Published online Dec 7, 2018. doi: 10.3748/wjg.v24.i45.5154
Peer-review started: September 2, 2018
First decision: October 14, 2018
Revised: October 22, 2018
Accepted: November 13, 2018
Article in press: November 13, 2018
Published online: December 7, 2018
Processing time: 98 Days and 10.6 Hours
To identify the clinicopathological characteristics of pT1N0 esophageal squamous cell carcinoma (ESCC) that are associated with tumor recurrence.
We reviewed 216 pT1N0 thoracic ESCC cases who underwent esophagectomy and thoracoabdominal two-field lymphadenectomy without preoperative chemoradiotherapy. After excluding those cases with clinical follow-up recorded fewer than 3 mo and those who died within 3 mo of surgery, we included 199 cases in the current analysis. Overall survival and recurrence-free survival were assessed by the Kaplan-Meier method, and clinicopathological characteristics associated with any recurrence or distant recurrence were evaluated using univariate and multivariate Cox proportional hazards models. Early recurrence (≤ 24 mo) and correlated parameters were assessed using univariate and multivariate logistic regression models.
Forty-seven (24%) patients had a recurrence at 3 to 178 (median, 33) mo. The 5-year recurrence-free survival rate was 80.7%. None of 13 asymptomatic cases had a recurrence. Preoperative clinical symptoms, upper thoracic location, ulcerative or intraluminal mass macroscopic tumor type, tumor invasion depth level, basaloid histology, angiolymphatic invasion, tumor thickness, submucosal invasion thickness, diameter of the largest single tongue of invasion, and complete negative aberrant p53 expression were significantly related to tumor recurrence and/or recurrence-free survival. Upper thoracic tumor location, angiolymphatic invasion, and submucosal invasion thickness were independent predictors of tumor recurrence (Hazard ratios = 3.26, 3.42, and 2.06, P < 0.001, P < 0.001, and P = 0.002, respectively), and a nomogram for predicting recurrence-free survival with these three predictors was constructed. Upper thoracic tumor location and angiolymphatic invasion were independent predictors of distant recurrence. Upper thoracic tumor location, angiolymphatic invasion, submucosal invasion thickness, and diameter of the largest single tongue of invasion were independent predictors of early recurrence.
These results should be useful for designing optimal individual follow-up and therapy for patients with T1N0 ESCC.
Core tip: Recurrences of pT1N0 esophageal squamous cell carcinoma (ESCC) after esophagectomy are usually metachronous regional lymph node or distant metastases. We analyzed 199 thoracic pT1N0 ESCC cases who underwent esophagectomy and thoracoabdominal two-field lymphadenectomy. Forty-seven (24%) patients had a recurrence during 3 to 178 (median, 33) mo. Upper thoracic tumor location, angiolymphatic invasion, and submucosal invasion thickness were independent predictors of tumor recurrence, and a nomogram for predicting recurrence-free survival with these three predictors was constructed. These results should be useful for designing optimal individual follow-up and therapy for patients with T1N0 ESCC.
- Citation: Xue LY, Qin XM, Liu Y, Liang J, Lin H, Xue XM, Zou SM, Zhang MY, Zhang BH, Hui ZG, Zhao ZT, Ren LQ, Zhang YM, Liu XY, Yuan YL, Ying JM, Gao SG, Song YM, Wang GQ, Dawsey SM, Lu N. Clinicopathological parameters predicting recurrence of pT1N0 esophageal squamous cell carcinoma. World J Gastroenterol 2018; 24(45): 5154-5166
- URL: https://www.wjgnet.com/1007-9327/full/v24/i45/5154.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i45.5154
Esophageal squamous cell carcinoma (ESCC) is one of the most common fatal malignancies worldwide, and is especially common in East Asia, including China and Japan. The prognosis of superficial (T1) ESCC is poor, compared with T1 gastric or colorectal cancer. The long longitudinally arranged collecting channels and plexuses of lymphatics in the esophageal submucosa account for the clinical observation that T1 esophageal cancer can metastasize not only to the mediastinal lymph nodes, but also to the cervical and abdominal lymph nodes far distant from the primary tumor, and to distant organs as well[1-3].
The presence of metastasis is the most important prognostic factor for ESCC. The unfavorable prognosis of patients with T1 ESCC is largely due to high rates of both synchronous and metachronous metastases. Recurrences of T1 ESCC after esophagectomy are usually metachronous regional lymph node or distant metastases, and are only infrequently due to anastomotic recurrences. When recurrence occurs, the prognosis is similar in patients who were node-negative or node-positive at the time of the original surgery[4]. Therefore, patients found to have a high risk of recurrence after esophagectomy need additional chemoradiotherapy. However, only a few studies have evaluated the clinicopathological characteristics associated with an increased risk of a postoperative recurrence in pT1N0 ESCC patients. These studies have shown that invasion depth of the primary tumor, lymphovascular invasion, histologic grade, and tumor length are associated with a high risk of recurrence[5-8]. No previous studies have separately evaluated the clinicopathological characteristics that are associated with distant recurrence or early recurrence in pT1N0 ESCC patients.
We previously reviewed 271 T1 ESCC esophagectomy cases, and established a set of clinicopathological and immunohistochemical indicators to identify patients with a high risk of synchronous regional lymph node metastasis[9]. However, recurrence was observed in quite a few pT1N0 ESCC cases. Thus, the identification of pT1N0 cases at high risk for recurrence is a very important and challenging aspect of the clinical management of these patients, to ensure appropriate use and maximum benefit of additional therapies. In the present study, we followed 199 pT1N0 thoracic ESCC cases in our original esophagectomy case series and investigated the clinicopathological characteristics that were associated with recurrence, distant recurrence, and early recurrence, in order to provide clues to optimal individual therapy.
Two hundred and sixteen pT1N0 thoracic ESCC patients received esophagectomy with thoracoabdominal lymphadenectomy, without preoperative chemoradiotherapy, at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, between February 1990 and January 2004. After excluding those cases with clinical follow-up recorded fewer than 3 mo (n = 12) and those who died within 3 mo of surgery (operative death, n = 5), we included 199 cases in the current analysis.
For lesions in the upper third of the thoracic segment, a three-phase abdominothoracic McKeown resection was generally performed through a right thoracotomy. For lesions in the middle and lower thirds, esophagectomy was performed on the left side using a single-incision Sweet approach. The tumor location was defined by the position of the center of the largest invasive lesion of each case (continuous invasive tongues were considered as one invasive lesion, but discontinuous invasive tongues separated by normal or dysplastic mucosa were considered as multiple invasive lesions). This study was approved by the Institutional Review Board of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (NCC 2014 G-47), and interpretation of anonymized data was exempted from review by the Office of Human Subject Research Protection of the NIH.
Macroscopic tumor types were defined as we previously described[9]. Briefly, we classified the lesions into six types, occult type (Paris classification 0-IIb), erosive type (Paris classification 0-IIc or 0-IIa + IIc), papillary type (Paris classification 0-Ip), plaque-like type (Paris classification 0-Is or 0-IIa), ulcerative type (Paris classification 0-III or 0-III + I), and intraluminal mass (fungating) type (Paris classification 0-Ip)[10,11]. The difference between the papillary type and the intraluminal mass type is that the largest diameter is < 3 cm and ≥ 3 cm, respectively[9].
All histopathological variables were first reviewed and graded independently by three pathologists (LX, SZ, and LR), and discordant cases were reviewed jointly until a consensus was reached. For the patients with multicentric esophageal carcinomas, the histopathological factors for the lesion with the greatest invasion depth were evaluated[9].
Maximum depth of invasion was classified into five levels: m2 (lamina propria mucosae), m3 (muscularis mucosae), sm1, sm2, and sm3 (superficial, middle, and deep thirds of the submucosa, respectively). Degree of differentiation was classified as well, moderate, poor, basaloid or spindle cell/sarcomatoid[12].
Tumor thickness (from the surface to the deepest invasive front of cancer nests), submucosal invasion thickness (from the bottom of the muscularis mucosae to the deepest invasive front of the cancer nests), and the diameter of the largest single tongue of invasion were measured microscopically. Submucosal invasion thickness was measured in submucosal cases, and defined as 0 in mucosal cases.
In our previous study[9], 3000 μm for tumor thickness, 2000 μm for submucosal invasion thickness, and 2 cm for the diameter of the largest single tongue of invasion were found to be the best cut points for predicting lymph node metastasis. Thus, we also used these cut points for categorizing these measurements in this study.
Details of the tissue microarray construction and the immunohistochemical staining and scoring for Cyclin D1, EGFR, and VEGF have been described previously[9]. We rescored p53 expression into three groups: weak or patchy (wild type), complete loss (nonsense, frameshift, or splice-site mutation type), and diffuse and strong (missense mutation type). The latter two groups were considered as aberrant p53 expression[13]. In the present study, the correlation between the expression levels of these four markers and tumor recurrence was further analyzed in the pT1N0 cases.
Follow-up and mortality data were mainly gathered from clinical notes. Patients were evaluated at return visits every 3 mo during the first 2 years after treatment, every 6 mo for the following 3 years, and annually thereafter according to hospital policy. At each visit, physical examination, endoscopic examination, and CT scan of the cervix, chest and abdomen were performed. Suspicious recurrences were biopsied. Confirmation of recurrence required imaging or pathological evaluation. Information about tumor recurrence was updated every time the patient came for a follow-up visit. For those patients who did not come for a follow-up visit, data were gathered by phone calls, and/or mail contact with patients or their next of kin. The patients were followed for a median of 72 mo and a maximum period of 263 mo.
Overall survival time was recorded as the number of months from the date of surgery to the date when death occurred, or to the time of last follow-up, at which point, the data were censored. Recurrence-free survival time was recorded as the number of months from the date of surgery to the date when recurrence occurred, or to the time of last follow-up, at which point, the data were censored.
Four cases underwent radiotherapy after esophagectomy, due to upper resection margins being involved by high grade dysplasia or as part of a randomized clinical trial.
Continuous variables such as age, tumor thickness, and submucosal invasion thickness were analyzed after categorization.
Overall and recurrence-free survival rates were calculated and survival curves were constructed using the Kaplan-Meier method, with significance evaluated by the log-rank test. The associations between clinicopathological characteristics and any recurrence or distant recurrence were determined using univariate Cox proportional hazards analysis. A backward stepwise multivariate Cox proportional hazards analysis was applied for factors achieving a significance level of 0.05 in univariate analysis. Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported.
The associations between clinicopathological parameters and early recurrence (≤ 24 mo after surgery) were evaluated similarly, except using logistic regression analysis.
All the above statistical analyses were performed using SPSS 16.0 for Windows (SPSS, Chicago, IL, United States), with a significance level of 0.05 on two-tailed P-values.
A nomogram based on independent predictors for the recurrence-free survival identified by multivariate Cox proportional hazards analysis was constructed using the rms package in R 3.4.2 software.
The clinicopathological features of the 199 pT1N0 ESCC patients are shown in Table 1. Seventy-one percent of the patients were men. The average age was 56 years, the median age was 57 years, and the age range was 34-77 years. Seventy-two percent of the tumors were found in the middle thoracic region. For all of the 199 patients, a total of 3197 lymph nodes (median, 14) were dissected.
| Characteristic | Patients | |
| Clinical variable | ||
| Sex | Male | 142 (71) |
| Female | 57 (29) | |
| Age (yr) | < 60 | 121 (61) |
| ≥ 60 | 78 (39) | |
| Symptoms | No | 13 (7) |
| Yes | 186 (93) | |
| Endoscopic variable | ||
| Tumor location | Upper thoracic | 31 (16) |
| Middle thoracic | 143 (72) | |
| Lower thoracic | 25 (13) | |
| Tumor size (measured endoscopically) | < 2 cm | 59 (30) |
| ≥ 2 cm | 140 (70) | |
| Macroscopic tumor type | Erosive | 73 (37) |
| Papillary | 26 (13) | |
| Plaque-like | 79 (40) | |
| Ulcerative | 9 (5) | |
| Intraluminal mass | 12 (6) | |
| Standard histopathological variable | ||
| Tumor invasion depth level | m2 | 21 (11) |
| m3 | 26 (13) | |
| sm1 | 18 (9) | |
| sm2 | 45 (23) | |
| sm3 | 89 (45) | |
| Degree of differentiation | Well | 39 (20) |
| Moderate | 76 (38) | |
| Poor | 56 (28) | |
| Basaloid | 19 (10) | |
| Spindle cell/sarcomatoid | 9 (5) | |
| Angiolymphatic invasion | No | 172 (86) |
| Yes | 27 (14) | |
| Multicentric invasive lesions | No | 183 (92) |
| Yes | 16 (8) | |
| Number of lymph nodes dissected | < 14 | 90 (45) |
| ≥ 14 | 109 (55) | |
| Measured histopathological variables | ||
| Tumor thickness | < 3000 μm | 85 (43) |
| ≥ 3000 μm | 114 (57) | |
| Submucosal invasion thickness | 0 | 47 (24) |
| 0-2000 μm | 85 (43) | |
| ≥ 2000 μm | 67 (34) | |
| Diameter of the largest single tongue of invasion | < 2 cm | 134 (67) |
| ≥ 2 cm | 65 (33) | |
| Immunohistochemical staining1 | ||
| P53 | Complete loss | 50 (39) |
| Weak, patchy | 41 (32) | |
| Diffuse, strong | 37 (29) | |
| Cyclin D1 | - | 38 (30) |
| 1+ | 39 (31) | |
| 2+ | 49 (39) | |
| EGFR | - | 52 (41) |
| 1+ | 44 (34) | |
| 2+ | 32 (25) | |
| VEGF | - | 54 (44) |
| 1+ | 34 (28) | |
| 2+ | 35 (28) |
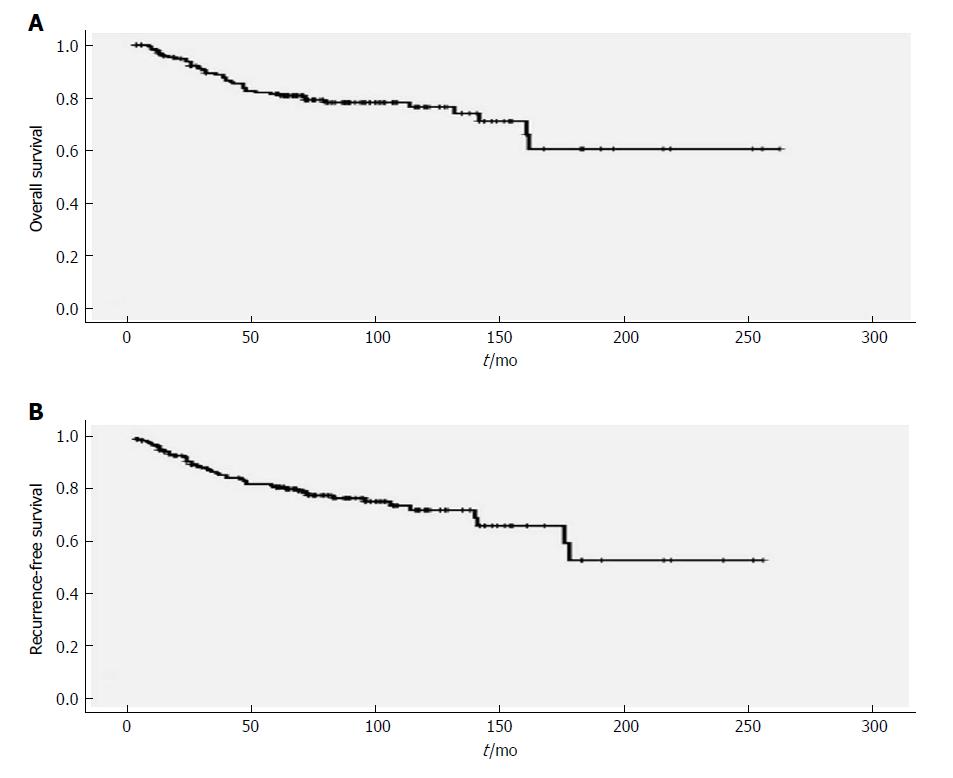
The 5-year and 10-year overall survival rates were 81.4% and 76.4%, respectively (Figure 1). Forty-seven (24%) patients had documented recurrences. These recurrences occurred during 3-178 mo, with a median of 33 mo. The 5-year and 10-year recurrence-free survival rates were 80.7% and 71.9%, respectively (Figure 1). Mediastinal lymph nodes (21 patients, 11%) were the most frequent site of recurrence, followed by cervical lymph nodes (19 patients, 10%, with 8 left, 10 right, and 1 bilateral) (Table 2).
| Site of recurrence | Total patients with recurrence | Patients by tumor location | Patients by macroscopic tumor type | |||
| Upper thoracic (n = 31) | Middle thoracic (n = 143) | Lower thoracic (n = 25) | Ulcerative or intraluminal (n = 21) | Erosive, papillary, or plaque-like (n = 178) | ||
| Local-regional recurrences1 | 33 (17) | 15 (48) | 14 (10) | 4 (16) | 8 (38) | 25 (14) |
| Anastomosis | 3 (2) | 3 (10) | 0 | 0 | 2 (10) | 1 (1) |
| Cervical node | 19 (10) | 10 (32) | 7 (5) | 2 (8) | 4 (19) | 15 (8) |
| Mediastinal node | 21 (11) | 9 (29) | 9 (6) | 3 (12) | 8 (38) | 13 (7) |
| Abdominal node | 0 | 0 | 0 | 0 | 0 | 0 |
| Distant recurrences1 | 16 (8) | 5 (16) | 8 (6) | 3 (12) | 4 (19) | 12 (7) |
| Lung | 7 (4) | 2 (6) | 5 (3) | 0 | 1 (5) | 6 (3) |
| Liver | 2 (1) | 1 (3) | 0 | 1 (4) | 0 | 2 (1) |
| Bone | 6 (3) | 0 | 4 (3) | 2 (8) | 2 (10) | 4 (2) |
| Brain | 1 (0) | 1 (3) | 0 | 0 | 0 | 1 (1) |
| Pleura | 3 (2) | 2 (6) | 1 (1) | 0 | 1 (5) | 2 (1) |
| Distant node | 0 | 0 | 0 | 0 | 0 | 0 |
| Multiple site recurrences | 15 (8) | 7 (23) | 6 (4) | 2 (8) | 5 (24) | 10 (6) |
| Mediastinal node and bone | 2 (1) | 0 | 1 (1) | 1 (4) | 1 (5) | 1 (1) |
| Mediastinal node, cervical node, and bone | 1 (1) | 0 | 0 | 1 (4) | 0 | 1 (1) |
| Mediastinal node, pleura, and bone | 1 (1) | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Mediastinal node and cervical node | 3 (1) | 2 (6) | 1 (1) | 0 | 1 (5) | 2 (1) |
| Cervical node and lung | 1 (1) | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Mediastinal node, cervical node, and anastomosis | 1 (1) | 1 (3) | 0 | 0 | 1 (5) | 0 |
| Mediastinal node, cervical node, anastomosis, and pleura | 1 (1) | 1 (3) | 0 | 0 | 1 (5) | 0 |
| Mediastinal node, cervical node, and lung | 2 (1) | 1 (3) | 1 (1) | 0 | 1 (5) | 1 (1) |
| Cervical node, bone, and lung | 1 (1) | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Mediastinal node, liver, and lung | 1 (1) | 1 (3) | 0 | 0 | 0 | 1 (1) |
| Mediastinal node and brain | 1 (1) | 1 (3) | 0 | 0 | 0 | 1 (1) |
| Unknown sites | 10 (5) | 1 (3) | 8 (6) | 1 (4) | 1 (5) | 9 (5) |
| Total recurrences | 47 (24) | 16 (52) | 25 (17) | 6 (24) | 10 (48) | 37 (21) |
Using the Kaplan-Meier method, preoperative clinical symptoms, tumor location, macroscopic tumor type, tumor invasion depth level, degree of differentiation, angiolymphatic invasion, tumor thickness, submucosal invasion thickness, and diameter of the largest single tongue of invasion were significantly associated with recurrence-free survival (P < 0.05) (Table 3).
| Parameter | Total | Recurrences | Kaplan-Meier analysis | Univariate Cox proportional hazards analysis | ||||||
| 5-yr RFS (%) | 10-yr RFS (%) | P-value | HR | 95%CI | Global P | P-for-trend | ||||
| Clinical variable | ||||||||||
| Sex | Male | 142 | 33 (23) | 81 | 71.6 | 0.97 | 1 | |||
| Female | 57 | 14 (25) | 80 | 72.7 | 1.011 | 0.54-1.89 | 0.97 | |||
| Age (yr) | < 60 | 121 | 28 (23) | 79.2 | 74.1 | 0.6 | 1 | |||
| ≥ 60 | 78 | 19 (24) | 82.9 | 66.4 | 1.17 | 0.65-2.10 | 0.6 | |||
| Symptoms | No | 13 | 0 | 100 | 100 | 0.03 | 1 | |||
| Yes | 186 | 47 (25) | 79.2 | 69.8 | 23.54 | 0.28-2017 | 0.16 | |||
| Endoscopic variable | ||||||||||
| Tumor location | Upper thoracic | 31 | 15 (48) | 55.9 | 44.7 | < 0.001 | 3.46 | 1.83-6.54 | < 0.001 | |
| Middle thoracic | 143 | 26 (18) | 85.2 | 78.5 | 1 | |||||
| Lower thoracic | 25 | 6 (24) | 86.5 | 65.4 | 1.49 | 0.61-3.63 | 0.38 | |||
| Tumor size (endoscopically) | < 2 cm | 59 | 16 (27) | 79.3 | 69.7 | 0.53 | 1 | |||
| ≥ 2 cm | 140 | 31 (22) | 81.2 | 73 | 0.82 | 0.45-1.51 | 0.53 | |||
| Macroscopic | Erosive | 73 | 12 (16) | 87.2 | 78 | 0.001 | 1 | |||
| tumor type | Papillary | 26 | 4 (15) | 90.2 | 90.2 | 0.98 | 0.32-3.06 | 0.98 | ||
| Plaque-like | 79 | 21 (27) | 75.8 | 68.6 | 1.64 | 0.81-3.25 | 0.13 | |||
| Ulcerative | 9 | 6 (67) | 53.3 | 26.7 | 6.06 | 2.26-16.26 | < 0.001 | |||
| Intraluminal mass | 12 | 4 (33) | 74.1 | 49.4 | 3.94 | 1.26-12.32 | 0.02 | |||
| Standard histopathological variable | ||||||||||
| Tumor invasion depth level | m2 | 21 | 1 (5) | 94.1 | 94.1 | 0.04 | 1.52 | 1.14-1.97 | 0.004 | |
| m3 | 26 | 3 (12) | 96.2 | 88.1 | ||||||
| sm1 | 18 | 4 (22) | 81.4 | 81.4 | ||||||
| sm2 | 45 | 11 (25) | 83.8 | 74.8 | ||||||
| sm3 | 89 | 28 (31) | 70.6 | 58.6 | ||||||
| Degree of differentiation | Well | 39 | 10 (26) | 79.5 | 65 | 0.02 | 1 | |||
| Moderate | 76 | 15 (20) | 84.7 | 82 | 0.73 | 0.33-1.63 | 0.44 | |||
| Poor | 56 | 13 (23) | 80.7 | 67.8 | 0.92 | 0.40-2.11 | 0.92 | |||
| Basaloid | 19 | 8 (42) | 58.3 | 35 | 2.88 | 1.13-7.38 | 0.03 | |||
| Spindle cell/sarcomatoid | 9 | 1 (11) | 88.9 | 88.9 | 0.54 | 0.07-4.21 | 0.55 | |||
| Angiolymphatic invasion | No | 172 | 33 (19) | 84.6 | 75.3 | < 0.001 | 1 | |||
| Yes | 27 | 14 (52) | 55.4 | 49.9 | 3.48 | 1.85-6.52 | < 0.001 | |||
| Multicentric invasive lesions | No | 183 | 45 (25) | 80.2 | 70.9 | 0.42 | 1 | |||
| Yes | 16 | 2 (13) | 86.5 | 86.5 | 0.56 | 0.14-2.32 | 0.42 | |||
| Number of lymph nodes dissected | < 14 | 90 | 24 (27) | 72.7 | 70.2 | 0.25 | 1 | |||
| ≥ 14 | 109 | 23 (21) | 87.2 | 73.8 | 0.72 | 0.41-1.27 | 0.26 | |||
| Measured histopathological variable | ||||||||||
| Tumor thickness | < 3000 μm | 85 | 13 (15) | 90 | 79.3 | 0.005 | 1 | |||
| ≥ 3000 μm | 114 | 34 (30) | 73.2 | 65.7 | 2.41 | 1.27-4.56 | 0.007 | |||
| Submucosal | 0 | 47 | 4 (9) | 95.5 | 90.5 | 0.001 | 2.24 | 1.44-3.46 | < 0.001 | |
| invasion thickness | 1-2000 μm | 85 | 19 (22) | 82.8 | 74.4 | |||||
| ≥ 2000 μm | 67 | 24 (36) | 67.4 | 56.4 | ||||||
| Diameter of the | < 2 cm | 134 | 27 (20) | 85.5 | 75.7 | 0.008 | 1 | |||
| largest single tongue of invasion | ≥ 2 cm | 65 | 20 (31) | 70.1 | 64.1 | 2.15 | 1.20-3.85 | 0.01 | ||
| Immunohistochemical staining1 | ||||||||||
| P53 | Complete loss | 50 | 19 (38) | 64.8 | 59.5 | 0.33 | 2.43 | 1.02-5.79 | 0.045 | |
| Weak, patchy | 41 | 7 (17) | 81.8 | 81.8 | 1 | |||||
| Diffuse, strong | 37 | 12 (33) | 88.6 | 78.2 | 1.88 | 0.74-4.77 | 0.19 | |||
| Cyclin D1 | - | 38 | 14 (37) | 71.3 | 56.4 | 0.88 | 0.93 | 0.64-1.36 | 0.72 | |
| 1+ | 39 | 11 (28) | 80.7 | 73.7 | ||||||
| 2+ | 49 | 14 (29) | 68.7 | 68.7 | ||||||
| EGFR | - | 52 | 13 (25) | 82.9 | 75.6 | 0.27 | 1.15 | 0.78-1.69 | 0.49 | |
| 1+ | 44 | 17 (39) | 65.6 | 51.3 | ||||||
| 2+ | 32 | 9 (28) | 70.2 | 68 | ||||||
| VEGF | - | 54 | 15 (28) | 76 | 67.3 | 0.59 | 1.05 | 0.72-1.54 | 0.79 | |
| 1+ | 34 | 12 (35) | 74.1 | 59.6 | ||||||
| 2+ | 35 | 10 (29) | 72.2 | 72.2 | ||||||
In univariate Cox regression, upper thoracic tumor location, ulcerative or intraluminal mass macroscopic tumor type, invasion depth level, basaloid histology, angiolymphatic invasion, tumor thickness, submucosal invasion thickness, diameter of the largest single tongue of invasion, and complete loss of p53 expression were significantly associated with tumor recurrence (P < 0.05) (Table 3). In multivariate Cox regression, upper thoracic tumor location, angiolymphatic invasion, and submucosal invasion thickness were independent significant predictors of recurrence (Table 4).
| Parameter | HR | 95%CI | Global P | P-for-trend | |
| Tumor location | Upper thoracic | 3.26 | 1.70-6.27 | < 0.001 | |
| Middle thoracic | 1 | ||||
| Lower thoracic | 1.05 | 0.43-2.59 | 0.91 | ||
| Angiolymphatic invasion | 3.42 | 1.80-6.52 | < 0.001 | ||
| Submucosal invasion thickness | 2.06 | 1.30-3.27 | 0.002 | ||
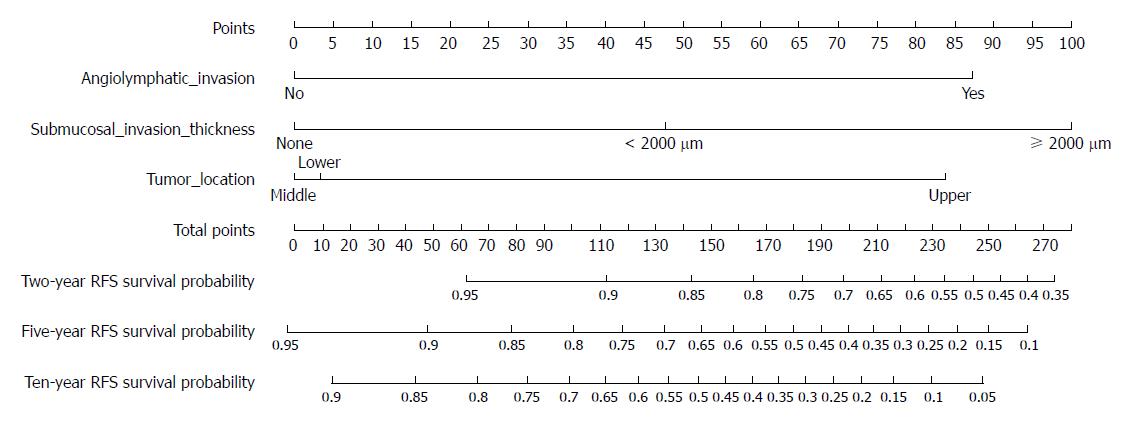
A nomogram for predicting tumor recurrence with these three independent significant predictors is shown in Figure 2. The nomogram had a concordance index of 0.752.
Sixteen cases had well-documented distant recurrences. The lung (7 patients, 4%) was the most frequent site of distant recurrence, followed by the bone (6 patients, 3%) (Table 2). The time to distant recurrence ranged from 3-192 mo, with a median of 39 mo.
In univariate Cox regression, upper thoracic tumor location, ulcerative or intraluminal mass macroscopic tumor type, basaloid histology, and angiolymphatic invasion were significantly associated with distant recurrence (P < 0.05). In multivariate Cox regression, upper thoracic tumor location and angiolymphatic invasion were independent predictors of distant recurrence (Table 5).
| Univariate Cox proportional hazards analysis | |||||||
| Parameter | Total | Distant recurrences (%) | HR | 95%CI | Global P | P-for-trend | |
| Clinical variables | |||||||
| Sex | Male | 136 | 14 (10) | 1 | |||
| Female | 53 | 2 (4) | 0.33 | 0.07-1.44 | 0.14 | ||
| Age (yr) | < 60 | 118 | 8 (7) | 1 | |||
| ≥ 60 | 71 | 8 (11) | 2.18 | 0.79-6.02 | 0.13 | ||
| Symptoms | No | 13 | 0 | 1 | |||
| Yes | 176 | 16 (9) | 23.59 | 0.01-40030 | 0.41 | ||
| Endoscopic variables | |||||||
| Tumor location | Upper thoracic | 30 | 5 (17) | 3.56 | 1.15-11.07 | 0.03 | |
| Middle thoracic | 135 | 8 (6) | 1 | ||||
| Lower thoracic | 24 | 3 (13) | 2.37 | 0.63-8.94 | 0.2 | ||
| Tumor size (measured endoscopically) | < 2 cm | 55 | 6 (11) | 1 | |||
| ≥ 2 cm | 134 | 10 (8) | 0.76 | 0.27-2.09 | 0.59 | ||
| Macroscopic type | Erosive | 71 | 5 (7) | 1 | |||
| Papillary | 26 | 2 (8) | 1.11 | 0.21-5.75 | 0.9 | ||
| Plaque-like | 72 | 5 (7) | 0.95 | 0.27-3.31 | 0.94 | ||
| Ulcerative | 8 | 2 (25) | 5.58 | 1.06-29.32 | 0.04 | ||
| Intraluminal mass | 12 | 2 (17) | 5.41 | 1.02-28.68 | 0.047 | ||
| Standard histopathological variable | |||||||
| Tumor invasion depth level | m2 | 21 | 0 | 1.42 | 0.91-2.21 | 0.13 | |
| m3 | 26 | 2 (8) | |||||
| sm1 | 16 | 1 (6) | |||||
| sm2 | 43 | 4 (9) | |||||
| sm3 | 83 | 9 (11) | |||||
| Degree of differentiation | Well | 36 | 1 (3) | 1 | |||
| Moderate | 73 | 5 (7) | 2.19 | 0.25-18.98 | 0.48 | ||
| Poor | 54 | 6 (11) | 3.93 | 0.47-32.89 | 0.21 | ||
| Basaloid | 17 | 3 (18) | 12.4 | 1.27-121.08 | 0.03 | ||
| Spindle cell/sarcomatoid | 9 | 1 (11) | 5.73 | 0.35 -92.90 | 0.22 | ||
| Angiolymphatic invasion | No | 166 | 11 (7) | 1 | |||
| Yes | 23 | 5 (22) | 3.38 | 1.15-9.93 | 0.03 | ||
| Multicentric invasive lesions | No | 173 | 15 (9) | 1 | |||
| Yes | 16 | 1 (6) | 0.92 | 0.12-7.00 | 0.93 | ||
| Number of lymph nodes dissected | < 14 | 85 | 7 (8) | 1 | |||
| ≥ 14 | 104 | 9 (9) | 0.95 | 0.36-2.57 | 0.93 | ||
| Measured histopathological variable | |||||||
| Tumor thickness | < 3000 μm | 82 | 6 (7) | 1 | |||
| ≥ 3000 μm | 107 | 10 (9) | 1.53 | 0.56-4.21 | 0.42 | ||
| Submucosal | 0 | 47 | 2 (4) | 1.88 | 0.91-3.90 | 0.09 | |
| invasion thickness | < 2000 μm | 80 | 7 (9) | ||||
| ≥ 2000 μm | 62 | 7 (11) | |||||
| Diameter of the | < 2 cm | 126 | 9 (7) | 1 | |||
| largest invasive lesion | ≥ 2 cm | 63 | 7 (11) | 2.56 | 0.92-7.15 | 0.07 | |
| Immunohistochemical staining2 | |||||||
| P53 | Complete loss | 47 | 6 (13) | 1.78 | 0.44-7.16 | 0.42 | |
| Weak, patchy | 40 | 3 (8) | 1 | ||||
| Diffuse, strong | 34 | 6 (18) | 2.31 | 0.58-9.25 | 0.24 | ||
| Cyclin D1 | - | 35 | 5 (14) | 0.94 | 0.51-1.74 | 0.84 | |
| + | 37 | 5 (14) | |||||
| ++ | 47 | 5 (11) | |||||
| EGFR | - | 52 | 9 (17) | 0.63 | 0.31-1.28 | 0.2 | |
| + | 40 | 4 (10) | |||||
| ++ | 29 | 2 (7) | |||||
| VEGF | - | 50 | 6 (12) | 0.95 | 0.50-1.81 | 0.88 | |
| + | 33 | 3 (9) | |||||
| ++ | 33 | 4 (12) | |||||
| Multivariate Cox proportional hazards analysis | |||||||
| Parameter | |||||||
| Tumor location | Upper thoracic | 3.83 | 1.23-11.96 | 0.02 | |||
| Middle thoracic | 1 | ||||||
| Lower thoracic | 1.95 | 0.50-7.54 | 0.34 | ||||
| Angiolymphatic invasion | 3.55 | 1.17-10.77 | 0.03 | ||||
Among the 47 cases with recurrences, 18 (38%) had early recurrences (≤ 24 mo after surgery).
In univariate logistic regression, upper thoracic tumor location, ulcerative or intraluminal mass macroscopic tumor type, angiolymphatic invasion, tumor invasion depth level, tumor thickness, submucosal invasion thickness, and diameter of the largest single tongue of invasion were significantly associated with early recurrence (P < 0.05). Multivariate logistic regression showed that upper thoracic tumor location, angiolymphatic invasion, submucosal invasion thickness, and diameter of the largest single tongue of invasion were independent predictors of early recurrence (Table 6).
| Univariate logistic regression | |||||||
| Parameter | Total | Early recurrence | Odds ratio | 95%CI | Global P | P-for-trend | |
| Clinical variable | |||||||
| Sex | Male | 142 | 12 (9) | 1 | |||
| Female | 57 | 6 (11) | 1.28 | 0.45-3.58 | 0.65 | ||
| Age (yr) | < 60 | 121 | 15 (12) | 1 | |||
| ≥ 60 | 78 | 3 (4) | 0.28 | 0.08-1.01 | 0.052 | ||
| Endoscopic variable | |||||||
| Tumor location | Upper thoracic | 31 | 9 (29) | 7.95 | 2.68-23.54 | < 0.001 | |
| Middle thoracic | 143 | 7 (5) | 1 | ||||
| Lower thoracic | 25 | 2 (8) | 1.69 | 0.33-8.64 | 0.53 | ||
| Tumor size (endoscopically) | < 2 cm | 59 | 4 (7) | 1 | |||
| ≥ 2 cm | 140 | 14 (10) | 1.53 | 0.48-4.85 | 0.47 | ||
| Macroscopic | Erosive | 73 | 4 (6) | 1 | |||
| tumor types | Papillary | 26 | 1 (4) | 0.69 | 0.07-6.47 | 0.75 | |
| Plaque-like | 79 | 7 (9) | 1.68 | 0.47-5.98 | 0.43 | ||
| Ulcerative | 9 | 3 (33) | 8.63 | 1.55-47.86 | 0.01 | ||
| Intraluminal mass | 12 | 3 (25) | 5.75 | 1.10-29.95 | 0.04 | ||
| Standard histopathological variable | |||||||
| Tumor invasion depth level | m2 | 21 | 0 | 1.78 | 1.05-3.01 | 0.03 | |
| m3 | 26 | 1 (4) | |||||
| sm1 | 18 | 2 (11) | |||||
| sm2 | 45 | 2 (4) | |||||
| sm3 | 89 | 13 (15) | |||||
| Degree of differentiation | Well | 39 | 3 (8) | 1 | |||
| Moderate | 76 | 6 (8) | 1.03 | 0.24-4.35 | 0.97 | ||
| Poor | 56 | 4 (7) | 0.92 | 0.20-4.38 | 0.92 | ||
| Basaloid | 19 | 4 (21) | 3.2 | 0.64-16.07 | 0.16 | ||
| Spindle cell/sarcomatoid | 9 | 1 (11) | 1.5 | 0.14-16.36 | 0.74 | ||
| Angiolymphatic invasion | No | 172 | 10 (6) | 1 | |||
| Yes | 27 | 8 (30) | 6.82 | 2.40-19.38 | < 0.001 | ||
| Multicentric invasive lesions | No | 183 | 17 (9) | 1 | |||
| Yes | 16 | 2 (13) | 0.65 | 0.08-5.24 | 0.69 | ||
| Number of lymph nodes dissected | < 14 | 90 | 11 (12) | 1 | |||
| ≥ 14 | 109 | 0.49 | 0.18-1.33 | 0.16 | |||
| Measured histopathological variable | |||||||
| Tumor thickness | < 3000 μm | 85 | 2(2) | 1 | |||
| ≥ 3000 μm | 114 | 16(14) | 6.78 | 1.51-30.33 | 0.01 | ||
| Submucosal invasion thickness | 0 | 47 | 1(2) | 4.02 | 1.66 -9.73 | 0.001 | |
| 0-2000 μm | 85 | 4(5) | |||||
| ≥ 2000 μm | 67 | 13(19) | |||||
| Diameter of the largest single tongue of invasion | < 2 cm | 134 | 5(4) | 1 | |||
| ≥ 2 cm | 65 | 13(20) | 6.45 | 2.19-19.00 | 0.001 | ||
| Immunohistochemical staining1 | |||||||
| P53 | Complete loss | 50 | 7 (14) | 1.34 | 0.36-4.98 | 0.66 | |
| Weak, patchy | 41 | 5 (12) | 1 | ||||
| Diffuse, strong | 37 | 4 (11) | 1.15 | 0.28-4.63 | 0.85 | ||
| Cyclin D1 | - | 38 | 4 (11) | 1.32 | 0.69-2.54 | 0.4 | |
| 1+ | 39 | 4 (10) | |||||
| 2+ | 49 | 8 (16) | |||||
| EGFR | - | 52 | 4 (8) | 1.32 | 0.69-2.54 | 0.4 | |
| 1+ | 44 | 8 (18) | |||||
| 2+ | 32 | 4 (13) | |||||
| VEGF | - | 54 | 4 (7) | 1.59 | 0.83-3.04 | 0.16 | |
| 1+ | 34 | 5 (15) | |||||
| 2+ | 35 | 6 (17) | |||||
| Multivariate logistic regression | |||||||
| Tumor location | Upper thoracic | 7.73 | 2.15-27.78 | 0.002 | |||
| Middle thoracic | 1 | ||||||
| Lower thoracic | 1.18 | 0.20-6.86 | 0.85 | ||||
| Angiolymphatic invasion | 5.75 | 1.63-20.24 | 0.006 | ||||
| Submucosal invasion thickness | 2.64 | 0.92-7.60 | 0.07 | ||||
| Diameter of the largest single tongue of invasion | 4.13 | 1.17-14.56 | 0.03 | ||||
We previously analyzed pT1 ESCC esophagectomy cases to identify predictors of synchronous regional lymph node metastatsis[5]. In the present study, we followed the pT1N0 thoracic ESCC cases further, for a median of 6 years, and investigated the risk of tumor recurrence and parameters predicting tumor recurrence. We studied the cases before 2004 when the endoscopic resection had not been performed yet in our hospital.
Consistent with our previous observation that all asymptomatic cases had no lymph node metastases, these cases also had no recurrence in our follow-up period. ESCC has a very good prognosis if detected when it is asymptomatic. This can be achieved by appropriate screening programs. However, few studies have analyzed the impact of symptoms on the prognosis of ESCC. Wang et al[14] observed the natural progression of untreated superficial ESCCs identified by screening in a high-risk area. Most of the patients were asymptomatic. It took a long time to progress from an early to an advanced stage, and most survived for over 5 years. Wang et al[15] also reported a 30-year experience with esophagectomy for superficial ESCCs identified in large-scale mass screenings in high-risk areas. Most patients were asymptomatic, and had a low recurrence rate. Natsugoe et al[16] also reported that asymptomatic esophageal carcinoma patients had a lower stage and a better prognosis.
Proximal tumors are known to have a more advanced stage, a lower resection rate, fewer R0 resections, more cervical and tracheobronchial lymph node metastases, and a poorer prognosis[17,18]. The 7th and 8th editions of the American Joint Committee on Cancer (AJCC) staging system include tumor location as a staging factor for T2-3N0M0 ESCC cases and T3N0M0 ESCC cases, respectively[19,20]. Few studies have focused on T1 proximal tumors. We found that the patients with upper thoracic tumors had much higher frequencies of cervical and mediastinal lymph node recurrences than other patients (Table 2), and these proximal tumors were significantly associated with an increased risk for any recurrence (Table 3), distant recurrence (Table 5), and early recurrence (Table 6). One reason for a higher frequency of cervical and mediastinal lymph node recurrences in upper thoracic cases is the characteristics of the lymphatic channels draining this area[1,2]. We also found that tumor thickness was greater in upper thoracic tumors (data not shown), which may be another reason. Upper thoracic tumor location was also one of the independent risk factors for any recurrence, distant recurrence, and early recurrence.
We need to say that the fact that most (33/37 = 89%) of the patients in whom the locations of the recurrences were recorded had recurrences in the cervical and/or mediastinal lymph nodes raises the question of whether (macroscopic or microscopic) tumor was present at the time of surgery and could have been removed if a three-field lymph node dissection (including the cervical lymph nodes) or a more extensive two-field lymph node dissection (including more mediastinal lymph nodes) had been done. In Japan, standard treatment for clinically submucosal ESCC is esophagectomy with three-field lymphadenectomy[21]. It is not yet known whether all patients would benefit from cervical lymphadenectomy, which often results in more severe complications. The optimal extent of lymph node dissection in esophagectomies is an ongoing discussion among surgeons, and our data can contribute to this discussion.
There is a macroscopic tumor type which looks like a large mushroom or a big polyp, and is commonly pedunculated. It belongs to the Paris classification 0-Ip[10,11], but it is different from other common 0-Ip cases. It can be called the intraluminal mass (fungating) type[9]. Most tumors of this type are spindle cell/sarcomatoid, basaloid, or poorly differentiated squamous cell carcinoma. Our previous study found that patients with ulcerative or intraluminal tumors had a high risk of lymph node metastasis[9]. We have now shown that they also have a significantly higher rate of recurrence.
In the current analysis, angiolymphatic invasion was significantly associated with tumor recurrence, distant recurrence, and early recurrence. We relied on HE staining to evaluate angiolymphatic invasion, and immunohistochemistry for endothelial cells was not routinely performed, in keeping with standard practice. Huang et al[8] reported that angiolymphatic invasion could act as a prognostic and staging factor in T1-3N0M0 ESCC.
In our previous study, patients with basaloid histology had a moderate risk of synchronous lymph node metastasis[9]. However, in the current study they had a high risk of recurrence, especially distant recurrence. Zhang et al[22] retrospectively analyzed 142 cases of basaloid ESCC, and found that the first site of recurrence was distant in 39 (54.9%) cases, distant plus loco-regional in 24 (33.8%) cases, and loco-regional alone in 8 (11.3%) cases. They concluded that basaloid ESCC frequently progresses via hematogenous metastasis rather than lymph node metastasis[22]. Saito et al[23] also reported that differentiated components of ESCC were most often found in sites of lymph node metastases, whereas basaloid components predominated in sites of hematogenous metastases. Thus, control of the hematogenous spread of basaloid components may lead to improved outcomes in these patients. Indeed, there is a case report of surgical intervention helping a basaloid ESCC patient with a solitary lung metastasis achieve a long-term survival[24].
It should be noticed that one m2 case had a cervical lymph node recurrence at nearly 5 years after esophagectomy. This case had a tumor thickness of 325 μm and no adverse parameters. Therefore, long-term follow-up is needed for all patients with T1 ESCC after endoscopic resection or esophagectomy, even when the patients have no known adverse parameters.
We have identified certain clinicopathological features that are associated with an increased risk of tumor recurrence in pT1N0 thoracic ESCC patients after esophagectomy and thoracoabdominal two-field lymphadenectomy: (1) Patients with an upper thoracic location, ulcerative or intraluminal mass tumor type, deeper tumor invasion level, basaloid histology, angiolymphatic invasion, greater tumor thickness, greater submucosal invasion thickness, greater diameter of the largest single tongue of invasion, and/or completely negative aberrant p53 expression are at greater risk of tumor recurrence. A nomogram including tumor location, angiolymphatic invasion, and submucosal invasion thickness can be used to predict the likelihood of recurrence-free survival at different times after surgery; (2) Patients with an upper thoracic tumor location and/or angiolymphatic invasion have a higher risk of distant recurrence; and (3) Patients with an upper thoracic tumor location, angiolymphatic invasion, submucosal invasion thickness, and diameter of the largest single tongue of invasion have a higher risk of early recurrence.
All patients with T1 ESCC need long-term follow-up after endoscopic resection or esophagectomy, even patients without any adverse parameters. But this analysis should help clinicians select a subset of these patients who need especially close postoperative surveillance and/or chemoradiotherapy. Additional long-term follow-up studies are needed to confirm these findings.
The prognosis of superficial (T1) esophageal squamous cell carcinoma (ESCC) is poor, compared with T1 gastric or colorectal cancer. The unfavorable prognosis of patients with T1 ESCC is due to high rates of both synchronous and metachronous metastases. Recurrences of T1 ESCC after esophagectomy are usually metachronous metastases. When recurrence occurs, the prognosis is similar in patients who were node-negative or node-positive at the time of the original surgery. However, only a few studies have evaluated the clinicopathological characteristics associated with an increased risk of a postoperative recurrence in pT1N0 ESCC patients. No previous studies have separately evaluated the clinicopathological characteristics that are associated with distant recurrence or early recurrence in pT1N0 ESCC patients.
The identification of pT1N0 ESCC cases at high risk for recurrence is a very important and challenging aspect of the clinical management of these patients, to ensure appropriate use and maximum benefit of additional therapies.
To investigate the clinicopathological characteristics that are associated with recurrence, distant recurrence, and early recurrence, in order to provide clues to optimal individual therapy.
Clinicopathological characteristics associated with any recurrence or distant recurrence were evaluated using univariate and multivariate Cox proportional hazards models. Early recurrence (≤ 24 mo) and correlated parameters were assessed using univariate and multivariate logistic regression models.
We have identified certain clinicopathological features that are associated with an increased risk of tumor recurrence in pT1N0 thoracic ESCC patients. A nomogram including tumor location, angiolymphatic invasion, and submucosal invasion thickness can be used to predict the likelihood of recurrence-free survival at different times after surgery. Patients with an upper thoracic tumor location and/or angiolymphatic invasion have a higher risk of distant recurrence. Patients with an upper thoracic tumor location, angiolymphatic invasion, submucosal invasion thickness, and an greater diameter of the largest single tongue of invasion have a higher risk of early recurrence. Additional long-term follow-up studies are needed to confirm these findings.
We evaluated the clinicopathological characteristics associated with an increased risk of a postoperative recurrence and separately evaluated the clinicopathological characteristics that are associated with distant recurrence or early recurrence in pT1N0 ESCC patients. This study should help clinicians select a subset of these patients who need especially close postoperative surveillance and/or chemoradiotherapy.
Risk of tumor recurrence in pT1N0 ESCC patients can be predicted using certain clinicopathological features. This should be confirmed in more prospective studies and multi-center studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Luyer MD, Otowa Y, Thota PN S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Liebermann-Meffert D. Anatomical basis for the approach and extent of surgical treatment of esophageal cancer. Dis Esophagus. 2001;14:81-84. [PubMed] |
| 2. | Mizutani M, Murakami G, Nawata S, Hitrai I, Kimura W. Anatomy of right recurrent nerve node: why does early metastasis of esophageal cancer occur in it? Surg Radiol Anat. 2006;28:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Kuge K, Murakami G, Mizobuchi S, Hata Y, Aikou T, Sasaguri S. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. J Thorac Cardiovasc Surg. 2003;125:1343-1349. [PubMed] |
| 4. | Ozawa Y, Kamei T, Nakano T, Taniyama Y, Miyagi S, Ohuchi N. Characteristics of Postoperative Recurrence in Lymph Node-Negative Superficial Esophageal Carcinoma. World J Surg. 2016;40:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Wang S, Chen X, Fan J, Lu L. Prognostic Significance of Lymphovascular Invasion for Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2016;23:4101-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Araki K, Ohno S, Egashira A, Saeki H, Kawaguchi H, Sugimachi K. Pathologic features of superficial esophageal squamous cell carcinoma with lymph node and distal metastasis. Cancer. 2002;94:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Song Z, Wang J, Lin B, Zhang Y. Analysis of the tumor length and other prognosis factors in pT1-2 node-negative esophageal squamous cell carcinoma in a Chinese population. World J Surg Oncol. 2012;10:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Huang Q, Luo K, Chen C, Wang G, Jin J, Kong M, Li B, Liu Q, Li J, Rong T. Identification and Validation of Lymphovascular Invasion as a Prognostic and Staging Factor in Node-Negative Esophageal Squamous Cell Carcinoma. J Thorac Oncol. 2016;11:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Xue L, Ren L, Zou S, Shan L, Liu X, Xie Y, Zhang Y, Lu J, Lin D, Dawsey SM. Parameters predicting lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. Mod Pathol. 2012;25:1364-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 11. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 646] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 12. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumors of the digestive system 4ed. Lyon: IARC 2010; . |
| 13. | Setia N, Agoston AT, Han HS, Mullen JT, Duda DG, Clark JW, Deshpande V, Mino-Kenudson M, Srivastava A, Lennerz JK. A protein and mRNA expression-based classification of gastric cancer. Mod Pathol. 2016;29:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Wang GQ, Wei WQ, Hao CQ, Zhang JH, Lü N. [Natural progression of early esophageal squamous cell carcinoma]. Zhonghua Zhong Liu Za Zhi. 2010;32:600-602. [PubMed] |
| 15. | Wang GQ, Jiao GG, Chang FB, Fang WH, Song JX, Lu N, Lin DM, Xie YQ, Yang L. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg. 2004;77:1740-1744. [PubMed] |
| 16. | Natsugoe S, Baba M, Shimada M, Kijima F, Kusano C, Yoshinaka H, Mueller J, Aikou T. Positive impact on surgical treatment for asymptomatic patients with esophageal carcinoma. Hepatogastroenterology. 1999;46:2854-2858. [PubMed] |
| 17. | Law S, Kwong DL, Kwok KF, Wong KH, Chu KM, Sham JS, Wong J. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg. 2003;238:339-347; discussion 347-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Li H, Zhang Q, Xu L, Chen Y, Wei Y, Zhou G. Factors predictive of prognosis after esophagectomy for squamous cell cancer. J Thorac Cardiovasc Surg. 2009;137:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Edge SB BD, Compton CC, FritzAG , Greene FL, Trotti A III. AJCC cancer staging manual. 7th ed. 7th ed. New York: Springer 2009; 103-115. |
| 20. | Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol. 2017;12:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 490] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 21. | Kosugi S, Kawaguchi Y, Kanda T, Ishikawa T, Sakamoto K, Akaike H, Fujii H, Wakai T. Cervical lymph node dissection for clinically submucosal carcinoma of the thoracic esophagus. Ann Surg Oncol. 2013;20:4016-4021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Zhang BH, Cheng GY, Xue Q, Gao SG, Sun KL, Wang YG, Mu JW, He J. Clinical outcomes of basaloid squamous cell carcinoma of the esophagus: a retrospective analysis of 142 cases. Asian Pac J Cancer Prev. 2013;14:1889-1894. [PubMed] |
| 23. | Saito S, Hosoya Y, Zuiki T, Hyodo M, Lefor A, Sata N, Nagase M, Nakazawa M, Matsubara D, Niki T. A clinicopathological study of basaloid squamous carcinoma of the esophagus. Esophagus. 2009;6:177-181. |
| 24. | Takemura M, Yoshida K, Fujiwara Y, Sakurai K, Takii M. A case of long-term survival after pulmonary resection for metachronous pulmonary metastasis of basaloid squamous cell carcinoma of the esophagus. Int J Surg Case Rep. 2012;3:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |