Published online Dec 14, 2018. doi: 10.3748/wjg.v24.i46.5246
Peer-review started: September 25, 2018
First decision: October 16, 2018
Revised: October 28, 2018
Accepted: November 13, 2018
Article in press: November 13, 2018
Published online: December 14, 2018
Processing time: 82 Days and 17.4 Hours
To investigate the antitumor effects and underlying mechanisms of (17R,18R)-2-(1-hexyloxyethyl)-2-devinyl chlorine E6 trisodium salt (YLG-1)-induced photodynamic therapy (PDT) on pancreatic cancer in vitro and in vivo.
YLG-1 is a novel photosensitizer extracted from spirulina. Its phototoxicity, cellular uptake and localization, as well as its effect on reactive oxygen species (ROS) production, apoptosis, and expression of apoptosis-associated proteins were detected in vitro. An in vivo imaging system (IVIS), the Lumina K imaging system, and mouse models of subcutaneous Panc-1-bearing tumors were exploited to evaluate the drug delivery pathway and pancreatic cancer growth in vivo.
YLG-1 was localized to the mitochondria, and the appropriate incubation time was 6 h. Under 650 nm light irradiation, YLG-1-PDT exerted a potent cytotoxic effect on pancreatic cancer cells in vitro, which could be abolished by the ROS scavenger N-acetyl-L-cysteine (NAC). The death mode caused by YLG-1-PDT was apoptosis, accompanied by upregulated Bax and cleaved Caspase-3 and decreased Bcl-2 expression. The results from the IVIS images suggested that the optimal administration route was intratumoral (IT) injection and that the best time to conduct YLG-1-PDT was 2 h post-IT injection. Consistent with the results in vitro, YLG-1-PDT showed great growth inhibition effects on pancreatic cancer cells in a mouse model.
YLG-1 is a potential photosensitizer for pancreatic cancer PDT via IT injection, the mechanisms of which are associated with inducing ROS and promoting apoptosis.
Core tip: Photodynamic therapy (PDT) has become a feasible treatment for advanced pancreatic neoplasms. Photosensitizers play a critical role in PDT. (17R,18R)-2-(1-hexyloxyethyl)-2-devinyl chlorine E6 trisodium salt (YLG-1) is a promising photosensitizer with high water solubility and phototoxicity. The functions of YLG-1-induced PDT (YLG-1-PDT) are still poorly understood. We found that YLG-1-PDT displayed great antitumor effects on pancreatic cancer cells in vitro and in vivo, the mechanisms of which involved inducing reactive oxygen species and promoting apoptosis. The optimal administration of YLG-1 towards pancreatic cancer is an intratumoral injection. Our study suggests that YLG-1 is a potential photosensitizer for pancreatic neoplasm PDT.
- Citation: Shen YJ, Cao J, Sun F, Cai XL, Li MM, Zheng NN, Qu CY, Zhang Y, Shen F, Zhou M, Chen YW, Xu LM. Effect of photodynamic therapy with (17R,18R)-2-(1-hexyloxyethyl)-2-devinyl chlorine E6 trisodium salt on pancreatic cancer cells in vitro and in vivo. World J Gastroenterol 2018; 24(46): 5246-5258
- URL: https://www.wjgnet.com/1007-9327/full/v24/i46/5246.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i46.5246
Pancreatic cancer is one of the most malignant diseases, with a 5-year survival rate less than 5%[1]. Despite advancements in therapies, the overall prognosis remains dismal. Local invasion, early metastasis, and tumor cell chemoresistance after radical surgery or various treatments account for the low survival rate[2]. Therefore, there is still a great need for additional therapies to improve the prognosis of pancreatic cancer.
Photodynamic therapy (PDT) is a minimally invasive ablation of local tumors and other diseases that impairs target cells or microorganisms via inducing the production of reactive oxygen species (ROS)[3,4]. Photosensitizer accumulation in target tissues, specific light wavelengths, and oxygen are at the core of PDT[5]. A growing body of studies indicate that PDT is a promising therapy for tumors in that it not only directly kills tumor cells and damages the tumor vasculature but also enhances antitumor immune responses to prevent recurrence[6,7]. However, the characteristics associated with pancreatic cancer, including its deep location and hypovascular nature, seriously affect optical accessibility and photosensitizer accumulation in tumor tissues, which limits the application of PDT in pancreatic cancer therapy. With the development of endoscopic ultrasonography (EUS) and EUS-guided fine needle aspiration (EUS-FNA), the problems associated with optical accessibility have been well resolved. Initially, EUS and EUS-FNA have been applied in pancreatic neoplasm diagnosis. In 2015, Choi et al[8] demonstrated that EUS-guided PDT with a flexible laser-light catheter inserted directly into the tumor is feasible and safe for locally advanced pancreaticobiliary malignancies. Thus, the selection of an appropriate photosensitizer is currently of critical importance for PDT.
Chlorins are second-generation photosensitizers with a longer absorption band (650 nm), more ROS generation, and lower skin sensitivity than commercially available porphyrin photosensitizers (630 nm) such as 5-aminolevulinic acid (5-ALA) and porfimer sodium (Photofrin)[5,9,10]. (17R,18R)-2-(1-hexyloxyethyl)-2-devinyl chlorine E6 trisodium salt (YLG-1) is a hydrophilic chlorine derivative extracted from spirulina chlorophyll. Spirulina is now being considered as a health supplement but has a light sensitivity as a side effect[11]. It has been used as the raw material for many photosensitizers including methyl 3-[1’-(m-iodobenzyloxy) ethyl] pyropheophorbide-a 2, selenium-containing phycocyanin, and chlorin e6 (Ce6)[12-14]. YLG-1 was initially approved as a disinfection product owing to its great antimicrobial effect under PDT. It possesses various characteristics including a high purity of 99.5%, high water-solubility, high chemical stability, high phototoxicity, low dark toxicity, and a low price according to the product manual. The advantages of YLG-1 make it a potential candidate for applications in clinical work. However, the functions of YLG-1-induced PDT (YLG-1-PDT) are still poorly understood, with no available publications. Thus, we wondered whether YLG-1-PDT displays antitumor efficacy.
Here, we evaluated the antitumor effects and associated mechanisms of YLG-1-PDT on pancreatic cancer cells in vitro and in vivo. YLG-1 was found to be localized to the mitochondria and exert great phototoxicity on pancreatic cancer cells in vitro, the mechanisms of which involved inducing ROS production and apoptosis. To ensure the photosensitizer to accumulate in pancreatic tumors in vivo, YLG-1 was administered by intratumoral (IT) injection according to in vivo imaging system (IVIS) images. In in vivo experiments, pancreatic tumor growth was potently inhibited by YLG-1-PDT. We thus proposed that YLG-1 is a potential photosensitizer in pancreatic cancer PDT. Our findings provided fundamental research for the clinical application of YLG-1 in pancreatic cancer therapies.
YLG-1 was provided by Guilin Huiang Biochemistry Pharmaceutical Co., Ltd (Guilin, China). It was dissolved in phosphate buffer solution (PBS; Solarbio, China) to obtain a 10 mg/mL solution and then stored at 4 °C. The 650-nm laser PDT instrument was provided by Guilin Xingda Photoelectric Medical Equipment Co., Ltd (Guilin, China).
The UV-Vis absorption spectrum of YLG-1 was measured on an ultraviolet visible spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan). The fluorescence (FL) spectra were detected using a fluorescence spectrometer (LS-55, PerkinElmer, Waltham, MA, United States). Slits were kept narrow to 1 nm for excitation and at 1 or 2 nm for emission.
The human pancreatic cancer cell lines SW1990 and Panc-1 were purchased from the cell bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). SW1990 and Panc-1 cells were cultured in RPMI 1640 medium (GIBCO, Grand Island, NY, United States) or DMEM (GIBCO), respectively, with 10% fetal bovine serum (FBS) (GIBCO) at 37 °C in a 5% CO2 atmosphere.
SW1990 or Panc-1 cells were seeded in 12-well plates and cultured to 70%-80% confluence. The cells were treated with serum-free culture medium containing 0.5 μg/mL YLG-1 at 37 °C for 0-12 h. After washing twice with PBS, the cells were treated with 500 μL cell lysate (0.3% Triton X-100 in PBS) for 30 min at 37 °C as described in a previous report[15]. Then, the cell lysate was centrifuged to acquire the supernatant. The FL intensity of 100 μL supernatant was determined using a Bio-Tek Synergy H1 (Ex = 397 nm and Em = 652 nm) (Bio-Tek, Winooski, VT, United States).
To locate the YLG-1, the cells were cultured to 40%-60% confluence, loaded with 0.5 μg/mL YLG-1 for 6 h in serum-free culture medium, and then incubated with 100 nmol/L MitoTracker Green FM (Yeasen Biotechnology, Shanghai, China) (Ex = 490 nm and Em = 516 nm) for 30 min to label mitochondria. The cells were then washed with PBS and replaced with fresh culture medium. Then, a confocal microscope (Leica, Wetzlar, Germany) was used to observe the FL.
Cells were incubated with YLG-1 at concentrations ranging from 0 to 5 μg/mL in serum-free culture at 37 °C or exposed to laser light doses of 0 to 10 J/cm2 at 650 nm. After 24 h, the cells were subjected to CCK-8 assay (Yeasen Biotechnology) according to the manufacturer’s protocol. The absorbance was measured at 450 nm with the Bio-Tek Synergy H1. In another experiment, the cells were incubated with the indicated dose of YLG-1 for an indicated time and then irradiated at 5 or 10 J/cm2 for CCK-8 assay after 24 h.
Cells were incubated with the indicated dose of YLG-1 for 6 h with or without exposure to laser light (10 J/cm2) and then divided into the following four groups: control (Con) group, light-only (Light) group, YLG-1 alone (YLG-1) group, and YLG-1-PDT (PDT) group. Before irradiation, the cells were incubated with 10 μmol/L DCFH-DA (ROS-sensitive probe; Yeasen Biotechnology) for 30 min at 37 °C. The intracellular ROS was detected by observing the DCF using the Leica DMI6000B Live Cell Application (Leica, Wetzlar, Germany) at 1 h postirradiation. To quantitatively detect the FL intensity of the DCF, the cells were treated with 0.3% Triton X-100 for 30 min at 37 °C and centrifuged to acquire the supernatant. The FL intensity was read with the Bio-Tek Synergy H1 (Ex = 488 nm and Em = 525 nm). In another experiment, the PDT group was preincubated with 5 mmol/L N-acetyl-L-cysteine (NAC) (Yeasen Biotechnology), an ROS scavenger, for 1 h before irradiation. The following steps were conducted as mentioned above. Cell viability was evaluated using the CCK-8 assay.
Cell grouping and culturing conditions were consistent with the aforementioned experiments. At 4 h after illumination, the cells were harvested for staining with Alexa Fluor 488 Annexin V and propidium iodide (PI) (BD apoptosis assay kit, BD Pharmingen, CA, United States) according to the manufacturer’s protocol. In total, 104 cells per group were analyzed immediately using a FACSCalibur flow cytometer (BD Biosciences, CA, United States). The data were analyzed using the FlowJo software.
To detect apoptosis-associated protein expression, cells were collected for Western blot assay at 8 h after illumination. In total, 20-40 μg protein/well was separated on 10% or 12% SDS-polyacrylamide gels and transferred to PVDF membranes. After blocking with 5% BSA (Yeasen Biotechnology), the blots were incubated with antibodies (Cell Signaling Technology, Danvers, United States) against β-actin (1:3000), Bax (1:1000), Bcl-2 (1:1000), and Caspase-3 (1:1000) overnight at 4 °C. The membranes were subjected to incubation with HRP-conjugated secondary antibodies (Yeasen Biotechnology) for 1 h. Finally, the proteins were detected using enhanced chemiluminescent reagents (Yeasen Biotechnology).
Female BALB/c nude mice at 6 wk of age were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences and reared in a specific pathogen-free facility (23 °C, 12 h/12 h light/dark cycle, 50% humidity, and ad libitum access to food and water). All experimental procedures were approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, with approved institutional protocols set by the China Association of Laboratory Animal Care. Panc-1 cells (5 × 106) suspended in 100 μL cold PBS were subcutaneously injected into the lower back region of mice. The tumor volume was calculated using the following formula: 1/2 × width2 × length. When tumors sizes reached approximately 100 mm3, the surviving mice were intraperitoneally injected with 60, 30, or 8 mg/kg YLG-1 or intratumorally with YLG-1 at doses of 30 or 8 mg/kg. At the indicated time points, the mice were intraperitoneally anesthetized with 10 mg/mL pentobarbital sodium (40 mg/kg) for imaging using an IVIS Lumina K Series III imaging system (PerkinElmer, United States) (Ex = 420 nm and Em = 670 nm; the optimal absorbance and emission peaks were unavailable using this IVIS imaging system). The FL intensity of YLG-1 in the tumor site was quantified under the same color scale (min = 6.35e8 and max = 6.31e9).
The Panc-1 tumor-bearing mice were randomly divided into the following four groups (n = 6) when the tumors volumes reached 100 mm3: Con group, Light group, YLG-1 group, and PDT group. Mice in the PDT group were intratumorally injected with 8 mg/kg YLG-1 followed by 650 nm laser-irradiation (100 J/cm2) at 2 h postinjection. The Light and YLG-1 groups received the same intensity of irradiation or the same dosage of YLG-1 as the PDT group. The subcutaneous tumors were measured within 14 d. Tumor tissue was embedded and sliced for staining with hematoxylin and eosin (H&E). Histopathological changes were observed under a light microscope (DMI4000 B, Leica Microsystems, Wetzlar, Germany). No body weight changes or other apparent adverse effects were observed on the mice during the study period.
All data are shown as the mean ± SD and replicated three or more times. All data were assessed by t-tests and one-way ANOVA (SPSS 17.0 software). P values < 0.05 were considered statistically significant.
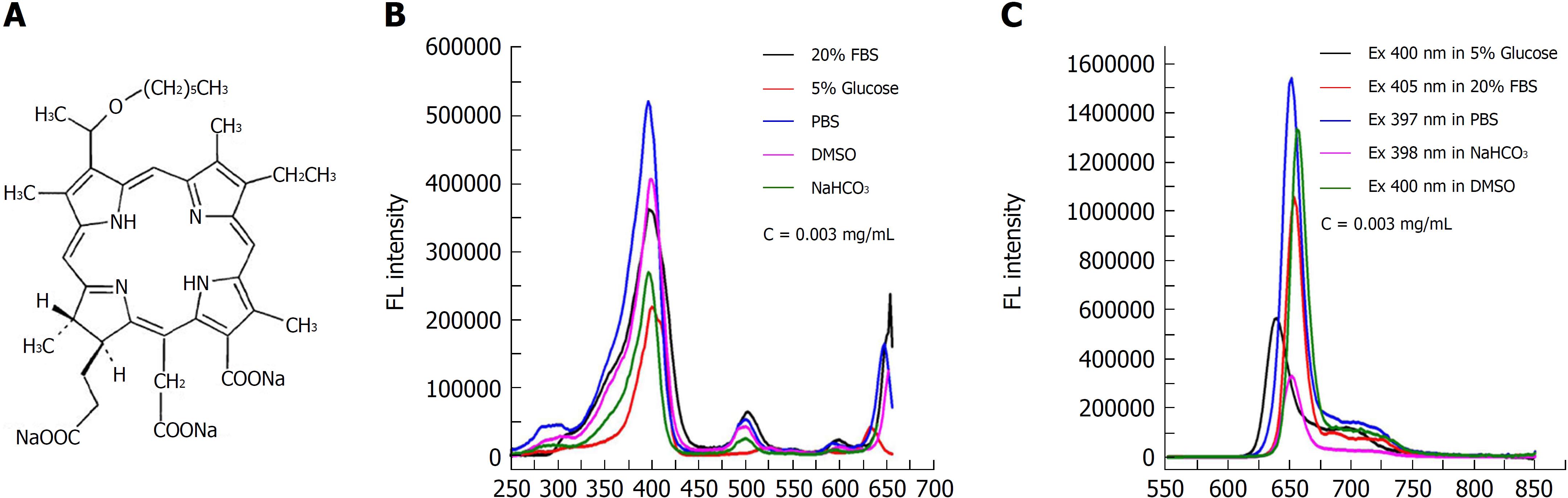
The chemical structure of the photosensitizer YLG-1 is shown in Figure 1A. YLG-1 dissolved in different solutions had similar spectral peak positions but at different values, suggesting the high chemical stability of YLG-1. It appeared that PBS was the optimal solution for YLG, with strong absorption and emission peaks (Figure 1B and C). In our study, YLG-1 was dissolved in PBS, which had several distinct absorption peaks at approximately 397 nm, 500 nm, 600 nm, and 652 nm; the maximum peak was at 397 nm. As shown in Figure 1C, the peak emission for FL was detected at approximately 652 nm. Considering the penetration depth of light, 650 nm laser irradiation was used in the PDT for pancreatic cancer.
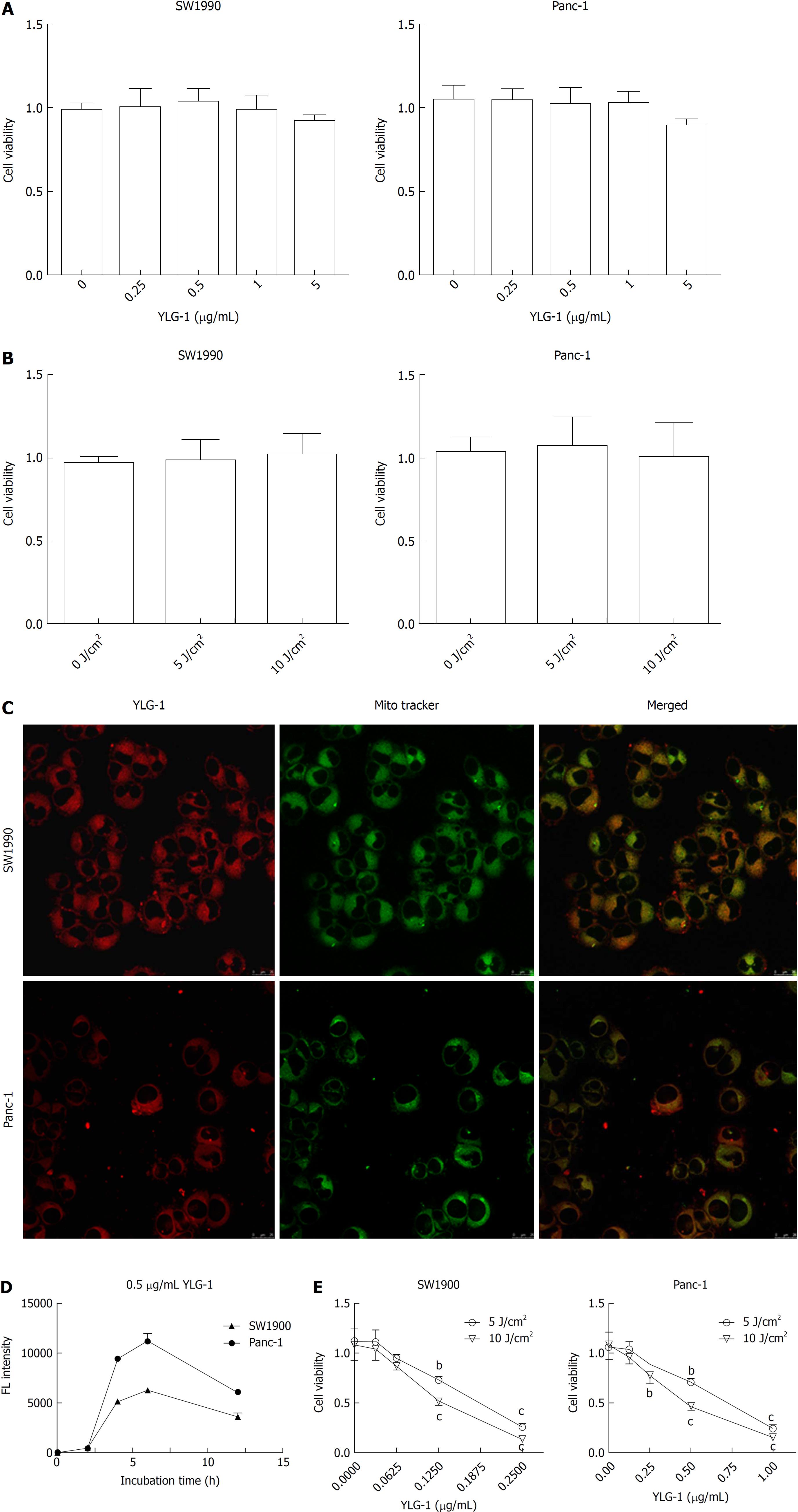
As shown in Figure 2A and B, YLG-1 (0-5 μg/mL) or light (0-10 J/cm2) alone had no significant cytotoxicity on the cells at 24 h posttreatment. To assess the appropriate incubation time for YLG-1 with cells, the cells were incubated with 0.5 μg/mL YLG-1 for 0-12 h. The YLG-1 FL intensity increased with time and peaked at 6 h in SW1990 and Panc-1 cells, before fading with time during the next 6-12 h (Figure 2C). Therefore, 6 h was selected as the incubation time in the following experiments.
Next, we investigated the subcellular localization of YLG-1 using mitochondrial FL probes. The cells were loaded with 0.5 μg/mL YLG-1 for 6 h and incubated with MitoTracker Green FM for 30 min. Yellow FL regions showed overlap between the mitochondria (green FL) and YLG-1 (red FL). The results showed that YLG-1 was primarily localized in mitochondria (Figure 2D).
To study the influence of YLG-1-PDT on cell viability, SW1990 or Panc-1 cells were incubated with 0-0.25 or 0-1 μg/mL YLG-1 for 6 h, respectively. As shown in Figure 2E, enhanced cell death occurred with higher YLG-1 concentrations and illumination doses. Cell viability was significantly decreased by YLG-1-PDT in SW1990 cells (IC50 = 0.18 μg/mL at 5 J/cm2 and 0.13 μg/ml at 10 J/cm2) and Panc-1 (IC50 = 0.67 μg/mL at 5 J/cm2 and 0.46 μg/mL at 10 J/cm2). Hence, 0.13 and 0.46 μg/mL YLG-1 at a light intensity of 10 J/cm2 was used in the following ROS production and apoptosis experiments.
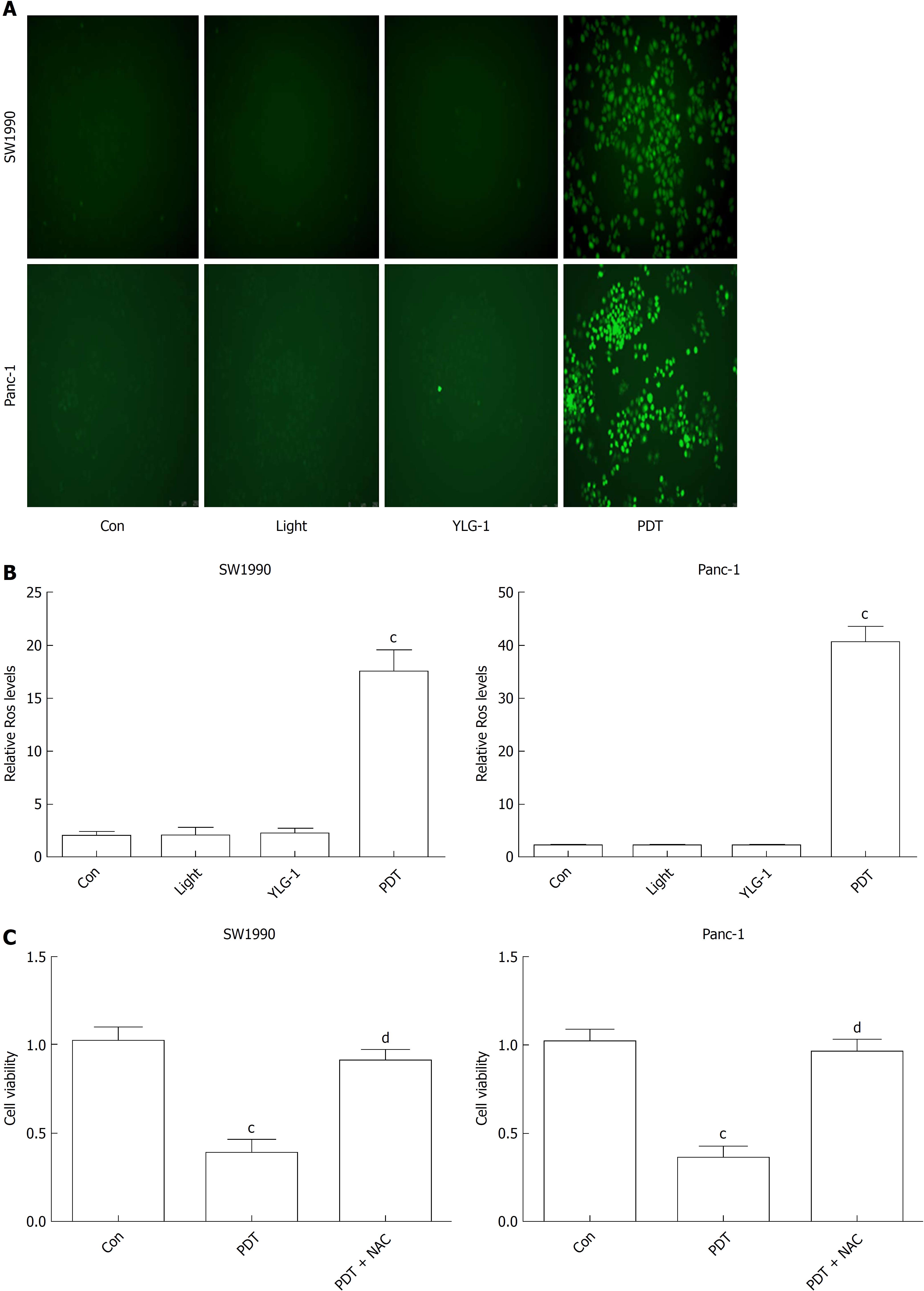
To understand the therapeutic mechanisms of YLG-1-PDT in killing pancreatic cancer, we detected cellular ROS production and the frequency of apoptosis in vitro. As shown in Figure 3A and B, ROS generation increased significantly in both the SW1990 and Panc-1 cell PDT groups (0.13 and 0.46 μg/mL, respectively, and 10 J/cm2) at 1 h postirradiation compared with the Con groups, while no significant changes in ROS contents were observed among the YLG-1, Light, and Con groups. To verify the role of ROS in YLG-1-PDT-induced cytotoxicity, we pretreated cells with ROS scavenger (5 mmol/L NAC) 1 h prior to irradiation. When ROS production was inhibited by NAC, pancreatic cancer cell viability was also restored (Figure 3C). These data indicated that YLG-1-PDT could kill pancreatic cancer cells by inducing the production of ROS.
To investigate the cell death mechanism of pancreatic cancer cells induced by YLG-1-PDT, flow cytometry was performed 4 h after treatment. As shown in Figure 4A, the rates of early and late apoptosis were dramatically higher in the SW1990 and Panc-1 cell PDT groups than in the Con groups (44.03% in the SW1990 PDT group vs 10.09% in the Con group and 29.2% in the Panc-1 PDT group vs 2.35% in the Con group, respectively), while those in the YLG-1, Light, and Con groups exhibited no significant changes. Moreover, the pro-apoptotic protein expression of Bax and cleaved Caspase-3 in both the SW1990 and Panc-1 PDT groups was significantly upregulated compared to the Con groups at 8 h postillumination, while anti-apoptotic Bcl-2 expression showed the opposite results (Figure 4B). Meanwhile, the YLG-1 and Light groups showed no obvious alterations in the expression of those proteins compared to the Con groups. Therefore, YLG-1-PDT promoted pancreatic tumor apoptosis by upregulating cleaved Caspase-3 and Bax expression and downregulating Bcl-2 expression.
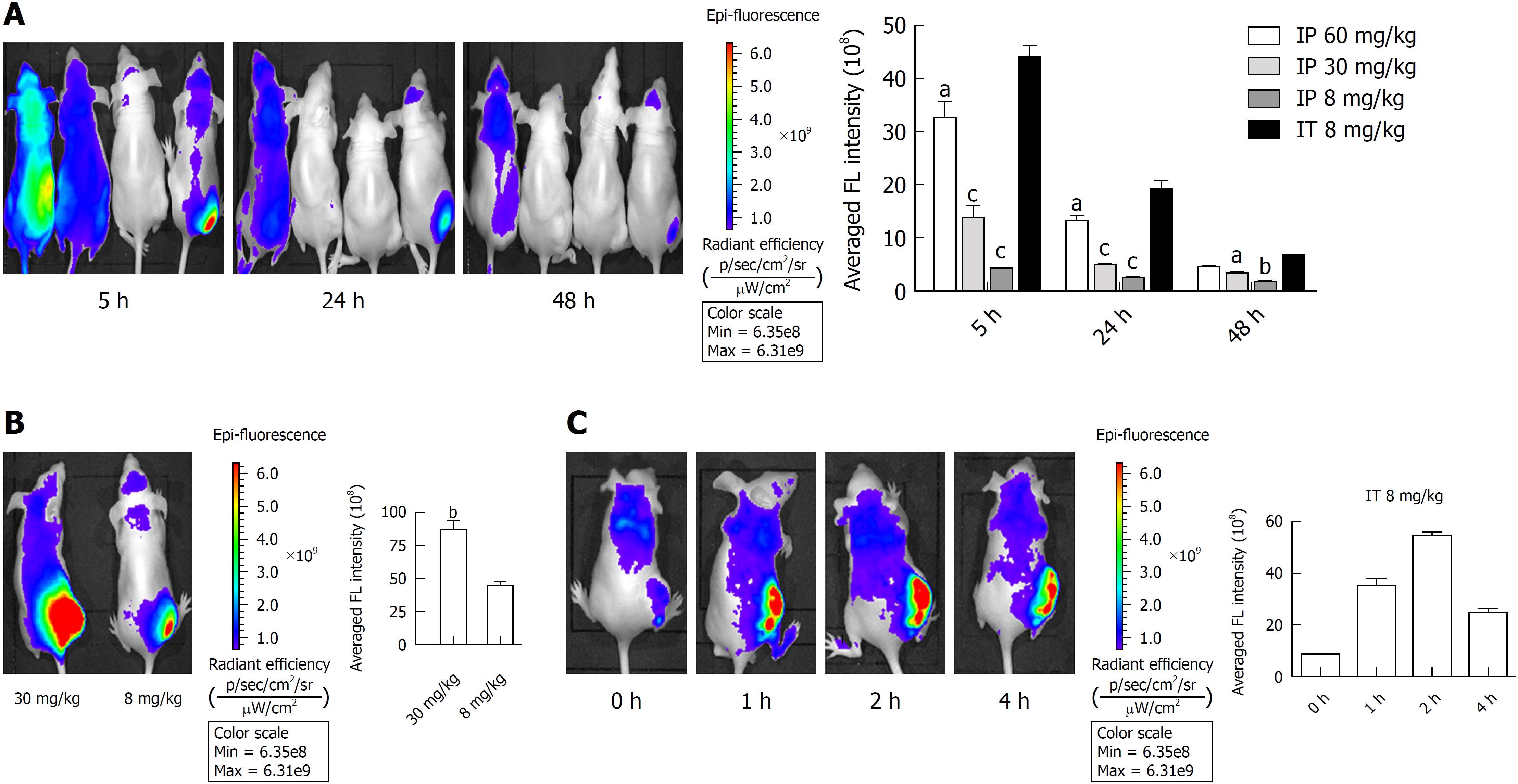
To select the appropriate YLG-1 administration route, we detected the biodistribution of YLG-1 via intraperitoneal (IP) and IT injection in Panc-1 tumor-bearing mice using an IVIS Lumina K imaging system. As displayed in Figure 5A, the mice listed from left to right were respectively administered with 60, 30, and 8 mg/kg YLG-1 via IP injection and 8 mg/kg YLG-1 by IT injection. IVIS images were captured at 5, 24, and 48 h postinjection. The images showed that the FL of YLG-1 was widely distributed throughout the mouse bodies 5 h after IP injection with three doses of YLG and did not show tumor-specific accumulation. In contrast, mice treated with YLG-1 IT injections showed strong FL signals concentrated in the tumor with weak signals in other parts of the body. Later, the YLG-1 FL signal in all the mice faded away. The FL signal in the mice with IT injections declined at a considerably slower rate compared with mice with IP injections. We believe that the low tumor-specific accumulation of YLG-1 by systemic administration was due to the rapid clearance of free small molecular compounds from the blood circulation. Thus, our data suggested the optimal drug delivery route for YLG-1 was IT injection.
As shown in Figure 5B, when the IT injection dosage of YLG-1 reached 30 mg/kg YLG-1, FL signal in the tumor area appeared oversaturated and the outline of the tumor could not be clearly depicted. This phenomenon indicated that YLG-1 overflowed into the surrounding region, which might damage the surrounding tissues and result in some complications when conducting PDT.
To better evaluate the FL biodistribution of YLG-1 at various time points, the tumor-bearing mice were intratumorally injected with 8 mg/kg YLG-1 and imaged at 0-4 h postinjection. The FL signal in the tumor could be clearly detected at 1 h after injection and rapidly peaked at 2 h (Figure 5C). Then, the average FL intensity declined, decreasing to approximately 50% at 4 h postinjection (from 5.46 × 109 ± 1.414 × 108 to 2.45 × 109 ± 1.56 × 108). Therefore, we determined that the best time to perform YLG-1-PDT was 2 h after IT injection.
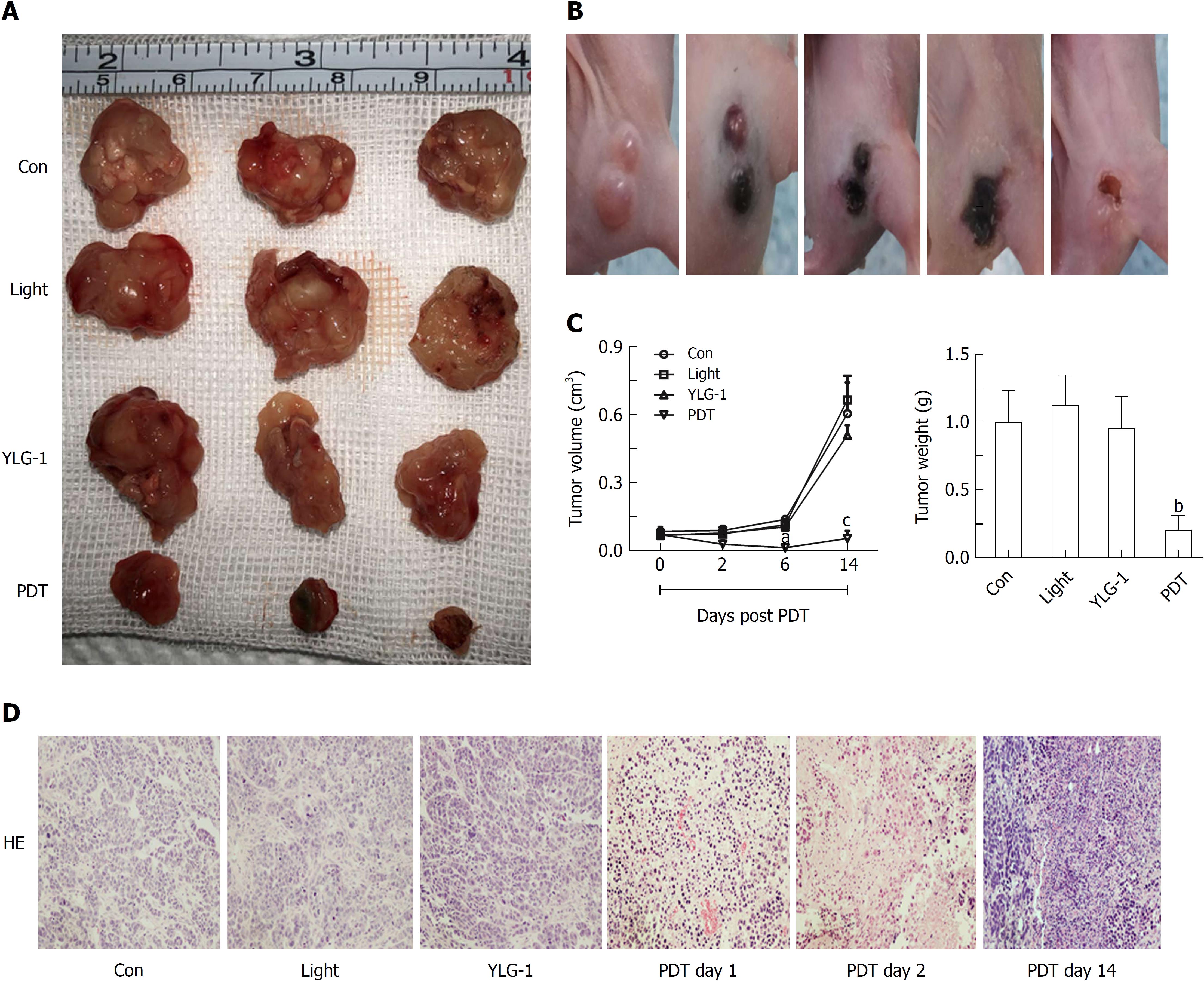
Encouraged by the antitumor effects of YLG-1-PDT in vitro, mice in the PDT group were treated with 8 mg/kg YLG-1 by IT injection and exposed to 650 nm laser-irradiation (100 J/cm2) at 2 h postinjection. Images were taken at 0-14 d post-PDT to show the dynamic changes. Tumors treated with YLG-1-PDT gradually became dark, shrunk within 6 d, and eventually formed a scab by 14 d (Figure 6B). In contrast, tumors in the Con, YLG-1, and Light groups continued to grow, and there were no significant differences among the tumor volumes in the three groups (Figure 6A). The PDT group exhibited significantly smaller tumors than the Con group during the 6-14 d posttreatment. Similarly, tumors weights in the PDT group were significantly lower than those in the Con group at 14 d posttreatment (Figure 6C). Tissues from the Con group displayed pleomorphic cell nuclei, compact tumor cells, and an intact structure. No remarkable morphological differences were observed between the YLG-1, Light, and Con groups. However, cellular swelling, nuclear fragments, and large areas of necrosis were observed in the PDT group (Figure 6D). These data further supported the antitumor effects of YLG-1-PDT on pancreatic neoplasms in vivo.
Effective treatment regimens remain an urgent need for pancreatic cancer due to its poor prognosis. PDT was demonstrated to inhibit the growth of pancreatic cancer cells in fundamental and clinical experiments[16-19]. Advancements in EUS have made PDT a feasible clinical therapy for pancreatic cancer. The development of appropriate photosensitizers is highly desired to improve the effects of PDT. Here, we demonstrated that YLG-1 had potent PDT effects for use in pancreatic cancer therapy.
The high phototoxic effect of a photosensitizer is an essential feature for PDT. In our study, we found that YLG-1 had a broad safe dose up to at least 5 μg/mL in pancreatic cancer. Due to its high water-solubility, YLG-1 could exert potent phototoxicity in both cells lines at less than 0.5 μg/mL (10 J/cm2). These data indicated that YLG-1 is a safe and efficient photosensitizer.
It has been widely accepted that PDT-induced cytotoxicity is attributed to the generation of ROS. Here, we detected a significant increase in ROS production at 1 h post-YLG-1-PDT treatment. The crucial role of ROS in YLG-1-PDT was further confirmed using the ROS scavenger NAC, which recovered pancreatic cancer cell viability. Next, we observed the distribution of YLG-1 in the mitochondria and YLG-1-PDT-induced apoptosis in pancreatic cancer cells. Because the subcellular localization of a photosensitizer can impact its mechanisms of action, we considered that the mitochondria-caspase pathway was probably involved in the apoptotic mechanism. It is well known that Bax and cleaved Caspase-3 (active Caspase-3) are pro-apoptotic proteins and Bcl-2 is an antiapoptotic protein[20-23]. As expected, Bax and cleaved Caspase-3 expression was significantly upregulated by YLG-1-PDT, while Bcl-2 expression exhibited the opposite results. Our data suggested that YLG-1-PDT could eradicate pancreatic cancer cells by inducing ROS and promoting apoptosis, accompanied by an increase in Bax and cleaved Caspase-3 expression and a decrease in Bcl-2 expression.
The highly hydrophilic properties of a photosensitizer are a double-edged sword. It can enhance their efficiency locally, but it is always accompanied by their rapid clearance from blood circulation, resulting in lacking tumor-targeting abilities. As we expected, YLG-1 did not exhibit tumor-specific accumulation via systemic IP injection (even up to 60 mg/kg). For photosensitizers with high water solubility, IT injection might be an appropriate way to promote their accumulation in tumor tissues. Administration via IT injection with only 8 mg/kg YLG-1 could achieve a strong and long-term FL intensity within the tumor area. This suggested that the optimal administration route for YLG-1 was IT injection. Indeed, IT injection is an acknowledged administration route applied in many studies[24-27]. Compared with conventional systematic administration, a small amount of photosensitizer could be effective via IT injection. We believe that IT injection should be attempted to apply clinical PDT, especially for hypovascular tumors such as pancreatic cancer. Inspired by the direct insertion of an EUS-mediated laser-light catheter into tumors to deliver sufficient illumination in pancreatic cancer PDT[8], we proposed that an EUS-guided IT injection method with a photosensitizer using a puncture needle might be a feasible and appropriate way for photosensitizer accumulation in pancreatic tumor tissue. Based on our experiments, we believe that YLG-1 might be suitable for superficial, luminal, and hypovascular tumors that require topical administration, and that YLG-1 might be a promising photosensitizer in pancreatic cancer PDT. Aside from changing the drug delivery route, modifying YLG-1 is another solution to improve the concentration of photosensitizer in tumors. Hu et al[28] developed nanoparticles by hierarchically assembling doxorubicin (DOX), Ce6, and colloidal manganese dioxide (MnO2) with poly (ε-caprolactone-co-lactide)-b-poly (ethylene glycol)-b-poly (ε-caprolactone-co-lactide) to treat breast cancer, which enhanced tumor uptake of the photosensitizer by prolonging its blood circulation. We wonder whether YLG-1 has the potential to be modified in that way; thus, more research focused on YLG-1 characteristics should be performed in the future.
We next demonstrated that YLG-1-PDT significantly decreased the growth of pancreatic tumors in vivo by IT administration. The tumors treated with PDT mostly disappeared at 6 d posttreatment and were significantly smaller than the untreated group during the 6-14 d. However, at 14 d posttreatment, the tumors returned, though with much smaller tumor volumes. Therefore, similar to other anticancer therapies, PDT could leave behind a significant number of surviving tumor cells, as cancer cells surrounding the focally treated area were always subjected to a low dose laser light. The surviving cells are likely to become the source for relapse and metastasis. This suggested that multipoint fiber insertion irradiation or multiple PDT or multimodal regimens were indispensable for clinical pancreatic neoplasm PDT.
In conclusion, our results demonstrate that YLG-1-PDT has a potent antitumor effect on pancreatic cancer cells via inducing ROS and apoptosis. Although YLG-1 lacks specific tumor-targeting accumulation due to its small and highly soluble nature, it could contribute to locally high efficiency via topical delivery. With regard to the hypovascular characteristics of pancreatic tumors, IT injection, rather than systemic administration, is the appropriate drug delivery route. Hence, we assume that YLG-1 is a potential photosensitizer for pancreatic neoplasm PDT. To apply YLG-1 in more cancers, further studies should focus on modifying YLG-1 to target tumor accumulation.
With the development of endoscopic ultrasonography (EUS) and EUS-guided fine needle aspiration (EUS-FNA), photodynamic therapy (PDT) has become a feasible treatment for advanced pancreatic neoplasms. The selection of an appropriate photosensitizer is of great importance in PDT. (17R,18R)-2-(1-hexyloxyethyl)-2-devinyl chlorine E6 trisodium salt (YLG-1) is hydrophilic chlorine derivative extracted from spirulina. It possesses characteristics including high purity, high water-solubility, high chemical stability, high phototoxicity, low dark toxicity as well as low price. Initially, YLG-1 was approved as a disinfection product for its great antimicrobial effect under illumination. However, the functions of YLG-1 in PDT are still poorly understood with no available publication.
Our findings will provide fundamental research for the clinical application of YLG-1 in pancreatic cancer therapy.
To explore the antitumor effects of YLG-1-induced PDT (YLG-1-PDT) on pancreatic cancer cells and its underlying mechanisms in vitro and in vivo.
The human pancreatic cancer cell lines SW1990 and Panc-1 were used to detect the effects of YLG-1. CCK-8 assay, Bio-Tek Synergy H1, confocal microscopy, DCFH-DA, flow cytometry, and Western blot were exploited to detected the phototoxicity, cellular uptake, localization, reactive oxygen species (ROS) production, apoptosis and apoptosis-associated proteins (Bax, Bcl-2, and cleaved Caspase-3) expression, respectively. An in vivo imaging system (IVIS), the Lumina K imaging system, and mouse models of subcutaneous Panc-1-bearing tumors were used to assess the drug-delivered way of YLG-1 and pancreatic tumor growth in vivo.
YLG-1 was located in mitochondria and the appropriated incubation time was 6 h. Under 650 nm illumination, YLG-1 exhibited a potent phototoxicity on pancreatic cancer cells with a great generation of ROS in vitro. Besides, YLG-1-PDT induced pancreatic cancer cell apoptosis, upregulated Bax and cleaved Caspase-3 expression and decreased Bcl-2 expression. IVIS images indicated the optimal administration of YLG-1 was intratumoral (IT) injection and the best time to perform PDT was 2 h post IT injection. In accordance with the results in vitro, YLG-1-PDT potently inhibited the growth of pancreatic cancer cells in a mouse model. Notably, due to its small and highly soluble nature, YLG-1 lacked specific tumor-targeting accumulation and had better be applied by topical administration.
YLG-1 is a potential photosensitizer for pancreatic cancer PDT via IT injection, the mechanisms of which are related with inducing ROS and promoting apoptosis. Hence, YLG-1-PDT might be a promising component of multimodality therapy of pancreatic neoplasms.
Our results demonstrated that YLG-1-PDT had a potent antitumor effect on pancreatic cancer cells via inducing ROS and apoptosis. Since YLG-1 is lack of specific tumor-targeting accumulation, it might be suitable for superficial, luminal, and hypovascular tumors that need topical administration. Thus, YLG-1 is a promising photosensitizer in pancreatic cancer PDT in terms of its hypovascular character. In order to apply YLG-1 in more cancers, further studies should focus on modifying YLG-1 for target tumor accumulation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ashida R, Guo XZ S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1716] [Article Influence: 190.7] [Reference Citation Analysis (1)] |
| 2. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1313] [Article Influence: 145.9] [Reference Citation Analysis (2)] |
| 3. | Mallidi S, Anbil S, Bulin AL, Obaid G, Ichikawa M, Hasan T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics. 2016;6:2458-2487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Cieplik F, Deng D, Crielaard W, Buchalla W, Hellwig E, Al-Ahmad A, Maisch T. Antimicrobial photodynamic therapy - what we know and what we don’t. Crit Rev Microbiol. 2018;44:571-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 520] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 5. | Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O, Kotowski K, Kulbacka J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 1215] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 6. | Yang Y, Hu Y, Wang H. Targeting Antitumor Immune Response for Enhancing the Efficacy of Photodynamic Therapy of Cancer: Recent Advances and Future Perspectives. Oxid Med Cell Longev. 2016;2016:5274084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 622] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 8. | Choi JH, Oh D, Lee JH, Park JH, Kim KP, Lee SS, Lee YJ, Lim YS, Song TJ, Lee SS. Initial human experience of endoscopic ultrasound-guided photodynamic therapy with a novel photosensitizer and a flexible laser-light catheter. Endoscopy. 2015;47:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Usuda J, Ichinose S, Ishizumi T, Hayashi H, Ohtani K, Maehara S, Ono S, Honda H, Kajiwara N, Uchida O. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin Cancer Res. 2010;16:2198-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Chang JE, Liu Y, Lee TH, Lee WK, Yoon I, Kim K. Tumor Size-Dependent Anticancer Efficacy of Chlorin Derivatives for Photodynamic Therapy. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Zeinalian R, Farhangi MA, Shariat A, Saghafi-Asl M. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: a randomized double blinded placebo controlled trial. BMC Complement Altern Med. 2017;17:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Jeon YM, Lee HS, Jeong D, Oh HK, Ra KH, Lee MY. Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life Sci. 2015;124:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Fu X, Huang W, Li C, Wang X, Huang B. Photodynamic effect and mechanism study of selenium-enriched phycocyanin from Spirulina platensis against liver tumours. J Photochem Photobiol B. 2018;180:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Srivatsan A, Pera P, Joshi P, Wang Y, Missert JR, Tracy EC, Tabaczynski WA, Yao R, Sajjad M, Baumann H. Effect of chirality on cellular uptake, imaging and photodynamic therapy of photosensitizers derived from chlorophyll-a. Bioorg Med Chem. 2015;23:3603-3617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Chen WH, Lecaros RL, Tseng YC, Huang L, Hsu YC. Nanoparticle delivery of HIF1α siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer. Cancer Lett. 2015;359:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Ding F, Li HJ, Wang JX, Tao W, Zhu YH, Yu Y, Yang XZ. Chlorin e6-Encapsulated Polyphosphoester Based Nanocarriers with Viscous Flow Core for Effective Treatment of Pancreatic Cancer. ACS Appl Mater Interfaces. 2015;7:18856-18865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, Kent E, Bown SG, Hasan T, Pogue BW. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110:1698-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 18. | Roh YJ, Kim JH, Kim IW, Na K, Park JM, Choi MG. Photodynamic Therapy Using Photosensitizer-Encapsulated Polymeric Nanoparticle to Overcome ATP-Binding Cassette Transporter Subfamily G2 Function in Pancreatic Cancer. Mol Cancer Ther. 2017;16:1487-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Li MM, Cao J, Yang JC, Shen YJ, Cai XL, Chen YW, Qu CY, Zhang Y, Shen F, Xu LM. Effects of arginine-glycine-aspartic acid peptide-conjugated quantum dots-induced photodynamic therapy on pancreatic carcinoma in vivo. Int J Nanomedicine. 2017;12:2769-2779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Li KT, Chen Q, Wang DW, Duan QQ, Tian S, He JW, Ou YS, Bai DQ. Mitochondrial pathway and endoplasmic reticulum stress participate in the photosensitizing effectiveness of AE-PDT in MG63 cells. Cancer Med. 2016;5:3186-3193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Shao J, Xue J, Dai Y, Liu H, Chen N, Jia L, Huang J. Inhibition of human hepatocellular carcinoma HepG2 by phthalocyanine photosensitiser PHOTOCYANINE: ROS production, apoptosis, cell cycle arrest. Eur J Cancer. 2012;48:2086-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Liu YQ, Meng PS, Zhang HC, Liu X, Wang MX, Cao WW, Hu Z, Zhang ZG. Inhibitory effect of aloe emodin mediated photodynamic therapy on human oral mucosa carcinoma in vitro and in vivo. Biomed Pharmacother. 2018;97:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Allison RR, Moghissi K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin Endosc. 2013;46:24-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 426] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 24. | Foster TH, Giesselman BR, Hu R, Kenney ME, Mitra S. Intratumor administration of the photosensitizer pc 4 affords photodynamic therapy efficacy and selectivity at short drug-light intervals. Transl Oncol. 2010;3:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Ai F, Wang N, Zhang X, Sun T, Zhu Q, Kong W, Wang F, Zhu G. An upconversion nanoplatform with extracellular pH-driven tumor-targeting ability for improved photodynamic therapy. Nanoscale. 2018;10:4432-4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Luo Z, Zheng M, Zhao P, Chen Z, Siu F, Gong P, Gao G, Sheng Z, Zheng C, Ma Y. Self-Monitoring Artificial Red Cells with Sufficient Oxygen Supply for Enhanced Photodynamic Therapy. Sci Rep. 2016;6:23393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Dou X, Nomoto T, Takemoto H, Matsui M, Tomoda K, Nishiyama N. Effect of multiple cyclic RGD peptides on tumor accumulation and intratumoral distribution of IRDye 700DX-conjugated polymers. Sci Rep. 2018;8:8126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Hu D, Chen L, Qu Y, Peng J, Chu B, Shi K, Hao Y, Zhong L, Wang M, Qian Z. Oxygen-generating Hybrid Polymeric Nanoparticles with Encapsulated Doxorubicin and Chlorin e6 for Trimodal Imaging-Guided Combined Chemo-Photodynamic Therapy. Theranostics. 2018;8:1558-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |