Published online May 28, 2018. doi: 10.3748/wjg.v24.i20.2163
Peer-review started: March 28, 2018
First decision: April 19, 2018
Revised: May 4, 2018
Accepted: May 18, 2018
Article in press: May 18, 2018
Published online: May 28, 2018
Processing time: 62 Days and 9.4 Hours
Helicobacter pylori (H. pylori) eradication can reduce gastric cancer. However, gastric cancer still develops after eradication, and cases who received eradication therapy are increasing. In this study, we have reviewed the characteristics and predictors of primary gastric cancer developing after H. pylori eradication. In terms of the characteristics, endoscopic, histologic, and molecular characteristics are reported. Endoscopically, gastric cancer after eradication is often depressed-type and shows a gastritis-like appearance, which sometimes makes the diagnosis difficult. Histologically, most gastric cancer after eradication is intestinal type, and non-neoplastic epithelium, also called epithelium with low-grade atypia, is frequently seen over the tumor, which is presumably the cause of the endoscopic gastritis-like appearance. As for molecular characteristics, some markers, such as Ki67, MUC2, and Wnt5a expression, are lower in cancer from patients in whom H. pylori has been eradicated. In terms of predictors, several Japanese studies have reported that severe endoscopic atrophy at eradication is a risk factor for gastric cancer development. Histologic intestinal metaplasia, especially in the corpus, and long-term use of proton pump inhibitors, are also reported as risk factors for gastric cancer after H. pylori eradication. These studies on the characteristics and predictors of gastric cancer development will become the cornerstone for establishing a novel surveillance program based on the gastric cancer risk stratification specific to H. pylori-eradicated patients.
Core tip: Gastric cancer develops even after successful Helicobacter pylori (H. pylori) eradication therapy. With the prevalence of eradication therapy, occurrence rates of gastric cancer detected after eradication are increasing and this is becoming an important clinical issue. We review the characteristics and predictors of primary gastric cancer after H. pylori eradication, and discuss the risk stratification of gastric cancer after eradication.
- Citation: Shichijo S, Hirata Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J Gastroenterol 2018; 24(20): 2163-2172
- URL: https://www.wjgnet.com/1007-9327/full/v24/i20/2163.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i20.2163
Gastric cancer is one of the deadliest malignancies, with 1 million cases annually around the world. In the past, the standard curative treatment was surgical resection with lymph node dissection, as the disease was usually diagnosed in the advanced stages in symptomatic subjects. To improve the prognosis of gastric cancer, diagnostic instruments and techniques were developed in Japan, where the occurrence of, and mortality by, gastric cancer were extremely high. Surveillance by endoscopy is one of the methods that enable early diagnosis of gastric cancer. Not only through diagnosis but also by its therapeutic properties[1] endoscopy has changed the management, and improved the prognosis, of gastric cancer. The discovery of the gastric pathogen, Helicobacter pylori (H. pylori), which was recognized as a group 1 carcinogen[2], dramatically changed the image of gastric cancer from a cryptogenic devastating disease to an infectious, predictable and preventable one[3].
Warren and Marshall isolated H. pylori from gastric tissue with gastritis[4]. Initially the pathogenesis of this bacterium was examined in peptic ulcer patients. Developments of diagnostic procedures and antibiotics for H. pylori have improved the treatment of peptic ulcers[5-7]. Further research on this pathogen revealed its pathogenesis in relation to chronic gastritis and gastric cancer as well, with early studies demonstrating that H. pylori infection increased the risk for gastric cancer[8,9]. Uemura et al[10] performed a cohort study of endoscopic surveillance of gastric cancer and found that all occurrences of gastric cancer in the cohort were in H. pylori-infected subjects. From these findings, H. pylori infection was incorporated into the previously proposed gastric carcinogenesis process known as Correa’s cascade[11-13]. Specifically, H. pylori infection initiates sequential histological changes such as non-atrophic gastritis, atrophic gastritis[14-16], intestinal metaplasia[15-18], dysplasia, and intestinal-type gastric cancer[19]. In contrast, such a sequential model was not applicable to diffuse-type gastric cancer, though diffuse-type gastric cancer is also associated with H. pylori infection[20-22].
Based on these findings, other studies examined the effect of H. pylori eradication on preventing gastric cancer. Fukase et al[23] reported that metachronous development of gastric carcinoma was reduced by H. pylori eradication after endoscopic resection of early gastric cancer in an open-label multicenter randomized controlled trial. Wong et al[24] performed a prospective, randomized, placebo-controlled, population-based study to examine the association of primary gastric cancer and eradication therapy. The incidence of gastric cancer development was similar between the eradication-treatment group and placebo group in this study. However, in the subgroup without precancerous lesions, eradication significantly decreased the development of gastric cancer. Recent systematic reviews and meta-analysis showed reduction of primary and metachronous gastric cancer by H. pylori eradication[25-27]. Although the effect of H. pylori eradication on the prognosis is not determined yet, it is expected that H. pylori eradication and elimination within society will lead to less gastric cancer cases and a reduction of medical costs[28].
These reports on the effect of H. pylori eradication for gastric cancer also elucidated another important fact. That is, gastric cancer did develop in H. pylori-eradicated patients[29,30], not only in gastric cancer-treated patients, who presumably possess the highest risk, but also in gastric-cancer naïve cases[24,31]. Time from eradication to cancer occurrence varied from several months to more than 10 years[31,32]. Therefore, identification of high-risk subjects, who would benefit from extensive surveillance, is an important clinical problem. Many earlier studies have investigated differences in gastric cancers that developed in H. pylori-infected and eradicated patients, to assist the early and accurate diagnosis in eradicated cases. Recent studies, which included a relatively large number of H. pylori-eradicated cohorts, enabled analysis of the risk factors of future gastric cancer development.
The purpose of this review article was to summarize the characteristics of gastric cancer that developed after H. pylori-eradication therapy, and also to identify the predictors of primary gastric cancer. Many previous studies have examined risk factors for metachronous gastric cancer development, in follow-up or cross-sectional studies of endoscopically removed gastric cancer cases[33-37]. Because these cohorts had already developed gastric cancer, they benefited from multiple, surveillance endoscopy as well as H. pylori eradication. There are many review articles on this specific topic[38-40]. However, these patients who once had gastric cancer are high-risk patients anyway, and close follow-up should be required. In contrast, a review article for the risk factors in gastric-cancer naïve cases after H. pylori eradication, which would be valuable for stratifying huge numbers of H. pylori-eradicated patients according to gastric cancer risk, has not been conducted thus far. The findings of this article will be useful for establishing a proper follow-up strategy for H. pylori-eradicated subjects.
Many studies have examined the endoscopic findings of primary gastric cancer after H. pylori eradication. Most of these studies were derived from early gastric cancer cases who underwent endoscopic treatment and, therefore, potentially include selection bias.
Depressed lesion: One of the notable endoscopic features of gastric cancer after H. pylori eradication is its depressed appearance. Kamada et al. reported that among 20 gastric cancer cases discovered in H. pylori-eradicated patients, 90% (18 cases) were ulcer type[41]. In a recent and relatively large propensity score-matching study of endoscopic submucosal dissection cases, 81% (78 of 96) of early gastric cancers from H. pylori-eradicated patients were depressed type, a significantly higher proportion than the 53% (51 of 96) in H. pylori-positive cases[42]. Many other studies, including case series[43], or case control studies[44,45] also indicated predominance of depressed or ulcer type (0-IIc) gastric cancer in H. pylori-eradicated cases (Table 1).
| Ref. | Number of gastric cancer after eradication/during infection | Study design | Case recruitment | Characteristics |
| Shichijo et al[31] | 21/NA | Case series | Surveillance | Intestinal type |
| Maehata et al[42] | 96/96 | Propensity score-matched study | ESD cases | Depressed |
| Nishizawa et al[43] | 34/NA | Case series | Surveillance | Depressed, intestinal type, relatively small |
| Matsuo et al[44] | 26/78 | Case control study | Surveillance | Male, intestinal type, flat-depressed, low MUC2 and Wnt5a |
| Yamamoto et al[45] | 18/36 | Case control study | Early stage cancer | Smaller, lower Ki-67 index, depression, complete gastric type or gastric predominant mixed type |
| Horiguchi et al[48] | 71/115 | Case control study | Case series | Non-tumorous epithelium Surface differentiation |
| Ito et al[52] | 29/NA | Case series | ESD cases | Normal columnar epithelium |
| Kitamura et al[53] | 27/27 | Case control study | Endoscopic resection cases | Low-grade atypia on the surface |
| Hori et al[54] | 59/152 | Case control study | Endoscopic resection cases | Non-neoplastic epithelium, flattening of tumors, muting of the whitish discoloration |
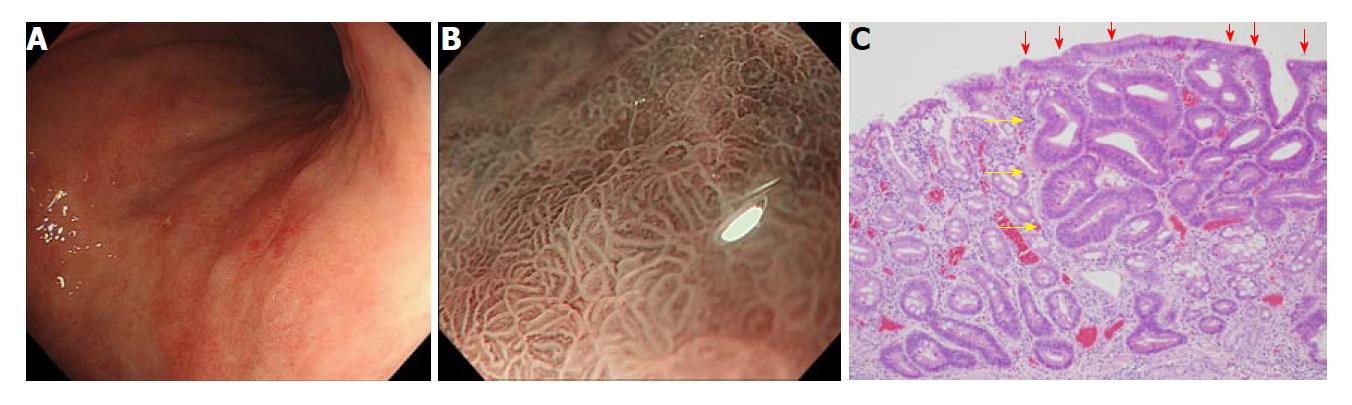
Gastritis like appearance: Another important characteristic of gastric cancer after H. pylori eradication is its gastritis-like appearance. This was initially reported by Kobayashi et al[46]. A “gastritis-like” appearance under narrow-band imaging with magnifying endoscopy was characterized by uniform papillae and/or tubular pits with a whitish border, regular or faint microvessels, and unclear demarcation, resembling the adjacent noncancerous mucosa (Figure 1). They examined retrospectively, differentiated-type early gastric cancer of 50 lesions after eradication, and 50 lesions without eradication. A “gastritis-like” appearance was more frequent for the eradication group (22/50) than the control group (2/50), and the “gastritis-like” appearance correlated with histological surface differentiation[46]. They also reported that the development of “gastritis-like” appearance was associated with less endoscopic atrophy[47]. These phenotypic characteristics or changes make the diagnosis of gastric cancer after H. pylori eradication difficult. In fact, it is reported that utilization of chromoendoscopy did not improve the diagnostic reliability of gastric cancer after H. pylori eradication[48]. These endoscopic characteristics of gastric cancer after eradication were associated with histological features that have been termed “non-neoplastic epithelium” (Discussed in the following chapter).
Other: Smaller tumor size is also reported as a characteristic of gastric cancer in H. pylori-eradicated cases. Yamamoto et al[45] reported that the average diameter of gastric cancer detected after successful eradication was smaller than that in non-eradicated, age, sex, and cancer-depth matched controls. However the control group did not undergo the routine follow-up examination that was performed in the eradicated group. Another propensity-matched study indicated similar tumor size in H. pylori-eradicated and infected patients[42].
Other characteristics of gastric cancer detected after H. pylori eradication by histological assessment.
Intestinal type: We have previously conducted a cohort study of 573 H. pylori-eradicated cases. During the 6.2 ± 4.8 years of the observation period, we found 21 cases of primary gastric cancer in these H. pylori-eradicated patients[31]. Among the 21 tumors, 20 (95%) were intestinal-type gastric cancer, while only one was diffuse type. We did not compare those cancers with non-eradicated cases; however, before eradication therapy, the numbers of intestinal and diffuse-type gastric cancers were roughly even[49]. We speculate that Helicobacter eradication could lead to the dominance of intestinal-type gastric cancer. This intestinal-type dominance (i.e., diffuse-type reduction) was also reported in other studies[35,50,51]. However, several studies did not show differences in histological type between H. pylori-eradicated and non-eradicated cases[42,45]. These studies analyzed only endoscopic treatment cases, which inevitably exclude diffuse-type cancers. This reduction of diffuse-type gastric cancer by H. pylori eradication will be clarified in future large-scale analysis.
Non-neoplastic epithelium: This histological characteristic was initially described in a study that evaluated the histological change of gastric tumors after Helicobacter pylori eradication[52]. They named non-neoplastic epithelium which often appeared on the surface of gastric cancer after eradication as epithelium with low-grade atypia (ELA). ELA was observed in 22 out of 27 gastric cancer cases detected after successful eradication where gastric-type mucin was frequently expressed[53]. Hori et al. compared 59 tumors detected after eradication and 152 detected while infected, and showed that the histological length ratio of non-neoplastic epithelium to the tumor was 8% for the eradicated group, and 0% for the infected group. The extension of non-neoplastic epithelium has been reported in several other studies (Table 1)[48,54].
Other: One study evaluated Ki67 staining, which is a molecular indicator of cell proliferation. The Ki67 index was lower in the eradicated group than in the non-eradicated group. Immunohistochemical phenotyping revealed that gastric cancer after eradication was more often gastric-predominant type[45]. Another study investigated mucus patterns and Wnt5a expression in gastric cancer specimens derived from H. pylori-eradicated and infected patients. The result showed MUC2 and Wnt5a expressions were significantly lower in gastric cancers from H. pylori-eradicated patients[44].
In this section, we summarize risk factors for gastric cancer development after H. pylori eradication. There are several cohort studies[31,32,55-58] and case-control studies[59-63] on this topic (Table 2). These studies have examined patients’ characteristics, endoscopic features, and histological findings associated with gastric cancer after H. pylori eradication.
| Ref. | Subject number | Study design | Diagnosis | Age | Follow-up period (yr) | Number of cancer | Risk factor |
| Shichijo et al[31] | 573 | Cohort study | CG/DU/GU | 58 | 6.4 | 21 | Endoscopic severe atrophy |
| Histologic intestinal metaplasia | |||||||
| Take et al[32] | 1674 | Cohort study | GU/DU | 51 | 5.6 | 28 | Endoscopic severe atrophy |
| Toyoshima et al[55] | 1232 | Cohort study | CG/DU/GU | 54 | 2.5 | 15 | Endoscopic severe atrophy |
| Sakitani et al[56] | 965 | Cohort study | CG/DU/GU | 63 | 4.5 | 21 | Endoscopic severe atrophy |
| Cheung et al[57] | 63397 | Cohort study | Helicobacter pylori infection (GU 2%, DU 3%) | 55 | 7.6 | 153 | Proton pump inhibitor |
| Takata et al[58] | 101 | Cohort study | CG/GU/GC | 56 | 5.3 | 8 | Age |
| Kodama et al[59] | 2355 | Matched control study | CG/DU/GU/GC | 63 | 4.1 | 21 | Endoscopic severe atrophy |
| OLGA staging | |||||||
| Histologic atrophy at the antrum | |||||||
| Histologic inflammation at the corpus | |||||||
| Histologic intestinal metaplasia at the corpus | |||||||
| Sugimoto et al[60] | 1200 | Cross- sectional study | NA | 70 | 4.6 | 79 | Endoscopic severe atrophy |
| Haneda et al[62] | 261 | Cross sectional study | CG/DU/GU/GC/MALToma/hyperplastic polyp | 57 | NA | 47 | Pepsinogen I/II ratio ≤ 4.5 |
| Maeda et al[63] | 177 | Cross sectional study | NA | NA | NA | 94 | Epigenetic marker |
The classification of endoscopic atrophy was first described by Kimura and Takemoto in 1969 to discriminate the histological border between the pyloric and fundic glands[64]. They found a close association between this boundary and gastritis. Later, Uemura et al. showed, in their important report, which indicated the critical involvement of H. pylori in gastric carcinogenesis, that severe endoscopic atrophy was a risk factor for primary gastric cancer development in H. pylori-infected cases[10].
As for patients in whom H. pylori had been eradicated, Take et al. investigated risk factors associated with gastric cancer development in 1674 H. pylori-eradicated peptic ulcer patients[32]. In their mean 5.6-year surveillance endoscopy program following H. pylori eradication, they found 28 cases of gastric cancer in patients with a mean age of 51 years. Patients with severe endoscopic gastric atrophy, which they defined as Kimura-Takemoto classification O2 and O3, before eradication had increased risk for gastric cancer (0.62% per year), compared to patients with mild (C1 and C2) and moderate (C3 and O1) atrophy (0.04% and 0.28% per year, respectively).
We also examined endoscopic atrophy for the prediction of gastric cancer in the above-mentioned study[31]. Multivariate analysis revealed that histologic intestinal metaplasia and severe endoscopic atrophy are independent risks for gastric cancer development. In our study, patients with O2 or O3 atrophy at eradication had 9.3-fold risk for developing gastric cancer compared to patients with no or mild atrophy (C0-C2) in multivariate analysis. Many other cohort studies[55,56] and case-control studies[59,60] showed similar results, that severe endoscopic atrophy is associated with gastric cancer development in H. pylori-eradicated patients (Table 2).
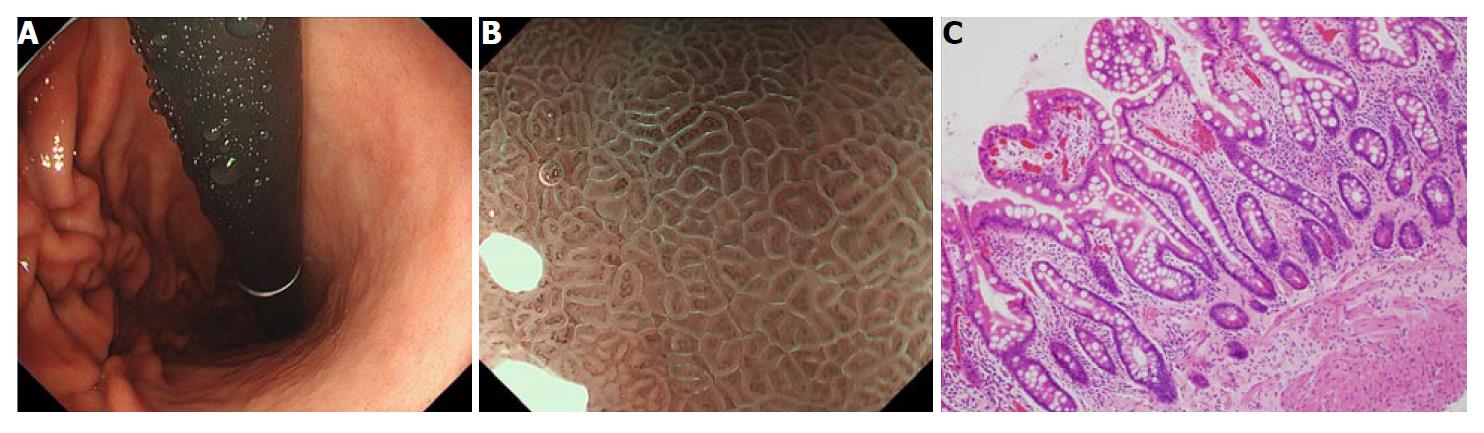
Another well-characterized gastric cancer risk is histological intestinal metaplasia, evaluated at the time of eradication therapy (Figure 2). It has been suggested that intestinal metaplasia precedes gastric cancer development, especially for intestinal-type cancer[11,13]. There have been debates on whether this histological change is a precancerous or a paracancerous lesion, which has not yet been completely clarified. Nonetheless, several observational studies have indicated that the presence of intestinal metaplasia in the background gastric tissue indicates a higher risk for accompanying or developing gastric cancer[10,17,18,65,66].
As described above, we have analyzed 573 endoscopy-based surveillance cases after H. pylori eradiation, in which 21 cases of gastric cancer were observed[31]. Before eradication, participants had been evaluated for the presence of intestinal metaplasia[17,18] and neutrophil infiltration using antral and corpus biopsies, and the degree of endoscopic atrophy. We classified patients into three groups according to the histological metaplasia distribution[17,18]. Compared to the group with no intestinal metaplasia, patients with metaplasia limited to antrum had a 4.5-fold increased risk, and patients with metaplasia in corpus had a 7.6-fold increased risk in univariate analysis. Multivariate analysis revealed that the presence of histologic intestinal metaplasia was an independent risk for gastric cancer development. To the best of our knowledge this is the first report that shows intestinal metaplasia as the predictor of future gastric cancer after H. pylori eradication.
Kodama et al[59] performed cross-sectional and case-control analyses of an H. pylori-eradicated cohort and reported that the histological intestinal metaplasia score in the corpus was significantly higher in gastric cancer cases than that in age- and sex- matched non-cancer controls. This study also evaluated the intestinal metaplasia score in the antrum, which did not show a statistical difference between the two groups. Taken together, the presence of intestinal metaplasia, especially in the corpus, might indicate a higher risk for developing gastric cancer.
Cheung et al[57] recently reported, based on a territory-wide health database of Hong Kong, that long term use of proton pump inhibitors (PPI) was associated with an increased gastric cancer risk in subjects after H. pylori-eradication therapy. Among 63397 eligible patients who received clarithromycin-based triple therapy between 2003 and 2012, 153 cases of gastric cancer developed before 2015. The risk increased with duration of PPI use (Hazard ratio 5.0, 6.7, and 8.3 for ≥ 1, 2, and 3 years, respectively). Many researchers quickly responded to this topic, and both affirming[67] and contradicting reports[68,69] followed this paper. Interestingly, another population-based study in Sweden also indicated the increased risk of gastric cancer (regardless of H. pylori infection or eradication) in maintenance therapy with PPI[70]. Contrary to the former study on H. pylori-eradicated cases[57], this study did not find that the risk increased with therapeutic duration. Therefore, this topic still requires more study before a consensus can be reached, but clinicians should take PPI use into account for future studies of gastric cancer risk assessment.
Age factor has also been reported in many studies. Most studies showed an older age at eradication is associated with an increased risk of developing cancer in univariate analysis[31,55,59]. However, age is also closely associated with other gastritis-related phenotypes, which often lead to less value in carcinogenesis under multivariate analysis. For example, a cohort study examined 101 histologically diagnosed corpus atrophic gastritis patients who underwent successful eradication therapy[58]. This study found eight gastric cancer cases (all intestinal type) during a mean follow-up period of 5.3 year, and the patients’ characteristics and serum biochemistry data were compared in the groups with and without cancer. Out of age, sex, the disease indicated for eradication (gastritis or gastric ulcer), prior gastric cancer, pepsinogen value, and gastrin value, only age (64 years vs 55 years) was statistically different between groups. However, lack of multivariate analysis or other important confounding factors, such as endoscopic atrophy or histological metaplasia, might have led to an immature conclusion in this study.
Endoscopic diagnosis associated with H. pylori infection has been examined as a risk or protective factor for gastric cancer development. In a H. pylori-persistent infection cohort, reduced risk for gastric cancer development was found specifically in duodenal ulcer patients (0 out of 275 for duodenal ulcer vs 36 out of 971 for other diseases)[10]. As for patients after eradication therapy, Kamada et al[41] reported that no gastric cancer developed in 654 duodenal ulcer patients, while 12 of 575 (2.1%), two of 453 (0.4%), and six of 105 (5.7%) cases were reported in gastric ulcer, atrophic gastritis and endoscopic resection for early gastric cancer patients, respectively. Kodama et al[59]. also reported only three gastric cancer cases, developed from 655 patients with duodenal ulcers (0.5%), while 10 of 902 (1.1%), 14 of 593 (2.4%), and 3 of 51 (5.9%) cases developed from patients with chronic gastritis, gastric ulcers, and gastric cancer, respectively. These reports indicate duodenal ulcer patients who received eradication therapy have less risk for future gastric cancer occurrence than do patients who have undergone eradication for other H. pylori-related diseases.
Pepsinogen (PG) methods are clinically used for the gastric-cancer screening program in Japan. As low PGI levels and low PGI/II ratios are correlated with mucosal atrophy, the efficacy of this screening method for identifying high-risk subjects of gastric cancer has been reported in multiple cohort studies[71,72]. However PG values and ratios change after H. pylori eradication[73], and the usefulness of the PG method in H. pylori-eradicated patients was not evident. Haneda et al[62]. examined PG levels in post-eradication cases with and without gastric cancer, and found that the optimal cut-off value for the PGI/II ratio was 4.5 (instead of the usual 3.0). The usefulness of this cut-off value in clinical practice needs to be confirmed in a cohort or a prospective study.
Finally, molecular indicators of gastric cancer risk have been investigated intensively. Recent research, focused on epigenetic markers, has revealed completely new types of gastric cancer risk predictors. In a case-control study consisting of eight cases without infection, 75 atrophic gastritis post-eradication cases and 94 gastric cancer post-eradication cases, nine candidate epigenetic markers, which showed elevated methylation levels in cancer cases, were isolated[63]. These new markers are now being evaluated in a prospective cohort study, which will elucidate the clinical usefulness of these molecular approaches in the near future.
Here we have reviewed characteristics and predictors of gastric cancer after H. pylori eradication. Knowledge of endoscopic characteristics, such as depressed and gastritis-like appearance, and an understanding of the histological non-neoplastic epithelium, will be helpful in detecting gastric cancer while screening subjects after eradication therapy. Reportedly, the tumors detected after H. pylori eradication seemed to be less proliferative and more gastric phenotype. This might be associated with a differentiation program by adult-tissue stem cells, and the mechanism of these molecular changes and the effect of H. pylori eradication will be an interesting research project. As for predictors, severe endoscopic atrophy, histologic intestinal metaplasia before eradication, and PPI use are reportedly risk factors for gastric cancer development after eradication. Cases with these risk factors should be carefully followed up by endoscopy, with special attention paid to the aforementioned characteristic endoscopic findings.
So far, most of the risk factors were evaluated before the eradication, which is helpful for identifying high-risk patients early so they can be invited into a surveillance program. However, risk stratification according to findings after eradication, not those before eradication, might be more practical, because information prior to eradication is not always available. For example, we proposed that histological intestinal metaplasia is an important risk factor for future gastric cancer development, but most of the H. pylori-eradicated subjects in the community did not receive a histological evaluation prior to eradication. If there is little change in the metaplasia after eradication, then assessment of histology after eradication may be used as a substitute, but this would need to be evaluated by independent studies. Risk stratification by pepsinogen levels after eradication has been reported[62], but further validation studies are necessary. Some researchers have focused on endoscopic changes after H. pylori eradication that was accompanied with cancer[74]. Map-like redness after eradication, which corresponds to intestinal metaplasia histologically, could be a predictor for metachronous gastric cancer. Importantly, recent retrospective epigenetic research used gastric samples collected from post-eradication cases, which is ideally applicable to all subjects[63]. Therefore the result of the ongoing prospective study is highly anticipated.
Eradication therapy is relatively new, and current studies are mostly limited to elder patients over 50 years old. Therefore, further long-term follow-up studies, over several decades, or a study of the young population should be required to form a consensus for an adequate surveillance program. Based on the results reviewed in this paper, it is safe to propose annual endoscopic surveillance for high-risk H. pylori-eradicated patients, such as those with severe endoscopic atrophic gastritis (O2 or O3) or histological intestinal metaplasia before eradication. Patients who require PPI treatment for any reason after eradication should also have an annual checkup for both gastric cancer surveillance and for the conditions requiring PPI. However, for other relatively low-risk eradicated patients, such as subjects with mild atrophy or no metaplasia, little evidence exists to propose a proper surveillance program. As these relatively low risk patients consist of the majority of H. pylori-eradicated cases, studies targeting these subjects will definitely be required. New studies, new modalities, and new concepts will lead to the establishment of a primary gastric cancer surveillance program suitable for all H. pylori-eradicated cases according to their cancer risk stratification.
In this review article, we have summarized the previous studies on the characteristics and predictors of gastric cancer which developed after successful H. pylori eradication. Gastric cancer surveillance program after H. pylori eradication according to risk stratification needs to be established in future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kravtsov V, Ozen H, Sun LM S- Editor: Gong ZM L- Editor: A E- Editor: Wang C
| 1. | Uedo N, Jung HY, Fujishiro M, Lee IL, Zhou PH, Chiu PW, Chang D, Goda K. Current situation of endoscopic submucosal dissection for superficial neoplasms in the upper digestive tract in East Asian countries: a questionnaire survey. Dig Endosc. 2012;24 Suppl 1:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177-240. [PubMed] |
| 3. | Rugge M, Genta RM, Di Mario F, El-Omar EM, El-Serag HB, Fassan M, Hunt RH, Kuipers EJ, Malfertheiner P, Sugano K. Gastric Cancer as Preventable Disease. Clin Gastroenterol Hepatol. 2017;15:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 4. | Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] |
| 5. | Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A meta-analysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15:1949-1958. [PubMed] |
| 6. | Ford A, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2004;4:CD003840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Gisbert JP, Pajares JM. Systematic review and meta-analysis: is 1-week proton pump inhibitor-based triple therapy sufficient to heal peptic ulcer? Aliment Pharmacol Ther. 2005;21:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1234] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 9. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 10. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3185] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 11. | Correa P, Cuello C, Duque E, Burbano LC, Garcia FT, Bolanos O, Brown C, Haenszel W. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57:1027-1035. [PubMed] |
| 12. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 13. | Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 14. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 15. | Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter. 2001;6:294-299. [PubMed] |
| 16. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [PubMed] |
| 17. | Sakitani K, Hirata Y, Watabe H, Yamada A, Sugimoto T, Yamaji Y, Yoshida H, Maeda S, Omata M, Koike K. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol. 2011;26:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Shichijo S, Hirata Y, Sakitani K, Yamamoto S, Serizawa T, Niikura R, Watabe H, Yoshida S, Yamada A, Yamaji Y. Distribution of intestinal metaplasia as a predictor of gastric cancer development. J Gastroenterol Hepatol. 2015;30:1260-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Hsu PI, Lai KH, Hsu PN, Lo GH, Yu HC, Chen WC, Tsay FW, Lin HC, Tseng HH, Ger LP. Helicobacter pylori infection and the risk of gastric malignancy. Am J Gastroenterol. 2007;102:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017;32:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Handa Y, Saitoh T, Kawaguchi M, Misaka R, Ohno H, Tsai CR, Tani Y, Tsurui M, Yoshida H, Morita S. Association of Helicobacter pylori and diffuse type gastric cancer. J Gastroenterol. 1996;31 Suppl 9:29-32. [PubMed] |
| 22. | Wang C, Yuan Y, Hunt RH. The association between Helicobacter pylori infection and early gastric cancer: a meta-analysis. Am J Gastroenterol. 2007;102:1789-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 935] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 24. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1046] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 25. | Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. 2014;19:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [PubMed] |
| 27. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 674] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 28. | Asaka M, Mabe K, Matsushima R, Tsuda M. Helicobacter pylori Eradication to Eliminate Gastric Cancer: The Japanese Strategy. Gastroenterol Clin North Am. 2015;44:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | de Vries AC, Kuipers EJ, Rauws EA. Helicobacter pylori eradication and gastric cancer: when is the horse out of the barn? Am J Gastroenterol. 2009;104:1342-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Jung S, Park CH, Kim EH, Shin SJ, Chung H, Lee H, Park JC, Shin SK, Lee YC, Lee SK. Preventing metachronous gastric lesions after endoscopic submucosal dissection through Helicobacter pylori eradication. J Gastroenterol Hepatol. 2015;30:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, Fukayama M, Koike K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. 2016;84:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 32. | Take S, Mizuno M, Ishiki K, Yoshida T, Ohara N, Yokota K, Oguma K, Okada H, Yamamoto K. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J Gastroenterol. 2011;46:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Yang HJ, Kim SG, Lim JH, Choi JM, Oh S, Park JY, Han SJ, Kim J, Chung H, Jung HC. Novel risk stratification for metachronous recurrence after curative endoscopic submucosal dissection for early gastric cancer. Gastrointest Endosc. 2018;87:419-428.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Hanaoka N, Uedo N, Shiotani A, Inoue T, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Haruma K, Tatsuta M. Autofluorescence imaging for predicting development of metachronous gastric cancer after Helicobacter pylori eradication. J Gastroenterol Hepatol. 2010;25:1844-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Mori G, Nakajima T, Asada K, Shimazu T, Yamamichi N, Maekita T, Yokoi C, Fujishiro M, Gotoda T, Ichinose M. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer. 2016;19:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Sugimoto T, Yamaji Y, Sakitani K, Isomura Y, Yoshida S, Yamada A, Hirata Y, Ogura K, Okamoto M, Koike K. Neutrophil infiltration and the distribution of intestinal metaplasia is associated with metachronous gastric cancer following endoscopic submucosal dissection. Can J Gastroenterol Hepatol. 2015;29:321-325. [PubMed] |
| 37. | Maeda M, Nakajima T, Oda I, Shimazu T, Yamamichi N, Maekita T, Asada K, Yokoi C, Ando T, Yoshida T. High impact of methylation accumulation on metachronous gastric cancer: 5-year follow-up of a multicentre prospective cohort study. Gut. 2017;66:1721-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Shiotani A, Haruma K, Graham DY. Metachronous gastric cancer after successful Helicobacter pylori eradication. World J Gastroenterol. 2014;20:11552-11559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Kobayashi M, Sato Y, Terai S. Endoscopic surveillance of gastric cancers after Helicobacter pylori eradication. World J Gastroenterol. 2015;21:10553-10562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Ohba R, Iijima K. Pathogenesis and risk factors for gastric cancer after Helicobacter pylori eradication. World J Gastrointest Oncol. 2016;8:663-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Kamada T, Hata J, Sugiu K, Kusunoki H, Ito M, Tanaka S, Inoue K, Kawamura Y, Chayama K, Haruma K. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: results from a 9-year prospective follow-up study in Japan. Aliment Pharmacol Ther. 2005;21:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Maehata Y, Nakamura S, Esaki M, Ikeda F, Moriyama T, Hida R, Washio E, Umeno J, Hirahashi M, Kitazono T. Characteristics of Primary and Metachronous Gastric Cancers Discovered after Helicobacter pylori Eradication: A Multicenter Propensity Score-Matched Study. Gut Liver. 2017;11:628-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Nishizawa T, Suzuki H, Arano T, Yoshida S, Yamashita H, Hata K, Kanai T, Yahagi N, Toyoshima O. Characteristics of gastric cancer detected within 1 year after successful eradication of Helicobacter pylori. J Clin Biochem Nutr. 2016;59:226-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Matsuo T, Ito M, Tatsugami M, Boda T, Takata S, Tanaka S, Chayama K. Gastric cancer development after Helicobacter pylori eradication therapy: a new form of gastric neoplasia. Digestion. 2012;85:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Yamamoto K, Kato M, Takahashi M, Haneda M, Shinada K, Nishida U, Yoshida T, Sonoda N, Ono S, Nakagawa M. Clinicopathological analysis of early-stage gastric cancers detected after successful eradication of Helicobacter pylori. Helicobacter. 2011;16:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Kobayashi M, Hashimoto S, Nishikura K, Mizuno K, Takeuchi M, Sato Y, Ajioka Y, Aoyagi Y. Magnifying narrow-band imaging of surface maturation in early differentiated-type gastric cancers after Helicobacter pylori eradication. J Gastroenterol. 2013;48:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Kobayashi M, Hashimoto S, Mizuno K, Takeuchi M, Sato Y, Watanabe G, Ajioka Y, Azumi M, Akazawa K, Terai S. Therapeutic or spontaneous Helicobacter pylori eradication can obscure magnifying narrow-band imaging of gastric tumors. Endosc Int Open. 2016;4:E665-E672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Horiguchi N, Tahara T, Kawamura T, Okubo M, Tahara S, Nagasaka M, Nakagawa Y, Shibata T, Ohmiya N. A Comparative Study of White Light Endoscopy, Chromoendoscopy and Magnifying Endoscopy with Narrow Band Imaging in the Diagnosis of Early Gastric Cancer after Helicobacter pylori Eradication. J Gastrointestin Liver Dis. 2017;26:357-362. [PubMed] |
| 49. | Grabsch HI, Tan P. Gastric cancer pathology and underlying molecular mechanisms. Dig Surg. 2013;30:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Yanaoka K, Oka M, Ohata H, Yoshimura N, Deguchi H, Mukoubayashi C, Enomoto S, Inoue I, Iguchi M, Maekita T. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer. 2009;125:2697-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Mabe K, Takahashi M, Oizumi H, Tsukuma H, Shibata A, Fukase K, Matsuda T, Takeda H, Kawata S. Does Helicobacter pylori eradication therapy for peptic ulcer prevent gastric cancer? World J Gastroenterol. 2009;15:4290-4297. [PubMed] |
| 52. | Ito M, Tanaka S, Takata S, Oka S, Imagawa S, Ueda H, Egi Y, Kitadai Y, Yasui W, Yoshihara M. Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a short-term follow-up. Aliment Pharmacol Ther. 2005;21:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Kitamura Y, Ito M, Matsuo T, Boda T, Oka S, Yoshihara M, Tanaka S, Chayama K. Characteristic epithelium with low-grade atypia appears on the surface of gastric cancer after successful Helicobacter pylori eradication therapy. Helicobacter. 2014;19:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Hori K, Watari J, Yamasaki T, Kondo T, Toyoshima F, Sakurai J, Ikehara H, Tomita T, Oshima T, Fukui H. Morphological Characteristics of Early Gastric Neoplasms Detected After Helicobacter pylori Eradication. Dig Dis Sci. 2016;61:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Toyoshima O, Yamaji Y, Yoshida S, Matsumoto S, Yamashita H, Kanazawa T, Hata K. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc. 2017;31:2140-2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Sakitani K, Hirata Y, Suzuki N, Shichijo S, Yanai A, Serizawa T, Sakamoto K, Akanuma M, Maeda S, Yamaji Y. Gastric cancer diagnosed after Helicobacter pylori eradication in diabetes mellitus patients. BMC Gastroenterol. 2015;15:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 58. | Takata S, Ito M, Yoshihara M, Tanaka S, Imagawa S, Haruma K, Chayama K. Host factors contributing to the discovery of gastric cancer after successful eradication therapy of Helicobacter pylori: preliminary report. J Gastroenterol Hepatol. 2007;22:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Kodama M, Murakami K, Okimoto T, Abe H, Sato R, Ogawa R, Mizukami K, Shiota S, Nakagawa Y, Soma W. Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer. Scand J Gastroenterol. 2013;48:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 61. | Majima A, Handa O, Naito Y, Dohi O, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T. Early-Stage Gastric Cancer Can Be Found in Improved Atrophic Mucosa over Time from Successful Helicobacter pylori Eradication. Digestion. 2017;95:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Haneda M, Kato M, Ishigaki S, Suzuki M, Takahashi M, Nakagawa M, Ono S, Mori Y, Mabe K, Nakagawa S. Identification of a high risk gastric cancer group using serum pepsinogen after successful eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2013;28:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Maeda M, Yamashita S, Shimazu T, Iida N, Takeshima H, Nakajima T, Oda I, Nanjo S, Kusano C, Mori A. Novel epigenetic markers for gastric cancer risk stratification in individuals after Helicobacter pylori eradication. Gastric Cancer. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (3)] |
| 65. | Rugge M, Fassan M, Pizzi M, Farinati F, Sturniolo GC, Plebani M, Graham DY. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J Gastroenterol. 2011;17:4596-4601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 66. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 586] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 67. | Niikura R, Hayakawa Y, Hirata Y, Yamada A, Fujishiro M, Koike K. Long-term proton pump inhibitor use is a risk factor of gastric cancer after treatment for Helicobacter pylori: a retrospective cohort analysis. Gut. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Suissa S, Suissa A. Proton-pump inhibitors and increased gastric cancer risk: time-related biases. Gut. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Gueta I, Halkin H, Markovits N, Loebstein R. Proton pump inhibitors and the risk for gastric cancer: possible confounding by serum vitamin B12. Gut. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Brusselaers N, Wahlin K, Engstrand L, Lagergren J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open. 2017;7:e017739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 71. | Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 72. | Oishi Y, Kiyohara Y, Kubo M, Tanaka K, Tanizaki Y, Ninomiya T, Doi Y, Shikata K, Yonemoto K, Shirota T. The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study. Am J Epidemiol. 2006;163:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Shirai N, Furuta T, Sugimoto M, Kanaoka S, Watanabe F, Takashima M, Yamada M, Futami H, Sato Y, Kubota H. Serum pepsinogens as an early diagnostic marker of H. pylori eradication. Hepatogastroenterology. 2008;55:486-490. [PubMed] |
| 74. | Moribata K, Iguchi JK, Nakachi K, Maeda Y, Shingaki N, Niwa T, Deguchi H, Inoue I, Maekita T, Tamai H. Endoscopic features associated with development of metachronous gastric cancer in patients who underwent endoscopic resection followed by Helicobacter pylori eradication. Dig Endosc. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |