Published online Jan 14, 2018. doi: 10.3748/wjg.v24.i2.297
- This article has been corrected.
- See: World J Gastroenterol. Dec 14, 2021; 27(46): 8031-8032
Peer-review started: October 20, 2017
First decision: November 8, 2017
Revised: November 15, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: January 14, 2018
Processing time: 86 Days and 20.4 Hours
Mass forming chronic pancreatitis is very rare. Diagnosis could be done by the pathologic findings of focal inflammatory fibrosis without evidence of tumor in pancreas. A 34-year-old man presented with right upper abdominal pain for a few weeks and slightly elevated bilirubin level on clinical findings. Radiological findings of multidetector-row computed tomography, magnetic resonance (MR) imaging with MR cholangiopancreatography and endoscopic ultrasonography revealed focal branch pancreatic duct dilatation with surrounding delayed enhancing solid component at uncinate process and head of pancreas, suggesting branch duct type intraductal papillary mucinous neoplasm. Surgery was done and pathology revealed the focal chronic inflammation, fibrosis, and branch duct dilatation. Herein, I would like to report the first case report of mass forming chronic pancreatitis mimicking pancreatic cystic neoplasm.
Core tip: Extremely unusual radiological manifestation of mass forming chronic pancreatitis mimicking pancreatic cystic neoplasm is the first case report in the English-written medical literature.
- Citation: Jee KN. Mass forming chronic pancreatitis mimicking pancreatic cystic neoplasm: A case report. World J Gastroenterol 2018; 24(2): 297-302
- URL: https://www.wjgnet.com/1007-9327/full/v24/i2/297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i2.297
Chronic pancreatitis represents a recurrent, prolonged inflammatory process and progressive fibrosis of the pancreas. These results in irreversible morphologic change of the pancreas, clinical symptoms of abdominal pain, and insufficiency of exocrine and endocrine function[1-3]. On computed tomography (CT) and magnetic resonance (MR) image, dilatation of the main pancreatic duct, parenchymal atrophy, pancreatic calcification or stone, focal pancreatic enlargement or inflammatory pancreatic mass, bile duct dilatation, attenuation change of peripancreatic fat and fluid collection are frequent findings[4-6].
Inflammatory mass in chronic pancreatitis retain a large degree of fibrosis like pancreatic carcinoma[7-9], and both lesions are shown as a gradual progressive enhancement on contrast-enhanced CT and dynamic MR imaging, making the discrimination of the two entities difficult[5,6,10].
In the case of mass forming chronic pancreatitis, diagnosis of inflammatory pancreatic mass could be almost impossible if associated radiological findings of chronic pancreatitis is not shown.
This paper presents a very unique case of mass forming chronic pancreatitis mimicking pancreatic cystic neoplasm.
A 34-year-old man complained for right upper abdominal pain for a few days. His laboratory findings including white blood cell count, C-reactive protein, alkaline phosphatase, liver enzyme level and tumor markers of carbohydrate antigen 19-9 and carcinoembryonic antigen were within normal range except slight elevation of total bilirubin (1.3 mg/dL, normal range of 0.2-1.2), gamma-glutamyl transferase (108 IU/L, normal range of 8-60) and lipase (90 U/L, normal range of 30-60). The patient had past medical history of admission due to acute alcoholic pancreatitis 13 years ago and social history of daily alcohol consumption for 15 years and having smoked 20 pack years.
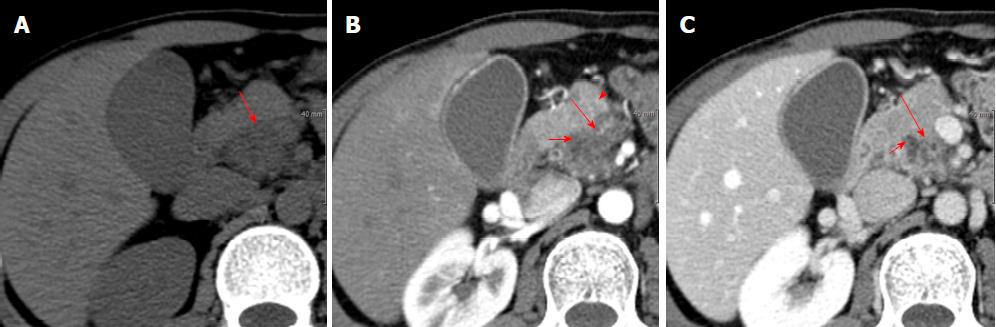
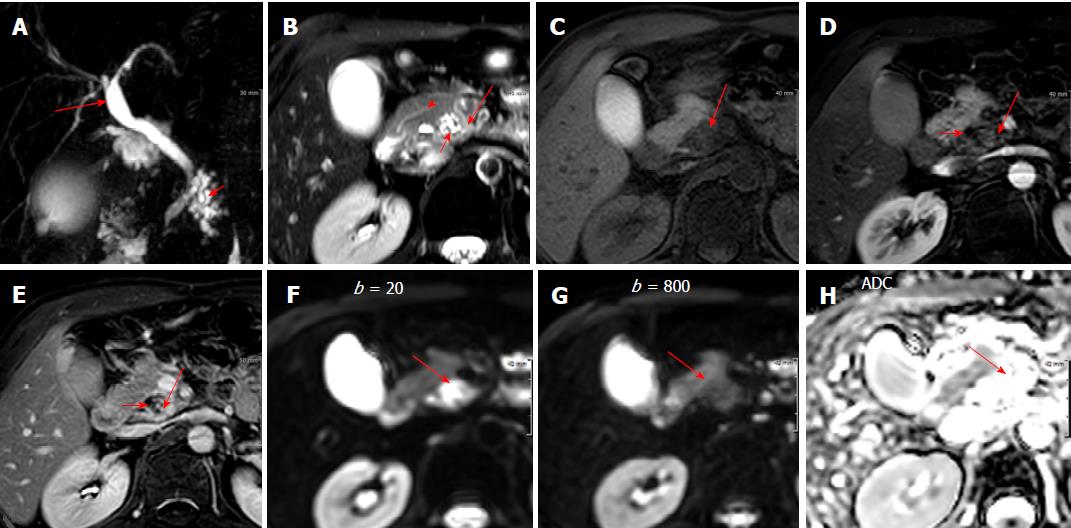
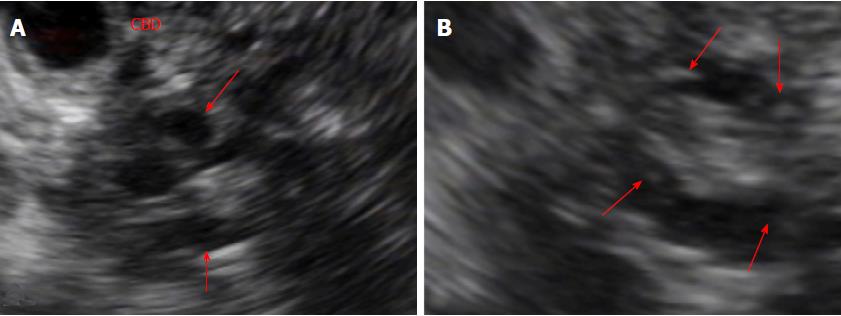
Unenhanced abdomen CT image showed slight low attenuating lesion involving pancreatic uncinate process and head (Figure 1A). Contrast-enhanced abdominal CT images showed a delayed enhancing solid portion surrounding a few tubular cystic attenuating lesion sized about 2.5 cm x 2.1 cm in pancreatic uncinate process and head, and mild dilatation of common bile duct (CBD) and gallbladder (Figure 1B and C). MR cholangiopancreatography showed branch pancreatic duct dilation in head and uncinate process causing extrinsic indentation and tapering of distal CBD, and mild dilatation of proximal CBD and gallbladder (Figure 2A). Fat-saturated T2-weighted MR image showed a slight high signal intensity solid component surrounding bright signal intensity branch duct dilatation in pancreatic uncinate process and head, with the lesion sized about 2.6 cm × 2.2 cm (Figure 1B). Fat-suppressed T1-weighted MR image showed a well-demarcated low signal intensity lesion in head and uncinate process of pancreas (Figure 2C), and delayed contrast-enhancing solid component surrounding low signal intensity branch-duct dilation in pancreatic uncinate process and head was shown on fat-suppressed T1-weighted dynamic gadolinium-enhanced MR images (Figure 2D and E). Diffusion-weighted MR images showed higher signal intensity on low b factor (b = 20 s/m2) image and low signal intensity on high b factor (b = 800 s/m2) image, suggesting no diffusion restriction on apparent diffusion coefficient map (Figure 2H), which reflecting the large area of cystic component of the lesion. Endoscopic ultrasonography (EUS) showed pruning pattern, anechoic branch duct dilatation containing a few small hyperechoic mural nodules (Figure 3A and B).
The lesion located in uncinate process and head of pancreas with indenting distal CBD and dilatation of proximal CBD, without dilatation of main pancreatic duct due to anatomic variation of pancreatic divisum which was detected on MR image (Figure 2B). Radiological diagnostic impression was branch duct type intraductal papillary mucinous neoplasm (IPMN) of pancreas. However, some worrisome features of delayed contrast-enhancing solid component around the wall of dilated branch duct on CT and MR images and small mural nodules in dilated branch ducts on EUS were shown. EUS guided fine needle aspiration (FNA) cytology was obtained from the solid component along the wall of dilated duct and suggested the possibility of intraductal-growing epithelial neoplasm.
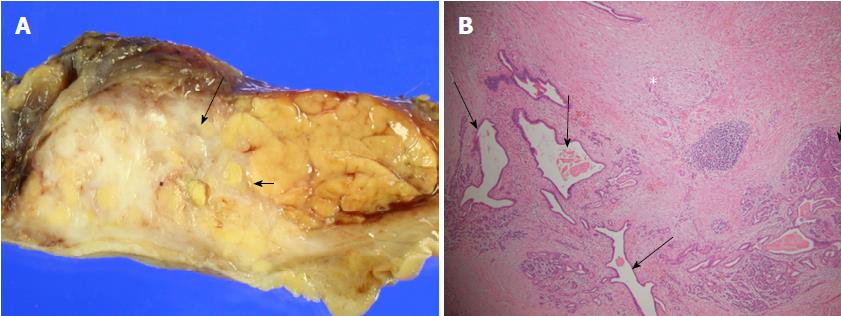
The patient underwent pylorus-preserving pancreaticoduodenectomy, due to considering FNA finding, imaging findings of CT, MRI, and EUS and aggravated right upper abdominal pain and persistent mild elevation of bilirubin and gamma-glutamyl transferase levels without response to conservative medical treatment for four weeks. The gross pathology of resected specimen showed whitish hard infiltrating lesion in pancreatic uncinate process and head portion (Figure 4A). The histopathologic report revealed periductal inflammation with fibrosis and mild dilatation of branch pancreatic ducts and intralobular fibrosis, consistent with chronic pancreatitis (Figure 4B).
Chronic pancreatitis is defined as inflammatory and fibrotic disease of pancreatic tissue, characterized by irreversible functional and morphologic change. Alcohol abuse is the most common (70%-80%) cause of chronic pancreatitis in the developed countries[1,2,3,7]. In addition, smoking, gene mutations, autoimmune syndromes, metabolic disturbances, environmental conditions and anatomical abnormalities are suggested as other associated factors with occurrence of the disease[3,11,12].
The pathology of advanced alcoholic chronic pancreatitis revealed a firm consistency of pancreas with an irregular contour without the normal lobulation[13]. The fibrosis may diffusely affect the entire gland, but occasionally it is unevenly distributed, with preserved normal lobular pattern in some areas. The severity of the duct changes depends on the extent of the surrounding fibrosis. Thus, the main duct may be focally or diffusely involved with obstruction, irregular dilatation and distortion[14,15]. Fibrosis in the pancreas head may cause a tapering stenosis of CBD[16].
In this case, initial clinical symptom of right upper abdominal pain was developed due to dilatation of gallbladder by stenosis of distal CBD, and the causative lesion of CBD obstruction was a focal mass lesion, including branch duct dilatation with surrounding solid component in uncinate process and head of pancreas, detected on CT, MRI and EUS findings. The diagnostic impression based on radiological imaging findings was branch duct type IPMN most likely and serous cystadenoma as a possible differential diagnosis. In thinking of branch duct type IPMN, analyses of imaging findings included the “worrisome features” of contrast-enhancing ductal margin on CT and MRI and mural nodules in dilated duct on EUS[17-19]. In addition, FNA suggested intraductal-growing epithelial neoplasm though scant cellularity. Surgery was the best choice at that time, considering aggravated clinical symptom, radiological findings, opinion of FNA, and patient’s young age. However, final pathologic result revealed interlobular and intralobular inflammation and fibrosis associated with branch duct dilatation, compatible with chronic pancreatitis. It was a totally unexpected one, even though considering patient’s past medical history of severe alcoholic pancreatitis and social history of frequent alcohol consumption and heavy smoking.
There have been many reports for the differentiation mass forming chronic pancreatitis from pancreatic adenocarcinoma such as dynamic enhancement of CT and MR imaging, Perfusion CT imaging, duel energy CT in spectral imaging mode, 18F fluorodeoxyglucose positron emission tomography/CT combined with carbohydrate antigen 19-9, and quantitative endoscopic ultrasound elastography, but still it is very difficult to distinguish accurately between the two[5,10,20-24]. MRI is much better than CT for detection and characterization of focal pancreatic lesion, but it could not differentiate mass forming chronic pancreatitis from pancreatic carcinoma, even using diffusion-weighted functional MR imaging technique[5,10,25,26]. In addition, none of the above mentioned papers included a case of a solid mass containing cystic lesion like this in their research of differentiation between mass forming pancreatitis and pancreatic carcinoma.
Among the papers on relationship between main pancreatic duct (MPD) and the mass, the “duct-penetrating” sign of MPD on MR cholangiopancreatography was reported to be helpful with relatively high sensitivity and specificity, and the result was smoothly stenotic or normal MPD penetrating a mass was seen more frequently in inflammatory pancreatic mass than in pancreatic carcinoma[27]. However, in this peculiar case, inflammatory mass possessed dilated branch pancreatic duct without stenosis.
In this very unique case, it could be comprehended uneven fibrosis and inflammation developed in localized area of uncinate process and head of pancreas, focal severe perilobular and interlobular fibrosis caused stricture and dilatation of branch pancreatic duct in uncinate process, and the these outbreaks led to very distinctive and peculiar radiological features of mass forming chronic pancreatitis and clinical symptoms of bile duct obstruction.
There has been no literature about focal fibrotic mass forming chronic pancreatitis containing branch duct dilatation, and incidentally this lesion showed almost typical imaging findings of pancreatic cystic neoplasm.
A 34-year-old man was referred to our hospital with right upper abdominal pain, and a pancreatic solid and cystic lesion found on computed tomography (CT), magnetic resonance (MR) image with MR cholangiography, and endoscopic ultrasonography (EUS).
Branch duct type intraductal papillary mucinous neoplasm.
Serous cystadenoma among solid and cystic pancreatic neoplasms.
Abnormal laboratory results included slightly elevated level of total bilirubin (1.3 mg/dL, normal range of 0.2-1.2) and gamma-glutamyl transferase (108 IU/L, normal range of 8-60).
CT and MR imaging showed a delayed contrast-enhanced solid lesion containing pruning-pattern branch duct dilatation in uncinate process and head of pancreas, with small hyperechoic mural nodules in the dilated branch ducts on EUS.
Microscopic findings of resected specimen revealed mass forming chronic pancreatitis including branch duct dilatation.
The patient was treated with pylorus-preserving pancreaticoduodenectomy.
There have been many reports for the discrimination between mass forming chronic pancreatitis and pancreatic adenocarcinoma using various imaging modalities.
There are no non-standard medical terms used in this manuscript.
The author presents this case to share the very unusual but important knowledge that mass forming chronic pancreatitis might include the branch duct dilatation.
I wish to thank to Drs. Sung Ho Cho (Department of Surgery, Dankook University Hospital) and Won-Ae Lee (Department of Pathology, Dankook University Hospital) for their comments and discussions about the case.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Agrawal S, Tandon RK S- Editor: Chen K L- Editor: A E- Editor: Ma YJ
| 1. | Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 418] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 2. | Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon. 2014;60:530-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 758] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 4. | Luetmer PH, Stephens DH, Ward EM. Chronic pancreatitis: reassessment with current CT. Radiology. 1989;171:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 91] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Kim T, Murakami T, Takamura M, Hori M, Takahashi S, Nakamori S, Sakon M, Tanji Y, Wakasa K, Nakamura H. Pancreatic mass due to chronic pancreatitis: correlation of CT and MR imaging features with pathologic findings. AJR Am J Roentgenol. 2001;177:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi--detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Imamura T, Iguchi H, Manabe T, Ohshio G, Yoshimura T, Wang ZH, Suwa H, Ishigami S, Imamura M. Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas. 1995;11:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Longnecker DS. Pancreas. Anderson’s Pathology. 10th ed. St. Louis: Mosby-Year Book; 1996; 1891-1916. |
| 9. | Ritchie AC. Pancreas. Boyd’s textbook of pathology, 9th ed. Philadelphia: Lea & Febiger; 1990; 1202-1234. |
| 10. | Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology. 1999;212:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Maisonneuve P, Lowenfels AB, Müllhaupt B, Cavallini G, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L, Frulloni L. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Ammann RW. The natural history of alcoholic chronic pancreatitis. Intern Med. 2001;40:368-375. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 13. | Klöppel G, Maillet B. The morphological basis for the evolution of acute pancreatitis into chronic pancreatitis. Virchows Arch A Pathol Anat Histopathol. 1992;420:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 107] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Ammann RW, Heitz PU, Klöppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996;111:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 207] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Klöppel G. Chronic pancreatitis of alcoholic and nonalcoholic origin. Semin Diagn Pathol. 2004;21:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Yadegar J, Williams RA, Passaro E Jr, Wilson SE. Common duct stricture from chronic pancreatitis. Arch Surg. 1980;115:582-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 18. | Goh BK, Tan DM, Ho MM, Lim TK, Chung AY, Ooi LL. Utility of the sendai consensus guidelines for branch-duct intraductal papillary mucinous neoplasms: a systematic review. J Gastrointest Surg. 2014;18:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Castellano-Megías VM, Andrés CI, López-Alonso G, Colina-Ruizdelgado F. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol. 2014;6:311-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Yin Q, Zou X, Zai X, Wu Z, Wu Q, Jiang X, Chen H, Miao F. Pancreatic ductal adenocarcinoma and chronic mass forming pancreatitis: Differentiation with dual-energy MDCT in spectral imaging mode. Eur J Radiol. 2015;84:2470-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Yadav AK, Sharma R, Kandasamy D, Pradhan RK, Garg PK, Bhalla AS, Gamanagatti S, Srivastava DN, Sahni P, Upadhyay AD. Perfusion CT - Can it resolve the pancreatic carcinoma versus mass forming chronic pancreatitis conundrum? Pancreatology. 2016;16:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Lu N, Feng XY, Hao SJ, Liang ZH, Jin C, Qiang JW, Guo QY. 64-slice CT perfusion imaging of pancreatic adenocarcinoma and mass forming chronic pancreatitis. Acad Radiol. 2011;18:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Gu X, Liu R. Application of 18F-FDG PET/CT combined with carbohydrate antigen 19-9 for differentiating pancreatic carcinoma from chronic mass forming pancreatitis in Chinese elderly. Clin Interv Aging. 2016;11:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Kim SY, Cho JH, Kim YJ, Kim EJ, Park JY, Jeon TJ, Kim YS. Diagnostic efficacy of quantitative endoscopic ultrasound elastography for differentiating pancreatic disease. J Gastroenterol Hepatol. 2017;32:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL, Hammond NA, Yaghmai V, Nikolaidis P. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics. 2011;31:E47-E64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Barral M, Taouli B, Guiu B, Koh DM, Luciani A, Manfredi R, Vilgrain V, Hoeffel C, Kanematsu M, Soyer P. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology. 2015;274:45-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 27. | Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |