expression relationship between NEK2 and ERK in clinical samples. The RT-qPCR result showed that NEK2 was positively correlated with ERK in GC tissues in terms of the expression level (Spearman, r = 0.594, P < 0.05) (Figure 2C). Collectively, all results suggest a correlation in expression patterns between NEK2 and ERK in GC.
Although the abnormal NEK2 expression is related to the therapeutic effect and prognosis in many cancers, the prognostic significance of NEK2 in GC patients remains unclear. We first classified 30 GC tissues into different groups according the expression of NEK2 and ERK (Figure 2D). The results showed that 56.67% (17/30) of the 30 GC samples showed strong NEK2 immunostaining, while in the high NEK2 expression samples, only 23.53% (4/17) of the tumors showed low ERK expression. In the samples with low expression of NEK2 (43.33%, 13/30), only five samples ex-hibited high REK expression. Whatever, the χ2 test revealed a significant expression relation between ERK and NEK2 (P = 0.035, Figure 2E). Analysis of the expression of NEK2 and ERK using the Kaplan-Meier method in GC patients indicated that patients with low NEK2 and low ERK expression levels had better overall survival than patients with high NEK2 and high ERK levels (Figure 3D). The above results suggest that co-expression of NEK2 and ERK in tissues may be involved in the pathological process of GC and significantly affect the prognosis of patients with GC.
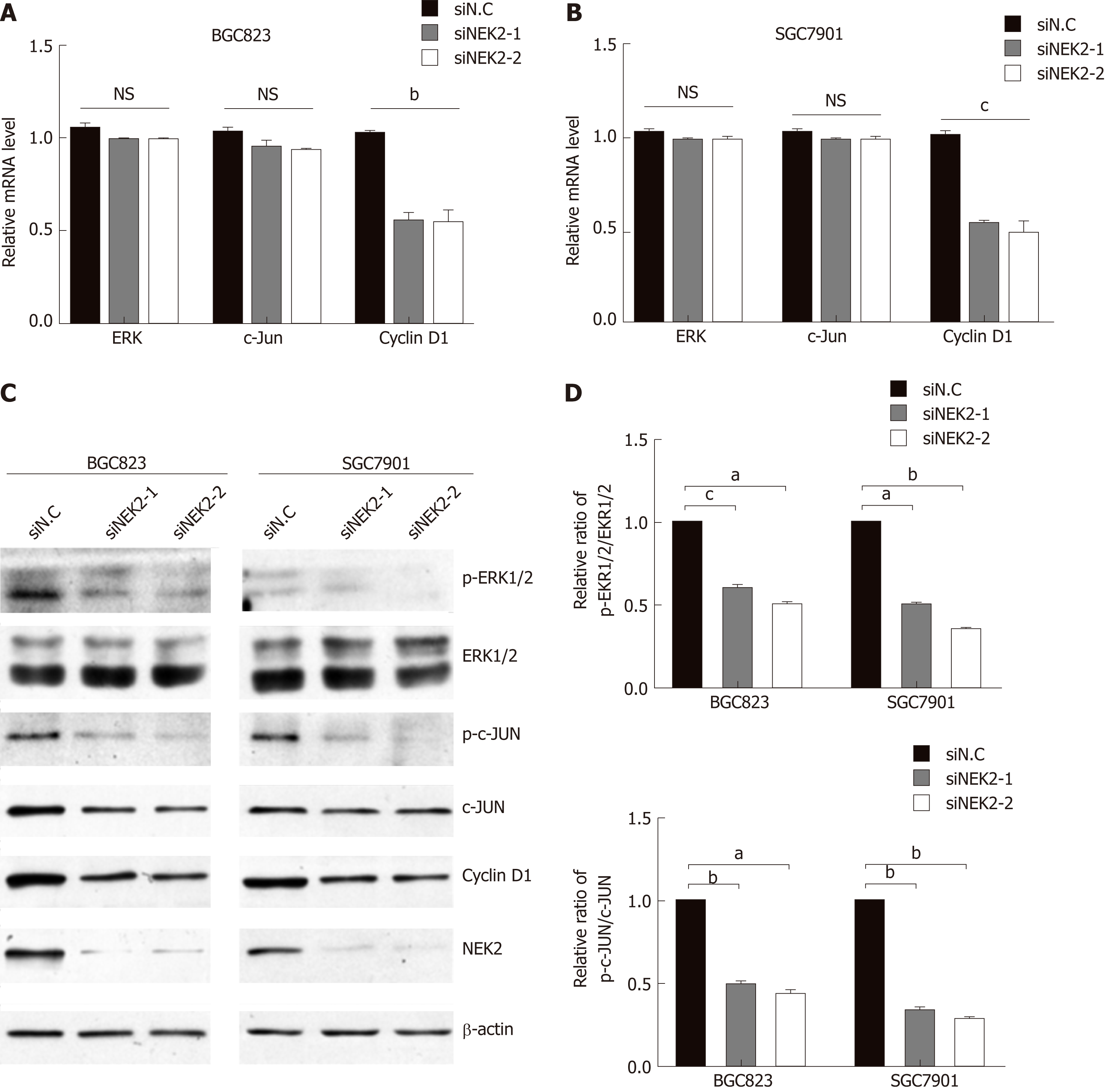
To elucidate the underlying molecular mechanisms by which NEK2 affects the pathogenesis of GC and the prognosis of patients with GC, we analyzed the effect of NEK2 on MEK/ERK signaling. Cell proliferation-related gene expression patterns were examined in BGC823 and SGC7901 cells after NEK2 silencing. We found that, when NEK2 expression in BGC823 and SGC7901 cells was significantly reduced (Figure 4B), the expression of key genes regulating cell proliferation was significantly down-regulated, including c-JUN and cyclin D1, which are considered to be the downstream molecule of the ERK/MAPK signaling pathway (Figure 4A).
In addition, NEK2 silencing resulted in decreased levels of c-JUN and ERK pho-sphorylation, as revealed by Western blot analysis, although c-JUN and ERK did not change significantly at the protein level. And grayscale comparison showed their phosphorylation levels were significantly reduced (Figure 4B and C). In addition, cyclin D1 protein expression was significantly reduced after down-regulation of NEK2 expression in BGC823 and SGC7901 cells (Figure 4B). These results revealed that the expression of cyclin D1 can be regulated by NEK2 through the ERK/c-JUN pathway in GC.
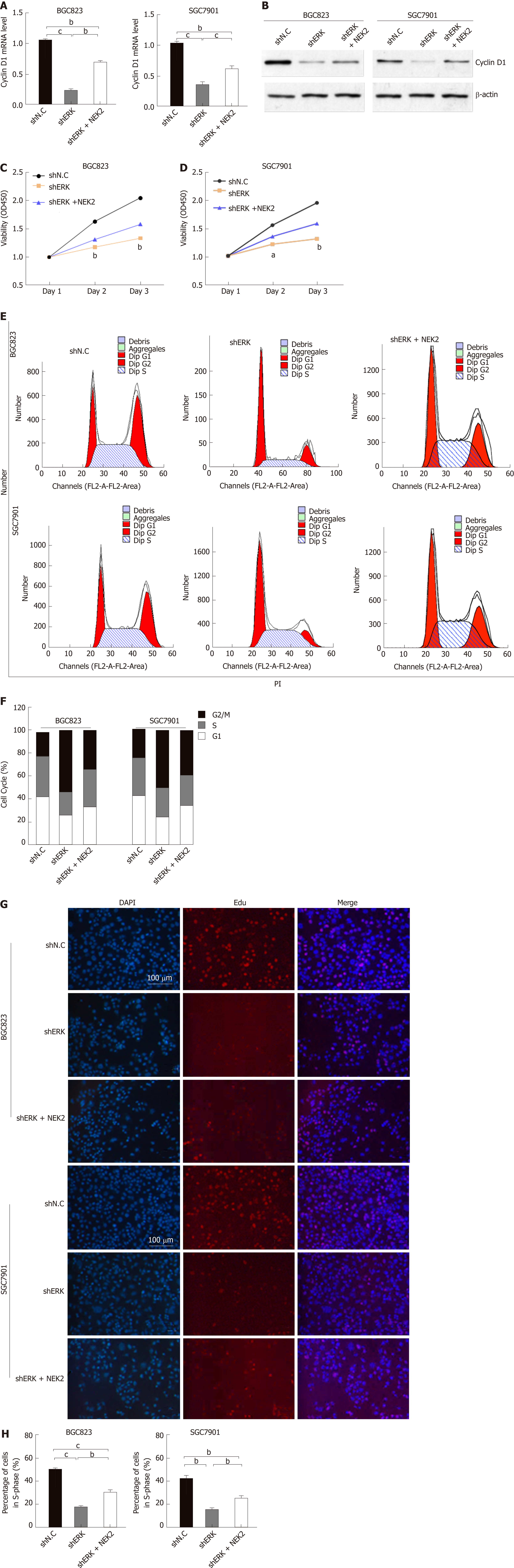
To evaluate whether NEK2 regulates potential phenotypic changes in GC cells via the ERK/c-JUN/cyclin D1 pathway, we used shRNA to establish BGC823 and SGC7901 cells with stable ERK knockdown. We found that cyclin D1 was significantly reduced both at the mRNA and protein levels in the NEK2 knockdown cell line compared to control cells (Figure 3A and B), while overexpression of NEK2 rescued the decreased cyclin D1 expression caused by ERK knockdown.
To explore how NEK2 mediates cell viability in GC, the CCK-8 assay was used to detect the viability of GC cells in BGC823 and SGC7901 cell lines with ERK knockdown. CCK8 assay showed that down-regulation of ERK significantly reduced the proliferation of BGC823 and SGC7901 cells after cell seeding, but cell viability was restored after stably expressing NEK2 in ERK-silenced BGC823 and SGC7901 cells (Figure 3C and D).
We next investigated the effect of silencing NEK2 on cell cycle progression by flow cytometry. Cell cycle distribution analysis showed that ERK silencing resulted in cell cycle arrest in the G0/G1 phase (Figure 4E and F), and the EdU incorporation assay showed that ERK silencing resulted in a decrease in the percentage of S phase cells, whereas both the cell cycle and DNA synthesis in BGC823 and SGC7901 cells were restored to some extent after overexpression of NEK2 (Figure 4G and H). Altogether, these data indicate that NEK2 can regulate GC cell cycle progression and cell pro-liferation via the ERK/c-JUN/cyclin D1 axis.
Previous studies have indicated that, unlike other kinases, NEK2 can promote cancer progression by participating in the regulation of centrosome activity during mitosis, leading to abnormal activation of mitosis. Therefore, the regulation of NEK2 is mainly involved in cancer during the advanced stage[30-32]. In the development of cancer at early stages, for example, NEK2 promotes tumor cell proliferation and participates in the invasion of tumor cells to epithelial tissues[9,16]. It is speculated that enhanced activity of centrosomes is a feature of advanced cell carcinogenesis, driving mutations to progeny cells and providing a source of genetic variation in tumorigenesis[33].
In this study, we for the first time showed that NEK2 overexpression promoted the activation of c-JUN and the up-regulation of cyclin D1 through the abnormally activated ERK/MAPK pathway in GC. We also found that NEK2 overexpression promoted the cell cycle progression in GC cell lines and participated in the regulation of proliferation of GC cells. In human myeloma, NEK2 promotes myeloma resistance via activating the ERK pathway, indirectly supporting our conclusion[34-36]. Thus, our results suggest that NEK2 may have a new and important role in tumor progression.
In many mammalian cell types, the ERK pathway can integrate cellular external stimulation signals into cells, acting as a significant regulator that mediates cell growth and proliferation[37,38]. It is well known that uncontrolled growth or malignant proliferation is a necessary and initial step in the development of tumors[39,40]. Furthermore, it has been shown that abnormal NEK2 expression obviously affected the process of mitosis of breast tumor cells, and in breast cancer, NEK2 over-expression was significantly associated with a poor prognosis, indicating that NEK2 may be an ideal target for therapy[9,41]. However, in our study, we demonstrated that NEK2 was highly expressed in GC and the up-regulated expression of NEK2 was associated with the activity of the ERK/MAPK pathway. NEK2 can promote pho-sphorylation of ERK and further promote cyclin D1 expression through the ERK/c-JUN pathway, regulating the mitosis of GC cells. In addition, the combined and elevated expression of NEK2 and ERK was found to be significantly associated with a poor prognosis in patients with GC, indicating that NEK2 is an essential factor that mediates the transmission of important signals in GC. Therefore, NEK2 has the potential to become an ideal target for GC therapy[41,42].
In conclusion, our study reveals a novel mechanism by which NEK2 promotes the growth of GC cells by activating the ERK/MAPK pathway. Although we have demonstrated that NEK2 can regulate the phosphorylation of c-JUN and ERK and cell cycle progression in GC cells, there may be other targets that may also affect cancer cell proliferation. Nonetheless, our finding that NEK2 targets the ERK/c-JUN/cyclin D1 pathway provides new insights into NEK2 function and reveals that NEK2 may be a potential target for developing optimal treatments for GC patients.
NIMA related kinase 2 (NEK2) is closely related to mitosis, and it is currently considered to be overexpressed frequently in many poorly prognostic cancers. However, the effect of up-regulated NEK2 expression on cellular signaling in tumors, such as gastric cancer (GC), is confusing.
To explore the treatment of GC and improve patient survival.
To determine the role of the up-regulation of NEK2 in GC.
To investigate the pathological significance of NEK2 in GC, the expression of NEK2 in GC was investigated based on the “Oncomain” database and compared between 30 pairs of cancer and adjacent tissues. The co-expression of NEK2 and ERK in GC was analyzed based on the TCGA database and confirmed in clinical samples by quantitative real-time PCR (qRT-PCR), and the survival curve was also plotted. Western blot or qRT-PCR was used to analyze the effect of NEK2 on the phosphorylation levels of ERK and c-JUN in BGC823 and SGC7901 cells, and the expression of the downstream effector cyclin D1. Furthermore, CCK8, EdU incorporation assay, and flow cytometry were used to detect the proliferative of BGC823 and SGC7901 cell lines with stable silencing of ERK.
In this study, we found that NEK2 was significantly up-regulated in human GC tissues. In addition, ERK was significantly associated with NEK2 expression in human clinical specimens, and combined overexpression of NEK2 and ERK potentially forecasted a poor prognosis and survival in GC patients. NEK2 knockdown in GC cells inhibited ERK and c-JUN pho-sphorylation and reduced transcription of cyclin D1. More interestingly, NEK2 can rescue the inhibition of cellular viability, proliferation, and cell cycle progression due to ERK knockdown.
NEK2 plays a carcinogenic role in the malignant proliferation of GC cells via the ERK/MAPK signaling, which is important for treating GC and improving patient survival.
Future research may further reveal the mechanism of action of NEK2 to enhance the sensitivity of cancer cells and promote its application in anti-cancer treatments. And the identification of the molecular pathway related to ERK/MAPK signaling may further elucidate the underlying mechanism.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jones G, Tanabe S S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Strand MS, Lockhart AC, Fields RC. Genetics of Gastric Cancer. Surg Clin North Am. 2017;97:345-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1615] [Cited by in RCA: 1759] [Article Influence: 219.9] [Reference Citation Analysis (0)] |
| 3. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3134] [Cited by in RCA: 3538] [Article Influence: 393.1] [Reference Citation Analysis (0)] |
| 4. | Mihmanli M, Ilhan E, Idiz UO, Alemdar A, Demir U. Recent developments and innovations in gastric cancer. World J Gastroenterol. 2016;22:4307-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Lansdorp-Vogelaar I, Kuipers EJ. Screening for gastric cancer in Western countries. Gut. 2016;65:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Liu Z, Gao P, Liu S, Zheng G, Yang J, Sun L, Hong L, Fan D, Zhang H, Feng F. Tumor volume increases the predictive accuracy of prognosis for gastric cancer: A retrospective cohort study of 3409 patients. Oncotarget. 2017;8:18968-18978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Schipper DL, Wagenmans MJ, Peters WH, Wagener DJ. Significance of cell proliferation measurement in gastric cancer. Eur J Cancer. 1998;34:781-790. [PubMed] |
| 8. | Rugge M, Genta RM, Di Mario F, El-Omar EM, El-Serag HB, Fassan M, Hunt RH, Kuipers EJ, Malfertheiner P, Sugano K, Graham DY. Gastric Cancer as Preventable Disease. Clin Gastroenterol Hepatol. 2017;15:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W, Xu H, Shetty S, Chen T, Zeng Z, Shi L, Zangari M, Miles R, Bearss D, Tricot G, Zhan F. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer Cell. 2013;23:48-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Cappello P, Blaser H, Gorrini C, Lin DC, Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, Youngson B, Done SJ, Mak TW. Role of Nek2 on centrosome duplication and aneuploidy in breast cancer cells. Oncogene. 2014;33:2375-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res. 2004;64:7370-7376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Ha Kim Y, Yeol Choi J, Jeong Y, Wolgemuth DJ, Rhee K. Nek2 localizes to multiple sites in mitotic cells, suggesting its involvement in multiple cellular functions during the cell cycle. Biochem Biophys Res Commun. 2002;290:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Fry AM, O'Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci. 2012;125:4423-4433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 14. | Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene. 2002;21:6184-6194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 2001;20:7117-7127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Kokuryo T, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res. 2007;67:9637-9642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Wang S, Li W, Liu N, Zhang F, Liu H, Liu F, Liu J, Zhang T, Niu Y. Nek2A contributes to tumorigenic growth and possibly functions as potential therapeutic target for human breast cancer. J Cell Biochem. 2012;113:1904-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH. Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem. 2002;277:49408-49416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 652] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 20. | Lee J, Gollahon L. Nek2-targeted ASO or siRNA pretreatment enhances anticancer drug sensitivity in triplenegative breast cancer cells. Int J Oncol. 2013;42:839-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1978] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 23. | Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14-21. [PubMed] |
| 24. | Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 1350] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 25. | Edmunds JW, Mahadevan LC. MAP kinases as structural adaptors and enzymatic activators in transcription complexes. J Cell Sci. 2004;117:3715-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Li S, Ma YM, Zheng PS, Zhang P. GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp Clin Cancer Res. 2018;37:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 27. | Lin CL, Lee CH, Chen CM, Cheng CW, Chen PN, Ying TH, Hsieh YH. Protodioscin Induces Apoptosis Through ROS-Mediated Endoplasmic Reticulum Stress via the JNK/p38 Activation Pathways in Human Cervical Cancer Cells. Cell Physiol Biochem. 2018;46:322-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Yan L, Liu X, Yin A, Wei Y, Yang Q, Kong B. Huaier aqueous extract inhibits cervical cancer cell proliferation via JNK/p38 pathway. Int J Oncol. 2015;47:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Di Agostino S, Rossi P, Geremia R, Sette C. The MAPK pathway triggers activation of Nek2 during chromosome condensation in mouse spermatocytes. Development. 2002;129:1715-1727. [PubMed] |
| 30. | Tsunoda N, Kokuryo T, Oda K, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as a novel molecular target for the treatment of breast carcinoma. Cancer Sci. 2009;100:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Hayward DG, Fry AM. Nek2 kinase in chromosome instability and cancer. Cancer Lett. 2006;237:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 33. | Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci USA. 2009;106:4713-4718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Wang X, Zhou YX, Qiao W, Tominaga Y, Ouchi M, Ouchi T, Deng CX. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25:7148-7158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Spalluto C, Wilson DI, Hearn T. Nek2 localises to the distal portion of the mother centriole/basal body and is required for timely cilium disassembly at the G2/M transition. Eur J Cell Biol. 2012;91:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. J Cell Biol. 2011;193:435-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 37. | Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem J. 2005;392:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005.0008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 405] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 39. | Zarich N, Oliva JL, Martínez N, Jorge R, Ballester A, Gutiérrez-Eisman S, García-Vargas S, Rojas JM. Grb2 is a negative modulator of the intrinsic Ras-GEF activity of hSos1. Mol Biol Cell. 2006;17:3591-3597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227-3239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 841] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 41. | Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 885] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 42. | Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R, Lau J, Chen PL, Lee WH. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res. 2008;68:8393-8399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |