Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.360
Peer-review started: October 17, 2017
First decision: October 31, 2017
Revised: November 7, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: January 21, 2018
Processing time: 94 Days and 6.9 Hours
To investigate the effect of ischaemia and reperfusion (I/R) injury on the Ca2+-ATPase activation in the intestinal tissue of a rat autologous orthotopic liver transplantation model and to determine if hypoxia preconditioning (HP) therapy induces HIF-1α to protect rat intestinal tissue against I/R injury.
Rats received non-lethal hypoxic preconditioning therapy to induce HIF-1α expression. We used an autologous orthotopic liver transplantation model to imitate the I/R injury in intestinal tissue. Then, we detected the microstructure changes in small intestinal tissues, Ca2+-ATPase activity, apoptosis, and inflammation within 48 h postoperatively.
HIF-1α expression was significantly increased in intestinal tissue at 12 h postoperatively in rats that were exposed to a hypoxic environment for 90 min compared with a non-HP group (HP vs AT, P = 0.0177). Pathological analysis was performed on the intestinal mucosa cells, and the cells in the HP group appeared healthier than the cells in the AT group. The Ca2+-ATPase activity in the small intestinal cells in the AT group was significantly lower after the operation, and the Ca2+-ATPase activity in the HP group recovered faster than that in the AT group at 6 h postoperatively (HP vs AT, P = 0.0106). BCL-2 expression in the HP group was significantly higher than that in the AT group at 12 h postoperatively (HP vs AT P = 0.0010). The expression of the inflammatory factors NO, SOD, IL-6, and TNF-α was significantly lower in the HP group than in the AT group.
Hypoxia-induced HIF-1α could protect intestinal mucosal cells against mitochondrial damage after I/R injury. HP could improve hypoxia tolerance in small intestinal mucosal cells and increase Ca2+-ATPase activity to reduce the apoptosis of and pathological damage to intestinal cells. HP could be a useful way to promote the earlier recovery of intestinal function after graft procedure.
Core tip: Ischaemia/reperfusion (I/R) injury affects the recovery of postoperative bowel function in liver transplantation. In our research, hypoxia-induced HIF-1α expression could protect mitochondrial function and Ca2+-ATPase activity against I/R injury to reduce the apoptosis and pathological damage to intestinal cells. Therefore, we suggest that hypoxic preconditioning therapy could improve the tolerance of small intestinal mucosal cell to hypoxia in rat autologous orthotopic liver transplantation.
- Citation: Ji ZP, Li YX, Shi BX, Zhuang ZN, Yang JY, Guo S, Xu XZ, Xu KS, Li HL. Hypoxia preconditioning protects Ca2+-ATPase activation of intestinal mucosal cells against R/I injury in a rat liver transplantation model. World J Gastroenterol 2018; 24(3): 360-370
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/360.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.360
During liver transplantation, intestinal ischaemia/reperfusion (I/R) injury is usually caused by the blockage of blood flow in the portal vein (PV). The digestive tract usually plays an important role in the pathophysiological process of trauma and shock due to its unique physiological environment, metabolic factors, network of mucosal blood vessels, and counter-current exchange mechanism. In particular, intestinal mucosal I/R injury is associated with systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) after shock or trauma[1]. The postoperative recovery of small intestinal mucosa cells is important in treatment and prognosis.
Under normal circumstances, the intestinal mucosa plays an important role in maintaining normal barrier function, absorbing nutrients, and resisting the invasion of local bacteria and toxins in the intestine[2]. The intestinal villus nutrient vessels resemble a hairpin that sits at the top of the intestinal villi, and they are extremely curved. Due to their high metabolism and villus microvascular structure characteristics, the tolerance of intestinal mucosa cells to I/R injury, especially the top of the intestinal villus epithelial cells, is much lower than that in other tissue cells; thus, these cells are particularly sensitive to hypo-perfusion[3]. Another cause of intestinal mucosal damage is the presence of hypoxia and acidosis in the gastrointestinal tract. Therefore, the intestinal villi can undergo ischaemic damage.
Non-lethal hypoxic preconditioning (HP) can increase tolerance to I/R injury and is effective in reducing damage to a variety of organs[4], including the liver and kidney[5]. For tumour cells, research has found that HIF-1α plays an important role in hypoxia conditioning, and HIF-1α is also the critical transcription factor that mediates cell hypoxia reactions[6]. In our previous study, we induced HIF-1α expression in liver tissue by exposing rats to a non-lethal hypoxia environment, and detected changes in the NF-κB and Erk pathways. Moreover, changes in glucose metabolism were also detected, and hypoxia-induced HIF-1α expression promoted HK2 and Glut1 expression, which could decrease liver inflammation and I/R injury after orthotopic liver transplantation. BCL-2 (B-cell lymphoma 2), encoded in humans by the Bcl-2 gene, is the founding member of the Bcl-2 family of regulator proteins that regulate cell death (apoptosis), by either inducing (pro-apoptotic) or inhibiting (anti-apoptotic) apoptosis[7,8]. BCL-2 is considered an important anti-apoptotic protein. Studies of human cancer cells also confirmed that the expression of BCL-2 was positively correlated with VEGF and HIF1A, which are target genes of miR-27a and miR-17, and the expression of VEGF and HIF1A was related to the poor prognosis of patients[9]. The BCL2 protein functions as an antiapoptotic protein and inhibits programmed cell death[10]. Both gene amplification and translocation are common mechanisms for BCL2 protein overexpression in diffuse large B-cell lymphoma (DLBCL). The clinical significance of BCL2 protein expression in DLBCL is still controversial[11]. Ca2+-ATPase damage is one of the early manifestations in intestinal mucosa cells during ischaemia-reperfusion injury. During intestinal ischaemia, calcium mobilization and extracellular calcium influx greatly increase the calcium ion concentration in the cytoplasm. Intracellular calcium activates proteolytic enzymes, which produce a large number of free radicals that are involved in cell injury during reperfusion. Oxygen free radicals damage cell membrane lipids, and the peroxidation of calcium channels can lead to the inactivation of the Na+-K+-ATP enzyme and the Ca2+/Na+ exchange, which can enhance Ca2+ influx, leading to intracellular calcium overload[12]. Cell damage is caused by mitochondrial oxygen utilization and the synthesis of ATP is further damaged. Acidic products are produced during anaerobic metabolism, which leads to a change in intracellular enzyme activity and a deficient transmembrane ion gradient. When the duration of tissue ischaemia exceeds a certain critical value, I/R injury will be irreversible and the tissue will become necrotic[13].
In this study, we investigated how I/R injury affects the Ca2+-ATPase activation in intestinal tissue in a rat autologous orthotopic liver transplantation model. Hypoxia-induced HIF-1α could protect against the I/R injury to mitochondria and preserve Ca2+-ATPase activity in rat intestinal tissue. HP can improve the tolerance of small intestine mucosal cells to hypoxia, and reduce the apoptosis by increasing BCL2 expression and pathological damage to intestinal cells. It could be a useful way to promote the earlier recovery of intestinal function after graft procedure.
Healthy 8-10-week-old male SD rats that weighed 225-275 g were provided by the Experimental Animal Center of Jiangsu Province. All studies were approved by our Institutional Animal Care and Use Committee. The homemade hypoxic device (referring to Vannucci’s and other methods[4]) consisted of a noninvasive vascular folder of 8% nitrogen-oxygen mixed gas (containing 8% oxygen and 92% nitrogen, provided by the Nanjing Flextronics Gas Co., Ltd.). Hypoxia equipment consisted of a high pressure tank filled with nitrogen-oxygen mixed gas, a 5-L sealed hypoxic disposal tank, an outflow of gas, water bottle, mask, oxygen valve, an oxygen flow metre, and connection tubes. The rats can be completely enclosed in the transparent low-disposal tank. Rats were maintained at atmospheric pressure for 90 min with an 8% nitrogen-oxygen gas mixture (containing 8% oxygen and 92% nitrogen) at a flow rate of 5 L/min. Eight hours later, we administered anaesthesia and began the autologous orthotopic liver transplantation surgery.
The healthy SD rats were randomly divided into three groups as follows: a normal control group (NC; n = 3, total n = 18), an autotransplantation group (AT; n = 3, total n = 18), or a HP group (HP; n = 3, total n = 18). The autologous orthotopic liver transplantation procedure was performed as follows: after rats were injected with 100 mg/kg ketamine and 0.03 mg atropine intraperitoneally, they were maintained on semi-open mask inhalation of ether for 10 min. After the abdominal cavity was opened, the falciform ligament was resected, and the blood vessel along the oesophagus was removed. The liver was dissected until the suprahepatic vena cava (SVC) was completely liberated. A homemade leash was prepared to guide the SVC for blockage. The PV was dissected from the convergence of the inferior mesenteric and splenic veins. The hepatic artery and biliary tract were freed together. Vascular clamps were used at the convergence of the inferior mesenteric and splenic veins, hepatic artery, SVC, and IVC. The PV was punctured with a No. 4 transfixion pin in preparation for reperfusion, and fixed with a vascular clamp. Ringer’s lactate solution was injected for reperfusion at 2.5 mL/min, and a 1-mm incision was made in the wall of the IVC as an outflow tract. The liver gradually turned yellow when reperfusion was successful. A total of 20-25 min passed to imitate the operation range of liver transplantation and the duration of ischaemia/reperfusion injury. At the end of the procedure, the rats received an injection of 1.6 million units of penicillin in the abdomen and 4 mL of Ringer’s lactate was infused through the abdominal wall vein, then the abdomen was sutured. After surgery, rats were kept in a 38 °C incubator[5].
Intestinal tissue samples were lysed and cell lysates were collected with radioimmuno-precipitation assay (RIPA) protein lysate buffer. The cytoplasm was centrifuged at 750 g at 4 °C for 5 min, then the supernatant was collected and centrifuged at 13000 rpm at 4 °C for 30 min to collect the sediment. A total of 30 μg of protein were loaded and run on a gel and then transferred to a PVDF membrane (Immobilon-P, Millipore, Bedford, MA, United States) for 1 h at 300 mA, followed by blocking with 5% non-fat dry milk in 0.1% TBST for 1 h. The membrane was incubated with primary antibodies overnight at 4 °C. The primary antibodies against HIF-1α, Caspase 3, cleaved Caspase 3, and cleaved PARP were purchased from Cell Signalling Technology (United States) and diluted 1:500. The secondary antibodies and β-actin antibody were purchased from Abcam Biotechnology Company.
Formalin-fixed paraffin-embedded tissue sections were subjected to immunostaining using the streptavidin-peroxidase technique, with diaminobenzidine as a chromogen. Haematoxylin and eosin (H and E) staining and immunohistochemistry were performed according to standard procedures. The protein expression of BCL-2 was evaluated by immunohistochemistry. The target protein was detected via the Elivison two-step immunohistochemical method. Briefly, tissues were dewaxed in xylene and hydrated using an alcohol gradient (ethanol, 95% ethanol in water, 85% ethanol in water, and 70% ethanol in water). A high-temperature plastic staining tray submerged in a beaker of antigen retrieval buffer was used for antigen retrieval. After endogenous peroxidase was inactivated, the primary was added. The sections were then incubated in 50 μL of universal IgG antibody-Fab-HRP polymer for 30 min. Subsequently, the glass slides underwent colour development, dehydration, and sealing. The expression of BCL-2 protein was represented by a blue colour in the cells. Image-Pro Plus 6.0 software was used to analyse the optical density of immunohistochemical results. In brief, the scoring was as follows: 0, <10% of cells stained; 1, 10% to 25% of cells stained; 2, 25% to 50% of cells stained; 3, > 50% of cells stained. For all cases, slides with a score ≥ 2 were considered positive.
ATPase (Ca2+-ATPase) activity was detected in 0.2 mL of the supernatant added to 0.8 mL of normal saline. One ATPase activity unit per hour is the amount of 1 μmol of inorganic phosphorus produced by the decomposition of ATP by per milligram of protein.
Blood samples were collected from rats at 0, 2, 6, 12, 24 and 48 h after the operation and centrifuged to separate sera, which were stored at -80 °C. All serum samples were analysed with ELISA assay kits, which were purchased from Kaiji Biology, Inc. (Nanjing, China). Nitric oxide (NO), superoxide dismutase (SOD), interleukin-6 (IL-6), and rat tumour necrosis factor-α(TNF-α) ELISA kits were purchased from Kaiji Biology Company, Nanjing, China.
Statistical analyses were performed with commercially available SPSS version 19.0 software (Chicago, IL, United States) and GraphPad Prism software (La Jolla, CA, United States). All experimental data were analysed via analysis of variance and are expressed as mean ± SD. Independent t-tests were used to analyse the differences between groups. P < 0.05 was considered statistically significant.
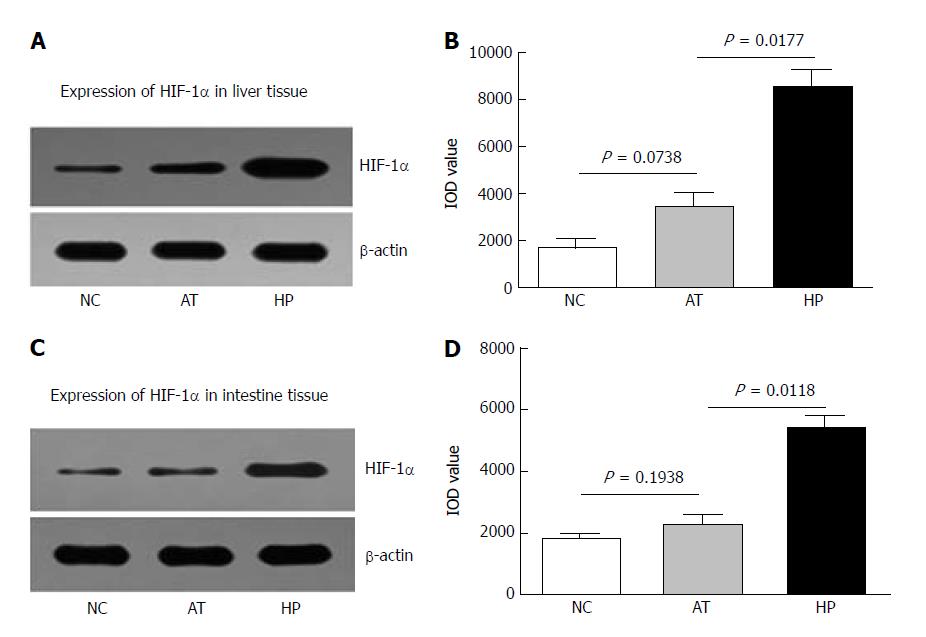
The rats in the HP group were exposed to a hypoxic environment for 90 min before the procedure; this experimental protocol had been used in our lab previously[14]. Then, the rats underwent autologous orthotopic liver transplantation. The changes in HIF-1α levels in the total cellular extract of liver and intestinal tissues were detected 12 h after the procedure. HIF-1α expression induced by HP was increased in rat liver tissue 12 h after the operation (Figure 1A). We also observed that HIF-1α expression in the AT group was higher than in the NC group, but the difference was not significant (AT vs NC, P = 0.0738, Figure 1B). The increased HIF-1α expression in ischaemia and hypoxia tissues may have been caused by the procedure, which includes clamping and blocking the blood circulation during the liver transplantation. HIF-1α expression was significantly higher after the HP therapy than in the non-HP group (HP vs AT, P = 0.0177, Figure 1B).
The intestinal tissues were also examined for changes in HIF-1α levels 12 h after the procedure via Western blot analysis. We observed that HIF-1α expression in intestinal tissues was significantly higher after HP therapy than in the non-HP group (HP vs AT, P = 0.0118, Figure 1D). HIF-1α level in the AT group was not significantly different compared to the NC group (AT vs NC, P = 0.1938, Figure 1D).
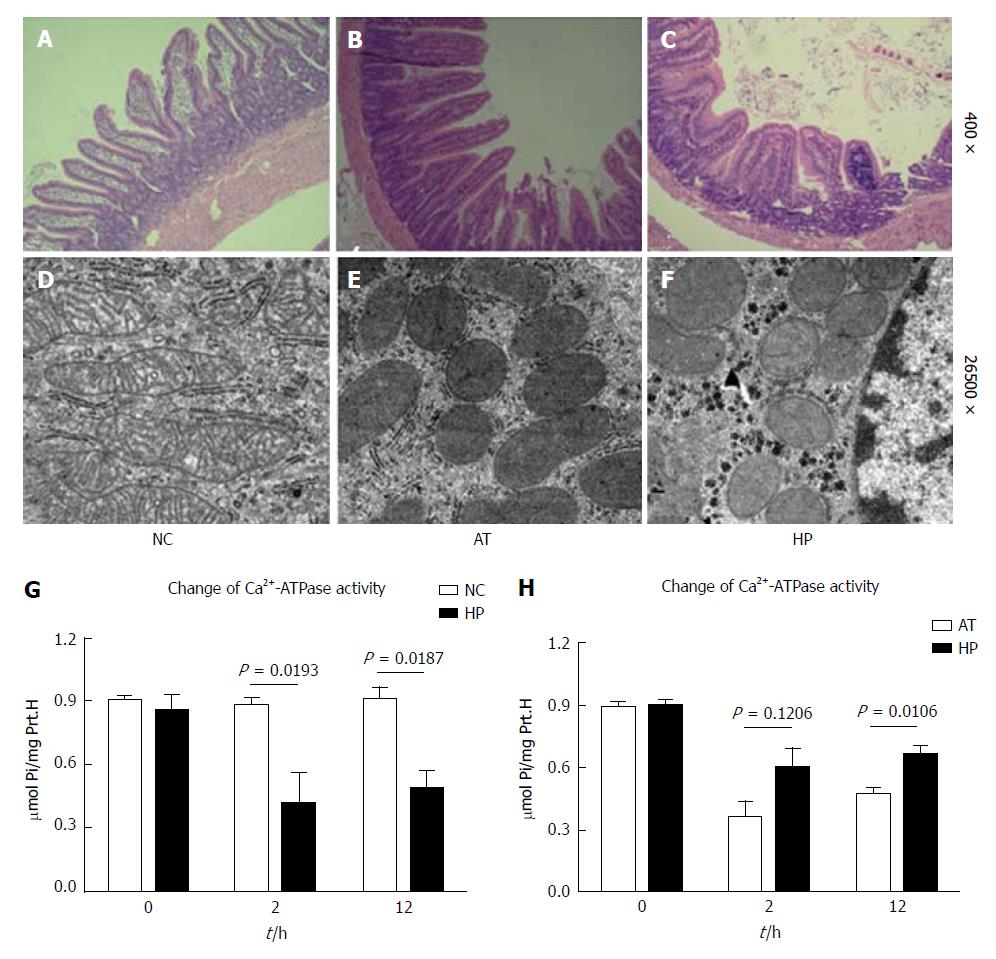
Intestinal cells, particularly mucosal cells, usually suffer I/R injury during the liver transplantation procedure. In this study, we collected rat intestinal tissues for pathological examination at 12 h after the operation. The intestinal mucosal cells in the AT group exhibited noticeable oedema, and capillary vessels were filled with red blood cells and blood clots. The villus epithelial cells were shedding, and glands were severely damaged and infiltrated with inflammatory cells (Figure 2B). Mitochondria are important cellular organs that affect the oxidative respiratory chain, and damage to mitochondria that occurs during the early stage of ischaemia/reperfusion injury leads to cell apoptosis. We observed that the mitochondria in the AT group appeared swollen, round, and degenerated 12 h after the operation, and the visible cristae appeared less fractured or even disappeared (Figure 2E). Our previous study showed that HP induced HIF-1α expression and decreased oxidative respiratory chain damage in mitochondria and protected against I/R injury in liver tissue[14]. In this study, we also observed that the intestinal cells appeared to have slight oedema, fewer inflammatory cells were present, and less damage was present in rat intestinal tissue after HP therapy compared with the AT group (Figure 2C). The mitochondria of intestinal cells appeared less swollen with fewer cristae. The structure of the endoplasmic reticulum was maintained (Figure 2F). The intestinal cells and mitochondria appeared normal in the NC group (Figure 2A and D).
The I/R injury to the intestinal tissue during the procedure may decrease Ca2+-ATPase activity, which was evaluated to assess the damage to intestinal cell membranes and to predict functional recovery.
Ca2+-ATPase activity in the AT group was significantly lower compared with the NC group, which decreased to the lowest level 2 h postoperatively (Figure 2G, AT vs NC, P = 0.0193) and then recovered. The same pattern was evident in the HP group, which exhibited decreased cellular Ca2+-ATPase activity after the procedure. However, we observed that the cellular Ca2+-ATPase activity in the HP group was significantly higher than that in the AT group 12 h postoperatively (Figure 2H, HP vs AT, P = 0.0106). To further detect the effect of HP on the activity of cellular Ca2+-ATPase, we analysed the enzyme bioactivity changes at different time points. Table 1 shows that the cellular Ca2+-ATPase activity was significantly higher in the HP group than in the AT group at 2, 12 and 24 h postoperatively (Table 1).
| Group | n | 1 h | 2 h | 12 h | 24 h | 48 h |
| HP | 12 | 0.923 ± 0.008a | 0.389 ± 0.014ac | 0.566 ± 0.013ac | 0.771 ± 0.011ac | 0.908 ± 0.014a |
| AT | 12 | 0.904 ± 0.06e | 0.323 ± 0.018 e | 0.374 ± 0.011e | 0.575 ± 0.033e | 0.871 ± 0.012 e |
| NC | 12 | 0.923 ± 0.004 | 0.916 ± 0.010 | 0.928 ± 0.034 | 0.932 ± 0.011 | 0.909 ± 0.010 |
| F | 3.075 | 501.688 | 616.699 | 274.498 | 3.109 | |
| P value | 0.0600 | < 0.001 | < 0.001 | < 0.001 | 0.0580 |
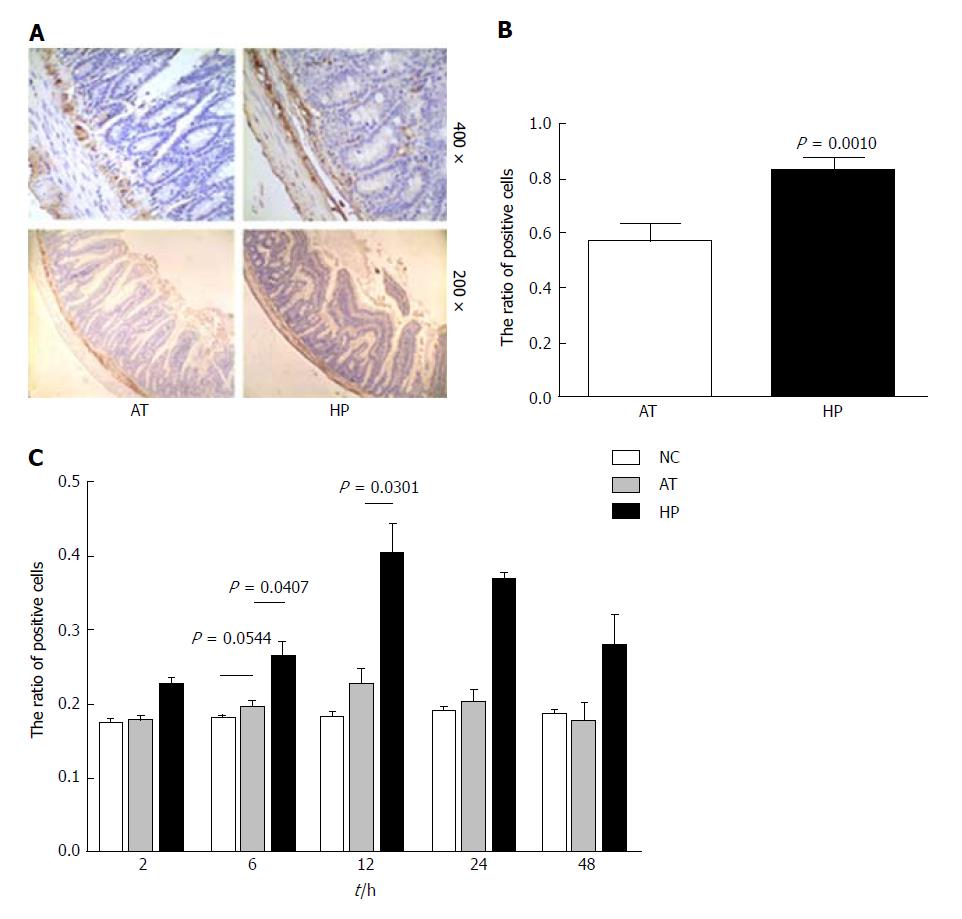
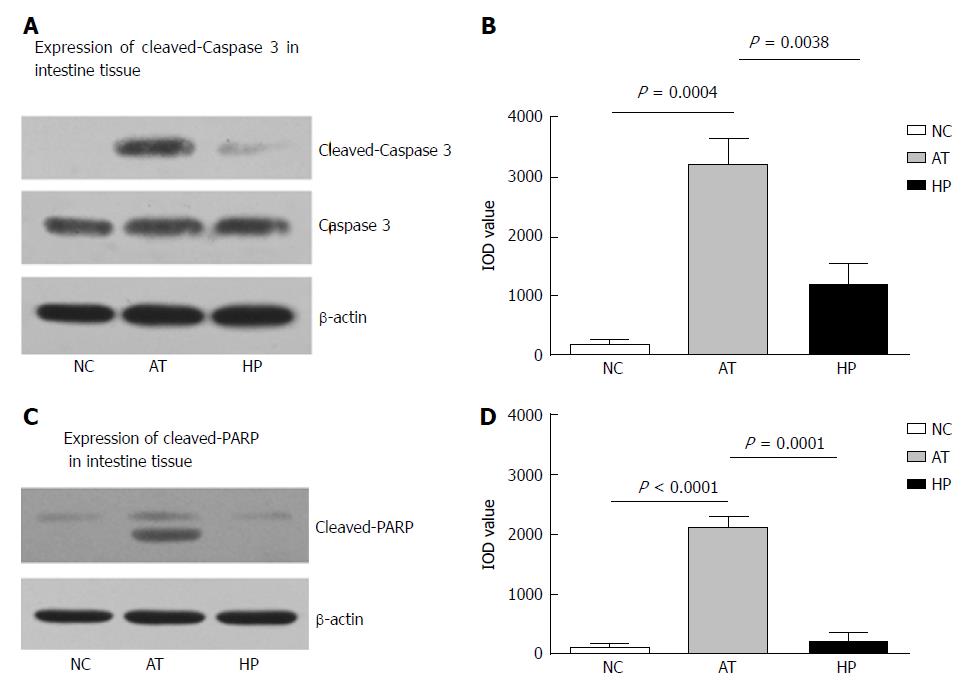
Our previous study showed that HP therapy induced HIF-1α expression and could protect against mitochondrial damage and apoptosis in liver tissue[14]. In this study, rat intestinal tissues were analysed by immunohistochemistry to detect BCL2 expression, which can affect cell apoptosis by regulating mitochondrial membrane permeability. Immunohistochemistry demonstrated that the ratio of BCL2 positive signal in the HP group was significantly higher than in the AT group at postoperative 12 h (Figure 3B, HP vs AT P = 0.0010). The ratio of positive BCL2 signal was detected at several time points after the operation (2, 6, 12, 24 and 48 h) in the NC, AT and HP groups. The expression of BCL2 in the HP group was increased after the operation, peaked at 12 h postoperatively, and was maintained at a high level. It was significantly higher in the HP group compared with the NC group and the AT group (Figure 3C, 6 h: HP vs AT, P = 0.0407; 12 h: HP vs AT, P = 0.0301). However, for the AT group, we observed that there was no significant difference in BCL2 expression compared with the NC group (Figure 3C, 6 h: AT vs NC, P = 0.0544). To confirm that BCL2 expression inhibited apoptosis in rat intestinal tissue, we detected the cleaved Caspase 3 and cleaved PARP expression levels by Western blot and found that cleaved Caspase 3 expression was increased in rat intestinal tissue that had undergone I/R injury at 24 h postoperatively. In the AT group, the level of cleaved Caspase 3 was significantly higher than that in the NC group (Figure 4B, AT vs NC, P = 0.0004), while cleaved Caspase 3 expression in the HP group was lower than that in the AT group at postoperative 24 h (Figure 4B, HP vs AT, P = 0.0038). The expression of cleaved PARP was increased in rat intestinal tissue that had undergone I/R injury at 24 h postoperatively. The level of cleaved PARP in the AT group was significantly higher than that in the NC group (Figure 4D, AT vs NC, P < 0.0001). Cleaved PARP expression in the HP group was lower than that in the AT group at postoperative 24 h (Figure 4D, HP vs AT, P = 0.0001).
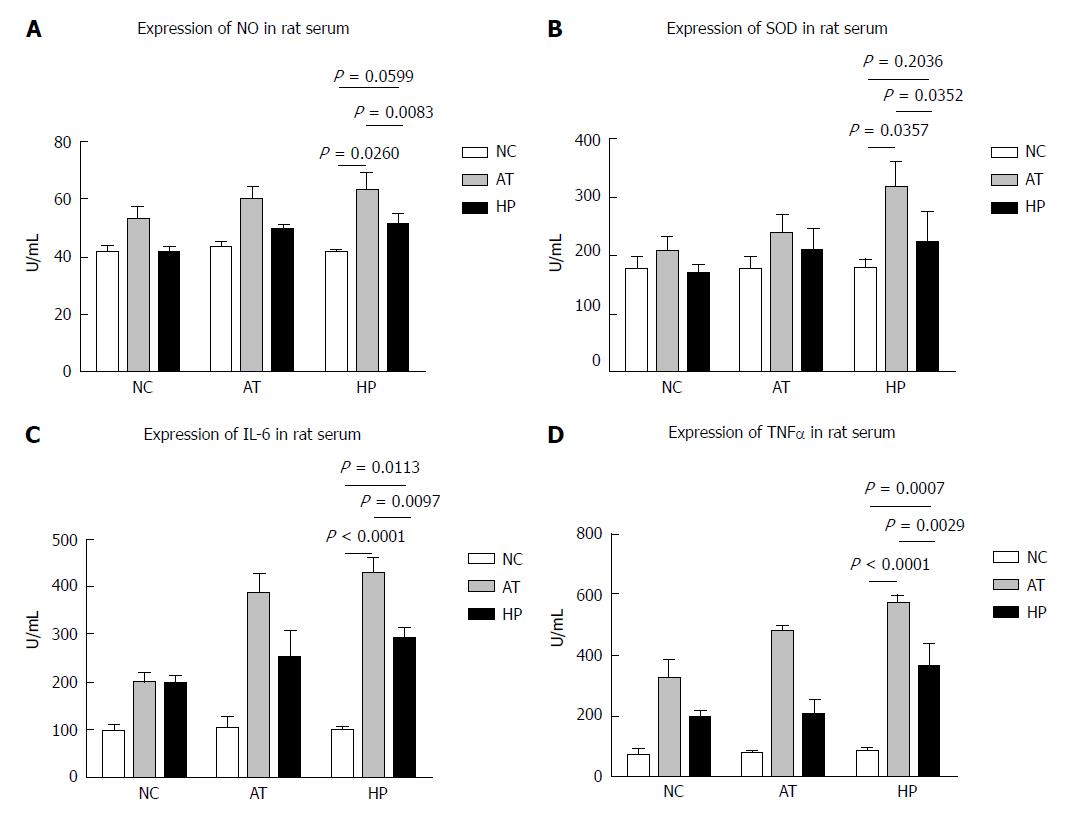
To investigate the ability of HP treatment to protect intestinal tissue from I/R injury, we monitored changes in the levels of NO, SOD, IL-6 and TNF-α in rat serum samples. In the HP group, serum NO and SOD levels increased after the operation, but they were lower compared with the AT group, in which NO and SOD levels stayed elevated after the operation (Figure 5A and B; NO, 24 h: AT vs NC, P = 0.0260; AT vs HP, P = 0.0083; HP vs NC, P = 0.0599; SOD, 24 h: AT vs NC, P = 0.0357; HP vs AT, P = 0.0352; HP vs NC, P = 0.2036). We measured the serum levels of IL-6 and TNF-α, and found that HP treatment appeared to relieve some of the inflammatory reaction due to the lower IL-6 and TNF-α levels that were detected (Figure 5C and D, IL-6, 24 h: AT vs NC, P < 0.0001; AT vs HP, P = 0.0097; HP vs NC, P = 0.0113; TNF-α, 24 h: AT vs NC, P < 0.0001; HP vs AT, P = 0.0029; HP vs NC, P = 0.0007).
There is a close relationship between the contractile activity of the small intestine and the intracellular concentration of Ca2+[15]. Ca2+-ATPase can be found in the plasma membrane, endoplasmic reticulum, and mitochondrial membrane. It can be activated and hydrolyse ATP to provide energy when the concentration of Ca2+ rises to a certain level. Therefore, Ca2+-ATPase levels can indicate ischaemia-reperfusion injury because they can reflect the severity of cell injury[16]. The results of the present study showed that the intestine suffered hypoxia with congestion after the occlusion of the PV during autologous orthotopic liver transplantation. The calcium balance system became dysfunctional because the activity of the Ca2+-ATPase in intestinal tissue was damaged due to the presence of toxins in the blood. We found that the Ca2+-ATPase activity in intestinal tissue which suffered the I/R injury after the procedure of autologous orthotopic liver transplantation was significantly lower compared with the non-surgery intestinal tissue. Therefore, the systolic and diastolic function of the intestinal smooth muscle was inhibited in the autologous orthotopic liver transplantation group, resulting in decreased peristalsis of the stomach and intestine.
Due to hypoxia, intestinal epithelial cells were shedding and necrosis occurred, increasing intestinal permeability and triggering intestinal bacteria and endotoxin translocation[17]. In a previous study, we showed that HIF-1α promoted HK2 and Glut1 expression could decrease the liver inflammation and I/R injury after orthotopic liver transplantation[14]. In this study, due to non-noticeably reduced Ca2+-ATPase activity in the liver transplantation group with HPtherapy, small intestinal tissue exhibited less apoptosis and hypoxia tolerance was increased after I/R injury and exposure to oxygen free radicals and toxins in the intestine. Our previous study found that HP protects mitochondria against I/R injury[18]. In this study, we observed that Ca2+-ATPase activity in intestinal tissue of the rat with HP therapy was not noticeably reduced compared to the non-surgery intestinal tissue. The cells could adapt to hypoxia and post-operative injury in a hypoxic environment was reduced.
BCL2 affects cell apoptosis by regulating mitochondrial membrane permeability. The mechanism may be that BCL2 changes the pores or channels in the mitochondrial membrane[19-21]. BCL2 is considered an important anti-apoptotic protein, and it can affect cell apoptosis by regulating mitochondrial membrane permeability.
Our previous studies found that HP was sufficient to activate the BCL2 signalling pathway and up-regulated the expression of BCL2 protein, a regulatory factor that restrains apoptosis, and it may regulate apoptosis by altering the configuration of mitochondria in liver tissue of the rat liver transplantation model[22]. In this study, HP therapy protected the rat intestinal tissue against apoptosis as revealed by a low level of cleaved Caspase 3 and cleaved PARP expression after the liver transplantation procedure compared to the rat with non-HP therapy. BCL2 expression was also increased in the HP therapy group and may be related with Ca2+-ATPase activity and mitochondrial protection after intestinal I/R injury in the rat autologous liver transplantation model. It requires further studies to show the relationship between HP and BCL2.
During HP, intestinal mucosal cells could be resistant to hypoxia caused by the blockage of blood flow during liver transplant, which would reduce apoptosis. HP therapy protected the intestinal mucosa cells against I/R injury. The characteristic morphological changes of intestinal mucosa cells undergoing HP therapy included slight oedema and fewer inflammatory cells. In addition, the tops of the epithelial cells were less damaged, the mitochondria showed less swelling and fewer cristae, and the endoplasmic reticulum structure was visible. However, the situation was worse in the autologous orthotopic liver transplantation group; cells were filled with red blood cells and blood clots in capillary vessels, intestinal mucosa cells exhibited obvious oedema, and infiltrated inflammatory cells surrounded the epithelial cells. The villus epithelial cells were shedding, had severely damaged glands, and had been infiltrated by inflammatory cells. The swollen mitochondria appeared round, had degenerated, and had severe visible cristae that had fewer fractures or had disappeared.
In summary, the early recovery of enteral nutrition is very important for intestinal prognosis after liver transplantation. It is essential to reduce the damage to small intestinal mucosal cells. In this study, we used a rat autologous orthotopic liver transplantation model to simulate intestinal I/R injury and observed that HP could protect Ca2+-ATPase and reduce small intestinal mucosal mitochondrial damage and apoptosis. The protective mechanism of Ca2+-ATPase against intestinal cristae I/R injury in rat autologous liver transplantation requires further study. I/R injury was decreased in the intestine, and inflammation was reduced. These findings may provide a theoretical basis for the clinical recovery and treatment in liver transplantation, but further experimental evidence is needed.
During liver transplantation, intestinal ischaemia/reperfusion (I/R) injury usually occurs due to the blockage of blood flow in the portal vein. Intestinal mucosal I/R injury is related with SIRS and MODS after shock or trauma. The postoperative recovery of small intestinal mucosal cells is important in treatment and prognosis. Non-lethal hypoxic preconditioning (HP) can increase tolerance to I/R injury and is effective in reducing damage to a variety of organs. In our previous study, we induced HIF-1α expression in liver tissue by exposing rats to a non-lethal hypoxia environment, and detected changes in the NF-κB and Erk pathways. Moreover, changes in glucose metabolism were also detected, and hypoxia-induced HIF-1α expression promoted HK2 and Glut1 expression, which could decrease liver inflammation and I/R injury after orthotopic liver transplantation. BCL-2 is considered an important anti-apoptotic protein. Ca2+-ATPase damage is one of the early manifestations in intestinal mucosa cells during ischaemia-reperfusion injury.
In this study, we investigated how I/R injury affects the Ca2+-ATPase activation in intestinal tissue in a rat autologous orthotopic liver transplantation model.
To investigate the effect of I/R injury on the Ca2+-ATPase activation in rat intestinal tissue in a rat autologous orthotopic liver transplantation model and to determine if HP therapy induced HIF-1α to protect rat intestinal tissue against I/R injury.
Non-lethal hypoxic preconditioning therapy was applied to induce HIF-1α expression. An autologous orthotopic liver transplantation model was established to imitate I/R injury to intestinal tissue. Then, we detected the microstructure changes in small intestinal tissues using histology and immunohistochemistry, the expression of HIF-1α, cleaved Caspase 3, and cleaved PARP by Western blot analysis, and the expression of inflammatory factors in rat serum by ELISA.
After HP therapy, HIF-1α expression was significantly increased in intestinal tissue of rats at 12 h postoperatively. Pathology of the intestinal mucosal cells appeared healthier in the HP group than in the AT group. The Ca2+-ATPase activity in small intestinal cells in the HP group recovered faster than that in the AT group. BCL-2 expression in the HP group was significantly higher than that in the AT group. The expression of the inflammatory factors NO, SOD, IL-6 and TNF-α was significantly lower in the HP group than in the AT group.
Hypoxia-induced HIF-1α could protect against the I/R injury to mitochondria and preserve Ca2+-ATPase activity in rat intestinal tissue. HP can improve the tolerance of small intestinal mucosal cells to hypoxia, and reduce the apoptosis by increasing BCL2 expression and pathological damage to intestinal cells.
Non-lethal HP could be a useful way to promote the earlier recovery of intestinal function after graft procedure.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Berger BM, Cetinkunar S, Gad EH S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Li D
| 1. | Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Moore-Olufemi SD, Kozar RA, Moore FA, Sato N, Hassoun HT, Cox CS Jr, Kone BC. Ischemic preconditioning protects against gut dysfunction and mucosal injury after ischemia/reperfusion injury. Shock. 2005;23:258-263. [PubMed] |
| 3. | Fujise T, Iwakiri R, Wu B, Amemori S, Kakimoto T, Yokoyama F, Sakata Y, Tsunada S, Fujimoto K. Apoptotic pathway in the rat small intestinal mucosa is different between fasting and ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2006;291:G110-G116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Schurr A, Reid KH, Tseng MT, Edmonds HL Jr, West CA, Rigor BM. Effect of electrical stimulation on the viability of the hippocampal slice preparation. Brain Res Bull. 1986;16:299-301. [PubMed] |
| 5. | Liang SH, Tao LD, Zhang PJ, Wang ML, Feng M, Liu XY, Zhang M. The effect of hypoxic preconditioning to rat liver function and organizational structure. Jiangsu Daxue Xuebao (Medical Sciences). 2007;17:220-221. [DOI] [Full Text] |
| 6. | Vannucci RC, Towfighi J, Vannucci SJ. Hypoxic preconditioning and hypoxic-ischemic brain damage in the immature rat: pathologic and metabolic correlates. J Neurochem. 1998;71:1215-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Zou C, Xu Q, Mao F, Li D, Bian C, Liu LZ, Jiang Y, Chen X, Qi Y, Zhang X. MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle. 2012;11:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878-15883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 735] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 9. | Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1470] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 10. | Nichols AC, Finkelstein DM, Faquin WC, Westra WH, Mroz EA, Kneuertz P, Begum S, Michaud WA, Busse PM, Clark JR. Bcl2 and human papilloma virus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clin Cancer Res. 2010;16:2138-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Iqbal J, Neppalli VT, Wright G, Dave BJ, Horsman DE, Rosenwald A, Lynch J, Hans CP, Weisenburger DD, Greiner TC. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med. 2000;28:1362-1369. [PubMed] |
| 13. | Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81:637-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 454] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Zhuonan Z, Sen G, Zhipeng J, Maoyou Z, Linglan Y, Gangping W, Cheng J, Zhongliang M, Tian J, Peijian Z. Hypoxia preconditioning induced HIF-1α promotes glucose metabolism and protects mitochondria in liver I/R injury. Clin Res Hepatol Gastroenterol. 2015;39:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Schreiber R, Faria D, Skryabin BV, Wanitchakool P, Rock JR, Kunzelmann K. Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflugers Arch. 2015;467:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Wei L, Chen WY, Hu T, Tang YX, Pan BB, Jin M, Kong GY. Effect and mechanism of propofol in hepatic ischemia/reperfusion injury of rat. Eur Rev Med Pharmacol Sci. 2017;21:3516-3522. [PubMed] |
| 17. | van der Heijden KM, van der Heijden IM, Galvao FH, Lopes CG, Costa SF, Abdala E, D’Albuquerque LA, Levin AS. Intestinal translocation of clinical isolates of vancomycin-resistant Enterococcus faecalis and ESBL-producing Escherichia coli in a rat model of bacterial colonization and liver ischemia/reperfusion injury. PLoS One. 2014;9:e108453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Zhuang Z, Lian P, Wu X, Shi B, Zhuang M, Zhou R, Zhao R, Zhao Z, Guo S, Ji Z. Abate Cytochrome C induced apoptosome to protect donor liver against ischemia reperfusion injury on rat liver transplantation model. Am J Transl Res. 2016;8:1738-1747. [PubMed] |
| 19. | Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 397] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331-342. [PubMed] |
| 21. | Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat Cell Biol. 2000;2:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 349] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Jin C, Zhang PJ, Wu XM, Zhou B, Li Y, Liu XY, Feng M, Tao LD. Impact of hypoxic preconditioning on apoptosis and its possible mechanism in orthotopic liver autotransplantation in rats. Hepatobiliary Pancreat Dis Int. 2009;8:40-45. [PubMed] |