Published online Dec 21, 2018. doi: 10.3748/wjg.v24.i47.5297
Peer-review started: October 19, 2018
First decision: November 22, 2018
Revised: November 27, 2018
Accepted: November 30, 2018
Article in press: November 30, 2018
Published online: December 21, 2018
Processing time: 63 Days and 7.8 Hours
Hepatitis C virus (HCV) infection commonly causes progressive liver diseases that deteriorate from chronic inflammation to fibrosis, cirrhosis and even to hepatocellular carcinoma. A long-term, persistent and uncontrolled inflammatory response is a hallmark of these diseases and further leads to hepatic injury and more severe disease progression. The levels of inflammatory cytokines and chemokines change with the states of infection and treatment, and therefore, they may serve as candidate biomarkers for disease progression and therapeutic effects. The mechanisms of HCV-induced inflammation involve classic pathogen pattern recognition, inflammasome activation, intrahepatic inflammatory cascade response, and oxidative and endoplasmic reticulum stress. Direct-acting antivirals (DAAs) are the first-choice therapy for effectively eliminating HCV, but DAAs alone are not sufficient to block the uncontrolled inflammation and severe liver injury in HCV-infected individuals. Some patients who achieve a sustained virologic response after DAA therapy are still at a long-term risk for progression to liver cirrhosis and hepatocellular carcinoma. Therefore, coupling with anti-inflammatory/hepatoprotective agents with anti-HCV effects is a promising therapeutic regimen for these patients during or after treatment with DAAs. In this review, we discuss the relationship between inflammatory mediators and HCV infection, summarize the mechanisms of HCV-induced inflammation, and describe the potential roles of anti-inflammatory/hepatoprotective drugs with anti-HCV activity in the treatment of advanced HCV infection.
Core tip: Inflammatory responses triggered by hepatitis C virus (HCV) infection lead to severe progressive liver diseases. Some inflammatory cytokines and chemokines may serve as biomarkers for the disease progression and therapeutic effect in chronic hepatitis C (CHC) patients. The inflammatory pathogenesis in HCV-infected patients is complicated, including classic pathogen pattern recognition, inflammasome activation, intrahepatic inflammatory cascade response, and oxidative and endoplasmic reticulum stress. Direct-acting antivirals (DAAs) are not sufficient to block the uncontrolled inflammation and disease progression in severe CHC patients. Therefore, coupling with anti-inflammatory/hepatoprotective agents with anti-HCV effects is a promising therapeutic regimen for advanced HCV-infected patients during or after treatment with DAAs.
- Citation: Li H, Huang MH, Jiang JD, Peng ZG. Hepatitis C: From inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J Gastroenterol 2018; 24(47): 5297-5311
- URL: https://www.wjgnet.com/1007-9327/full/v24/i47/5297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i47.5297
Hepatitis C virus (HCV) belongs to the genus Hepacivirus in the family Flaviviridae and is a positive single-stranded RNA virus that is approximately 9.6 kb. The HCV genome encodes three structural (Core, E1 and E2) and seven non-structural (NS) proteins (NS1 or P7, NS2, NS3, NS4A, NS4B, NS5A and NS5B)[1]. As a kind of hepatic tropism virus, HCV mainly replicates in the hepatocyte cytoplasm and frequently causes acute or chronic hepatitis C (CHC), which has an estimated prevalence of 71 million people and is responsible for approximately 399000 deaths annually[1,2]. CHC patients generally experience liver diseases ranging from liver fibrosis and cirrhosis to hepatocellular carcinoma (HCC) and suffer from metabolic disorders such as lipid abnormalities, steatosis, insulin resistance, and iron load dysregulation[3]. These abnormalities are aggravated by long-term hepatic inflammatory responses. Upon HCV infection, the immune responses in the liver are initiated by parenchymal cells (hepatocytes), non-parenchymal liver cells [Kupffer cells (KCs) and hepatic stellate cells (HSCs)] and immune cells (macrophages, mast cells, dendritic cells and natural killer cells) recruited to the liver, resulting in the spontaneous elimination of acute HCV infection[4]. However, in 70%-80% of cases, the immune responses fail to eliminate the virus during the acute phase, leading to chronic infection[4]. Persistent HCV replication in hepatocytes leads to uncontrolled inflammation and chemokine production. The excessive cytokines, as inflammatory agents, further cause inflammation in the liver, which eventually exacerbates tissue damage and liver disease progression[5].
Direct antiviral treatment is undoubtedly the first choice for the treatment of HCV infection. Currently, several direct-acting antivirals (DAAs) have been approved for clinical use, including NS3/4A, NS5A and NS5B inhibitors and fixed-dose combined agents[6]. The combinational use of these DAAs has become the standard treatment regimen for the treatment of HCV infection, which greatly improves sustained virologic response (SVR) rates to over 90%, shortens the treatment duration and reduces adverse effects, when compared with the traditional interferon (IFN) plus ribavirin treatment[6]. However, HCV is just the initiator for pathophysiological processes, while persistent inflammatory cytokine storms (known as hypercytokinaemia) and HCV-induced hepatocyte damage exacerbate the progression of severe liver diseases[7-10]. DAAs primarily control viral replication but are not sufficient to restore HCV-induced liver dyshomeostasis and advanced liver diseases. Clinically, there are different conclusions about the contribution of current DAA therapy to reducing cirrhosis and HCC, and a subset of patients are still subjected to the risk of cirrhosis, HCC and liver failure even after achieving an SVR[6,7,11]. Given these limitations of DAA therapy, anti-inflammatory and hepatoprotective drugs with anti-HCV effects become a good choice for those individuals. These drugs have advantages in suppressing inflammation/oxidative stress, reducing hepatocyte injury and alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels and preventing the development of liver fibrosis[12]. Therefore, although anti-inflammatory/hepatoprotective drugs would not replace DAAs, they can be used as a supplement to DAA therapy for preventing HCV relapse and liver disease progression during or after DAA therapy.
Most inflammatory cytokine and chemokine levels are positively correlated with the HCV load and decline after antiviral therapy. Although the role of inflammatory mediators in HCV infection after treatment with DAAs has not received much attention, previous research still reported that one or more of these inflammatory mediators might serve as inflammatory biomarkers in CHC patients (Table 1). For instance, Costantini et al[13,14] revealed that several serum mediators, including interleukin (IL)-6 and IL-8, C-X-C motif ligand (CXCL)-9, CXCL-10, CXCL-12 and macrophage migration inhibitory factor (MIF), might be used as potential markers for evaluating the progression of chronic hepatitis to cirrhosis. Hepatic tumor necrosis factor (TNF)-α was associated with increased inflammatory activity, hepatic fibrosis and liver injury in CHC[15-17]. Meanwhile, TNF-α has been identified as the key molecule promoting the development of insulin resistance and diabetes during HCV infection, and patients with severe liver disease and HCC have a higher TNF-α/IL-10 ratio[18,19]. In patients co-infected with HCV and hepatitis B virus (HBV), plasma IL-6 concentrations were positively correlated with illness duration and viral load, whereas the IL-18 concentration was positively correlated with ALT and AST levels and might evaluate the effect of IFN on the immune state[20,21]. The above results suggest that the level of inflammatory factors in hepatitis C patients might be used as a reference for disease progression during HCV infection and antiviral therapy.
| Biomarker | Clinical relevance | Ref. |
| TNF-α | Promoting development of insulin resistance and diabetes during HCV infection; Hepatic TNF-α was associated with increased inflammatory activity, hepatic fibrosis, liver injury and HCC | [15-19] |
| IL-6 | Evaluating the progression of CHC to cirrhosis; Plasma IL-6 positively correlated with illness duration and viral load in HBV/HCV co-infected patients | [13,20] |
| IL-8 | Associated with interferon therapy non-response and high histologic activities in CHC patients | [29,30] |
| IL-18 | Plasma IL-18 concentration was positively correlated with ALT and AST levels in HBV/HCV co-infected patients; A marker for evaluating the effect of IFN on the immune state | [20,21] |
| CXCL-9 | Potential marker for evaluating the progression of CHC to cirrhosis | [13,14] |
| CXCL-10 | CXCL-10 level in hepatocytes correlated with histological severity and hepatic lobule inflammation; A marker of viral response and therapeutic outcome | [5,13,14,23,24] |
Chemokines play a more extensive role in the pathogenesis of CHC-related liver diseases and are even considered markers and therapeutic targets in CHC[22]. Among the various chemokines, human IFN-induced protein (CXCL-10) is the most widely studied. In CHC patients, the levels of peripheral blood and liver CXCL-10, along with C-C motif ligand (CCL)-5, IFN-inducible T cell α chemo attractant (I-TAC) and macrophage inflammatory protein (MIP)-1α/1β, were increased markedly[5,23], whereas the level of CXCL-10 in hepatocytes was correlated with histological severity and hepatic lobule inflammation[14,24,25]. CXCL-10 also serves as a marker of viral response and therapeutic outcome since a high pretreatment level of CXCL-10 indicated the inhibition of CXC chemokine receptor (CXCR) 3-expressing T cell response, which leads to therapeutic non-responses[5,23]. This phenomenon is also confirmed in the following cases where the virus was controlled during treatment with an increased number of CD8+ cells expressing high CXCR3 levels[26]. In addition, monocyte chemotactic protein-1 (MCP-1), soluble adhesion molecule (sAM), CCL-20 and CXCL-9 were reported to predict the outcome of antiviral therapy in CHC patients[27,28]. Chemokine IL-8 was also induced by HCV, and patients who were biochemical non-responders to IFN therapy had higher pretreatment levels of IL-8 or high histologic activities[29,30]. Therefore, chemokine levels are important reference values for monitoring the natural course and progression of HCV-related liver diseases and even identifying different treatment response rates before treatment[5].
However, these studies investigating the utility of inflammatory cytokines and chemokines for the prediction of treatment responses were based only on a small number of patients infected with limited HCV subtypes, most were based on IFN or IFN plus ribavirin therapy regimens, and the results did not preclude an epiphenomenon associated with the effects of IFN[23,27]. Therefore, the use of inflammatory cytokines and chemokines as indicators of the pathological progression of HCV infection and treatment efficacy has yet to be supported by more data from larger multivariate studies of patients, especially those treated with DAAs.
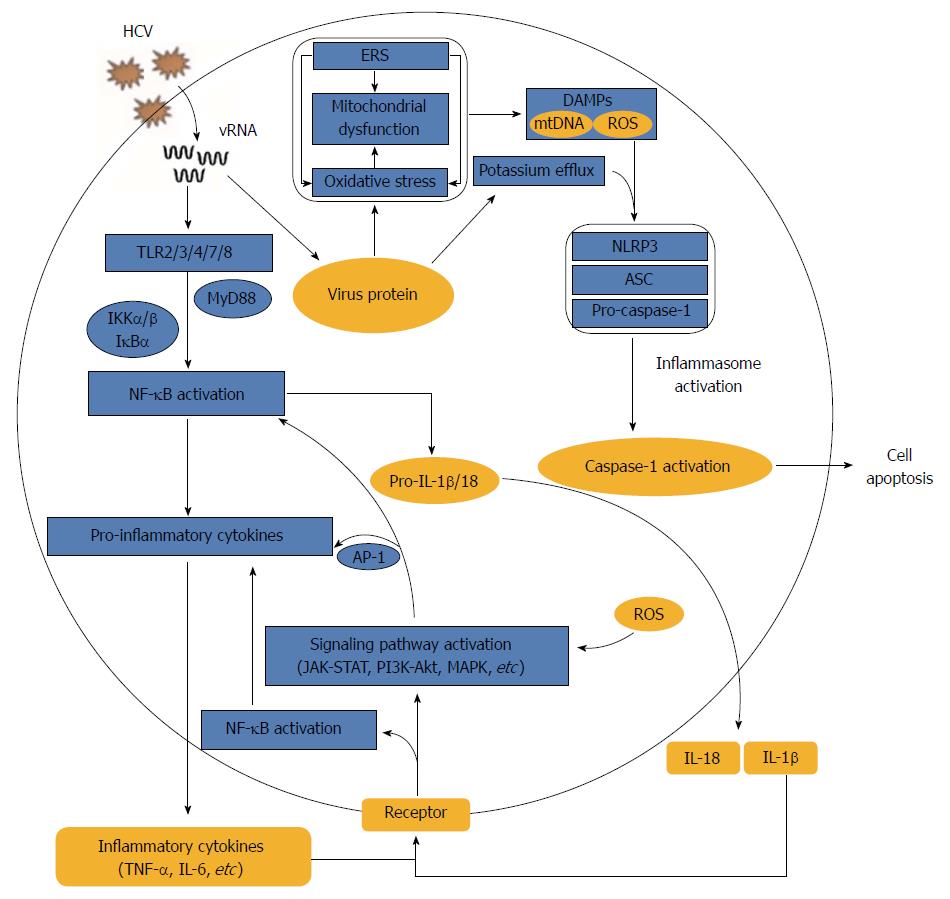
Because of T-cell-mediated autologous hepatocytotoxicity, the spontaneous clearance of HCV infection is not only difficult to achieve but also increases the risk of progression to chronic hepatitis and liver injury[31]. In addition to this T-cell-mediated cytotoxic response, HCV infection alone also triggers inflammatory responses through a variety of strategies to aggravate the progression of liver diseases (Figure 1).
Unlike receptor-mediated HCV entry processes, the occurrence of inflammation in HCV infection relies on the recognition, binding and interaction of HCV RNA and protein components with pathogen pattern recognition receptors (PRRs) or other host cellular structures, which in turn activate downstream immune- and inflammation-associated signal transduction pathways.
Cell surface or internal PRRs, such as Toll-like receptors (TLRs) 1-10, can discern pathogens and activate canonical signalling pathways during immune and inflammatory responses. Virus-derived pathogen-associated molecular patterns (PAMPs), HCV RNA and viral proteins could induce pro-inflammatory cytokine and chemokine production via several PRRs. For example, TLR3 recognized the HCV double-stranded RNA produced during HCV replication and activated TLR3 signalling, resulting in high releases of IL-8, CCL-5, MIP-1 and CXCL-10[32,33]. Macrophages uptake HCV RNA through clathrin-mediated endocytosis, which is independent of receptor and productive infection, and therefore trigger myeloid differentiation primary response (MyD) 88-mediated TLR7 signalling to induce pro-IL-1β mRNA expression[34,35]. In addition, a conformation-dependent interaction between the TLR2 and HCV core or NS3 proteins triggers the TLR2-specific inflammatory pathway[36]. NS5A specifically activated the promoter of the TLR4 gene in both hepatocytes and B cells, thereby activating the signal transduction cascades from MyD88 to IFN regulatory factor (IRF)-3 and stimulating nuclear factor (NF)-κB-mediated IFN-β and IL-6 secretion[37].
In addition to the host PRRs, HCV proteins can also interact with other cellular structures and activate inflammatory pathways. The binding of HCV E2 to CD81 induced CCL-5 secretion possibly through activating mitogen-activated protein kinase (MAPK)[38]. The transient expression of HCV NS5B in mouse liver and human hepatocytes catalysed the production of small RNA species, which activated innate immune signalling via TANK-binding kinase (TBK) 1, IRF-3 and NF-κB, and eventually induced the production of IFNs and inflammatory cytokines[39]. HCV core protein, which is mostly implicated in liver disorders, activated signal transducer and activator of transcription (STAT) 3 in human hepatocytes, leading to subsequent immune activation, inflammation and tumorigenesis[40]. In short, the over-replication of HCV in host cells is accompanied by a broad and complex interaction of host and HCV components, eventually unbalancing signal transduction pathways, causing uncontrolled excessive inflammatory responses and further exacerbating disease progression.
An inflammasome is a large, multi-protein cytosolic complex that perceives intracellular danger signals, such as microbial pathogens, inflammatory diseases, cancers and metabolic and autoimmune disorders via Nod-like receptors (NLRs) and ultimately stimulates the production of the inflammatory cytokines IL-1β and IL-18[41,42]. Four NLR families, NLRP1, NLRP3, NLRC4 and AIM2, have been characterized to date. Among them, NLRP3, as the most extensively studied, responded to the host- and environment-derived molecules and pathogen-associated activators[41]. Increased levels of plasma IL-1β and IL-18 in hepatitis C patients indicated an activation of the inflammasome during HCV infection[43].
Activation of the inflammasome and secretion of mature IL-1β and IL-18 during HCV infection require the integration of two signals. The first is known as signal 1, which occurs when the virus is detected by a PRR or cytokine receptor, resulting in the activation of NF-κB and consequent upregulation of pro-IL-1β and pro-IL-18 mRNAs. Signal 2 is that NLRP3 senses HCV, recruits the adaptor protein ASC (apoptosis-associated speck-like protein containing CARD) and induces the recruitment and autocatalytic activation of caspase-1. The activated caspase-1 processes cytosolic cytokines IL-1β and IL-18 precursors into mature secretory proteins[34]. Although HCV infection was reported to activate the inflammasome[35], the conclusions and proposed mechanisms differ across studies. Michael et al[34] demonstrated that HCV infection induced inflammasome activation and IL-18 and IL-1β secretion in monocytes and macrophages through the recognition of viral single-stranded RNA by TLR7 but failed to stimulate the inflammasome and cytokine production by lymphocytes, dendritic cells or hepatocytes. Other studies reported that no inflammasome activation was detected in HCV-infected Huh-7 cells[44,45], whereas Burdette et al[46] reported HCV-induced secretion of IL-1β in Huh7.5 cells via induction of inflammasome complex assembly involving NALP3, ASC and caspase-1. In addition, although a previous report suggested that reactive oxygen species (ROS) are not inflammasome effectors in HCV-infected hepatocytes[45], Chen et al[44] demonstrated that HCV-RNA transfected monocytes and THP-1 derived macrophages could activate the NLRP3 inflammasome in a ROS-dependent manner, and the process was independent on retinoic acid-inducible gene 1 (RIG-1). Alternative mechanisms for inducing inflammasomes were also reported in HCV infection. For instance, after macrophage phagocytosis of HCV, HCV induced potassium efflux and activated the NLRP3 inflammasome for the processing and secretion of IL-1β[35]. The HCV P7 protein is a kind of pH-sensitive proton channel, and the decrease of extracellular pH could enhance P7 activity and then stimulate signal 2 to induce the maturation and secretion of IL-1β from RAW264.7 macrophages[47], whereas a report showed that high expression of P7 protein in Huh7.5 cells failed to induce IL-1β production[46].
Altogether, inflammasome activation triggered by HCV infection might depend on HCV RNA and the secondary effects during HCV replication, such as ROS generation, potassium efflux and P7 activity. However, the phenomenon of inflammasome activation and detailed mechanisms in response to HCV infection might vary across different cell types, and these data suggest that monocytes and macrophages are the main effector cells activated by the inflammasome after HCV infection.
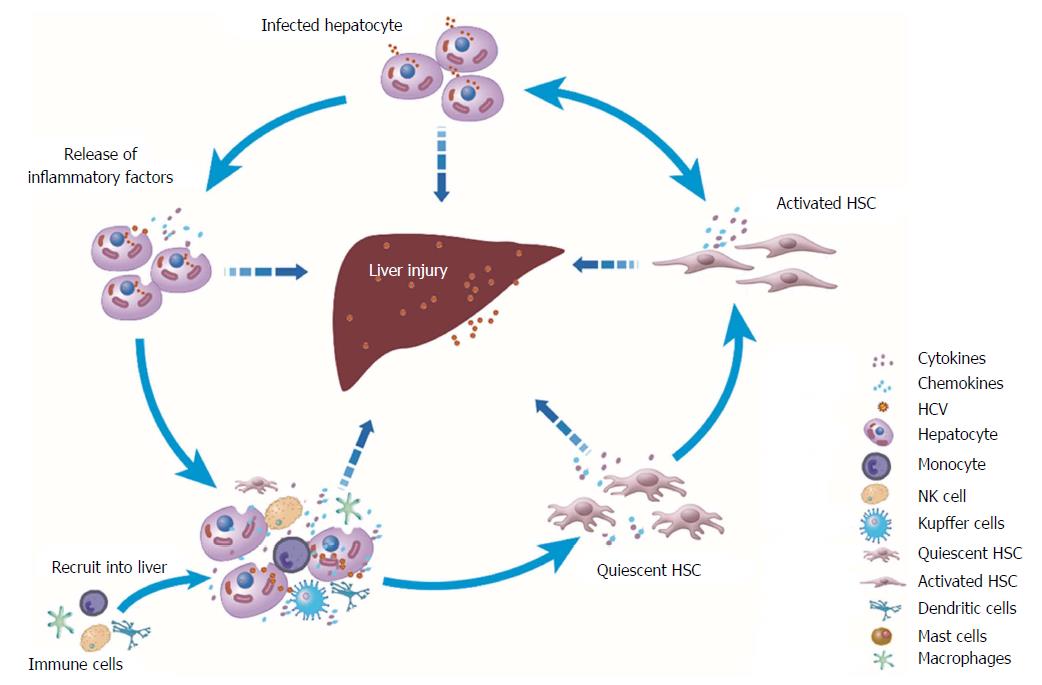
Although hepatocytes are the major cell population in the liver and the targets of cells for HCV entry and replication, non-parenchymal cells in the liver, such as KCs and HSCs, also play key roles in HCV-induced liver diseases. KCs, which are hepatic macrophages and account for approximately 15% of the total cells in the liver[48], exhibit limited internalization of HCV via phagocytosis, leading to the production of pro-inflammatory cytokines and chemokines[35]. HSCs are activated after HCV infection and are associated with liver fibrogenesis, including collagen deposition and abnormal extracellular matrix remodelling[49]. Crosstalk among these three kinds of liver cells plus the recruited immune cells during HCV infection in the hepatic microenvironment mediates inflammatory cascade signalling and exacerbates disease progression (Figure 2). The icons in the figure are shared by Reactome[50].
The interaction between HCV-infected hepatocytes and HSCs enhances the inflammatory response to HCV infection[31]. For example, in the HCV-infected hepatocyte and HSC co-culture system, IL-1α secreted by HSCs enhanced CCAAT-enhancer-binding protein β-targeted downstream gene expression, leading to enhanced expression of IL-6, IL-8 and MIP-1α/1β[31]. Additionally, in the co-culture system of HCV replicon cells and HSCs, HCV replicon cells released transforming growth factor (TGF)-β1 into conditioned medium and thereby induced fibrogenesis in HSCs, which was characterized by increased production of procollagen α1 (I) and procollagen α1 (III) and decreased expression of fibrinolytic matrix metalloproteinase (MMP)[51]. Exosomes, which are endosomal-derived vesicles, also mediate communication between hepatocytes and HSCs. Although exosomes secreted from HCV-infected hepatocytes (HCV-exo) could transport small amounts of HCV RNA into HSCs, the quantity of HCV RNA carried into HSCs is gradually decreased because HSCs do not support HCV infection and replication[52,53]. However, one study found that the miR-19a carried by HCV-exo could target suppressor of cytokine signalling 3 (SOCS3) in HSCs, leading to STAT3-mediated TGF-β signalling activation, profibrotic marker high expression and enhanced inflammatory responses[52].
After HCV infection, the inflammatory cascades between HCV-stimulated macrophages and HSCs are also activated in the hepatic microenvironment. To mimic the effect of HCV in the blood circulation on hepatic non-parenchymal cells, Negash et al[35] exposed human THP-1 cell-derived macrophages and KCs to HCV in vitro and showed that they activated caspase-1 expression and enhanced IL-1β/-18 secretion. Similarly, conditioned medium derived from HCV-exposed human THP-1 macrophages and KCs increased the expression of inflammatory (NLRP3, TNF-α, IL-1β, IL-6 and CCL-5) and profibrogenic (TGF-β1, collagen 4A1, MMP2 and α-smooth muscle actin) markers in primary human and immortalized HSCs (LX2 cells)[54]. Further study identified that the chemokine CCL-5 in this conditioned medium induced inflammasome activation and fibrotic marker expression in HSCs, whereas TNF-α but not IL-1β could only induce inflammasome markers[54]. These studies emphasize that inflammatory cascade reactions could occur between macrophages and HSCs through highly expressed inflammatory mediators during HCV infection.
Moreover, chemokines secreted by HCV-infected hepatocytes and HCV-internalized KCs recruit immune cells to the site of infection, leading to aggravation of the inflammatory response and even liver damage in CHC[47,55]. Other molecules, such as ROS and lipid peroxidation products produced by activated KCs or injured hepatocytes, could also induce the activation of quiescent HSCs[49]. The interactions between the HSCs and the immune cells recruited into the liver and the HCV-infected or exposed hepatocytes/macrophages mediate inflammation-related cellular signalling, together establishing a microenvironment of the liver in an excessively inflammatory state and continuing to exacerbate disease progression.
Oxidative stress is characterized by excessive ROS accumulation in vivo. ROS includes radicals, such as superoxide anion (O2-∙), hydroxyl radical (OH•) and hydrogen peroxide (H2O2). Superoxide anions are mainly derived from the mitochondrial electron transport chain and catalysed into H2O2 and OH• by superoxide dismutase[56]. Hepatocytes have abundant mitochondria, which are the main source of ROS. Oxidative stress is more severe in HCV infection than in HBV infection[57]. Upon HCV infection, oxidative stress is thought to occur because of the expression of viral proteins, changes in the activity of oxidative enzymes, depletions of antioxidants and the ensuing chronic inflammation[58]. HCV core protein interacts with the mitochondrial protein chaperone prohibitin, leading to impairment of mitochondrial respiratory chain function accompanied by ROS overproduction[59]. The intracellular expression of E1, E2, NS3 and NS5A also potently enhances ROS levels by increasing intracellular calcium influx and decreasing mitochondrial transmembrane potential[58]. In addition to mitochondria-derived ROS, cellular nicotinamide adenine dinucleotide phosphate (NADPH) oxidase also acts as an important source of ROS, generating superoxide anions by catalysing the oxidation of NADPH[60]. Among the seven Nox enzymes (Nox1-5, Dual oxidase Duox1 and Duox2), Nox1 and Nox4 induced in hepatocytes by infecting with HCV phenotype 2a and 1b or expressing HCV proteins contribute to ROS production[60,61]. In brief, ROS accumulation caused by HCV infection in the liver further leads to activation of the signalling factors phosphoinositide 3-kinase (PI3K), Janus kinase, MAPK pathways or transcription factor NF-κB, activator protein-1 (AP-1), STAT3, HIF-1α, PPAR-γ and Nrf2, and those pathways induce downstream inflammatory and immune responses[62-65].
HCV protein expression is also accompanied by strong endoplasmic reticulum stress (ERS). HCV structural and NS proteins are continuously processed in ER-derived membrane structures[66,67], perturbing normal ER functions and inducing ER stress[67,68]. The unfolded protein response (UPR) is that cells respond to ERS by activating an adaptive cellular programme and alleviate ER stress by inducing protein folding and degradation in the ER and decreasing overall protein synthesis[66]. The UPR is mediated by three ER transmembrane proteins: cleavage of activating transcription factor 6 (ATF-6), inositol-requiring enzyme 1 (IRE1) and PKR-like endoplasmic reticulum kinase (PERK) and their downstream factors X-box binding protein 1 (XBP-1) and eukaryotic initiation factor 2α (EIF2α)[66]. The UPR is initially activated in HCV-infected Huh7.5.1 cells and HCV-transgenic mice, resulting in the phosphorylation of IRE1, EIF2α and ATF-6, and splicing of XBP-1[69]. However, HCV RNA or protein per se also exerts the resistance for UPR, such as XBP-1 activity downregulation in HCV-expressing cells and PERK activity inhibition by HCV E2, leading to a reduction in ER-associated protein degradation and UPR-associated translational attenuation, respectively[66]. Virtually, there is an interaction between ERS and inflammation, and ERS might be both a trigger and a consequence of chronic inflammation[70]. NS5A expression in the ER triggers ERS and ultimately leads to the activation of STAT3 and NF-κB, and this pathway is sensitive to mitochondrial calcium uptake inhibitors, calcium chelators and antioxidants, therefore providing evidence for the role of ERS and oxidative stress in the activation of the inflammatory response during HCV infection[71]. Under severe ERS, the UPR activates the JNK/AKT pathway and phosphorylates NF-κB protein IκB kinase (IKK), leading to cleavage of IκBα and activation of NF-κB[70]. UPR-independent Ca2+ release and excessive ROS and ER chaperone GRP78 that leak into the cytosol were also proposed to activate NF-κB to induce inflammation[70]. In turn, inflammatory factors also exacerbate ERS and oxidative stress. For example, TNF-α induced intracellular excessive generation of ROS, which in turn induced ERS, whereas IL-1β and TNF-α also increased ERS in a nitric oxide dependent manner[72,73].
The ERS and oxidative stress networks interact with each other and therefore play a key role in local and systemic inflammatory responses. HCV infection triggers ERS and disrupts mitochondrial signalling and cytosolic redox homeostasis, thereby inducing oxidative stress and inflammation. ERS and oxidative stress might individually or concurrently stimulate or exacerbate inflammatory responses, and vice versa.
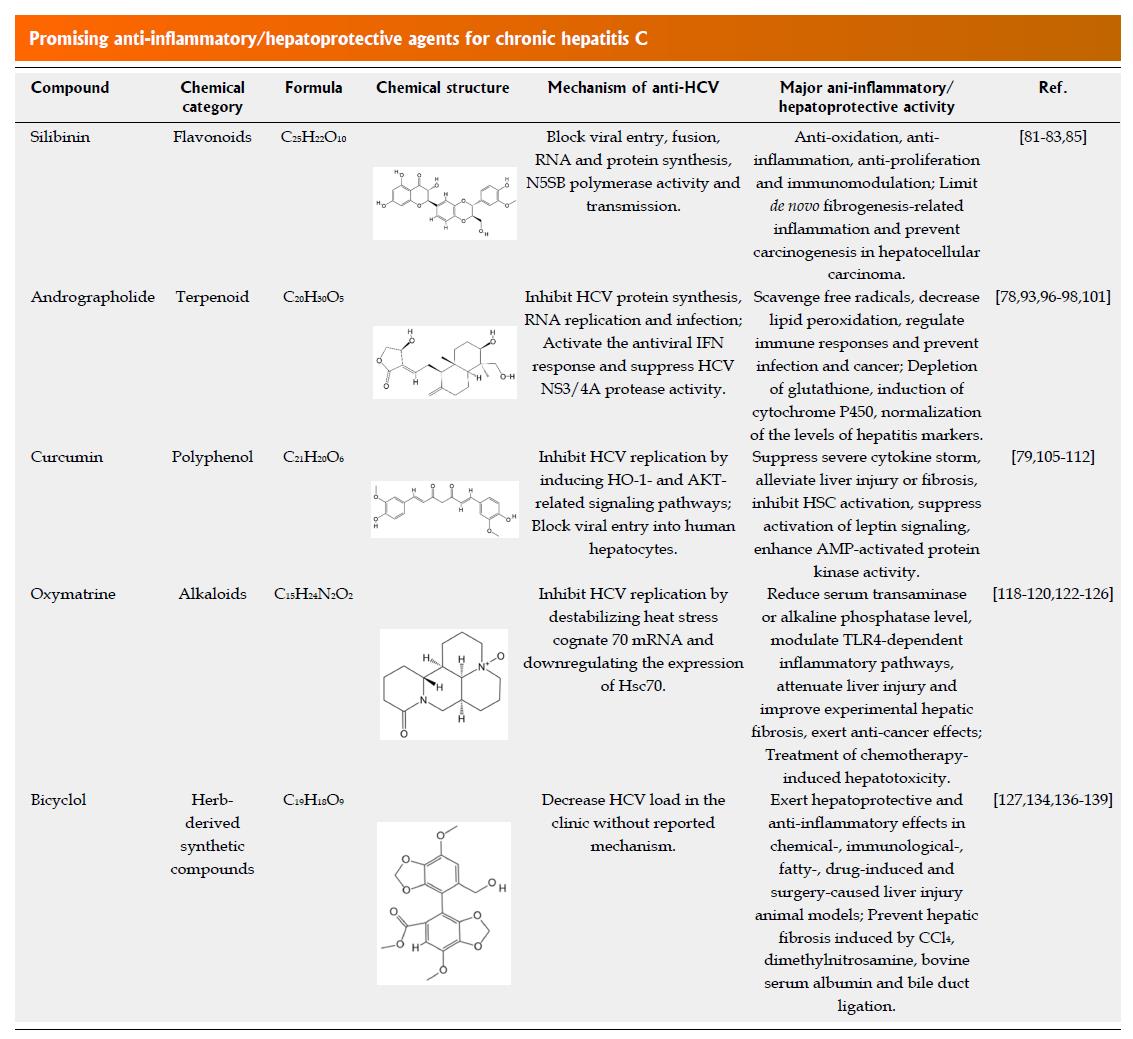
DAAs have been used clinically for several years, and some limitations were reported, including drug-resistance, low efficacy in cirrhotic patients, drug interactions, liver toxicities, HBV reactivation and skin reactions[6,74-77]. Anti-inflammatory/hepatoprotective agents have fewer side effects for the treatment of HCV-induced liver injury, fibrosis, cirrhosis or even HCC, as demonstrated by their long-term use in liver health cares in many Asian countries[77-80]. Most of these agents are natural or naturally derived compounds, and many have anti-HCV effects. After DAA treatment, some patients are still subject to persistent disease progression and HCV relapse[81]. Therefore, anti-inflammatory/hepatoprotective agents with anti-HCV activity might be a better treatment choice for preventing CHC progression to severe liver diseases when used in combination with or subsequent to DAA therapy. According to the chemical category and practical application in the clinic, we mainly focus on certain representative plant or plant derivate active constituents considered to be the most promising for anti-inflammatory/hepatoprotective therapy in HCV infection, including the flavonoid compound silibinin, terpenoid compound andrographolide, polyphenols compound curcumin, alkaloids oxymatrine and herb-derived antioxidant bicyclol (Figure 3)[77,78,80].
Flavonoids or bioflavonoids are found in numerous plants, and over 4500 flavonoids or their subgroups have been identified to date, of which silymarin and silibinin are representative compounds[77,80]. Silymarin is extracted from the seeds of the milk thistle Silybum marianum and comprises at least seven flavonolignans (silybin A, silybin B, isosilybin A, isosilybin B, silychristin, isosilychristin and silydianin) and one flavonoid (taxifolin)[81,82]. Silibinin (formerly named silybin) is the major bioactive compound of silymarin and is widely used for the treatment of insulin resistance, alcoholic/non-alcoholic liver disease and viral hepatitis because of its antioxidative, anti-inflammatory, anti-proliferative and immunomodulatory properties[81-84]. In vitro and in vivo studies showed that silymarin/silibinin could stimulate the expression of lysophosphatidylcholine acyltransferase, reduce the level of liver cirrhotic platelet-activating factor or block the activation of major signalling pathways, such as NF-κB and TGF-β signalling, thus limiting de novo fibrogenesis-related inflammation and preventing carcinogenesis in HCC[82,85]. Moreover, silymarin and silibinin were found to inhibit HCV infection in cell cultures by blocking viral entry, fusion, RNA and protein synthesis, N5SB polymerase activity and transmission[84]. Although oral administration of silymarin had little effect on liver enzyme activity and viral load in HCV-infected patients due to its rapid metabolism and low bioavailability[86-88], intravenous injection of water-soluble, succinate-conjugated silibinin formulations showed significant antiviral effects in CHC patients who failed to treatment with the standard pegylated IFN/ribavirin therapy[89,90].
Other flavonoids, such as epigallocatechin-3-gallate, naringenin, quercetin, luteolin and apigenin, also have potential anti-HCV activities, hepatoprotective and anti-inflammatory activities[77,91,92]. Therefore, these agents are expected to be developed for the treatment of hepatitis C patients with advanced liver diseases.
Among the various secondary metabolites produced by plants, terpenoids are phytochemicals with potential therapeutic applications for the treatment of liver cancer, of which labdane diterpenoid compound andrographolide (C20H30O5) is isolated from the stem and leaf of Andrographis paniculata and was initially used to treat upper respiratory tract infections with good safety but was later reported to have anti-inflammatory/hepatoprotective effects[78,79]. Its mechanisms of action are mainly via scavenging free radicals, decreasing lipid peroxidation, regulating immune responses and preventing infection and cancer[78,93]. In vitro and in vivo, the anti-inflammatory activities of andrographolide were attributed to the attenuation of the protein kinase C, extracellular signal-regulated kinase (ERK)1/2 or PI3K/AKT pathways, leading to the inhibition of NF-κB signalling pathway activation[93-95]. The anti-hepatotoxic activities of andrographolide were correlated with depletion of glutathione, induction of cytochrome P450 or normalization of the levels of hepatitis markers such as alkaline phosphatase and glutamic pyruvate transaminase[96-98]. The synthetic analogues of andrographolide exhibit analgesic, antipyretic and anti-inflammatory effects without notable toxicity in animal models[99,100]. Moreover, andrographolide was reported to inhibit HCV protein synthesis, RNA replication and infection[101]. Detailed mechanisms showed that andrographolide activates p38 MAPK phosphorylation and stimulates Nrf2-mediated heme oxygenase (HO)-1 expression, thereby increasing the amounts of its metabolite biliverdin, which was found to activate the antiviral IFN response and suppress HCV NS3/4A protease activity[101,102]. These findings support a clinical trial of andrographolide and its derivatives for the treatment of severe CHC.
Curcumin, a hydrophobic polyphenol derived from the rhizome of the herb turmeric (Curcuma longa), has been widely used as a spice and colorant in foods[103]. To date, extensive clinical studies have proven its pharmacological properties of anti-inflammatory, antioxidant, antiviral, anticancer, hypoglycaemic, wound-healing and antimicrobial activities and showed that curcumin is safe and well tolerated[104]. In view of these observations, curcumin is predominantly used to treat inflammatory diseases via multiple mechanisms involving inflammatory transcription factors, cytokines, redox status or protein kinases[105]. Curcumin suppresses the severe cytokine storm caused by infection with severe viruses, such as HIV, HSV, HBV and HCV, and might be potentially useful to treat inflammation induced by Ebola virus infection[106]. For its hepatoprotective property, curcumin alleviates liver injury or fibrosis by targeting platelet-derived growth factor-β receptor, TGF-β, TLRs, MMPs and peroxisome proliferator-activated receptors and decreasing inflammatory cytokines[78]. In vitro studies showed that curcumin inhibits HSC activation via preventing leptin from increasing intracellular glucose levels[107], suppressing advanced glycation end-product-dependent activation of leptin signalling[108] or enhancing AMP-activated protein kinase (AMPK) activity[109] and therefore reduces liver fibrosis. In addition, evidence suggests that curcumin has anticarcinogenic and chemopreventive effects through arresting the cell cycle and/or inducing apoptosis in a p53 dependent manner[103], as well as by activating caspase cascades[105]. Curcumin also inhibits HCV replication by inducing HO-1- and AKT-related signalling pathways[110,111] or by blocking viral entry into human hepatocytes[112]. Therefore, based on the available pharmacological data obtained from in vitro and in vivo studies, as well as clinical trials, there is an opportunity to translate curcumin into clinics for therapy of CHC with hepatic cirrhosis and HCC in the near future.
Oxymatrine and its active metabolite matrine are the major alkaloid aqueous extracts from the root of Sophora flavescens, Sophora tonkinensis and Sophora alopecuroides[113]. Clinically, oxymatrine has been used to treat chronic hepatitis B and leukopenia caused by tumour radiotherapy and chemotherapy in China[113,114]. In recent years, many laboratory and clinical trials have also shown the antiviral activity of oxymatrine against HCV in cell cultures and human studies[115-117]. Oxymatrine or its derivatives destabilize heat stress cognate 70 (Hsc70) mRNA and thereby downregulate the expression of Hsc70[118-120]. As Hsc70 is packaged into HCV particles and becomes a structural component of the virus in the assembly process, oxymatrine or its derivatives could inhibit HCV replication[118,121]. Oxymatrine could also inhibit inflammatory activity as defined by reducing serum transaminase or alkaline phosphatase levels, modulating TLR4-dependent inflammatory pathways, attenuating liver injury and improving experimental hepatic fibrosis by downregulating fibrosis-related gene expression, decreasing collagen deposits, inducing apoptosis of HSCs or inhibiting lipid peroxidation[122-126]. Furthermore, accumulating research suggests that oxymatrine has anticancer effects, which might have therapeutic effects on HCC caused by HCV infection, but could also be used in the treatment of chemotherapy-induced hepatotoxicity[127,128]. Therefore, oxymatrine is expected to be used in CHC patients with liver inflammation or injury and could improve chemotherapy-induced hepatotoxicity or increase the survival rate of HCC patients.
To date, although the effects of ROS and the host redox system on HCV replication remain unclear[56,63] and different antioxidants show controversial anti-HCV effects[63,129-131], clinical evidence suggests that antioxidant therapy might alleviate necroinflammation and fibrosis progression[131,132]. Classical antioxidants, such as glutathione (GSH)[63], N-acetyl cysteine (NAC)[133] and vitamin E[130], have been reported to treat hepatitis C with efficacy. In addition, bicyclol (4,4’-dimethoxy-5,6,5’,6’-bis(methylenedioxy)-2-hydroxymethyl-2’-methoxycarbonyl biphenyl), a synthetic compound derived from schizandrin C extracted from the Chinese medicinal herb Fructus schisandrae, has potent antioxidative and certain anti-HCV effects in the clinic with safety[134,135]. Clinically, bicyclol tablets are used in many countries to treat various non-viral hepatitis and chronic hepatitis B and C accompanied by mild and moderate serum aminotransferase abnormality. Preclinical pharmacological experiments also showed that bicyclol exerts hepatoprotective and anti-inflammatory effects in chemical-, immunological-, fatty-, drug-induced and surgery-caused liver injury animal models[135]. Bicyclol also prevents hepatic fibrosis induced by CCl4, dimethylnitrosamine, bovine serum albumin and bile duct ligation[136-139]. Although the detailed mechanism varies in different models, the overall effect of bicyclol is derived from stabilizing mitochondrial and hepatocyte membranes, scavenging free radicals, reducing lipid peroxides, enhancing antioxidant gene expression or activity or inhibiting liver cell apoptosis, and thus achieves anti-inflammatory, antioxidant and liver cell-protective activities [135,140]. These effects of bicyclol and its antiviral activities make bicyclol a promising drug for treating CHC patients with liver injury or co-infection with HBV.
Inflammation is a common feature of most liver diseases, and inflammatory cytokines and chemokines produced after HCV infection accelerate hepatocyte damage and liver disease progression. HCV infection triggers inflammation through various mechanisms including pathogen pattern recognition, inflammasome activation and intrahepatic inflammatory cascades, while oxidative and ER stress coexist with and exacerbate inflammation and liver injury (Figures 1 and 2). HCV infection is a predisposing factor in the pathological process, but the long-term inflammatory responses and oxidative stress induced by the virus might further destroy the liver microenvironment and cause irreversible liver tissue damage[7,11]. Direct antiviral therapy might not be sufficient to stop the progression of liver disease in the context of inflammation, oxidative stress, liver tissue damage and metabolic dysregulation. Although anti-inflammatory/hepatoprotective drugs might not provide a fast-acting remedy for the treatment of HCV infection as DAAs do, they can serve as an adjunct to DAAs by exerting comprehensive effects. These effects include the following: (1) decreasing uncontrolled inflammatory cytokine and chemokine levels; (2) directly protecting against oxidative stress or enhancing antioxidant gene expression; (3) restoring mitochondrial function, regulating liver enzyme levels and protecting against liver cell damage; (4) inhibiting HCV replication; and (5) improving the efficacy of IFN antiviral therapy in vivo[80,129,141]. Given the status of excessive inflammation and liver microenvironment dyshomeostasis in chronic HCV-infected patients, care for hepatitis C should extend beyond merely achieving an SVR to encompass an anti-inflammatory/hepatoprotective strategy concurrently with or after DAA therapy. Meanwhile, their anti-HCV effects could prevent HCV relapse after the DAA treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciccone M, Tenca A S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Rebbani K, Tsukiyama-Kohara K. HCV-Induced Oxidative Stress: Battlefield-Winning Strategy. Oxid Med Cell Longev. 2016;2016:7425628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Perlin CM, Ferreira VL, Borba HHL, Wiens A, Ivantes CAP, Lenzi L, Pontarolo R. Quality of life in Brazilian patients with treated or untreated chronic hepatitis C. Rev Inst Med Trop Sao Paulo. 2017;59:e81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Wong RJ, Gish RG. Metabolic Manifestations and Complications Associated With Chronic Hepatitis C Virus Infection. Gastroenterol Hepatol (NY). 2016;12:293-299. [PubMed] |
| 4. | Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol. 2014;61:S14-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 5. | Zeremski M, Petrovic LM, Talal AH. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat. 2007;14:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct Acting Anti-hepatitis C Virus Drugs: Clinical Pharmacology and Future Direction. J Transl Int Med. 2017;5:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 699] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 8. | Huang JF, Yu ML, Dai CY, Chuang WL. Glucose abnormalities in hepatitis C virus infection. Kaohsiung J Med Sci. 2013;29:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | D’Elia RV, Harrison K, Oyston PC, Lukaszewski RA, Clark GC. Targeting the “cytokine storm” for therapeutic benefit. Clin Vaccine Immunol. 2013;20:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 10. | Huang CF, Hsieh MY, Yang JF, Chen WC, Yeh ML, Huang CI, Dai CY, Yu ML, Lin ZY, Chen SC. Serum hs-CRP was correlated with treatment response to pegylated interferon and ribavirin combination therapy in chronic hepatitis C patients. Hepatol Int. 2010;4:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 12. | Hsu CC, Lien JC, Chang CW, Chang CH, Kuo SC, Huang TF. Yuwen02f1 suppresses LPS-induced endotoxemia and adjuvant-induced arthritis primarily through blockade of ROS formation, NFkB and MAPK activation. Biochem Pharmacol. 2013;85:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Costantini S, Capone F, Guerriero E, Maio P, Colonna G, Castello G. Serum cytokine levels as putative prognostic markers in the progression of chronic HCV hepatitis to cirrhosis. Eur Cytokine Netw. 2010;21:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 14. | Zeremski M, Dimova R, Brown Q, Jacobson IM, Markatou M, Talal AH. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis. 2009;200:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, Romano C, Adinolfi LE. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 16. | Neuman MG, Schmilovitz-Weiss H, Hilzenrat N, Bourliere M, Marcellin P, Trepo C, Mazulli T, Moussa G, Patel A, Baig AA. Markers of inflammation and fibrosis in alcoholic hepatitis and viral hepatitis C. Int J Hepatol. 2012;2012:231210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Jonsson JR, Barrie HD, O’Rourke P, Clouston AD, Powell EE. Obesity and steatosis influence serum and hepatic inflammatory markers in chronic hepatitis C. Hepatology. 2008;48:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Aroucha DC, do Carmo RF, Moura P, Silva JL, Vasconcelos LR, Cavalcanti MS, Muniz MT, Aroucha ML, Siqueira ER, Cahú GG. High tumor necrosis factor-α/interleukin-10 ratio is associated with hepatocellular carcinoma in patients with chronic hepatitis C. Cytokine. 2013;62:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Knobler H, Schattner A. TNF-{alpha}, chronic hepatitis C and diabetes: a novel triad. QJM. 2005;98:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 20. | Falasca K, Ucciferri C, Dalessandro M, Zingariello P, Mancino P, Petrarca C, Pizzigallo E, Conti P, Vecchiet J. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci. 2006;36:144-150. [PubMed] |
| 21. | Jia H, Du J, Zhu S, Ma Y, Cai H. Clinical observation of serum IL-18, IL-10 and sIL-2R levels in patients with chronic hepatitis C pre- and post antiviral treatment. Chin Med J (Engl). 2003;116:605-608. [PubMed] |
| 22. | Fallahi P, Ferrari SM, Giuggioli D, Sebastiani M, Colaci M, Ferri C, Antonelli A. Chemokines in the Pathogenesis and as Therapeutical Markers and Targets of HCV Chronic Infection and HCV Extrahepatic Manifestations. Curr Drug Targets. 2017;18:786-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Larrubia JR, Benito-Martínez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14:7149-7159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | You CR, Park SH, Jeong SW, Woo HY, Bae SH, Choi JY, Sung YC, Yoon SK. Serum IP-10 Levels Correlate with the Severity of Liver Histopathology in Patients Infected with Genotype-1 HCV. Gut Liver. 2011;5:506-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, Marinos G, Lloyd AR. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Larrubia JR, Calvino M, Benito S, Sanz-de-Villalobos E, Perna C, Pérez-Hornedo J, González-Mateos F, García-Garzón S, Bienvenido A, Parra T. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, Charles ED, Talal AH, Jacobson IM, Rice CM. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Yamauchi K, Akbar SM, Horiike N, Michitaka K, Onji M. Increased serum levels of macrophage inflammatory protein-3alpha in chronic viral hepatitis: prognostic importance of macrophage inflammatory protein-3alpha during interferon therapy in chronic hepatitis C. J Viral Hepat. 2002;9:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Kaplanski G, Farnarier C, Payan MJ, Bongrand P, Durand JM. Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C. Correlation with cytokine concentrations and liver inflammation and fibrosis. Dig Dis Sci. 1997;42:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Fukuda R, Ishimura N, Ishihara S, Chowdhury A, Morlyama N, Nogami C, Miyake T, Niigaki M, Tokuda A, Satoh S. Intrahepatic expression of pro-inflammatory cytokine mRNAs and interferon efficacy in chronic hepatitis C. Liver. 1996;16:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Nishitsuji H, Funami K, Shimizu Y, Ujino S, Sugiyama K, Seya T, Takaku H, Shimotohno K. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J Virol. 2013;87:8169-8178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Wagoner J, Austin M, Green J, Imaizumi T, Casola A, Brasier A, Khabar KS, Wakita T, Gale M Jr, Polyak SJ. Regulation of CXCL-8 (interleukin-8) induction by double-stranded RNA signaling pathways during hepatitis C virus infection. J Virol. 2007;81:309-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW 3rd, Blankson JN, Pardoll D, Cox AL. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014;10:e1004082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 36. | Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Nattermann J, Nischalke HD, Feldmann G, Ahlenstiel G, Sauerbruch T, Spengler U. Binding of HCV E2 to CD81 induces RANTES secretion and internalization of CC chemokine receptor 5. J Viral Hepat. 2004;11:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Yu GY, He G, Li CY, Tang M, Grivennikov S, Tsai WT, Wu MS, Hsu CW, Tsai Y, Wang LH. Hepatic expression of HCV RNA-dependent RNA polymerase triggers innate immune signaling and cytokine production. Mol Cell. 2012;48:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Basu A, Meyer K, Lai KK, Saito K, Di Bisceglie AM, Grosso LE, Ray RB, Ray R. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes. Virology. 2006;349:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1286] [Cited by in RCA: 1283] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 42. | Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4047] [Cited by in RCA: 4688] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 43. | Capone F, Guerriero E, Colonna G, Maio P, Mangia A, Castello G, Costantini S. Cytokinome profile evaluation in patients with hepatitis C virus infection. World J Gastroenterol. 2014;20:9261-9269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 44. | Chen W, Xu Y, Li H, Tao W, Xiang Y, Huang B, Niu J, Zhong J, Meng G. HCV genomic RNA activates the NLRP3 inflammasome in human myeloid cells. PLoS One. 2014;9:e84953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Shrivastava S, Mukherjee A, Ray R, Ray RB. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages. J Virol. 2013;87:12284-12290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Burdette D, Haskett A, Presser L, McRae S, Iqbal J, Waris G. Hepatitis C virus activates interleukin-1β via caspase-1-inflammasome complex. J Gen Virol. 2012;93:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Farag NS, Breitinger U, El-Azizi M, Breitinger HG. The p7 viroporin of the hepatitis C virus contributes to liver inflammation by stimulating production of Interleukin-1β. Biochim Biophys Acta Mol Basis Dis. 2017;1863:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 49. | Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Sidiropoulos K, Viteri G, Sevilla C, Jupe S, Webber M, Orlic-Milacic M, Jassal B, May B, Shamovsky V, Duenas C. Reactome enhanced pathway visualization. Bioinformatics. 2017;33:3461-3467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 51. | Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 53. | Florimond A, Chouteau P, Bruscella P, Le Seyec J, Mérour E, Ahnou N, Mallat A, Lotersztajn S, Pawlotsky JM. Human hepatic stellate cells are not permissive for hepatitis C virus entry and replication. Gut. 2015;64:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Sasaki R, Devhare PB, Steele R, Ray R, Ray RB. Hepatitis C virus-induced CCL5 secretion from macrophages activates hepatic stellate cells. Hepatology. 2017;66:746-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431-7436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 56. | Brault C, Levy PL, Bartosch B. Hepatitis C virus-induced mitochondrial dysfunctions. Viruses. 2013;5:954-980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Fujita N, Sugimoto R, Ma N, Tanaka H, Iwasa M, Kobayashi Y, Kawanishi S, Watanabe S, Kaito M, Takei Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat. 2008;15:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 59. | Tsutsumi T, Matsuda M, Aizaki H, Moriya K, Miyoshi H, Fujie H, Shintani Y, Yotsuyanagi H, Miyamura T, Suzuki T. Proteomics analysis of mitochondrial proteins reveals overexpression of a mitochondrial protein chaperon, prohibitin, in cells expressing hepatitis C virus core protein. Hepatology. 2009;50:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | de Mochel NS, Seronello S, Wang SH, Ito C, Zheng JX, Liang TJ, Lambeth JD, Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 61. | Boudreau HE, Emerson SU, Korzeniowska A, Jendrysik MA, Leto TL. Hepatitis C virus (HCV) proteins induce NADPH oxidase 4 expression in a transforming growth factor beta-dependent manner: a new contributor to HCV-induced oxidative stress. J Virol. 2009;83:12934-12946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 62. | Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4137] [Cited by in RCA: 3704] [Article Influence: 246.9] [Reference Citation Analysis (0)] |
| 63. | Kuroki M, Ariumi Y, Ikeda M, Dansako H, Wakita T, Kato N. Arsenic trioxide inhibits hepatitis C virus RNA replication through modulation of the glutathione redox system and oxidative stress. J Virol. 2009;83:2338-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Presser LD, McRae S, Waris G. Activation of TGF-β1 promoter by hepatitis C virus-induced AP-1 and Sp1: role of TGF-β1 in hepatic stellate cell activation and invasion. PLoS One. 2013;8:e56367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Machida K, Cheng KT, Lai CK, Jeng KS, Sung VM, Lai MM. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J Virol. 2006;80:7199-7207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005;13:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 67. | von dem Bussche A, Machida R, Li K, Loevinsohn G, Khander A, Wang J, Wakita T, Wands JR, Li J. Hepatitis C virus NS2 protein triggers endoplasmic reticulum stress and suppresses its own viral replication. J Hepatol. 2010;53:797-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Liberman E, Fong YL, Selby MJ, Choo QL, Cousens L, Houghton M, Yen TS. Activation of the grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J Virol. 1999;73:3718-3722. [PubMed] |
| 69. | Merquiol E, Uzi D, Mueller T, Goldenberg D, Nahmias Y, Xavier RJ, Tirosh B, Shibolet O. HCV causes chronic endoplasmic reticulum stress leading to adaptation and interference with the unfolded protein response. PLoS One. 2011;6:e24660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90:260-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 71. | Waris G, Tardif KD, Siddiqui A. Endoplasmic reticulum (ER) stress: hepatitis C virus induces an ER-nucleus signal transduction pathway and activates NF-kappaB and STAT-3. Biochem Pharmacol. 2002;64:1425-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Kacheva S, Lenzen S, Gurgul-Convey E. Differential effects of proinflammatory cytokines on cell death and ER stress in insulin-secreting INS1E cells and the involvement of nitric oxide. Cytokine. 2011;55:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917-33925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 332] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 74. | Federico A, Aitella E, Sgambato D, Savoia A, De Bartolomeis F, Dallio M, Ruocco E, Pezone L, Abbondanza C, Loguercio C. Telaprevir may induce adverse cutaneous reactions by a T cell immune-mediated mechanism. Ann Hepatol. 2015;14:420-424. [PubMed] |
| 75. | Kim S, Han KH, Ahn SH. Hepatitis C Virus and Antiviral Drug Resistance. Gut Liver. 2016;10:890-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Dyson JK, Hutchinson J, Harrison L, Rotimi O, Tiniakos D, Foster GR, Aldersley MA, McPherson S. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 77. | Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, Wu V, Wong A, Wang Y, Zhang X. Hepatitis due to Reactivation of Hepatitis B Virus in Endemic Areas Among Patients With Hepatitis C Treated With Direct-acting Antiviral Agents. Clin Gastroenterol Hepatol. 2017;15:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 78. | Lam P, Cheung F, Tan HY, Wang N, Yuen MF, Feng Y. Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-Inflammatory and Anti-Oxidative Activities. Int J Mol Sci. 2016;17:465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 79. | Chua LS. Review on liver inflammation and antiinflammatory activity of Andrographis paniculata for hepatoprotection. Phytother Res. 2014;28:1589-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Domitrović R, Potočnjak I. A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch Toxicol. 2016;90:39-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 81. | Kriss M, Burchill M. HCV and nonhepatic malignancy: Is pre-emptive direct-acting antiviral therapy indicated prior to treatment? Hepatology. 2018;67:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Federico A, Dallio M, Loguercio C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 83. | Neha , Jaggi AS, Singh N. Silymarin and Its Role in Chronic Diseases. Adv Exp Med Biol. 2016;929:25-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Polyak SJ, Ferenci P, Pawlotsky JM. Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology. 2013;57:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Stanca E, Serviddio G, Bellanti F, Vendemiale G, Siculella L, Giudetti AM. Down-regulation of LPCAT expression increases platelet-activating factor level in cirrhotic rat liver: potential antiinflammatory effect of silybin. Biochim Biophys Acta. 2013;1832:2019-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJ, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50:434-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 88. | Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos. 2008;36:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schöniger-Hekele M, Holzmann H, Steindl-Munda P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 90. | Rutter K, Scherzer TM, Beinhardt S, Kerschner H, Stättermayer AF, Hofer H, Popow-Kraupp T, Steindl-Munda P, Ferenci P. Intravenous silibinin as ‘rescue treatment’ for on-treatment non-responders to pegylated interferon/ribavirin combination therapy. Antivir Ther. 2011;16:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 92. | Lee MH, Yoon S, Moon JO. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull. 2004;27:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Chao WW, Lin BF. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin Med. 2010;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 94. | Tsai HR, Yang LM, Tsai WJ, Chiou WF. Andrographolide acts through inhibition of ERK1/2 and Akt phosphorylation to suppress chemotactic migration. Eur J Pharmacol. 2004;498:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Lim JC, Chan TK, Ng DS, Sagineedu SR, Stanslas J, Wong WS. Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 96. | Chatuphonprasert W, Jarukamjorn K, Kondo S, Nemoto N. Synergistic increases of metabolism and oxidation-reduction genes on their expression after combined treatment with a CYP1A inducer and andrographolide. Chem Biol Interact. 2009;182:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Jaruchotikamol A, Jarukamjorn K, Sirisangtrakul W, Sakuma T, Kawasaki Y, Nemoto N. Strong synergistic induction of CYP1A1 expression by andrographolide plus typical CYP1A inducers in mouse hepatocytes. Toxicol Appl Pharmacol. 2007;224:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Kapil A, Koul IB, Banerjee SK, Gupta BD. Antihepatotoxic effects of major diterpenoid constituents of Andrographis paniculata. Biochem Pharmacol. 1993;46:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 128] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Suebsasana S, Pongnaratorn P, Sattayasai J, Arkaravichien T, Tiamkao S, Aromdee C. Analgesic, antipyretic, anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Arch Pharm Res. 2009;32:1191-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 100. | Li J, Huang W, Zhang H, Wang X, Zhou H. Synthesis of andrographolide derivatives and their TNF-alpha and IL-6 expression inhibitory activities. Bioorg Med Chem Lett. 2007;17:6891-6894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Lee JC, Tseng CK, Young KC, Sun HY, Wang SW, Chen WC, Lin CK, Wu YH. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br J Pharmacol. 2014;171:237-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 102. | Chandramohan V, Kaphle A, Chekuri M, Gangarudraiah S, Bychapur Siddaiah G. Evaluating Andrographolide as a Potent Inhibitor of NS3-4A Protease and Its Drug-Resistant Mutants Using In Silico Approaches. Adv Virol. 2015;2015:972067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 103. | Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin--from molecule to biological function. Angew Chem Int Ed Engl. 2012;51:5308-5332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 614] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 104. | Mantzorou M, Pavlidou E, Vasios G, Tsagalioti E, Giaginis C. Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytother Res. 2018;32:957-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 105. | Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors. 2013;39:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 106. | Sordillo PP, Helson L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo. 2015;29:1-4. [PubMed] |
| 107. | Tang Y, Zheng S, Chen A. Curcumin eliminates leptin’s effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology. 2009;150:3011-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Suyasunanont D, Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol. 2009;2009:981963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 109. | Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 110. | Chen MH, Lee MY, Chuang JJ, Li YZ, Ning ST, Chen JC, Liu YW. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int J Mol Med. 2012;30:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Kim K, Kim KH, Kim HY, Cho HK, Sakamoto N, Cheong J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010;584:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 112. | Anggakusuma , Colpitts CC, Schang LM, Rachmawati H, Frentzen A, Pfaender S, Behrendt P, Brown RJ, Bankwitz D, Steinmann J. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 113. | Azzam HS, Goertz C, Fritts M, Jonas WB. Natural products and chronic hepatitis C virus. Liver Int. 2007;27:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Yu YY, Wang QH, Zhu LM, Zhang QB, Xu DZ, Guo YB, Wang CQ, Guo SH, Zhou XQ, Zhang LX. A clinical research on oxymatrine for the treatment of chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2002;10:280-281. [PubMed] |
| 115. | Chen N, Liu YH, Liu XJ, Chen YR, Guo YH, Liu M. [Oxymatrine inhibits target cell infection in the HCVcc system]. Zhonghua Gan Zang Bing Za Zhi. 2016;24:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 116. | Chen Y, Li J, Zeng M, Lu L, Qu D, Mao Y, Fan Z, Hua J. [The inhibitory effect of oxymatrine on hepatitis C virus in vitro]. Zhonghua Gan Zang Bing Za Zhi. 2001;9 Suppl:12-14. [PubMed] |
| 117. | Li J, Li C, Zeng M. [Preliminary study on therapeutic effect of oxymatrine in treating patients with chronic hepatitis C]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1998;18:227-229. [PubMed] |
| 118. | Li YH, Wu ZY, Tang S, Zhang X, Wang YX, Jiang JD, Peng ZG, Song DQ. Evolution of matrinic ethanol derivatives as anti-HCV agents from matrine skeleton. Bioorg Med Chem Lett. 2017;27:1962-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Basile A, Pascale M, Franceschelli S, Nieddu E, Mazzei MT, Fossa P, Turco MC, Mazzei M. Matrine modulates HSC70 levels and rescues ΔF508-CFTR. J Cell Physiol. 2012;227:3317-3323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Du NN, Li X, Wang YP, Liu F, Liu YX, Li CX, Peng ZG, Gao LM, Jiang JD, Song DQ. Synthesis, structure-activity relationship and biological evaluation of novel N-substituted matrinic acid derivatives as host heat-stress cognate 70 (Hsc70) down-regulators. Bioorg Med Chem Lett. 2011;21:4732-4735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Peng ZG, Fan B, Du NN, Wang YP, Gao LM, Li YH, Li YH, Liu F, You XF, Han YX. Small molecular compounds that inhibit hepatitis C virus replication through destabilizing heat shock cognate 70 messenger RNA. Hepatology. 2010;52:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Zhao HW, Zhang ZF, Chai X, Li GQ, Cui HR, Wang HB, Meng YK, Liu HM, Wang JB, Li RS. Oxymatrine attenuates CCl4-induced hepatic fibrosis via modulation of TLR4-dependent inflammatory and TGF-β1 signaling pathways. Int Immunopharmacol. 2016;36:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP, Wu BY. Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells. World J Gastroenterol. 2012;18:4199-4206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 124. | Wu XL, Zeng WZ, Jiang MD, Qin JP, Xu H. Effect of Oxymatrine on the TGFbeta-Smad signaling pathway in rats with CCl4-induced hepatic fibrosis. World J Gastroenterol. 2008;14:2100-2105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 125. | Shi GF, Li Q. Effects of oxymatrine on experimental hepatic fibrosis and its mechanism in vivo. World J Gastroenterol. 2005;11:268-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Yang W, Zeng M, Fan Z, Mao Y, Song Y, Jia Y, Lu L, Chen CW, Peng YS, Zhu HY. [Prophylactic and therapeutic effect of oxymatrine on D-galactosamine-induced rat liver fibrosis]. Zhonghua Gan Zang Bing Za Zhi. 2002;10:193-196. [PubMed] |
| 127. | Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q, Su C. Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol. 2014;35:5111-5119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 128. | Lao Y. Clinical study on effect of matrine injection to protect the liver function for patients with primary hepatic carcinoma after trans-artery chemo-embolization (TAE). Zhong Yao Cai. 2005;28:637-638. [PubMed] |
| 129. | Weiskirchen R. Hepatoprotective and Anti-fibrotic Agents: It’s Time to Take the Next Step. Front Pharmacol. 2016;6:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 130. | Huang H, Chen Y, Ye J. Inhibition of hepatitis C virus replication by peroxidation of arachidonate and restoration by vitamin E. Proc Natl Acad Sci USA. 2007;104:18666-18670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 131. | Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, Hemed N, Rowe M, Ohana H, Zabrecky G. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J Clin Gastroenterol. 2005;39:737-742. [PubMed] |
| 132. | Tee HP, Kaffes AJ. Non-small-bowel lesions encountered during double-balloon enteroscopy performed for obscure gastrointestinal bleeding. World J Gastroenterol. 2010;16:1885-1889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 133. | Beloqui O, Prieto J, Suárez M, Gil B, Qian CH, García N, Civeira MP. N-acetyl cysteine enhances the response to interferon-alpha in chronic hepatitis C: a pilot study. J Interferon Res. 1993;13:279-282. [PubMed] |
| 134. | Yang XY, Zhuo Q, Wu TX, Liu GJ. Bicyclol for chronic hepatitis C. Cochrane Database Syst Rev. 2007;CD004994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 135. | Liu GT. Bicyclol: a novel drug for treating chronic viral hepatitis B and C. Med Chem. 2009;5:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 136. | Zhen YZ, Li NR, He HW, Zhao SS, Zhang GL, Hao XF, Shao RG. Protective effect of bicyclol against bile duct ligation-induced hepatic fibrosis in rats. World J Gastroenterol. 2015;21:7155-7164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 137. | Gu Y, Zhao J, Yao XM, Li Y. Effects of bicyclol on immunological liver fibrosis in rats. J Asian Nat Prod Res. 2010;12:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 138. | Hu QW, Liu GT. Effects of bicyclol on dimethylnitrosamine-induced liver fibrosis in mice and its mechanism of action. Life Sci. 2006;79:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Li Y, Li Y, Liu GT. [Protective effects of bicyclol on liver fibrosis induced by carbon tetrachloride]. Zhonghua Yi Xue Za Zhi. 2004;84:2096-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 140. | Lou XE, Xu N, Yao HP, Chen Z. Bicyclol attenuates pro-inflammatory cytokine and chemokine productions in CpG-DNA-stimulated L02 hepatocytes by inhibiting p65-NF-kappaB and p38-MAPK activation. Pharmazie. 2010;65:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 141. | Paracha UZ, Fatima K, Alqahtani M, Chaudhary A, Abuzenadah A, Damanhouri G, Qadri I. Oxidative stress and hepatitis C virus. Virol J. 2013;10:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |