Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2354
Peer-review started: March 7, 2019
First decision: April 11, 2019
Revised: April 16, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 21, 2019
Processing time: 74 Days and 22.2 Hours
The individual performances and the complementarity of Crohn’s disease (CD) activity index (CDAI), C-reactive protein (CRP) and faecal calprotectin (Fcal) to monitor patients with CD remain poorly investigated in the era of “tight control” and “treat to target” strategies.
To assess CDAI, CRP and Fcal variation, alone or combined, after 12 wk (W12) of anti-tumor necrosis factor (TNF) therapy to predict corticosteroids-free remission (CFREM = CDAI < 150, CRP < 2.9 mg/L and Fcal < 250 μg/g with no therapeutic intensification and no surgery) at W52.
CD adult patients needing anti-TNF therapy with CDAI > 150 and either CRP > 2.9 mg/L or Fcal > 250 μg/g were prospectively enrolled.
Among the 40 included patients, 13 patients (32.5%) achieved CFREM at W52. In univariable analysis, CDAI < 150 at W12 (P = 0.012), CRP level < 2.9 mg/L at W12 (P = 0.001) and Fcal improvement at W12 (Fcal < 300 μg/g; or, for patients with initial Fcal < 300 μg/g, at least 50% decrease of Fcal or normalization of Fcal (< 100 μg/g) (P = 0.001) were predictive of CFREM at W52. Combined endpoint (CDAI < 150 and CRP ≤ 2.9 mg/L and FCal improvement) at W12 was the best predictor of CFREM at W52 with positive predictive value = 100.0% (100.0-100.0) and negative predictive value = 87.1% (75.3-98.9). In multivariable analysis, Fcal improvement at W12 [odd ratio (OR) = 45.1 (2.96-687.9); P = 0.03] was a better predictor of CFREM at W52 than CDAI < 150 [OR = 9.3 (0.36-237.1); P = 0.145] and CRP < 2.9 mg/L (0.77-278.0; P = 0.073).
The combined monitoring of CDAI, CRP and Fcal after anti-TNF induction therapy is able to predict favorable outcome within one year in patients with CD.
Core tip: The CALM trial reported that a tight control of inflammation achieved better outcomes than conventional monitoring, but did not explore specifically the value of each biomarker. In this multicentre study, we investigated the performances of Crohn’s disease (CD) activity index (CDAI), C-reactive protein (CRP) and faecal calprotectin (Fcal) variation, alone or combined, after 12 wk of anti-tumor necrosis factor (TNF) therapy to predict corticosteroids-free remission (CFREM) at one year, in CD patients treated with anti-TNF. We showed the complementarity of the variation of CDAI, CRP and Fcal after anti-TNF induction therapy, to predict CFREM at one year, and confirmed that Fcal was the most effective predictor among these three markers.
- Citation: Sollelis E, Quinard RM, Bouguen G, Goutte M, Goutorbe F, Bouvier D, Pereira B, Bommelaer G, Buisson A. Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. World J Gastroenterol 2019; 25(19): 2354-2364
- URL: https://www.wjgnet.com/1007-9327/full/v25/i19/2354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i19.2354
Crohn’s disease (CD) is a chronic and disabling disorder that can highly affect quality of life[1]. The natural history of CD can lead to cumulative bowel damage such as stricture, fistula or intestinal resection[2,3]. In this context, mucosal healing is recognized hitherto as the best therapeutic endpoint in patients with CD, as it is associated with sustained clinical remission, reduced rates of subsequent hospitalization and surgery[4]. In daily practice, this endpoint is limited by the potential risks[5] and the need of repeated endoscopic procedures, which is felt as a burden by patients with CD[6].
Faecal calprotectin (Fcal) is a well-accepted monitoring tool and a surrogate marker of mucosal healing[6-8] and could then be an alternative. However, the STRIDE guidelines considered that Fcal was not a target because of insufficient evidence to recommend treatment optimization using biomarkers alone[4]. Recently, the CALM trial compared two ways of monitoring patients with inflammatory bowel disease (IBD) treated with adalimumab[9]. In the first arm (conventional care), the patients had a therapeutic intensification if the CD activity index (CDAI) did not decrease of at least 70 points. In the second group so-called “tight control group”, the therapies were upgraded in cases of CDAI > 150 or C-reactive protein (CRP) > 5 mg/L or Fcal > 250 µg/g[9]. The authors reported that the group monitored using a tight control of inflammation with objective markers of disease activity and clinical symptoms to drive treatment decisions (second group), achieved better endoscopic and clinical outcomes than conventional monitoring[9]. In a post-hoc analysis of this study, the authors reported that most of the therapeutic intensification were related to increased level of Fcal in the tight control group. However, even though the conclusion of this landmark trial encourages IBD physicians to use Fcal testing in daily practice, the authors did not explore specifically the value of each marker, i.e., CDAI, CRP and Fcal.
In this study, we aimed to investigate the performances of CDAI, CRP and Fcal variation, alone or combined, after 12 wk of anti-tumor necrosis factor (TNF) therapy to predict corticosteroids-free remission (CFREM) at one year, in CD patients treated with anti-TNF.
The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements. The study was approved by local Ethics Committee (#2014/CE 72).
We conducted an observational multicenter study in three French IBD centers. All the patients with CD older than 18 years-old requiring anti-TNF therapy according to the physician’s judgement with CDAI > 150, and CRP > 5 mg/L or Fcal > 250 µg/g were consecutively and prospectively enrolled. Patients who presented with usual contra-indications to anti-TNF or who received anti-TNF therapy to prevent endoscopic postoperative recurrence or to treat isolated perianal lesions were excluded. The patients were treated with infusion of infliximab (5 mg/kg at W0, W2 and W6 and then every 8 wk) or subcutaneous injection of adalimumab (160 mg at W0, 80 mg at W2 and 40 mg every other week) according to the usual guidelines. The choice of the type of anti-TNF agent and the use of concomitant immunosuppressive therapy (azathioprine from 2 to 2.5 mg/kg or methotrexate from 15 mg to 25 mg SC) were free and based on the physician’s judgment. No systematic drug level monitoring was performed during the study as routinely in our centers.
Clinical parameters including the CDAI are detailed in Table 1 and were collected before starting anti-TNF therapy (W0), at W12 and W52. Blood samples were taken and used to measure high- sensitive serum CRP level by immunonephelemetric method (Vista; Siemens, Berlin, Germany) at W0, W12 and W52.
| n = 40 patients | |
| Age at the time of inclusion, mean ± SD (yr) | 34 .0 ± 13.6 |
| Disease duration, median (IQR) (yr) | 4 (0.8-11.3) |
| Female gender, n (%) | 21 (52.5) |
| Current smokers, n (%) | 15 (37.5) |
| Prior bowel resection, n (%) | 7 (17.5) |
| Montreal classification | |
| Location | |
| L1, n (%) | 18 (45.0) |
| L2, n (%) | 3 (7.5) |
| L3, n (%) | 19 (47.5) |
| Behaviour | |
| B1, n (%) | 13 (32.5) |
| B2, n (%) | 16 (40.0) |
| B3, n (%) | 11 (27.5) |
| Perianal lesions, n (%) | 7 (17.5) |
| Anti-TNF-naïve patients, n (%) | 24 (60.0) |
| Type of anti-TNF | |
| Infliximab, n (%) | 16 (40.0) |
| Adalimumab, n (%) | 24 (60.0) |
| Concomitant medications | |
| Immunosuppressive therapies, n (%) | 21 (52.5) |
| Steroids, n (%) | 7 (17.5) |
| Faecal calprotectin level at baseline, median (IQR) (µg/g) | 1010.5 (357.8-1800.0) |
| CRP level at baseline, median (IQR) (mg/L) | 13.2 (5.2-25.9) |
Stools samples were collected at W0, W12 and W52, in the morning to reduce intra-individual variation, and immediately stored at 4 °C. Patients were instructed to transport the stool samples in a dedicated container at 4 °C. Faecal samples were immediately transferred, upon patient arrival, to the Clermont-Ferrand hospital Biochemistry Laboratory. Stool cultures were performed on all samples to exclude gastrointestinal infection. Calprotectin was measured, as routinely performed in our IBD centre, using quantitative immunochromatographic test Quantum Blue High Range (Bühlmann Laboratories AG, Schönenbuch, Switzerland), according to the manufacturer’s instructions. Laboratory personnel, who were blinded from the current clinical disease activity of the patients, performed the analyses. The lower and the upper limits of detection for calprotectin were 100 and 1800 μg/g, respectively. Consequently, all calprotectin levels < 100 and > 1800 μg/g were considered as equal to 100 and 1800 μg/g, respectively. Results were given in μg/g.
CFREM at W52 was defined as: CDAI < 150 and CRP < 2.9 mg/L (normal value according to the manufacturer’s instruction) and faecal calprotectin < 250 μg/g, with no switch or swap of biologics and no bowel resection, and with no therapeutic intensification between W12 and W52. Therapeutic intensification was defined as an increase of anti-TNF dose or a decrease of interval between two infusions/injections or as an addition of another CD-specific medication (steroids or immunosuppressant therapy). Therapeutic intensification was based on clinical activity (CDAI > 150) and not on CRP or Fcal level.
Sample size estimation has been performed in order to assess our primary endpoint. Overall, 40 patients were necessary for a type I error at 5% and a statistical power greater than 80% to detect a true absolute difference higher than 50% to predict CFREM at week 52 using CDAI, CRP, or Fcal, alone or in combination. Consequently, we planned to include 40 patients.
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Clermont-Ferrand University Hospital[10]. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Statistical analysis was performed using Stata software (version 13, StataCorp LP, College Station, TX, United States). The tests were two-sided, with a type I error set at α = 0.05. Continuous data were presented as mean ± standard-deviation or median (interquartile range) according to statistical distribution (assumption of normality assessed using the Shapiro-Wilk test). Categorical parameters were presented as frequencies and associated percentages. To assess the factors associated with CFREM at W52, univariable analyses were realized using usual statistical tests: for continuous outcomes Student t-test or Mann-Whitney test when assumptions of t-test were not met (normality, homoscedasticity assessed by the Fisher-Snedecor test) and for categorical data chi-squared or Fisher’s exact tests. Regarding Fcal, ROC curve analyses were performed to determine the best thresholds to predict CFREM. The optimal threshold was determined according to clinical relevance and usual indexes reported in medical literature (Youden, Liu and efficiency). According to univariable results, a multivariable analysis (logistic regression) was carried out considering an adjustment on relevant clinical parameters. The results were expressed as odds-ratios and 95% confidence intervals.
Overall, 40 patients with CD were enrolled in this study. Their baseline characteristics are detailed in Table 1.
Among them, 16 patients (40%) and 24 patients (60%) were treated with IFX and ADA, respectively. Twenty-one patients received concomitant thiopurines therapy (52.5%). At baseline the median CDAI was 215 (166-282) and the median levels of Fcal and CRP were 1010.5 (357.8-1800.0) µg/g and 13.2 (5.2-25.9) mg/L, respectively. Among the 40 patients treated with anti-TNF, the levels of CDAI [111 (55-198) vs 215 (166-282); P < 0.0001], CRP [3.0 (1.0-17.0) mg/L vs 13.2 (5.2-25.9) mg/L; p=0.011] and Fcal [374.0 (103.0-969.0) vs 1010.5 (357.8-1800.0) µg/g; p = 0.001] were significantly diminished after 12 wk of anti-TNF agents.
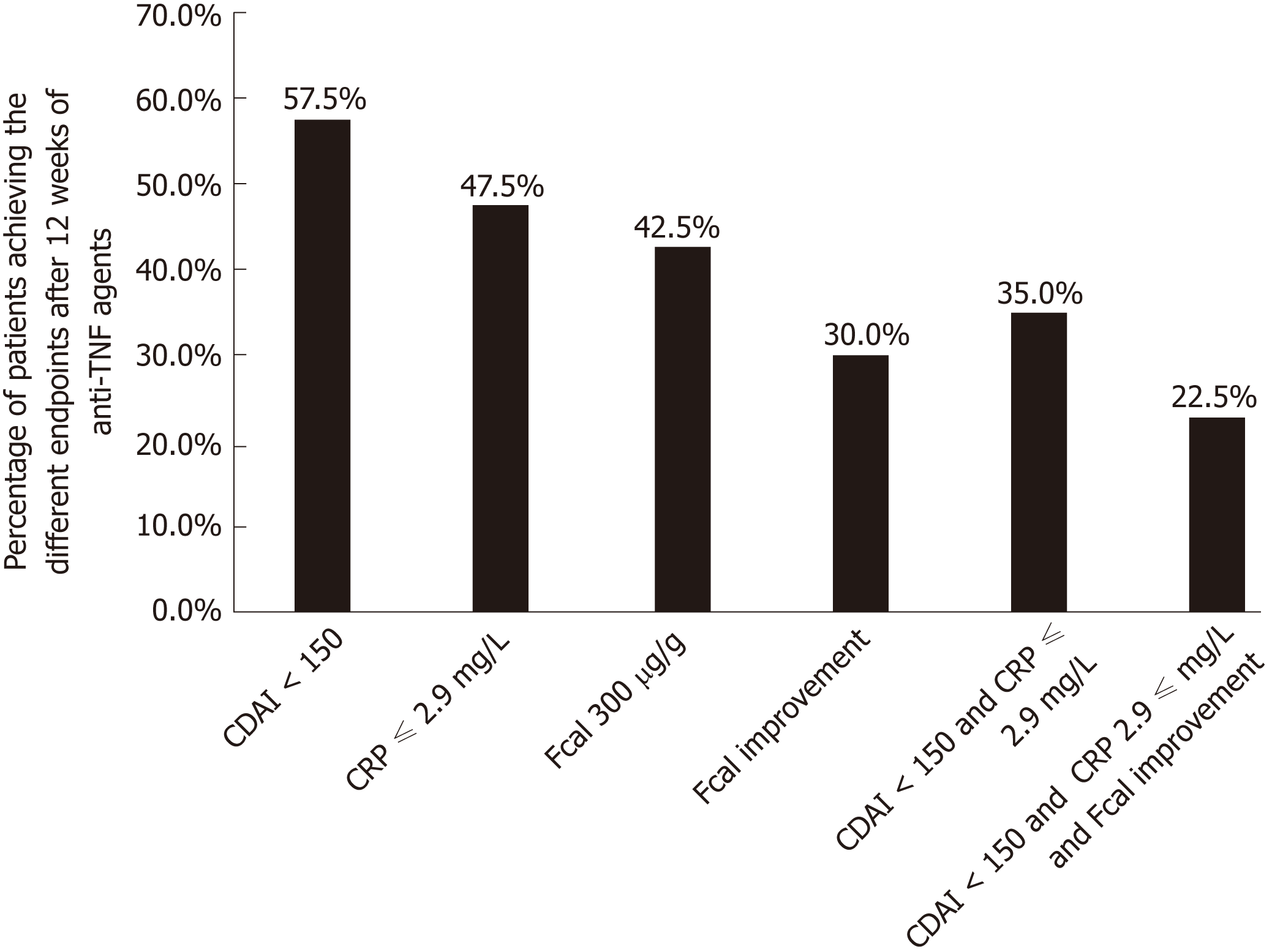
Overall, 13 patients (32.5%) achieved CFREM at W52. The proportion of patients achieving the different therapeutic endpoints at W12 is reported in Figure 1.
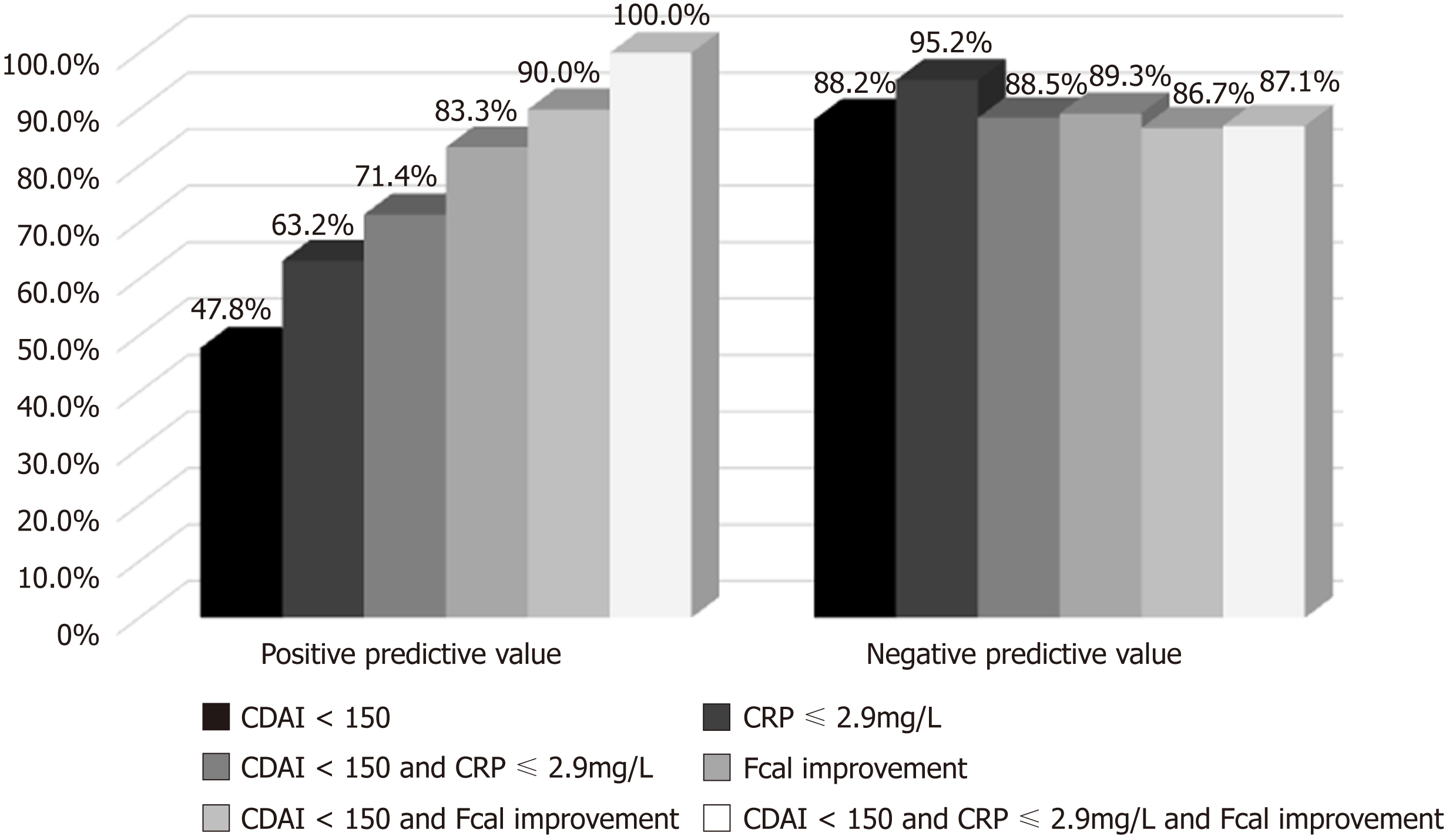
The median CDAI at W12 was significantly lower in patients presenting with CFREM at one year[46 (14-64)] compared to those who did not [165 (83-265)] (P < 0.001). In univariable analysis, CDAI < 150 at W12 was associated with higher likelihood of CFREM at W52 (47.9% vs 11.8%, P = 0.012). Clinical remission at W12 (CDAI < 150) predicted CFREM at W52 with the following performances: sensitivity = 84.6% (56.3-96.6), specificity = 55.6% (37.3-72.4), positive predictive value (PPV) = 47.8% (27.4-68.2), negative positive value (NPV) = 88.2% (72.9-100.0), positive likelihood ratio (LR+) = 1.904 (1.177-3.081) and negative likelihood ratio (LR-) = 0.277 (0.074-1.035) (Table 2).
| Endpoints | Sensitivity | Specificity | PPV | NPV | LR+ | LR- | Accuracy |
| CDAI < 150 | 84.6% (56.3-96.6) | 55.6% (37.3-72.4) | 47.8% (27.4-68.2) | 88.2% (72.9-100.0) | 1.904 (1.177-3.081) | 0.277 (0.074-1.035) | 60.5% (50.2-79.8) |
| CRP ≤ 2.9 mg/L | 92.3% (64.2-100.0) | 74.1% (55.0-86.9) | 63.2% (41.5-84.8) | 95.2% (86.1-100.0) | 3.560 (1.846-6.865) | 0.104 (0.016-0.692) | 80.0% (67.6-92.4) |
| CDAI < 150 and CRP ≤ 2.9 mg/L | 76.9% (48.9-92.2) | 85.2% (66.7-94.6) | 71.4% (47.8-95.1) | 88.5% (76.2-100.0) | 5.192 (2.004-13.456) | 0.271 (0.099-0.740) | 82.5% (70.7-94.3) |
| Fcal < 300 µg/g | 84.6% (56.3-96.6) | 77.8% (58.8-89.6) | 64.7% (42.0-87.4) | 91.3% (79.8-100%) | 3.808 (1.812-8.003) | 0.198 (0.054-0.719) | 80.0% (67.6-92.4) |
| Fcal improvement | 76.9% (48.9-92.2) | 92.6% (75.3-98.9) | 83.3% (62.2-100.0) | 89.3% (77.8-100.0) | 10.385 (2.648-40.721) | 0.249 (0.092-0.676) | 87.5% (77.3-97.7) |
| CDAI < 150 and Fcal improvement | 69.2% (42.0-87.4) | 96.3% (79.9-100.0) | 90.0% (71.4-100.0) | 86.7% (74.5-98.8) | 18.692 (2.640-132.328) | 0.320 (0.141-0.725) | 87.5% (77.3-97.7) |
| CDAI < 150 and CRP ≤ 2.9 mg/L and Fcal improvement | 69.2% (42.0-87.4) | 100.0% (84.9-100.0) | 100.0% (100.0-100.0) | 87.1% (75.3-98.9) | NA | 0.308 (0.136-0.695) | 90.0% (80.7-99.3) |
The level of CRP dropped at W12 in patients with CFREM at one year [1.0 mg/L (1.0-2.9) vs 8.4 mg/L (2.9-29.5); P = 0.001]. In univariable analysis, a CRP level < 2.9 mg/L (normalized CRP) at W12 was associated with higher rate of CFREM at W52 (61.2% vs 4.8%, P < 0.001). CRP level below 2.9 mg/L at W12 predicted CFREM at W52 with the following performances: sensitivity = 92.3% (64.2-100.0), specificity = 74.1% (55.0-86.9), PPV = 63.2% (41.5-84.8), NPV = 95.2% (86.1-100.0), LR+ = 3.560 (1.846-6.865), LR- = 0.104 (0.016-0.692) (Table 2).
The combined performances of both CDAI < 150 and CRP ≤ 2.9 mg/L at W12 to predict CFREM at W52 were sensitivity = 76.9% (48.9-92.2), specificity = 85.2% (66.7-94.6), PPV = 71.4% (47.8-95.1), NPV = 88.5% (76.2-100.0), LR+ = 5.192 (2.004-13.456), LR- = 0.271 (0.099-0.740) (Table 2).
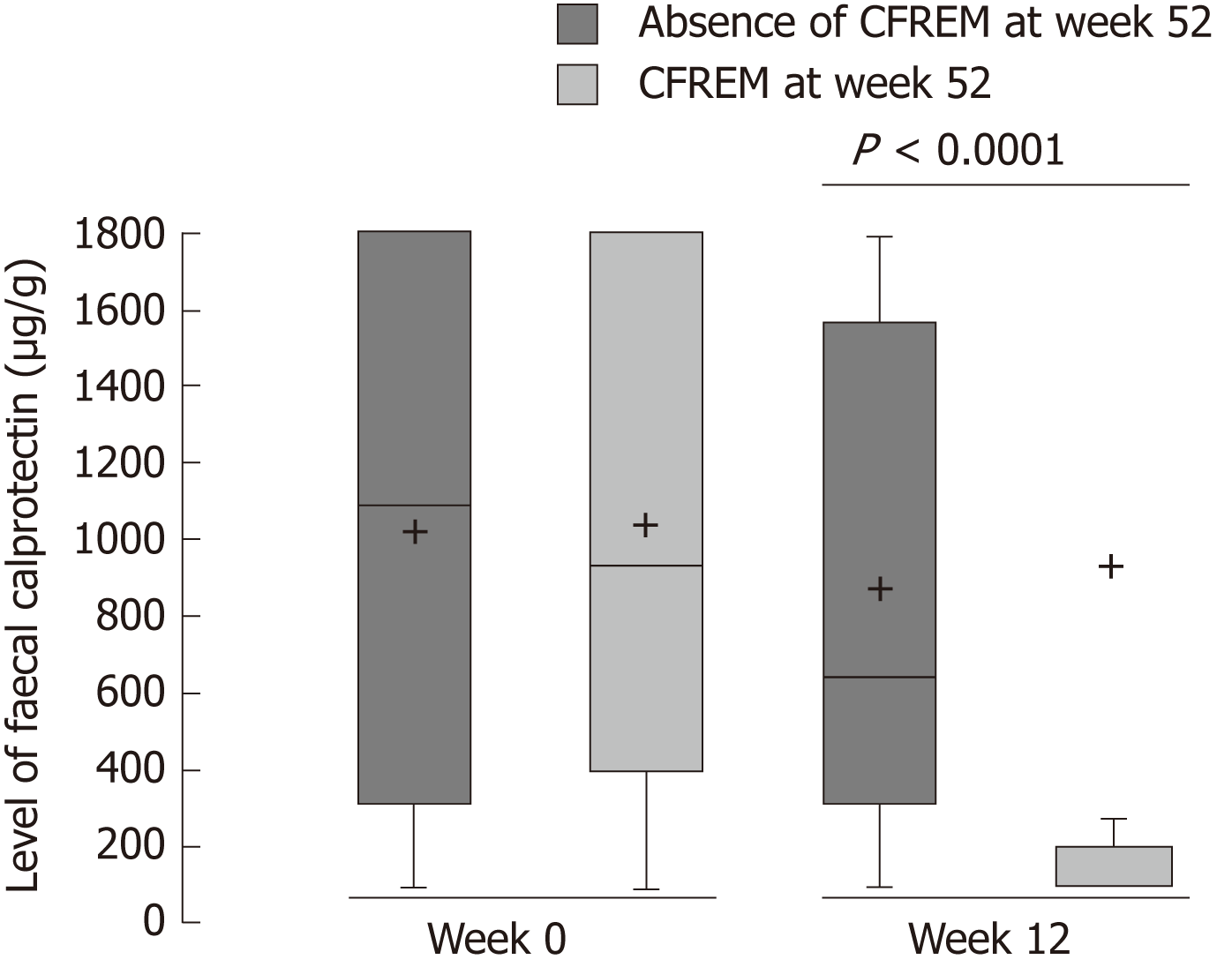
In patient with CFREM at one year, the median Fcal level at W12 was significantly lower [100.0 μg/g (100.0-193.0)] vs compared to those who were not [646.0 μg/g (315.0-1567.0)] (P < 0.001) (Figure 2).
We also observed that the relative decrease of Fcal between W0 and W12 was higher [83.3% (33.6%-83.3%) vs 0.0% (0.0%-33.2%), P = 0.001] in the patients achieving CFREM at W52 compared to the patients who failed to obtain CFREM at W52. Based on ROC curve analyses, we determined that the best threshold of Fcal after anti-TNF induction therapy (W12) to predict CFREM at one year was 300 μg/g [area under the curve (AUC) = 0.848]. The performances of this cut-off value were: sensitivity = 84.6% (56.3-96.6), specificity = 77.8% (58.8-89.6), PPV = 64.7% (42.0-87.4), NPV = 91.3% (79.8-100%), LR+ = 3.808 (1.812-8.003), LR- = 0.198 (0.054-0.719) (Table 2).
Using a ROC curve (AUC = 0.82), a decreased > 50% of Fcal was also predictive of CFREM at one year with sensitivity = 61.5% (35.4-82.2), specificity = 85.2% (66.7-94.6), PPV = 66.7% (40.0-93.3), NPV = 82.1% (68.0-96.3), LR+ = 4.154 (1.526-11.307), LR- = 0.452 (0.223-0.914).
We also studied the complementary of these two thresholds by creating a composite criterion so-called Fcal improvement [Fcal < 300 μg/g at W12; or, for patients with initial Fcal < 300 μg/g, at least 50% decrease of Fcal or normalization of Fcal (< 100 μg/g)]. Fcal improvement predicted CFREM at 1 year with the following performances: sensitivity = 76.9% (48.9-92.2), specificity = 92.6% (75.3-98.9), PPV = 83.3% (62.2-100.0), NPV = 89.3% (77.8-100.0), LR+ = 10.385 (2.648-40.721), LR- = 0.249 (0.092-0.676) (Table 2).
Concomitant CDAI < 150 and FCal improvement at W12 was predictive of CFREM at W52 with the following performances: sensitivity = 69.2% (42.0-87.4), specificity = 96.3% (79.9-100.0), PPV = 90.0% (71.4-100.0), NPV = 86.7% (74.5-98.8), LR+ = 18.692 (2.640-132.328), LR- = 0.320 (0.141-0.725) (Table 2).
Combined endpoints such as CDAI < 150 and CRP ≤ 2.9 mg/L and FCal improvement at W12 predicted CFREM at W52 with the following performances sensitivity = 69.2% (42.0-87.4), specificity = 100.0% (84.9-100.0), PPV = 100.0% (100.0-100.0), NPV = 87.1% (75.3-98.9), LR+ = not applicable, LR- = 0.308 (0.136-0.695) (Table 2).
In univariable analysis, we did not observe any additional factors associated with CFREM. In multivariable analysis including disease duration, smoking status, CDAI and CRP values, Fcal improvement at W12 was independently associated with CFREM at W52 [odd ratio (OR) = 45.1 (2.96-687.9); P = 0.03] and was a better predictor than CDAI < 150 [9.3 (0.36-237.1); P = 0.145] and CRP < 2.9 mg/L (0.77-278.0; P = 0.073) (Figure 3).
In this prospective study, we showed the complementarity of the variation of CDAI, CRP and Fcal after anti-TNF induction therapy, to predict CFREM at one year, and confirmed that Fcal was the most effective predictor among these three markers.
According to the results of the CALM trial, we decided to use the same composite endpoint at W52 i.e., CDAI < 150 and CRP normalization (< 2.9 mg/L using our assay) and Fcal < 250 µg/g, so-called CFREM, which has been shown to be associated with clinical and endoscopic remission[9]. We added the absence of therapeutic intensification including the absence of CD-related surgery to assess the impact of these biomarkers on CD outcomes and also to avoid a complete overlap between the criteria assessed at W12 and the composite primary endpoint at W52. We aimed to investigate what was the best predictor among CDAI, CRP, FCal, or combination of these biomarkers assessed at W12 to predict CFREM at W52.
In this study, we confirmed that CDAI alone is not suitable to monitor CD patients as we found a low PPV (47.3%) of clinical remission (CDAI < 150) at W12 to predict CFREM at W52. The lack of correlation between clinical symptoms and objective markers of activity is now well admitted. A post-hoc analysis from the SONIC trial showed that almost one half of the patients with CDAI < 150 presented with significant endoscopic lesions[11]. The STRIDE guidelines considered that resolution of symptoms alone is not a sufficient target and that objective evidence of bowel inflammation is necessary when making clinical decisions[4]. As expected, we also found that the patients, who did not achieve clinical remission (CDAI < 150) at W12, had a very high likelihood of treatment failure (with NPV of 88.2%) reminding that clinical remission is a necessary but not sufficient therapeutic goal in patients with CD.
We also investigated the measurement of CRP value at W12 in patients receiving anti-TNF agents as predictor of mid-term CFREM (W52). While CRP normalization after 12 wk demonstrated moderate performances (PPV = 63.2%) to predict CFREM at W52, the absence of CRP normalization at W12 was highly predictive of treatment failure (NPV = 95.2%). Our data seems slightly different from two studies dedicated to CRP normalization as predictor of remission. Reinisch et al[12] reported in a post-hoc analysis of the landmark ACCENT 1 trial that normalisation of CRP at week 14 led to a higher probability of maintained response or remission during one-year of infliximab maintenance therapy (P < 0.001) with PPV of 51.8% and NPV of 68.0%. Kiss et al[13] showed that CD patients who had normalised CRP at week 12 were associated with clinical efficacy at 12 mo with PPV ranging from 67% to 79% and NPV from 73% to 80%. This discrepancy could be partly explained by our sample size but also by the choice of our combined endpoint, which is probably more stringent than the two former studies and could have improve the NPV.
As the capability of Fcal to change under treatment remains poorly inve-stigated[14-16], the STRIDE guidelines did not consider Fcal as a therapeutic target[4]. Our results highlighted the performances of Fcal improvement [defined as Fcal < 300 μg/g at W12 or, for patients with baseline Fcal < 300 μg/g, at least 50% decrease or normalization of Fcal (< 100 μg/g)] to predict CFREM at 1 year: PPV = 83.3% and NPV = 89.3%. We chose to also enroll patients with moderate elevation of Fcal (100-300 µg/g) as it reflects some real-life clinical situations in daily practice. Our data are in line with the three studies available to date on this topic. In a French prospective study including 32 patients with CD receiving anti-TNF therapy, Fcal level above 82 µg/g at W14 demonstrated PPV = 85% and NPV = 87% to predict clinical remission within one year using quantitative monoclonal antibody-based enzyme-linked immunosorbent assay (Bühlmann, Schönenbuch, Switzerland). Guidi and colleagues (n = 44 patients with CD), post-induction level of Fcal ≤ 168 µg/g using enzyme-linked immunosorbent assay (ELISA, Calprest, Eurospitals.p.a., Trieste, Italy) was predictive of sustained clinical response with PPV = 81% and NPV = 77%[15]. Eventually, Molander et al[16] found that a post-induction level of Fcal < 139 µg/g [measured by a quantitative enzyme immunoassay (PhiCal Test, Calpro, Oslo, Norway)] predicted the risk of relapse in 34 patients with CD treated with anti-TNF agents. The heterogeneity of these thresholds is explained by the difference of assays used across the studies. IBD physicians have to be aware of these variations between the different assays to measure FCal when they make a decision based on Fcal cut-off values. We strongly encourage IBD physicians to use the same assays when assessing the variation of Fcal under treatment in a same patient. A study comparing six commercially available assays underlined that Fcal level may vary with up to 5-fold quantitative differences between assays[17].
The CALM trial was designed considering that the use of biomarkers could improve patients’ outcomes without real evidence of it. Since the publication of this study, the authors insisted on the role of FCal to monitor patients with CD. However, what is the part of Fcal, CRP and CDAI remains questionable. It is why we decided to investigate the specific role and the potential complementarity of these three biomarkers. In our study, we observed that achieving CDAI < 150, CRP normalization and Fcal improvement was the best combination and led to a PPV of 100% with substantial NPV (87.1%) for the prediction of CFREM at W52. This result could mean that the surveillance scheduled every three months in the CALM trial could be slightly lightened in patients achieving this endpoint after induction therapy and extended to every 6 mo.
The main limitations of our study are the lack of endoscopic evaluation at W52 and the relative small number of patients even though our sample size calculation showed that it was appropriate. However, we investigated prospectively with a suitable power the performances of each item of the CALM criteria and their combinations to predict favorable outcomes in patients with CD.
In conclusion, the combined monitoring of CDAI, CRP and FCal after anti-TNF induction therapy is able to predict favorable outcome within one year in patients with CD. The most impactful biomarker was Fcal among these three biomarkers. Our results should lead IBD physicians to monitor patients with CD using a tight control strategy based on CDAI, CRP and Fcal in daily practice.
Crohn’s disease (CD) is a chronic and disabling disorder that can highly affect quality of life. Faecal calprotectin (Fcal) is a well-accepted monitoring tool and a surrogate marker of mucosal healing and could then be an alternative to endoscopy. Recently, the CALM trial compared two ways of monitoring patients with inflammatory bowel disease (IBD) treated with adalimumab. The authors reported that the group monitored using a tight control of inflammation with objective markers of disease activity and clinical symptoms to drive treatment decisions, achieved better endoscopic and clinical outcomes than conventional monitoring. In a post-hoc analysis of this study, the authors reported that most of the therapeutic intensification were related to increased level of Fcal in the tight control group. However, even though the conclusion of this landmark trial encourages IBD physicians to use Fcal testing in daily practice, the authors did not explore specifically the value of each marker, i.e., CD activity index (CDAI), C-reactive protein (CRP) and Fcal.
Understanding the value of each monitoring biomarker to guide physicians to manage patients with inflammatory bowel disease is a key point.
In this study, we aimed to investigate the performances of CDAI, CRP and Fcal variation, alone or combined, after 12 wk of anti-tumor necrosis factor (TNF) therapy to predict corticosteroids-free remission (CFREM) at one year, in CD patients treated with anti-TNF.
It was a multicentre prospective observational study.
Among the 40 included patients, 13 patients (32.5%) achieved CFREM at W52. In univariable analysis, CDAI < 150 at W12 (P = 0.012), CRP level < 2.9 mg/L at W12 (P = 0.001) and Fcal improvement at W12 [Fcal < 300 μg/g; or, for patients with initial Fcal < 300 μg/g, at least 50% decrease of Fcal or normalization of Fcal (<100 μg/g)] (P = 0.001) were predictive of CFREM at W52. Combined endpoint (CDAI < 150 and CRP ≤ 2.9 mg/L and FCal improvement) at W12 was the best predictor of CFREM at W52 with PPV = 100.0% (100.0-100.0) and NPV = 87.1% (75.3-98.9). In multivariable analysis, Fcal improvement at W12 [odd ratio (OR) = 45.1 (2.96-687.9); P = 0.03] was a better predictor of CFREM at W52 than CDAI < 150 [OR = 9.3 (0.36-237.1); P = 0.145] and CRP < 2.9 mg/L (0.77-278.0; P = 0.073).
The combined monitoring of CDAI, CRP and FCal after anti-TNF induction therapy is able to predict favorable outcome within one year in patients with CD. The most impactful biomarker was Fcal among these three biomarkers. Our results should lead IBD physicians to monitor patients with CD using a tight control strategy based on CDAI, CRP and Fcal in daily practice.
Additional studies from independent cohorts should be conducted to confirm these data.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lv XP, Sachar DB, Yang MS S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T, Kostanjsek N, Stucki G, Colombel JF; International Programme to Develop New Indexes for Crohn's Disease (IPNIC) group. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012;61:241-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 2. | Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D'Haens G, Feagan BG, Hibi T, Hommes DW, Irvine EJ, Kamm MA, Loftus EV, Louis E, Michetti P, Munkholm P, Oresland T, Panés J, Peyrin-Biroulet L, Reinisch W, Sands BE, Schoelmerich J, Schreiber S, Tilg H, Travis S, van Assche G, Vecchi M, Mary JY, Colombel JF, Lémann M. Development of the Crohn's disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 463] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 3. | Pariente B, Mary JY, Danese S, Chowers Y, De Cruz P, D'Haens G, Loftus EV, Louis E, Panés J, Schölmerich J, Schreiber S, Vecchi M, Branche J, Bruining D, Fiorino G, Herzog M, Kamm MA, Klein A, Lewin M, Meunier P, Ordas I, Strauch U, Tontini GE, Zagdanski AM, Bonifacio C, Rimola J, Nachury M, Leroy C, Sandborn W, Colombel JF, Cosnes J. Development of the Lémann index to assess digestive tract damage in patients with Crohn's disease. Gastroenterology. 2015;148:52-63.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 4. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1406] [Article Influence: 140.6] [Reference Citation Analysis (115)] |
| 5. | Buisson A, Chevaux JB, Hudziak H, Bresler L, Bigard MA, Peyrin-Biroulet L. Colonoscopic perforations in inflammatory bowel disease: a retrospective study in a French referral centre. Dig Liver Dis. 2013;45:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, Pariente B, Flamant M, Trang-Poisson C, Bonnaud G, Mathieu S, Thevenin A, Duruy M, Filippi J, Lʼhopital F, Luneau F, Michalet V, Genès J, Achim A, Cruzille E, Bommelaer G, Laharie D, Peyrin-Biroulet L, Pereira B, Nachury M, Bouguen G; ACCEPT study group. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, Van Olmen G, Rutgeerts P. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 621] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 8. | Goutorbe F, Goutte M, Minet-Quinard R, Boucher AL, Pereira B, Bommelaer G, Buisson A. Endoscopic Factors Influencing Fecal Calprotectin Value in Crohn's Disease. J Crohns Colitis. 2015;9:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, Danalioglu A, Novacek G, Armuzzi A, Hébuterne X, Travis S, Danese S, Reinisch W, Sandborn WJ, Rutgeerts P, Hommes D, Schreiber S, Neimark E, Huang B, Zhou Q, Mendez P, Petersson J, Wallace K, Robinson AM, Thakkar RB, D'Haens G. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 668] [Article Influence: 83.5] [Reference Citation Analysis (1)] |
| 10. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36664] [Article Influence: 2291.5] [Reference Citation Analysis (0)] |
| 11. | Peyrin-Biroulet L, Reinisch W, Colombel JF, Mantzaris GJ, Kornbluth A, Diamond R, Rutgeerts P, Tang LK, Cornillie FJ, Sandborn WJ. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut. 2014;63:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 12. | Reinisch W, Wang Y, Oddens BJ, Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Kiss LS, Szamosi T, Molnar T, Miheller P, Lakatos L, Vincze A, Palatka K, Barta Z, Gasztonyi B, Salamon A, Horvath G, Tóth GT, Farkas K, Banai J, Tulassay Z, Nagy F, Szenes M, Veres G, Lovasz BD, Vegh Z, Golovics PA, Szathmari M, Papp M, Lakatos PL; Hungarian IBD Study Group. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn's disease. Aliment Pharmacol Ther. 2011;34:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Boschetti G, Garnero P, Moussata D, Cuerq C, Préaudat C, Duclaux-Loras R, Mialon A, Drai J, Flourié B, Nancey S. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn's disease. Inflamm Bowel Dis. 2015;21:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Guidi L, Marzo M, Andrisani G, Felice C, Pugliese D, Mocci G, Nardone O, De Vitis I, Papa A, Rapaccini G, Forni F, Armuzzi A. Faecal calprotectin assay after induction with anti-Tumour Necrosis Factor α agents in inflammatory bowel disease: Prediction of clinical response and mucosal healing at one year. Dig Liver Dis. 2014;46:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Molander P, af Björkesten CG, Mustonen H, Haapamäki J, Vauhkonen M, Kolho KL, Färkkilä M, Sipponen T. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFα blocking agents. Inflamm Bowel Dis. 2012;18:2011-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Labaere D, Smismans A, Van Olmen A, Christiaens P, D'Haens G, Moons V, Cuyle PJ, Frans J, Bossuyt P. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J. 2014;2:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |