Published online Jul 28, 2018. doi: 10.3748/wjg.v24.i28.3192
Peer-review started: April 19, 2018
First decision: May 30, 2018
Revised: June 17, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: July 28, 2018
Processing time: 99 Days and 20.6 Hours
Stent migration, which causes issues in stent therapy for esophageal perforations, can counteract the therapeutic effects and lead to complications. Therefore, techniques to regulate stent migration are important and lead to effective stent therapy. Here, in these cases, we placed a removable fully covered self-expandable metallic stent (FSEMS) in a 52-year-old man with suture failure after surgery to treat Boerhaave syndrome, and in a 53-year-old man with a perforation in the lower esophagus due to acute esophageal necrosis. At the same time, we nasally inserted a Sengstaken-Blakemore tube (SBT), passing it through the stent lumen. By inflating a gastric balloon, the lower end of the stent was supported. When the stent migration was confirmed, the gastric balloon was lifted slightly toward the oral side to correct the stent migration. In this manner, the therapy was completed for these two patients. Using a FSEMS and SBT is a therapeutic method for correcting stent migration and regulating the complete migration of the stent into the stomach without the patient undergoing endoscopic rearrangement of the stent. It was effective for positioning a stent crossing the esophagogastric junction.
Core tip: Here, we report two cases of stent migrations that were managed using Sengstaken-Blakemore tubes, which prevented complete migration to the stomach. The correction of the stent position was easy, and did not require endoscopic guidance. By regulating the stents, nutrition management became possible through a nasoenteric feeding tube. Both patients were discharged after oral ingestion became possible.
- Citation: Sato H, Ishida K, Sasaki S, Kojika M, Endo S, Inoue Y, Sasaki A. Regulating migration of esophageal stents - management using a Sengstaken-Blakemore tube: A case report and review of literature. World J Gastroenterol 2018; 24(28): 3192-3197
- URL: https://www.wjgnet.com/1007-9327/full/v24/i28/3192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i28.3192
The efficacy of stent placement as a nonsurgical treatment for perforation and suture failures in benign and malignant esophageal diseases has been shown[1,2]. However, a major problem with stent placement is stent migration. Stents that cross the esophagogastric junction (EGJ) are 7.5 times more likely to migrate than stents that do not cross the EGJ[3]. Migration can cause complications and requires repositioning with endoscopic guidance, which are disadvantageous to the therapeutic course. Endoscopic sutures[4] and clipping[5] can reduce migration, but stent migration is difficult to prevent completely. The challenges in achieving the efficacy and therapeutic effects of a stent include sustainable placement at the perforated site and the prevention of stent migration to the stomach. We used a Sengstaken-Blakemore tube (SBT) to position a fully covered self-expandable metallic stent (FSEMS) crossing the EGJ for perforation of the distal esophagus, which enabled correction of stent migration without endoscopic rearrangement. This treatment proved effective for regulating complete migration of the stent into the stomach, so we are reporting our findings.
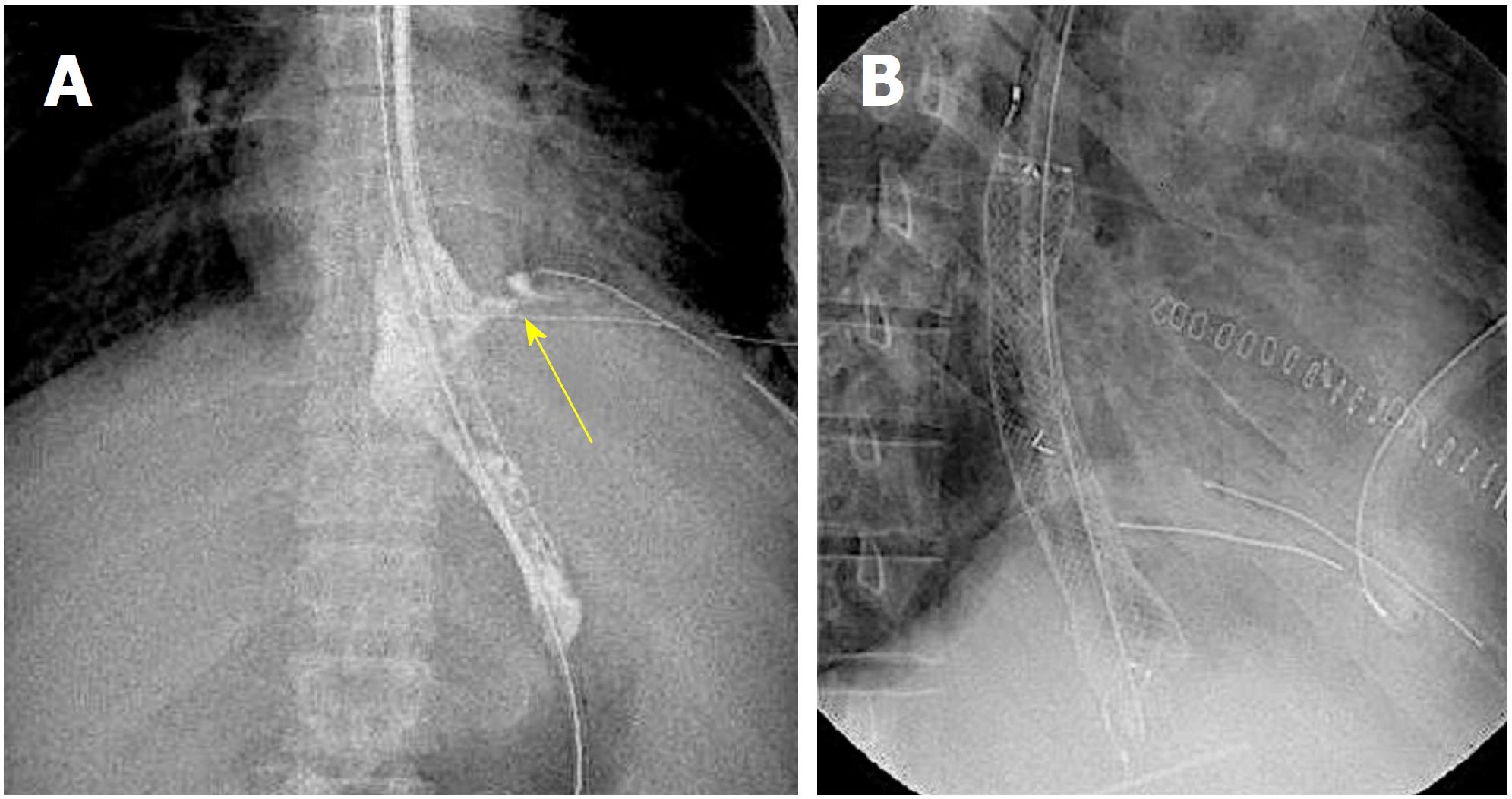
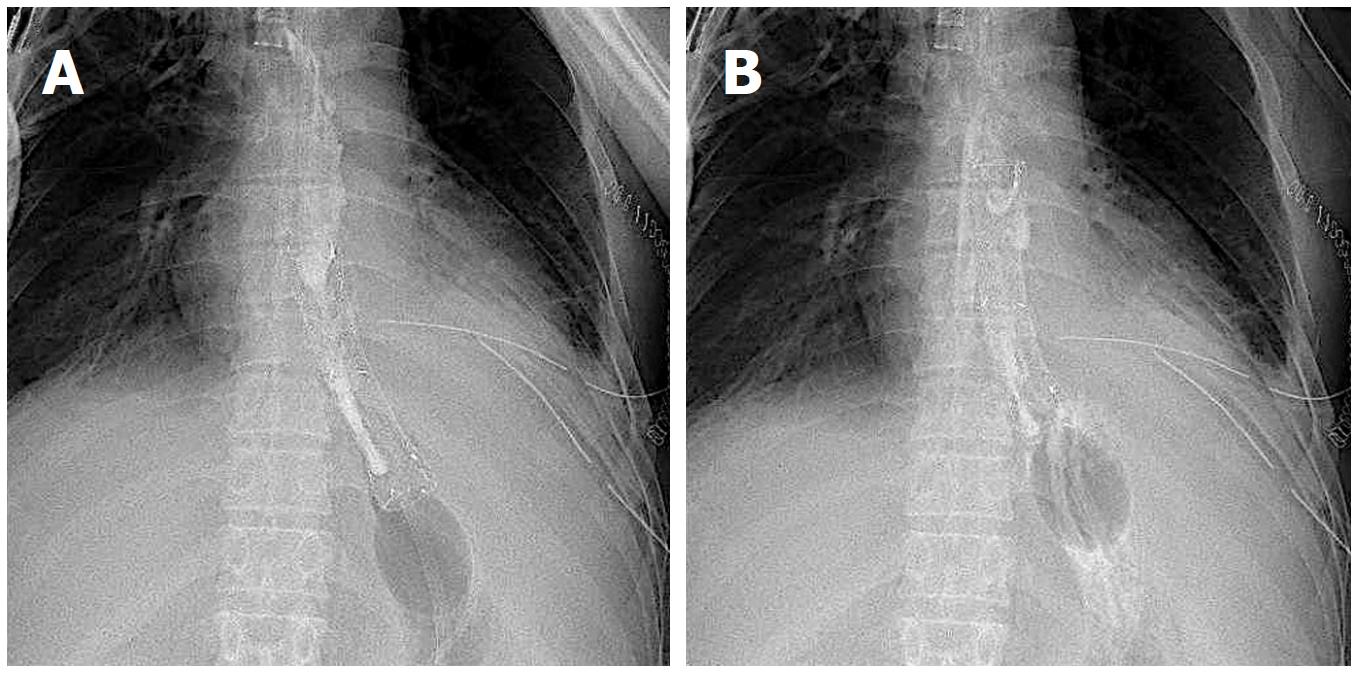
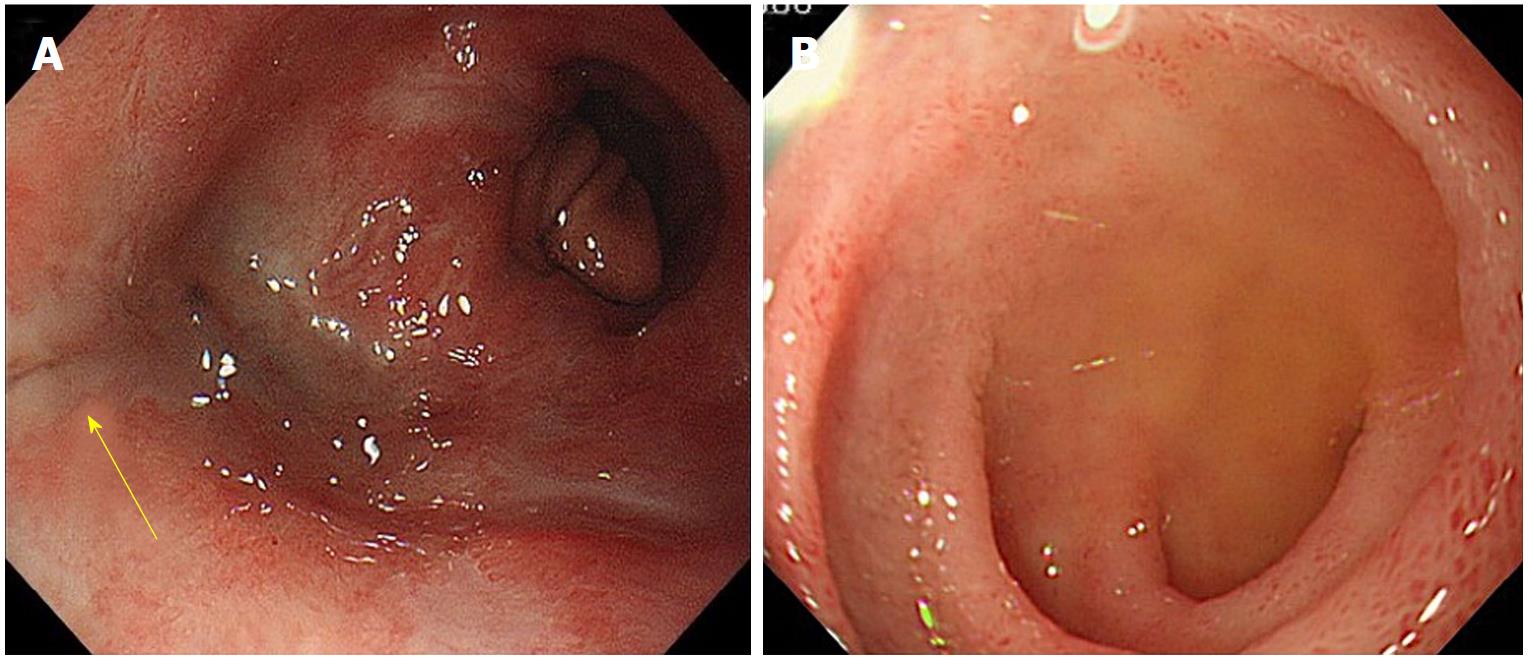
A 52-year-old man with a history of alcoholic liver disease and gastric ulcer presented with complaints of chest pain after vomiting. Esophagogastroduodenoscopy (EGD) confirmed a rupture in the left wall of the lower esophagus, which led to a diagnosis of Boerhaave syndrome. As the focus was confined within the mediastinum, nonsurgical management through fasting was selected. Two days into his hospitalization, leakage to the thoracic cavity due to a mediastinal perforation and changes in the respiratory and circulatory dynamics were confirmed; thus, surgery was selected. The perforated site was sutured, and a drain was placed via a left thoracotomy. Postoperatively, he exhibited fever with a body temperature of 40 °C or higher and respiratory condition exacerbation, which was confirmed to have led to suture failure, as observed on the contrast imaging (Figure 1A). Therefore, a FSEMS (Flexella-J; ELLA-CS., Ltd. Hradec Kralove, Czech Republic) of 110 mm in length and 23 mm in diameter and SBT (Sumitomo Bakelite Co., Ltd.) were inserted (Figure 1B). At the same time, a nasoenteric feeding tube was passed through the stent lumen and placed in the jejunum (Figure 2A). The bottom of the stent was supported by the SBT gastric balloon that was positioned in the stomach and inflated slightly larger than the stent diameter to prevent migration into the stomach (Figure 2B). During the course of this treatment, the stent migration was confirmed. While supporting the bottom (anal side) of the stent by inflating the SBT gastric balloon, the stent was lifted toward the oral side and repositioned (Figure 3A and B). After the stent placement, the fever began to decrease, along with decreasing discharge from the drain. The stent was removed 66 d after placement.
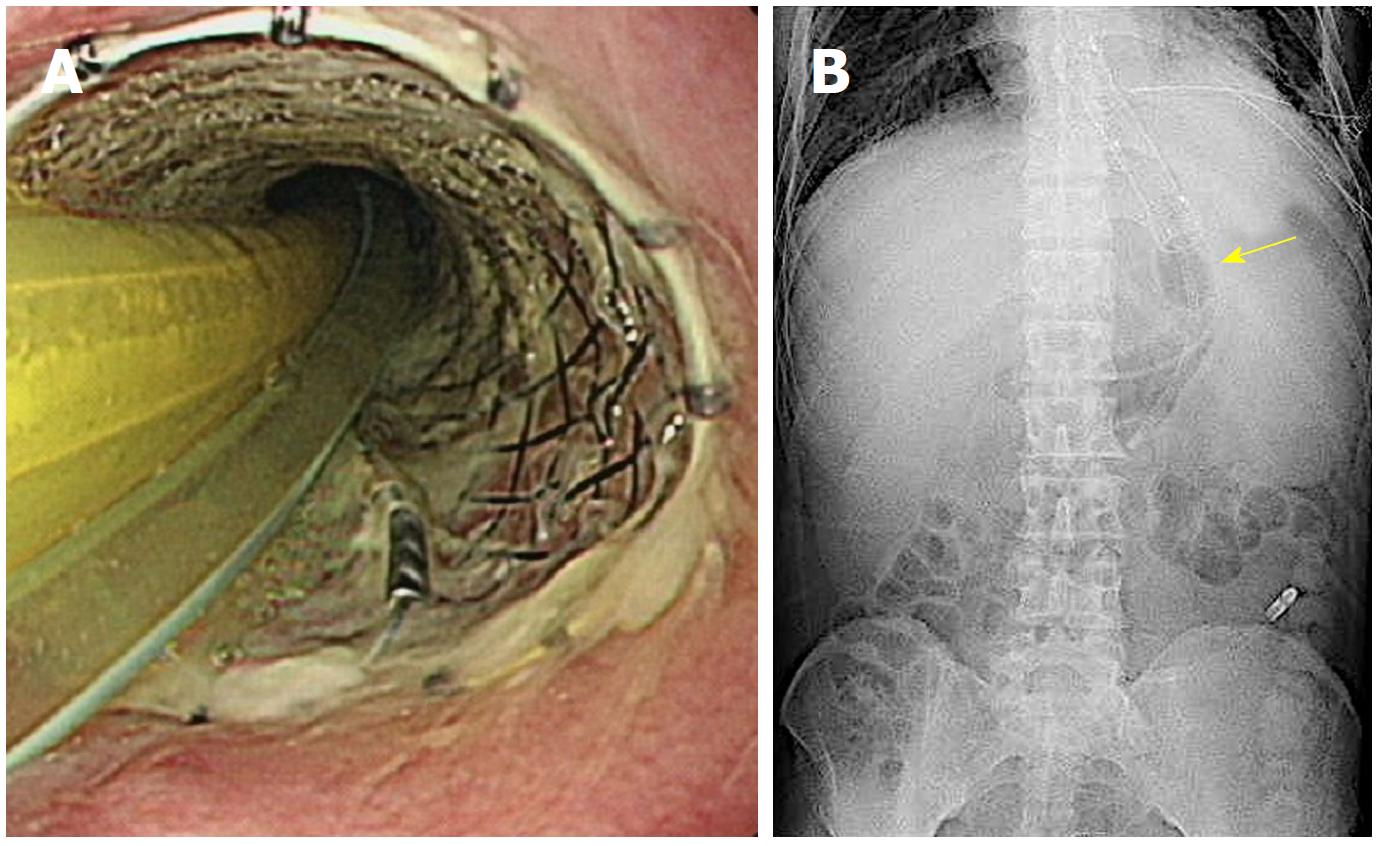
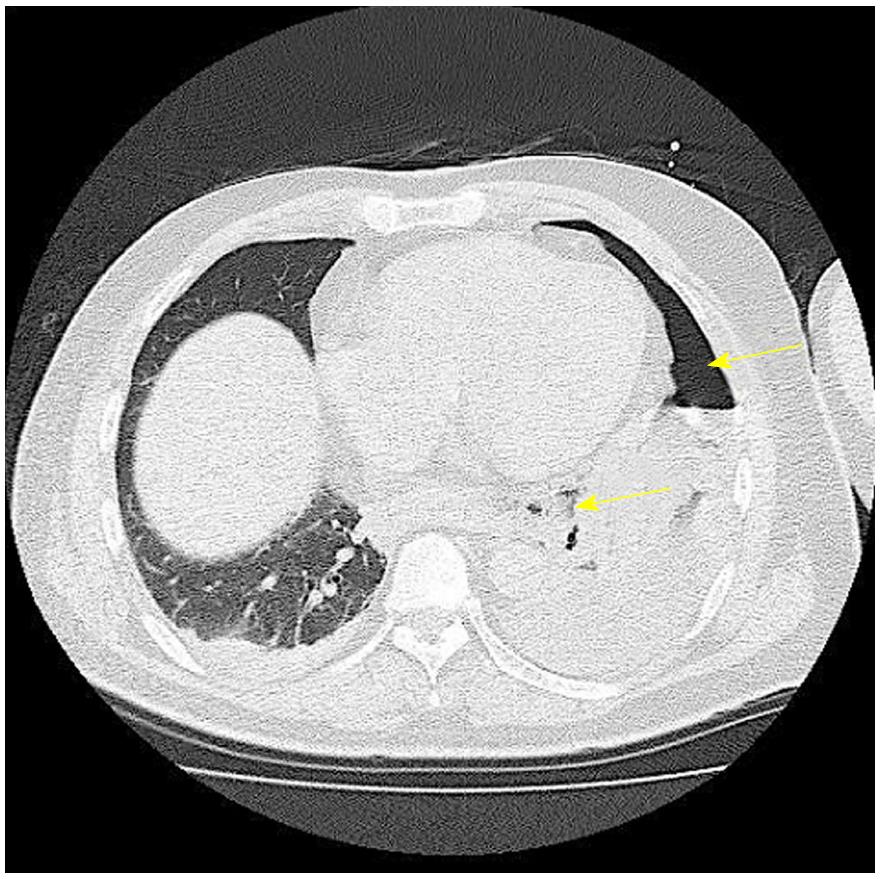
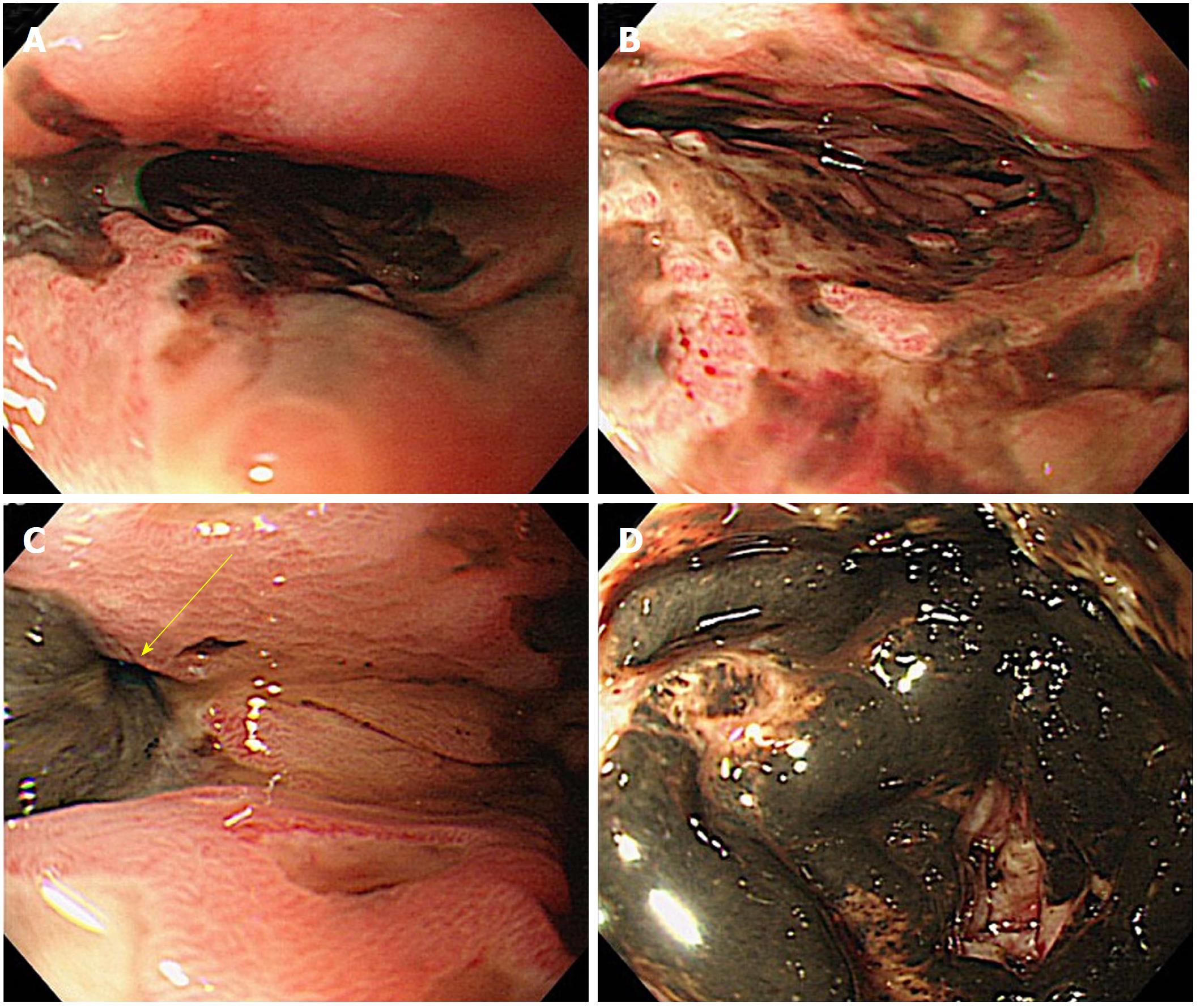
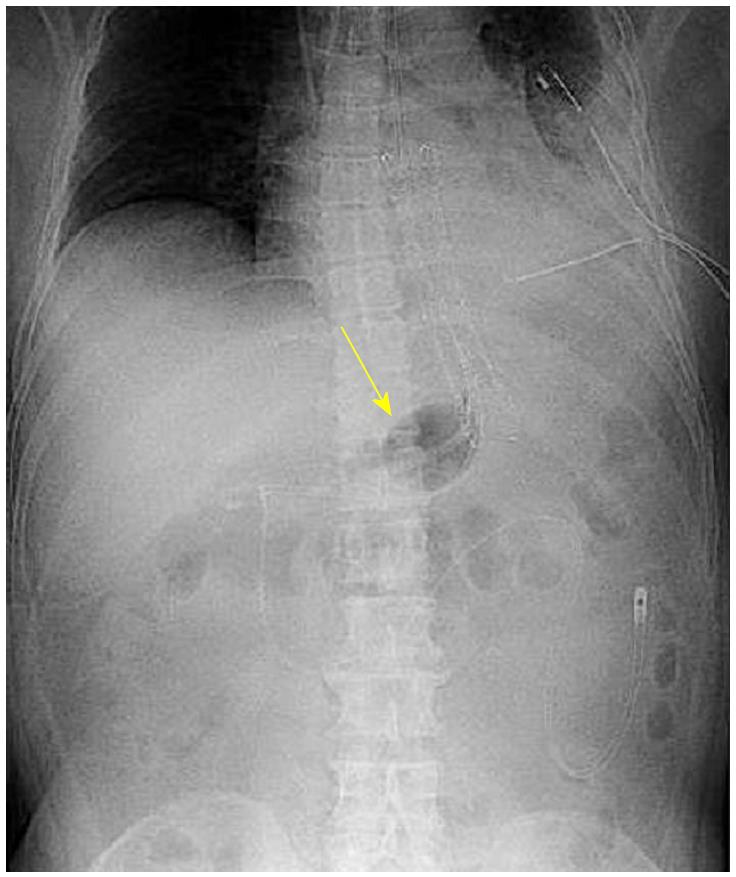
A 53-year-old man sought medical care in another hospital because of shock, with a systolic blood pressure of 7.9 kPa (60 mmHg). The patient could not accurately specify the site of his pain, and he was managed in the hospital’s cardiovascular unit. The patient was transferred to our cardiovascular unit because the cause of shock remained unknown, even after he received a vasoactive agonist. A chest computed tomography (CT) scan revealed mediastinal emphysema and a left pneumothorax (Figure 4). The EGD confirmed black discoloration from the esophagus to the gastric junction (Figure 5A and B) and the site of the esophageal perforation (Figure 5C). In addition, the duodenum showed black discoloration (Figure 5D). His blood test result showed a creatinine level of 3.83 mg/dL. Anuria continued from his previous hospitalization, and an acute kidney injury was confirmed. With the administration of 80% oxygen, the partial of arterial oxygen/fraction of inspired oxygen ratio was 72 mmHg, and his respiratory and circulatory dynamics were unstable. A thoracic cavity drain was placed, and a FSEMS (Hanaro stent; M.I. Tech Co.Ltd) of 150 mm in length and 24 mm in diameter and SBT were inserted to treat the esophageal perforation. At the same time, respiratory management using a high-flow nasal cannula, and circulatory management through fluid resuscitation were started. A nasoenteric feeding tube was inserted through the stent lumen, and an inflated SBT gastric balloon was placed to regulate the stent migration (Figure 6). Perforation closure (Figure 7A) and an improvement in the ischemic change in the duodenum (Figure 7B) were confirmed 38 d after the stent placement, and the stent was removed.
The incidence of esophageal perforation is rare, and the treatment experience at each facility is limited[6]. In recent years, the reports that support the usefulness of stent therapy for esophageal perforation and the reports on stent fixation have accumulated[7-10]. Stent therapy is also recommended by a set of guidelines[11]. For effective stent placement, continuously covering the leakage from the perforated or suture site is a prerequisite. Stent migration is a serious issue that could counteract therapeutic effects and cause complications.
The incidence of stent migration is 18% to 40%[1,2,12,13]. However, stent migration in an esophageal perforation often involves malignant diseases, postoperative suture failure, Boerhaave syndrome, and iatrogenic causes, and it is difficult to evaluate the treatment for a specific cause or disease. In cases of benign diseases, the lack of stenosis to support a stent is the cause of stent migration, but when using a stent for hemostasis in esophageal variceal bleeding, migration has been confirmed in 21.6% of the cases[14]. As a therapeutic strategy against such stent migration, the effectiveness of a clip fixation[5] or migration prevention with silk threads has been shown[15]. However, stent migration is confirmed in 15.9% of the cases of SEMS fixed with endoscopic sutures[10] and in 13% of the cases in clip stent fixations[5]. When a stent migration is confirmed, the stent is often adjusted or repositioned endoscopically[1,8,12,13]; thus, removal or repositioning is required to the maximum extent allowed. Unfortunately, repetitive stent placements reduces the cost effectiveness[5]. Furthermore, stent migration away from the esophagus could lead to extraction due to migration to the stomach, intestinal obstruction due to migration to the small intestine[16], and injury to the spleen due to migration to the abdominal cavity[17].
The stents used in the present cases were FSEMS, but an FSEMS migrates more easily than a partially covered SEMS[6,8,11]. As the whole stent is covered, it easily slides against the esophageal mucosa. In addition, when readjusting the position of the stent with an SBT[18], we found that its slippery nature was an advantage. The initial insertion of the stent and SBT was performed with fluoroscopy, after administering a sedative. During hospitalization, the patients maintained normal levels of consciousness. Passing a SBT through the stent was simple, and no migration occurred through contact with the stent.
After inserting the SBT, a gastric balloon was inflated slightly larger than the diameter of the stent at the bottom to support and fix the stent in position. If the balloon is not inflated enough, it cannot retain the stent, but if it is inflated too much, it tends to be affected by gastric peristalsis. Thus, it is essential to check under fluoroscopy the amount of inflation that can retain the stent, which is slightly larger than the caliber of the bottom end of the stent, and fix the stent in place. SBT fixation was implemented by fixing with a nasal tape only, without traction. The gastric aspiration and esophageal openings decompressed the esophagus and stomach, and the stent migration was corrected by slowly pulling the SBT toward the oral side while keeping the gastric balloon inflated. The patient did experience nasal discomfort but had no chest or abdominal pain. The stent migration was confirmed with a routine morning radiographic examination. Stent migration due to gastrointestinal peristalsis was found in the present treatment in which the stent movement was regulated by the SBT balloon. However, the migration from the leakage site was not complete because it was within the area where the stent therapy was still effective. When the migration was confirmed, complete migration to the stomach was prevented, and the optimum repair site for the leakage could be identified without using endoscopy. Overall, controlling a stent with an SBT is more cost-effective than repositioning with an endoscope. It should be noted that gastric balloons occasionally collapse naturally; thus, stent positions and gastric balloons must be checked under radiography. Before the treatment, we offered an explanation that we would be using a SBT, which is intended for hemostasis in esophageal variceal rupture. This is to regulate stent migration, however this incurs risks such as bleeding during repositioning and tissue damage, such as that caused by exacerbation of perforation. Treatment began after obtaining informed consent.
Nutrient pathways for patients with esophageal perforations, such as gastrostomy, jejunostomy, and non-oral feeding, are available[10]. In the present cases, the nutrients were administered by inserting a tube through the nose and positioning it in the jejunum. By passing the nasoenteric feeding tube through the stent lumen, the leakage site and nasoenteric feeding tube did not come in contact with each other; thus, there were no disadvantages. The balloon was not inflated immediately after insertion of the SBT, but rather after insertion of the nasoenteric feeding tube. No obstruction of the feeding tube due to compression by the balloon and stent, and no migration of the feeding tube due to the stent adjustment were observed.
The acute esophageal necrosis in Case 2 was a rare syndrome characterized by a diffuse black appearance around the circumference of the esophageal mucosa[19-22], which tends to develop in the distal third of the esophagus. Acute esophageal necrosis has been suggested to be caused by relatively low vascularity[23], and a duodenal lesion complication could develop at the same time. This compromises blood vessels diverging from the axis of the abdominal cavity, which provide the common blood supply to the distal part of the esophagus and duodenum[21,23,24]. In the present cases, although duodenal lesions were found, no complications such as bleeding caused by the nasoenteric feeding tube were observed. During the treatment with insertion of a SBT and nasoenteric feeding tube, no complication occurred other than an ulcer at the fixation site on the nose. The patient was discharged after oral ingestion was possible without transvenous or enteral nutrition, and without disturbance in the activities of daily living.
This case report has problems. First, with Case 1, if surgery and drainage had been selected as the initial treatment at admission, stent therapy may have been avoided. Second, closure of the postoperative suture leakage with clips may have been more effective than this treatment. Third, management using a stent and SBT may not be effective for proximal and central esophageal perforations. Lastly, this method does not completely prevent stent migration.
We herein provided SBT treatment for stent migration, which is the biggest problem in stent therapy. This treatment is effective for positioning stents that cross the EGJ in distal esophageal perforation. Early treatment is important for esophageal perforations, and stent therapy is not the only influence on prognosis. A multilateral treatment approach, including the appropriate management of breathing, circulation, infection, nutrition, drainage of the mediastinum, and thoracic cavity control, is necessary.
Using a fully covered self-expandable metallic stent (FSEMS) and Sengstaken-Blakemore tube (SBT) is a therapeutic method for correcting stent migration and regulating the complete migration of the stent into the stomach without the patient undergoing endoscopic rearrangement of the stent. It was effective for positioning a stent crossing the esophagogastric junction (EGJ).
The diagnoses were postoperative suture failure and perforation due to acute esophageal necrosis.
When Case 2 was hospitalized, treatment was started under the suspicion of cardiovascular disease.
In terms of the data before starting stent therapy, Case 1 had a white blood count of 7260 μL, CRP level of 11.8 mg/dL, and procalcitonin level of 1.5 ng/mL. Pseudomonas species were detected from the drain. Case 2 had a white blood cell count of 9850 μL, CRP level of 43.3 mg/dL, and procalcitonin level of 7.7 ng/mL. Klebsiella species were detected in the drain fluid sample.
Case 1 had contrast leakage from the suture site. Case 2 had a pneumothorax found on a computed tomography scan. An esophagogastroduodenoscopy revealed a blackened mucosal lesion, and perforations were found from the central to the lower part of the esophagus.
No pathological examination was performed.
A FSEMS was used for the stent therapy, and stent migration was controlled using a SBT.
No reports have described stent management using a SBT for esophageal perforation.
SBTs are generally used to control bleeding from esophageal varices. Acute esophageal necrosis is a rare syndrome characterized by esophageal mucosa with a diffuse blackened appearance, and tends to occur in the distal one-third of the esophagus.
This treatment is effective for positioning stents that cross the EGJ in distal esophageal perforation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
P- Reviewer: Aurello P, Guo YM, Osawa S, Paduani G, Tseng PH S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Yin SY
| 1. | van Heel NC, Haringsma J, Spaander MC, Bruno MJ, Kuipers EJ. Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol. 2010;105:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Gonzalez JM, Servajean C, Aider B, Gasmi M, D’Journo XB, Leone M, Grimaud JC, Barthet M. Efficacy of the endoscopic management of postoperative fistulas of leakages after esophageal surgery for cancer: a retrospective series. Surg Endosc. 2016;30:4895-4903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol. 2009;104:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Kantsevoy SV, Bitner M. Esophageal stent fixation with endoscopic suturing device (with video). Gastrointest Endosc. 2012;76:1251-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Vanbiervliet G, Filippi J, Karimdjee BS, Venissac N, Iannelli A, Rahili A, Benizri E, Pop D, Staccini P, Tran A. The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc. 2012;26:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Biancari F, D’Andrea V, Paone R, Di Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg. 2013;37:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Sharma P, Kozarek R; Practice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105:258-273; quiz 274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Law R, Prabhu A, Fujii-Lau L, Shannon C, Singh S. Stent migration following endoscopic suture fixation of esophageal self-expandable metal stents: a systematic review and meta-analysis. Surg Endosc. 2018;32:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Spaander MC, Baron TH, Siersema PD, Fuccio L, Schumacher B, Escorsell À, Garcia-Pagán JC, Dumonceau JM, Conio M, de Ceglie A, Skowronek J, Nordsmark M, Seufferlein T, Van Gossum A, Hassan C, Repici A, Bruno MJ. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 12. | Aryaie AH, Singer JL, Fayezizadeh M, Lash J, Marks JM. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg Endosc. 2017;31:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation. Ann Thorac Surg. 2007;83:2003-7; discussion 2007-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Shao XD, Qi XS, Guo XZ. Esophageal Stent for Refractory Variceal Bleeding: A Systemic Review and Meta-Analysis. Biomed Res Int. 2016;2016:4054513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Shim CS, Cho YD, Moon JH, Kim JO, Cho JY, Kim YS, Lee JS, Lee MS. Fixation of a modified covered esophageal stent: its clinical usefulness for preventing stent migration. Endoscopy. 2001;33:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Sabharwal T, Gulati MS, Fotiadis N, Dourado R, Botha A, Mason R, Adam A. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: proton pump inhibitor combination for prevention of post-stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. J Gastroenterol Hepatol. 2008;23:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Khan S, George N, Tharian B. Extraluminal migration of an esophageal metal stent causing splenic injury. Endoscopy. 2016;48:E326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Sengstaken rw, Blakemore AH. Balloon tamponage for the control of hemorrhage from esophageal varices. Ann Surg. 1950;131:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Gurvits GE, Cherian K, Shami MN, Korabathina R, El-Nader EM, Rayapudi K, Gandolfo FJ, Alshumrany M, Patel H, Chowdhury DN. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (2)] |
| 24. | Del Hierro PM. Acute Necrotizing Esophagitis Followed by Duodenal Necrosis. Gastroenterology Res. 2011;4:286-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |