Published online Nov 28, 2018. doi: 10.3748/wjg.v24.i44.5005
Peer-review started: September 11, 2018
First decision: October 11, 2018
Revised: October 20, 2018
Accepted: November 2, 2018
Article in press: November 2, 2018
Published online: November 28, 2018
To develop a novel rat model of heterogeneous hepatic injury.
Seventy male Sprague-Dawley rats were randomly divided into a control group (n = 10) and a colchicine group (n = 60). A 0.25% colchicine solution (0.4 mL/kg) was injected via the splenic vein in the colchicine group to develop a rat model of heterogeneous hepatic injury. An equal volume of normal saline was injected via the splenic vein in the control group. At days 3, 7, and 14 and weeks 4, 8, and 12 after the operation, at least seven rats of the colchicine group were selected randomly for magnetic resonance imaging (MRI) examinations, and then they were euthanized. Ten rats of the control group underwent MRI examinations at the same time points, and then were euthanized at week 12. T2-weighted images (T2WI) and diffusion weighted imaging (DWI) were used to evaluate the heterogeneous hepatic injury. The heterogeneous injury between the left and right hepatic lobes was assessed on liver sections according to the histological scoring criteria, and correlated with the results of MRI study.
Obvious pathological changes occurred in the hepatic parenchyma in the colchicine group. Hepatic injury scores were significantly different between the left and right lobes at each time point (P < 0.05). There was a significant difference in apparent diffusion coefficient (ADC) of DWI and liver-to-muscle ratio (LMR) of T2WI between the left and right lobes of rats in the colchicine group (P < 0.05) at each time point, and similar results were observed between the colchicine and control groups. Besides, there was a significant correlation between hepatic injury scores and ADC values or LMR (r = -0.682, P = 0.000; r = -0.245, P = 0.018).
Injection with colchicine via the splenic vein can be used to successfully develop a rat model of heterogeneous hepatic injury. DWI and T2WI may help evaluate the heterogeneous injury among liver lobes.
Core tip: In this study, injection with colchicine via the splenic vein is shown to successfully develop a rat model of heterogeneous hepatic injury. Obvious pathological changes occurred in the hepatic parenchyma in the colchicine group. A significant difference was observed in liver injury scores, apparent diffusion coefficient values, and liver-to-muscle ratios of T2-weighted images (T2WI) between the left and right lobes in the colchicine group (P < 0.05). Our study suggested that diffusion weighted imaging and T2WI can be used to evaluate the heterogeneous injury among liver lobes.
- Citation: Zhang YY, Zhang CX, Li Y, Jiang X, Wang YF, Sun Y, Wang J, Ji WY, Liu Y. Development of a novel rat model of heterogeneous hepatic injury by injection with colchicine via the splenic vein. World J Gastroenterol 2018; 24(44): 5005-5012
- URL: https://www.wjgnet.com/1007-9327/full/v24/i44/5005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i44.5005
Heterogeneous hepatic injury is often manifested in patients with hepatic tumors, especially those accompanied by hepatitis and cirrhosis. Estimation of total and regional hepatic function is essential for preventing postoperative liver failure and devising an effective treatment plan for patients with hepatic tumors[1]. The current technological limitations that preclude whole-organ assessment of heterogeneous hepatic injury present a clinical challenge. Unfortunately, the lack of an ideal animal model of heterogeneous hepatic injury has hindered the development of such assessment methods[2]. Therefore, it is urgent to establish a practical and reproducible animal model of heterogeneous hepatic injury to provide a manipulable in vivo tool for future development of simple, safe, and effective whole liver assessment methods.
Several animal models have been established to evaluate liver function, but they are limited in their ability to reflect homogeneous hepatic injury. The most popular of these models induce hepatic injury subcutaneous injection with a mixture of CCl4 and olive oil to induce hepatic injury[3] and daily gavage with colchicine consecutively for 4 wk (in mice)[4].
Colchicine is an antimitotic cytotoxic agent derived from the Colchicum autumnale plant. Although the exact mechanism of hepatotoxicity of colchicine remains unclear, in the present study we injected rats with colchicine via the splenic vein to develop a practical model of heterogeneous hepatic injury. The heterogeneous injury between the left and right hepatic lobes was assessed on liver sections according to the histological scoring criteria, which was then correlated with the results of magnetic resonance imaging (MRI) using the sequences reported for evaluating hepatic injury[5,6].
All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. This study was approved by the animal care committee of our university.
Seventy male Sprague-Dawley rats of SPF grade, weighing 280 ± 20 g, were purchased form Changsheng Laboratory, Benxi, Liaoning (certification number: SCXK-2015-0001). Before the start of experiments, the rats were fed normally in separated cages. The animals were randomly divided into a control group (n = 10) and a colchicine group (n = 60). All animals were fed a standard diet before the operation.
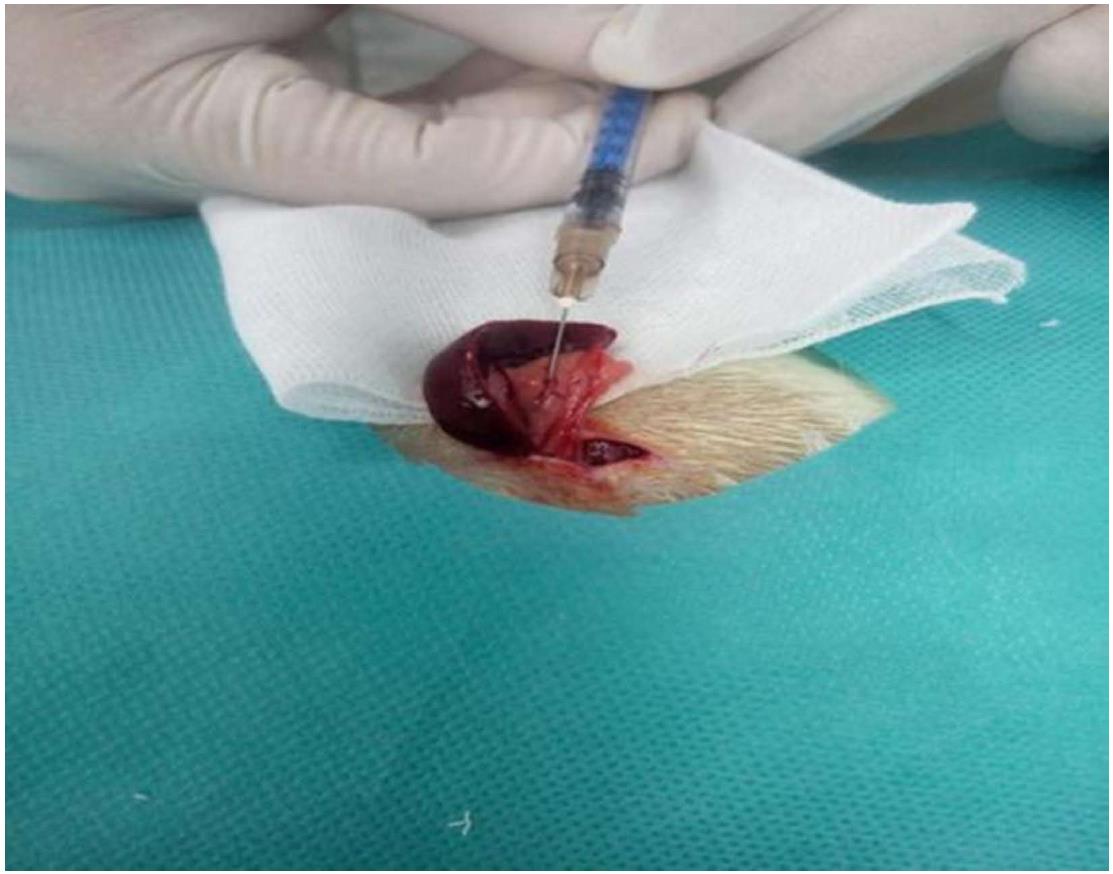
All rats were fasted for at least 6 hours before the operation. Pentobarbital sodium (1%; 40 mg/kg) was injected intraperitoneally for anesthesia. For rats in the colchicine group, after opening the peritoneal cavity, 0.25% colchicine (Nanjng Zelang Medical Technology Co. Ltd., Nanjing, China; purity: > 98%; injection rate: 0.1 mL/s) was injected at a dose of 0.4 mL/kg via the splenic vein. For rats in the control group, the peritoneal cavity was opened similarly and an equal volume of normal saline was injected via the splenic vein (Figure 1). After the operation, all animals were fed a normal diet. Twelve rats in the colchicine group died after 24 h and were excluded from further experiments.
At days 3, 7, and 14 and week 4 after the operation, seven rats of the colchicine group, and at weeks 8 and 12, ten rats of the colchicine group were randomly selected for MRI examinations, and then the rats were euthanized by over-anesthesia. One rat died from an overdose of anesthetic at week 8, and a second one died at week 12. Ten rats of the control group underwent MRI examinations at the same time points, and were euthanized at week 12 after MRI examinations. Liver tissue was fixed in 4% paraformaldehyde.
Prior to MRI examinations, the selected rats were fasted for about 8 h. After anesthesia by intraperitoneal injection with 1% pentobarbital sodium (40 mg/kg), MRI was performed using a GE Signa HDxT 3.0T magnet scanner with a wrist coil. The detailed scanning settings are as follows: T2-weighted images (T2WI): TR, 3840 ms; TE, 85 ms; field of view, 14 cm; NEX, 4; matrix, 256 × 192; slice thickness, 3 mm; flip angle, 90°; scan time, 3 min and 12 s; and diffusion weighted imaging (DWI): b = 500 s/mm2; TR, 5000 ms; TE, 77.3 ms; field of view, 14 cm; NEX, 2; matrix, 128 × 128; slice thickness, 3 mm; flip angle, 90°; scan time, 40 s.
Image analysis was performed independently by two radiologists with more than 5 years of clinical experience, who were blinded to the histopathologic results. The largest regions of interest (ROIs) as possible were defined in both the left and right lobes of the liver in three successive slices on T2WI and apparent diffusion coefficient (ADC) maps, and the vessels and artifacts were excluded when positioning the ROI. The signal intensities of the erector spinae muscles on T2WI were detected on the same slices simultaneously, and the average values of these measurements on the three successive slices were calculated. Based on the average values, the liver-to-muscle ratio (LMR) on T2WI, ΔLMR (the difference of LMR on T2WI between the left and right lobes of the liver), and ΔADC (the difference of ADC values between the left and right lobes of the liver) were calculated[7,8].
Liver tissue was fixed, paraffin-embedded, and sliced (5.0 μm). After conventional hematoxylin and eosin (H&E) staining, the sections were examined under a light microscope. Masson’s trichrome staining was used to assess fibrosis. Scoring for liver injury was conducted according to the following criteria: no hepatocellular necrosis, edema, or inflammatory cell infiltration, 0; mild, 1; moderate, 2; severe, 3. Liver fibrosis was scored as no fibrosis, 0; fibrous portal expansion, 1; bridging fibrosis, 2; bridging fibrosis with architectural distortion, 3; liver cirrhosis, 4[9,10].
Data are expressed as mean ± standard deviation. Normal distribution was assessed by the Kolmogorov-Smirnov test. Differences in liver injury, ΔLMR, and ΔADC between the two groups were compared using the Student’s t-test or Mann-Whitney U-test. Statistical significance was defined as P < 0.05. Correlations between LMR, ADC values, and liver injury scores in the colchicine group were assessed using the Spearman’s correlation coefficient by rank test. Statistically significant correlations were defined as P < 0.05.
All animals awoke within 1 h after the operation. Rats in the colchicine group exhibited fatigue, reduced food and water consumption, and slow movement after the operation. Although the activity of rats in the control group was slightly decreased, their general state was normal upon awaking. Rats in the control group all survived the operation. Twelve rats in the colchicine group did not survive the procedure and died after 24 h of the operation. One rat died from an overdose of anesthetic at week 8 wk during the MRI examination, and a second one died at week 12.
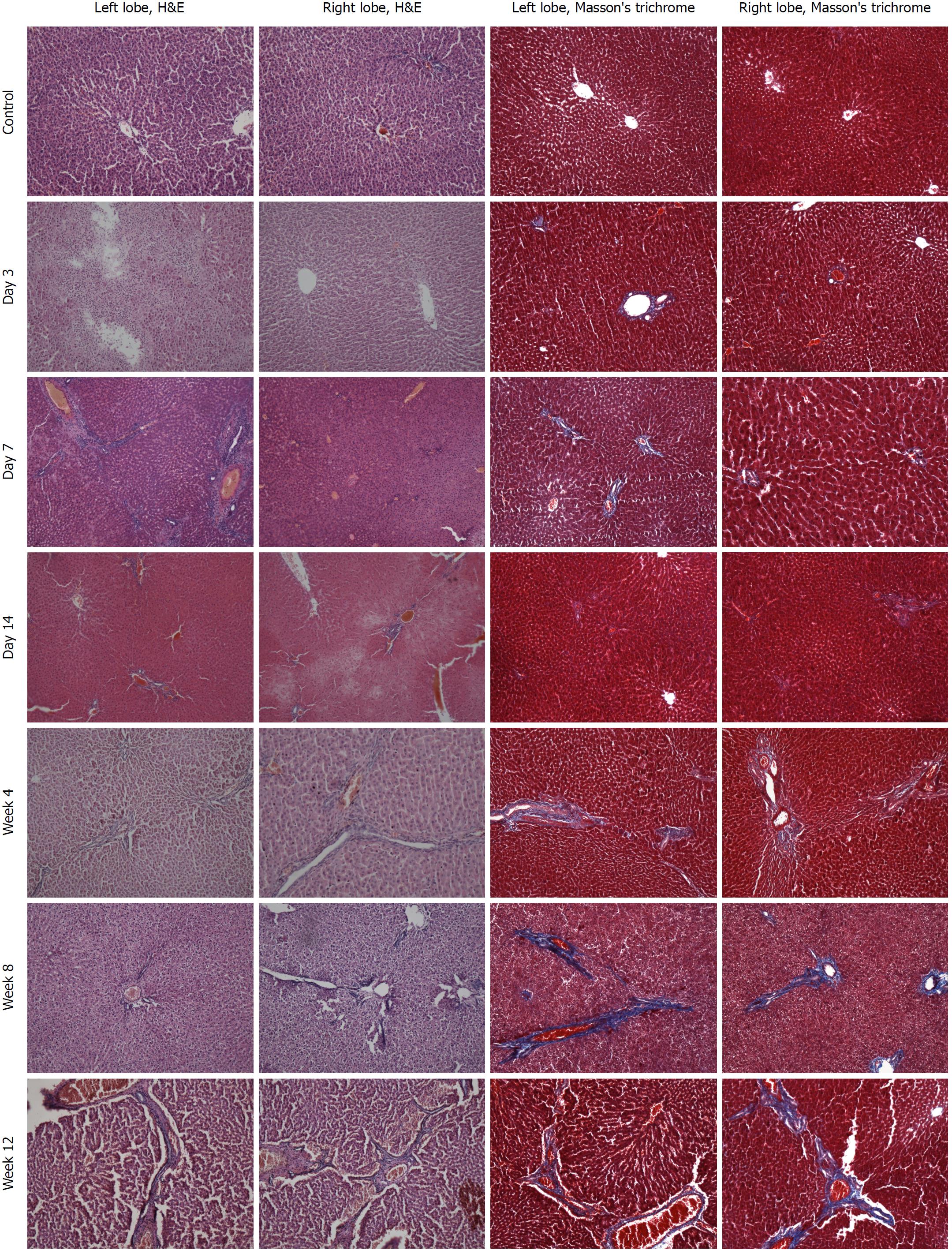
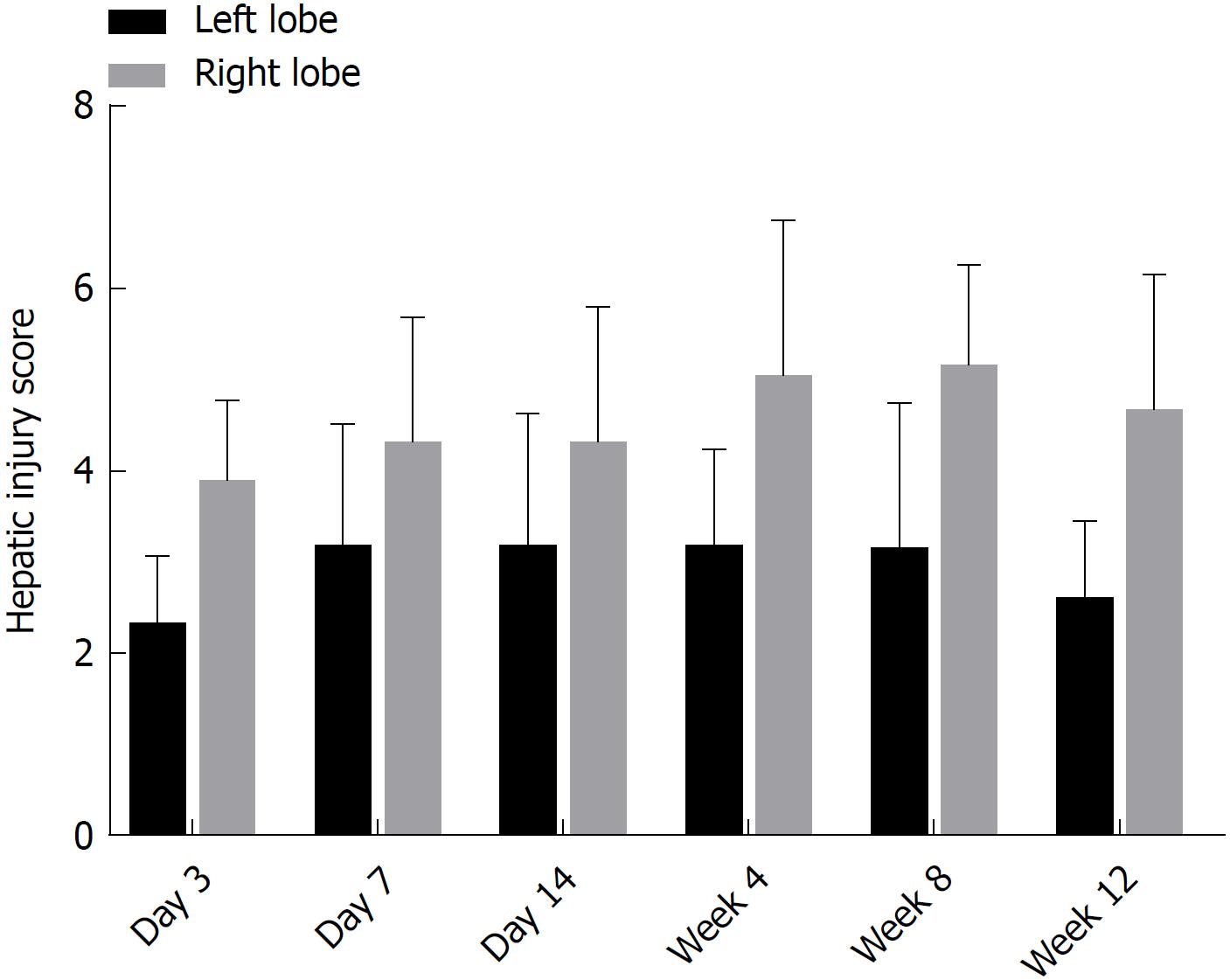
There were no obvious pathological changes of rat liver tissue in the control group under a light microscope. In the colchicine group, hepatocellular necrosis, inflammatory cell infiltration, hepatocellular edema, and liver fibrosis were observed, accompanied by hepatic cord disappearance and nuclear dissolution. At day 3 after colchicine injection, there was massive inflammatory cell infiltration, hepatocellular edema, and mild liver necrosis, without apparent fibrosis. At day 14, reduced inflammation and increased necrosis were observed, while fibrosis was not detected. At week 4, cholestasis and early fibrosis were observed. At weeks 8 and 12, there was further fibrosis (Figure 2). Based on the scoring criteria[9,10], hepatic injury scores were significantly different between the left and right lobes at each time point (P < 0.05, Figure 3).
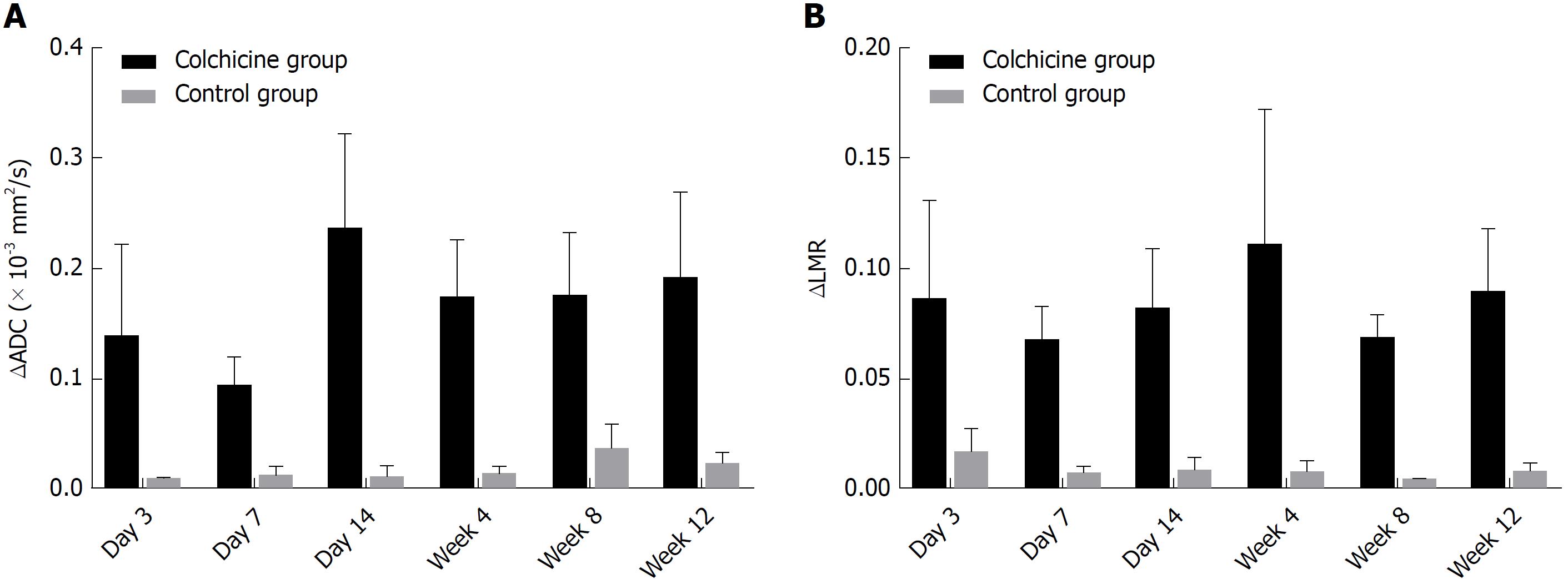
ΔADC between the right and left hepatic lobes differed significantly between the colchicine group and the control group (P < 0.05; Figure 4A). A statistically significant difference was also noted in ΔLMR between the right and left hepatic lobes from the T2WI of the colchicine group compared to that of the control group at each time point (P < 0.05; Figure 4B).
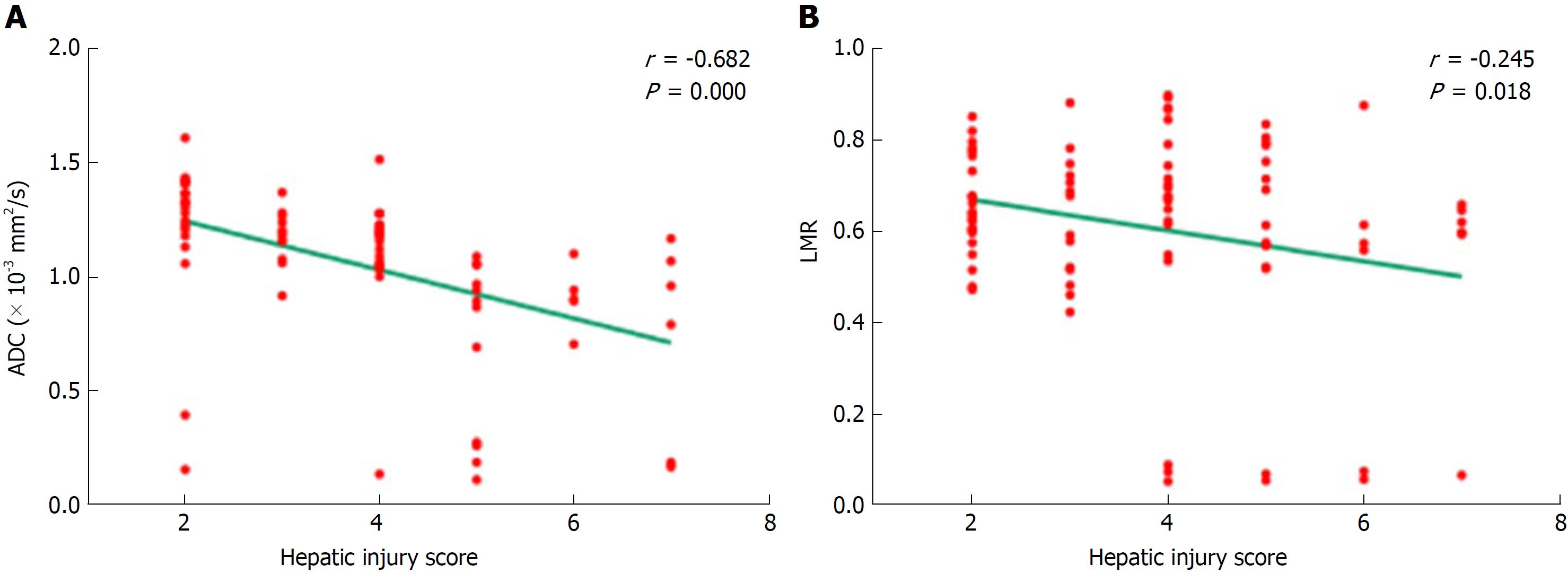
The relationship between ADC values and hepatic injury scores is shown in Figure 5A. The ADC values decreased as hepatic injury scores increased, and the correlation was statistically significant (r = -0.682; P = 0.000). LMR and hepatic injury scores also demonstrated a negative correlation (r = -0.245; P = 0.018) (Figure 5B).
Patients who will undergo liver resection always exhibit heterogeneous hepatic injury induced by chemotherapy, metabolic syndrome, or cirrhosis[1,11-16], and this often leads to postoperative liver failure, which has become the leading cause of mortality after liver resection[17-19]. Therefore, assessment of the uneven distribution of hepatic function and prediction of reserved liver function are essential for preventing postoperative liver failure[12]. The development of a practical animal model of heterogeneous liver injury is the basis for further studies to curtail or eliminate this dire situation. Although there have been some animal models of hepatic injury, such as administration of thioacetamide solution in drinking water and a choline-deficient diet[20] and subcutaneous injection with a mixture of CCl4 and olive oil[3], these models show homogeneous hepatic injury and cannot be used to investigate the heterogeneous liver injury condition that exists in human patients. Therefore, it is urgent to develop a simple, noninvasive, and reliable method to estimate liver regional function in patients with liver diseases.
In this study, we successfully developed an animal model of heterogeneous liver injury by injection with colchicine via the splenic vein in rats. The toxicity of colchicine may affect all cells in the body and causes multi-organ toxicity[21]. Colchicine binds to the intracellular tubule, arresting its polymerization of alpha and beta forms into microtubules. Proteins of the Golgi apparatus, endocytosis, exocytosis, cellular shape, and motility are therefore impaired. Mitosis is also disrupted in metaphase because of compromised microtubule-dependent functions in chromosome separation[22,23]. Colchicine has been used to induce conspicuous hepatotoxicity diseases including liver necrosis and steatosis in animals[4,24]. On the other hand, it was found that portal vein blood, which comes from the superior mesenteric vein and the splenic vein, is unevenly distributed in different lobes of the liver after merging into the portal vein. Thus, we injected rats with colchicine via the splenic vein to introduce inhomogeneous hepatic injury, and a statistically significant difference in pathological changes between the left and right hepatic lobes was observed. The histological results showed heterogeneous hepatocellular necrosis, edema, inflammatory cell infiltration, and liver fibrosis after colchicine injection, as well as cord disappearance, fibrous septa collapse, and nuclear dissolution. These findings support the point that this rat model can be used for future studies of hepatic injury, such as quantitative analysis of regional liver function.
In this model, the pathological changes of hepatic parenchyma mirrored the findings of previous studies[6], namely, the decreased inflammation of hepatic parenchyma within 2 wk after colchicine injection and the progressive, irreversible development of fibrosis. The current gold standard for estimation of liver injury is liver biopsy, yet liver specimens obtained by needle biopsy represent only a very small part of the liver parenchyma[25]. Moreover, liver biopsy is associated with the possibility of sampling errors, invasiveness, interobserver variability, and risk of complications. Therefore, liver biopsy is not practicable for estimating inhomogeneous hepatic injury[26]. In addition, there are currently no serum markers or clinical signs that can accurately assess liver regional function[20].
Recently, several MRI methods have been developed to characterize the processes of various liver diseases and grade the extent of liver disease[27]. In addition, several studies have confirmed the value of magnetic resonance sequences in evaluating liver injury[5,6,28]. Therefore, in this study we used the T2WI and DWI to assess heterogeneous liver injury in our model. The ADC values from DWI can be used to measure the diffusion of random molecular motions[29]. As such, in the early stage of chemical hepatic injury, decreased ADC values may reflect a reduced ratio of extracellular/intracellular water volume caused by cytotoxic intracellular edema, as well as decreased intracellular proton movement resulting from energy loss. As fibrosis progresses, narrowed sinusoids and restricted water mobility caused by accumulation of collagen fibers, glycosaminoglycans, and proteoglycan lead to even lower ADC values for the liver parenchyma. This is similar to other reports showing that the ADC values decreased as liver disease progressed and fibrotic scores increased[30]. A previous study showed that hepatic injury resulted in increased T2 relaxation time and heightened T2WI sensitivity to necrosis[31]. Others also reported that T2WI can be used to monitor in vivo hepatotoxicity over time[32]. In our study, the difference of histological changes between the left and right lobes caused by uneven injury was reflected by LMR calculated from T2WI and ADC value from DWI. Both ADC values and LMR decreased as hepatic injury scores increased, and the correlations were statistically significant. The correlation coefficient between the ADC value and hepatic injury score was significantly higher than that between the LMR and hepatic injury score. The results of this study support the notion that both ADC value and LMR are potentially useful for evaluating heterogeneous hepatic injury.
There are several limitations to the present study that must be considered when interpreting or generalizing our findings. First, the stability of uneven hepatic injury is influenced by individual differences of animals. Second, none of the MRI parameters was obtained over time to evaluate longitudinal changes. Third, although the ADC value from DWI enables noninvasive prediction of heterogeneous hepatic injury, it is limited by its relatively poor spatial resolution. Further studies are needed to explore other techniques such as gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI, T1 mapping, and T2 mapping for assessing regional liver function in this model[28,33].
In summary, a novel rat model with uneven hepatic injury has been developed by injection with colchicine via the splenic vein. Data generated using this model suggested that DWI and T2WI can potentially evaluate heterogeneous injury between liver lobes.
We appreciate the help from Professor Xuyong Lin (Pathology, The First Hospital of China Medical University) for pathology diagnosis.
Heterogeneous hepatic injury is often exhibited in patients who will undergo liver resection, especially those accompanied by hepatitis and cirrhosis. Assessment of uneven hepatic function is essential for preventing postoperative liver failure. Until now, there has been no simple, safe, and effective method to evaluate heterogeneous hepatic injury due to the absence of an ideal animal model.
The development of a practical, reproducible animal model of heterogeneous hepatic injury is the basis for future studies that will ultimately benefit human clinical practice. In the present study, a novel rat model was established by injection with colchicine via the splenic vein, aiming at developing a practical model of heterogeneous hepatic injury. The heterogeneous injury between the left and right hepatic lobes was assessed on liver sections according to the histological scoring criteria, which was then correlated with the results of magnetic resonance imaging (MRI) using the sequences reported for evaluating hepatic injury.
To develop a practical rat model of heterogeneous hepatic injury which can be used for the future studies into human clinical parameters, such as quantitative analysis of the regional liver function, by injection with colchicine via the splenic vein.
Seventy male Sprague-Dawley rats were randomly divided into a control group and a colchicine group. Colchicine (0.25%) was injected via the splenic vein to develop a rat model of heterogeneous hepatic injury. An equal volume of normal saline was injected via the splenic vein in the control group. After the operation, rats of the colchicine group were selected randomly for MRI examinations. Rats of the control group underwent MRI examinations. T2-weighted images (T2WI) and diffusion weighted imaging (DWI) were used to evaluate the heterogeneous hepatic injury. The heterogeneous injury between the left and right hepatic lobes was assessed on liver sections according to the histological scoring criteria, which was then correlated with the results of MRI study.
Obvious pathological changes of hepatic parenchyma were observed over time in the colchicine group. Hepatic injury scores were significantly different between the left and right lobes at each time point. There was a significant difference in apparent diffusion coefficient (ADC) of DWI and liver-to-muscle ratio (LMR) of T2WI between the left and right lobes in the colchicine group at each time point, and similar result was also observed between the colchicine and control groups. Besides, there were significant correlations between hepatic injury scores and ADC values or LMR. Some problems, such as the stability of the uneven hepatic injury influenced by individual differences of animals, and longitudinal changes that can be evaluated using MRI parameters obtained over time, remain to be solved.
In this study, it was found that injection with colchicine via the splenic vein can be used to successfully develop a rat model of heterogeneous hepatic injury. The results of this study support that DWI and T2WI can potentially evaluate heterogeneous injury among liver lobes.
Using this model, future studies are needed to explore other new techniques for assessing the uneven distribution of hepatic function and predicting the reserved liver function.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kositamongkol P, Lee S, Svein D S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Nilsson H, Karlgren S, Blomqvist L, Jonas E. The inhomogeneous distribution of liver function: possible impact on the prediction of post-operative remnant liver function. HPB (Oxford). 2015;17:272-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Ma C, Liu A, Wang Y, Geng X, Hao L, Song Q, Sun B, Wang H, Zhao G. The hepatocyte phase of Gd-EOB-DTPA-enhanced MRI in the evaluation of hepatic fibrosis and early liver cirrhosis in a rat model: an experimental study. Life Sci. 2014;108:104-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Guo X, Lin D, Li W, Wang K, Peng Y, Zheng J. Electrophilicities and Protein Covalent Binding of Demethylation Metabolites of Colchicine. Chem Res Toxicol. 2016;29:296-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Cassinotto C, Feldis M, Vergniol J, Mouries A, Cochet H, Lapuyade B, Hocquelet A, Juanola E, Foucher J, Laurent F. MR relaxometry in chronic liver diseases: Comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. Eur J Radiol. 2015;84:1459-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Zhao F, Wang YX, Yuan J, Deng M, Wong HL, Chu ES, Go MY, Teng GJ, Ahuja AT, Yu J. MR T1ρ as an imaging biomarker for monitoring liver injury progression and regression: an experimental study in rats with carbon tetrachloride intoxication. Eur Radiol. 2012;22:1709-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Shimamoto D, Nishie A, Asayama Y, Ushijima Y, Takayama Y, Fujita N, Shirabe K, Hida T, Kubo Y, Honda H. MR Prediction of Liver Function and Pathology Using Gd-EOB-DTPA: Effect of Liver Volume Consideration. Biomed Res Int. 2015;2015:141853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Katsube T, Okada M, Kumano S, Imaoka I, Kagawa Y, Hori M, Ishii K, Tanigawa N, Imai Y, Kudo M. Estimation of liver function using T2* mapping on gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid enhanced magnetic resonance imaging. Eur J Radiol. 2012;81:1460-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Lu Y, Liu P, Fu P, Chen Y, Nan D, Yang X. Comparison of the DWI and Gd-EOB-DTPA-enhanced MRI on assessing the hepatic ischemia and reperfusion injury after partial hepatectomy. Biomed Pharmacother. 2017;86:118-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Tomimaru Y, Sasaki Y, Yamada T, Eguchi H, Ohigashi H, Ishikawa O, Imaoka S. Fibrosis in non-cancerous tissue is the unique prognostic factor for primary hepatocellular carcinoma without hepatitis B or C viral infection. World J Surg. 2006;30:1729-1735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Tsujino T, Samarasena JB, Chang KJ. EUS anatomy of the liver segments. Endosc Ultrasound. 2018;7:246-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Nilsson H, Blomqvist L, Douglas L, Nordell A, Janczewska I, Näslund E, Jonas E. Gd-EOB-DTPA-enhanced MRI for the assessment of liver function and volume in liver cirrhosis. Br J Radiol. 2013;86:20120653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | de Graaf W, Häusler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJ, Kullak-Ublick GA, Hesselmann R, van Gulik TM, Stieger B. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54:738-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Arun J, Jhala N, Lazenby AJ, Clements R, Abrams GA. Influence of liver biopsy heterogeneity and diagnosis of nonalcoholic steatohepatitis in subjects undergoing gastric bypass. Obes Surg. 2007;17:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1376] [Cited by in F6Publishing: 1416] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 17. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-discussion 829. [PubMed] [Cited in This Article: ] |
| 18. | Capussotti L, Viganò L, Giuliante F, Ferrero A, Giovannini I, Nuzzo G. Liver dysfunction and sepsis determine operative mortality after liver resection. Br J Surg. 2009;96:88-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862; discussion 862-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 471] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 20. | Tsuda N, Matsui O. Signal profile on Gd-EOB-DTPA-enhanced MR imaging in non-alcoholic steatohepatitis and liver cirrhosis induced in rats: correlation with transporter expression. Eur Radiol. 2011;21:2542-2550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Smilde BJ, Woudstra L, Fong Hing G, Wouters D, Zeerleder S, Murk JL, van Ham M, Heymans S, Juffermans LJ, van Rossum AC. Colchicine aggravates coxsackievirus B3 infection in mice. Int J Cardiol. 2016;216:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Deng M, Zhao F, Yuan J, Ahuja AT, Wang YX. Liver T1ρ MRI measurement in healthy human subjects at 3 T: a preliminary study with a two-dimensional fast-field echo sequence. Br J Radiol. 2012;85:e590-e595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Unal E, Idilman IS, Karçaaltıncaba M. Multiparametric or practical quantitative liver MRI: towards millisecond, fat fraction, kilopascal and function era. Expert Rev Gastroenterol Hepatol. 2017;11:167-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Declèves X, Niel E, Debray M, Scherrmann JM. Is P-glycoprotein (ABCB1) a phase 0 or a phase 3 colchicine transporter depending on colchicine exposure conditions? Toxicol Appl Pharmacol. 2006;217:153-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36:S57-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Kose S, Ersan G, Tatar B, Adar P, Sengel BE. Evaluation of Percutaneous Liver Biopsy Complications in Patients with Chronic Viral Hepatitis. Eurasian J Med. 2015;47:161-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Wibmer A, Nolz R, Trauner M, Ba-Ssalamah A. [Functional MR imaging of the liver]. Radiologe. 2015;55:1057-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Poynard T, Lenaour G, Vaillant JC, Capron F, Munteanu M, Eyraud D, Ngo Y, M’Kada H, Ratziu V, Hannoun L. Liver biopsy analysis has a low level of performance for diagnosis of intermediate stages of fibrosis. Clin Gastroenterol Hepatol. 2012;10:657-663.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med. 2015;128:461-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 30. | Roubille F, Kritikou E, Busseuil D, Barrere-Lemaire S, Tardif JC. Colchicine: an old wine in a new bottle? Antiinflamm Antiallergy Agents Med Chem. 2013;12:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Shankar S, Kalra N, Bhatia A, Srinivasan R, Singh P, Dhiman RK, Khandelwal N, Chawla Y. Role of Diffusion Weighted Imaging (DWI) for Hepatocellular Carcinoma (HCC) Detection and its Grading on 3T MRI: A Prospective Study. J Clin Exp Hepatol. 2016;6:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Koinuma M, Ohashi I, Hanafusa K, Shibuya H. Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J Magn Reson Imaging. 2005;22:80-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Lisotti A, Serrani M, Caletti G, Fusaroli P. EUS liver assessment using contrast agents and elastography. Endosc Ultrasound. 2018;7:252-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |