Published online Jan 28, 2018. doi: 10.3748/wjg.v24.i4.484
Peer-review started: October 29, 2017
First decision: November 30, 2017
Revised: December 10, 2017
Accepted: December 20, 2017
Article in press: December 20, 2017
Published online: January 28, 2018
Processing time: 89 Days and 19.2 Hours
To identify clinical biomarkers that could early predict improved survival in patients with advanced-stage hepatocellular carcinoma (HCC) treated with transarterial chemoembolization combined with sorafenib (TACE-S).
We retrospectively evaluated the medical records of consecutive patients with advanced-stage HCC who underwent TACE-S from January 2012 to December 2015. At the first follow-up 4-6 wk after TACE-S (median, 38 d; range, 33-45 d), patients exhibiting the modified Response Evaluation Criteria in Solid Tumors (mRECIST)-evaluated complete response, partial response, and stable disease were categorized as early disease control. At this time point, multiple variables were analyzed to identify the related factors affecting survival.
Ninety-five patients were included in this study, and 60 of these patients achieved early disease control, with an overall disease control rate (DCR) of 63.2%. Patients who got sorafenib at the first TACE (no previous TACE) and patients without portal vein tumor thrombus (PVTT) had a higher DCR than those who underwent previous TACE before TACE-S (72.4% vs 48.6%, P = 0.019) and those with PVTT (75.5% vs 50.0%, P = 0.010). Early disease control after TACE-S, no previous TACE, and no PVTT were the independent prognostic factors for survival in the uni- and multivariate analyses.
The first follow-up 4-6 wk after TACE-S can be used as the earliest time point to assess the response to TACE-S, and patients with mRECIST-evaluated early disease control, no previous TACE, and no PVTT had better survival.
Core tip: There are no clinical data/markers to early predict improved survival in patients with advanced-stage hepatocellular carcinoma treated with transarterial chemoembolization combined with sorafenib (TACE-S). In this study, we found that mRECIST-evaluated disease control (complete response, partial response, and stable disease) at the first follow-up 4-6 wk after TACE-S can be used as an early indicator of better survival from TACE-S. We also found that patients with previous TACE and portal vein tumor thrombus had a poor survival.
- Citation: Meng XC, Chen BH, Huang JJ, Huang WS, Cai MY, Zhou JW, Guo YJ, Zhu KS. Early prediction of survival in hepatocellular carcinoma patients treated with transarterial chemoembolization plus sorafenib. World J Gastroenterol 2018; 24(4): 484-493
- URL: https://www.wjgnet.com/1007-9327/full/v24/i4/484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i4.484
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the fourth most prevalent cause of tumor-related deaths[1-4]. Although the surveillance programs for the early detection of HCC have been recommended to high-risk populations, some HCC patients are still diagnosed at an advanced stage, with vascular invasion or distant metastasis. The prognosis of patients with advanced-stage HCC is very poor, with a very short median survival time (less than 6 mo)[5-7]. The Barcelona Clinic Liver Cancer (BCLC) group recommended the tyrosine kinase inhibitor sorafenib as a standard therapy for patients with advanced-stage HCC (BCLC stage C)[8-10]. However, the tumor response rate to sorafenib monotherapy is modest with survival prolonged only for less than three months compared with placebo[9,10]. Recently, a new treatment modality, the combination of delaying intrahepatic tumor progression with transarterial chemoembolization (TACE) and targeting systemic disease (e.g., vascular invasion or extrahepatic metastasis) with sorafenib, is recommended as an alternative for patients with advanced-stage HCC[11-13], and indeed, some studies have demonstrated favorable safety profiles and survival benefits conferred by TACE combined with sorafenib (hereafter, TACE-S)[12-17].
The first follow-up assessment after TACE-S, usually at 4-6 wk after TACE-S, which is considered the earliest assessment time point, may directly guide the decisions about subsequent therapies. However, to date, there has been no specific baseline or clinical biomarker (clinical, radiologic, and/or biochemical) used at the first follow-up assessment to identify those patients who would benefit most from this combination treatment. The modified Response Evaluation Criteria in Solid Tumors (mRECIST) has been proposed for assessing the response to therapy in patients with HCC[18-22]. Indeed, some studies have demonstrated that patients with mRECIST objective responses [complete response (CR) and partial response (PR)] to TACE alone at the first follow-up assessment 4-6 wk after TACE have better survival[18,19]. However, what is the situation after combination therapy with TACE and sorafenib? As sorafenib is part of the combination therapy, the majority of sorafenib adverse events (AEs) appear within the first month of sorafenib treatment. Zhao et al[14] demonstrated that ≥ grade 2 early sorafenib-related dermatologic AEs within the first month of sorafenib initiation could determine the efficacy of TACE-S[23]. This finding implies that sorafenib has had an effect in targeting HCC cells and/or inhibiting tumor angiogenesis within the first month of sorafenib initiation. Thus, we speculated that patients obtaining survival benefits from the combination therapy may include not only patients with mRECIST-evaluated CR and PR at 4-6 wk after TACE-S but also those patients with mRECIST-evaluated stable disease (SD) because sorafenib might have a tumor stabilizing effect in delaying tumor progression. In fact, in two phase III randomized controlled trials of sorafenib in patients with advanced-stage HCC[9,10], the main benefit of sorafenib monotherapy is from the prolonged disease stabilization, which leads to improvement in overall survival (OS). Therefore, in the present study, we designed the first follow-up assessment at 4-6 wk after TACE-S as the earliest observation time point and included mRECIST-evaluated disease control (CR + PR + SD) as one of the early indicators for investigating which patients might benefit the most from TACE-S.
This study was a retrospective study in which patients with advanced-stage HCC (BCLC stage C) who had been treated with TACE-S between January 2012 and December 2015 were consecutively enrolled at our institution. HCC was diagnosed according to the non-invasive criteria following the European Association for the Study of Liver/American Association for the Study of Liver Disease guidelines[24]. The inclusion criteria for the study population were: (1) being between 18 and 75 years of age; (2) having an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1; (3) having Child-Pugh class A or B liver disease; (4) having total bilirubin < 51 μmol/ L; and (5) having an abdominal and chest CT or magnetic resonance (MR) scan one week before treatment (at baseline), and by mRECIST criteria[25-27] having at least one target lesion that confirmed the diagnosis of HCC. Patients were excluded from this study if they: (1) had complete main portal vein obstruction without collateral circulation around the portal trunk; (2) had undergone radiofrequency ablation, surgery, or liver transplantation; (3) had undergone other treatments (radiofrequency ablation or 125I seed implantation) besides TACE during this study; (4) had infiltrative lesions not suitable for imaging assessment; (5) had serious medical comorbidities; or (6) had current or a history of malignant tumors in addition to HCC. The study was approved by our institutional review board. Written informed consent was obtained from all patients before treatment.
TACE was performed with a five-French catheter or microcatheter as selectively as possible through the lobar or segmental arteries, depending on the tumor distribution. Initially, a solution of lobaplatin at a concentration of 0.5 mg/mL was infused into the tumor feeder vessels. The total level of lobaplatin ranged from 20 to 50 mg depending on the patient’s body weight. Then, an emulsion of 2-10 mL of lipiodol (Lipiodol Ultrafluido, Guerbet, France) and 20-60 mg of doxorubicin hydrochloride was administered into the feeder vessels. Finally, gelatin sponge particles or polyvinyl alcohol particles (Cook, Bloomington, IN, United States) that were mixed with contrast material were administered into the feeder vessels until stasis of arterial flow was achieved. After embolization, angiography was performed to determine the extent of vascular occlusion.
Sorafenib 400 mg was orally administered twice daily 3-5 d after TACE, and patients were treated with continuous sorafenib with no breaks before or after repeated TACE. Sorafenib dose reduction was based on the presence of toxicity. If grade 3/4 hematological toxicity, skin toxicity, gastrointestinal toxicity, hypertension, or hepatic dysfunction defined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03 occurred[28], a dose adjustment (400 mg once daily) was required until the AEs were alleviated or eliminated. After dose adjustment, if grade 3/4 AEs continued, sorafenib treatment was halted until the adverse effects were alleviated or disappeared.
All patients treated in our institution for HCC required follow-up according to our institutional protocol. Each follow-up session included a detailed history and physical examination, laboratory tests, and abdominal contrast material-enhanced three-phase dynamic spiral CT or MR imaging. Laboratory tests included hematological and biochemical analyses, such as complete blood cell count, prothrombin time, α-fetoprotein, aspartate aminotransferase, alanine aminotransferase, total bilirubin, serum albumin, and creatinine. Follow-up of all patients was conducted at a 4-6-wk interval after previous TACE. Patients with intrahepatic residual viable tumor or recurrent tumor on CT/MR imaging underwent repeated TACE, if the Child-Pugh status remained at class A or B and there was no evidence of hepatic decompensation (e.g., uncontrolled ascites or hepatic encephalopathy).
The clinical, laboratory, and radiologic records were reviewed. Side effects of sorafenib and TACE were reported according to NCI-CTCAE version 4.03[28]. Follow-up contrast-enhanced CT or MR was performed 4-6 wk after previous TACE to assess the tumor response and to guide timely decision-making for subsequent therapies. Tumor response was assessed according to the overall mRECIST[25-27], which included a combined assessment of target lesions, nontarget lesions, and new lesions. At baseline, measurable lesions with diameters 1 cm or greater, suitability for repeat measurement, and intratumoral arterial enhancement on contrast-enhanced CT or MR imaging were qualified as target lesions. The longest diameter of the viable tumor (defined as the enhanced area during the arterial phase) was measured on contrast-enhanced CT or MR imaging. Non-enhancing atypical lesions and extrahepatic lesions were assessed using RECIST criteria. The presence or absence of nontarget lesions and the appearance of new lesions were assessed during follow-up. Overall responses were classified into the following four categories: CR, PR, SD, and progressive disease (PD). Patients exhibiting CR, PR, or SD at the first follow-up assessment 4-6 wk after TACE-S were categorized as early disease control, whereas those with PD were classified as non-early disease control. The early disease control rate (DCR) was defined as the percentage of patients who achieved CR, PR, and SD at 4-6 wk after TACE-S. Furthermore, we analyzed the OS. OS was calculated for all patients from the date of their first TACE, with or without sorafenib, until their death or the last follow-up.
All statistical analyses were performed using SPSS version 16.0 software. To determine significant differences in DCR between baseline characteristics, chi-square tests were used. OS was compared using Kaplan-Meier curves with the log-rank test. A Cox proportional hazards model was used to examine risk factors associated with survival. A two-tailed P-value less than 0.05 was considered statistically significant. Chen BH and Cai MY, who had learned about biostatistics, performed the statistical analyses together.
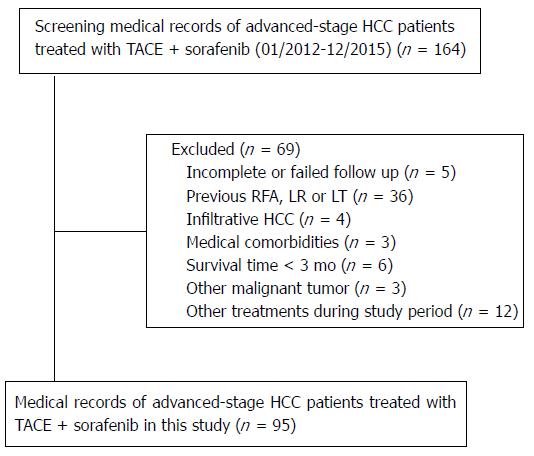
Of the 164 patients initially recruited, 69 were excluded from the study because they met the exclusion criteria (Figure 1). Ultimately, 95 HCC patients were enrolled in this study. The detailed baseline characteristics of these patients are summarized in Table 1. The patient population consisted of 88 (92.6%) men with an age range of 19-73 years (mean, 48.2 years). Among 95 patients who received TACE-S, 58 (61.1%) got sorafenib therapy 3-5 d after the first TACE (no previous TACE); the remaining 37 (38.9%) patients, who had undergone one or more TACE treatments before TACE-S (previous TACE), received the combination of TACE and sorafenib because of tumor progression after previous TACE. Ninety (94.7%) patients presented with hepatitis B, and 80 (84.2%) patients were classified with Child-Pugh A disease. Forty-six (48.4%) patients had PVTT, including 36 (37.9%) at the portal vein branch and 10 (10.5%) at the main portal vein. Twenty-six (27.4%) patients had extrahepatic metastasis, including 14 patients with extrahepatic metastasis in the lymph nodes, 7 patients in the lung, 3 patients in the bones, and 2 patients in the suprarenal gland. The mean duration of follow-up was 14.6 mo (range, 2-28 mo). The median duration of sorafenib treatment was 13.1 mo (range, 2-26 mo). Eighty-six of the 95 patients (90.5%) underwent repeated TACE after TACE-S, with the mean number of TACE procedures per patient of 3.1 (range, 1-5).
| Characteristic | Overall (n = 95) | CR + PR + SD (n = 60) | DCR (63.2) | P value2 |
| Sex | 1.000 | |||
| Male | 88 | 56 | 63.6 | |
| Female | 7 | 4 | 57.1 | |
| Age (yr)1 | 48.2 ± 11 | 0.390 | ||
| < 60 | 80 | 52 | 65 | |
| ≥ 60 | 15 | 8 | 53.3 | |
| α-fetoprotein level (ng/mL) | 0.822 | |||
| < 400 | 42 | 26 | 61.9 | |
| ≥ 400 | 53 | 34 | 64.2 | |
| ECOG performance | 0.752 | |||
| 0 | 66 | 41 | 62.1 | |
| 1 | 29 | 19 | 65.5 | |
| Hepatitis B | 0.745 | |||
| No | 5 | 4 | 80 | |
| Yes | 90 | 56 | 62.2 | |
| Previous TACE | 0.019 | |||
| No | 58 | 42 | 72.4 | |
| Yes | 37 | 18 | 48.6 | |
| Ascites | 0.719 | |||
| Absent | 52 | 32 | 61.5 | |
| Present | 43 | 28 | 65.1 | |
| Child-Pugh classification | 0.373 | |||
| A | 80 | 49 | 61.3 | |
| B | 15 | 11 | 73.3 | |
| PVTT | 0.010 | |||
| Absent | 49 | 37 | 75.5 | |
| Present | 46 | 23 | 50 | |
| Extrahepatic metastasis | 0.103 | |||
| No | 69 | 47 | 68.1 | |
| Yes | 26 | 13 | 50 | |
| Number of tumor | 0.952 | |||
| 1 | 16 | 10 | 62.5 | |
| ≥ 2 | 79 | 50 | 63.3 | |
| Maximum tumor diameter (cm)1 | 9.5 ± 4.5 | 1.000 | ||
| ≤ 3 | 9 | 6 | 66.7 | |
| > 3 | 86 | 54 | 62.8 | |
Among all the 95 patients, 3 (3.2%) achieved CR, 35 (36.8%) achieved PR, and 22 (23.2%) achieved SD at 4-6 wk (median, 38 d; range, 33-45 d) after TACE-S. Thus, a total of 60 patients achieved early disease control (CR + PR + SD), with an overall DCR of 63.2%. The basic characteristics of these 60 patients are shown in Table 1. It was observed that patients who got sorafenib at the first TACE (no previous TACE) had higher DCR than those who underwent one or more TACE treatments before TACE-S (DCR: 72.4% vs 48.6%; P = 0.019). Similarly, patients without PVTT had higher DCR than those with PVTT (DCR: 75.5% vs 50.0%; P = 0.010).
Eighty-one (85.3%) of the 95 patients died during the observation period. The 0.5-, 1-, and 2-year cumulative OS rates were 89.5%, 51.3%, and 16.2%, respectively, and the median OS was 12.7 mo (95%CI: 9.4-15.9 mo).
The univariate Cox proportional hazards regression analysis revealed that no previous TACE, the absence of PVTT, the absence of extrahepatic metastasis, and early disease control were significantly associated with a better OS (Table 2). Based on these findings, previous TACE, PVTT, extrahepatic metastasis, and early disease control were included in the multivariate analysis. The multivariate Cox proportional hazards regression analysis found that previous TACE, PVTT, and early disease control were identified as independent prognostic factors for OS (Table 3).
| Factor | HR (95%CI) | P value |
| Sex (Male/Female) | 1/0.723 (0.314-1.666) | 0.446 |
| Age (< 60/≥ 60 yr) | 1/1.233 (0.698-2.179) | 0.470 |
| α-fetoprotein (< 400/≥ 400 ng/mL) | 1/1.279 (0.821-1.995) | 0.277 |
| ECOG performance (0/1) | 1/1.058 (0.645-1.735) | 0.824 |
| Hepatitis B (No/Yes) | 1/2.665 (0.653-10.874) | 0.172 |
| Previous TACE (No/Yes) | 1/2.997 (1.831-4.903) | < 0.001 |
| Ascites (Absent/ Present) | 1/1.440 (0.922-2.250) | 0.109 |
| Child-Pugh classification (A/B) | 1/1.342 (0.751-2.400) | 0.321 |
| PVTT (Absent/ Present) | 1/2.678 (1.697-4.227) | < 0.001 |
| Absent | 1.000 | |
| Main PVTT | 19.206 (8.436-43.727) | < 0.001 |
| Branch PVTT | 2.246 (1.386-3.639) | 0.001 |
| Extrahepatic metastasis (No/Yes) | 1/1.910 (1.182-3.087) | 0.008 |
| Number of tumor (1/≥ 2) | 1/1.125 (0.620-2.043) | 0.698 |
| Maximum tumor diameter (≤ 3/> 3 cm) | 1/1.029 (0.472-2.244) | 0.944 |
| Early disease control (No/Yes) | 1/0.362 (0.227-0.577) | < 0.001 |
| Factor | HR (95%CI) | P value |
| Previous TACE | ||
| No | 1 | |
| Yes | 2.552 (1.477-4.412) | 0.001 |
| PVTT | ||
| Absent | 1 | |
| Present | 2.582 (1.608-4.146) | < 0.001 |
| Early disease control | ||
| No | 1 | |
| Yes | 0.564 (0.339-0.936) | 0.027 |
| Extrahepatic metastasis | ||
| No | 1 | |
| Yes | 1.193 (0.680-2.092) | 0. 538 |
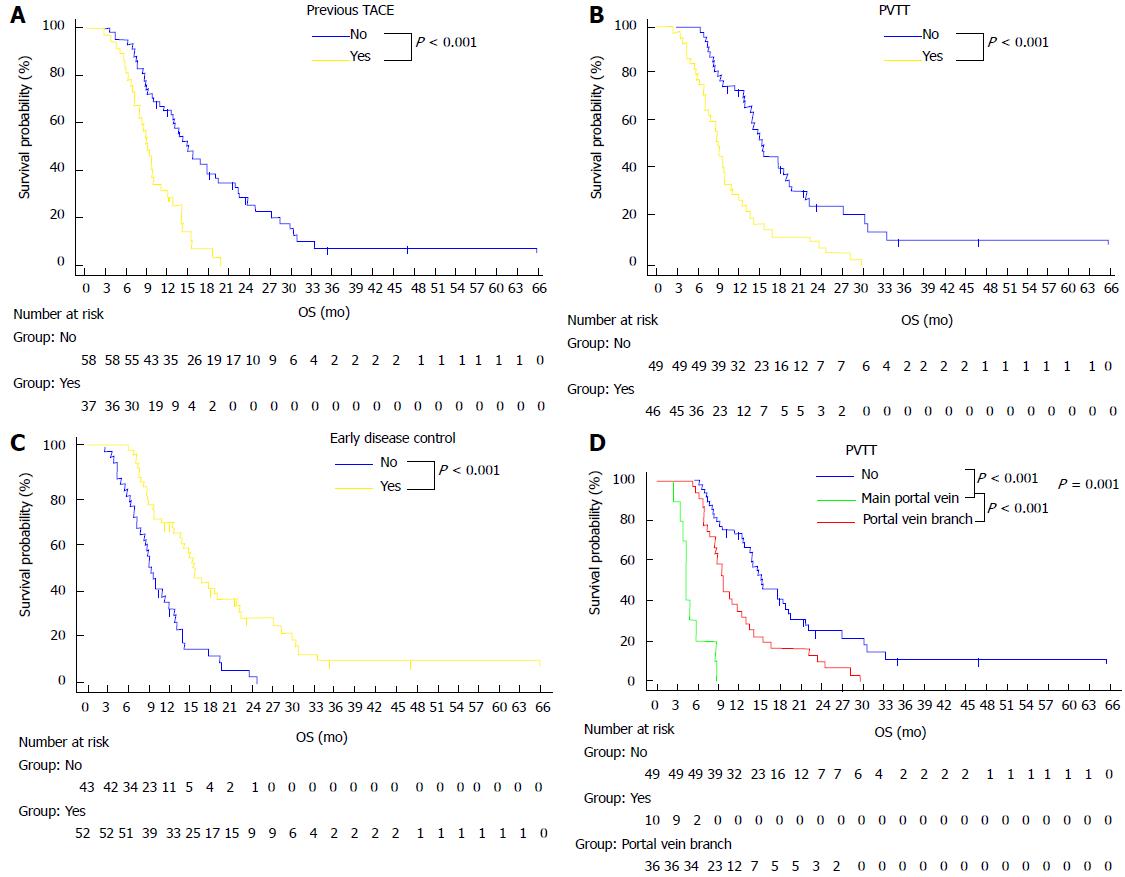
Based on the above three factors, Kaplan-Meier survival curves were analyzed (Figure 2A-C). The median OS of patients who got sorafenib at the first TACE (no previous TACE) was 14.9 mo (95%CI: 12.4-17.4 mo), which was significantly longer than the 9.1 mo (95%CI: 7.8-10.3 mo) observed for patients who had received previous TACE (Figure 2A) (P < 0.001). The median OS of patients without PVTT was 15.4 mo (95%CI: 11.9-19.1 mo), which was significantly longer than the 8.9 mo (95%CI: 7.9-9.9 mo) observed for patients with PVTT (Figure 2B) (P < 0.001). The median OS of patients with early disease control after combined therapy was 15.5 mo (95%CI: 13.7-17.3 mo), which was significantly longer than the 9.1 mo (95%CI: 7.9-10.2 mo) observed for patients without early disease control after combined therapy (Figure 2C) (P < 0.001).
The median OS of patients without PVTT was 15.4 mo (95%CI: 11.9-19.1 mo), which was longer than the 4.3 mo (95%CI: 3.8-4.9 mo) observed for patients with main PVTT (P < 0.001) and the 9.7 mo (95%CI: 9.2-10.2 mo) observed for patients with branch PVTT (P = 0.001). There were also significant differences in OS between the patients with main PVTT and patients with branch PVTT (P < 0.001) (Figure 2D).
The most common AEs after sorafenib treatment observed in this study (Table 4) were hand-foot skin reaction (82.1%), diarrhea (74.7%), alopecia (30.5%), and fatigue (30.5%). Most of these adverse reactions were grade 1/2. Grade 3/4 AEs occurred in 29 (30.9%) patients, all of whom required sorafenib dose reductions or interruption. The sorafenib dose was reduced to 400 mg once daily for grade 3 hand-foot skin reactions in 11 (11.6%) patients, grade 3/4 diarrhea in 9 (9.5%) patients, and grade 3/4 hypertension in 2 (2.1%) patients. There were 7 (7.4%) patients with interrupted sorafenib for gastrointestinal hemorrhage. Common AEs associated with TACE in the combination treatment (Table 5) were liver dysfunction (31.6%), new ascites (26.3%), and pleural effusion (10.5%). Most of these AEs were well tolerated because they were grade 1/2 adverse reactions to TACE, suggesting that combination therapy does not increase TACE-related adverse reactions. No treatment-related deaths occurred in this study.
| Adverse event | All events | Grade 1-2 events | Grade 3 or higher events |
| Hand-foot skin reactions | 78 (82.1) | 67 (70.5) | 11 (11.6) |
| Diarrhea | 71 (74.7) | 62 (65.3) | 9 (9.5) |
| Hypertension | 10 (10.5) | 8 (8.4) | 2 (2.1) |
| Alopecia | 29 (30.5) | 29 (30.5) | 0 |
| Fatigue | 29 (30.5) | 29 (30.5) | 0 |
| Voice change | 1 (1.1) | 1 (1.1) | 0 |
| Gastrointestinal hemorrhage | 7 (7.4) | 0 | 7 (7.4) |
| Epistaxis | 1 (1.1) | 1 (1.1) | 0 |
| Adverse event | All events | Grade 1-2 events | Grade 3 or higher events |
| New ascites | 25 (26.3) | 18 (19.0) | 7 (7.3) |
| Liver dysfunction | 30 (31.6) | 22 (23.2) | 8 (8.4) |
| Pleural effusion | 10 (10.5) | 8 (8.4) | 2 (2.1) |
| Spontaneous bacterial peritonitis | 6 (6.3) | 3 (3.2) | 3 (3.2) |
| Gastrointestinal hemorrhage | 6 (6.3) | 6 (6.3) | |
| Inguinal haematoma | 5 (5.3) | 5 (5.3) | |
| Hepatorenal syndrome | 1 (1.1) | 1 (1.1) | |
| Ischemic cholecystitis | 1 (1.1) | 1 (1.1) |
In this retrospective study, we found that previous TACE, PVTT, and mRECIST-evaluated disease control (CR, PR, and SD) at the first follow-up assessment 4-6 wk after TACE-S were independent prognostic factors for OS. To the best of our knowledge, this is the first study to use the first follow-up assessment 4-6 wk after TACE-S as the earliest observation time point to predict survival in patients with advanced-stage HCC treated with TACE-S therapy. In prior reports, Prajapati et al[19] and Gillmore et al[20] demonstrated that patients with mRECIST-evaluated objective responses (CR and PR) at the first follow-up assessment after TACE monotherapy had better survival. In our study, we not only showed that mRECIST-evaluated responses (CR and PR) at the first follow-up assessment were associated with improved survival but also found that patients with mRECIST-evaluated SD had better survival from TACE-S therapy. These results imply that TACE may induce extensive intrahepatic tumor necrosis to reduce the tumor burden, whereas sorafenib may improve local tumor control by blocking HCC cell proliferation and/or inhibiting tumor angiogenesis[29], which may present as a tumor stabilizing agent that delays tumor progression. Our study further proved the results of sorafenib monotherapy in patients with advanced-stage HCC[9,10], and the survival benefit of sorafenib came mainly from the prolonged disease stabilization. Another study[30] supported our results and found a relationship between early tumor growth rate (eTGR) and OS in HCC patients who received sorafenib. eTGR was found to be an independent prognostic factor for OS, and eTGR in patients receiving sorafenib was significantly lower than that in patients receiving the placebo, indicating that sorafenib slowed tumor progression. Tumor shrinkage and tumor stabilization have similar OS outcomes. Our result further supported that SD is an important indicator for improving survival in patients with HCC who were treated with TACE-S.
Wang et al[22] combined mRECIST with dermatologic AEs to stratify prognosis in patients with unresectable HCC receiving TACE-S. They found that the earliest time at which mRECIST-evaluated objective responses (CR and PR) and dermatologic responses correlated with survival was 2 mo after TACE-S. Our results advanced the evaluation time point forward to the first follow-up assessment after TACE-S (median, 38 d; range: 33-45 d) and found that mRECIST-evaluated disease control (CR, PR, and SD) could be used as an indicator of better survival with TACE-S. Consequently, we believe that patients with mRECIST-evaluated CR, PR, and SD at the first follow-up assessment 4-6 wk after TACE-S should be considered candidates for continued TACE-S.
Importantly, our study further confirmed that patients who underwent previous TACE treatment had lower survival than those who received timely sorafenib treatment 3-5 d after the first TACE. This result may be attributed to the low DCR in patients who underwent previous TACE. This low DCR indicated that the residual tumor or tumor progression after TACE may be more difficult to treat with TACE-S, possibly because TACE-induced residual tumor angiogenesis is difficultly controlled by TACE-S or resistant to repeated TACE. Arizumi et al[31] also noted that repeated TACE could cause tumor resistance to chemotherapy drugs, thereby increasing the risk of tumor recurrence and metastasis. Therefore, we believe that sorafenib should be orally administered early after the first TACE, which may lead to a greater survival benefit.
PVTT has a profound adverse effect on prognosis, resulting in a very short median survival time (2-4 mo)[5,32]. In our study, the classification of PVTT played an important role in determining disease outcomes, and those patients with main PVTT had worse survival than those with branch PVTT. The results of this study are consistent with our previous findings[14], which showed that PVTT involving the main portal vein was the most important prognostic factor for survival. For HCC patients with main PVTT, the combination of TACE and sorafenib is not recommended because the combined therapy may exacerbate liver function damage in these patients. However, for HCC patients with PVTT confined to portal vein branches, TACE-S had acceptable side effects and may improve OS.
Considering that the cause of death of HCC patients with extrahepatic metastasis is mainly intrahepatic HCC or hepatic failure, rather than extrahepatic metastasis[33,34], a local treatment modality such as TACE is often performed at some centers[14,33]. Our result showed that extrahepatic metastasis was not an independent prognostic factor for worse survival. This implied that the combination of delaying intrahepatic tumor progression with TACE and targeting extrahepatic metastasis with sorafenib might be benefit for survival, although further trials are required to confirm this finding. This study showed that TACE-S in patients with HCC is safe and well tolerated, with the most common drug-related AEs including hand-foot skin reaction, diarrhea, alopecia, fatigue, and hypertension, which were similar to those reported in previous studies with sorafenib as monotherapy[9,10] and with sorafenib in combination with TACE[14]. Furthermore, patients tolerated TACE well, which was similar to that observed in a previous study[15], suggesting that the combination therapy does not increase TACE-related adverse reactions.
Our study had several limitations. First, this study was a single-institution, retrospective study. Therefore, the strength of our conclusions is limited by the retrospective nature of the results. Second, the population used in this study was heterogeneous with regard to the frequency of patients with Child-Pugh B, previous TACE, and different PVTT classifications. However, our population is similar to that of patients who are treated in routine clinical practice. Third, the evaluation of mRECIST may be biased because of investigator-independent factors. However, every evaluation was independently assessed by at least two clinicians, and when there was a discrepancy, a consensus was reached by a panel of clinicians to reduce the error caused by the observers.
In conclusion, our study demonstrated that the first follow-up 4-6 wk after TACE-S can be used as the earliest time point at which the response to TACE-S should be evaluated in patients with advanced-stage HCC. Moreover, mRECIST-evaluated disease control (CR, PR, and SD) was an independent predictor for OS at this early time point and could be considered a valuable early indicator for making subsequent therapeutic decisions and predicting long-term survival. In addition, we found that patients who received previous TACE and patients with main PVTT had worse outcomes. Sorafenib should be orally administered early after the first TACE. We do not, however, recommend the combination of TACE and sorafenib for patients with advanced HCC complicated by main PVTT.
Recently, some studies recommended that the combination of transarterial chemoembolization (TACE) and sorafenib (TACE-S) may be used as an alternative for patients with advanced-stage HCC. However, it is still uncertain which patients can obtain survival benefits from TACE-S treatment.
The aim of this study was to find some clinical biomarkers that can early predict improved survival in patients with advanced-stage HCC treated with TACE-S therapy, which will be beneficial to the choice of the patients who received TACE-S therapy.
The objective of this study was to identify which clinical biomarkers that could early predict improved survival in patients with advanced-stage HCC treated with TACE-S. This may help us make decisions about subsequent therapies and choose the timing of sorafenib treatment.
A retrospective study was performed. The mRECIST-evaluated early disease control (including complete response, partial response, and stable disease) and multiple clinical variables at the first follow-up 4-6 wk after TACE-S were analyzed to identify the factors affecting survival.
No previous TACE, the absence of portal vein tumor thrombus (PVTT), and mRECIST-evaluated disease control at the first follow-up assessment 4-6 wk after TACE-S were independent prognostic factors for better survival. The incidence and severity of adverse events are similar to that observed in previous study.
The first follow-up 4-6 wk after TACE-S can be used as the earliest time point at which the response to TACE-S should be evaluated in patients with advanced-stage HCC. At this point, mRECIST-evaluated disease control could be considered a valuable early indicator for making subsequent therapeutic decisions and predicting long-term survival. In addition, patients who received previous TACE or had main PVTT had worse outcomes.
A further prospective study is needed to confirm mRECIST-evaluated disease control at the first follow-up 4-6 wk after TACE-S as an early indicator for predicting improved survival in patients with advanced-stage HCC treated with TACE-S therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sakaguchi T, Vij M S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 698] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3597] [Article Influence: 276.7] [Reference Citation Analysis (4)] |
| 3. | Breen DJ, Lencioni R. Image-guided ablation of primary liver and renal tumours. Nat Rev Clin Oncol. 2015;12:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 907] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 6. | Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 398] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 9. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10272] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 11. | Abou-Alfa GK. TACE and sorafenib: a good marriage? J Clin Oncol. 2011;29:3949-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960-3967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 13. | Cosgrove DP, Reyes DK, Pawlik TM, Feng AL, Kamel IR, Geschwind JF. Open-Label Single-Arm Phase II Trial of Sorafenib Therapy with Drug-eluting Bead Transarterial Chemoembolization in Patients with Unresectable Hepatocellular Carcinoma: Clinical Results. Radiology. 2015;277:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Zhao Y, Wang WJ, Guan S, Li HL, Xu RC, Wu JB, Liu JS, Li HP, Bai W, Yin ZX. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013;24:1786-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, Cai M, Shan H. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology. 2014;272:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, Woo SM, Nam BH. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, Suh DJ. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 19. | Prajapati HJ, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, Chen Z, Kim HS. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol. 2013;24:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Liu L, Wang W, Chen H, Zhao Y, Bai W, Yin Z, He C, Jia J, Yang M, Xia J. EASL- and mRECIST-evaluated responses to combination therapy of sorafenib with transarterial chemoembolization predict survival in patients with hepatocellular carcinoma. Clin Cancer Res. 2014;20:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Wang W, Bai W, Wang E, Zhao Y, Liu L, Yang M, Cai H, Xia D, Zhang L, Niu J. mRECIST response combined with sorafenib-related adverse events is superior to either criterion alone in predicting survival in HCC patients treated with TACE plus sorafenib. Int J Cancer. 2017;140:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zhao Y, Li H, Bai W, Liu J, Lv W, Sahu S, Guan S, Qin X, Wang W, Ren W. Early sorafenib-related adverse events predict therapy response of TACE plus sorafenib: A multicenter clinical study of 606 HCC patients. Int J Cancer. 2016;139:928-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 25. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3305] [Article Influence: 220.3] [Reference Citation Analysis (36)] |
| 26. | Shim JH, Lee HC, Won HJ, Shin YM, Kim KM, Lim YS, Suh DJ. Maximum number of target lesions required to measure responses to transarterial chemoembolization using the enhancement criteria in patients with intrahepatic hepatocellular carcinoma. J Hepatol. 2012;56:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Kim BK, Kim SU, Kim MJ, Kim KA, Kim DY, Park JY, Ahn SH, Han KH, Chon CY. Number of target lesions for EASL and modified RECIST to predict survivals in hepatocellular carcinoma treated with chemoembolization. Clin Cancer Res. 2013;19:1503-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | National Cancer Institute. Common terminology criteria for adverse events, version 4.03. Available from: http://ctep.cancer.gov/reporting/ctc.html. |
| 29. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1206] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 30. | Meinhardt G, Bruix J, Llovet J, Cheng A, Kappeler C. Analysis of tumor growth rate for advanced hepatocellular cancer patients receiving placebo or sorafenib in the phase 3 SHARP and Asia Pacific trials. Ann Oncol. 2016;27:vi207-vi242. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer. 2015;4:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 32. | Han K, Kim JH, Ko GY, Gwon DI, Sung KB. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: A comprehensive review. World J Gastroenterol. 2016;22:407-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY, Yoon HK, Sung KB, Lee JL, Kang YK, Lim YS. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (1)] |