Published online Sep 14, 2018. doi: 10.3748/wjg.v24.i34.3821
Peer-review started: May 21, 2018
First decision: June 13, 2018
Revised: June 22, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: September 14, 2018
Processing time: 116 Days and 4 Hours
Recently, diabetic gastroparesis (DGP) has received much attention as its prevalence is increasing in a dramatic fashion and management of patients with DGP represents a challenge in the clinical practice due to the limited therapeutic options. DGP highlights an interrelationship between the gastric emptying and pancreatic secretory function that regulate a wide range of digestive and metabolic functions, respectively. It well documented that both gastric emptying and pancreatic secretion are under delicate control by multiple neurohormonal mechanisms including extrinsic parasympathetic pathways and gastrointestinal (GI) hormones. Interestingly, the latter released in response to various determinants that related to the rate and quality of gastric emptying. Others and we have provided strong evidence that the central autonomic nuclei send a dual output (excitatory and inhibitory) to the stomach and the pancreas in response to a variety of hormonal signals from the abdominal viscera. Most of these hormones released upon gastric emptying to provide feedback, and control this process and simultaneously regulate pancreatic secretion and postprandial glycemia. These findings emphasize an important link between gastric emptying and pancreatic secretion and its role in maintaining homeostatic processes within the GI tract. The present review deals with the neurohormonal-coupled mechanisms of gastric emptying and pancreatic secretory function that implicated in DGP and this provides new insights in our understanding of the pathophysiology of DGP. This also enhances the process of identifying potential therapeutic targets to treat DGP and limit the complications of current management practices.
Core tip: Prevalence of diabetic gastroparesis (DGP) is increasing in a dramatic fashion, however there are still gaps in our understanding of the pathophysiology of DGP. It well documented that gastric emptying and subsequent pancreatic secretion are interrelated and regulated by several neurohormonal mechanisms. Dysfunction of these mechanisms affects gastric emptying, pancreatic secretion and postprandial glycemia. Therefore, the present article reviews the neurohormonal-coupled mechanisms that control gastric emptying and pancreatic secretion and their plausible involvement in DGP. This will help in identification of novel therapeutic targets to treat DGP with minimal adverse effects on postprandial glycemia.
- Citation: Mussa BM, Sood S, Verberne AJ. Implication of neurohormonal-coupled mechanisms of gastric emptying and pancreatic secretory function in diabetic gastroparesis. World J Gastroenterol 2018; 24(34): 3821-3833
- URL: https://www.wjgnet.com/1007-9327/full/v24/i34/3821.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i34.3821
Gastroparesis (or stomach paralysis) is a chronic and symptomatic disorder characterized by a complex pathogenesis which mainly includes delayed gastric emptying in the absence of mechanical obstruction[1,2]. It may also involve reduced antral contraction, impaired gastric accommodation, slow wave dysrhythmia and partial loss of the interstitial cells of Cajal (ICCs)[3-5]. Therefore, comprehensive criteria have been recommended to evaluate and diagnose gastoparesis. Documented delay in gastric emptying is one of the main requirements to confirm the diagnosis of gastoparesis and this can be achieved by measuring gastric retention of solids by scintigraphy[6].
Given the fact that more than 30% of gastroparesis cases are related to diabetes mellitus (DM), several studies have investigated the pathophysiological nature of diabetic gastroparesis (DGP)[7]. Understanding of the relationship between DM and gastoparesis has evolved during the last decade as a result of several research studies and initiatives such as the Gastoparesis Clinical Research Consortium[8].
High prevalence of DGP has been reported in Type 1 DM (approximately 40%) and Type 2 DM (approximately 30%) and it was found that in a cohort of unselected patients with DM, DGP was present in 28% of cases[9]. The prevalence of DGP seems to be significantly dependent on the duration of DM and gender. Compared to newly diagnosed patients with DM, patients with long-standing DM are more likely to experience DGP[10,11]. Similarly, the prevalence of DGP is higher among females compared to males and, although the reason for this gender-difference is unknown, the fact that gastric emptying is slower in females may explain this observation[12,13].
High rate of mortality is not directly related to DGP however, quality of life seems to be impaired independently of several factors including age and type of DM[14]. In addition, poor glycemic control is one of the main challenges that the patients with DGP face during the course of the disease[15]. It has been found that delayed gastric emptying leads to time mismatch between blood glucose and insulin secretion jeopardizing the regulation of postprandial glycemia[16]. Several studies have demonstrated that patients with DGP experience a blunted postprandial glucose response and hypoglycemia which further complicates the management of DM in this group of patients. These findings highlight an important aspect about the interrelationship between gastric emptying and pancreatic secretory function.
Although the prevalence of gastroparesis dramatically increased among DM patients with consequent adverse effects on glycemic control, the exact pathophysiology of DGP is yet to be determined. Multiple gastrointestinal (GI) hormonal mechanisms, autonomic neuropathy with loss of the ICCs as well as myopathy, have been proposed[17]. The role of the ICCs and myopathy is beyond the scope of the present review and other investigators including Bashashati’s group have comprehensively reviewed the involvement of Cajal-opathy in gastroparesis[18].
We believe that identification of the exact pathophysiological processes that are involved in DGP is a crucial step towards development of potential targets for management of DGP. The hormonal coupled mechanisms of gastric emptying and pancreatic secretory function may be an important element that requires further characterization.
The focus of this review is to discuss the extrinsic neural pathways and the neurohormonal mechanisms that regulate both gastric emptying and pancreatic secretory function and the interrelationship between these two elements. In addition, the review sheds light on how the dysfunction of these processes may contribute to development of DGP.
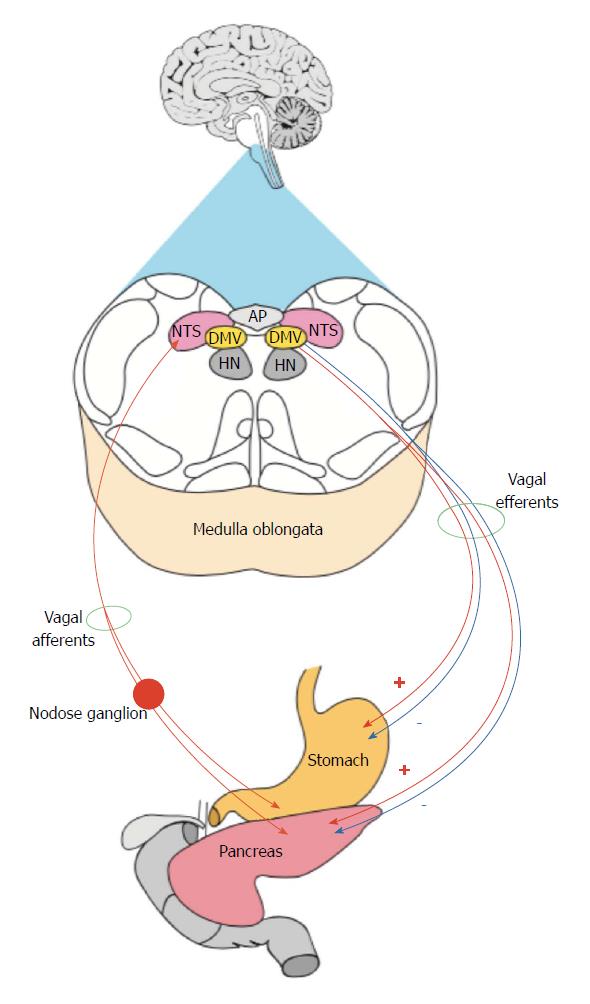
Digestion is an essential homeostatic process that is involved in maintenance of homeostasis and general health. It is a complex phenomenon, consists of multiple phases that eventually lead to absorption, assimilation and uptake of nutrients. Digestion starts with the smell or the taste of food and this sensory information is conveyed to the central nervous system (CNS) via trigeminal, facial, glossopharyngeal and vagal afferents which innervate different parts of the digestive tract including the tongue, pharynx, esophagus, stomach, intestine and pancreas[19]. The majority of vagal afferents terminate in the nucleus of the solitary tract (NTS) for sensory signals integration. Subsequently, this information is conveyed to motor neurons such as those in the dorsal motor nucleus of the vagus (DMV) which then transforms the information into motor output. The vagal efferent fibres which originate in the DMV, in turn, control subsequent phases of digestion including the cephalic, gastric and intestinal components of pancreatic secretion (PS)[19,20]. It is noteworthy that the phases of PS strongly correlated with the phases of digestion highlighting the importance of the former in the digestive process. During the cephalic phase of digestion, the pancreatic exocrine acinar cells are stimulated by a vagal mechanism to secrete digestive enzymes. However, the latter remain inactive due to the low pH environment and inadequate levels of bicarbonate. This phase followed by the gastric phases that include an increase in the number of digestive zymogens that release the active digestive enzymes when pH rises after bicarbonate secretion. Gastric emptying of stomach contents into the small intestine is described as the intestinal phase and represents the final phase of PS and is controlled mainly by vagovagal pathways and GI hormones such as cholecystokinin (CCK) and secretin[19,21]. It is noteworthy that gastric emptying is strongly coupled to the neurohormonal mechanisms that control PS. Interestingly, most of the GI hormones and agents that control PS are also involved in regulation of gastric emptying.
As early as 1642, the pancreatic ducts were identified by Virsung and in the same century the first collection of PS via a pancreatic fistula was made by Regner de Graaf[22]. However, it took more than two centuries to appreciate the significance of PS in digestion[22]. Later, Pavlov highlighted the role of the CNS in control of PS[23]. Subsequent discovery of various GI hormones and peptides such as secretin, modified Pavlov’s theory[24,25]. However, it was not until the late 1970s that there was a renewed focus on the relationship between the CNS and PS[26-28]. The results of these investigations showed for the first time the importance of vagovagal reflexes and PS as common factors in controlling different GI functions including gastric emptying. Since then several lines of evidence have implicated various interacting factors including hormones, paracrine mediators and vagovagal reflexes in regulation of gastric emptying[29]. The latter represents one of the significant determinants of postprandial glycemia in health and in glycemic disorders including DM. Therefore, delayed gastric emptying (gastroparesis) that is associated with DM affects several aspects of glycemic control in patients with DM.
Gastric emptying defined as the process of ejecting the stomach’s content (chyme) into the duodenum. The rate of gastric emptying is dependent on several physiological factors including fundal relaxation, pyloric control of flow into the duodenum and antro-duodenal coupling. In addition, the physical nature and the composition of the chyme are important determinants of the rate of gastric emptying[30].
This process is precisely tuned to react to various intrinsic and extrinsic signals and therefore it is not surprising that highly complex systems are involved in the regulation of gastric emptying. This includes (1) intrinsic neural plexuses (2) extrinsic autonomic factors and (3) neurohormonal mechanisms[30]. Although, it seems that intrinsic neural pathways have some degree of independence in regulating GI functions, the extrinsic control of the parasympathetic and sympathetic pathways are still the predominant players that modulate various gastric processes along with the output of the intrinsic plexuses[31]. In particular, regulation of gastric motility is largely dependent on excitatory (cholinergic) inputs and inhibitory (nitrergic) inputs[17]. In addition, ICCs are also involved to some extent in electrical control of gastric motility. Given the PS has two main types: (1) Exocrine secretion and (2) endocrine secretion, it is considered to be one of the main factors that regulates both digestive and metabolic processes.
Hormonal regulation of PS was demonstrated as early as 1902 when the first hormone, secretin, was discovered by Bayliss and Starling[24]. Strong evidence has shown that the shortest circulation times for maximal doses of GI hormones are significantly longer than the observed latency of pancreatic responses to nutrient stimuli[27]. Moreover, this latency increased 10-fold when neuronal influences were excluded, supporting the theory of neurohormonal regulation of PS (for review see Niebergall-Roth[29]). This theory proposes that hormonal and neural factors, which were previously thought to act separately, act together to regulate PS. Although both divisions of the autonomic nervous system; the parasympathetic nervous system (PNS) and sympathetic nervous system (SNS), are known to innervate pancreatic exocrine and endocrine tissues, the parasympathetic (vagal) pathways have the greatest influence on PS[32]. While not wishing to diminish the important role of the SNS, which is mainly concerned with GI smooth muscle function, blood flow, and mucosal secretion, the PNS is the principal regulator of gastric emptying and secretion. Therefore, dysfunction of the latter is always associated with disruption of the parasympathetic pathways[33].
To understand the potential pathophysiological mechanisms that underpin the delayed gastric emptying noted in DGP, it is very important to identify and characterize these mechanisms. The following sections discuss these systems in detail and highlight the relationship between gastric emptying and pancreatic secretory function in each process.
The involvement of the vago-vagal pathways and reflexes in control of gastric emptying and pancreatic secretory function is well-documented[33-36]. It facilitates the complex processes that are associated with gastric emptying and also explains the interrelationship between gastric emptying and pancreatic secretory function.
The vago-vagal model consists of three major components (1) vagal afferents, (2) central neurons in the NTS and the DMV and (3) vagal efferents (Figure 1). These reflexes seem mediated by GI hormones such as secretin, serotonin (5-hydroxytryptamine, 5-HT), glucagon-like peptide-1 (GLP-1) and CCK[37-40].
The vagal afferents respond to different components of the chyme including nutrients, osmotic pressure and chemicals[41]. Therefore, the vagal innervation plays a major role in monitoring and regulating the gastric emptying process. On the other hand, the vagal innervation controls PS by responding to various pancreatic secretagogues, such as CCK and 5-HT that are secreted from the intestinal enteroendocrine and enterochromaffin cells, respectively. It is well-documented that these agents provoke excitatory influences on PS via vagal mechanisms[42]. Thus, it is reasonable to postulate that GI hormones mediate their actions on gastric emptying and PS via activation of gastric and pancreatic vagal afferents, respectively[38,40,43,44]. It is noteworthy that a wide range of GI hormones are engaged in the dual control of PS and gastric emptying however, CCK and 5-HT represent an important classic neurohormonal examples that have been studied extensively.
In addition, it is evident that CCK and 5-HT mediate their physiological effects on gastric and pancreatic vagal afferents via activation of CCK and 5-HT receptors, respectively. These findings are in good agreement with previous reports which have shown that under physiological conditions CCK1 and 5-HT3 receptors on the vagus nerve are the main players in the regulation of PS and gastric emptying[39,45].
All GI vagal afferents terminate in and activate NTS neurons mainly via glutamatergic transmission[46-49]. NTS neurons, in turn, integrate and assimilate this sensory information and eventually influence DMV neurons mainly via GABAergic transmission although blockade of GABAA receptors indirectly enhances glutamatergic transmission[34,50]. A large body of evidence supports a role for GABAergic inputs from the NTS to the DMV in the regulation of the vagal efferent output from the DMV to the GI tract. DMV neurons are the main source of vagal motor output to various GI organs including the pancreas and the stomach[49-53].
The hypothesis that GABA receptors in the DMV are involved in modulation of PS was tested. Mussa et al[42]. have shown that blockade of GABAA receptors using bilateral microinjection of Bicuculline methionine (GABAA receptor blocker) into the DMV produced pronounced excitatory effects on both pancreatic exocrine secretion and glucose-induced insulin secretion[53,54]. Interestingly, the excitatory effects of chemical activation of the DMV were sensitive to muscarinic acetylcholine receptor blockade, confirming the involvement of a peripheral cholinergic pathway. These findings support the hypothesis that pancreatic secretagogues activate pancreatic vagal afferent input into the NTS, which in turn, stimulates cholinergic efferent output from the DMV possibly via inhibition of GABAergic transmission. The excitatory effects of GABAA receptor blockade in the DMV on glucose-induced insulin secretion are enhanced in the presence of the nitric oxide (NO) synthase inhibitor L-NAME. This suggests that a nitrergic inhibitory pathway is involved in pancreatic insulin secretion[54,55].
Similarly, a considerable number of studies have shown that blockade of GABA receptors within the DMV has a profound effect on gastric emptying. Stimulation of the DMV by GABAA receptor blockade led to a significant increase in gastric motility suggesting that GABAergic transmission in the DMV is involved in control of gastric emptying. Therefore, gastric emptying is very sensitive to any sort of disruption of GABAergic transmission to the GI tract[56,57].
Functional studies in vivo have emphasized the relationship between the DMV and the pancreas by showing that electrical and chemical stimulation of dorsal vagal motor neurons activates both pancreatic endocrine and exocrine secretion[53,58,59]. In addition, several in vitro studies have documented that all vagal efferents that project to the pancreas originate from the DMV[52,60,61]. However, major questions regarding the exact details of these pathways remain to be elucidated. Nevertheless, the electrophysiological and morphological characteristics of DMV pancreatic preganglionic neurons (PPNs) using whole cell patch clamp recording techniques have been described[62]. There were identifiable differences between the gastric and other preganglionic neurons and heterogeneity of the PPNs confirmed by this and other studies. This supports the finding that PS regulated by heterogeneous vagal efferent output from the DMV.
As mentioned previously, CCK and 5-HT are powerful stimulatory agents of the pancreatic secretion therefore their effects on DMV PPNs have been investigated[63]. The results of this investigation has shown that all DMV preganglionic neurons, the origin of the pancreatic vagal efferent, were activated in response to stimulation of the pancreatic branch of the vagus nerve and had axonal conduction velocities in the C-fibre range. This is not surprising since most of the subdiaphragmatic vagal efferents are of C-fibre type[64]. However, stimulation of peripheral CCK1 and 5-HT3 receptors produced differential effects on the firing rates of these neurons. The majority of the preganglionic neurons within the intermediate DMV inhibited whereas the preganglionic neurons within the caudal and rostral DMV were activated or insensitive, respectively. This lends strong support to previous findings that emphasized heterogeneity of DMV PPNs. However, these results cast doubt on the hypothesis that pancreatic secretagogues, which known for their excitatory effects on pancreatic vagal afferents, would also activate the majority of DMV PPNs. Another possibility is that an inhibitory pathway is involved in modulation of the motor output from the DMV to the pancreas. This suggestion is supported further by the observation that nitrergic inhibitory inputs were actively involved in the regulation of pancreatic secretory function (Figure 1).
Interestingly, previous reports have shown that some gastric functions including gastric emptying are also under the control of both excitatory and inhibitory motor inputs from the DMV[65]. It has been found that, reflex-induced fundus relaxation is mainly controlled by the inhibitory pathways that originate in the DMV[66]. Studies in cats and rats have demonstrated that different regions within the DMV are involved in regulating gastric emptying.
The inhibitory pathway consists of cholinergic and nitrergic preganglionic neurons, and noncholinergic and nonadrenergic postganglionic neurons[65]. Given that postganglionic nitrergic nerves are involved in innervation of the stomach and the pancreas, it is possible that these nerves somehow inhibit the release of acetylcholine (Ach). This does not exclude the possibility that nitrergic nerves are tonically involved in control of gastric and pancreatic functions[67].
One of the most significant responses of the duodenum to gastric emptying is the release of several GI hormones and interestingly, this response depends on the composition of the chyme. For instance, CCK is released from the duodenum in response to the presence of nutrients, particularly fat and proteins. The role of neurohormonal mechanisms in regulation of gastric emptying and pancreatic secretion is well documented. Therefore, it has been hypothesized that dysfunction of these mechanisms is closely related to abnormally delayed gastric emptying. The holistic contribution of the GI hormones in regulation of the gastric emptying been emphasized by various findings. Importantly, it has been demonstrated that hypersensitivity to, and hypersecretion of, GI hormones were common features of the delayed gastric emptying that is associated with different metabolic disorders[68,69].
It is important to note that almost all the GI hormones that are released from the intestine in response to gastric emptying activate a feedback loop to control gastric emptying and simultaneously influence pancreatic secretory function. These findings highlight a critical interrelationship between gastric emptying and pancreatic secretory function that mainly controlled by neurohormonal processes. There are many GI hormones that are involved in regulation of gastric motility and pancreatic secretion including motilin, somatostatin, xenin, orexin A and B, ghrelin, gastrin, CCK, leptin, enterostatin, peptide YY (PYY), apolipoprotein A-IV, glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), glucose-dependent insulinotropic polypeptide (GIP), pancreatic polypeptide, oxyntomodulin and amylin[69]. Taking into account all the studies that have discussed the neurohormonal involvement of CCK, 5-HT and GLP-1 in the GI activities, the present review focuses on the dual functions of CCK, 5-HT and GLP-1 in regulation of gastric emptying and pancreatic secretion.
Under normal physiological conditions, CCK inhibits gastric emptying, stimulates the secretion of the digestive enzymes from the pancreas and bile from the gallbladder and regulates intestinal motility. These actions allow a slow delivery of food into the small intestine and provide enough time for the digestion and absorption of nutrients that have already been in the duodenum[70]. It has been known for more than 40 years that CCK inhibits gastric emptying via two main mechanisms; relaxation of the proximal stomach and contraction of the pyloric sphincter.
It is believed that CCK acts directly on pancreatic tissue to mediate PS in rodents. High and low affinity CCK receptors were detected in pancreatic acini and they possess high sensitivity to low levels of CCK[71]. In addition, the correlation between the increased CCK plasma levels after food ingestion and the elevation in PS well documented. In vitro studies support the hypothesis that CCK acts as a circulating hormone to stimulate PS by showing that activation of CCK1 receptors on rat pancreatic cells by CCK elevates intracellular Ca2+ levels and subsequently PS. In addition, it has been shown that blockade of muscarinic receptors did not produce a significant change in pancreatic responses to CCK whereas CCK receptor antagonists were able to block the excitatory effects of CCK on PS[19]. The excitatory effects of CCK on pancreatic endocrine secretion were also reported in rats and dogs. Glucose-induced insulin secretion was enhanced in a dose-dependent manner after infusion of caerulein, a CCK analogue, in perfused rat pancreas[72]. In addition, it has been demonstrated that in perfused dog pancreas, pancreatic α-, β-, δ-cell secretion was stimulated in a dose-dependent fashion in response to CCK[73].
Previously, it was thought that CCK receptors in the human pancreas were undetectable or absent and thus the possibility of a direct action of CCK on the pancreas to mediate PS in human was excluded. However, Murphy and his group have demonstrated the presence of CCK receptors within the human exocrine pancreas[74].
5-HT is a potent activator of vagal afferent fibres that innervate the stomach and proximal intestine of different species and it has several types and subtypes of receptors. The 5-HT1A receptor subtype has been detected in pancreatic neurons and 5-HT3 receptor is abundant on sensory vagal afferents[75-77].
It is well documented that 5-HT is directly and indirectly involved in regulation of intestinal and gastric motility. It has been demonstrated that under normal physiological conditions, 5-HT reduces the rate of the gastric emptying and stimulates intestinal motility[78-80].
Fibres containing 5-HT were also found in different parts of the pancreas including the wall of the pancreatic blood vessels, ducts, acini and islets and thus 5-HT is one of the main factors that are involved in regulation of PS[81]. Studies in rats have shown that 5-HT2 and 5-HT3 receptor antagonists were able to inhibit approximately 94% of PS that was evoked by intragastric administration of rodent chow[39]. It has been found that luminal and mechanical factors stimulate PS via activation of 5-HT2 and 5-HT3 receptors which are present in intestinal vagal afferents. In addition, electrophysiological studies have shown that endogenously released and intraluminally perfused 5-HT activated vagal afferent neurons within the nodose ganglion. On the other hand, 5-HT is considered as one of the key factors that regulates food intake and mediates satiety due to its wide distribution within the GI tract[82].
GLP-1-(7-36) and GLP-1-(7-37) amides are signaling peptides that are produced in the enteroendocrine L-cells of the intestinal mucosa and released postprandially in response to luminal nutrients including fat and carbohydrates[83,84]. It stimulates and inhibits insulin and glucagon, respectively, in a glucose-independent manner[83,85-87]. Interesting findings have demonstrated the involvement of TRPV2 ion channel in Lysophosphatidylinositol-induced GLP-1 secretion from enteroendocrine L cells[88].
Several studies have demonstrated the presence of GLP-1 receptors in various tissues including the pancreas, GIT and the brain[89,90]. The unique and powerful stimulatory effects of GLP-1 on insulin secretion in response to postprandial hyperglycemia have well documented using GLP-1 receptor agonists and antagonists. Interestingly, the application of the latter was sufficient to block insulin secretion in response to orally- and intraduodenally administered glucose[91,92]. In addition to the potent insulinotropic effects of GLP-1, a deceleration of gastric emptying was observed in response to GLP-1 administration. This observation was documented in healthy and Type 2 DM subjects supporting the fact that GLP-1 possesses an inhibitory influence in gastric emptying under physiological conditions[93,94]. Furthermore, additional experiments have shown that diversion of the duodenal delivery of nutrients affected the synergistic effects of GLP-1 on insulin secretion[95,96]. This finding not only emphasized the importance of the dual effects of GI hormones in gastric emptying and pancreatic secretion, but also sheds light on the involvement of neurohormonal factors in control of postprandial glycemia.
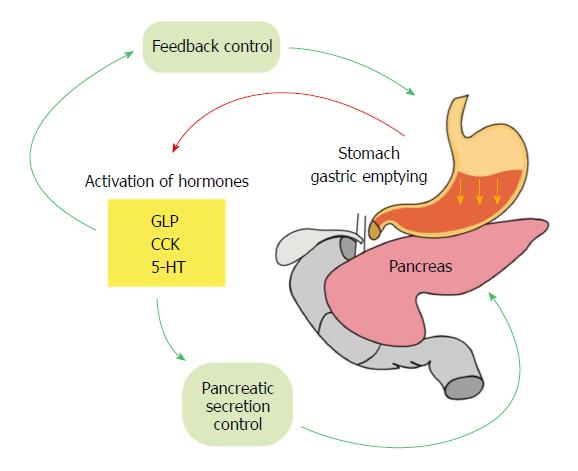
The influence of gastric emptying on postprandial glycemia is evident and it is not surprising that the coupled mechanisms that are involved in regulation of gastric emptying also control postprandial glycemia[97-99]. The latter emphasizes the link between the digestive processes and pancreatic secretory function. The composition and rate of chyme emptying into the intestine precisely monitored and determined the feedback systems that control postprandial glycemia and pancreatic secretory functions. Interestingly, most of the neurohormonal processes that inhibit or decrease the rate of the gastric emptying simultaneously increase insulin secretion.
The initial involvement of gastric emptying in modulating postprandial glycemia started prior to the digestive process. This hypothesis is supported by the fact that the composition of each meal determines the rate of gastric emptying for that specific meal. High glucose content in a meal or infusion of glucose into the duodenum inhibits gastric emptying in a dose- dependent fashion[100]. The second important checkpoint for control of postprandial glycemia is the neurohormonal mechanisms and feedback that are triggered as the result of the interaction between the nutrients and the cells of the intestine (Figure 2). As mentioned previously, the hormones that are released from L and K cells of the intestine such as CCK and GLP-1, are able to feedback and control gastric emptying and at the same time modulate insulin secretion[101]. The integrity of these mechanisms are well maintained in healthy subjects and therefore any increase in digested glucose (hyperglycemia) stimulates insulin secretion and reduces glucagon levels. Similarly, feedback mechanisms are initiated to control the hormones that are involved directly in control of gastric emptying. A good example is ghrelin, which under normal physiological conditions, increases gastric emptying. However, during postprandial hyperglycemia, the secretion of this hormone is suppressed so that the gastric emptying is inhibited[102].
Taking into account the significance of the physiological conditions that control postprandial glycemia, it is not surprising to know that pronounced hyperglycemia in both Type 1 DM and Type 2 DM is associated with several abnormalities in gastric motility including DGP[103,104]. It proposed that DGP also occurs in response to the high level of insulin as a compensatory process. However, this proposal was challenged by the fact that patients with Type 1 DM also experienced delayed gastric emptying in response to hyperglycemia[105,106]. On the other hand, it was found that insulin-induced hypoglycemia was sufficient to provoke a counter-regulatory mechanism which involves acceleration of gastric emptying[107].
One of the key findings that emphasizes the central role of gastric emptying in the integrity of the response to postprandial glycemia, is that both healthy patients and patients with DM experienced an increase in almost all of the hormones that are insulin secretagogues in response to intraduodenal infusion of high loads of glucose[108].
A considerable number of studies have shown that NO produces inhibitory effects on insulin secretion and this has been demonstrated in several species[109-114]. The involvement of nitrergic pathways is strongly supported by the finding that peripheral inhibition of NO enhanced the excitatory effects of chemical stimulation of the DMV on insulin secretion[54]. In addition, a significant glucose uptake was reported as result of NO inhibition suggesting that NO is also involved in glucose metabolism.
A number of studies have documented the distribution of nitrergic postganglionic neurons within the pancreas. NO and nitric oxide (NOS) were localized within the pancreatic tissue or ganglia of a wide range of species including human, pig, monkey, dog, cat, rat, chick and kitten[109-114]. In particular, it is evident that NOS is localized within the endocrine islets and nerves as well as in the pancreatic β-cell line HIT-T15 from rat and mouse[115-120]. Interestingly, it has been found that NO evoked fast excitatory postsynaptic potentials in the majority of neurons within the cat pancreatic ganglia supporting the hypothesis that postganglionic nitrergic neurons are modulators of pancreatic function[113].
Noncholinergic and nonadrenergic (NANC) neurons play a critical role in regulation of gastric motility, in particular gastric emptying, therefore any neural loss or dysfunction is always considered a major contributory factor to gastropathy[121]. Both cholinergic and nitrergic pathways are involved in regulation of the gastric fundic tone and imbalance between these two factors lead to dysfunction in the accommodation reflex and gastric emptying[122,123]. This hypothesis was strengthened by the finding that administration of NO inhibitors such as L-NAME in cats produced a significant increase in the fundic tone and these effects were reversible in the presence of L-arginine[124]. Several lines of evidence in different species including humans have shown that NO is a potent inhibitory neurotransmitter that mediates gastric relaxation and is considered a vital part of the accommodation reflex. In addition, recent reports have shown that mechanosensitive TRPV2 ion channel is co-expressed in nNOS-expressing inhibitory motor neurons in mouse stomach emphasizing the contribution of these inhibitory neurons gastric adaptive relaxation and gastric emptying in mice[125].
It is well documented that the effects of nitrergic inputs are mediated via a vagovagal reflex and NANC pathways[126]. This was strongly supported by several findings, which demonstrated that vagotomy led to significantly impaired accommodation and gastric emptying[127,128]. Vagotomy is used as a classic model to study the processes that are involved in delayed gastric emptying. However, studies in animals have shown that in vagotomized dogs, local gastric stimulation was able to improve gastric accommodation and emptying via a nitrergic pathway emphasizing the significance of local nitrergic inputs[129].
Interestingly, experiments in a diabetic gastroparesis model have provided evidence for loss of NOS neurons in this condition thus further emphasizing the key role of nitrergic neurons in regulating gastric emptying. Moreover, pharmacological studies have demonstrated that inhibition of NOS and knockout of NOS genes led to gastroparesis, gastric stasis and enlarged stomachs[130].
Taking this findings into account, we can propose that both pancreatic secretion and gastric emptying are under extrinsic and intrinsic inhibitory nitrergic neurotransmission.
In DM, the exocrine pancreas loses its ability to secrete adequate amounts of pancreatic enzymes and to digest carbohydrates leading to Exocrine Pancreatic Insufficiency (EPI)[131,132]. It is important to emphasize that dysfunction of the neuronal pathways that innervate different organs within the GI tract produce negative impact on the interrelated functions of these organs. Normal gastric emptying is one of the critical determinants of the subsequent exocrine pancreatic secretion. It is not surprising to know that insufficiency of the exocrine pancreatic secretion is very prevalent in DM and strongly associated with DGP. Although it has been a long-standing debate that EPI is a cause or a sequel to DM, several studies have demonstrated that EPI is a complication of DM[132]. The exocrine pancreas is normally exposed to high concentrations of islet hormones since the blood flow from the endocrine pancreas pass through the exocrine pancreas in a very extensive manner and therefore, any changes in the levels of the endocrine hormones, including insulin, will affect the exocrine pancreatic secretory function[133]. It is well documented that insulin is a strong trophic factor for the exocrine pancreatic tissue and increases pancreatic enzyme output and this may partially explain the dysfunction of exocrine pancreas in DM[132,134]. However, given the fact that a considerable number of patients with Type 1 DM who experience a total loss of insulin still have normal exocrine pancreatic function, it remains unclear as to which factors are most important in development of EPI[132]. Autonomic neuropathy, on the other hand, may explain the etiology of diabetic EPI and its association with gastroparesis. Malfunction of the autonomic nervous system is one of the common complications, which can occur at any time during at the DM course. It affects several functions of the body including gastric emptying and pancreatic secretory function[133]. Since intact vagovagal reflexes and hormonal secretion play an important role in the regulation of these two digestive processes, interruption of this neurohormonal model interferes with the gastric emptying into the duodenum and, in turn, the feedback control of exocrine pancreatic secretion[135].
Chronic pancreatitis is another important progressive fibro-inflammatory disorder of the pancreas, which affects the digestive processes significantly and is associated with poor prognosis. The most common symptoms of this disorder include malabsorption, malnutrition, and abdominal pain[136]. Delayed gastric emptying is one of the hallmark features of pancreatitis and it has founded that the prevalence of gastroparesis in chronic pancreatitis is considerably high. Although the associative pathogenesis of the latter remains poorly understood, there are two main proposed etiological mechanisms[135]. The first mechanism involves increased levels of CCK that are a well-documented feature of chronic pancreatitis. Previous studies have shown that infusion of postprandial concentrations of CCK produced a marked delay in gastric emptying[137]. In addition, the involvement of CCK as contributory factor further supported by animal studies. It was found that the CCK failed to inhibit gastric emptying in CCKA receptor gene knockout rat model suggesting that the action of CCK in gastric emptying is mediated via CCKA receptors[138]. The second mechanism is autonomic neuropathy that has received much attention due to the fact it explains the pathological background of severe abdominal pain. This type of pain is considered as one of the most problematic symptoms of chronic pancreatitis[139]. The concept of central sensitization which revolutionized the classic response to nociceptive stimuli has explained how the intensive nerve damage that is present in the chronic pancreatitis increases the efficiency of synaptic communication leading to severe pain sensation[140]. Interestingly, twenty years ago a study by Nakamura et al[141] has demonstrated that delayed gastric emptying that is associated with chronic pancreatitis is due to dysfunction of autonomic nerves.
Impaired awareness of hypoglycemia is another important aspect that highlights the interrelationship between the neurohormonal components and the postprandial glycemic control.
Previous research has shown that hypoglycemia is not only associated with transient impairment of cognition but also with high rates of functional mortality and morbidity[142]. It is well documented that type 1 DM patients experience at least two episodes of hypoglycemia per week and this represents a significant challenge in the clinical practice to achieve optimal therapeutic targets that involves insulin regimens[143].
A normal response to hypoglycemia includes an activation of a complex and sensitive counter-regulatory response leads eventually to a suppression of endogenous insulin and an increase in glucagon secretion. It has been found that in DM, the pancreatic α-cells, which are the main source of endogenous glucagon, loose their ability to secrete this hormone[143]. The notion that the pathophysiology of DM depends on the sole mechanism of insulin malfunction or resistance, has been revolutionized by the finding that loss of the glucagon response is a significant feature in DM[144].
Interestingly, recent studies have demonstrated that diabetic patients with DGP experience episodes hypoglycemia more frequent. This due to several factors including delayed gastric emptying and subsequent slow absorption of food. Insulin is an important therapeutic agent mainly for patients with type 1 DM however, type 2 DM patients also use insulin to improve their glycemic control. Dosing and administration of insulin in patients with DGP is almost impossible due to the delayed gastric emptying and slow absorption of food. This, in turn, leads to frequent episodes of postprandial hypoglycemia.
The dramatic increase in the prevalence of DGP has directed significant attention to the pathophysiology of DGP. In addition, the strong association between DGP and abnormal glycemic profile has highlighted the involvement of coupled mechanisms that control gastric emptying and endocrine and exocrine pancreatic secretion. Therefore, characterization of these mechanisms will enhance the understating of the etiology of DGP and in turn, this will facilitate the process of identifying novel therapeutic targets. The latter will further ease the burden of complex and challenging DGP management.
Under normal conditions, there is a delicate balance between the neurohormonal mechanisms that control gastric emptying and pancreatic secretion. Malfunction of any of these mechanisms affects the metabolic profile adversely and this has been strongly proven in DM where the delayed gastric emptying is associated with pancreatic secretory dysfunction.
This article has reviewed the neurohormonal-coupled mechanisms that control gastric emptying and pancreatic secretory function to identify the potential components and pathways that are involved in DGP and this will stimulate the development of novel therapeutic approaches hopefully for this disorder.
The authors would like to thank Professor Joe Proietto for his valuable feedback and inputs regarding the clinical aspects of diabetic gastroparesis. The authors would also like to thank Ms. Judy Bastaty for her help in designing the Figures.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Arab Emirates
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mihara H S- Editor: Wang XJ L- Editor: A E- Editor: Bian YN
| 1. | Waseem S, Moshiree B, Draganov PV. Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15:25-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 2. | Falk GW. Gastroparesis. Gastroenterol Clin North Am. 2015;44:xiii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SS. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. 2008;20:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Karamanolis G, Caenepeel P, Arts J, Tack J. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: gastric emptying rate or proximal stomach dysfunction? Gut. 2007;56:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, Ordög T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-37; quiz 38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 7. | Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 380] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 8. | Marathe CS, Rayner CK, Jones KL, Horowitz M. Novel insights into the effects of diabetes on gastric motility. Expert Rev Gastroenterol Hepatol. 2016;10:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hasler WL. Gastroparesis--current concepts and considerations. Medscape J Med. 2008;10:16. [PubMed] |
| 10. | Parkman HP, Fass R, Foxx-Orenstein AE. Treatment of patients with diabetic gastroparesis. Gastroenterol Hepatol (N Y). 2010;6:1-16. [PubMed] |
| 11. | Krishnan B, Babu S, Walker J, Walker AB, Pappachan JM. Gastrointestinal complications of diabetes mellitus. World J Diabetes. 2013;4:51-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 12. | Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med. 1987;28:1204-1207. [PubMed] |
| 13. | Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Lyrenås EB, Olsson EH, Arvidsson UC, Orn TJ, Spjuth JH. Prevalence and determinants of solid and liquid gastric emptying in unstable type I diabetes. Relationship to postprandial blood glucose concentrations. Diabetes Care. 1997;20:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Mantides A, Stefanides G, Kioulanis J, Tzovaras G, Epanomeritakis E, Xynos E. Cutaneous electrogastrography for the assessment of gastric myoelectrical activity in type I diabetes mellitus. Am J Gastroenterol. 1997;92:1190-1193. [PubMed] |
| 17. | Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Bashashati M, McCallum RW. Is Interstitial Cells of Cajal-opathy Present in Gastroparesis? J Neurogastroenterol Motil. 2015;21:486-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271:E808-E813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Konturek SJ, Pepera J, Zabielski K, Konturek PC, Pawlik T, Szlachcic A, Hahn EG. Brain-gut axis in pancreatic secretion and appetite control. J Physiol Pharmacol. 2003;54:293-317. [PubMed] |
| 22. | Bernard C, Memoir on the pancreas and on the role of pancreatic juice in digestive processes, particularly in the digestion of neutral fat. By Claude Bernard. 1856. Translated by John Henderson. Monogr Physiol Soc. 1985;42:1-131. [PubMed] |
| 23. | Pavlov IP. The work of the digestive glands. (Translated by Thompson WH). 1910;Charles Griffin and Company Ltd. |
| 24. | Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 732] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 25. | Chey WY, Chang TM. Neural control of the release and action of secretin. J Physiol Pharmacol. 2003;54 Suppl 4:105-112. [PubMed] |
| 26. | Singer MV, Solomon TE, Grossman MI. Effect of atropine on secretion from intact and transplanted pancreas in dog. Am J Physiol. 1980;238:G18-G22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Singer MV, Solomon TE, Wood J, Grossman MI. Latency of pancreatic enzyme response to intraduodenal stimulants. Am J Physiol. 1980;238:G23-G29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Solomon TE, Grossman MI. Effect of atropine and vagotomy on response of transplanted pancreas. Am J Physiol. 1979;236:E186-E190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Niebergall-Roth E, Singer MV. Enteropancreatic reflexes mediating the pancreatic enzyme response to nutrients. Auton Neurosci. 2006;125:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol Ther. 2008;27:724-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | McMenamin CA, Travagli RA, Browning KN. Inhibitory neurotransmission regulates vagal efferent activity and gastric motility. Exp Biol Med (Maywood). 2016;241:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Roze C. Central regulation of pancreatic secretion. Brain-gut interactions. 1991;CRC Press: 187-198. |
| 33. | Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 34. | Travagli RA, Browning KN. Central autonomic control of the pancreas. Central regulation of autonomic functions. 2011;Oxford University Press. |
| 35. | Hornby PJ. Receptors and transmission in the brain-gut axis. II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1055-G1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Vanormelingen C, Tack J, Andrews CN. Diabetic gastroparesis. Br Med Bull. 2013;105:213-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Li Y, Owyang C. Pancreatic secretion evoked by cholecystokinin and non-cholecystokinin-dependent duodenal stimuli via vagal afferent fibres in the rat. J Physiol. 1996;494:773-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology. 1994;107:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Li Y, Hao Y, Zhu J, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology. 2000;118:1197-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G916-G923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Beyak MJ, Bulmer DCE, Jiang W, Keating C, Rong W, Grundy D. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. Physiology of the gastrointestinal tract. 2006;Elsevier Academic Press: 685-725. |
| 42. | Mussa BM, Sartor DM, Verberne AJ. Activation of cholecystokinin (CCK 1) and serotonin (5-HT 3) receptors increases the discharge of pancreatic vagal afferents. Eur J Pharmacol. 2008;601:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Li Y, Owyang C. Vagal afferent pathway mediates physiological action of cholecystokinin on pancreatic enzyme secretion. J Clin Invest. 1993;92:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Li Y, Hao Y, Owyang C. High-affinity CCK-A receptors on the vagus nerve mediate CCK-stimulated pancreatic secretion in rats. Am J Physiol. 1997;273:G679-G685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | McCann MJ, Rogers RC. Functional and chemical neuroanatomy of a gastric vago-vagal reflex. Innervation of the Gut: Pathophysiological Implications, edited by Tache Y, Wingate DL and Burks TF 1994; CRC Press. |
| 47. | Niebergall-Roth E, Singer MV. Central and peripheral neural control of pancreatic exocrine secretion. J Physiol Pharmacol. 2001;52:523-538. [PubMed] |
| 48. | Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Streefland C, Maes FW, Bohus B. Autonomic brainstem projections to the pancreas: a retrograde transneuronal viral tracing study in the rat. J Auton Nerv Syst. 1998;74:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2011;300:G21-G32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Berthoud HR, Powley TL. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of Dil. Brain Res. 1991;553:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Rinaman L, Miselis RR. The organization of vagal innervation of rat pancreas using cholera toxin-horseradish peroxidase conjugate. J Auton Nerv Syst. 1987;21:109-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Mussa BM, Verberne AJ. Activation of the dorsal vagal nucleus increases pancreatic exocrine secretion in the rat. Neurosci Lett. 2008;433:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Mussa BM, Sartor DM, Rantzau C, Verberne AJ. Effects of nitric oxide synthase blockade on dorsal vagal stimulation-induced pancreatic insulin secretion. Brain Res. 2011;1394:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Alm P, Ekström P, Henningsson R, Lundquist I. Morphological evidence for the existence of nitric oxide and carbon monoxide pathways in the rat islets of Langerhans: an immunocytochemical and confocal microscopical study. Diabetologia. 1999;42:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 58. | Berthoud HR, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Physiol. 1990;258:R523-R530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Ionescu E, Rohner-Jeanrenaud F, Berthoud HR, Jeanrenaud B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve. Endocrinology. 1983;112:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Fox EA, Powley TL. Tracer diffusion has exaggerated CNS maps of direct preganglionic innervation of pancreas. J Auton Nerv Syst. 1986;15:55-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Loewy AD, Haxhiu MA. CNS cell groups projecting to pancreatic parasympathetic preganglionic neurons. Brain Res. 1993;620:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Browning KN, Coleman FH, Travagli RA. Characterization of pancreas-projecting rat dorsal motor nucleus of vagus neurons. Am J Physiol Gastrointest Liver Physiol. 2005;288:G950-G955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Mussa BM, Sartor DM, Verberne AJ. Dorsal vagal preganglionic neurons: differential responses to CCK1 and 5-HT3 receptor stimulation. Auton Neurosci. 2010;156:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 820] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 65. | Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R291-R307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Hermann GE, Travagli RA, Rogers RC. Esophageal-gastric relaxation reflex in rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1570-R1576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Toda N, Herman AG. Gastrointestinal function regulation by nitrergic efferent nerves. Pharmacol Rev. 2005;57:315-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Sanger GJ, Lee K. Hormones of the gut-brain axis as targets for the treatment of upper gastrointestinal disorders. Nat Rev Drug Discov. 2008;7:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Khoo J, Rayner CK, Feinle-Bisset C, Jones KL, Horowitz M. Gastrointestinal hormonal dysfunction in gastroparesis and functional dyspepsia. Neurogastroenterol Motil. 2010;22:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Allescher H-D, Ahmad S. Postulated physiological and pathophysiological roles on motility. Neuropeptide Function in the Gastrointestinal Tract. 1991;CRC Press: 311-371. |
| 71. | Owyang C. Physiological mechanisms of cholecystokinin action on pancreatic secretion. Am J Physiol. 1996;271:G1-G7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Martindale R, Levin S, Alfin-Slater R. Effects of caerulein and bombesin on insulin and glucagon secretion from the isolated, perfused rat pancreas. Regul Pept. 1982;3:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Hermansen K. Effects of cholecystokinin (CCK)-4, nonsulfated CCK-8, and sulfated CCK-8 on pancreatic somatostatin, insulin, and glucagon secretion in the dog: studies in vitro. Endocrinology. 1984;114:1770-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Raraty MG, Ghaneh P. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68:325-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 248] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 76. | Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine (5-HT) on the discharge of vagal mechanoreceptors and motility in the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Hendrix TR, Atkinson M, Clifton JA, Ingelfinger FJ. The effect of 5-hydroxytryptamine on intestinal motor function in man. Am J Med. 1957;23:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 71] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Misiewicz JJ, Waller SL, Eisner M. Motor responses of human gastrointestinal tract to 5-hydroxytryptamine in vivo and in vitro. Gut. 1966;7:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Bornstein JC. Serotonin in the gut: what does it do? Front Neurosci. 2012;6:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Chey WY, Chang T. Neural hormonal regulation of exocrine pancreatic secretion. Pancreatology. 2001;1:320-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Aja S. Serotonin-3 receptors in gastric mechanisms of cholecystokinin-induced satiety. Am J Physiol Regul Integr Comp Physiol. 2006;291:R112-R114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Fehmann HC, Göke R, Göke B. Glucagon-like peptide-1(7-37)/(7-36)amide is a new incretin. Mol Cell Endocrinol. 1992;85:C39-C44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Schirra J, Göke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept. 2005;128:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 624] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 86. | Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 314] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 87. | Fehmann HC, Göke R, Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev. 1995;16:390-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 350] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 88. | Harada K, Kitaguchi T, Kamiya T, Aung KH, Nakamura K, Ohta K, Tsuboi T. Lysophosphatidylinositol-induced activation of the cation channel TRPV2 triggers glucagon-like peptide-1 secretion in enteroendocrine L cells. J Biol Chem. 2017;292:10855-10864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 89. | Nakatani Y, Maeda M, Matsumura M, Shimizu R, Banba N, Aso Y, Yasu T, Harasawa H. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. 2017;43:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 90. | Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4:718-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 91. | Kolligs F, Fehmann HC, Göke R, Göke B. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9-39) amide. Diabetes. 1995;44:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Wang Z, Wang RM, Owji AA, Smith DM, Ghatei MA, Bloom SR. Glucagon-like peptide-1 is a physiological incretin in rat. J Clin Invest. 1995;95:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 93. | Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 473] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 94. | Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1287] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 96. | Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 115] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Meyer JH, Gu YG, Jehn D, Taylor IL. Intragastric vs intraintestinal viscous polymers and glucose tolerance after liquid meals of glucose. Am J Clin Nutr. 1988;48:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Thompson DG, Wingate DL, Thomas M, Harrison D. Gastric emptying as a determinant of the oral glucose tolerance test. Gastroenterology. 1982;82:51-55. [PubMed] |
| 99. | Woodyatt RT, Sansum WD, Wilder RM. Prolonged and accurately timed intravenous injection of sugar. JAMA. 1915;65:2067-2070. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Tambascia MA, Malerbi DA, Eliaschewitz FG. Influence of gastric emptying on the control of postprandial glycemia: physiology and therapeutic implications. Einstein (Sao Paulo). 2014;12:251-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Vinik A, Nakave A, Chuecos Mdel P. A break in the brake mechanism in diabetes: a cause of postprandial hyperglycemia. Diabetes Care. 2008;31:2410-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 103. | Eliasson B, Björnsson E, Urbanavicius V, Andersson H, Fowelin J, Attvall S, Abrahamsson H, Smith U. Hyperinsulinaemia impairs gastrointestinal motility and slows carbohydrate absorption. Diabetologia. 1995;38:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643-1648. [PubMed] |
| 105. | Sims MA, Hasler WL, Chey WD, Kim MS, Owyang C. Hyperglycemia inhibits mechanoreceptor-mediated gastrocolonic responses and colonic peristaltic reflexes in healthy humans. Gastroenterology. 1995;108:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Schvarcz E, Palmér M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 291] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 107. | Russo A, Stevens JE, Chen R, Gentilcore D, Burnet R, Horowitz M, Jones KL. Insulin-induced hypoglycemia accelerates gastric emptying of solids and liquids in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2005;90:4489-4495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Ma J, Pilichiewicz AN, Feinle-Bisset C, Wishart JM, Jones KL, Horowitz M, Rayner CK. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med. 2012;29:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Liu HP, Leong SK, Tay SS. Localization of NADPH-diaphorase positive neurons in the pancreas of the mouse, rat, chick, kitten and monkey. J Hirnforsch. 1994;35:501-510. [PubMed] |
| 110. | Liu HP, Tay SS, Leong SK. Nitrergic neurons in the pancreas of newborn guinea pig: their distribution and colocalization with various neuropeptides and dopamine-beta-hydroxylase. J Auton Nerv Syst. 1996;61:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 111. | De Giorgio R, Parodi JE, Brecha NC, Brunicardi FC, Becker JM, Go VL, Sternini C. Nitric oxide producing neurons in the monkey and human digestive system. J Comp Neurol. 1994;342:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Shimosegawa T, Abe T, Satoh A, Asakura T, Yoshida K, Koizumi M, Toyota T. Histochemical demonstration of NADPH-diaphorase activity, a marker for nitric oxide synthase, in neurons of the rat pancreas. Neurosci Lett. 1992;148:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Sha L, Miller SM, Szurszewski JH. Nitric oxide is a neuromodulator in cat pancreatic ganglia: histochemical and electrophysiological study. Neurosci Lett. 1995;192:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Umehara K. [Localization of NADPH-diaphorase activity and NOS immunoreactivity in the pancreas of rat and dog]. Nihon Shokakibyo Gakkai Zasshi. 1995;92:1161-1168. [PubMed] |
| 115. | Corbett JA, Wang JL, Misko TP, Zhao W, Hickey WF, McDaniel ML. Nitric oxide mediates IL-1 beta-induced islet dysfunction and destruction: prevention by dexamethasone. Autoimmunity. 1993;15:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Vincent SR. Nitric oxide and arginine-evoked insulin secretion. Science. 1992;258:1376-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Panagiotidis G, Akesson B, Alm P, Lundquist I. The nitric oxide system in the endocrine pancreas induces differential effects on secretion of insulin and glucagon. Endocrine. 1994;2:787-792. |
| 118. | Panagiotidis G, Alm P, Lundquist I. Inhibition of islet nitric oxide synthase increases arginine-induced insulin release. Eur J Pharmacol. 1992;229:277-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 119. | Schmidt HH, Warner TD, Ishii K, Sheng H, Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992;255:721-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 311] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 120. | Salehi A, Carlberg M, Henningson R, Lundquist I. Islet constitutive nitric oxide synthase: biochemical determination and regulatory function. Am J Physiol. 1996;270:C1634-C1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Gangula PR, Chinnathambi V, Hale AB, Mukhopadhyay S, Channon KM, Ravella K. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice. Neurogastroenterol Motil. 2011;23:773-e335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Lidums I, Hebbard GS, Holloway RH. Effect of atropine on proximal gastric motor and sensory function in normal subjects. Gut. 2000;47:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 123. | Azpiroz F, Malagelada JR. Importance of vagal input in maintaining gastric tone in the dog. J Physiol. 1987;384:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 124. | Coulie B, Tack J, Sifrim D, Andrioli A, Janssens J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am J Physiol. 1999;276:G373-G377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Mihara H, Suzuki N, Yamawaki H, Tominaga M, Sugiyama T. TRPV2 ion channels expressed in inhibitory motor neurons of gastric myenteric plexus contribute to gastric adaptive relaxation and gastric emptying in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G235-G240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Di Stefano M, Miceli E, Mazzocchi S, Tana P, Corazza GR. The role of gastric accommodation in the pathophysiology of functional dyspepsia. Eur Rev Med Pharmacol Sci. 2005;9:23-28. [PubMed] |
| 127. | Petrakis IE, Vrachassotakis N, Vassilakis SJ, Sciacca V, Chalkiadakis G. Erythromycin enhances solid-phase gastric emptying in induced-hyperglycemia in patients with truncal vagotomy and pyloroplasty. Dig Dis Sci. 2000;45:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 128. | Howlett PJ, Sheiner HJ, Barber DC, Ward AS, Perez-Avila CA, Duthie HL. Gastric emptying in control subjects and patients with duodenal ulcer before and after vagotomy. Gut. 1976;17:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Chen J, Koothan T, Chen JD. Synchronized gastric electrical stimulation improves vagotomy-induced impairment in gastric accommodation via the nitrergic pathway in dogs. Am J Physiol Gastrointest Liver Physiol. 2009;296:G310-G318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Rivera LR, Poole DP, Thacker M, Furness JB. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil. 2011;23:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 131. | Piciucchi M, Capurso G, Archibugi L, Delle Fave MM, Capasso M, Delle Fave G. Exocrine pancreatic insufficiency in diabetic patients: prevalence, mechanisms, and treatment. Int J Endocrinol. 2015;2015:595649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 132. | Hardt PD, Brendel MD, Kloer HU, Bretzel RG. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care. 2008;31 Suppl 2:S165-S169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 133. | Hardt PD, Ewald N. Exocrine pancreatic insufficiency in diabetes mellitus: a complication of diabetic neuropathy or a different type of diabetes? Exp Diabetes Res. 2011;2011:761950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 134. | Ewald N, Raspe A, Kaufmann C, Bretzel RG, Kloer HU, Hardt PD. Determinants of Exocrine Pancreatic Function as Measured by Fecal Elastase-1 Concentrations (FEC) in Patients with Diabetes mellitus. Eur J Med Res. 2009;14:118-122. [PubMed] |
| 135. | Chowdhury RS, Forsmark CE, Davis RH, Toskes PP, Verne GN. Prevalence of gastroparesis in patients with small duct chronic pancreatitis. Pancreas. 2003;26:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 136. | Czul F, Coronel E, Donet JA. [Update on chronic pancreatitis: review article]. Rev Gastroenterol Peru. 2017;37:146-155. [PubMed] |
| 137. | Liddle RA, Morita ET, Conrad CK, Williams JA. Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest. 1986;77:992-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Shoji E, Okumura T, Onodera S, Takahashi N, Harada K, Kohgo Y. Gastric emptying in OLETF rats not expressing CCK-A receptor gene. Dig Dis Sci. 1997;42:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Poulsen JL, Olesen SS, Malver LP, Frøkjær JB, Drewes AM. Pain and chronic pancreatitis: a complex interplay of multiple mechanisms. World J Gastroenterol. 2013;19:7282-7291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (3)] |
| 140. | Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3346] [Cited by in RCA: 2988] [Article Influence: 213.4] [Reference Citation Analysis (0)] |
| 141. | Nakamura T, Takebe K, Ishii M, Kasai F, Arai Y, Tando Y, Yamada N, Terada A, Suda T. Study of gastric emptying in patients with pancreatic diabetes (chronic pancreatitis) using acetaminophen and isotope. Acta Gastroenterol Belg. 1996;59:173-177. [PubMed] |
| 142. | Frier BM. The incidence and impact of hypoglycemia in type 1 and type 2 diabetes. International Diabetes Monitor. 2009;210-218. |
| 143. | Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 458] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 144. | Siafarikas A, Johnston RJ, Bulsara MK, O’Leary P, Jones TW, Davis EA. Early loss of the glucagon response to hypoglycemia in adolescents with type 1 diabetes. Diabetes Care. 2012;35:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |