Published online Apr 28, 2019. doi: 10.3748/wjg.v25.i16.1950
Peer-review started: February 17, 2019
First decision: March 5, 2019
Revised: March 12, 2019
Accepted: March 24, 2019
Article in press: March 25, 2019
Published online: April 28, 2019
Processing time: 69 Days and 22.4 Hours
Chronic hepatitis B is a highly heterogeneous disease that can be divided into four phases: Immune tolerant (IT), immune active (IA), inactive carrier (IC) and hepatitis B envelope antigen (HBeAg)-negative hepatitis (ENEG).
To investigate the immune status of natural killer (NK) and T cells in different phases of chronic hepatitis B.
The frequency, phenotype and function of circulating NK cells, as well as nonantigen-specific and hepatitis B virus (HBV)-specific T cell responses were detected by flow cytometry in healthy and HBV-infected subjects.
The ability of NK cells to produce IFN-γ was markedly attenuated in HBV-infected patients overall but was less compromised in IC patients. Patients in the IT and IA phases also displayed significantly lower TNF-α production compared to healthy subjects. NK cells were phenotypically activated in the IA and ENEG phases, as evidenced by the upregulation of NKp44 in CD56bright NK cells and CD69 in CD56dim NK cells. Furthermore, global T-cells from the ENEG phase displayed a proinflammatory cytokine profile with upregulated IFN-γ and TNF-α expression, while this profile was suppressed in IT and IA patients. Finally, core and S antigen-specific T cell responses were significantly stronger after in vitro expansion in the IC phase compared to other phases.
Our findings demonstrate the changes in immune response pattern during the natural history of HBV infection. Both NK and T cells are functionally impaired in the IT and IA phases. With the spontaneous clearance of HBeAg and hepatitis B surface antigen decline, NK cell cytokine production and HBV-specific T responses are partially restored in IC phase, and the ENEG phase is dominated by nonantigen-specific T cell responses.
Core tip: Chronic hepatitis B is a highly heterogeneous disease, which can be divided into four phases: immune tolerant, immune active (IA), inactive carrier and hepatitis B envelope antigen (HBeAg)-negative hepatitis (ENEG). Natural killer (NK) and virus-specific T cells are two key effector cells of cellular immunity. Our study demonstrates the conversion of the immune response pattern along the natural history of chronic hepatitis B virus infection. NK cells were phenotypically activated in the clinical phases (IA and ENEG) with biochemical liver damage. NK, non-specific and virus-specific T cells were functionally impaired in immune tolerant and IA phases. With the spontaneous clearance of HBeAg and hepatitis B surface antigen decline, NK cell cytokine production and HBV-specific T cell responses were partially restored in the inactive carrier phase, and the ENEG phase was primarily dominated by nonantigen-specific T cell responses.
- Citation: Wang WT, Zhao XQ, Li GP, Chen YZ, Wang L, Han MF, Li WN, Chen T, Chen G, Xu D, Ning Q, Zhao XP. Immune response pattern varies with the natural history of chronic hepatitis B. World J Gastroenterol 2019; 25(16): 1950-1963
- URL: https://www.wjgnet.com/1007-9327/full/v25/i16/1950.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i16.1950
Worldwide, an estimated 240 million people are chronically infected with hepatitis B virus (HBV), which places them at relatively high risks of developing liver cirrhosis, hepatic decompensation and hepatocellular carcinoma[1]. According to the natural course and clinical manifestations, chronic HBV infection can be categorized into four different phases: Immune tolerant [IT, also termed hepatitis B envelope antigen (HBeAg)-positive chronic infection], immune active (IA, also termed HBeAg-positive chronic hepatitis), inactive carrier (IC, also termed HBeAg-negative chronic infection), and HBeAg-negative hepatitis (ENEG) phases[1-3]. The IT and IA phases manifest disparate degrees of liver injury, although viral replication is at extremely high levels in both clinical stages. Furthermore, the levels of serum hepatitis B surface antigen (HBsAg), HBV-DNA and intrahepatic covalently closed circular DNA (cccDNA) differ markedly across the four phases[4,5]. However, the underlying mechanisms or immune features distinguishing these phases are still obscure.
HBV is a non-cytopathic virus, and it is generally accepted that immune factors are indispensably involved in the occurrence and development of liver diseases. Among these, natural killer (NK) cells are greatly enriched in the liver, the site of HBV replication, and serve as an early warning system for viral infection[6]. Functional disturbance of NK cells has been described in chronic HBV infection, potentially contributing to ineffective antiviral immunity and the pathogenesis of liver injury. However, multiple studies regarding NK cell phenotype or function have revealed partially conflicting results[7-12]. For example, circulating NK cells from HBV-infected patients expressed higher levels of the activating receptors CD69, NKp46, NKp44[10] and lower levels of the inhibitory receptor KIR3DL1[11] than those from healthy individuals. However, others have reported that NK cells exhibited an inhibitory phenotype, with elevated expression of the inhibitory receptor NKG2A and downregulated expression of the activating receptors CD16 and NKp30 in chronic HBV infection[12]. This discrepancy may be attributed to the high heterogeneity of the disease profile in chronic HBV infection, but this interpretation remains to be verified.
HBV-specific T cell responses are vigorous, polyclonal and multi-specific in patients with resolved HBV infections[13], and their experimental depletion obviously delayed viral clearance in chimpanzee models[14]. On the contrary, virus-specific T cells displayed an exhausted phenotype with the upregulated expression of the inhibitory receptor programmed death 1 (PD-1) and Tim-3 and severely impaired antiviral ability in chronic HBV infection[6,15]. In a report from Webster et al[16], serum HBV-DNA levels of < 107 copies/mL appeared to be the threshold for consistent detection of circulating HBV-specific CD8+ T cells, suggesting that despite the overall suppression in chronic HBV infection, the hierarchy of HBV-specific T cell responses may also be influenced by virus replication and liver disease activity. It is not clear whether the magnitude of NK and T cell responses vary along the natural history of chronic HBV infection. The objective of this study was to analyze the characteristics of NK cells, nonantigen-specific and virus-specific T cells, which may define the different clinical phases.
This study was approved by the ethics committee of Huazhong University of Science and Technology and Tongji Hospital, and all participants provided informed consent. All procedures conformed to the Helsinki Declaration. Peripheral blood samples were obtained at the Infectious Disease Clinic of Tongji Hospital. All enrolled patients with HBV infections met the following criteria: HBsAg positive for at least 6 mo; absence of human immunodeficiency virus, hepatitis C virus (commonly known as HCV) or hepatitis D virus co-infections; without evidence of hepatic decompensation and hepatocellular carcinoma; absence of autoimmune disease, immunosuppressive agents, organ transplant or other comorbid illnesses that may impact immune response; absence of current antiviral therapy; without evidence for alcoholic hepatitis, nonalcoholic fatty liver disease and overt liver cirrhosis. In total, 92 cases of HBV-infected patients were enrolled in this study. The clinical phases were further assigned by at least two clinicians based on available history and laboratory results, using classification criteria described in clinical practice guidelines[1-3]. In addition, 23 healthy individuals were enrolled as the control group.
Liver functions were measured with routine automated techniques [normal upper limit for both alanine aminotransferase (ALT) and aspartate aminotransferase (AST): 40 U/L]. Serum HBsAg titers were quantified using the Architect HBsAg assay (Abbott Laboratories, Abbott Park, IL, United States; range 0.05–250 IU/mL). Serum HBeAg titers were quantified using AXSYM HBe 2.0 (Abbott Laboratories; range 0.15–100 PEIU/mL). Serial dilutions were performed when HBsAg or HBeAg titers exceeded the reference range. Serum HBV-DNA levels were detected by a standard generic HBV-DNA assay (ACO Biotech Co. Ltd, Hangzhou, China; detection limit: 500 copies/mL) or COBAS TaqMan HBV Test (Roche Molecular Systems, Meylan, France; detection limit: 20 IU/mL).
Peripheral blood mononuclear cells (PBMCs) from HBV-infected and healthy subjects were isolated by Ficoll-Hypaque density gradient centrifugation and were suspended in RPMI 1640 medium supplemented with 2 mM/L L-glutamine and 10% fetal calf serum (Hyclone, United States). For NK cell phenotypic analysis, freshly isolated PBMCs were stained with fluorochrome-conjugated antibodies to CD3-PerCP/Cy5.5, CD56-FITC, NKp46-PE, NKp30-APC, NKG2A-PE, NKG2D-APC, NKp44-PE, CD69-APC, CD4-FITC, CD8-FITC or isotype matched controls. All antibodies were purchased from Biolegend (San Jose, CA, United States) except NKG2A-PE (R and D Systems, Minneapolis, MN, United States). Finally, stained cells were acquired on Canto II flow cytometer (Becton Dickinson, United States) and analyzed using FlowJo analysis software (Treestar, Ashland, OR, United States).
PBMCs were stimulated with recombinant human IL-12 (10 ng/mL; Peprotech, United States) and IL-18 (50 ng/mL; SAB, United States) for 21 h at 37 ˚C, and then 2 µM/L monensin (MultiSciences, China) was added for the final 3 h. Cells were fixed and permeabilized, followed by intracellular staining for IFN-γ-APC (Biolegend, CA, United States).
NK cell cytotoxic activity was assessed by CD107a degranulation. Briefly, after overnight stimulation with recombinant human IL-12 (10 ng/mL) and IL-18 (50 ng/mL), or only culture medium, PBMCs were incubated for 3 h at 37 °C with K562 target cells (E:T = 5:1) in the presence of anti-CD107a-PE antibody (Biolegend, CA, United States) and monensin (2 µM). Cells were then labeled with CD3-PerCP/Cy5.5 and CD56-FITC before flow cytometry analysis.
PBMCs were incubated with 20 ng/mL phorbol myristate acetate plus 1 μg/mL ionomycin (MultiSciences, China) for 1 h, and then 2 µmol/L monensin was added for the final 4 h. Cells were stained with fluorochrome-conjugated antibodies to CD3-PerCP/Cy5.5, CD56-FITC or CD8-FITC (Biolegend, San Diego, CA, United States) at 4 ˚C in the dark. Afterwards, cells were fixed and permeabilized followed by intracellular staining for IFN-γ-PE, TNF-α-PE, IL-2-APC, perforin-APC and granzyme B-PE and isotype matched controls (IL-2-APC from eBioscience, United States; others from Biolegend, CA, United States) at 4 ˚C in the dark. Finally, cells were detected using a Canto II flow cytometer.
For HBV-specific T cell expansion, a panel of 15-mer peptides overlapping by ten residues were pooled in two mixtures covering core (35) and S (44) proteins (GenScript, United States). Briefly, frozen PBMCs were thawed and resuspended in AIM-V medium with 5% human AB serum (Gibco, Invitrogen, United States). Then, PBMCs were incubated with the core or S peptide pool, and recombinant IL-2 (20 IU/mL; Peprotech, United States) was replenished every 3 d. Finally, virus-specific T cell responses were measured by intracellular cytokine staining, as mentioned above, on day 10. Positive responses were determined when the frequency of T cells producing IFN-γ or IL-2 exceeded at least twice the proportion found in unstimulated cells and 0.1% of total T cells.
All data were analyzed with SPSS 13.0 for Windows (SPSS, Inc. Chicago, IL, United States). Serum HBV-DNA, HBeAg and HBsAg levels were log-transformed. Continuous variables are presented as mean ± SD. Baseline clinical characteristics across multiple groups were compared using the Kruskal-Wallis test for continuous variables and chi-square (χ2) test for categorical variables. Comparisons between two groups were performed with the Mann-Whitney U test. Correlations between variables were evaluated with the Spearman rank correlation test. P < 0.05 was considered statistically significant.
The demographic, biochemical and virologic data of study population are illustrated in Table 1. In accordance with the natural course, IT and IA patients were younger than IC and ENEG patients (P < 0.05). The levels of serum HBsAg and HBV-DNA decreased successively from the IT, IA, ENEG to IC phase. HBeAg titers were significantly higher in the IT phase than those in the IA phase (P < 0.05). Moreover, serum ALT and AST levels were markedly higher in IA and ENEG patients than those observed in IT, IC and healthy subjects.
| Parameters | HD, n = 23 | IT, n = 20 | IA, n = 27 | IC, n = 22 | ENEG, n = 18 | P value |
| Age, yr (Median, range) | 29 (22-39) | 25.5 (20-33) | 27 (18-36) | 33.5 (23-51) | 39.5 (26-56) | < 0.001 |
| Male/female | 13/10 | 8/12 | 15/12 | 10/12 | 11/7 | 0.653 |
| Log 10 HBV-DNA, copies/mL | negative | 7.88 ± 0.53 | 7.49 ± 0.83 | < 4a | 5.97 ± 0.80 | < 0.001b |
| Log 10 HBsAg, IU/mL | negative | 4.72 ± 0.19 | 4.34 ± 0.32 | 3.06 ± 0.49 | 3.66 ± 0.56 | < 0.001b |
| Log 10 HBeAg, PEIU/mL | negative | 2.89 ± 0.26 | 2.34 ± 0.56 | negative | negative | 0.001c |
| ALT, U/L | 21.7 ± 6.9 | 21.2 ± 7.0 | 132.3 ± 70.5 | 21.1 ± 7.1 | 137.8 ± 105.6 | < 0.001 |
| AST, U/L | 19.0 ± 6.5 | 18.9 ± 5.1 | 75.9 ± 41.3 | 22.0 ± 5.3 | 68.3 ± 37.2 | < 0.001 |
| Tbil, µmol/L | 14.9 ± 5.3 | 14.8 ± 6.8 | 14.8 ± 4.4 | 13.4 ± 4.1 | 12. 7 ± 2.9 | 0.501 |
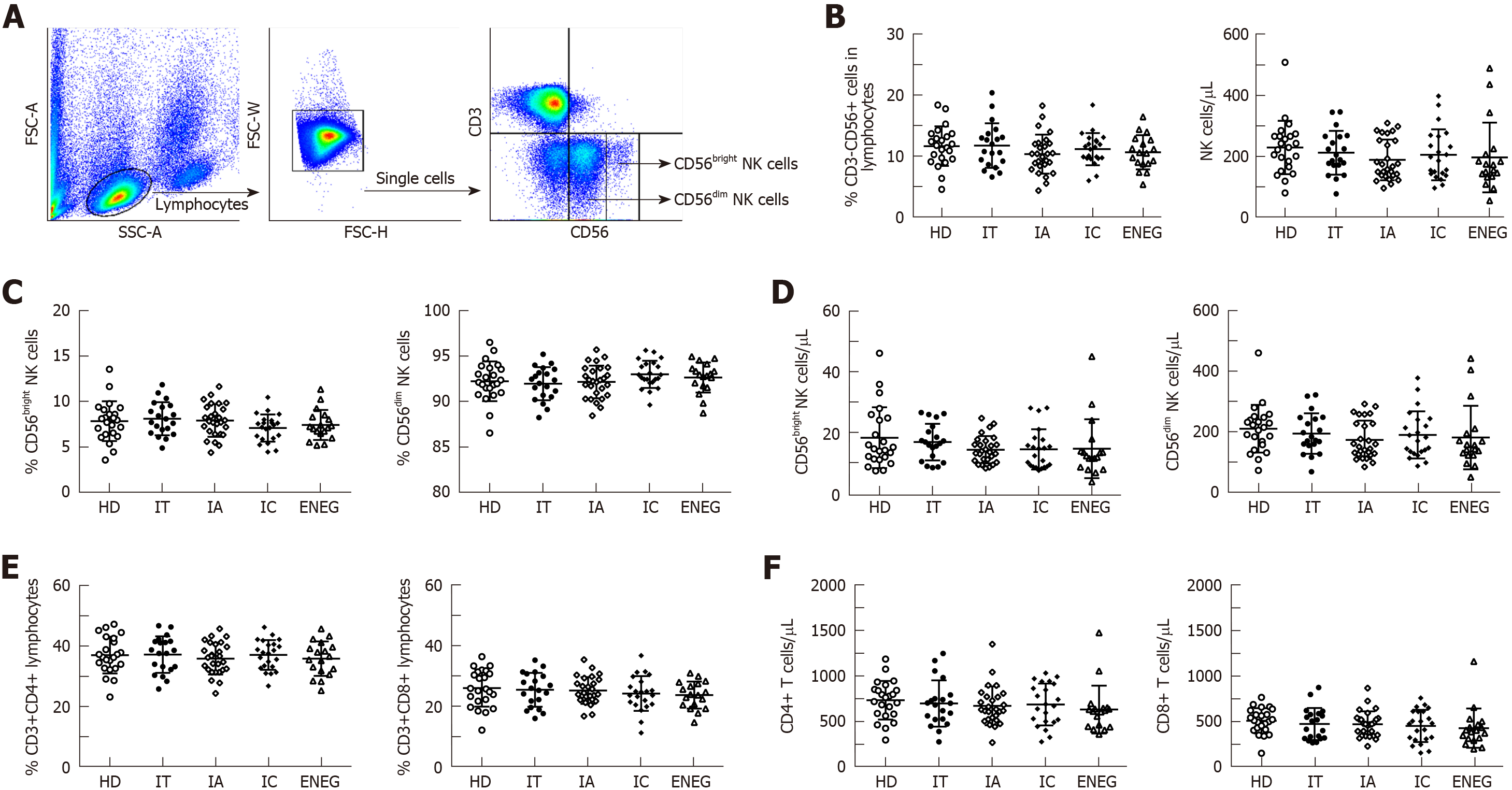
As shown in Figure 1, the proportion and absolute number of circulating CD3-CD56+ NK cells, CD56bright NK cells, CD56dim NK cells, global CD4+ and CD8+ T cells were similar in healthy donors (HD) and patients with different clinical phases, suggesting that direct measurement of NK and T cell frequencies and numbers did not provide distinct immune signatures for the clinical phases.
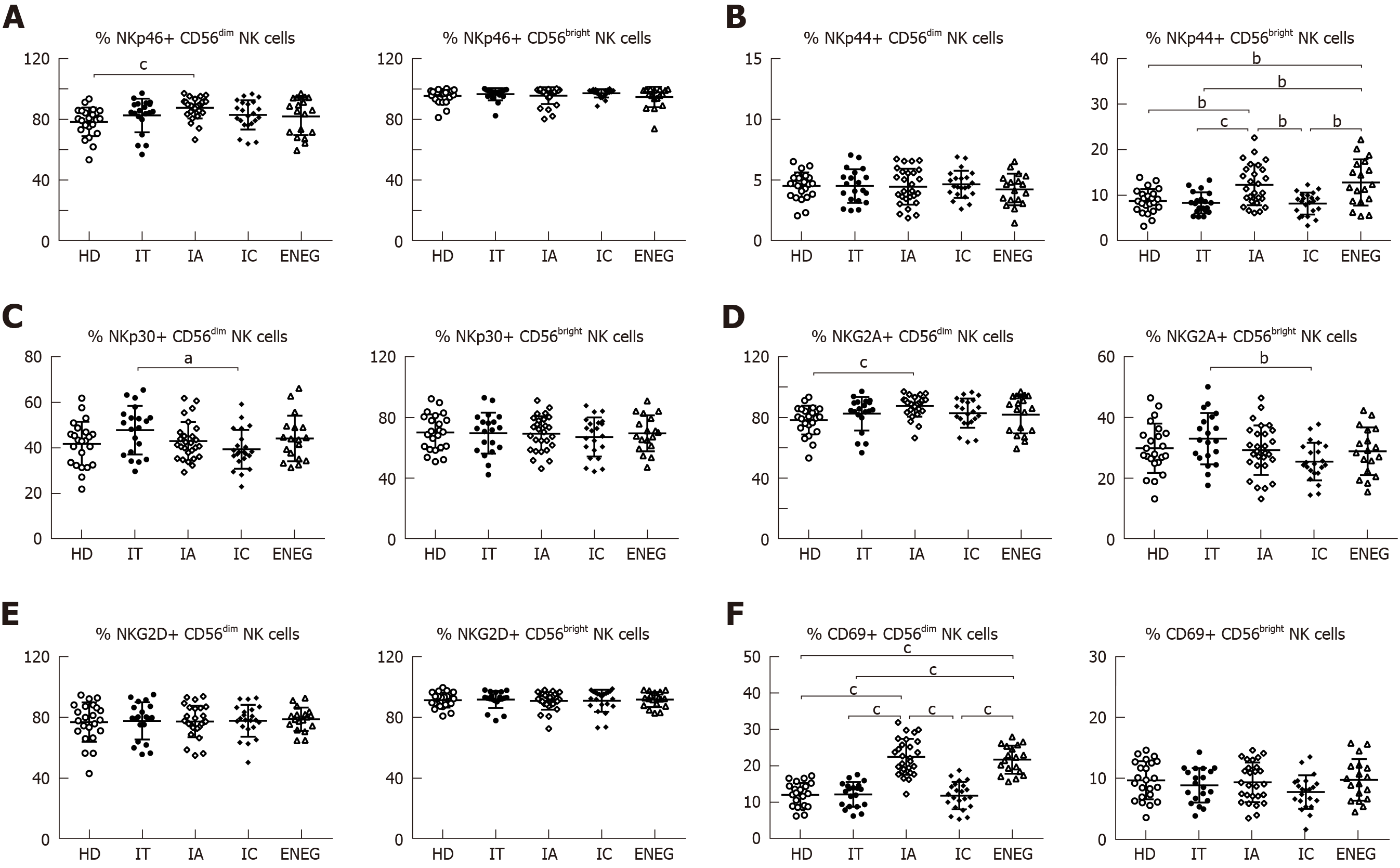
The effects of different clinical phases on NK cell phenotypes were investigated (Figure 2A-F). Compared with HD, NKG2A expression in CD56dim NK cells was downregulated in the IC phase (P < 0.05), while NKp46 expression in CD56dim NK cells was upregulated in the IA phase (P < 0.05). NKp30 expression in CD56dim NK cells and NKG2A expression in CD56bright NK cells were higher in the IT phase than the IC phase (P < 0.05). Furthermore, the frequencies of CD56bright NK cells expressing NKp44 and CD56dim NK cells expressing CD69 were increased in the IA and ENEG phases in comparison to those in the IT, IC and healthy subjects (P < 0.05).
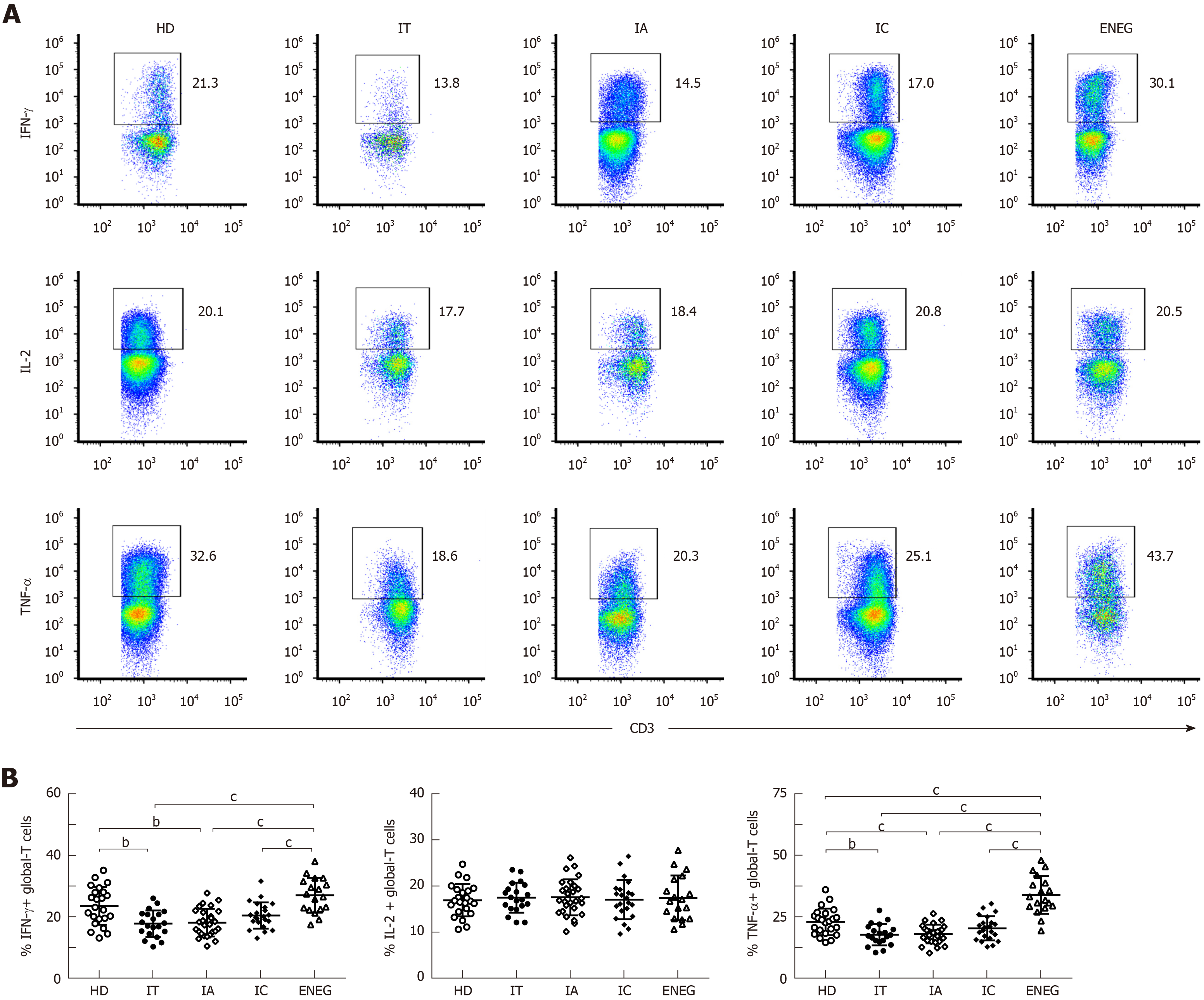
As shown in Figure 3A and B, compared with HD, the ability of IFN-γ-producing NK cells was markedly attenuated in chronic HBV-infected patients, irrespective of any clinical phases. Of note, IFN-γ production was less compromised in the IC phase than in the IT and ENEG phases (P < 0.05). Patients in the IT and IA phases also displayed significantly lower TNF-α production compared to HD (P < 0.05). Cytolytic activity was measured by CD107a degranulation after stimulation with IL-12+18 and K562 target cells and the expression of perforin and granzyme B in NK cells (Figure 3A, C and D). However, NK cell cytolytic activity was retained in chronic HBV-infected patients compared with HD, and no significant difference in CD107a, perforin or granzyme B expression was observed between the four phases (P > 0.05).
The frequencies of global-T cells producing IFN-γ, IL-2 and TNF-α are shown in Figure 4. Compared with HD, the ability of global T cells to produce IFN-γ and TNF-α was significantly decreased in the IT and IA phase (Figure 4B and D). On the contrary, IFN-γ and TNF-α production by global-T cells was dramatically increased in the ENEG phase compared to the other clinical phases, and TNF-α expression was even higher than that in HD. No statistical difference was observed for IL-2 expression in HD and patients at different clinical stages (Figure 4C).
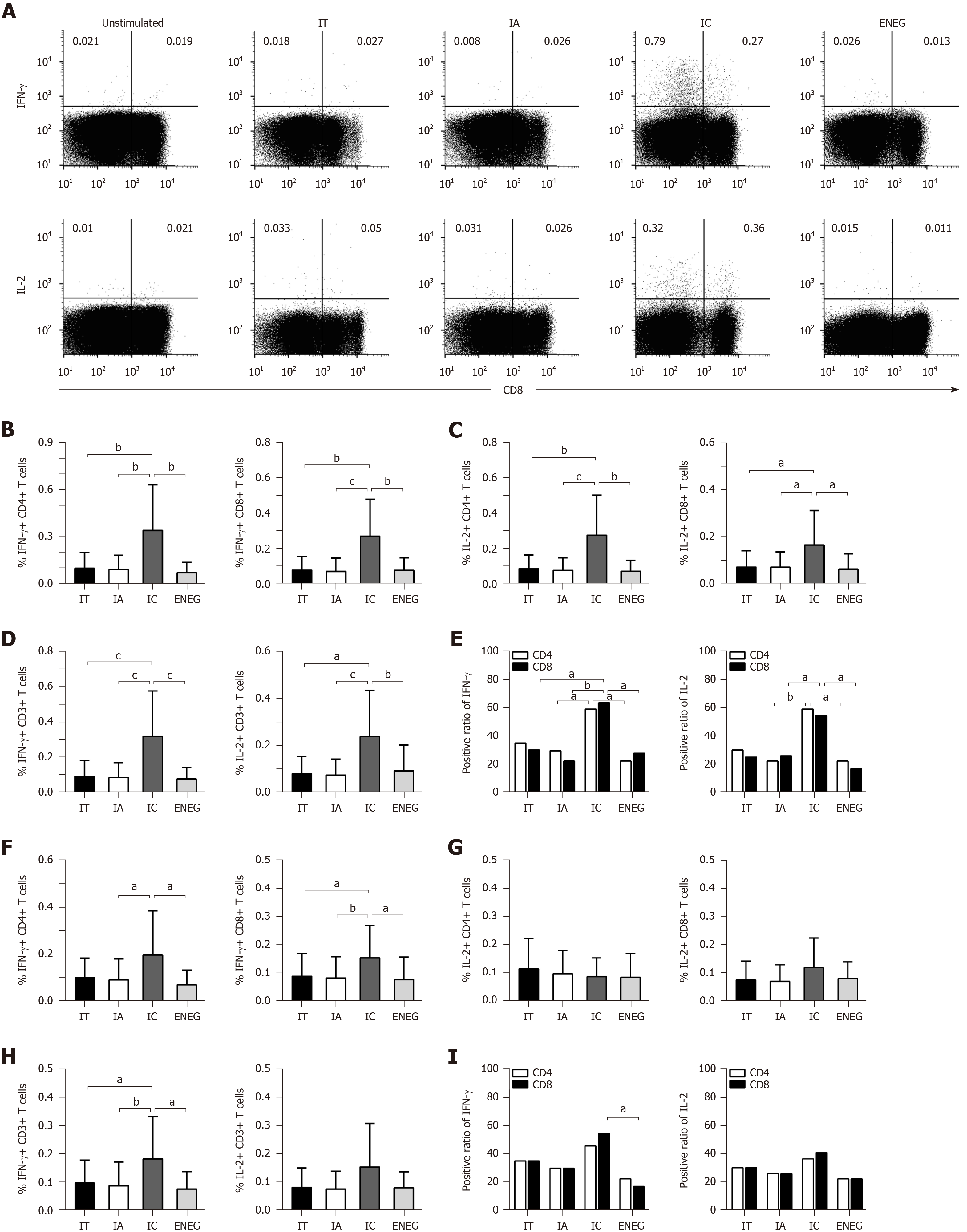
PBMCs were stimulated by HBV core or S peptide pools. After 10 d in vitro expansion, virus-specific T cell responses were determined by detecting the frequency of T cells producing IFN-γ or IL-2. In response to the core peptide pool, IFN-γ and IL-2 production by CD4+, CD8+ and CD3+ T cells significantly increased in the IC phase than in other phases (Figure 5A-D). The positive responses of IFN-γ and IL-2 in both CD4+ and CD8+ T cells were significantly higher in the IC phase than in the IA and ENEG phases, and the positive response of CD8+ T cells producing IFN-γ was significantly higher in the IC phase than in the IA phase (Figure 5E). In response to the S peptide pool (Figure 5F-I), IFN-γ production by CD8+ and CD3+ T cells was significantly increased in the IC phase than those in other phases. IFN-γ production by CD4+ T cells was significantly increased in the IC phase than in the IA and ENEG phases. IL-2 production by CD4+, CD8+ and CD3+ T cells did not significantly differ across the four phases. In addition, the positive response of CD8+ T cells-producing IFN-γ was higher in the IC phase than in the ENEG phase.
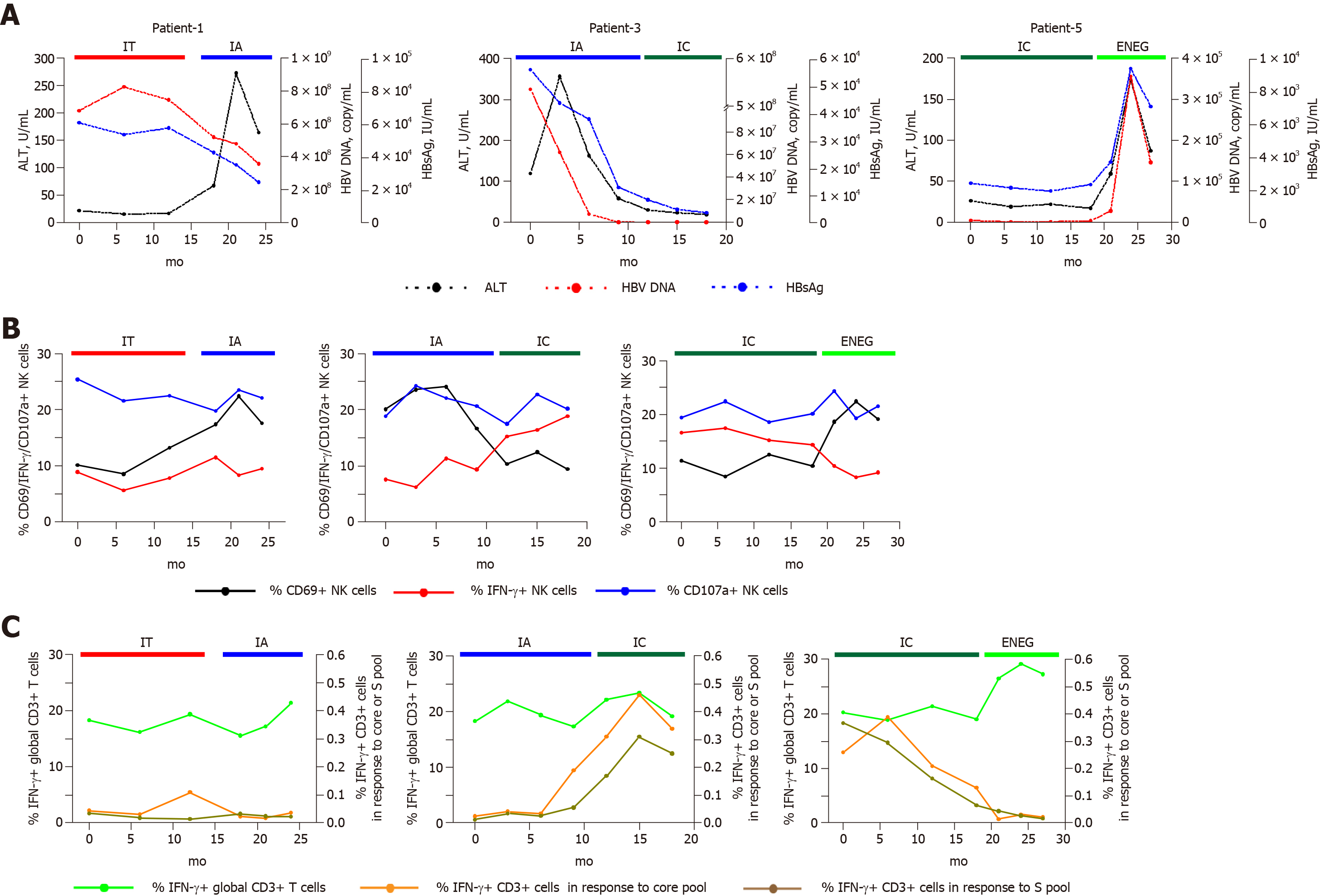
We identified several individuals who transitioned from one disease phase to another. As shown in Figure 6, IFN-γ production from NK and global-T cells was relatively stable in a patient that transitioned from the IT to IA phase, and the frequency of CD3+ T cells-producing IFN-γ in response to the core or S peptide pool remained low. In contrast, core and S-specific T cell responses improved in a patient who experienced spontaneous HBeAg clearance (from IA to IC phase). When virus reactivation occurred (from IC to ENEG phase), NK cell IFN-γ production and HBV-specific T cell responses were prominently inhibited, but IFN-γ production from global T cells was enhanced. These longitudinal findings were consistent with results from the cross-sectional analysis.
Chronic HBV infection is a dynamic state of complex equilibrium among virus, hepatocytes and host immune cells[13]. In previous reports and this study[4,17], the levels of serum HBsAg, HBV-DNA and intrahepatic cccDNA markedly differ across four phases. In accordance with this, our study further emphasized the distinct immune response pattern in different clinical phases.
Firstly, our study demonstrated that certain NK cell receptors are differentially expressed among the different clinical phases. Although disease progression and HBV replication status are relatively stable in both IT and IC phases, NKp30 expression in CD56dim NK cells and NKG2A expression in CD56bright NK cells were significantly higher in the high viremia IT phase than in the IC phase. Yoshioka et al[18] reported that the frequency of the NKp46highNKG2Ahigh subset was higher in high viremia patients, and this subset exhibited higher cytotoxicity but lower IFN-γ production. Similarly, simultaneous up-regulation of NKp30 and NKG2A expression may lead to more skewed NK cell function in the IT phase. In agreement with reports by Zhang et al[8] and Ghosh et al[10], our study found that NK cells were relatively activated in the IA and ENEG phases, as evidenced by the upregulated expression of NKp44 in CD56bright NK cells and CD69 in CD56dim NK cells. IA and ENEG phases were characterized by the fluctuation of ALT levels and liver disease progression, suggesting that NK cells played an important role in hepatitis activity.
Secondly, the ability of IFN-γ-producing NK cells was markedly impaired in HBV-infected patients, regardless of clinical stage. Notably, IFN-γ production was less compromised in the IC phase than in the IT and ENEG phases. Meanwhile, TNF-α production was inhibited in the IT and IA phases compared to HD. The effector function of NK cells is regulated by the balance of activating and inhibitory receptors, as well as cytokine milieu. Lower NKG2A expression in the IC phase may facilitate the restoration of cytokine production in NK cells. Moreover, circulating concentrations of IL-10 and TGF-β were increased in patients with active and immunotolerant phases, respectively[7,19], which represented a feedback mechanism that limits exuberant NK cell response but profoundly suppresses IFN-γ production. What’s more, the decrease in virus load may in turn have improved NK cell activity, which has been demonstrated in HCV patients receiving direct-acting antiviral therapy[20]. However, NK cell cytotoxicity was not correspondingly weakened and did not differ among the four clinical phases. Our results were in line with the report of Oliviero et al[11], in which NK cells were functionally dichotomous. This phenomenon may be attributed to the preferential pSTAT1-dependent cytotoxicity over pSTAT4-dependent IFN-γ production when exposed to endogenous IFN-α[6].
Subsequently, upregulated, downregulated or unchanged global-T cell responses were observed in different phases. Global-T cells primarily refer to nonantigen-specific T cell subsets, considering that the quantity of HBV-specific T cells is negligible in comparison to global-T cells. IT and IA phases were characterized by impaired nonantigen-specific T cell responses. In contrast, global-T cells from the ENEG phase displayed a proinflammatory cytokine profile with upregulated IFN-γ and TNF-α expression, which is relevant to the severity of liver injury (data not shown). IFN-γ and TNF-α had the potential to inhibit HBV replication and degrade cccDNA in a non-cytolytic fashion[21]. Nevertheless, intrahepatic proinflammatory IFN-γ and TNF-α overexpression by global-T cells significantly correlated with immune pathogenesis in HBV-associated liver failure[22].
Finally, core and S antigen-specific T cell responses after in vitro expansion were significantly stronger in the IC phase than in other phases. Our findings are in accordance with a report by Liang et al[23], in which the magnitude of core or S antigen-specific CD4+ and CD8+ T cell responses was significantly more intense in the IC phase relative to the IA phase. However, ex vivo lymphoproliferation response and ELISPOT assays showed that the pattern of antiviral T-cell responses didn’t clearly distinguish between the different clinical phases[24]. This discrepant result between ex vivo and in vitro experiment was also described in patients who achieved HBsAg loss after nucleos(t)ide analogue therapy[25]. We speculate that in vitro expansion may render some ex vivo T-cell hyporesponsiveness into effective T-cell responses. Given that persistent exposure to high titers of viral antigens may be a primary factor for the frequency decline and functional exhaustion of virus-specific T cells[26], HBsAg decrease may facilitate the functional reconstruction of virus-specific T cells in the IC phase. Similarly, HBsAg loss after nucleos(t)ide analogue therapy was significantly associated with the improved virus-specific T cell responses[25]. However, HBV-specific T cells played an indispensable role in clearing HBsAg, thereby accelerating the transition to a more stable disease state.
Our study found that both NK and T cell functions were repressed in the IT and IA phases. In a study of the peripheral blood transcriptome, B-cell related and interferon-stimulated genes were significantly upregulated in IT and IA phases[27]. Given this, IT and IA phases may be primarily dominated by immune types other than NK and T cells, such as type I IFN and B cell responses. With the spontaneous clearance of HBeAg and HBsAg decline, NK and T cells played a more important role in immune pathogenesis of chronic hepatitis B. In particular, NK cell cytokine production and HBV-specific T cell responses were partially restored in the IC phase, which may be associated with viral control in this low-replication phase. When virus reactivation occurred, NK and virus-specific T cell responses were inhibited, again likely due to the increased exposure to antigenic stimuli and immunosuppressive cytokines, but nonantigen-specific T cell responses were simultaneously activated. Elevated nonantigen-specific T responses may facilitate viral clearance to some extent, although not as effective as HBV-specific T responses, but concurrently aggravate liver inflammation by secreting the proinflammatory cytokines IFN-γ and TNF-α[28]. Our findings countered the previous opinion that innate and adaptive responses were synchronously inhibited or enhanced, which may result from the regulatory mechanisms underlying them. Functional activation of nonantigen-specific T cells is required to trigger factors other than excessive antigenic drive via MHC/peptide interactions. Putative factors affecting nonantigen-specific T cells included the bystander pathway, cytokine milieu, depletion of essential nutrients and accumulation of toxic metabolites[28]. But for virus-specific T cell responses, the fluctuation of antigen titers with the natural history disease progression may have a direct influence on HBV-specific T cells. In addition, virus replication and hepatitis activity affected the levels of regulatory T cells, arginine, suppressive cytokines-IL-10 and TGF-β, and suppressive surface receptors-PD-1 and Tim-3, which may further mediate the intensity of virus-specific T cell responses[6,29].
A limitation of our study is the lack of access to hepatic tissue samples. Therefore, we cannot exclude the local activation or inhibition of NK and T cell responses compartmentalized within the liver. It has been previously reported that although intrahepatic NK cells were more activated and HBV-specific CD8+ T cells displayed a more exhausted phenotype than their peripheral counterparts, the immunologic changes in peripheral blood could closely mirror those in the liver[8,30]. Another caveat to our study is that we cannot follow-up with an individual through the whole natural disease progression. However, we identified several individuals who experienced a transition from one phase to another, which may reflect the dynamic change of NK and T cell responses.
In conclusion, our findings demonstrate the conversion of immune response patterns along the natural course of chronic HBV infection. NK cells were phenotypically activated in clinical phases (IA and ENEG) with biochemical liver damage. NK, non-specific and virus-specific T cells were functionally impaired in the IT and IA phases. With the spontaneous clearance of HBeAg and HBsAg decline, NK cell cytokine production and HBV-specific T responses were partially restored in the IC phase, and the ENEG phase was primarily dominated by nonantigen-specific T cell responses. Our results highlight the complicated roles of NK and T cells, which were extremely helpful in understanding the immune status underlying different clinical stages and high heterogeneity of disease profile in chronic HBV infection.
Chronic hepatitis B is a highly heterogeneous disease, which can be divided into four phases: Immune tolerant (IT), immune active (IA), inactive carrier (IC) and hepatitis B envelope antigen (HBeAg)-negative hepatitis (ENEG). Accordingly, clinical manifestations and disease progression markedly differ among these phases. Natural killer (NK) and hepatitis B virus (HBV)-specific T cells are two key effectors of cellular immunity, which play an important role in mediating liver damage and inhibiting viral replication. Therefore, the identification of immune features in clinical phases are helpful in better understanding the highly heterogeneous disease state of chronic HBV infection. However, the associations between host immunity and clinical phases are obscure.
The motivation of this study is to explore the immunological mechanisms underlying different clinical phases.
The objective of this study is to analyze the features of NK cells, nonantigen-specific and HBV-specific T cells in different clinical phases.
The frequency, subpopulation and phenotype of circulating NK cells were detected by flow cytometry through direct surface staining. NK cell functions, including cytokine production and cytotoxic activity, and nonantigen-specific T cell responses were measured by flow cytometry after stimulant incubation and intracellular staining. For HBV-specific T-cell responses, PBMCs were stimulated by HBV core or S peptide pools. After 10 d of in vitro expansion, HBV-specific T cell responses were determined by detecting the frequency of T cells producing IFN-γ or IL-2. In addition, clinical phases were assigned according to the available history and laboratory results, using classification criteria described in clinical practice guidelines.
NK cells were phenotypically activated in the clinical phases (IA and ENEG) with biochemical liver damage. Both NK and T cells were functionally impaired in the IT and IA phases. With the spontaneous clearance of HBeAg and hepatitis B surface antigen decline, NK cell cytokine production and HBV-specific T responses were partially restored in the IC phase, and ENEG phase was primarily dominated by nonantigen-specific T cell responses.
In the cross-sectional analysis and longitudinal observation from several representative individuals, our findings depicted the shift of immune response pattern along the natural history of chronic HBV infection. Importantly, NK cells, nonantigen-specific and HBV-specific T cells may play a distinct role in different clinical phases. Our findings countered the previous opinion from the single perspective that innate and adaptive responses were synchronously inhibited or enhanced in some disease status. Our results emphasized the complicated roles of NK and T cells, which were extremely helpful in understanding the immunological mechanisms underlying different clinical stages in chronic HBV infection. Furthermore, the better understanding of immune features in different phases will lead towards individualized therapy.
Future research should be conducted to investigate the regulatory mechanisms of NK cells, nonantigen-specific and HBV-specific T cells in distinct phases of chronic HBV infection. In the future, virologic and immunological mechanisms under distinct phases will still be one of the most focused issues due to the highly heterogeneous disease profile until chronic HBV infection is completely solved. Virologic factors, such HBV-RNA, HBcAg, cccDNA and gene variation, and host factors, such as hepatocyte clone and other types of immune responses, are other perspectives for future research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Allam N, Ciccone MM, Dourakis SP S-Editor: Yan JP L-Editor: Filipodia E-Editor: Song H
| 1. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 3. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2844] [Article Influence: 406.3] [Reference Citation Analysis (0)] |
| 4. | Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE 4th, Xiong S, Brosgart CL, Chen SS, Gibbs CS, Zoulim F. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 734] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 5. | Zeng LY, Lian JS, Chen JY, Jia HY, Zhang YM, Xiang DR, Yu L, Hu JH, Lu YF, Zheng L, Li LJ, Yang YD. Hepatitis B surface antigen levels during natural history of chronic hepatitis B: A Chinese perspective study. World J Gastroenterol. 2014;20:9178-9184. [PubMed] |
| 6. | Rehermann B. Pathogenesis of chronic viral hepatitis: Differential roles of T cells and NK cells. Nat Med. 2013;19:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 7. | Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, Ellis G, Khanna P, Dusheiko G, Gilson RJ, Maini MK. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Zhang Z, Zhang S, Zou Z, Shi J, Zhao J, Fan R, Qin E, Li B, Li Z, Xu X, Fu J, Zhang J, Gao B, Tian Z, Wang FS. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology. 2011;53:73-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Li Y, Wang JJ, Gao S, Liu Q, Bai J, Zhao XQ, Hao YH, Ding HH, Zhu F, Yang DL, Zhao XP. Decreased peripheral natural killer cells activity in the immune activated stage of chronic hepatitis B. PLoS One. 2014;9:e86927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Ghosh S, Nandi M, Pal S, Mukhopadhyay D, Chakraborty BC, Khatun M, Bhowmick D, Mondal RK, Das S, Das K, Ghosh R, Banerjee S, Santra A, Chatterjee M, Chowdhury A, Datta S. Natural killer cells contribute to hepatic injury and help in viral persistence during progression of hepatitis B e-antigen-negative chronic hepatitis B virus infection. Clin Microbiol Infect. 2016;22:733.e9-733.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151-1160, 1160.e1-1160.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 12. | Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 13. | Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 764] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 15. | Tan AT, Koh S, Goh V, Bertoletti A. Understanding the immunopathogenesis of chronic hepatitis B virus: An Asian prospective. J Gastroenterol Hepatol. 2008;23:833-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (3)] |
| 16. | Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: Implications for immunotherapy. J Virol. 2004;78:5707-5719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 330] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 17. | Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: A perspective on Asia. J Hepatol. 2010;52:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 18. | Yoshioka T, Tatsumi T, Miyagi T, Mukai K, Nishio K, Nishio A, Yokoyama Y, Suda T, Kegasawa T, Shigekawa M, Hikita H, Sakamori R, Takehara T. Frequency and role of NKp46 and NKG2A in hepatitis B virus infection. PLoS One. 2017;12:e0174103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Wang XX, Luo BF, Jiang HJ, Cong X, Jin Q, Ma DL, Wei L, Feng B. Recovery of natural killer cells is mainly in post-treatment period in chronic hepatitis C patients treated with sofosbuvir plus ledipasvir. World J Gastroenterol. 2018;24:4554-4564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng X, Zhang K, Chou WM, Wettengel JM, Malo A, Bohne F, Hoffmann D, Eyer F, Thimme R, Falk CS, Thasler WE, Heikenwalder M, Protzer U. Interferon-γ and Tumor Necrosis Factor-α Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016;150:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 22. | Zou Z, Li B, Xu D, Zhang Z, Zhao JM, Zhou G, Sun Y, Huang L, Fu J, Yang Y, Jin L, Zhang W, Zhao J, Sun Y, Xin S, Wang FS. Imbalanced intrahepatic cytokine expression of interferon-gamma, tumor necrosis factor-alpha, and interleukin-10 in patients with acute-on-chronic liver failure associated with hepatitis B virus infection. J Clin Gastroenterol. 2009;43:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Liang M, Ma S, Hu X, Zhou B, Zhang J, Chen J, Wang Z, Sun J, Zhu X, Abbott W, Hou J. Cellular immune responses in patients with hepatitis B surface antigen seroclearance induced by antiviral therapy. Virol J. 2011;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N, Lau DT, Kim WR, Smith C, Carithers RL, Torrey KW, Keith JW, Levine DL, Traum D, Ho S, Valiga ME, Johnson GS, Doo E, Lok AS, Chang KM; Hepatitis B Research Network. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology. 2016;150:684-695.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 25. | Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G, Colombo M, Missale G, Ferrari C. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963-973.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 26. | Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2009;106:8623-8628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 27. | Vanwolleghem T, Hou J, van Oord G, Andeweg AC, Osterhaus AD, Pas SD, Janssen HL, Boonstra A. Re-evaluation of hepatitis B virus clinical phases by systems biology identifies unappreciated roles for the innate immune response and B cells. Hepatology. 2015;62:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT, Alexander G, Finney H, Lawson A, Plunkett FJ, Bertoletti A, Akbar AN, Maini MK. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med. 2008;205:2111-2124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Croagh CM, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol. 2014;20:10395-10404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 30. | Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G, Ferrari C. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682-693, 693.e1-693.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |