Published online Jul 28, 2018. doi: 10.3748/wjg.v24.i28.3130
Peer-review started: April 9, 2018
First decision: May 9, 2018
Revised: May 22, 2018
Accepted: June 25, 2018
Article in press: June 25, 2018
Published online: July 28, 2018
Processing time: 109 Days and 5.4 Hours
To investigate the effect and mechanism of moxibustion in rats with ulcerative colitis.
A rat colitis model was established by administering 4% dextran sulphate sodium solution. Seventy male rats were randomly divided into seven groups: Healthy controls (HC), ulcerative colitis model group (UC), UC with 7 d of moxibustion (UC-7), UC with 14 d of moxibustion (UC-14), UC with mesalazine gavage (UC-W), HC with 7 d of moxibustion (HC-7), HC with 14 d of moxibustion (HC-14). Moxibustion was applied to the bilateral Tianshu (ST25). Gut microbiome profiling was conducted by 16S rRNA amplicon sequencing, and PCR and ELISA determined the expression of inflammatory cytokines in colon mucosa and serum, respectively.
Moxibustion treatment restored the colonic mucosa and decreased submucosal inflammatory cell infiltration in colitis rats. Rats treated with moxibustion and mesalazine had significantly lower levels of the dominant phyla Proteobacteria and the genera Saccharibacteria, Sphingomonas and Barnesiella than colitis rats, and they could restore the microbiome to levels similar to those observed in healthy rats. UC rats had reduced alpha diversity, which could be alleviated by moxibustion therapy, and UC-7 had a higher alpha diversity than UC-14. This finding suggests that short-term (7 d) but no longer term (14 d) moxibustion treatment may significantly affect the gut microbiome. The potential bacterial functions affected by moxibustion may be ascorbate and aldarate metabolism, and amino acid metabolism. Compared with HC group, the levels of the cytokines interleukin-12 (IL-12) (P < 0.05) and IL-6, IL-17, IL-23, interferon-γ, lipopolysaccharide, IgA, tumour necrosis factor-α and its receptors 1 (TNFR1) and TNFR2 (P < 0.01) were all increased, whereas anti-inflammatory cytokine IL-2 and IL-10 (P < 0.01) and transforming growth factor-β (P < 0.05) were decreased in UC rats. These changes were reversed by moxibustion.
Our findings suggest that moxibustion exerts its therapeutic effect by repairing mucosal tissue damage and modulating the gut microbiome and intestinal mucosal immunity.
Core tip: Ulcerative colitis (UC) is a form of inflammatory bowel disease and microbial dysbiosis is an important pathological factor of UC. Moxibustion treatment can repair mucosal tissue damage and regulate immune function in patients with UC associated with gut microbiome changes. In this study, moxibustion can significantly reduce the colonic tissue damage and the levels of pro-inflammatory cytokines and anti-inflammatory cytokines in rats with 4% dextran sulphate sodium-induced ulcerative colitis, relieve intestinal inflammation, and promote the restoration of impaired colonic tissue. In addition, moxibustion can increase the diversity of the gut microbiome and the UC with 7 d of moxibustion had a higher alpha diversity than UC with 14 d of moxibustion.
- Citation: Qi Q, Liu YN, Jin XM, Zhang LS, Wang C, Bao CH, Liu HR, Wu HG, Wang XM. Moxibustion treatment modulates the gut microbiota and immune function in a dextran sulphate sodium-induced colitis rat model. World J Gastroenterol 2018; 24(28): 3130-3144
- URL: https://www.wjgnet.com/1007-9327/full/v24/i28/3130.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i28.3130
The gut microbiome has been referred to as a “forgotten organ”[1] due to its capacity to influence host physiology. The gut microbiome is incredibly diverse, with dominant phyla including Firmicutes, Bacteroidetes, Proteobacteria, Verrucomicrobia, Actinobacteria and Fusobacteria[2,3]. Gut microbiome composition varies substantially among individuals and is thought to be a key determinant of susceptibility to several diseases[4-6]. The gut microbiome provides signals that promote immune cell maturation and ensure the normal development of immune function[7], which is crucial for human life and profoundly influences human physiology and metabolism[8,9].
Ulcerative colitis (UC) is a form of inflammatory bowel disease that can lead to bloody diarrhoea, passage of pus and/or mucus, and abdominal cramping during bowel movements[10,11]. Immune dysfunction plays a key role in driving inflammatory responses during UC development and progression. UC is commonly treated with therapeutics such as the anti-inflammatory drugs mesalazine and sulphasalazine, with steroid enemas[12] or with surgical intervention. The aetiology and pathogenesis of UC have not been fully elucidated but are thought to result from detrimental interactions among intestinal microbiota, the gut epithelium, and the host immune system in genetically susceptible individuals[13,14]. Several studies have recently reported decreased gut microbial diversity in patients with active UC[15,16]. Microbial dysbiosis is an important pathological factor of UC that affects host mucosal immunity[17-19]. Pathogenic bacteria and opportunistic pathogens interact with the intestinal mucosa directly or indirectly via secreted toxins, which may lead to an inappropriate intestinal mucosal immune response.
Studies have demonstrated that moxibustion, a traditional Chinese therapy that treats and prevents diseases using moxa (floss of Artemisia argyi), is a safe and effective treatment for UC[20-23]. Moxibustion exerts its therapeutic effects by regulating immune function[24,25], apoptosis[26,27], and protein expression at acupuncture points and in colon tissues[28,29]. UC is associated with changes in the gut microbiome, and moxibustion modulates the imbalance of intestinal flora[30]. Nevertheless, the gut microbiome is a complex community with only a small proportion of culturable organisms, and the effect of moxibustion on the gut microbiome remains unknown. Recently developed culture-independent high-throughput sequencing methods allow gut microbiome profiling and assessments of microbial gene abundance and community structure to be performed more easily[31].
Here, we used 16S rRNA gene sequencing to assess the gut microbial composition and community structure in faecal samples of UC rats treated with moxibustion or mesalazine gavage. Our aims were to determine the impact of moxibustion on intestinal microbial community structure, the role of the intestinal microbiota in UC pathogenesis, and the molecular mechanism underlying the effect of moxibustion in UC treatment.
Seventy specific pathogen-free male Sprague Dawley rats (body weight 180-220 g) were provided by the Department of Laboratory Animal Science of Fudan University (Shanghai Slack Laboratory Animal Co., LTD. license: SCXK (Shanghai) 2012-0002 and SYXK (Shanghai) 2009-0082). All rats were raised under standard conditions: 25 ± 1 °C, 50%-70% humidity, and 12 h light/dark cycle. Rats were allowed to acclimatize for one week before the start of the trial. Rats (n = 70) were divided into seven groups: healthy controls (HC), healthy controls with seven days of moxibustion (HC-7), healthy controls with fourteen days of moxibustion (HC-14), UC model group (UC), UC model with seven days of moxibustion (UC-7), UC model with fourteen days of moxibustion (UC-14), and UC model with mesalazine gavage (UC-W). UC was induced with 4% dextran sulphate sodium (DSS; molecular weight: 36000-50000 Da; Art.No.9011-18-1; MP Biomedicals) supplied in drinking water. Animal breeding, care and all experiments were performed according to procedures approved by the University Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine (IACUC protocol number: SYXK (shanghai) 2009-0082) to relieve pain and avoid injury. All protocols were performed in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, as formulated by the Ministry of Science and Technology of the People’s Republic of China[32]. We tried all efforts to minimize animal suffering.
UC was induced with 4% DSS in drinking water for seven consecutive days as described previously[33]. After seven days, two rats were randomly selected to confirm successful establishment of the animal model. Rats were provided 1% DSS in the drinking water for seven consecutive days in the UC, UC-7 and UC-W groups and fourteen consecutive days in the UC-14 group.
For the HC-7 (n = 10) and UC-7 (n = 10) groups, moxa cones (0.5 cm in diameter and 0.6 cm high) (Nanyang Hanyi Moxa Co., Ltd. China) were placed on the herb cake, and the herb cake was placed on Tianshu (ST25, bilateral) with moxibustion for 10 min once a day for seven days, the herb cakes were prepared with Chinese medicine powder and yellow wine into size of 1.0 cm in diameter and 0.5 cm in thickness, and the skin temperature of acupuncture points was 43 ± 1 °C. ST25 is 5 mm lateral to anterior midline at the navel level[34]. The HC-14 (n = 10) and UC-14 (n = 10) groups received the same treatment as the HC-7 and UC-7 groups, respectively, but for fourteen days. The UC-W group (n = 10) received mesalazine enteric-coated tablets (Heilongjiang Tianhong Pharmaceutical Co., Ltd; Lot number: H20103359) by gavage administration twice daily for seven consecutive days with a dose calculated from that specified for an adult of 70 kg and a weight ratio of 1:0.018 for rats (200 g). The HC (n = 10) and UC (n = 10) groups did not receive any treatment but received the same fixation (to fix the hands and feet on the fixed mount).
Following treatment, rats were anaesthetized via intraperitoneal injection with 2% pentobarbital sodium for tissue collection. Blood samples were collected by abdominal aortic, after one hour standing, the samples were centrifuged at 3000 rpm for ten minutes at 4 °C to separate the plasma. The obtained plasma was stored at -80 °C. Colon samples were rapidly removed and separated from the large intestine (6 cm distal to the anus). Then, 5 g of faecal matter was collected in cryogenic vials and stored at -80 °C. Colon samples were flushed with physiological saline to remove the surrounding connective tissue and fat. Colonic mucosal injury was observed with the naked eye. Then, the colon was divided into two segments: one segment was fixed in 10% paraformaldehyde for haematoxylin and eosin (HE) staining for histological observation and the histological grade was scored with the bland method[30] (Table 1), whereas the other segment was stored at -80 °C.
| Histopathological manifestation | score | |
| Ulcer | No ulcer | 0 |
| Ulcer area < 3 cm | 1 | |
| Ulcer area > 3 cm | 2 | |
| Inflammation | No inflammation | 0 |
| Mild inflammation | 1 | |
| Severe inflammation | 2 | |
| Granuloma | No granuloma | 0 |
| Granuloma | 1 | |
| Lesion depth | No lesion | 0 |
| Submucosa | 1 | |
| Muscular layer | 2 | |
| Serosa layer | 3 | |
| Fibrosis | No fibrosis | 0 |
| Mild fibrosis | 1 | |
| Severe fibrosis | 2 | |
Colon samples fixed with 10% paraformaldehyde were dehydrated and immersed in wax using an automatic tissue dehydrating machine, embedded in paraffin and sectioned at 4 μm thickness with a microtome. Slices were incubated with xylene I and II for 20 min each and rehydrated in absolute alcohol, 95% alcohol, 85% alcohol and 75% alcohol (3 min each time). Slices were stained with haematoxylin for 1 min, followed by 1% hydrochloric acid-alcohol for 30 s, and counterstained with eosin for 5 min. Sample histology was observed by electron microscope.
Genomic DNA was extracted from faecal samples using the QIAamp DNA Mini Kit (QIAGEN, Germany). Briefly, 20 μL of proteinase K solution (20 mg/mL) and 100 mg of zirconium beads (0.1 mm) were added to the pellet. The mixture was agitated three times with a Mini-Beadbeater (FastPrep, Thermo Electron Corporation, United States); buffer AL was added to the mixture, which was then incubated for 10 min at 70 °C. Next, 200 μL of ethanol (96%) was added. This mixture was then loaded onto a QIAamp Mini spin column and centrifuged at 8000 g for 1 min. The column material was washed with the first washing buffer (buffer AW1, 500 μL) followed by the second washing buffer (buffer AW2, 500 μL) provided with the kit. Finally, DNA was eluted with 100 μL of buffer AE. DNA integrity was assessed by electrophoresis on a 1% agarose gel containing 0.5 mg/mL ethidium bromide. DNA concentrations were determined using a NanoDrop ND-2000 spectrophotometer (Thermo Electron Corporation).
The 16S rRNA gene hypervariable V3-V4 region was amplified by PCR using the barcode-tag universal bacterial modified primers. The prototype primers were 341F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACVVGGGTATCTAATC-3’). Amplicon sequencing was performed using Illumina MiSeq (PE300). Raw reads were demultiplexed and filtered according to read length and quality. Chimeric reads were removed, and OTU selection was performed as previously described[35]. The filtered reads were dereplicated to unique sequences and sorted by abundance before being subjected to OTU clustering at 97% similarity. Taxonomy was assigned to the representative sequence of each OTU using the Ribosomal Database Project classifier[36].
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt 1.0.0)[37] was used to predict metagenome function from the 16S rRNA data. Statistical Analysis of Metagenomic Profiles (STAMP)[38] was used to analyse the predicted metagenome and to identify pathways associated with UC. The Benjamini-Hochberg procedure was used to control the false discovery rate due to multiple testing.
Total RNA was isolated using TRIzol® LS (Invitrogen Corp.) according to the manufacturer’s instructions. The integrity of the RNA samples was assessed via agarose gel (10 g/L) electrophoresis, and RNA concentrations were determined using a NanoDrop ND-2000 Spectrophotometer (Thermo Electron Corporation). Two micrograms of total RNA was used for RT-PCR in a 20 μL reverse transcription reaction system. Two micrograms of RNA, up to 8 μL of DEPC, 0.5 μL (50 U/μL) of RNase inhibitor and 2 μL of oligomer (dT)15 and primer were mixed and placed in a 65 °C water for 5 min. Then, the mixture was placed at room temperature for 10 min. After a 5 s centrifugation, the following reagents were added to the reactions: 0.5 μL (50 U/μL) of RNase inhibitor, 4 μL of 5 × RT buffer solution, 2 μL of dNTPs (10 mmol/L for each), 2 μL of DTT, and 1 μL of AMV reverse transcriptase (200 U/μL). The solution was mixed and placed in water at 40 °C for 1 h, heated to 90 °C in water for 5 min, and centrifuged for 5 s.
The primers used were designed with Primer Express Software v2.0 (Applied Biosystems, United States) and synthesized at the Beijing Genomics Institute. Gene sequences of the primers are presented in Table 2.
| Target gene | Forward sequence | Reverse sequence |
| IL-6 | GCCAGAGTCATTCAGAGCAA | TTGGTCCTTAGCCACTCCTT |
| IL-10 | CTGTCATCGATTTCTCCCCT | CAAACTCATTCATGGCCTTG |
| IL-12 | CCCTCAAGTTCTTCGTCCGC | TTTGCATTGGACTTCGGCAG |
| IL-17 | ATCCAGCAAGAGATCCTGGT | CAATAGAGGAAACGCAGGTG |
| IL-23 | TGTGGTGATGGTTGTGATCC | TGTGAAGATGTCCGAGTCCA |
| TNF-α | GAAAGCATGATCCGAGATGT | CAGGAATGAGAAGAGGCTGA |
| TNFR1 | TGAGACGCATTTCCAGTGTG | AGAATCCTGCGTGGCAGTTA |
| TNFR2 | CATGTCAACGTCACCTGCAT | TCATCCTTTGGAGACCCTGA |
| rACTIN | ACTTCGAGCAGGAGATGGCC | CCCAGGAAGGAAGGCTGGAA |
Each 16 μL PCR included: 8 μL of 2 × SYBR Green PCR mix, 1 μL of each sense and antisense primer, 1 μL of reverse transcription product (cDNA), and 5 μL of H2O. RT-PCR was performed using a ViiA 7 Real Time PCR System (Applied Biosystems, United States). The reaction conditions were set at 95 °C for 2 min, 94 °C for 10 s, 60 °C for 10 s, and 72 °C for 40 s for 40 cycles.
The levels of interleukin-2, 10, 12, 17, 23, interferon-γ (IFN-γ), lipopolysaccharide (LPS), immunoglobulin A (IgA), transforming growth factor-β (TGF-β), tumour necrosis factor-α (TNF-α), TNF receptor 1 (TNFR1) and TNFR2 in all serum samples were tested by sandwich ELISA. According to manufacturer’s instructions, IL-2, IL-10, IL-12, 17, IL-23, IFN-γ, LPS, IgA, TGF-β, TNF-α, TNFR1 and TNFR2 were measured using corresponding ELISA kits (Biotech well, Shanghai, China). The detection limits were: 17 pg/mL for IL-2, IL-12, IL-23, and TNF-α, 16 pg/mL for IL-10, 15 pg/mL for IL-17, and TGF-β, 12 pg/mL for IFN-γ, 8 pg/mL for LPS, 8ng/mL for IgA, 6 pg/mL for TNFR1, and 5 pg/mL for TNFR2.
Differential abundance testing between groups was performed using the Kruskal-Wallis test in R 3.2.3 (http://cran.r-project.org). Alpha diversity was measured with Simpson and Shannon diversity indices. Beta diversity was determined using unweighted unifrac distance and was summarized by PCoA. A principal components analysis (PCA) plot of Kyoto Encyclopaedia of Genes and Genomes (KEGG) modules with significant differences in mean proportion differences was created using STAMP[39] (v2.1.3). Body weight gain, RT-PCR and ELISA analysis data are presented as the mean ± SD. Histopathological score data are expressed as M (Q25-Q75). ANOVA and Least Significant Difference multiple comparison tests were performed using IBM SPSS statistics 21.0 (IBM Co., Armonk, NY, United States). P < 0.05 was considered significant.
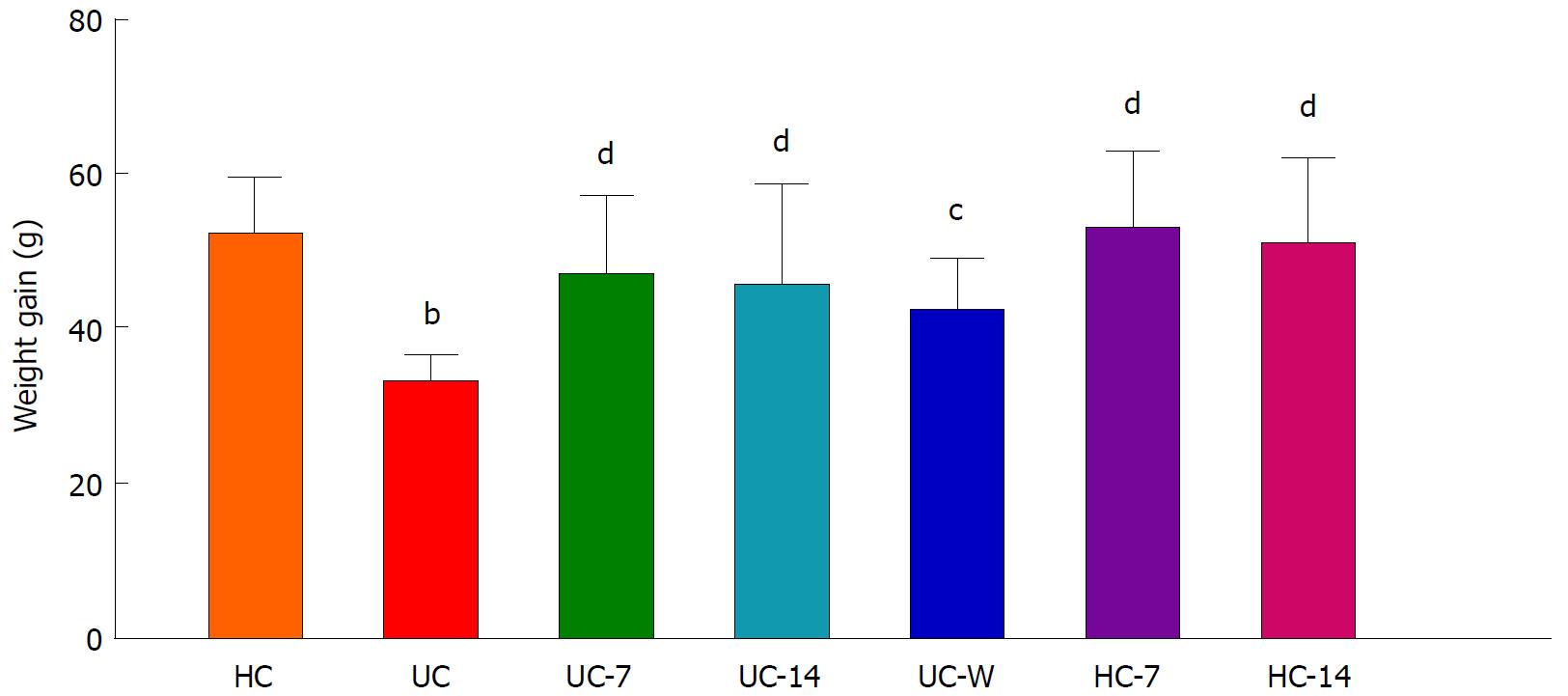
Two rats in the UC-W group died after gavage during the experiment. The body weight gain in the UC group was lower than that in the HC group (P < 0.01). After treatment, the body weight gains in the UC-7, UC-14, HC-7, HC-14 (P < 0.01) and UC-W (P < 0.05) groups were higher than that in the UC group (Figure 1).
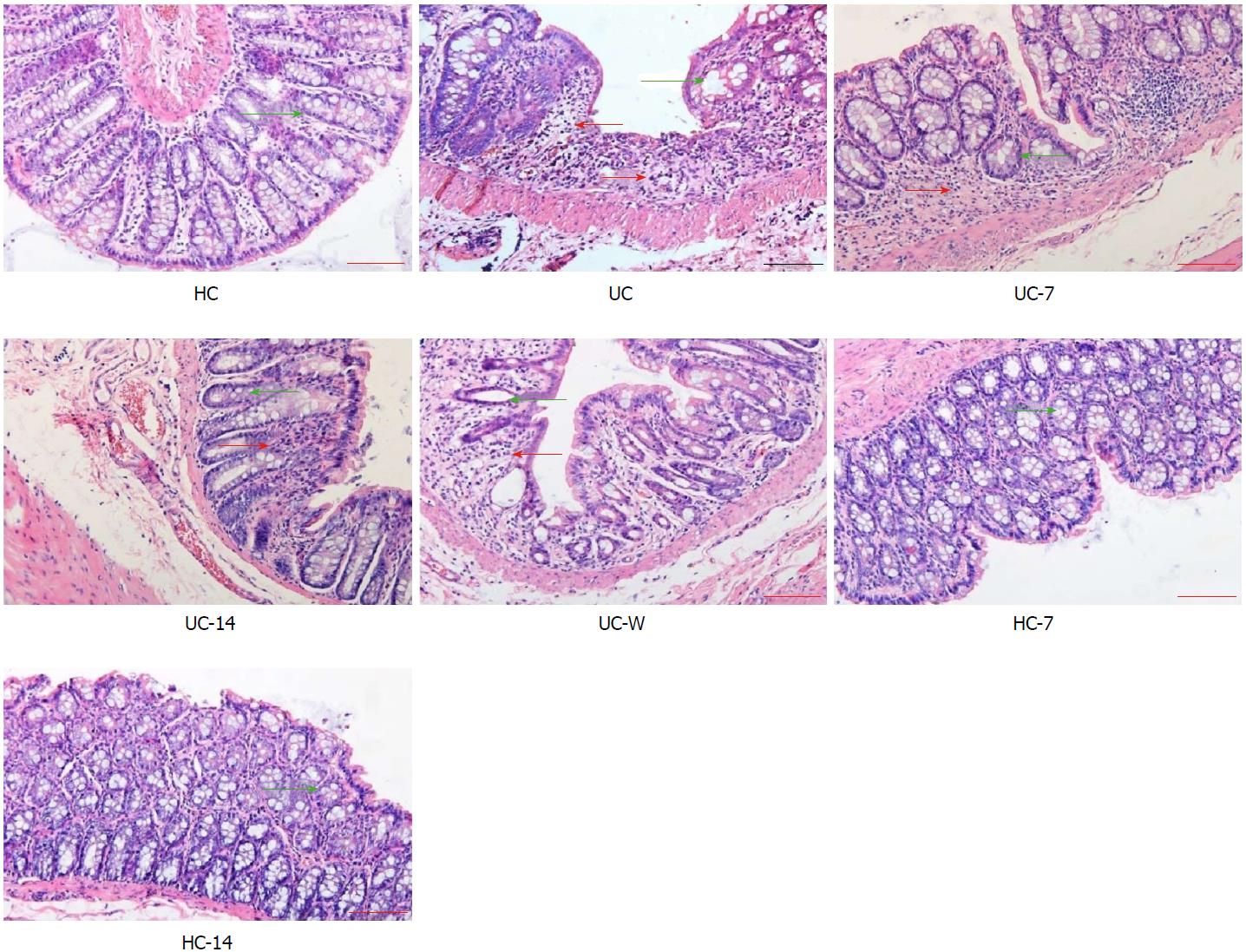
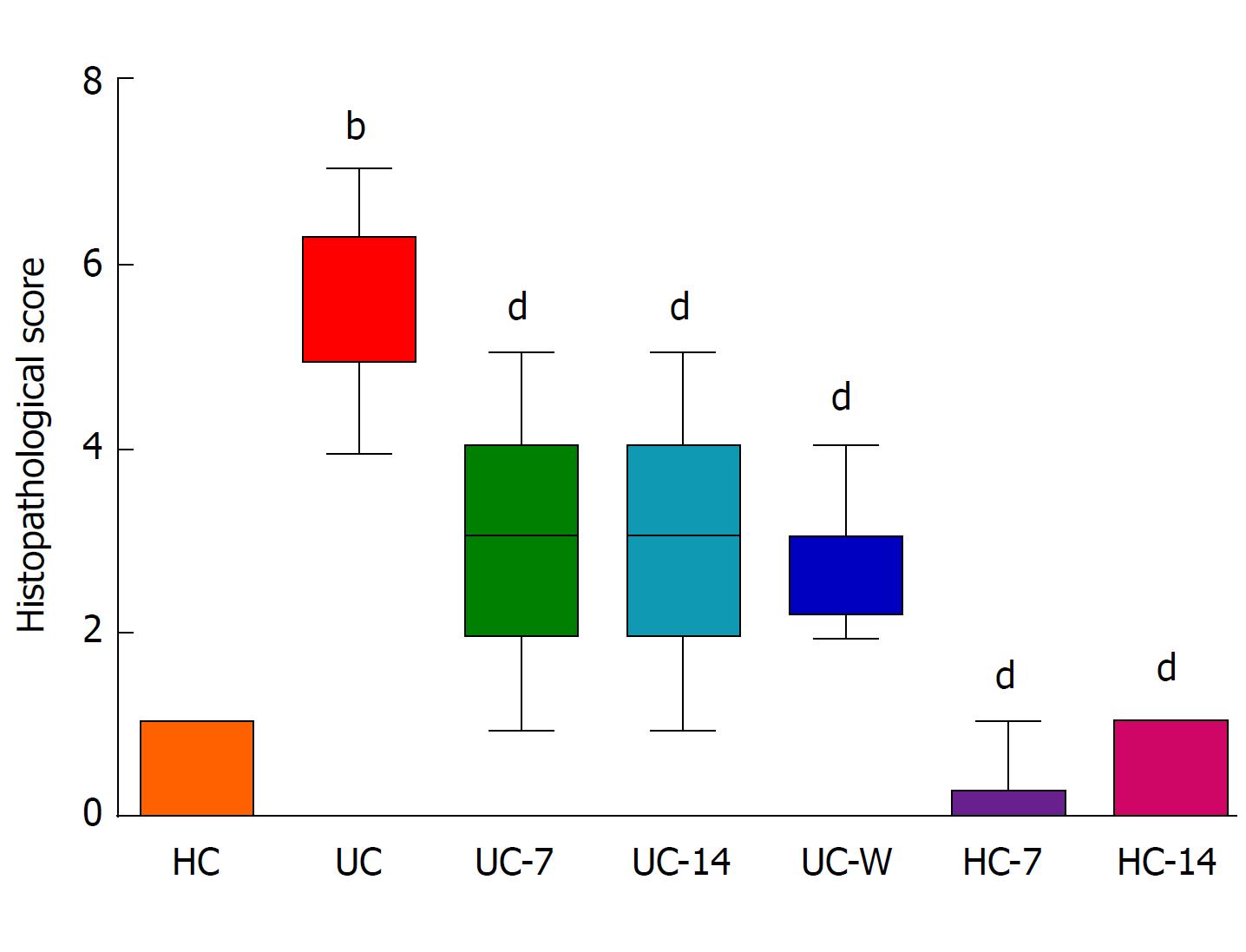
Rat colons were observed by light microscopy (Figure 2). The HC, HC-7, and HC-14 groups had similar histopathologies, with intact colonic mucosal epithelia, regularly arranged glands, and evenly distributed mesenchyme. No congestion, oedema, ulcers or other pathological abnormalities were noted. By contrast, UC rats exhibited colonic mucosa damage, with a reduced number of glands and substantial inflammatory cell infiltration in the mucosa and submucosa. These abnormalities could be alleviated by moxibustion treatment, which restored the colonic mucosa to the level of healthy rats, and decreased submucosal inflammatory cell infiltration and congestion in the UC-7 and UC-14 groups. However, in the UC-W group, mucosal and submucosal glands were disorganized, with abundant inflammatory cell infiltration. The histopathological scores for the colon tissues in the different groups are presented in Figure 3. The histopathological scores in the UC group were significantly higher than those in the HC group (P < 0.01). After treatment, the scores were decreased in the UC-7, UC-14, UC-W, HC-7 and HC-14 groups compared with those in the UC group (P < 0.01).
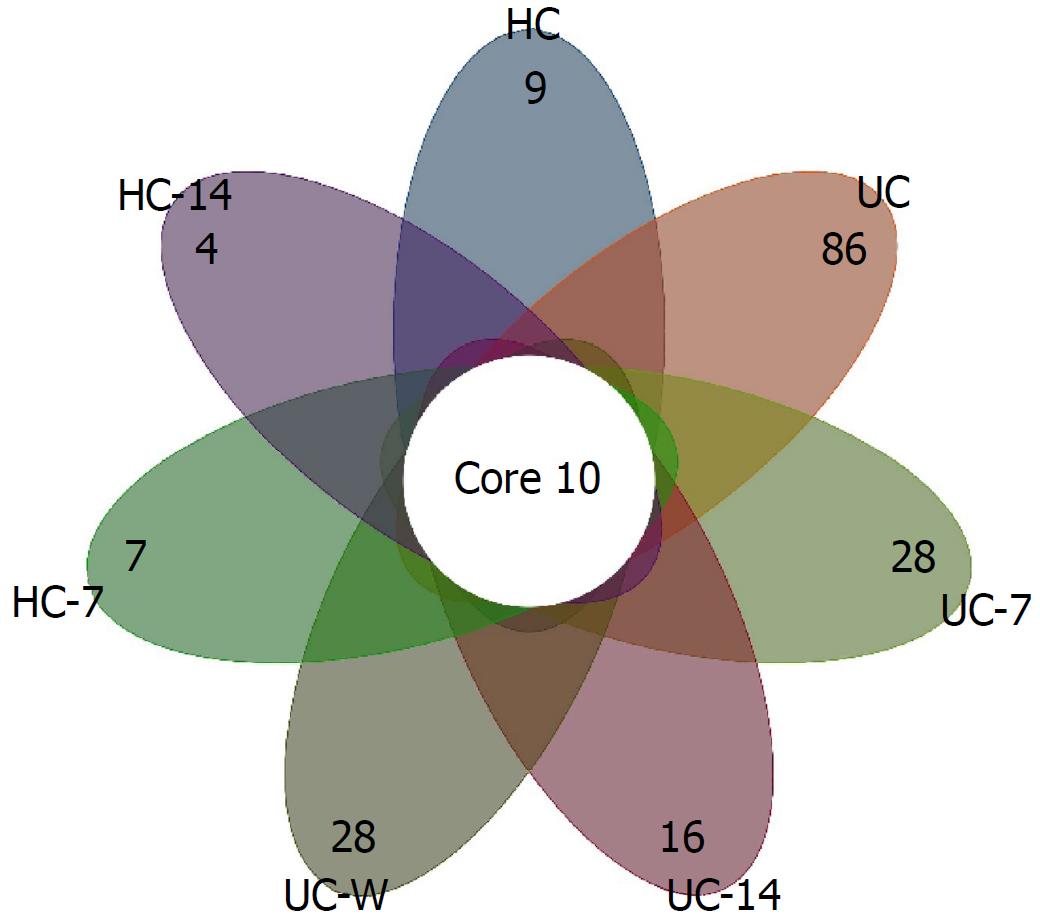
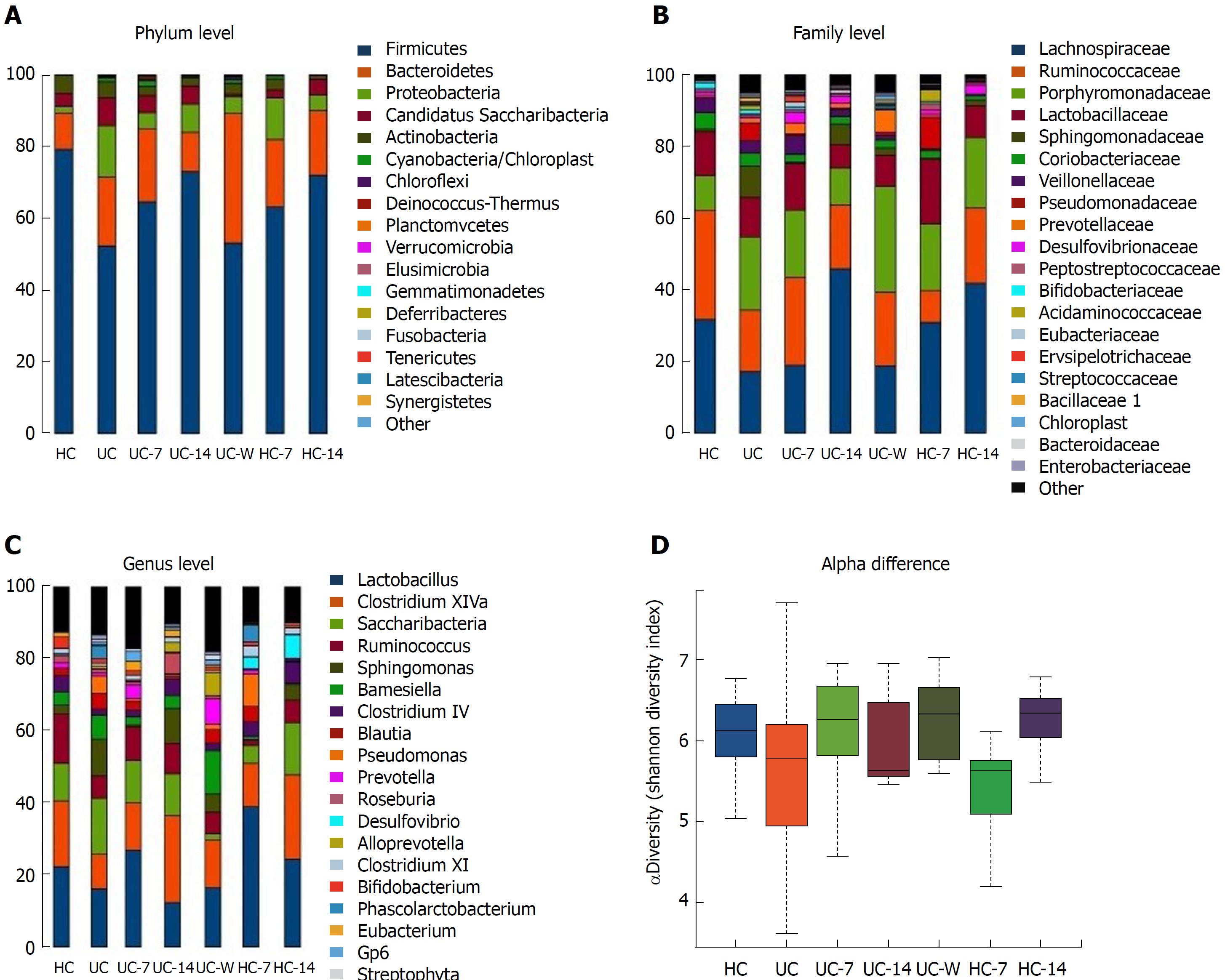
Approximately 3.9 million high-quality (> 220 bp) sequence reads were obtained using the MiSeq system (Illumina, San Diego, CA, United States), which led to the identification of 1545 operational taxonomic units (OTUs), with an average read length of 451.58 bp. Overall, the relative abundance levels of 18 dominant phyla were identified in the entire model system. Among these phyla, five (Firmicutes, Bacteroidetes, Proteobacteria, Candidatus Saccharibacteria, and Actinobacteria) were present at ≥ 1% and were the dominant phyla in all groups, but relative abundance varied by treatment. A total of 186 families were detected, and 100 families were commonly present, of which Lachnospiraceae, Ruminococcaceae, Porphyromonadaceae, Lactobacillaceae, Sphingomonadaceae, Veillonellaceae and Coriobacteriaceae were the dominant families detected. A total of 325 OTUs were identified at the genus level, of which 10 core OTUs were present in all groups. The highest average number of OTUs identified at the genus level (134) was detected in the UC-14 rats, whereas the lowest (78) was found in HC rats. As shown in Figure 4, the UC group had the most unique genera, even after treatment with either moxibustion or mesalazine gavage.
There were significant differences in the microbiome between healthy controls and UC rats. Although Firmicutes was the predominant phylum in all samples, UC rats had increased relative abundance levels of Proteobacteria, Candidatus and Saccharibacteria and decreased relative abundance levels of Firmicutes and Actinobacteria compared with HC rats. At the family level, UC rats had decreased Lactobacillaceae and Ruminococcaceae and increased Sphingomonadaceae, Pseudomonadaceae and Porphyromonadaceae (Figure 5B). In addition, Lactobacillus, Clostridium XIVa and Ruminococcus were increased and Saccharibacteria and Sphingomonas were decreased in HC compared with UC rats (Figure 5C). UC rats had lower alpha diversity than HC rats, and the Shannon Diversity Index was reduced (Figure 5D), consistent with previous reports[15,40].
In HC-7 and HC-14 groups, Firmicutes were decreased and Bacteroidetes and Proteobacteria were increased compared with the HC group (Figure 5A). At the family level, Porphyromonadaceae was increased and Ruminococcaceae and Veillonellaceae were decreased in moxibustion-treated groups (Figure 5B). Compared with the HC-7 group, the HC-14 group had increased abundance levels of Lachnospiraceae and Ruminococcaceae and a decreased abundance of Lactobacillaceae. At the genus level, Lactobacillus and Desulfovibrio were significantly increased, and Ruminococcus and Barnesiella were significantly decreased in both HC-7 and HC-14 groups relative to HCs. In particular, significant differences between HC-7 and HC-14 were noted regarding the abundance levels of Lactobacillus, Saccharibacteria, and Blautia. HC-7 exhibited increased abundance levels of Lactobacillus and Blautia and a reduced abundance of Saccharibacteria compared with those in the HC-14 group. Lactobacillus and Pseudomonas were significantly enriched in HC-7 (but not HC-14) compared with the HC group. Interestingly, Saccharibacteria was decreased in HC-7 but increased in HC-14 compared with HCs (Figure 5C). It is not clear why the alpha diversity was decreased in HC-7 but increased in HC-14 compared with HCs (Figure 5D).
Moxibustion treatment (but not mesalazine gavage) for seven days or fourteen days was associated with an increase in Firmicutes compared with the UC group, whereas Proteobacteria and Candidatus and Saccharibacteria were decreased to varying degrees in both the moxibustion- and mesalazine-treated groups compared with the UC group. Bacteroidetes was reduced in UC-14 but substantially increased in the UC-W group compared with the UC group (Figure 5A). At the family level, UC-7 was similar to UC-W but significantly different from UC-14. UC-14 exhibited increased Sphingomonadaceae and decreased Porphyromonadaceae compared with UC-7 and UC-W. Coriobacteriaceae, Pseudomonadaceae and Bifidobacteriaceae were significantly reduced in UC-7, UC-14 and UC-W groups compared with the UC group (Figure 5B). UC-14 had relatively low levels of Lactobacillus but relatively high levels of Clostridium XIVa and Sphingomonas with a dominant position in all groups. In the UC-W group, Saccharibacteria was decreased and Barnesiella, Prevotella, and Alloprevotella were increased significantly compared with all of the other groups (Figure 5C).
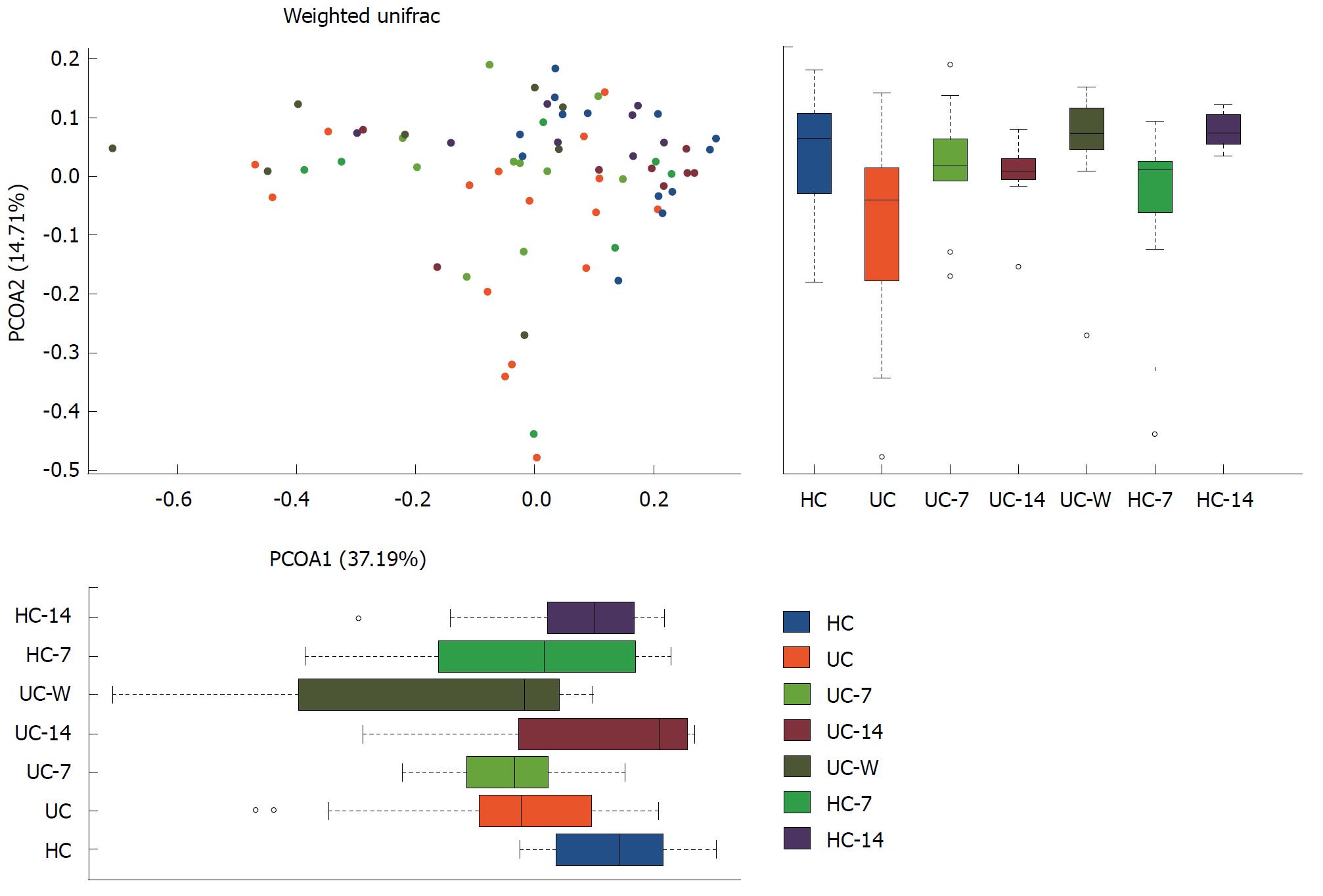
Alpha diversity was estimated through rarefaction analysis and the Shannon and Chao1 indexes. The lowest OTU richness was observed in the HC groups. Multiple rarefaction curves analysis indicated variation among samples and exhibited sufficient data coverage for diversity in the samples. The Shannon Wiener index showed the lowest value of evenness in HC rats. Surprisingly, HC-7 also showed a lower value. Interestingly, after seven days of treatment with moxibustion, the genus level diversity was comparable to that of HCs but notably different from the UC-14 and UC-W groups. UC-7 and UC-W had the highest median alpha diversity, which was restored to the normal level of HC and HC-14 (Figure 5D). This finding suggests that both moxibustion treatment and mesalazine gavage increased colonic microbial diversity. In terms of beta-diversity, UC samples clustered separately from HC samples in a principal coordinates analysis (PCoA), indicating that disease state was the primary factor influencing community differences (Figure 6). UC -7 was more similar to UC-W on the first axis, whereas HC-7 and HC-14 were more similar to HC and UC-14. Using non-metric multidimensional scaling (nMDS) instead of PCoA produced similar results (Figure 7).
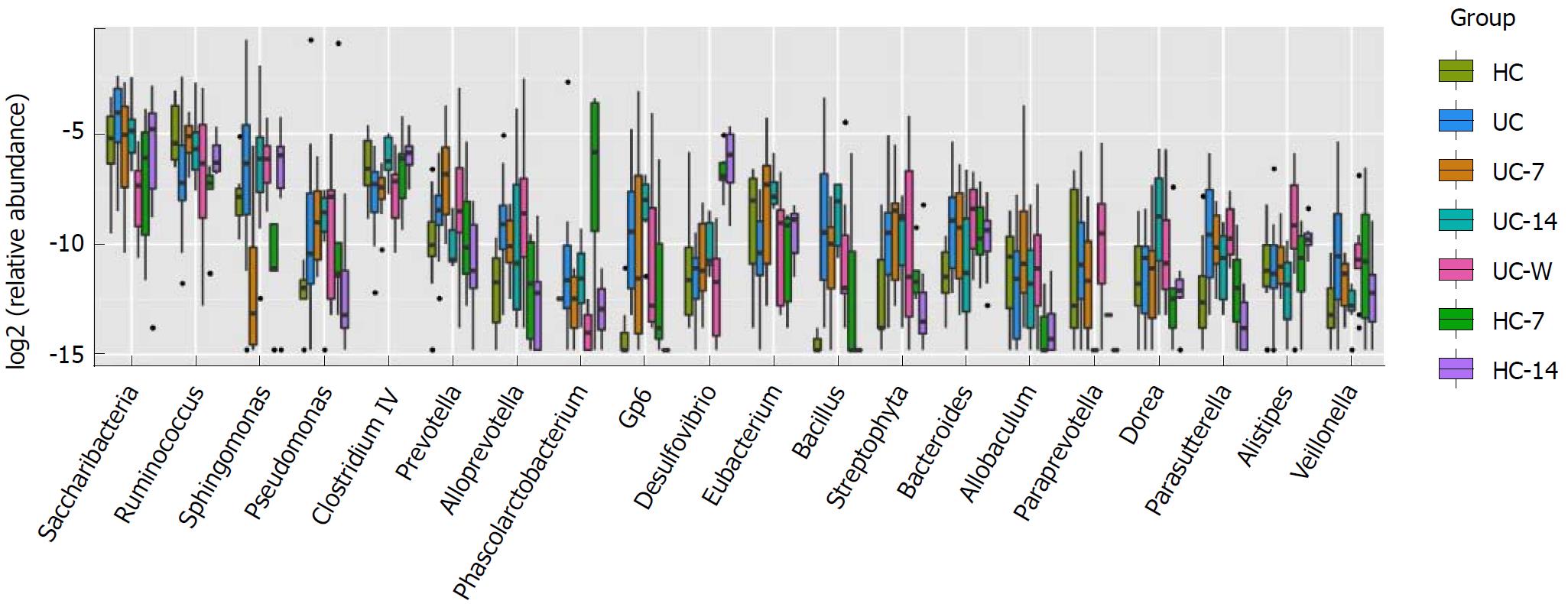
To establish which taxa were contributing most to the apparent differences in diversity between groups, genus level abundance was assessed. Saccharibacteria, Ruminococcus, Sphingomonas, Pseudomonas, Clostridium IV, Prevotella and Alloprevotella were the most affected genera (Figure 8). These opportunistic pathogens can cause infection and may be important contributing factors in UC pathogenesis. Indeed, both Bacteroidetes[41] and Clostridium IV[42] are associated with UC. In our study, we found that UC rats had a higher density of Bacteroidetes and reduced Clostridium IV compared with the control.
Community function was inferred from 16S compositional data using PICRUSt. In the KEGG pathway analysis, ascorbate and aldarate metabolism were significantly increased in both UC and HC rats treated with moxibustion for fourteen days (Supplement Table 1), which suggests that long-term moxibustion treatment could increase ascorbate and aldarate metabolism. Furthermore, compared with the HC group, the HC-7 and HC-14 groups had significant increases in genes belonging to the amino acid metabolism pathway. Compared with the UC group, these genes were increased in the UC-7 and UC-14 groups, but no significant differences were noted. However, the roles of these metabolic pathways in UC are unclear.
Levels of IL-6, IL-10, IL-12, IL-17, IL-23, TNF-α, TNFR1 and TNFR2 mRNA in colon mucosa
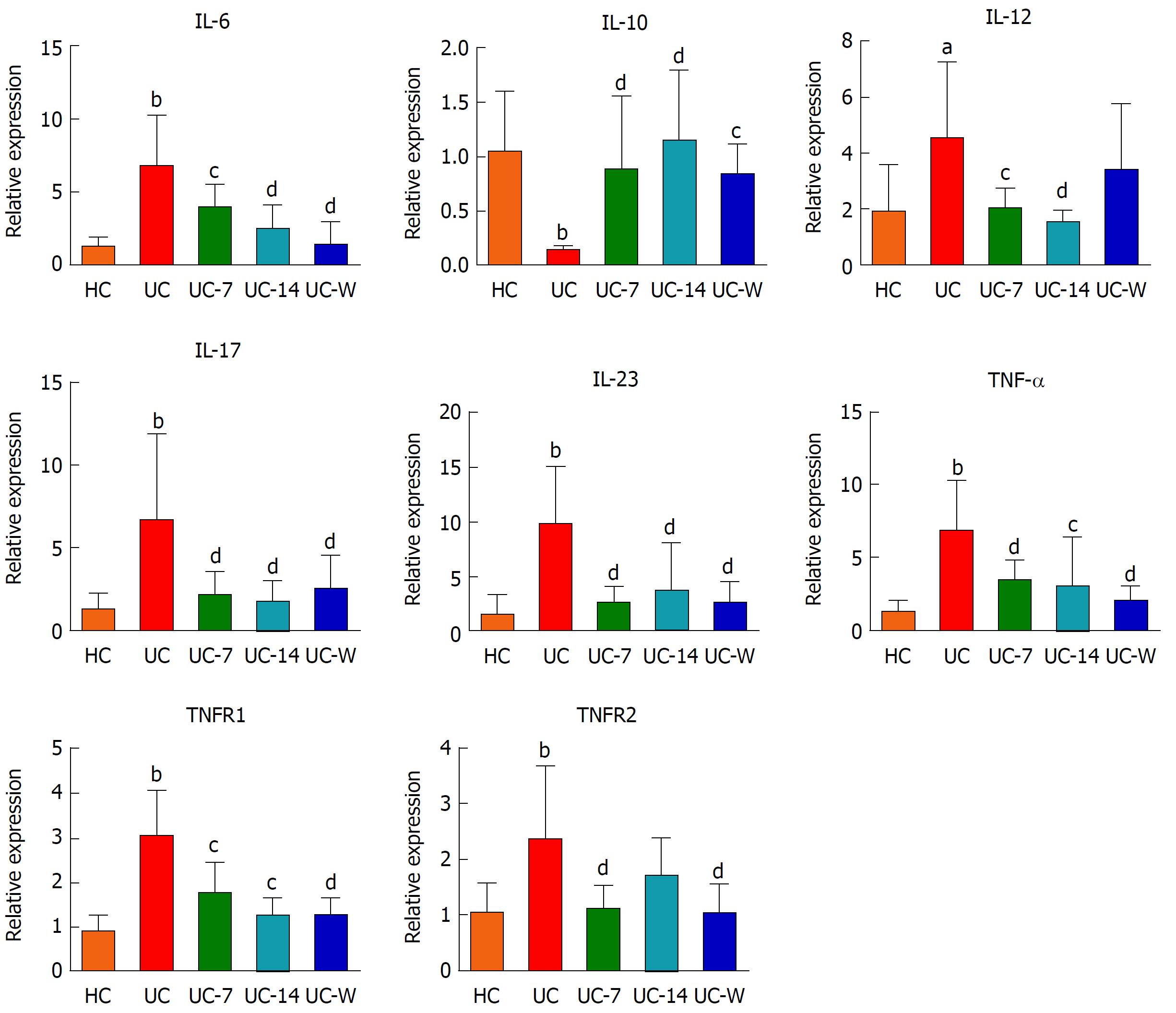
IL-6, IL-17, IL-23, TNF-α, TNFR1 and TNFR2 (P < 0.01) and IL-12 mRNA (P < 0.05) exhibited significant increases and IL-10 mRNA (P < 0.01) exhibited a significant decrease in the UC rats compared with their expression levels in HC rats (Figure 9). We found significantly lower levels of IL-17, IL-23, TNF-α, TNFR2 (P < 0.01), IL-6, IL-12, and TNFR1 mRNA (P < 0.05) in the UC-7 group compared to the UC group. The relative expression levels of IL-6, IL-12, IL-17, IL-23 (P < 0.01), TNF-α and TNFR1 mRNA (P < 0.05) were also lower in the UC-14 group than in the UC group, and IL-10 mRNA was both higher in the UC-7 group (P < 0.01), the UC-14 group (P < 0.01) and the UC-W group (P < 0.05) than in the UC group. However, no significant differences in the relative expression levels of TNFR2 mRNA were noted between the UC-14 and UC groups (P > 0.05). Significant decreases in IL-6, IL-17, IL-23, TNF-α, TNFR1, and TNFR2 mRNA (P < 0.01) were also noted in the UC-W group compared with the UC group.
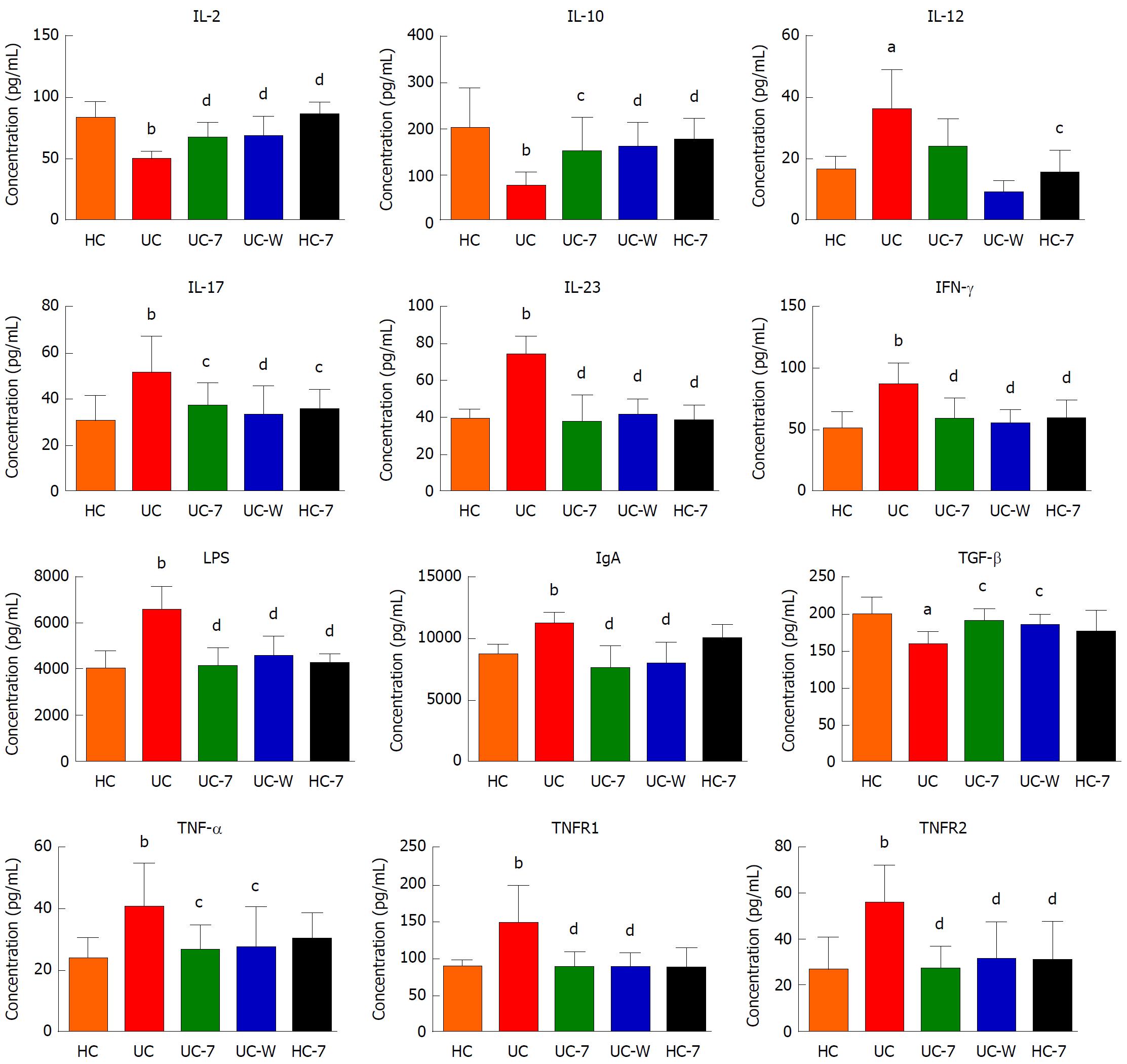
The levels of IL-2, IL-10, IL-12, IL-17, IL-23, IFN-γ, LPS, IgA, TGF-β, TNF-α, TNFR1 and TNFR2 in serum
As shown in Figure 10, we noted lower levels of IL-2, IL-10 (P < 0.01) and TGF-β (P < 0.05) and higher levels of IL-12 (P < 0.05), IL-17, IL-23, IFN-γ, LPS, IgA, TNF-α, TNFR1 and TNFR2 (P < 0.01) in the UC group compared with HC group. Interestingly, we also noted significantly higher levels of IL-2 (P < 0.01), IL-10 (UC-7, P < 0.05; UC-W and HC-7, P < 0.01) and TGF-β (UC-7 and UC-W, P < 0.05; HC-7, P > 0.05) in treatment groups than the UC group. Similarly, the levels of IL-17 (UC-7 and HC-7, P < 0.05; UC-W, P < 0.01), IL-23, IFN-γ, LPS, IgA, TNFR1, TNFR2 (P < 0.01), TNF-α (P < 0.05) in serum were lower in the UC-7 and UC-W groups. However, there were no significant differences in the level of IL-12 between the UC group and the UC-7 group and between the UC group and the UC-W group (P > 0.05). The results were not completely consistent with the PCR. ELISA is a quantitative method to detect proteins, and PCR is the detection of the target gene from the transcript level. Due to post-transcriptional and post-translation regulation, gene expression and protein expression do not necessarily correlate.
In the last decade, several studies have revealed a strong association between gut microbiome dysbiosis and UC[17,43-45]. According to the traditional Chinese medicine theory, the Tianshu (ST25) acupoint is the Mu point of large intestine and can regulate gastrointestinal functions, and is usually used to treat abdominal pain and diarrhoea[46]. Moxibustion is an effective and safe treatment for UC, but the underlying mechanism remains unknown. Moxibustion treatment regulates intestinal flora and inhibits TNF-α and IL-12 expression in the colon tissues of UC rats, which can regulate the colonic immune response[30]. However, no study has investigated the effects of moxibustion on the intestinal flora using whole microbiome analysis. Therefore, we employed a 16S rRNA amplicon sequencing approach to determine the effects of moxibustion treatment on the microbiome in a UC rat model.
We found that moxibustion treatment increased Firmicutes abundance and decreased the abundance levels of Bacteroidetes and Proteobacteria. In terms of alpha diversity, HC-14 was more similar to HC than HC-7, which was confirmed by PCoA. In terms of alpha diversity, UC-14 was also more similar to UC than UC-7. This finding suggests that short-term (seven days) but not longer term (fourteen days) moxibustion treatment may significantly affect the gut microbiome. Overall, the relative abundance levels of beneficial bacteria were decreased, and the growth of several opportunistic pathogenic species was increased in the UC rats.
We found that gut microbiome diversity was significantly reduced in UC rats compared with healthy controls, consistent with previous reports. The abundance levels of Firmicutes, Bacteroidetes and Proteobacteria Candidatus varied between treatment groups. In particular, Firmicutes was less abundant in UC rats. At the family level, this effect translated to reductions in host beneficial taxa, including Ruminococcaceae and Lactobacillaceae, which are closely associated with UC[47]. At the genus level, Lactobacillus, Bifidobacterium and Ruminococcus were decreased in UC rats, consistent with the literature[15,46]. Several studies have reported that Lactobacillus and Bifidobacterium played key roles in treating UC patients or rats by inhibiting Toll-like receptor 4 expression to reduce TNF-α expression[47] and enhancing anti-inflammatory cytokine IL-10 production[48]. UC pathogenesis may be associated with the decreased levels of Lactobacillus and Bifidobacterium, which consequently led to increases in inflammatory cytokines and decreases in anti-inflammatory cytokines. Lactobacillus and Bifidobacterium can down-regulate inflammatory cytokines[49] and stimulate anti-inflammatory cytokines, such as IL-10[50], which may protect the host from mucosal inflammation. Interestingly, we found that the expression levels of inflammatory cytokines, such as IL-6, IL-12, IL-17, IL-23, IFN-γ, TNF-α, TNFR1 and TNFR2, were increased and that anti-inflammatory cytokine IL-10 and TGF-β expression was decreased in UC rats compared with HC rats. These results indicate that gut microbial variation may affect mucosal immunity through up-regulating inflammatory cytokines and down-regulating anti-inflammatory cytokines. However, the specific relationship between the gut microbiome and mucosal immunity needs further investigation. After moxibustion treatment, the levels of these inflammatory cytokines were significantly decreased, and anti-inflammatory cytokines were increased. These results demonstrated that moxibustion treatment may repair intestinal mucosal by down-regulating inflammatory cytokines and up-regulating anti-inflammatory cytokines. In an animal model of colitis, butyrate-producing bacteria alleviated intestinal inflammation and necrosis in colitis rats[50]. UC patients have fewer butyrate-producing bacteria, such as Clostridium leptum and Clostridium coccoides[51,52], which is supported by our findings.
In UC rats, both moxibustion and mesalazine gavage for seven days led to increases in microbial diversity. Several studies have reported that mesalazine can improve the clinical symptoms and colon mucosal condition in UC patients[53,54]. These results demonstrate that moxibustion could potentially be used as an alternative treatment to mesalazine. It is not clear why the alpha diversity was decreased in UC rats treated with moxibustion for fourteen days. It is possible that moxibustion is not suitable for long-term treatment of UC, and determining whether moxibustion has side effects requires further study. PCoA analysis showed that UC rats had distinct microbiome profiles compared with HC rats, and an increased similarity in PCoA axis scores was noted between the HC and UC-14 groups. These results indicate that moxibustion could possibly decrease distinct bacteria, such as opportunistic pathogens.
High-throughput sequencing offers a new tool for studying the effect of moxibustion on the gut microbiome and its mechanism of action. Here, we report reduced diversity, gut microbial dysbiosis, increased inflammatory cytokines and decreased anti-inflammatory cytokines in DSS-induced colitis rats and show that these effects could be alleviated by moxibustion treatment. Our findings suggest that moxibustion exerts its therapeutic effect by modulating the microbiome and intestinal mucosal immunity. Bacterial functions possibly associated with moxibustion treatment include ascorbate and aldarate metabolism and amino acid metabolism.
The aetiology and pathogenesis of ulcerative colitis (UC) are not clear. In recent years, there has been growing evidence that the gut microbiota has a key role in the development of UC. Our previous study has shown that there are an imbalanced states of the beneficial bacteria Bifidobacterium, Lactobacillus, and harmful bacteria E. coli and Fusobacterium in the intestine of UC rats, and moxibustion can regulate the balance of beneficial bacteria and harmful bacteria in the intestine of UC rats.
Many studies have confirmed the efficacy of moxibustion in UC, however, the role of gut microbiota in the pathogenesis of UC and the role of moxibustion in the regulation of the UC gut microbiota remain unclear.
The aim of this work is to investigate the effect and mechanism of moxibustion on gut microbiota in UC rats.
UC was induced with 4% DSS in drinking water for seven consecutive days. Moxibustion was applied to the Tianshu (ST25, bilateral). Haematoxylin and eosin staining was assessed to evaluate the changes in the colon histopathology. Gut microbiome profiling was conducted by 16S rRNA amplicon sequencing, and PCR and ELISA determined the expression of inflammatory cytokines.
Compared with the healthy controls (HC) group, the UC group had increased histopathological scores. After treatment, the scores were decreased in the UC-7, UC-14, UC-W, HC-7 and HC-14 groups compared with those in the UC group. The relative abundance of some of the bacteria in the UC group was changed at both the phylum, family and genus level. In addition, UC rats had reduced alpha diversity, which all could be alleviated by moxibustion therapy. Moxibustion can significantly reduce the levels of pro-inflammatory cytokines IL-6, IL-12, IL-17, IL-23, IFN-γ, LPS, TNF-α, TNFR1 and TNFR2 and increase anti-inflammatory cytokines IL-2 and TGF-β in rats with ulcerative colitis and reduce the intestinal inflammation.
Our findings suggest that moxibustion exerts its therapeutic effect by modulating the microbiome and intestinal mucosal immunity.
The present study may provide a certain experimental basis and scientific basis for the clinical curative effect of the treatment of UC.
We want to thank Realbio Genomics Institute for 16S rRNA sequencing and data analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Raghow R, Suzuki H Watanabe T S- Editor: Wang JL L- Editor: Filipodia E- Editor: Yin SY
| 1. | O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1786] [Article Influence: 94.0] [Reference Citation Analysis (2)] |
| 2. | Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012;33:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Khan I, Yasir M, Azhar EI, Kumosani T, Barbour EK, Bibi F, Kamal MA. Implication of gut microbiota in human health. CNS Neurol Disord Drug Targets. 2014;13:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2236] [Cited by in RCA: 2560] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 5. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2501] [Cited by in RCA: 2749] [Article Influence: 183.3] [Reference Citation Analysis (1)] |
| 6. | Ayres JS. Cooperative Microbial Tolerance Behaviors in Host-Microbiota Mutualism. Cell. 2016;165:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Núñez G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity. 2016;44:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 306] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 8. | Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3003] [Cited by in RCA: 2988] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 9. | Dorrestein PC, Mazmanian SK, Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity. 2014;40:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 10. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1357] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 11. | M’Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ, Wu JC. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Goyal N, Rana A, Ahlawat A, Bijjem KR, Kumar P. Animal models of inflammatory bowel disease: a review. Inflammopharmacology. 2014;22:219-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Braun J, Wei B. Body traffic: ecology, genetics, and immunity in inflammatory bowel disease. Annu Rev Pathol. 2007;2:401-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Michail S, Durbin M, Turner D, Griffiths AM, Mack DR, Hyams J, Leleiko N, Kenche H, Stolfi A, Wine E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis. 2012;18:1799-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 896] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 17. | Rajilić-Stojanović M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013;19:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Kump PK, Gröchenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, Deutschmann A, Wenzl HH, Petritsch W, Krejs GJ. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 19. | Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1335] [Article Influence: 121.4] [Reference Citation Analysis (3)] |
| 20. | Ji J, Lu Y, Liu H, Feng H, Zhang F, Wu L, Cui Y, Wu H. Acupuncture and moxibustion for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:158352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Wu HG, Zhou LB, Shi DR, Liu SM, Liu HR, Zhang BM, Chen HP, Zhang LS. Morphological study on colonic pathology in ulcerative colitis treated by moxibustion. World J Gastroenterol. 2000;6:861-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Joos S, Wildau N, Kohnen R, Szecsenyi J, Schuppan D, Willich SN, Hahn EG, Brinkhaus B. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Mu JP, Wu HG, Zhang ZQ, Liu HR, Zhu Y, Shi Z, Wang XM. [Meta-analysis on acupuncture and moxibustion for treatment of ulcerative colitis]. Zhongguo Zhen Jiu. 2007;27:687-690. [PubMed] |
| 24. | Han Y, Ma TM, Lu ML, Ren L, Ma XD, Bai ZH. Role of moxibustion in inflammatory responses during treatment of rat ulcerative colitis. World J Gastroenterol. 2014;20:11297-11304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Wang X, Liu Y, Dong H, Wu L, Feng X, Zhou Z, Zhao C, Liu H, Wu H. Herb-Partitioned Moxibustion Regulates the TLR2/NF-κB Signaling Pathway in a Rat Model of Ulcerative Colitis. Evid Based Complement Alternat Med. 2015;2015:949065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Wu HG, Huang Z, Liu HR, Zhang W, Shi Y, Zhu Y, Cui YH, Liu SM. [Experimental study on influence of acupuncture and moxibustion therapy on apoptosis of colonic epithelial cells in rats of ulcerative colitis]. Zhongguo Zhen Jiu. 2005;25:119-122. [PubMed] |
| 27. | Wu HG, Liu HR, Tan LY, Gong YJ, Shi Y, Zhao TP, Yi Y, Yang Y. Electroacupuncture and moxibustion promote neutrophil apoptosis and improve ulcerative colitis in rats. Dig Dis Sci. 2007;52:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Huang Y, Zhao JM, Guan X, Zhang JB, Dou CZ, Shi Z, Liu HR, Wang XM, Wu HG. Effect of Warming Moxibustion on P2RX7 Regulation in Tianshu Acupoint Skin of Rats with Ulcerative Colitis. Shijie Kexuejishu - Zhongyiyao Xiandaihua. 2016;18:389-394. |
| 29. | Zhou EH, Liu HR, Wu HG, Shi Z, Zhang W, Zhu Y, Shi DR, Zhou S. Down-regulation of protein and mRNA expression of IL-8 and ICAM-1 in colon tissue of ulcerative colitis patients by partition-herb moxibustion. Dig Dis Sci. 2009;54:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Wang XM, Lu Y, Wu LY, Yu SG, Zhao BX, Hu HY, Wu HG, Bao CH, Liu HR, Wang JH. Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis. World J Gastroenterol. 2012;18:6819-6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1296] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 32. | The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006; Available from: http://www.most.gov.cn/fggw/zfwj/zfwj2006/200609/t20060930_54389.htm. |
| 33. | Fichna J, Dicay M, Lewellyn K, Janecka A, Zjawiony JK, MacNaughton WK, Storr MA. Salvinorin A has antiinflammatory and antinociceptive effects in experimental models of colitis in mice mediated by KOR and CB1 receptors. Inflamm Bowel Dis. 2012;18:1137-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods. 2008;5:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 911] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 36. | Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141-D145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3794] [Cited by in RCA: 3483] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 37. | Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5694] [Cited by in RCA: 6300] [Article Influence: 525.0] [Reference Citation Analysis (0)] |
| 38. | Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123-3124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2193] [Cited by in RCA: 2720] [Article Influence: 247.3] [Reference Citation Analysis (0)] |
| 39. | Parks DH, Beiko RG. Identifying biologically relevant differences between metagenomic communities. Bioinformatics. 2010;26:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 691] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 40. | Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 41. | Lucke K, Miehlke S, Jacobs E, Schuppler M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol. 2006;55:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 570] [Article Influence: 51.8] [Reference Citation Analysis (1)] |
| 43. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1376] [Article Influence: 80.9] [Reference Citation Analysis (1)] |
| 44. | Trier JS. Mucosal flora in inflammatory bowel disease: Intraepithelial bacteria or endocrine epithelial cell secretory granules? Gastroenterology. 2002;123:955; author reply 956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Hanning IB, Lingbeck JM, Ricke SC. Probiotics and Heart Health: Reduction of Risk Factors Associated with Cardiovascular Disease and Complications Due to Foodborne Illnesses. Bioactive Foods in Promoting Health: Probiotics and Prebiotics. Amsterdam: Elsevier Inc 2010; 423-439. [DOI] [Full Text] |
| 46. | Zhang D, Ren YB, Wu HG, Yang YT, Wu LJ, Zhang J, Shi Z, Ma XP. [Effect of Different Doses of Herbal Cake-partitioned Moxibustion on Histopathological Changes of Colon Tissue in Ulcerative Colitis Rats]. Zhen Ci Yan Jiu. 2018;43:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Yang X, Fu Y, Liu J, Ren HY. Impact of probiotics on toll-like receptor 4 expression in an experimental model of ulcerative colitis. J Huazhong Univ Sci Technolog Med Sci. 2013;33:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Imaoka A, Shima T, Kato K, Mizuno S, Uehara T, Matsumoto S, Setoyama H, Hara T, Umesaki Y. Anti-inflammatory activity of probiotic Bifidobacterium: enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J Gastroenterol. 2008;14:2511-2516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espín-Basany E, Guarner F, Malagelada JR. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm Bowel Dis. 2009;15:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731-16736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2747] [Cited by in RCA: 3200] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 51. | Andoh A, Imaeda H, Aomatsu T, Inatomi O, Bamba S, Sasaki M, Saito Y, Tsujikawa T, Fujiyama Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol. 2011;46:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 52. | Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1677] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 53. | Liu B, Piao X, Guo L, Wang G, Sun W, Gao L, Zheng X, Fang Y. A New Chinese Medicine Intestine Formula Greatly Improves the Effect of Aminosalicylate on Ulcerative Colitis. Evid Based Complement Alternat Med. 2017;2017:7323129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, Bernklev T, Henriksen M, Sauar J, Vatn MH. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (0)] |