Published online Jun 21, 2018. doi: 10.3748/wjg.v24.i23.2441
Peer-review started: March 26, 2018
First decision: April 18, 2018
Revised: May 4, 2018
Accepted: May 18, 2018
Article in press: May 18, 2018
Published online: June 21, 2018
Processing time: 81 Days and 21.9 Hours
Liver transplantation (LT) is one of the most effective treatments for end-stage liver disease caused by related risk factors when liver resection is contraindicated. Additionally, despite the decrease in the prevalence of hepatitis B virus (HBV) over the past two decades, the absolute number of HBsAg-positive people has increased, leading to an increase in HBV-related liver cirrhosis and hepatocellular carcinoma. Consequently, a large demand exists for LT. While the wait time for patients on the donor list is, to some degree, shorter due to the development of living donor liver transplantation (LDLT), there is still a shortage of liver grafts. Furthermore, recipients often suffer from emergent conditions, such as liver dysfunction or even hepatic encephalopathy, which can lead to a limited choice in grafts. To expand the pool of available liver grafts, one option is the use of organs that were previously considered “unusable” by many, which are often labeled “marginal” organs. Many previous studies have reported on the possibilities of using marginal grafts in orthotopic LT; however, there is still a lack of discussion on this topic, especially regarding the feasibility of using marginal grafts in LDLT. Therefore, the present review aimed to summarize the feasibility of using marginal liver grafts for LDLT and discuss the possibility of expanding the application of these grafts.
Core tip: There are few reviews concerning the feasibility of using marginal liver grafts in living donor liver transplantation (LDLT). We reviewed more than 300 articles, summarized new findings, and confirmed that marginal grafts are a feasible option for expanding options for patients on liver transplant waiting lists in emergency situations in LDLT (e.g., liver failure or hepatic encephalopathy). However, such grafts place the recipients at greater risk for adverse events. Although some indispensable treatments are needed to address the deficiencies of these grafts, recipients can receive a favorable prognosis, similar to that of patients who receive standard liver grafts, under these treatments.
- Citation: Lan X, Zhang H, Li HY, Chen KF, Liu F, Wei YG, Li B. Feasibility of using marginal liver grafts in living donor liver transplantation. World J Gastroenterol 2018; 24(23): 2441-2456
- URL: https://www.wjgnet.com/1007-9327/full/v24/i23/2441.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i23.2441
The high mortality of patients on waiting lists due to the shortage of cadaveric donors is a major challenge in liver transplantation (LT)[1]. This challenge has led to the emergence of living donor liver transplantation (LDLT) after the first successful procedure in 1989[2,3]. However, following a sharp increase in recipients who suffer from emergency situations, the wide gap between the demands of patients and suitable living donors is gradually increasing[4,5]. Therefore, the transplantation community has focused on the search for strategies to increase the pool of available liver grafts, including the use of organs that were previously considered “unusable” by many and often labeled “marginal” organs[6].
An accepted definition of marginal donors remains unclear in LDLT. These expanded-criteria grafts have the potential to increase the risk of poor graft function or primary nonfunction and are referred to as “marginal” organs[7]. In this review, we define marginal liver grafts for LDLT as small-for-size grafts, older donors, moderate or severe steatosis of liver grafts, chronic hepatitis, and grafts with tumors. The survival of recipients with marginal organs can be the same as that of patients with high-quality liver grafts under proper treatment[8].
Many previous studies have reported on the possibilities of using marginal grafts in orthotopic liver transplantation (OLT), but there is still a lack of discussion on this topic, especially regarding the feasibility of using marginal grafts in LDLT. Therefore, the present review aimed to summarize and discuss the possibility of expanding the application of marginal grafts in LDLT.
Choosing to use a liver graft can be a remarkably complex decision. There is an increasing trend of patients dying while on waiting lists due to the everyday risk of death or serious complications while waiting; this risk must be balanced against the use of a marginal graft, which may not be feasible. Size mismatching between the graft and the recipient is a critical predictor of the so-called “marginal liver grafts” in LDLT recipients. A small-for-size graft has become the main reason for unsuitability for liver donation in some transplantation centers[9]. The most common index with which to evaluate graft size matching is the graft-to-recipient weight ratio (GRWR) or graft volume (GV)/standard liver volume (SLV). The GRWR was first reported to require a safety range of above 1%; otherwise, the rate of graft survival could decrease[10]. With the increased demand for LDLT and the improvement of surgical techniques, however, many expanded-criteria grafts are used. Accordingly, the accepted arbitrary requirement for GRWR was reduced to 0.8%, and the GV/SLV value was 40%[11,12]. As many transplantation centers accumulated experience on small-for-size grafts for LDLT, grafts with a GRWR < 0.8% were used and reported to be as safe as those with a GRWR ≥ 0.8%[13-17]. After challenging the boundary of GRWR = 0.8%, the acceptable minimum GRWR has been continuously lowered. Lee SD et al[18] reported that a GRWR as low as 0.7% is safe and that there is no need to modulate portal pressure in adult-to-adult LDLT using the right-lobe in favorable conditions, such as a low Model for End-Stage Liver Disease (MELD) score. Furthermore, Alim A et al[19] even suggested that a GRWR as low as 0.6% may be safe if the MELD score is < 20, donor age is < 45, and there is no evidence of liver steatosis in the donor graft during portal inflow modulation performed according to the portal flow. To date, the reported lowest GRWR of grafts that have been successfully used is between 0.40% and 0.46% (Table 1)[20].
| Ref. | Recommended minimum GRWR | n (small vs large) | One-year survival (small vs large) | Five-year survival (small vs large) | Study type |
| Kiuchi et al[10] (1999) | 1% | 276 (49 vs 215) | 61.2% vs 92.6% | NS | RS |
| Lee et al[11] (2003) | 0.8% | 141 (10 vs 131) | Univariate and multiple analysis | NS | RS |
| Moon et al[13] (2010) | Less than 0.8% | 427 (35 vs 392) | 87.8% vs 90.7% | 74.1% vs 79.4% | RS |
| Lan et al[14] (2009) | Less than 0.8% | 89 (15 vs 74) | 73.3% vs 71.6% | NS | RS |
| Selzner et al[15] (2009) | Less than 0.8% | 271 (22 vs 249) | 91.0% vs 89.0% | 83.0% vs 87% | RS |
| Chen et al[16] (2014) | Less than 0.8% | 196 (45 vs 151) | 82.2% vs 81.4% | 71.1% vs 75.5% | RS |
| She et al[17] (2017) | Left lobe graft vs right lobe graft | 218 (19 vs 199) | 89.5% vs 95.9% | 89.5% vs 86.8% | RS |
| Lee et al[18] (2014) | Less than 0.7% | 317 (23 vs 294) | 100% vs 93.2% | NS | RS |
| Alim et al[19] (2016) | 0.6% | 649 | Seven patients had GRWR of 0.6%. If MELD score was below 20, donor age below 45, and no signs for any hepatosteatosis, GRWR of 0.6% was safe | RS | |
| Lee et al[20] (2015) | 0.40% | NS | Lowest GRWR of 0.40% had been successfully used | RS | |
Small-for-size syndrome (SFSS), including small-for-size dysfunction (SFSD) and small-for-size nonfunction (SFSNF), is a concerning and life-threatening complication in patients receiving grafts with a GRWR < 0.8%[21,22]. The incidence of SFSS varies from 4.7% to 27.5% in different LT centers[23-30]. Specifically, the syndrome rate can be as high as 50%-75% in left-lobe LDLT or small-for-size grafts group and as low as 8.4% in right-lobe LDLT[31,32]. Graft size is the only independent predictor of SFSS[31]. However, other studies have described that SFSS can occur even in the presence of a normal GRWR[16]. Regardless of the definition used for SFSS, it seems clear that other key factors should be considered in addition to a mismatched graft size. The incidence of SFSS is listed in Table 2.
| Ref. | n | SFSS (Incidence) | Factors to SFSS | Study type |
| Goldaracena et al[21] (2017) | NS | NS | A graft GRWR < 0.8% of predisposes the graft to SFSS | RE |
| Graham et al[22] (2014) | NS | NS | GRWR of 0.8 to 1.0 was established as a lower limit to prevent SFSS | RE |
| Botha et al[23] (2010) | 21 | 1 (4.7) | Hemi-portocaval shunt can decrease SFSS incidence | RS |
| Goralczyk et al[24] (2011) | 22 | 5 (22.7) | Posterior cavoplasty can decrease SFSS incidence | RS |
| Soejima et al[25] (2003) | 36 | 8 (22.2) | Cirrhosis predisposes the graft to SFSS | RS |
| Ben-Haim et al[26] (2001) | 40 | 5 (8) | Child’s class B or C with received grafts of GRWR < 0.85% predisposes the graft to SFSS | RS |
| Sudhindran et al[27] (2012) | NS | 10%-20% | Left lobe grafts predisposes the graft to SFSS | RE |
| Yi et al[28] (2008) | 29 | 8 (27.5) | Left lobe grafts predisposes the graft to SFSS | RS |
| Soejima et al[29] (2012) | 312 | 43 (15.3) | Left lobe grafts predisposes the graft to SFSS | RS |
| Gruttadauria et al[30] (2015) | 83 | 13 (15.7) | Non-surgical modulation of the portal inflow can decrease SFSS incidence | RS |
| Shoreem et al[31] (2017) | 174 | 20 (11.5) | Left lobe grafts predisposes the graft to SFSS | RS |
| Lauro et al[32] (2007) | 8 | 4 (50) | Surgical modulation of the portal inflow can decrease SFSS incidence | RS |
Middle hepatic vein (MHV) or outflow reconstruction of the liver graft is associated with size mismatch. A small-for-size graft without MHV reconstruction can lead to various degrees of congestion of the anterior segment and a greater loss of hepatocellular function[33]. In our early observational studies with small sample sizes, we recommended a GRWR > 1.0%[34] or even 1.2%[35] as a security threshold for patients without MHV reconstruction. Asakuma M et al[36] established an algorithm known as the estimated congestion ratio (ECR, ECR = regional volume of v5 + v8 / right-lobe volume) to estimate whether MHV should be reconstructed for low-GRWR grafts. A liver with an ECR > 0.4 is an MHV-dominant liver, and higher GRWR grafts should be used. However, it is still unknown how far we can lower the GRWR following the improvement of postoperative management and surgical technique if there is no reconstruction of outflow. In addition to outflow reconstruction, the inflow of grafts, including portal hypertension following reperfusion and the hyperdynamic splanchnic state, is reported as a major factor that can trigger SFSS[37-39]; however, these views are controversial[40]. Enhanced cholestasis, hepatocyte ballooning, disruption of the sinusoidal line, and transformation of activated Ito cells into fibroblasts are observed under the conditions of portal hypertension, or overperfusion[41,42]. Recipients with a final portal vein pressure (PVP) ≤ 15 mmHg or a pressure gradient of PVP-central vein pressure (CVP) ≤ 5 mmHg have a better prognosis[43]. In another study, liver-graft-to-spleen-volume ratio was used to predict early graft function in children and young adults undergoing LDLT, in which < 0.88 predicted portal hyperperfusion[44]. Moreover, a MELD score > 20[45], a decline in the platelet (PLT) count at post operation day (POD) 3 > 56%[46] and donor age > 45 years are also risk factors for a poor prognosis in recipients of small-for-size grafts[19].
To increase the safety of the expanded use of small-for-size grafts, some treatments are recommended. Graft inflow or PVP modulation is at the forefront of these treatments. Portosystemic shunting techniques or preservation of collateral veins[19,47-50], as well as splenectomy or splenic artery ligation/embolization[51-53], are effective ways to address post-transplantation portal hyperperfusion. In cases where the GRWR of grafts is very low, dual grafts can be considered[54]. Moreover, autologous stem cell implantation[55] and auxiliary partial LDLT (a second transplant) are also reported to treat SSFS[56]. Remedies when using small-for-size grafts are listed in Table 3.
| Ref. | n | Remedy for using small-for-size graft | Study type |
| Botha et al[23] (2010) | 21 | Hemi-portocaval shunt can decrease SFSS incidence | RS |
| Goralczyk et al[24] (2011) | 22 | Posterior cavoplasty can decrease SFSS incidence | RS |
| Kim et al[47] (2017) | 160 | Preserving collateral veins on small-for-size grafts | RS + PSM |
| Hessheimer et al[48] (2011) | NS | Portocaval shunt | AE |
| Xiao et al[49] (2012) | 1 | Transjugular intrahepatic portosystemic shunt | CR |
| Sato et al[50] (2008) | 4 | Portocaval shunt using ligamentum teres | CR |
| Nutu et al[51] (2018) | 2 | Complete splenic embolization | CR |
| Badawy et al[52] (2017) | 164 | Splenectomy | RS |
| Troisi et al[53] (2016) | NS | Splenic artery ligation, splenectomy, meso-caval shunt, spleno-renal shunt, portocaval shunt, and splenic artery embolization | SR |
| Xu et al[54] (2015) | NS | Dual grafts | RE |
| Gao et al[55] (2017) | NS | Adipose-derived mesenchymal stem cells tranplantation | AE |
| Kobayashi et al[56] (2009) | 5 | Auxiliary partial liver transplantation | CR |
Generally, a GRWR < 0.8% is no longer a critical predictor for recipients and can even be lowered to 0.5%-0.6% if there are accompanying factors of PVP ≤ 15 mmHg, MHV reconstruction, or young donor age.
Because LDLT allows more choices in the use of a suitable liver graft compared with OLT, elderly donors were rarely considered in the early years of transplantation. However, following the increasing demands for LDLT and the urgent need to save the lives of patients suffering from hepatic encephalopathy, the use of elderly liver grafts has been reported more frequently in recent years as a means to increase the donor pool and address high waiting list mortality[57]. In Japan, the percentages of donors older than 50 and 60 years were 18.1% and 4%, respectively[58]. It is expected that the number of older donors will increase in the future because of the continuing donor shortage[59].
The definition of older donors is quite different in different transplantation centers. In the present review, we define older donors as donors older than 50 years. Controversy exists regarding the use of livers from older donors. The liver regeneration rate is impaired in older donors (donor age ≥ 50 years) compared with young donors (donor age < 30 years), according to computed tomography (CT) volumetric data after LDLT at POD 7[60], and donor age (≥50 years) was independently correlated with impaired remnant liver regeneration at 3[61] and 6 mo in right-lobe LDLT[58]. Kawano Y et al[62] analyzed telomeres in the hepatocytes of 12 paired donor-recipients and found that donor age was a crucial factor affecting the sustainability of telomere length in hepatocytes after pediatric LDLT. Based on the conclusion that older donors were significantly associated with impaired liver regeneration, some researchers found that the recipients of grafts from donors older than 45-50 years old, along with a GW/SLV ratio < 35%-40%, had worse outcomes[63,64]. Yoshizumi T et al[65] established the following formula, called a predictive score, to evaluate the impact of donor age, graft size, and MELD score on prognosis: predictive score = 0.011 × graft weight (%) - 0.016 × donor age - 0.008 × MELD score - 0.15 × shunt (if present) - 1.757. Patients with a predictive score ≥ 1.3 had a lower incidence of postoperative complications and a better prognosis.
Additionally, more studies have shown that LDLT using older donors could induce more serious postoperative complications and higher mortality rates than transplants using younger donors[66-70]; similarly, having a donor older than the recipient by > 20 years is problematic[68]. Moreover, it has been reported that fibrosis progression in patients with recurrent hepatitis C tended to be faster after LDLT with grafts from older donors[71]. Donor age is an independent, strong prognostic factor in LDLT. However, other researchers found that grafts from older donors can be used safely, even though the regenerative capacity of older grafts is impaired when the donor age is ≥ 50 years[72-75] or even ≥ 55 years[76]. The impact of older donors on the 1- and 5-year survival of recipients is shown in Table 4.
| Ref. | Definition of older donors | n (older vs young) | One-year survival (older vs young) | Five-year survival (older vs young) | Study type |
| Tanemura et al[58] (2012) | 50 yr old | 101 (24 vs 77) | Older donor livers might have impaired regenerative ability | RS | |
| Ono et al[60] (2011) | 50 yr old | 15 (6 vs 9) | Liver regeneration is impaired with age after donor hepatectomy | RS | |
| Akamatsu et al[61] (2007) | 50 yr old | 299 (62 vs 237) | 85.0% vs 93.0% | 72.0% vs 87.0% | RS |
| Kawano et al[62] (2014) | NS | 12 | Donor age is a crucial factor affecting telomere length sustainability in hepatocytes after pediatric LDLT | PS | |
| Imamura et al[63] (2017) | NS | 198 | A worse outcome might be associated with aging of the donor | RS | |
| Dayangac et al[64] (2011) | 50 yr old | 150 (28 vs 122) | 78.6% vs 83.4% | NS | RS |
| Yoshizumi et al[65] (2008) | NS | 28 | Graft size, donor age, and patient status are the indicators of early graft function | RS | |
| Han et al[66] (2014) | 55 yr old | 604 (26 vs 578) | Median OS (M): 31.2 ± 31.3 vs 50.6 ± 40.6 | RS | |
| Kamo et al[67] (2015) | 60 yr old | 1597 (69 vs 1528) | 69.5% vs 81.2% | 62.0% vs 79.3% | RS |
| Shin et al[68] (2013) | Donor-recipient age gradient > 20 | 821 | Worse graft survival was observed if the donor is older than the recipient by > 20 | RS | |
| Kubota et al[69] (2017) | 50 yr old | 315 (126vs 189) | 73.0% vs 80.9% | 39.7% vs 47.1% | RS |
| Katsuragawa et al[70] | NS | 24 | G/SLV and donor age were independent factors that affected graft survival rates | RS | |
| Wang et al[72] (2015) | 50 yr old | 159 (10 vs 149) | 100% vs 93.0% | 90.0% vs 87.0% | RS |
| Ikegami et al[73] (2008) | 50 yr old | 232 (32 vs 200) | 80.0% vs 81.7% | 73.8% vs 76.7% | RS |
| Li et al[74] (2012) | 50 yr old | 129 (21 vs 108) | 90.0% vs 86.0% | 66.0% vs 75% | RS |
| Goldaracena et al[75] (2016) | 50 yr old | 469 (91 vs 378) | 92.0% vs 96.0% | 83.0% vs 79.0% | RS |
| Kim et al[76] (2017) | 55 yr old | 540 (42 vs 498) | 95.2% vs 94.6% | NS | RS |
While donor age is a controversial topic, the impaired regenerative capacity of older grafts has been confirmed in some studies. According to these previous studies, older liver grafts can be prudent candidates but cannot be used in the presence of other marginal conditions (e.g., small-for-size grafts or moderate and severe steatosis). More high-quality and prospective studies are needed on this topic.
Although more high-quality liver grafts are available for patients in LDLT than in OLT, donors are restricted to family members or domestic relationships in many transplantation centers because of ethical norms. ABO-incompatible LTs are performed only in emergencies, when ABO-compatible grafts are unavailable. Therefore, breaking ABO blood group barriers becomes inevitable. ABO-incompatible LT was first performed and reported by Starzl et al[77], and no acute rejections were observed after transplantation. Subsequently, ABO-incompatible LT gradually began to be performed in some LT centers, and hyperacute rejection was commonly reported[78,79].
In addition to antibody-mediated rejection, ABO-incompatible LDLT can involve other complications. Thrombotic microangiopathy (TMA) is a rare complication following LT, but it is reported to have a slightly higher incidence in ABO-incompatible LDLT[80-82]. ABO incompatibility, cyclophosphamide and recipient blood group (type O) are closely correlated with the occurrence of TMA[80,82]. The incidence of TMA is 37.9% following ABO-incompatible LDLT and 0.0%-2.8% following ABO-compatible LDLT (OR = 44.7)[80]. The elevation of fibrinolytic function markers, such as plasminogen activator inhibitor type 1, can be considered a predictor of TMA following LDLT. The incidence of biliary tract complications is more common than that of TMA. Biliary strictures are one of the most important complications associated with ABO incompatibility, with reported incidence rates between 15.8% and 20.7%[83,84]. An isoagglutinin attack on the graft vascular endothelium can result in ischemic cholangiopathy, and isoagglutinin can even directly attack the endothelium of the graft bile duct[85,86]. CT scans can provide a clear indication of biliary strictures in ABO-incompatible LDLT[87]. Yamada Y et al[88] reported a case of idiopathic hypereosinophilic syndrome following ABO-incompatible LDLT. The patient suffered from portal vein thrombosis on postoperative day 10, and the histopathological findings of the thrombus revealed dense eosinophilic deposition. Studies on the impact of ABO incompatibility on LDLT are listed in Table 5.
| Ref. | n | Complications | Incidence of related complication (%) | Risk factors | Study type |
| Miyata et al[80] (2007) | 57 | Thrombotic microangiopathy | 7.0 | ABO-incompatibility, CPA, and recipient blood group (type O) | RS |
| Oya et al[81] (2008) | 1 | Thrombotic microangiopathy | NS | ABO-incompatible LDLT (type B to O) | CR |
| Kishida et al[82] (2016) | 129 | Thrombotic microangiopathy | 10.1 | ABO-incompatible, tacrolimus | RS |
| Song et al[83] (2014) | 1102 | Biliary stricture | 15.8 | ABO-incompatible, acute cellular rejection | RS |
| Ikegami et al[84] (2016) | 408 | Biliary stricture | 20.4 | ABO-incompatible | RS |
| Yamada et al[88] (2010) | 1 | Idiopathic hypereosinophilic | NS | ABO-incompatible | CR |
Despite serious complications, ABO-incompatible LDLT can be a feasible option for patients if certain essential treatments are included[89,90]. Rituximab, an anti-CD20 immunoglobulin (IgG)1 terminating B-lymphocytes with an affinity for IgG Fc receptor (FcγR), is a critical strategy in the regimens for desensitization for ABO-incompatible LDLT and yields outcomes for ABO-incompatible LDLT that are similar to those for ABO-compatible LDLT[91,92]. Rituximab is given for 3 d[93], 3 wk, or even as soon as a suitable donor that is ABO-compatible is selected[94] at a dosage of 375 mg/m2. In the early stage of transplantation, rituximab was usually given along with one or more other protocols, such as a splenectomy[95,96], plasma exchanges[97-102], intravenous IgG[100,103], and intrahepatic arterial infusion of prostaglandin E1[92,104,105]. In some recent studies, pre-transplant rituximab and/or basiliximab monotherapy, without additional treatments, also yielded outcomes that are comparable to those of procedures with additional treatments[106]. The affinity between IgG Fcγ Receptor (FcγR) and rituximab, however, is influenced by the single-nucleotide polymorphisms (SNPs) of FcγR. SNPs of FCGR2A (131H/R) and FCGR3A (158F/V) are the alleles that encode FcγR. FCGR2A (131H/H) had a higher affinity for IgG1 than FCGR2A (131H/R or R/R). Accordingly, patients with FCGR2A (131H/H) have a better reaction to the effects of rituximab on B cells[91]. The treatment results of ABO-compatible LDLT are summarized in Table 6.
| Ref. | n | Immunosuppression strategy | Remedies | Conclusion | Study type |
| Kawagishi et al[89] (2009) | 105 | TAC + MP + AZ | Rituximab | ABO-incompatible LDLT can be feasible used if humoral rejection are overcome | RS |
| Yoon et al[90] (2018) | 918 | TAC + MP + steroids | Rituximab and PE | ABO-incompatible LDLT is a feasible option under remedies | RS |
| Sakai et al[91] (2017) | 20 | TAC+ MP | Rituximab and PE | FCGR SNPs influence the effect of rituximab on B-cells | PS |
| Egawa et al[92] (2017) | 33 | TAC | Rituximab, PE, local infusion, splenectomy and immunoglobulins | Only rituximab dose is a significantly favorable factor for AMR | RS |
| Ikegami et al[93] (2007) | 1 | TAC + MP + steroids | Rituximab and PE | Rituximab and plasma exchanges seemed ineffective | CR |
| Ikegami et al[94] (2009) | 7 | TAC + MP + steroids | Rituximab, IVIG, and PE | Rituximab, IVIG, and PE seems to be a safe treatment | RS |
| Usui et al[95] (2007) | 73 | TAC + MP + steroids | Rituximab, PE and splenectomy | Bone suppression is a big challenge when using rituximab | RS |
| Chen et al[96] (2017) | 2 | TAC + MP + steroids | Basiliximab combine with splenectomy | ABO-i LDLT with splenectomy is undoubtedly life-saving | CR |
| Uchiyama et al[97] (2011) | 15 | TAC + MP + steroids | Rituximab and PE | Isoagglutinin mediated-rejection should be more concerned | RS |
| Soin et al[98] (2014) | 3 | TAC + MP + steroids | Rituximab and PE | ABO-incompatible LDLT is a feasible option under remedies | CR |
| Rummler et al[99] (2017) | 10 | TAC + MP + steroids | PE | Immunosuppression only combining with PE is feasible | RS |
| Kim et al[100] (2016) | 182 | TAC + MP + steroids | Rituximab, IVIG, and PE | ABO-incompatible LDLT can be safely performed under remedies | RS |
| Kim et al[101] (2013) | 22 | TAC + MP + steroids | Rituximab and PE | ABO-incompatible LDLT can be safely performed under remedies | RS |
| Kawagishi et al[102] (2005) | 3 | TAC + MP + steroids | Rituximab and PE | ABO-incompatible LDLT can be safely performed under remedies | CR |
| Kim et al[103] (2017) | 43 | TAC + MP + steroids | Rituximab and IVIG | A simplified protocol using rituximab and IVIG for ABO-I LDLT is safe | RS |
| Yoshizawa et al[104] (2005) | 8 | TAC + MP + cyclophosphamide | Rituximab and PGE1 infusion | Rituximab prophylaxis and HA infusion therapy is feasible | RS |
| Egawa et al[105] (2008) | 118 | TAC + steroids | Methylprednisolone and PGE1 infusion | Recipients with preexisting high effector CD8 T- cells are unfavorable candidates for ABO-I LDLT | RS |
| Yamamoto et al[106] (2018) | 40 | TAC + MP + steroids | Rituximab monotherapy | Rituximab monotherapy is feasible | RS |
These findings reveal that rituximab monotherapy in ABO-compatible LDLT is feasible, but it is better to test the SNPs of FcγR; otherwise, multiple treatments, such as plasma exchanges and intravenous IgG, must be performed in addition to rituximab if there is a lower affinity between IgG FcγR and rituximab. There is still a lack of more persuasive evidence to confirm the feasibility of splenectomy in conjunction with ABO-compatible LDLT treatments.
Steatosis is a common feature used to identify marginal liver function, and reports on the utility of steatotic liver grafts in clinical practice have yielded controversial results. The use of steatotic liver grafts has been confirmed to have a significant relationship with increased complications and poorer outcomes[107,108]. Traditionally, steatotic livers with > 60% fat must be discarded. Livers with < 30% fat are feasible and anticipated to have good function. Livers with 30%-60% fat have poor results, with decreased graft survival and decreased patient survival[109]. Moreover, hepatic steatosis is reported to be a leading cause of donor rejection in LDLT[110]. In some transplantation centers, approximately 40% of donor grafts are discarded because of severe liver steatosis[9]. Because of the release of inflammatory cytokines and inhibition of the capacity to differentiate steatosis hepatocytes, the early regenerative capacity of the remnant liver is injured, and, as a result of impaired hepatocyte replication, compensatory expansion of hepatic progenitor cells occurs during steatotic liver regeneration after LDLT[111]. Furthermore, Cho et al[112] confirmed that hepatic steatosis is associated with intrahepatic cholestasis and transient hyperbilirubinemia during regeneration after LDLT. In this study, 67 LDLT recipients examined on POD 10 were scored based on the numbers of portal tracts per area of liver tissue and intrahepatic cholestasis, and the preoperative degree of macrovesicular steatosis was found to be independently associated with cholestasis after LDLT. However, these researchers also found that the long-term capacity of hepatocyte regeneration was not impaired after LDLT with mild macrovesicular steatosis grafts[113]. Based on this finding, some recent studies have found that moderately steatotic liver grafts and donors with a BMI ≥ 30 kg/m2 are not contraindications for LDLT, and complications and survival are not significantly different compared with those associated with non-steatosis grafts[114,115]. Moreover, the risk of steatosis was determined by the presence of microsteatosis and macrosteatosis, rather than the total quantitative degree of steatosis. The grafts with high microsteatosis (30%) mixed with macrosteatosis showed no significant difference in postoperative biochemical liver function, 2-wk graft regeneration, postoperative complications, and 5-year survival[116]. The studies on the impact of graft steatosis on LDLT outcomes are listed in Table 7.
| Ref. | n | Conclusion | Study type |
| Dirican et al[9] (2015) | 161 | Approximately 40% of donor grafts are discarded because of severe liver steatosis | RS |
| Perkins et al[109] (2006) | NS | Typically steatotic livers with > 60% fat are not transplanted; with < 30% fat are usable and anticipated to have good function; with 30%-60% fat give poor results | Comments |
| Kotecha et al[110] (2013) | 340 | Hepatic steatosis is a leading cause of donor rejection in LDLT | PS |
| Cho et a[111]l (2010) | 54 | Hepatocyte replication is impaired during steatotic liver regeneration after LDLT | PS |
| Cho et al[112] (2006) | 67 | Hepatic steatosis is associated with intrahepatic cholestasis and transient hyperbilirubinemia during regeneration | PS |
| Cho et al[113] (2005) | 55 | Mildly steatotic graft did not increase the risk of graft dysfunction or morbidity in LDLT | PS |
| Gao et al[114] (2009) | 24 | Moderately steatotic (30%-60%) liver grafts provide adequate function in the first phase after transplantation and can be used for transplantation | RS |
| Knaak et al[115] (2017) | 105 | Donors with BMI > 30, in the absence of graft steatosis, are not contraindicated for LDLT | RS |
| Han et al[116] (2015) | 211 | The risk of steatosis may be determined by the relative composition of MiS and MaS, rather than the total quantitative degree | RS |
To decrease the risk associated with fatty liver grafts, especially with severe steatosis, some treatments are suggested (Table 8). According to Oshita et al[117], donors who are diagnosed with hepatic steatosis pre-transplantation should undergo a diet treatment consisting of an 800-1400 kcal/d diet and a 100-400 kcal/d exercise regimen without drug treatment with a target body mass index of 22 kg/m². After these strategies, the average BMI was reduced from 23.3 ± 0.6 to 21.9 ± 0.4 kg/m². The liver biopsy results of most of these donors showed stage 0/1 fibrosis and minimal/mild steatosis after the diet therapy. In addition, surgical outcomes and overall survival did not significantly differ between the recipients of grafts from non-steatosis and diet-treated donors (with steatosis). In another study, bezafibrate (400 mg/d) was used along with a protein-rich (1000 kcal/d) diet and exercise (600 kcal/d) for 2-8 wk[118]. Even severely steatotic livers could be used for LDLT grafting subsequent to this short-term treatment regimen. Furthermore, a 1200 kcal/d diet and a minimum of 60 min/d of moderate cardio training are also recommended to rapidly reverse liver steatosis in donors[119]. In addition to lifestyle and dietary changes, dual-graft LDLT was reported when one donor had severe liver steatosis and another had a low GRWR[120].
| Ref. | n | Treatments | Study type |
| Oshita et al[117] (2012) | 128 | Diet treatment consisting of an 800 to 1400 kcal/d diet and a 100 to 400 kcal/d exercise regimen without drug treatment, targeting body mass index of 22 kg/m² | RS |
| Nakamuta et al[118] (2013) | 11 | Bezafibrate (400 mg/d) was used along with a protein-rich (1000 kcal/d) diet and exercise (600 kcal/d) for 2-8 wk | RS |
| Choudhary et al[119] (2015) | 16 | 1200 kcal/d and a minimum of 60 min/d of moderate cardio training are also recommended to rapidly reverse liver steatosis in donors | PS |
| Moon et al[120] (2006) | 2 | Dual-graft living donor liver transplantation for severe graft steatosis | CR |
In conclusion, steatosis in the donor must be thoroughly evaluated before LDLT, either by biopsy or imaging diagnosis. The proportion of macrosteatosis is now considered a crucial predictor of the prognosis of recipients. If there are no further options, donors with hepatic steatosis can reach donation criteria through lifestyle and dietary changes in a short time.
The use of liver grafts that test positive for chronic hepatitis or other blood disseminated diseases found in epidemic areas is usually inevitable in cases of organ shortages associated with OLT. However, because LDLT recipients, to some degree, have more choices regarding his/her donors, there are a few studies reporting on HBsAg or HBcAb(+) liver grafts, while no studies refer to HCV-positive living liver grafts.
HBsAg(-) LDLT patients who have received HBsAg or HBcAb(+) grafts have a high risk of de novo HBV infection after transplantation (Table 9). However, these grafts are still considered to be safe and feasible with antiviral prophylaxis in both adult and pediatric LDLT[121-126]. Patients were given HBV vaccinations to achieve anti-HBs > 1000 IU/L pre-transplantation and > 100 IU/L post-transplantation, with a standard post-transplantation treatment regimen of high-dose hepatitis B IgG, lamivudine and/or adefovir (in cases of lamivudine resistance)[126]. Specifically, some studies have proposed a new strategy; specifically, patients with a pre-transplantation anti-HB titer > 1000 IU/L do not need post-transplantation prophylaxis; patients with a low pre-transplantation titer, < 1000 IU/L, should be given lamivudine post-transplantation (at a dose of 100 mg/d or 3 mg/kg/d for at least 2 years after transplantation) or adefovir prophylaxis (with lamivudine at a dose of 10 mg/d if a mutant strain for lamivudine is identified) and, hopefully, will respond appropriately to post-transplantation vaccinations by maintaining anti-HB titers > 100 IU/L; and low titer non-responders (anti-HB titer < 100 IU/L despite vaccination) should be given continuous lamivudine or adefovir indefinitely[121]. In some transplantation centers, nucleotide analogs (lamivudine) are routinely used first if HBsAg(-) LDLT patients receive HBsAg or HBcAb(+) grafts, regardless of the anti-HB titer, for at least 2 years. Moreover, patients who had a YMDD mutation were given adefovir combined with lamivudine[123]. Hara Y et al[127] reported one patient who experienced spontaneous eradication of de novo HBV after LDLT with an HBcAb(+) graft without any treatment. This 8-year-old female patient (HBsAg-negative) underwent LDLT, received an HBcAb(+) left-lobe graft, and was subsequently infected with HBV. Sixteen years after LDLT, her serological HBV status was as follows: HbsAg(-), HBsAb(+), HBeAb(-), HBeAb(+), HbcAb(+), and HBV DNA(-). In another study, recipients with HCV genotype 2 infections who had received an HBcAb(+) graft were given sofosbuvir and ribavirin, along with hepatitis B IgG to prevent recurrence of HCV and HBV[128].
| Ref. | Donor | Incidence of de novo HBV infection (%) | Prevention of de novo HBV infection | Study type |
| Wang[121] (2017) | HBcAb(+) | 4.2 | HBV vaccinations with the aim of achieving anti-HBs > 1000 IU/L pre-transplant and > 100 IU/L post-transplant | RS |
| Xi et al[122] (2013) | HBcAb(+) | 23.9 | No prophylaxis, adefovir, and lamivudine are given to de novo patients | RS |
| Dong et al[123] (2017) | HBcAb(+) | 7.9 | HBIG 100 IU/kg during the operation and lamivudine 3 mg/kg per day after the surgery for at least 1 year until HBV vaccine reaction | RS |
| Loggi et al[124] (2016) | HBsAg(+) | NS | HBIG and lamivudine, adefovir or tenofovir | SR |
| Lei et al[125] (2013) | HBcAb(+) | 15.0 | No specific prophylaxis | RS |
| Lin et al[126] (2007) | HBcAb(+) | 3.3 | Lamivudine monoprophylaxis, HBV vaccinations | RS |
| Hara et al[127] (2016) | HBcAb(+) | NS | Lamivudine first and adefovir dipivoxil were combined with lamivudine 2 yr later | CR |
In HbsAg(+) LDLT patients who receive HBsAg or HBcAb(+) grafts, the antiviral protocol must be performed as for HBsAg(-) LDLT patients to maintain the HBV DNA at a low or negative level, despite the persistence of the HBV marker (HBsAg). High-dose HBV IgG, lamivudine, famciclovir, and interferon were recommended (Table 10)[129-131].
| Ref. | Donor | Incidence of de novo HBV infection | Prevention of De Novo HBV infection | Study type |
| Hwang et al[129] (2006) | HBsAg(+) | NS | High-dose HBIG and lamivudine, famciclovir and interferon; a final regimen of lamivudine and adefovir | CR |
| Soejima et al[130] (2007) | HBsAg(+) | NS | lamivudine and adefovir dipivoxil | CR |
| Jeng et al[131] (2015) | HBsAg(+) | NS | Entecavir 0.5 mg once daily | RS |
Populations with HBsAg-negative/HBcAb-positive and undetectable serum HBV DNA have been gradually increasing over the past several decades. Most patients are now considered to have a covert HBV infection and have a high risk of HBV reactivation when treated with a robust immunosuppressive agent[132]. Therefore, the use of HBsAg-negative/HBcAb-positive liver grafts has a high risk of de novo HBV for HBsAg(-) recipients. However, with active immunization and an antiviral protocol, the HBsAg-negative/HBcAb-positive liver grafts can be transplanted safely.
Usually, there are rare recipients of LDLT or doctors who are willing to make an active choice to use a graft with an undetermined tumor. This is not only an ethical issue but also indicates a high risk for recipients to face rapid dysfunction of their liver grafts. However, if recipients are in an emergency situation and have no other proper donors, grafts with benign tumors may be a last choice. Li G et al[133] recently reported on 15 consecutive recipients using an otherwise discarded, partial liver resection graft with a benign hepatic tumor. These benign tumors are as follows: Cavernous hemangioma, perivascular epithelioid cell tumor, inflammatory pseudotumor, and focal nodular hyperplasia. One patient died from a pulmonary embolism, and the other 14 patients had a good prognosis. Additionally, a vanishing tumor in a liver graft from an HBV(+) donor was observed. Contrast-enhanced magnetic resonance imaging (MRI) showed hypervascularity in the arterial phase and in the hepatobiliary phase, the tumor showed a low intensity, findings similar to those in HCC. Regardless, the graft with suspected HCC was accepted by the recipient, and the tumor disappeared completely within several months after LDLT[134].
For LDLT patients using grafts with a benign hepatic tumor, only two observational studies with a small sample size are present in the literature (Table 11). It seems that grafts with benign tumors are feasible in some conditions, but more studies with long-term follow-ups are needed to evaluate the safety of these marginal grafts.
| Ref. | n | Type of tumors in grafts | Prognosis | Study type |
| Li et al[133] (2017) | 15 | Cavernous hemangioma, perivascular epithelioid cell tumor, inflammatory pseudotumor, and focal nodular hyperplasia | One patient died from pulmonary embolism | OS |
| Fuchino et al[134] (2017) | 1 | HBsAg(+) and inflammatory pseudotumor | Tumor vanished after 3 yr | CR |
To our knowledge, this is the first review on marginal donors specifically for LDLT. This review, which includes cohort studies, case-control studies, and case reports on marginal liver grafts in LDLT, demonstrated that marginal grafts are a feasible way to expand the options for patients on LT waiting lists in emergency situations (e.g., liver failure or hepatic encephalopathy); however, these grafts place the recipients at a greater risk of liver dysfunction. Some indispensable treatments are needed to address the deficiencies of these grafts.
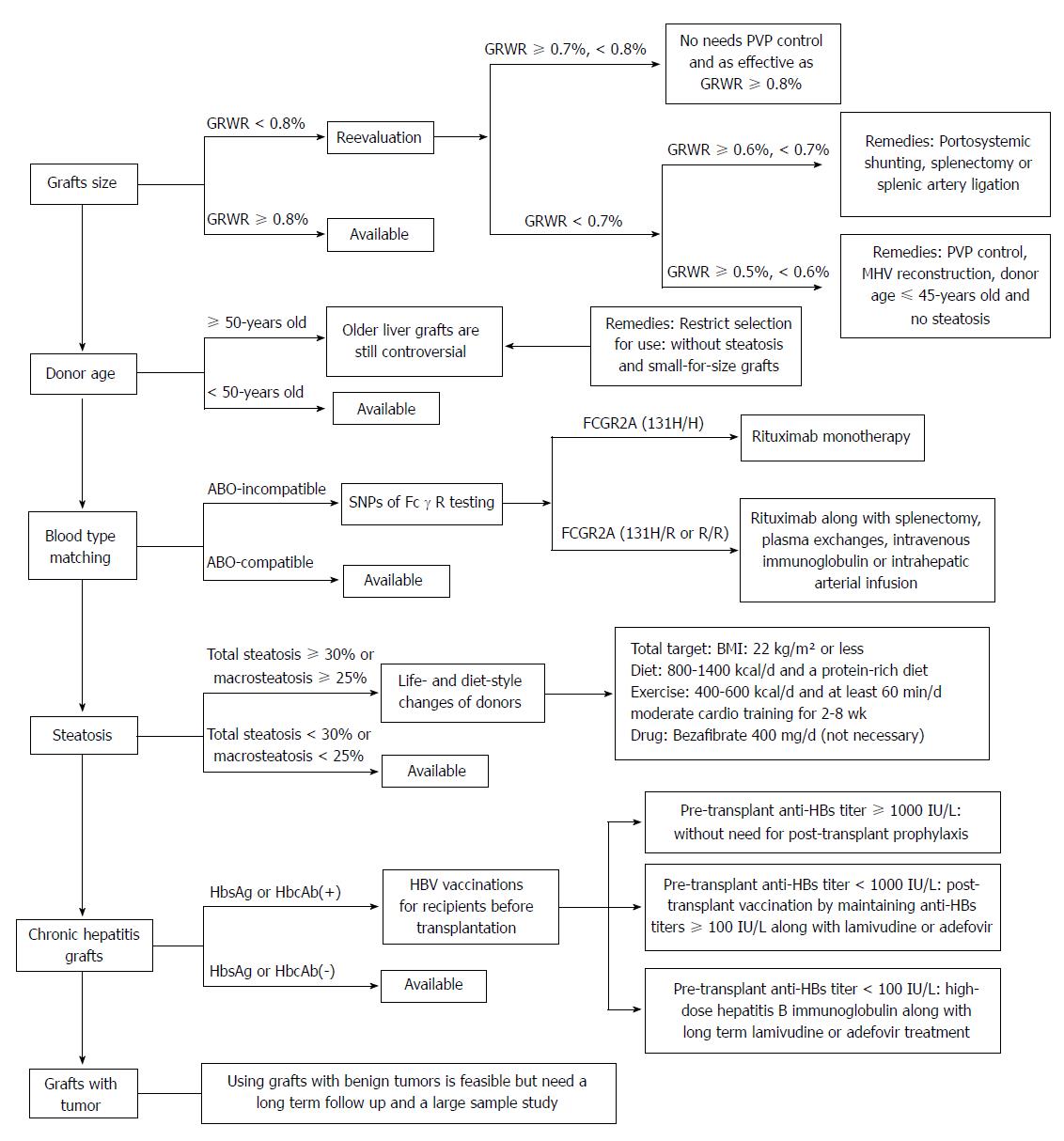
There are some new findings in this review: (1) It is permissible for the GRWR to be as low as 0.5%-0.6% (not 0.8%, as currently specified) if PVP is controlled under 15 mmHg; otherwise, outflow reconstruction is needed. (2) There is controversy surrounding older liver grafts. These grafts can be used prudently, but other marginal conditions must be absent (e.g., small-for-size grafts or moderate and severe steatosis). (3) Splenectomy is no longer necessary when an ABO-incompatible LDLT is performed. Rituximab monotherapy is even confirmed to be an effective treatment if there is a high affinity between IgG FcγR and rituximab. (4) Total steatosis of liver grafts is not a proper predictor of prognosis. Instead, the presence of microsteatosis and macrosteatosis is a crucial factor. Donors with steatosis of the liver can meet the donation criteria through lifestyle and dietary changes before surgery. (5) HbsAg or HbcAb(+) grafts increase the risk of de novo HBV infection after transplantation in HBsAg(-) LDLT patients but can also be used safely with active immunotherapy. And (6) Grafts with benign tumors that have been discarded from other patients are feasible, but the long-term prognosis cannot be determined.
According to the new findings of this review listed above, we summarized a selection of strategies for different types of marginal liver grafts in LDLT and their related treatments (Figure 1). With this review, based on more than 100 references, we expect that the transplantation pool can be effectively and safely expanded in the situation of organ shortage.
We thank Dr. Lunan Yan (Department of Liver Surgery and Liver Transplantation Center, West China Hospital of Sichuan University) for the assistance with data collection.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiu KW, Kanda T, Qi XS S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Fisher RA. Living donor liver transplantation: eliminating the wait for death in end-stage liver disease? Nat Rev Gastroenterol Hepatol. 2017;14:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Makuuchi M, Kawasaki S, Noguchi T, Hashikura Y, Matsunami H, Hayashi K, Harada H, Kakazu T, Takayama T, Kawarasaki H. Donor hepatectomy for living related partial liver transplantation. Surgery. 1993;113:395-402. [PubMed] |
| 3. | Makuuchi M, Kawarazaki H, Iwanaka T, Kamada N, Takayama T, Kumon M. Living related liver transplantation. Surg Today. 1992;22:297-300. [PubMed] |
| 4. | Routh D, Sharma S, Naidu CS, Rao PP, Sharma AK, Ranjan P. Comparison of outcomes in ideal donor and extended criteria donor in deceased donor liver transplant: a prospective study. Int J Surg. 2014;12:774-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Nure E, Lirosi MC, Frongillo F, Bianco G, Silvestrini N, Fiorillo C, Sganga G, Agnes S. Overextended Criteria Donors: Experience of an Italian Transplantation Center. Transplant Proc. 2015;47:2102-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Laing RW, Mergental H, Yap C, Kirkham A, Whilku M, Barton D, Curbishley S, Boteon YL, Neil DA, Hübscher SG. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open-label, non-randomised, prospective, single-arm trial. BMJ Open. 2017;7:e017733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Nemes B, Gelley F, Zádori G, Piros L, Perneczky J, Kóbori L, Fehérvári I, Görög D. Outcome of liver transplantation based on donor graft quality and recipient status. Transplant Proc. 2010;42:2327-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Giretti G, Barbier L, Bucur P, Marques F, Perarnau JM, Ferrandière M, Tellier AC, Kerouredan V, Altieri M, Causse X. Recipient Selection for Optimal Utilization of Discarded Grafts in Liver Transplantation. Transplantation. 2018;102:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Dirican A, Baskiran A, Dogan M, Ates M, Soyer V, Sarici B, Ozdemir F, Polat Y, Yilmaz S. Evaluation of Potential Donors in Living Donor Liver Transplantation. Transplant Proc. 2015;47:1315-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321-327. [PubMed] |
| 11. | Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, Kim SJ, Choi SH, Heo JS, Hyon WS, Kim GS. Factors affecting graft survival after living donor liver transplantation. Transplant Proc. 2004;36:2255-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Fan ST, Lo CM, Liu CL, Yong BH, Wong J. Determinants of hospital mortality of adult recipients of right lobe live donor liver transplantation. Ann Surg. 2003;238:864-869; discussion 869-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Moon JI, Kwon CH, Joh JW, Jung GO, Choi GS, Park JB, Kim JM, Shin M, Kim SJ, Lee SK. Safety of small-for-size grafts in adult-to-adult living donor liver transplantation using the right lobe. Liver Transpl. 2010;16:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Lan X, Li B, Wang XF, Peng CJ, Wei YG, Yan LN. [Feasibility of small size graft following living donor liver transplantation]. Zhonghua Wai Ke Za Zhi. 2009;47:1218-1220. [PubMed] |
| 15. | Selzner M, Kashfi A, Cattral MS, Selzner N, Greig PD, Lilly L, McGilvray ID, Therapondos G, Adcock LE, Ghanekar A. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Chen PX, Yan LN, Wang WT. Outcome of patients undergoing right lobe living donor liver transplantation with small-for-size grafts. World J Gastroenterol. 2014;20:282-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | She WH, Chok KS, Fung JY, Chan AC, Lo CM. Outcomes of right-lobe and left-lobe living-donor liver transplantations using small-for-size grafts. World J Gastroenterol. 2017;23:4270-4277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Lee SD, Kim SH, Kim YK, Lee SA, Park SJ. Graft-to-recipient weight ratio lower to 0.7% is safe without portal pressure modulation in right-lobe living donor liver transplantation with favorable conditions. Hepatobiliary Pancreat Dis Int. 2014;13:18-24. [PubMed] |
| 19. | Alim A, Erdogan Y, Yuzer Y, Tokat Y, Oezcelik A. Graft-to-recipient weight ratio threshold adjusted to the model for end-stage liver disease score for living donor liver transplantation. Liver Transpl. 2016;22:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 21. | Goldaracena N, Echeverri J, Selzner M. Small-for-size syndrome in live donor liver transplantation-Pathways of injury and therapeutic strategies. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Graham JA, Samstein B, Emond JC. Early Graft Dysfunction in Living Donor Liver Transplantation and the Small for Size Syndrome. Curr Transplant Rep. 2014;1:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Botha JF, Langnas AN, Campos BD, Grant WJ, Freise CE, Ascher NL, Mercer DF, Roberts JP. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl. 2010;16:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Goralczyk AD, Obed A, Beham A, Tsui TY, Lorf T. Posterior cavoplasty: a new approach to avoid venous outflow obstruction and symptoms for small-for-size syndrome in right lobe living donor liver transplantation. Langenbecks Arch Surg. 2011;396:389-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Soejima Y, Shimada M, Suehiro T, Hiroshige S, Ninomiya M, Shiotani S, Harada N, Hideki I, Yonemura Y, Maehara Y. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl. 2003;9:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl. 2001;7:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Sudhindran S, Menon RN, Balakrishnan D. Challenges and Outcome of Left-lobe Liver Transplants in Adult Living Donor Liver Transplants. J Clin Exp Hepatol. 2012;2:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Yi NJ, Suh KS, Cho YB, Lee HW, Cho EH, Cho JY, Shin WY, Kim J, Lee KU. The right small-for-size graft results in better outcomes than the left small-for-size graft in adult-to-adult living donor liver transplantation. World J Surg. 2008;32:1722-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Soejima Y, Shirabe K, Taketomi A, Yoshizumi T, Uchiyama H, Ikegami T, Ninomiya M, Harada N, Ijichi H, Maehara Y. Left lobe living donor liver transplantation in adults. Am J Transplant. 2012;12:1877-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Gruttadauria S, Pagano D, Liotta R, Tropea A, Tuzzolino F, Marrone G, Mamone G, Marsh JW, Miraglia R, Luca A. Liver Volume Restoration and Hepatic Microarchitecture in Small-for-Size Syndrome. Ann Transplant. 2015;20:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Shoreem H, Gad EH, Soliman H, Hegazy O, Saleh S, Zakaria H, Ayoub E, Kamel Y, Abouelella K, Ibrahim T. Small for size syndrome difficult dilemma: Lessons from 10 years single centre experience in living donor liver transplantation. World J Hepatol. 2017;9:930-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Lauro A, Diago Uso T, Quintini C, Di Benedetto F, Dazzi A, De Ruvo N, Masetti M, Cautero N, Risaliti A, Zanfi C. Adult-to-adult living donor liver transplantation using left lobes: the importance of surgical modulations on portal graft inflow. Transplant Proc. 2007;39:1874-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Kim DG, Moon IS, Kim SJ, Lee YJ, Lee MD. Effect of middle hepatic vein reconstruction in living donor liver transplantation using right lobe. Transplant Proc. 2006;38:2099-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Peng CJ, Wang XF, Li B, Wei YG, Yan LN, Wen TF, Yang JY, Wang WT, Zhao JC. Efficacy of middle hepatic vein reconstruction in adult right-lobe living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:135-138. [PubMed] |
| 35. | Wu H, Yan LN, Li B, Zeng Y, Wen TF, Zhao JC, Wang WT, Yang JY, Xu MQ, Chen ZY. Hepatic venous outflow reconstruction in right lobe graft without middle hepatic vein. Hepatol Res. 2007;37:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Asakuma M, Fujimoto Y, Bourquain H, Uryuhara K, Hayashi M, Tanigawa N, Peitgen HO, Tanaka K. Graft selection algorithm based on congestion volume for adult living donor liver transplantation. Am J Transplant. 2007;7:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Navasa M, Feu F, García-Pagán JC, Jiménez W, Llach J, Rimola A, Bosch J, Rodés J. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993;17:355-360. [PubMed] |
| 38. | Matsudaira S, Ishizaki Y, Yoshimoto J, Fujiwara N, Kawasaki S. Risk Factors for Intractable Ascites After Adult-to-Adult Living Donor Liver Transplantation Using Left Lobe. Transplant Direct. 2017;3:e138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Mukhtar A, Dabbous H. Modulation of splanchnic circulation: Role in perioperative management of liver transplant patients. World J Gastroenterol. 2016;22:1582-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 40. | Kanetkar AV, Balakrishnan D, Sudhindran S, Dhar P, Gopalakrishnan U, Menon R, Sudheer OV. Is Portal Venous Pressure or Porto-systemic Gradient Really A Harbinger of Poor Outcomes After Living Donor Liver Transplantation? J Clin Exp Hepatol. 2017;7:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Ikegami T, Shirabe K, Yoshizumi T, Aishima S, Taketomi YA, Soejima Y, Uchiyama H, Kayashima H, Toshima T, Maehara Y. Primary graft dysfunction after living donor liver transplantation is characterized by delayed functional hyperbilirubinemia. Am J Transplant. 2012;12:1886-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, Tan HP, Shaw-Stiffel T, Boig L, Novelli P. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986-993. [PubMed] |
| 43. | Uemura T, Wada S, Kaido T, Mori A, Ogura Y, Yagi S, Fujimoto Y, Ogawa K, Hata K, Yoshizawa A. How far can we lower graft-to-recipient weight ratio for living donor liver transplantation under modulation of portal venous pressure? Surgery. 2016;159:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Takahashi Y, Matsuura T, Yoshimaru K, Yanagi Y, Hayashida M, Taguchi T. Liver graft-to-spleen volume ratio as a useful predictive factor of the early graft function in children and young adults transplanted for biliary atresia: a retrospective study. Transpl Int. 2018;31:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Karademir S, Astarcioglu H, Ozbilgin M, Ozkardesler S, Yilmaz T, Akarsu M, Akan M, Ozzeybek D, Obuz F, Astarcioglu I. Efficacy of right anterior sector drainage reconstruction in right-lobe live-donor transplantation. Transplant Proc. 2006;38:3582-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Mori S, Park MS, Kim H, Choi Y, Hong G, Yi NJ, Lee KW, Suh KS. Dysfunction in Patients With Small-for-Size Grafts After Living Donor Liver Transplantation. Int Surg. 2015;100:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Kim SH, Lee EC, Park SJ. Impact of preserved collateral veins on small-for-size grafts in living donor liver transplantation. Hepatol Res. 2018;48:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Hessheimer AJ, Fondevila C, Taurá P, Muñoz J, Sánchez O, Fuster J, Rimola A, García-Valdecasas JC. Decompression of the portal bed and twice-baseline portal inflow are necessary for the functional recovery of a “small-for-size” graft. Ann Surg. 2011;253:1201-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Xiao L, Li F, Wei B, Li B, Tang CW. Small-for-size syndrome after living donor liver transplantation: successful treatment with a transjugular intrahepatic portosystemic shunt. Liver Transpl. 2012;18:1118-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Sato Y, Oya H, Yamamoto S, Kobayashi T, Hara Y, Kokai H, Hatakeyama K. Method for spontaneous constriction and closure of portocaval shunt using a ligamentum teres hepatis in small-for-size graft liver transplantation. Transplantation. 2010;90:1200-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Nutu OA, Justo Alonso I, Marcacuzco Quinto AA, Calvo Pulido J, Jiménez Romero LC. Complete splenic embolization for the treatment of refractory ascites after liver transplantation. Rev Esp Enferm Dig. 2018;110:257-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Badawy A, Hamaguchi Y, Satoru S, Kaido T, Okajima H, Uemoto S. Evaluation of safety of concomitant splenectomy in living donor liver transplantation: a retrospective study. Transpl Int. 2017;30:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Troisi RI, Berardi G, Tomassini F, Sainz-Barriga M. Graft inflow modulation in adult-to-adult living donor liver transplantation: A systematic review. Transplant Rev (Orlando). 2017;31:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Xu Y, Chen H, Yeh H, Wang H, Leng J, Dong J. Living donor liver transplantation using dual grafts: Experience and lessons learned from cases worldwide. Liver Transpl. 2015;21:1438-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Gao W, Zhang L, Zhang Y, Sun C, Chen X, Wang Y. Adipose-derived mesenchymal stem cells promote liver regeneration and suppress rejection in small-for-size liver allograft. Transpl Immunol. 2017;45:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Kobayashi T, Sato Y, Yamamoto S, Oya H, Hara Y, Watanabe T, Kokai H, Hatakeyama K. Feasibility of auxiliary partial living donor liver transplantation for fulminant hepatic failure as an aid for small-for-size graft: single center experience. Transplant Proc. 2009;41:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Dasari BVM, Schlegel A, Mergental H, Perera MTPR. The use of old donors in liver transplantation. Best Pract Res Clin Gastroenterol. 2017;31:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Tanemura A, Mizuno S, Wada H, Yamada T, Nobori T, Isaji S. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World J Surg. 2012;36:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Abdelfattah MR, Elsiesy H. Reappraisal of upper age limit for adult living-donor liver transplantation using right lobe grafts: an outcome analysis. Eur J Gastroenterol Hepatol. 2015;27:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Ono Y, Kawachi S, Hayashida T, Wakui M, Tanabe M, Itano O, Obara H, Shinoda M, Hibi T, Oshima G. The influence of donor age on liver regeneration and hepatic progenitor cell populations. Surgery. 2011;150:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Akamatsu N, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Impact of live donor age on liver transplantation. Transplant Proc. 2007;39:3189-3193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Kawano Y, Ishikawa N, Aida J, Sanada Y, Izumiyama-Shimomura N, Nakamura K, Poon SS, Matsumoto K, Mizuta K, Uchida E. Q-FISH measurement of hepatocyte telomere lengths in donor liver and graft after pediatric living-donor liver transplantation: donor age affects telomere length sustainability. PLoS One. 2014;9:e93749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Imamura H, Hidaka M, Soyama A, Kitasato A, Adachi T, Ono S, Natsuda K, Hara T, Kugiyama T, Baimakhanov Z. A Donor Age-Based and Graft Volume-Based Analysis for Living Donor Liver Transplantation in Elderly Recipients. Transplant Direct. 2017;3:e168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Dayangac M, Taner CB, Yaprak O, Demirbas T, Balci D, Duran C, Yuzer Y, Tokat Y. Utilization of elderly donors in living donor liver transplantation: when more is less? Liver Transpl. 2011;17:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Yoshizumi T, Taketomi A, Uchiyama H, Harada N, Kayashima H, Yamashita Y, Soejima Y, Shimada M, Maehara Y. Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation. Liver Transpl. 2008;14:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Han JH, You YK, Na GH, Kim EY, Lee SH, Hong TH, Kim DG. Outcomes of living donor liver transplantation using elderly donors. Ann Surg Treat Res. 2014;86:184-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Kamo N, Kaido T, Hammad A, Ogawa K, Fujimoto Y, Uemura T, Mori A, Hatano E, Okajima H, Uemoto S. Impact of elderly donors for liver transplantation: A single-center experience. Liver Transpl. 2015;21:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Shin M, Moon HH, Kim JM, Park JB, Kwon CH, Kim SJ, Joh JW. Importance of donor-recipient age gradient to the prediction of graft outcome after living donor liver transplantation. Transplant Proc. 2013;45:3005-3012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Kubota T, Hata K, Sozu T, Ueda Y, Hirao H, Okamura Y, Tamaki I, Yoshikawa J, Kusakabe J, Tanaka H. Impact of Donor Age on Recipient Survival in Adult-to-Adult Living-donor Liver Transplantation. Ann Surg. 2018;267:1126-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Katsuragawa H, Yamamoto M, Katagiri S, Yoshitoshi K, Ariizumi S, Kotera Y, Takahashi Y, Takasaki K. Graft size and donor age are independent factors for graft loss in adult-to-adult living-donor liver transplantation using the left liver. J Hepatobiliary Pancreat Surg. 2009;16:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, Therapondos G, Adeyi O, McGilvray I, Cattral M, Greig PD. The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl. 2008;14:1778-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Wang K, Jiang WT, Deng YL, Pan C, Shen ZY. Effect of donor age on graft function and long-term survival of recipients undergoing living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14:50-55. [PubMed] |
| 73. | Ikegami T, Taketomi A, Ohta R, Soejima Y, Yoshizumi T, Shimada M, Maehara Y. Donor age in living donor liver transplantation. Transplant Proc. 2008;40:1471-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Li C, Wen TF, Yan LN, Li B, Yang JY, Xu MQ, Wang WT, Wei YG. Safety of living donor liver transplantation using older donors. J Surg Res. 2012;178:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Goldaracena N, Sapisochin G, Spetzler V, Echeverri J, Kaths M, Cattral MS, Greig PD, Lilly L, McGilvray ID, Levy GA. Live Donor Liver Transplantation With Older (≥50 Years) Versus Younger Donors: Does Age Matter? Ann Surg. 2016;263:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Kim SH, Lee EC, Shim JR, Park SJ. Right lobe living donors ages 55 years old and older in liver transplantation. Liver Transpl. 2017;23:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Starzl TE, Koep LJ, Halgrimson CG, Hood J, Schroter GP, Porter KA, Weil R 3rd. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375-388. [PubMed] |
| 78. | Sanchez-Urdazpal L, Sterioff S, Janes C, Schwerman L, Rosen C, Krom RA. Increased bile duct complications in ABO incompatible liver transplant recipients. Transplant Proc. 1991;23:1440-1441. [PubMed] |
| 79. | Gugenheim J, Samuel D, Fabiani B, Saliba F, Castaing D, Reynes M, Bismuth H. Rejection of ABO incompatible liver allografts in man. Transplant Proc. 1989;21:2223-2224. [PubMed] |
| 80. | Miyata R, Shimazu M, Tanabe M, Kawachi S, Hoshino K, Wakabayashi G, Kawai Y, Kitajima M. Clinical characteristics of thrombotic microangiopathy following ABO incompatible living donor liver transplantation. Liver Transpl. 2007;13:1455-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Oya H, Sato Y, Yamamoto S, Nakatsuka H, Kobayashi T, Watanabe T, Kokai H, Hatakeyama K. Thrombotic microangiopathy after ABO-incompatible living donor liver transplantation: a case report. Transplant Proc. 2008;40:2549-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Kishida N, Shinoda M, Itano O, Obara H, Kitago M, Hibi T, Yagi H, Abe Y, Matsubara K, Odaira M. Increased Incidence of Thrombotic Microangiopathy After ABO-Incompatible Living Donor Liver Transplantation. Ann Transplant. 2016;21:755-764. [PubMed] |
| 83. | Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Kang SH. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014;61:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Ikegami T, Yoshizumi T, Soejima Y, Uchiyama H, Shirabe K, Maehara Y. Feasible usage of ABO incompatible grafts in living donor liver transplantation. Hepatobiliary Surg Nutr. 2016;5:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 85. | Sanchez-Urdazpal L, Batts KP, Gores GJ, Moore SB, Sterioff S, Wiesner RH, Krom RA. Increased bile duct complications in liver transplantation across the ABO barrier. Ann Surg. 1993;218:152-158. [PubMed] |
| 86. | Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Markus B, Mroczek E. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988;132:489-502. [PubMed] |
| 87. | Choi SH, Kim KW, Kim SY, Kim JS, Kwon JH, Song GW, Lee SG. Computed tomography findings in ABO-incompatible living donor liver transplantation recipients with biliary strictures. Eur Radiol. 2018;28:2572-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Yamada Y, Hoshino K, Shimojima N, Shinoda M, Obara H, Kawachi S, Fuchimoto Y, Tanabe M, Kitagawa Y, Morikawa Y. Idiopathic hypereosinophilic syndrome in a case with ABO-incompatible liver transplantation for biliary atresia complicated by portal vein thrombosis. Pediatr Transplant. 2010;14:e49-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 89. | Kawagishi N, Takeda I, Miyagi S, Satoh K, Akamatsu Y, Sekiguchi S, Satomi S. Long-term outcome of ABO-incompatible living-donor liver transplantation: a single-center experience. J Hepatobiliary Pancreat Surg. 2009;16:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Yoon YI, Song GW, Lee SG, Hwang S, Kim KH, Kim SH, Kang WH, Cho HD, Jwa EK, Kwon JH. Outcome of ABO-incompatible adult living-donor liver transplantation for patients with hepatocellular carcinoma. J Hepatol. 2018;68:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 91. | Sakai H, Tanaka Y, Tazawa H, Shimizu S, Verma S, Ohira M, Tahara H, Ide K, Ishiyama K, Kobayashi T. Effect of Fc-γ Receptor Polymorphism on Rituximab-Mediated B Cell Depletion in ABO-Incompatible Adult Living Donor Liver Transplantation. Transplant Direct. 2017;3:e164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Egawa H, Umeshita K, Uemoto S. Optimal dosage regimen for rituximab in ABO-incompatible living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2017;24:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Ikegami T, Taketomi A, Soejima Y, Iguchi T, Sanefuji K, Kayashima H, Yoshizumi T, Harada N, Maehara Y. Successful ABO incompatible living donor liver transplantation in a patient with high isoagglutinin titer using high-dose intravenous immunoglobulin. Transplant Proc. 2007;39:3491-3494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Harada N, Iguchi T, Hashimoto N, Maehara Y. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009;88:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 95. | Usui M, Isaji S, Mizuno S, Sakurai H, Uemoto S. Experiences and problems pre-operative anti-CD20 monoclonal antibody infusion therapy with splenectomy and plasma exchange for ABO-incompatible living-donor liver transplantation. Clin Transplant. 2007;21:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Chen G, Sun J, Wei S, Chen Y, Tang G, Xie Z, Xu H, Chen J, Zhao H, Yuan Z. Simultaneous ABO-incompatible living-donor liver transplantation and splenectomy without plasma exchange in China: Two case reports. J Int Med Res. 2017;45:2146-2152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Uchiyama H, Mano Y, Taketomi A, Soejima Y, Yoshizumi T, Ikegami T, Shirabe K, Maehara Y. Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange. Transplantation. 2011;92:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Soin AS, Raut V, Mohanka R, Rastogi A, Goja S, Balachandran M, Saigal S, Saraf N, Bhangui P, Sumana KR. Use of ABO-incompatible grafts in living donor liver transplantation--first report from India. Indian J Gastroenterol. 2014;33:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Rummler S, Bauschke A, Baerthel E, Juette H, Maier K, Malessa C, Barz D, Settmacher U. ABO-Incompatible Living Donor Liver Transplantation in Focus of Antibody Rebound. Transfus Med Hemother. 2017;44:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Kim JD, Choi DL, Kim SG, Lee AJ. Single-Center Experience of ABO-Incompatible Living-Donor Liver Transplantation With a New Simplified Intravenous Immunoglobulin Protocol: A Propensity Score-Matching Analysis. Transplant Proc. 2016;48:1134-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Kim JM, Kwon CH, Joh JW, Kang ES, Park JB, Lee JH, Kim SJ, Paik SW, Lee SK, Kim DW. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol. 2013;59:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 102. | Kawagishi N, Satoh K, Enomoto Y, Akamatsu Y, Sekiguchi S, Fukumori T, Fujimori K, Satomi S. New strategy for ABO-incompatible living donor liver transplantation with anti-CD20 antibody (rituximab) and plasma exchange. Transplant Proc. 2005;37:1205-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Kim SH, Lee EC, Shim JR, Park SJ. A simplified protocol using rituximab and immunoglobulin for ABO-incompatible low-titre living donor liver transplantation. Liver Int. 2018;38:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Yoshizawa A, Sakamoto S, Ogawa K, Kasahara M, Uryuhara K, Oike F, Ueda M, Takada Y, Egawa H, Tanaka K. New protocol of immunosuppression for liver transplantation across ABO barrier: the use of Rituximab, hepatic arterial infusion, and preservation of spleen. Transplant Proc. 2005;37:1718-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008;47:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Yamamoto H, Uchida K, Kawabata S, Isono K, Miura K, Hayashida S, Oya Y, Sugawara Y, Inomata Y. Feasibility of Monotherapy by Rituximab Without Additional Desensitization in ABO-incompatible Living-Donor Liver Transplantation. Transplantation. 2018;102:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Jiménez-Castro MB, Negrete-Sánchez E, Casillas-Ramírez A, Gulfo J, Álvarez-Mercado AI, Cornide-Petronio ME, Gracia-Sancho J, Rodés J, Peralta C. The effect of cortisol in rat steatotic and non-steatotic liver transplantation from brain-dead donors. Clin Sci (Lond). 2017;131:733-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Xue M, Lv C, Chen X, Liang J, Zhao C, Zhang Y, Huang X, Sun Q, Wang T, Gao J. Donor liver steatosis: A risk factor for early new-onset diabetes after liver transplantation. J Diabetes Investig. 2017;8:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Perkins JD. Saying “Yes” to obese living liver donors: short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Liver Transpl. 2006;12:1012-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Kotecha HL, Saraf N, Saigal S, Choudhary NS, Yadav A, Mohanka R, Rastogi A, Menon PB, Goja S, Soin AS. NAFLD is the leading cause of donor rejection in living donor liver transplantation. J Clin Exp Hepatol. 2013;3:S116. [DOI] [Full Text] |
| 111. | Cho JY, Suh KS, Shin WY, Lee HW, Yi NJ, Kim MA, Jang JJ, Lee KU. Expansion of hepatic progenitor cell in fatty liver graft after living donor liver transplantation. Transpl Int. 2010;23:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 112. | Cho JY, Suh KS, Lee HW, Cho EH, Yang SH, Cho YB, Yi NJ, Kim MA, Jang JJ, Lee KU. Hepatic steatosis is associated with intrahepatic cholestasis and transient hyperbilirubinemia during regeneration after living donor liver transplantation. Transpl Int. 2006;19:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Cho JY, Suh KS, Kwon CH, Yi NJ, Cho SY, Jang JJ, Kim SH, Lee KU. The hepatic regeneration power of mild steatotic grafts is not impaired in living-donor liver transplantation. Liver Transpl. 2005;11:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Gao F, Xu X, Ling Q, Wu J, Zhou L, Xie HY, Wang HP, Zheng SS. Efficacy and safety of moderately steatotic donor liver in transplantation. Hepatobiliary Pancreat Dis Int. 2009;8:29-33. [PubMed] |
| 115. | Knaak M, Goldaracena N, Doyle A, Cattral MS, Greig PD, Lilly L, McGilvray ID, Levy GA, Ghanekar A, Renner EL. Donor BMI; 30 Is Not a Contraindication for Live Liver Donation. Am J Transplant. 2017;17:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Han S, Ha SY, Park CK, Joh JW, Kwon CH, Kwon GY, Kim G, Gwak MS, Jeong WK, Ko JS. Microsteatosis may not interact with macrosteatosis in living donor liver transplantation. J Hepatol. 2015;62:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 117. | Oshita A, Tashiro H, Amano H, Kobayashi T, Onoe T, Ide K, Takaki S, Takahashi S, Arihiro K, Chayama K. Safety and feasibility of diet-treated donors with steatotic livers at the initial consultation for living-donor liver transplantation. Transplantation. 2012;93:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 118. | Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi S, Yoshimitsu K, Enjoji M, Kotoh K, Taketomi A. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 2005;80:608-612. [PubMed] |
| 119. | Choudhary NS, Saraf N, Saigal S, Gautam D, Lipi L, Rastogi A, Goja S, Menon PB, Bhangui P, Ramchandra SK. Rapid Reversal of Liver Steatosis With Life Style Modification in Highly Motivated Liver Donors. J Clin Exp Hepatol. 2015;5:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 120. | Moon D, Lee S, Hwang S, Kim K, Ahn C, Park K, Ha T, Song G. Resolution of severe graft steatosis following dual-graft living donor liver transplantation. Liver Transpl. 2006;12:1156-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 121. | Wang SH, Loh PY, Lin TL, Lin LM, Li WF, Lin YH, Lin CC, Chen CL. Active immunization for prevention of De novo hepatitis B virus infection after adult living donor liver transplantation with a hepatitis B core antigen-positive graft. Liver Transpl. 2017;23:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Xi ZF, Xia Q, Zhang JJ, Chen XS, Han LZ, Zhu JJ, Wang SY, Qiu DK. De novo hepatitis B virus infection from anti-HBc-positive donors in pediatric living donor liver transplantation. J Dig Dis. 2013;14:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Dong C, Gao W, Ma N, Sun C, Zheng WP, Wang K, Shen ZY. Risks and treatment strategies for de novo hepatitis B virus infection from anti-HBc-positive donors in pediatric living donor liver transplantation. Pediatr Transplant. 2017;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 124. | Loggi E, Conti F, Cucchetti A, Ercolani G, Pinna AD, Andreone P. Liver grafts from hepatitis B surface antigen-positive donors: A review of the literature. World J Gastroenterol. 2016;22:8010-8016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 125. | Lei J, Yan L, Wang W. A comprehensive study of the safety of using anti-hepatitis B core (Hbc) positive subjects in living donor liver transplants. Hepatogastroenterology. 2013;60:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 126. | Lin CC, Chen CL, Concejero A, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant. 2007;7:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 127. | Hara Y, Tokodai K, Nakanishi C, Miyagi S, Kawagishi N. Spontaneous resolution of de novo hepatitis B after living donor liver transplantation with hepatitis B core antibody positive graft: a case report. Surg Case Rep. 2016;2:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 128. | Sasaki R, Kanda T, Ohtsuka M, Yasui S, Haga Y, Nakamura M, Yokoyama M, Wu S, Nakamoto S, Arai M. Successful Management of Graft Reinfection of HCV Genotype 2 in Living Donor Liver Transplantation from a Hepatitis B Core Antibody-Positive Donor with Sofosbuvir and Ribavirin. Case Rep Gastroenterol. 2016;10:366-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Oh HB, Moon DB, Ha TY, Lim YS, Jung DH. Five-year follow-up of a hepatitis B virus-positive recipient of hepatitis B surface antigen-positive living donor liver graft. Liver Transpl. 2006;12:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Soejima Y, Shimada M, Taketomi A, Yoshizumi T, Uchiyama H, Ikegami T, Nakamuta M, Maehara Y. Successful living donor liver transplantation using a graft from a hepatitis B surface antigen-positive donor. Liver Int. 2007;27:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 131. | Jeng LB, Thorat A, Yang HR, Yeh CC, Chen TH, Hsu CH, Hsu SC, Poon KS, Li PC, Lai HC. Successful use of hepatitis B surface antigen-positive liver grafts - an effective source for donor organs in endemic areas: a single-center experience. Ann Transplant. 2015;20:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Tang Z, Li X, Wu S, Liu Y, Qiao Y, Xu D, Li J. Risk of hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients with undetectable serum HBV DNA after treatment with rituximab for lymphoma: a meta-analysis. Hepatol Int. 2017;11:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 133. | Li G, Mu X, Huang X, Qian X, Qin J, Tan Z, Zhang W, Xu X, Tan S, Zhu Z. Liver transplantation using the otherwise-discarded partial liver resection graft with hepatic benign tumor: Analysis of a preliminary experience on 15 consecutive cases. Medicine (Baltimore). 2017;96:e7295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 134. | Fuchino M, Tajiri K, Minemura M, Sugiyama T. Vanishing Tumor in a Liver Graft from a Hepatitis B Virus Surface Antigen-Positive Donor. Case Rep Gastroenterol. 2017;11:610-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |