Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.351
Peer-review started: September 8, 2017
First decision: September 20, 2017
Revised: November 27, 2017
Accepted: December 4, 2017
Article in press: December 4, 2017
Published online: January 21, 2018
Processing time: 133 Days and 4.3 Hours
To compare the capacity of newly developed epidermal growth factor receptor (EGFR)-targeted immune magnetic liposomes (EILs) vs epithelial cell adhesion molecule (EpCAM) immunomagnetic beads to capture colorectal circulating tumor cells (CTCs).
EILs were prepared using a two-step method, and the magnetic and surface characteristics were confirmed. The efficiency of capturing colorectal CTCs as well as the specificity were compared between EILs and EpCAM magnetic beads.
The obtained EILs had a lipid nanoparticle structure similar to cell membrane. Improved binding with cancer cells was seen in EILs compared with the method of coupling nano/microspheres with antibody. The binding increased as the contact time extended. Compared with EpCAM immunomagnetic beads, EILs captured more CTCs in peripheral blood from colorectal cancer patients. The captured cells showed consistency with clinical diagnosis and pathology. Mutation analysis showed same results between captured CTCs and cancer tissues.
EGFR antibody-coated magnetic liposomes show high efficiency and specificity in capturing colorectal CTCs.
Core tip: Epidermal growth factor receptor-targeted immune magnetic liposomes (EILs) were prepared by a two-step method. The binding with capturing tumor cells (CTCs) in peripheral blood from colorectal cancer patients was compared between EILs and epithelial cell adhesion molecule (EpCAM) immunomagnetic beads. We found that EILs captured more CTCs than EpCAM immunomagnetic beads with a higher efficiency and specificity.
- Citation: Kuai JH, Wang Q, Zhang AJ, Zhang JY, Chen ZF, Wu KK, Hu XZ. Epidermal growth factor receptor-targeted immune magnetic liposomes capture circulating colorectal tumor cells efficiently. World J Gastroenterol 2018; 24(3): 351-359
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.351
Metastasis contributes most to cancer-related deaths in patients with solid tumors. Mounting evidence suggests that circulating tumor cells (CTCs), which can shed from a primary tumor mass at the earliest stages of malignant progression, play a critical role in cancer metastasis[1-3]. As a “liquid biopsy” for tumor, CTCs provide an insight into tumor biology in a critical window where intervention could actually make a difference[4-7].
Detection and characterization of CTCs are challenging owing to their extreme scarcity in blood[8-11]. Several methods have been evolving, including immunomagnetic separation based on capture reagent-labeled magnetic beads, microfluidics-based technologies that enhance cell-surface contacts, and microfilter devices that isolate CTCs based on size difference[9,12-14]. However, the sensitivity of the aforementioned methods relies greatly on the degree of enrichment of CTCs and current techniques used are far from satisfactory[15-18].
Clinically, the CellSearch System, approved by the United States Food and Drug Administration recently, is deemed as the standard method[19,20]. Epithelial cell adhesion molecule (EpCAM) antibody is used to couple the magnetic beads in the system. However, EpCAM positive cells have been found in healthy persons[21]. Moreover, epithelial-mesenchymal transition (EMT), which is vital for metastasis, could result in loss of epithelial antigens in some CTCs[22-26].
Recently, a new method, in which an EGFR antibody-coupled magnetic liposome with a bilayer membrane structure was used, was developed[27,28]. Studies showed that this method had a significant improvement in cell-capture efficiency owing to its enhanced interactions between the deformable antibody receptor-lipid bilayer structure and nanoscale cellular surface components[29,30]. Such a high-affinity cell assay can be employed to recover cancer cells from spiked whole-blood samples in a stationary magnetic separation device[31,32]. On the basis of this stationary cell-capture assay, we hypothesized that further improvement of cell-capture efficiency can be achieved by increasing the surface activity sites of magnetic beads for tumor cells.
Chitosan (molecular weight, 5 × 104) was supplied by Yuhuan Aoxing Biochemistry (Zhejiang, China) with a deacetylation degree of above 99%. Octadecyl quaternized carboxymethyl chitosan (OQCMC), hydrophobic magnetic nanoparticles (BM), and hydrophilic magnetic nanoparticles (LM) were all prepared in our lab. All other chemicals were of reagent grade and were used as received. The study was approved by local institutional review board and informed consent was obtained from all recruited patients.
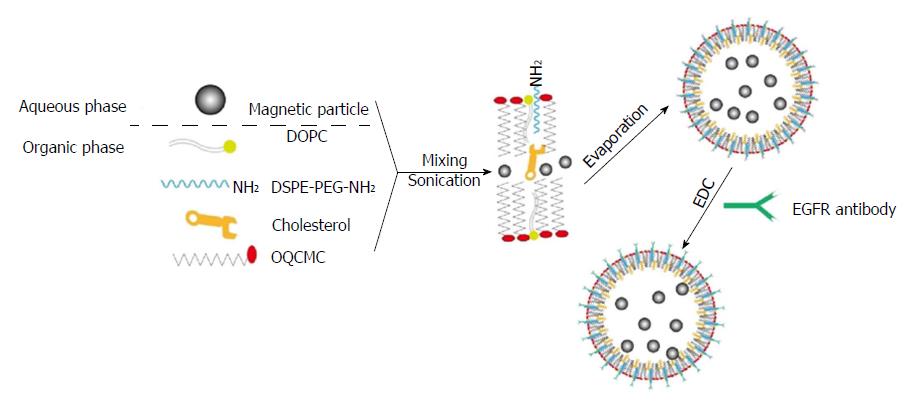
Modified DSPE-PEG-EGFR (1.0 mg) was weighed. After 3.0 mg of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 2.0 mg of OQCMC, and 6.0 mg of cholesterol (Chol) were dissolved in 5.0 mL of dichloromethane (CH2Cl2), bare magnetic nanobeads (3 mL) were added and incubated for 10 min, followed by the addition of a certain volume of phosphate buffer solution (PBS, pH = 7.4). The echo probe was used for phacoemulsification during ice bathing. After formation of a uniform emulsion, the organic phase was removed by rotary evaporation. After magnetic separation, PBS was used for washing for three times. At this stage, immune magnetic liposomes (IMLs) were obtained.
A certain quantity of IMLs was dispersed in PBS solution. After that, EDC was added, vortexed, and incubated for 3 h. After the unreacted coupling agent was removed, EGFR-targeted immune liposomes (EILs) were obtained.
The particle size and distribution were determined by quasielastic laser light scattering with a Malvern Zetasizer (Malvern Instruments, United Kingdom) at 25 °C. About 0.2 mL of EIL suspension were diluted in 2.5 mL water immediately after preparation. Each experiment was repeated three times. Zeta potential was measured using a Malvern Zetasizer (Malvern Instruments). Zeta limits ranged from -150 to 150 V. The magnetic properties of EILs were determined through using a vibrating samples magnetometer (LDJ9600-1, LDJ Electronics Inc., United States).
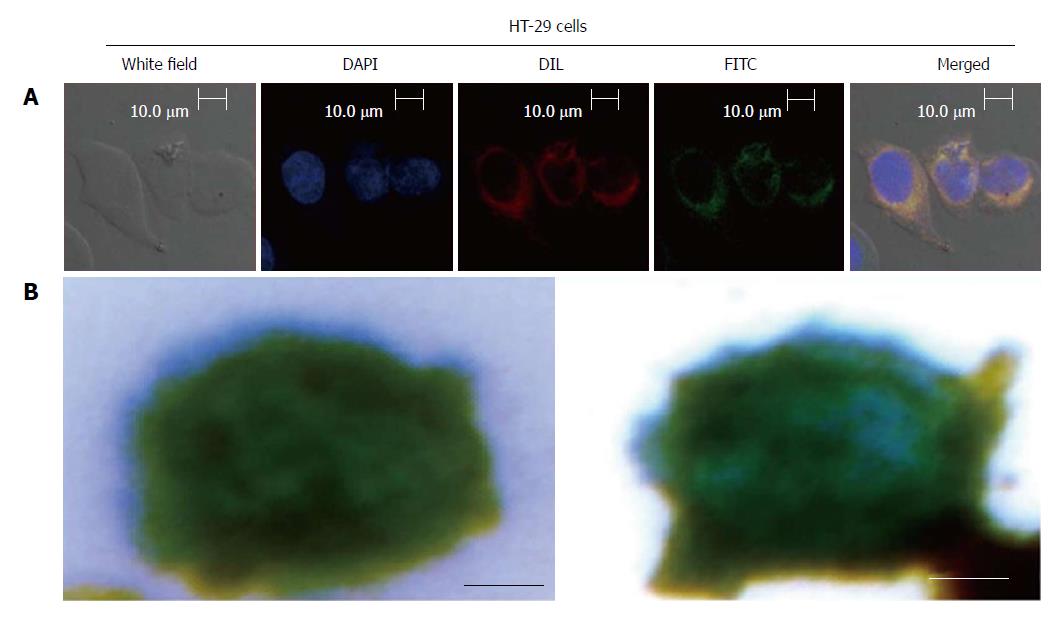
HT-29 cells (8 × 104) at logarithmic phase were plated on sterile round cover glasses in a 24-well plate with 0.5 mL of culture medium containing 10% serum and incubated at 37 °C with 5% CO2 overnight. When cell confluence reached > 70%, 20 μL of fluorescein (FITC)-labeled EILs were added. After culture for 30 min, the liquid was discarded and the pellet was washed with PBS three times before shaking for 2 min once. After that, 4% paraformaldehyde was added for cell fixation for 20 min, followed by washing with PBS three times again. Then, DAPI (4′,6-diamidino-2-phe-nylindole) dye liquor was added in each well and incubated for 10 min away from light to stain nuclei. After washing three times with PBS, the cell membrane probe Dil was added in each well and incubated for 10 min. After washing three times with PBS, the cover glasses were removed. The fluorescence decay sealing agents were adhered on glass slides. The distributions of the immune magnetic beads inside and outside the cell membrane were observed under a laser confocal microscope.
A three-color immunocytochemistry method was applied to identify and enumerate CTCs from non-specifically trapped white blood cells (WBCs). The markers included PE-labeled anti-CD45 (a marker for WBCs), FITC-labeled anti-CK19 (Cytokeratin, a protein marker for epithelial cells), and DAPI for nuclear staining. Fluorescence microscopy was employed to quantify DAPI intensity and expression levels of CK19 and CD45 in individual cells. The combined information was utilized to delineate CTCs (DAPI+/CK+/CD45-, cell size > 8 μm) from WBCs (DAPI+/CK-/CD45+, size < 15 μm) and cellular debris (DAPI-).
The CTC capture and identification methods were similar to those of CellSearch, but manually operated instead. Specifically, whole blood (7.5 mL) was collected and centrifuged at 1000 rpm/min for 10 min. After that, the upper-middle layer was transferred to a new tube and equal volume of PBS was added and mixed evenly. Then, 120 μL of EILs were added and incubated at room temperature for 30 min. The mixture was mixed once every 10 min and the tube was inserted into the magnetic separation frame to allow absorbing for 5 min. After that, the supernatant was removed and the tube was taken out. The captured CTCs were washed once with PBS, followed by staining with 30 μL of DAPI, 30 μL of CK19-FITC, and 10 μL of CD45-PE in a mixture for 15 min. Deionized water was then used to wash twice in the magnetic separation frame. Finally, 30 μL of deionized water was added into the tube for re-suspending the cells. The blending liquid was then evenly coated in the center of a slide. After the liquid drop was dried, the cells were photographed and counted under a fluorescent microscope.
The captured cells were put into a 10 μL PCR buffer with proteinase K and incubated at 60 °C overnight. After inactivating proteinase K at 95 °C for 10 min, agarose gel electrophoresis and sequencing were performed to determine mutations of the KRAS gene. Meanwhile, DNA extraction from peripheral blood of seven colorectal cancer patients was performed to analyze the KRAS mutations.
Statistical analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA, United States). An unpaired Student’s t-test was used to compare hydrodynamic size, diffuse efficient, and zeta potential between IMLs and EILs. A paired Student’s t-test was used to detect differences in the number of CTCs captured by EpCAM immunomagnetic beads in comparison with EILs. A P-value < 0.05 was considered statistically significant.
A two-step method was used to prepare EILs, which was achieved through encapsulating hydrophilic or hydrophobic magnetic nanoparticles into polymeric surfactant/cholesterol vesicles. Figure 1 illustrates the preparation procedure. The as-synthesized LM can be encapsulated into the aqueous core of polymeric liposomes. Furthermore, evaporation of organic solvent can transfer BM into the aqueous phase by an interfacial process driven by the hydrophobic van der Waals interactions between the primary alkane of the stabilizing ligand in oleic acid and the secondary alkane of the OQCMC. In addition, Chol can modulate membrane fluidity, elasticity, and permeability by stabilizing the polymeric liposome system.
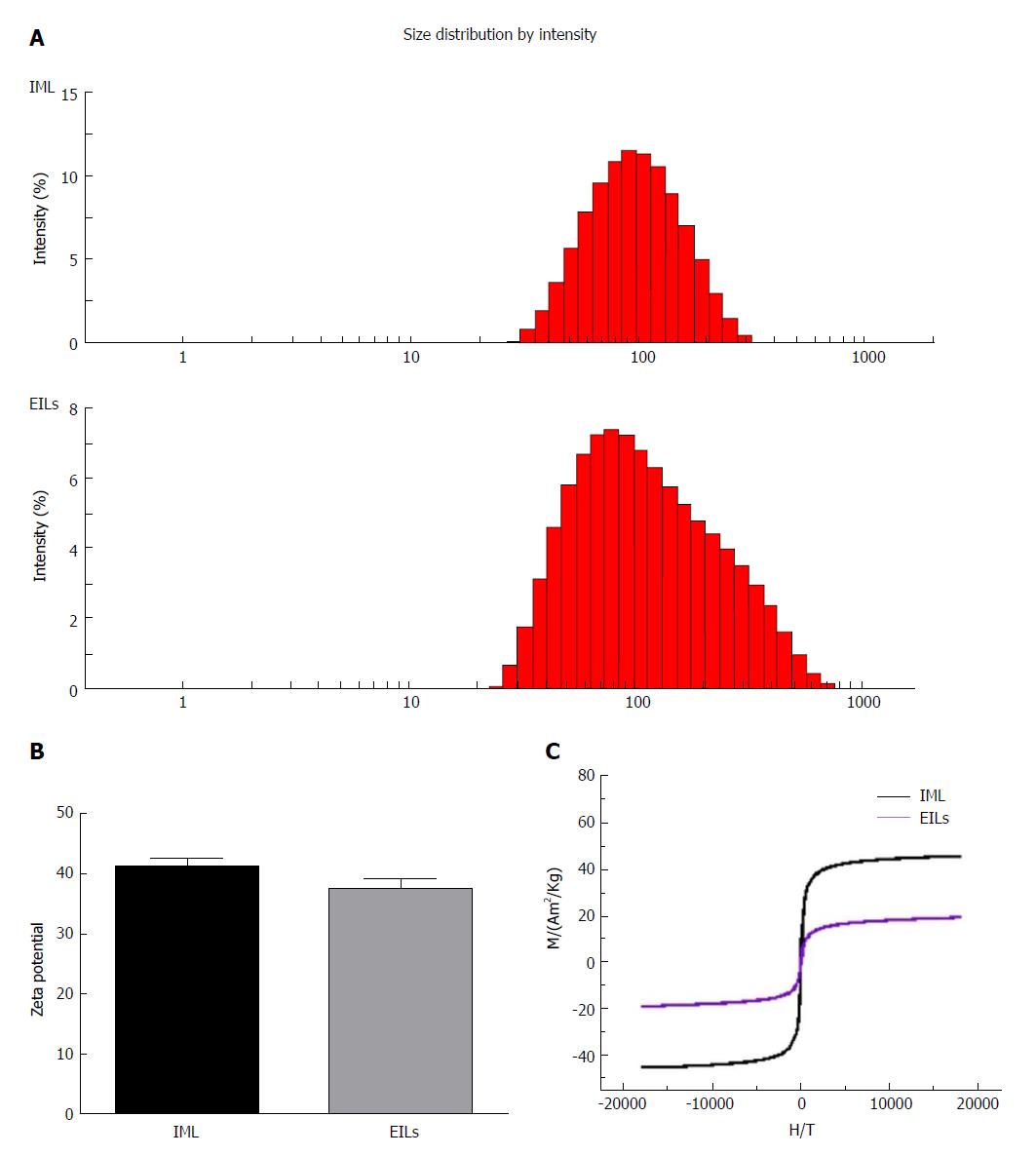
Figure 2A shows the hydrodynamic diameters of IMLs and EILs. The results indicated that the mean hydrodynamic size of IMLs was 83.1 ± 1.5 nm, whereas the diffusion coefficient was 1.3758E-14 m2/s. The mean hydrodynamic size of EILs was 104.5 ± 1.3 nm with a diffusion coefficient of 1.5870E-14 m2/s.
After encapsulating with OQCMC/Chol polymeric liposomes, the surface zeta potential of hydrophilic Fe3O4 nanoparticles could reach +41 mv. After reaction with EGFR antibody, the surface zeta potential of IMLs could reach +37 mv. There was no significant difference in zeta potential between them (Figure 2B).
Figure 2C shows the magnetization curves of IML and EILs at room temperature. Both samples showed a typical superparamagnetic behavior without any hysteresis loop. The saturation magnetization values of IMLs and EILs were 40 emu/g and 20 emu/g at 300 K, respectively. The volume fraction of magnetite can be further increased by increasing the number of layer of lipid coating.
As shown in Figure 3A, the captured CTCs were DAPI+/CK+/CD45-, with cell sizes larger than 8 μm, which are consistent with the CTC criterion. In Figure 3B, the magnetic bead was stained blue by Prussian blue.
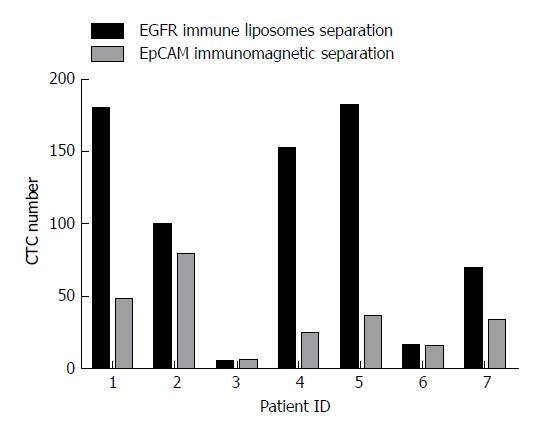
Blood samples from seven colorectal cancer patients without chemotherapy were collected for CTC capture analysis. For each patient, 7.5 mL of blood was divided equally for capturing CTCs by EpCAM magnetic beads and EILs. The number of CTCs captured by EpCAM magnetic beads was 15 to 79 and that by EILs was 5 to 181. A significant difference was found between the two methods. As shown in Figure 4, the numbers of CTCs captured by EILs in patients 1, 2, 4, 5, 6, and 7 were higher than those captured by EpCAM magnetic balls.
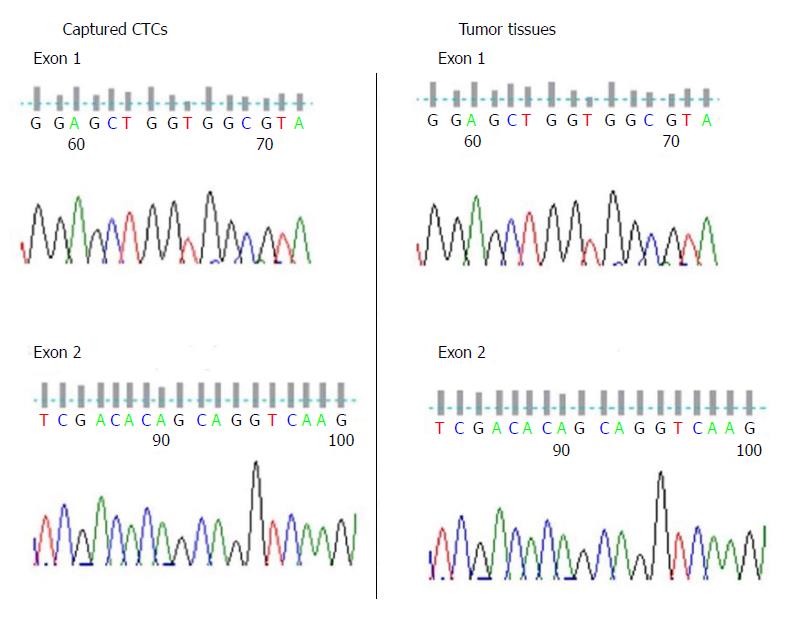
KRAS has been recognized as a marker for diagnosis and treatment of colorectal cancer. Mutations of KRAS in CTCs from the seven colorectal cancer patients were compared. Five of the seven DNA samples were successfully amplified and sequenced. We further amplified and sequenced their tumor tissue DNA, and found the results were coincident (Figure 5 and Table 1).
| Sample ID | Tumor type | Clinical stage | CTC DNA | Tissue DNA | ||
| KRAS Exon 1 | KRAS Exon 2 | KRAS Exon 1 | KRAS Exon 2 | |||
| 1 | CRC | IV | WT | WT | WT | WT |
| 2 | CRC | III | WT | WT | WT | WT |
| 3 | CRC | I | NAP | NAP | WT | WT |
| 4 | CRC | IV | WT | WT | WT | WT |
| 5 | CRC | IV | WT | WT | WT | WT |
| 6 | CRC | II | NAP | NAP | WT | WT |
| 7 | CRC | III | WT | WT | WT | WT |
In the current study, we developed new EGFR-targeted EILs for capturing colorectal CTCs. The EILs obtained showed similarity to cell membrane and could more efficiently capture colorectal CTCs compared with EpCAM immunomagnetic beads.
The higher efficiency of EILs compared to EpCAM immunomagnetic beads might be explained by the following facts. First, the obtained IMLs displayed a lipid nanoparticle structure similar to cell membrane, which can enhance contact with cancer cells[33-35]. Second, characteristics of the EILs were similar to those of IMLs (including mean hydrodynamic size, zeta potential, magnetization curves, and saturation magnetization value), which suggested that EILs could effectively bind CTC cells[30,32,36]. Third, expression of EpCAM on CTCs is dynamic[24,37]. Some cells might not express EpCAM and did not get captured using EpCAM immunomagnetic beads[22,38,39].
However, we should not ignore that in one patient, the number of CTCs captured by EILs was lower than that by EpCAM magnetic beads. This patient had stage I disease and the number of CTCs in the peripheral blood might be much fewer than those at advanced stages, which may be below the detection limit of EILs. Other factors such as operating mistakes might also be possible explanations. More studies with larger sample sizes are needed to validate the current findings.
The feasibility of capturing of CTCs by EILs was evaluated by mutation analysis, especially the KRAS gene. Five of the seven DNA samples were successfully amplified and sequenced. We found that mutations detected in CTCs were the same as those in tumor tissues. Considering that KRAS was reported to be a marker for diagnosis and predicting treatment outcomes of colorectal cancer[28,40-42], the current results suggested that detecting KRAS mutations in CTCs through EILs capture might be of practical use.
In 2005, Kullberg and colleagues first reported the use of magnetic liposomes modified by EGFR antibody for drug delivery to cancer cells[31]. Recently, Wang et al[43] found that magnetic liposomes modified by dual antibody (the nuclear protein Ki-67 and EGFR antibody) were potentially useful in helping treat tumor cells with proliferative characteristics. Our current study further confirmed the feasibility of EILs in capturing CTCs. These findings suggested that EGFR-targeted magnetic liposomes might be of more clinical significance in the future.
There were at least two limitations in this study. First, the number of patients included in our study was small. Second, all of the colorectal cancer patients included in the study were EGFR positive, which might cause a great bias to our results as a previous study reported that the sensitivity and specificity of EGFR were lower than those of EpCAM for colorectal cancer patients[44]. Liu et al[45] also reported that the positive expression rate of EGFR was only 64% (45/70). Future studies might include several specific molecular targets to improve efficiency[46]. For example, Myung et al[47] successfully enhanced tumor cell isolation by a biomimetic combination of E-selectin and anti-EpCAM. Besides, combining mechanical and molecular filtration seems to be another choice to better enrich CTCs[48-51].
In conclusion, we designed a new CTC-capture platform that combines a high-affinity cell enrichment assay based on cell capture agent (antibody)-coated nanostructured substrates and a cell membrane structure capable of improving CTC/substrate contact frequency. The synergistic effects led to better CTC capture performance in clinical blood samples compared with traditional EpCAM immunomagnetic beads. The significantly improved sensitivity of our new CTC capture technology might be useful in early detection of cancer metastasis and isolation of rare populations of cells.
Metastasis contributes most to cancer-related deaths in patients with solid tumors. Mounting evidence suggests that circulating tumor cells (CTCs) which can shed from a primary tumor mass at the earliest stages of malignant progression, play a critical role in cancer metastasis. Detection and characterization of CTCs are challenging owing to their extreme scarcity in blood. However, the sensitivity of the aforementioned methods relies greatly on the degree of enrichment of CTCs and current techniques used are far from satisfactory. Recently, a new method, in which an epidermal growth factor receptor (EGFR) antibody-coupled magnetic liposome with a bilayer membrane structure was used, was developed. Studies showed that this method had a significant improvement in cell-capture efficiency owing to its enhanced interactions between the deformable antibody receptor-lipid bilayer structure and nanoscale cellular surface components. Such a high-affinity cell assay can be employed to recover cancer cells from spiked whole-blood samples in a stationary magnetic separation device.
The main topics are to compare the capacity of newly developed EGFR-targeted immune magnetic liposomes (EILs) vs epithelial cell adhesion molecule (EpCAM) immunomagnetic beads to capture colorectal circulating tumor cells (CTCs).
The main objectives were to compare the capacity of newly developed EILs vs EpCAM immunomagnetic beads to CTCs. And the significantly improved sensitivity of our new CTC capture technology might be useful in early detection of cancer metastasis and isolation of rare populations of cells.
EILs were prepared using a two-step method, and the magnetic and surface characteristics were confirmed. The efficiency and specificity of EILs and EpCAM magnetic beads in capturing colorectal CTCs were compared. Statistical analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA, United States). An unpaired Student’s t-test was used to compare hydrodynamic size, diffuse efficient, and zeta potential between IMLs and EILs. A paired Student’s t-test was used to detect differences in the number of CTCs captured by EpCAM immunomagnetic beads in comparison with EILs. A P-value < 0.05 was considered statistically significant.
The obtained EILs have a lipid nanoparticle structure similar to cell membrane. Improved binding with cancer cells was seen in EILs compared with the method of coupling nano/microspheres with antibody. The binding increased as the contact time extended. Compared with EpCAM immunomagnetic beads, EILs captured more CTCs in peripheral blood from colorectal cancer patients. The captured cells showed consistency with clinical diagnosis and pathology. Mutation analysis showed same results between captured CTCs and cancer tissues.
Improved binding with cancer cells was seen in EILs compared with the method of coupling nano/microspheres with antibody. EILs were prepared using a two-step method and the magnetic and surface characteristics were confirmed. The efficiency and specificity in capturing colorectal CTCs were compared between EILs and EpCAM magnetic beads. EGFR-coated magnetic liposomes showed high efficiency and specificity in capturing colorectal CTCs. The captured cells showed consistency with clinical diagnosis and pathology. Mutation analysis showed same results between captured CTCs and cancer tissues. The significantly improved sensitivity of our new CTC capture technology might be useful in early detection of cancer metastasis and isolation of rare populations of cells.
The significantly improved sensitivity of our new CTC capture technology might be useful in early detection of cancer metastasis and isolation of rare populations of cells.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kane S, McCarthy M, Provenzale D S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2016;35:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 2. | Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2015;7:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 3. | Hardingham JE, Grover P, Winter M, Hewett PJ, Price TJ, Thierry B. Detection and Clinical Significance of Circulating Tumor Cells in Colorectal Cancer--20 Years of Progress. Mol Med. 2015;21 Suppl 1:S25-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Li N, Xiao T, Zhang Z, He R, Wen D, Cao Y, Zhang W, Chen Y. A 3D graphene oxide microchip and a Au-enwrapped silica nanocomposite-based supersandwich cytosensor toward capture and analysis of circulating tumor cells. Nanoscale. 2015;7:16354-16360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Rink M, Chun FK, Dahlem R, Soave A, Minner S, Hansen J, Stoupiec M, Coith C, Kluth LA, Ahyai SA. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol. 2012;61:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Rydén L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer. 2016;16:433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Gazzaniga P, Raimondi C, Nicolazzo C, Carletti R, di Gioia C, Gradilone A, Cortesi E. The rationale for liquid biopsy in colorectal cancer: a focus on circulating tumor cells. Expert Rev Mol Diagn. 2015;15:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Lee GW, Kim JY, Koh EH, Kang D, Choi DS, Maeng KY, Lee JS. Plasma human mammaglobin mRNA associated with poor outcome in patients with breast cancer. Genet Mol Res. 2012;11:4034-4042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Alix-Panabières C, Pantel K. Technologies for detection of circulating tumor cells: facts and vision. Lab Chip. 2014;14:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Chen JF, Zhu Y, Lu YT, Hodara E, Hou S, Agopian VG, Tomlinson JS, Posadas EM, Tseng HR. Clinical Applications of NanoVelcro Rare-Cell Assays for Detection and Characterization of Circulating Tumor Cells. Theranostics. 2016;6:1425-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Lin M, Chen JF, Lu YT, Zhang Y, Song J, Hou S, Ke Z, Tseng HR. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res. 2014;47:2941-2950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Lu Y, Liang H, Yu T, Xie J, Chen S, Dong H, Sinko PJ, Lian S, Xu J, Wang J. Isolation and characterization of living circulating tumor cells in patients by immunomagnetic negative enrichment coupled with flow cytometry. Cancer. 2015;121:3036-3045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Qian W, Zhang Y, Chen W. Capturing Cancer: Emerging Microfluidic Technologies for the Capture and Characterization of Circulating Tumor Cells. Small. 2015;11:3850-3872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Zheng S, Lin HK, Lu B, Williams A, Datar R, Cote RJ, Tai YC. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices. 2011;13:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 15. | Ankeny JS, Court CM, Hou S, Li Q, Song M, Wu D, Chen JF, Lee T, Lin M, Sho S. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016;114:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Wang FB, Yang XQ, Yang S, Wang BC, Feng MH, Tu JC. A higher number of circulating tumor cells (CTC) in peripheral blood indicates poor prognosis in prostate cancer patients--a meta-analysis. Asian Pac J Cancer Prev. 2011;12:2629-2635. [PubMed] |
| 17. | Barriere G, Riouallon A, Renaudie J, Tartary M, Rigaud M. Mesenchymal characterization: alternative to simple CTC detection in two clinical trials. Anticancer Res. 2012;32:3363-3369. [PubMed] |
| 18. | Karl A, Tritschler S, Hofmann S, Stief CG, Schindlbeck C. Perioperative search for circulating tumor cells in patients undergoing radical cystectomy for bladder cancer. Eur J Med Res. 2009;14:487-490. [PubMed] |
| 19. | Coumans F, Terstappen L. Detection and Characterization of Circulating Tumor Cells by the CellSearch Approach. Methods Mol Biol. 2015;1347:263-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Huang X, Gao P, Song Y, Sun J, Chen X, Zhao J, Xu H, Wang Z. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer. 2015;15:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Steinarsdóttir M, Jónasson JG, Vidarsson H, Júlíusdóttir H, Hauksdóttir H, Ogmundsdóttir HM. Cytogenetic changes in nonmalignant breast tissue. Genes Chromosomes Cancer. 2004;41:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 23. | Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014;25:1506-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Gires O, Stoecklein NH. Dynamic EpCAM expression on circulating and disseminating tumor cells: causes and consequences. Cell Mol Life Sci. 2014;71:4393-4402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JW, Gratama JW, Sleijfer S, Foekens JA. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101:61-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 26. | Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 379] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 27. | Limasale YD, Tezcaner A, Özen C, Keskin D, Banerjee S. Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. Int J Pharm. 2015;479:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Baas JM, Krens LL, Guchelaar HJ, Morreau H, Gelderblom H. Concordance of predictive markers for EGFR inhibitors in primary tumors and metastases in colorectal cancer: a review. Oncologist. 2011;16:1239-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Zhai J, Scoble JA, Li N, Lovrecz G, Waddington LJ, Tran N, Muir BW, Coia G, Kirby N, Drummond CJ. Epidermal growth factor receptor-targeted lipid nanoparticles retain self-assembled nanostructures and provide high specificity. Nanoscale. 2015;7:2905-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Nobuto H, Sugita T, Kubo T, Shimose S, Yasunaga Y, Murakami T, Ochi M. Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnet. Int J Cancer. 2004;109:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Kullberg M, Mann K, Owens JL. Improved drug delivery to cancer cells: a method using magnetoliposomes that target epidermal growth factor receptors. Med Hypotheses. 2005;64:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Kulshrestha P, Gogoi M, Bahadur D, Banerjee R. In vitro application of paclitaxel loaded magnetoliposomes for combined chemotherapy and hyperthermia. Colloids Surf B Biointerfaces. 2012;96:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Onuki Y, Obata Y, Kawano K, Sano H, Matsumoto R, Hayashi Y, Takayama K. Membrane Microdomain Structures of Liposomes and Their Contribution to the Cellular Uptake Efficiency into HeLa Cells. Mol Pharm. 2016;13:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Liang X, Shi B, Wang K, Fan M, Jiao D, Ao J, Song N, Wang C, Gu J, Li Z. Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials. 2016;82:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Fleischer CC, Payne CK. Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes. Acc Chem Res. 2014;47:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 398] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 36. | Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283-318. [PubMed] |
| 37. | Driemel C, Kremling H, Schumacher S, Will D, Wolters J, Lindenlauf N, Mack B, Baldus SA, Hoya V, Pietsch JM. Context-dependent adaption of EpCAM expression in early systemic esophageal cancer. Oncogene. 2014;33:4904-4915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Shigdar S, Qian C, Lv L, Pu C, Li Y, Li L, Marappan M, Lin J, Wang L, Duan W. The use of sensitive chemical antibodies for diagnosis: detection of low levels of EpCAM in breast cancer. PLoS One. 2013;8:e57613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Yoshida GJ, Saya H. EpCAM expression in the prostate cancer makes the difference in the response to growth factors. Biochem Biophys Res Commun. 2014;443:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Alves S, Castro L, Fernandes MS, Francisco R, Castro P, Priault M, Chaves SR, Moyer MP, Oliveira C, Seruca R. Colorectal cancer-related mutant KRAS alleles function as positive regulators of autophagy. Oncotarget. 2015;6:30787-30802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Chang YY, Lin JK, Lin TC, Chen WS, Jeng KJ, Yang SH, Wang HS, Lan YT, Lin CC, Liang WY. Impact of KRAS mutation on outcome of patients with metastatic colorectal cancer. Hepatogastroenterology. 2014;61:1946-1953. [PubMed] |
| 42. | Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol. 2014;53:852-864. [PubMed] |
| 43. | Wang S, Hüttmann G, Scholzen T, Zhang Z, Vogel A, Hasan T, Rahmanzadeh R. A light-controlled switch after dual targeting of proliferating tumor cells via the membrane receptor EGFR and the nuclear protein Ki-67. Sci Rep. 2016;6:27032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Mourtzikou A, Stamouli M, Kroupis C, Christodoulou S, Skondra M, Kastania A, Pectasides D, Athanasas G, Dimas C. Evaluation of carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule EpCAM (GA733-2), and carbohydrate antigen 19-9 (CA 19-9) levels in colorectal cancer patients and correlation with clinicopathological characteristics. Clin Lab. 2012;58:441-448. [PubMed] |
| 45. | Liu J, Zhou Q, Xu J, Wang J, Zhang Y. Detection of EGFR expression in patients with colorectal cancer and the therapeutic effect of cetuximab. J BUON. 2016;21:95-100. [PubMed] |
| 46. | Gorges TM, Stein A, Quidde J, Hauch S, Röck K, Riethdorf S, Joosse SA, Pantel K. Improved Detection of Circulating Tumor Cells in Metastatic Colorectal Cancer by the Combination of the CellSearch® System and the AdnaTest®. PLoS One. 2016;11:e0155126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Myung JH, Launiere CA, Eddington DT, Hong S. Enhanced tumor cell isolation by a biomimetic combination of E-selectin and anti-EpCAM: implications for the effective separation of circulating tumor cells (CTCs). Langmuir. 2010;26:8589-8596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Meunier A, Hernández-Castro JA, Turner K, Li K, Veres T, Juncker D. Combination of Mechanical and Molecular Filtration for Enhanced Enrichment of Circulating Tumor Cells. Anal Chem. 2016;88:8510-8517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Bhana S, Wang Y, Huang X. Nanotechnology for enrichment and detection of circulating tumor cells. Nanomedicine (Lond). 2015;10:1973-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Hanssen A, Wagner J, Gorges TM, Taenzer A, Uzunoglu FG, Driemel C, Stoecklein NH, Knoefel WT, Angenendt S, Hauch S. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci Rep. 2016;6:28010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 51. | Tulley S, Zhao Q, Dong H, Pearl ML, Chen WT. Vita-Assay™ Method of Enrichment and Identification of Circulating Cancer Cells/Circulating Tumor Cells (CTCs). Methods Mol Biol. 2016;1406:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |