Published online Jan 14, 2018. doi: 10.3748/wjg.v24.i2.257
Peer-review started: November 21, 2017
First decision: December 6, 2017
Revised: December 8, 2017
Accepted: December 13, 2017
Article in press: December 13, 2017
Published online: January 14, 2018
Processing time: 54 Days and 11.9 Hours
To evaluate whether the neoadjuvant chemotherapy (NACT)-surgery interval time significantly impacts the pathological complete response (pCR) rate and long-term survival.
One hundred and seventy-six patients with gastric cancer undergoing NACT and a planned gastrectomy at the Chinese PLA General Hospital were selected from January 2011 to January 2017. Univariate and multivariable analyses were used to investigate the impact of NACT-surgery interval time (< 4 wk, 4-6 wk, and > 6 wk) on pCR rate and overall survival (OS).
The NACT-surgery interval time and clinician T stage were independent predictors of pCR. The interval time > 6 wk was associated with a 74% higher odds of pCR as compared with an interval time of 4-6 wk (P = 0.044), while the odds ratio (OR) of clinical T3vs clinical T4 stage for pCR was 2.90 (95%CI: 1.04-8.01, P = 0.041). In Cox regression analysis of long-term survival, post-neoadjuvant therapy pathological N (ypN) stage significantly impacted OS (N0vs N3: HR = 0.16, 95%CI: 0.37-0.70, P = 0.015; N1vs N3: HR = 0.14, 95%CI: 0.02-0.81, P = 0.029) and disease-free survival (DFS) (N0vs N3: HR = 0.11, 95%CI: 0.24-0.52, P = 0.005; N1vs N3: HR = 0.17, 95%CI: 0.02-0.71, P = 0.020). The surgical procedure also had a positive impact on OS and DFS. The hazard ratio of distal gastrectomy vs total gastrectomy was 0.12 (95%CI: 0.33-0.42, P = 0.001) for OS, and 0.13 (95%CI: 0.36-0.44, P = 0.001) for DFS.
The NACT-surgery interval time is associated with pCR but has no impact on survival, and an interval time > 6 wk has a relatively high odds of pCR.
Core tip: The impact of interval time between completion of neoadjuvant chemotherapy and surgery on pathological complete response (pCR) had been proved in colorectal cancer and esophageal cancer. However, no such research was found in gastric cancer. To evaluate whether the interval time impacts efficiency of neoadjuvant chemotherapy, 176 patients with gastric cancer were recruited. The interval time and clinical T stage were proved predictors of pCR. Post-neoadjuvant therapy pathological N stage and surgical procedure have a significant impact on the long-term survival. An interval time > 6 wk was associated with a higher odds of pCR.
- Citation: Liu Y, Zhang KC, Huang XH, Xi HQ, Gao YH, Liang WQ, Wang XX, Chen L. Timing of surgery after neoadjuvant chemotherapy for gastric cancer: Impact on outcomes. World J Gastroenterol 2018; 24(2): 257-265
- URL: https://www.wjgnet.com/1007-9327/full/v24/i2/257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i2.257
Surgery is the only curative treatment for gastric cancer (GC). Although standard surgery has been performed in recent years, overall survival (OS) at 5 years for GC patients remains at 20%-30%[1]. Since more and more clinical trials have validated the survival benefit of preoperative chemotherapy[2-4], neoadjuvant chemotherapy (NACT) has been gradually accepted by clinicians.
Making patients experience significant tumor downstaging and even a pathologic complete response (pCR) is the most important goal of NACT. It has been proven that patients who have a pCR may achieve superior OS and fewer local or systemic recurrence than those with a partial or no response[5,6]. Therefore, every potential way has been explored to maximize the possibility of attaining a pCR. Since the Lyon R90-01 trial found that patients undergoing surgery at an interval of 6-8 wk after NACT showed improvement in clinical tumor response and pathologic downstaging compared with a 2-3-wk interval[7], a growing number of studies have proven that a longer interval is significantly related to increased pCR rates, increased tumor downstaging, and potential superior OS in rectal cancer[8-11]. However, in esophageal cancer, results are conflicting. Some studies found that a longer interval was associated with higher pCR rates that might improve the prognosis[12,13]; even intervals beyond 12 wk have been thought to be safe[14]. Yet, other studies failed to validate the connection between longer intervals and pCR rates, and found that longer intervals were disadvantageous to long-term OS[15,16]. To our knowledge, the optimal timing of performing surgery after NACT has never been studied in GC. An interval time of 4-6 wk was first practiced in some NACT clinical trials[17,18]. However, an interval of 4-6 wk has never been validated as being optimal. Thus, the aim of this study was to assess the link between NACT-surgery interval time and pCR rates and/or OS.
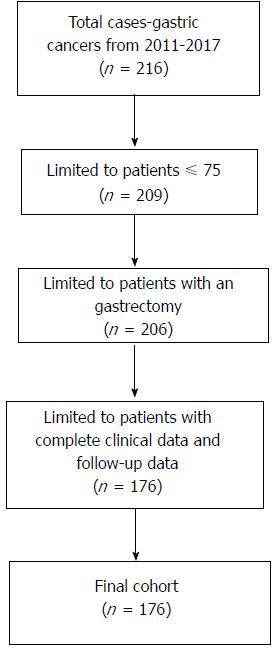
This was a retrospective study for which we recruited 216 patients with GC who underwent NACT at the Chinese PLA General Hospital from January 2011 to January 2017. The criteria for inclusion were: (1) GC was diagnosed using endoscopy and a biopsy; (2) Patients who underwent NACT and a planned gastrectomy; and (3) All clinical pathological information was available, including NACT relevant information, surgical parameters, imaging information, pathological diagnosis, perioperative therapy, and follow-up data. The exclusion criteria were: (1) Patients older than 75 years; and (2) Patients who ever received chemoradiotherapy. Finally, only 176 patients were included (Figure 1). Before NACT, endoscopic ultrasound (EUS) and contrast-enhanced computed tomography (CE-CT) had been performed to assess clinical stage and confirm that patients had T2-4N0-3M0 GC, according to the Japanese classification of gastric carcinoma[19].
Most patients (n = 167) received 2-4 cycles of a SOX regimen (S-1 80 mg/m2 per day, PO, days 1-14, and oxaliplatin 130 mg/m2 per day, IV, infusion on day 1), which is widely used in Asia[20]; the remaining patients (n = 9) received a XELOX regimen (capecitabine 1000 mg/m2 per day, PO, days 1-14, and oxaliplatin 130 mg/m2 per day, IV, infusion on day 1). After two cycles of chemotherapy, the curative effect was evaluated using EUS and CT according to RECIST1.1[21]. A gastrectomy was carried out immediately when imaging showed an observable increase in tumor size or tumor disappearance. If imaging indicated a decrease in tumor size, another one or two cycles of chemotherapy could be performed. The planned operations after NACT were conducted by experienced surgeons. Patients without evidence of metastasis underwent a gastrectomy with a D2 lymphadenectomy. For other patients, the type of operation was decided by a multidisciplinary team. The location of the primary tumor determined whether a proximal, distal, or total gastrectomy was selected.
The same pathologist microscopically analyzed all resected specimens. Patients with post-neoadjuvant therapy pathological (yp)T0N0M0 GC were defined as having a pCR and all others were defined as not having a pCR[11]. Clinical examinations and abdominal CT were performed every 6 mo for 3 years. Digestive endoscopy was performed at least once a year. In March 2017, we confirmed the survival status of patients and the median follow-up time was 42 mo (range, 2-74 mo). Follow-up data were completed for all recruited patients.
The primary objective was to evaluate the impact of NACT-surgery interval time on pCR rate and the optimal timing of operation. The secondary objective was to determine the association between NACT-surgery interval time and 3-year OS or disease-free survival (DFS). For that purpose, of the 171 patients who were admitted from January 2011 to March 2014, 121 were selected.
We used the Chi-squared test or Fisher’s exact test for binary and categorical variables, and ANOVA or t-tests for continuous variables, as appropriate. Patient and tumor characteristics were compared between the three groups at baseline and postsurgery. A bivariate analysis of patients, tumors and surgical characteristics, and pCR status was conducted. Tumor or treatment characteristics that achieved a P-value < 0.2 in univariate analysis were included in the multivariable analysis. Logistic regression was used to model the effects of optimal interval time on the odds of having a pCR, and factors independently associated with pCR were determined using a stepwise procedure. The Kaplan-Meier method was used to estimate survivor functions and the log-rank test was used for the comparison of survival curves. Multivariate analysis using Cox proportional hazards regression analysis with a stepwise procedure was performed to investigate independent factors of survival.
All the statistical analyses were performed using IBM SPSS Statistics version 22.0 software. The hazard ratio (HR) and 95% confidence interval (95%CI) were reported and used to assess the relationship between pCR rate and survival for each independent factor.
Among the 176 patients, 111 (63%) had an NACT-surgery interval time < 4 wk, 48 (27%) had an interval time of 4-6 wk, and 17 (9.7%) had an interval time > 6 wk. The median age was 57 years (range, 21-75 years) and the male to female ratio was 3.5/1. Characteristics of the study cohort are summarized in Table 1. Patient characteristics, tumor characteristics, and surgical procedure were compared among the three groups (< 4 wk, 4-6 wk, and > 6 wk). Age (P = 0.014), tumor differentiation (before NACT) (P = 0.000), clinical T stage (P = 0.006), and ypT stage (P = 0.045) were significantly different among the three groups. Forty (22.7%) patients had achieved a pCR; the pCR rate was 67.5% for those with a NACT-surgery interval time < 4 wk, 15% for those with a NACT-surgery interval time of 4-6 wk, and 17.5% for those with a NACT-surgery interval time > 6 wk.
| < 4 wk (n = 111) | 4-6 wk (n = 48) | > 6 wk (n = 17) | P value | pCR (n = 40) | No pCR (n = 136) | P value | |
| Age, yr, mean ± SD | 55.5585 ± 10.8079 | 59.7916 ± 9.7891 | 61.5882 ± 9.5985 | 0.014 | 57.375 ± 9.862354 | 57.27206 ± 10.88013 | 0.908 |
| Sex | 0.974 | 0.174 | |||||
| Male | 87 (78.38) | 37 (77.08) | 13 (76.47) | 28 (70.00) | 109 (80.15) | ||
| Female | 24 (21.62) | 11 (22.92) | 4 (23.53) | 12 (3.00) | 27 (19.85) | ||
| Chemotherapy cycles | 0.692 | 1.000 | |||||
| < 4 | 39 (35.14) | 17 (35.42) | 4 (23.53) | 14 (35.00) | 46 (33.82) | ||
| ≥ 4 | 72 (64.86) | 31 (64.58) | 13 (76.47) | 26 (65.00) | 90 (66.18) | ||
| ASA, yr, mean ± SD | 0.083 | 0.467 | |||||
| 1 | 8 (7.21) | 1 (2.8) | 2 (11.76) | 4 (10.00) | 7 (5.15) | ||
| 2 | 97 (87.39) | 39 (81.25) | 15 (88.24) | 32 (80.00) | 119 (87.50) | ||
| 3 | 6 (5.40) | 8 (16.67) | 0 | 4 (10.00) | 10 (7.35) | ||
| Histology (before NACT) | 0.398 | 0.658 | |||||
| Tubular adenocarcinoma | 90 (81.08) | 40 (83.33) | 15 (88.24) | 34 (85.00) | 111 (81.62) | ||
| Mucinous | 10 (9.01) | 1 (2.08) | 0 (0.00) | 1 (2.50) | 10 (7.35) | ||
| Signet ring cell | 9 (9.11) | 4 (8.33) | 1 (5.88) | 3 (7.50) | 11 (8.09) | ||
| mixed type1 | 2 (1.80) | 3 (6.25) | 1 (5.88) | 2 (5.00) | 4 (2.94) | ||
| Differentiation (before NACT) | 0.000 | 0.032 | |||||
| Well | 2 (1.80) | 0 (0.00) | 15 (88.24) | 2 (5.00) | 0 (0.00) | ||
| Moderate | 28 (25.23) | 10 (20.83) | 1 (5.88) | 10 (25.00) | 35 (25.74) | ||
| Poor | 81 (72.97) | 38 (79.17) | 1 (5.88) | 28 (79.00) | 101 (74.26) | ||
| Clinical T stage | 0.006 | 0.027 | |||||
| 2 | 31 (27.93) | 17 (35.42) | 6 (35.29) | 15 (37.50) | 39 (28.68) | ||
| 3 | 24 (21.62) | 19 (39.58) | 8 (47.06) | 16 (40.00) | 35 (19.85) | ||
| 4 | 56 (50.45) | 12 (25.00) | 3 (17.65) | 9 (22.50) | 62 (51.47) | ||
| Clinical N stage | 0.170 | 0.012 | |||||
| Positive | 89 (80.18) | 33 (68.75) | 11 (64.71) | 24 (60.00) | 109 (88.97) | ||
| Negative | 22 (19.82) | 15 (31.25) | 6 (35.29) | 16 (40.00) | 27 (11.03) | ||
| Tumor location | 0.650 | 0.044 | |||||
| Upper | 45 (40.54) | 23 (47.92) | 6 (35.29) | 10 (25.00) | 64 (47.06) | ||
| Middle | 16 (14.41) | 7 (14.58) | 2 (11.76) | 6 (15.00) | 19 (13.97) | ||
| Lower | 45 (40.54) | 14 (29.17) | 7 (41.18) | 22 (55.00) | 44 (32.35) | ||
| Diffuse type2 | 5 (4.51) | 4 (8.33) | 2 (11.76) | 2 (5.00) | 9 (6.62) | ||
| Tumor diameter (before NACT) | 0.134 | 0.069 | |||||
| ≤ 2 cm | 15 (13.51) | 8 (16.67) | 2 (11.76) | 7 (17.50) | 18 (2.21) | ||
| 2-5 cm | 50 (45.05) | 21 (43.75) | 13 (76.47) | 24 (60.00) | 60 (69.85) | ||
| ≥ 5 cm | 46 (41.44) | 19 (39.58) | 2 (11.76) | 9 (12.50) | 58 (27.94) | ||
| Surgical procedure | 0.363 | 0.002 | |||||
| Proximal gastrectomy | 21 (18.92) | 10 (20.83) | 2 (11.76) | 9 (22.50) | 24 (17.65) | ||
| Distal gastrectomy | 32 (28.83) | 10 (20.83) | 8 (47.06) | 19 (47.50) | 31 (22.79) | ||
| Total gastrectomy | 58 (52.25) | 28 (58.33) | 7 (41.18) | 12 (30.00) | 81 (59.56) | ||
| NACT-surgery interval time | 0.043 | ||||||
| < 4 wk | 27 (67.50) | 84 (61.76) | |||||
| 4-6 wk | 6 (15.00) | 42 (30.88) | |||||
| > 6 wk | 7 (17.50) | 10 (7.35) | |||||
| ypT stage | 0.045 | ||||||
| 0 | 27 (24.32) | 6 (12.50) | 7 (41.18) | ||||
| 1 | 7 (6.31) | 9 (18.75) | 3 (17.65) | ||||
| 2 | 25 (22.52) | 6 (12.50) | 2 (11.76) | ||||
| 3 | 38 (34.23) | 15 (31.25) | 4 (23.53) | ||||
| 4 | 14 (12.61) | 12 (25.00) | 1 (5.88) | ||||
| ypN stage | 0.187 | ||||||
| 0 | 67 (60.30) | 23 (47.92) | 14 (82.35) | ||||
| 1 | 7 (6.31) | 7 (14.58) | 2 (11.76) | ||||
| 2 | 16 (14.41) | 5 (10.42) | 1 (5.88) | ||||
| 3a | 14 (12.61) | 8 (16.67) | 0 | ||||
| 3b | 7 (6.31) | 5 (10.42) | 0 |
Table 1 also shows the bivariate association between pCR and patient characteristics, tumor characteristics, and surgical procedure. NACT-surgery interval time (P = 0.043), tumor differentiation (before NACT) (P = 0.032), clinical T stage (P = 0.027), clinical N stage (P = 0.012), tumor location (P = 0.044), and surgical procedure (P = 0.002) were significantly different between patients with and without pCR.
Factors that have achieved a P-value < 0.2 in univariate analysis were selected for multivariate analysis, including gender, NACT-surgery and interval time, clinical T stage, clinical N stage, tumor diameter. The multivariate analysis (Table 2) showed that a NACT-surgery interval time of 4-6 wk was associated with a 74% lower change of having a pCR as compared with an NACT-surgery interval time > 6 wk (P = 0.044), while the OR of clinical T3vs clinical T4 stage for pCR was 2.90 (95%CI: 1.04-8.01, P = 0.041).
| Factor | OR | 95%CI | P value |
| Sex | |||
| Male vs female | 1.76 | 0.74-4.18 | 0.201 |
| NACT-Surgery interval time | |||
| < 4 wk vs > 6 wk | 0.69 | 0.22-2.13 | 0.521 |
| 4-6 wk vs > 6 wk | 0.26 | 0.07-0.96 | 0.044 |
| Clinical T stage | |||
| T2 vs T4 | 1.99 | 0.70-5.68 | 0.200 |
| T3 vs T4 | 2.90 | 1.04-8.01 | 0.041 |
| Clinical N stage | |||
| Positive vs negative | 2.12 | 0.90-4.97 | 0.086 |
| Tumor diameter (before NACT) | |||
| ≤ 2 cm vs ≥ 5 cm | 1.60 | 0.44-5.80 | 0.472 |
| 2-5 cm vs ≥ 5 cm | 1.58 | 0.60-4.14 | 0.354 |
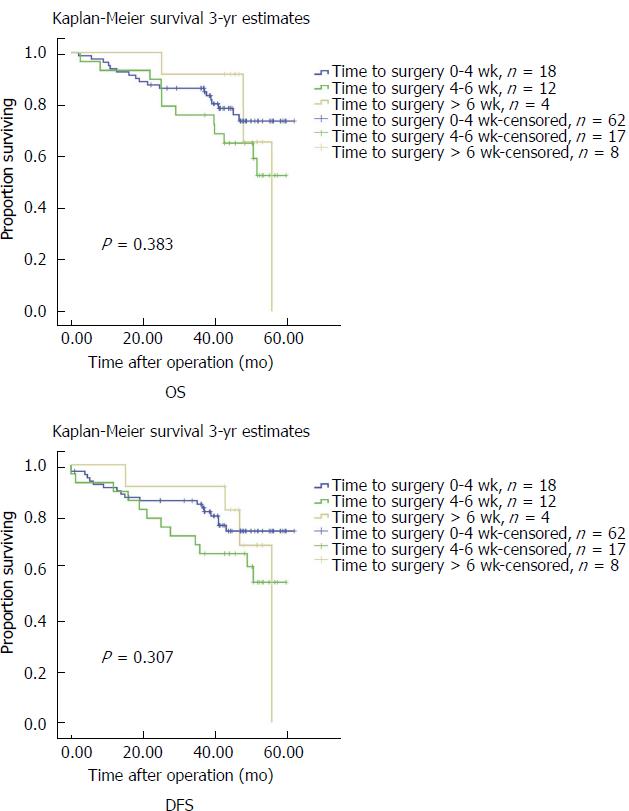
Kaplan-Meier analyses for 3-year OS and DFS are presented in Figure 2. There was no significant difference among the three survival curves for both OS and DFS according to the log-rank test. The median OS was 41.5 mo (range, 20.0-61.8 mo) and median DFS was 39.5 mo (range, 0-61.8 mo).
Recurrence was experienced by 29.5% of patients. As shown in Table 3, NACT-surgery interval time was not found to be independently associated with OS or DFS. Independent factors associated with OS were ypN stage (N0vs N3: HR = 0.16, 95%CI: 0.37-0.70, P = 0.015; N1vs N3: HR = 0.14, 95%CI: 0.02-0.81, P = 0.029) and surgical procedure (distal gastrectomy vs total gastrectomy: HR = 0.12, 95%CI: 0.33-0.42, P = 0.001). For DFS, independent factors were also ypN stage and surgical procedure.
| Independent predictor | 3-yr estimate (overall survival) | 3-yr estimate (disease-free survival) | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| NACT-Surgery interval time | ||||||
| < 4 wk vs > 6 wk | 0.49 | 0.11-2.129 | 0.340 | 0.43 | 0.10-1.85 | 0.258 |
| 4-6 wk vs > 6 wk | 0.99 | 0.24-4.06 | 0.985 | 0.93 | 0.23-3.80 | 0.922 |
| Age | ||||||
| ≤ 60 vs > 60 | 0.90 | 0.34-2.37 | 0.833 | 0.84 | 0.32-2.19 | 0.720 |
| Sex | ||||||
| Female vs male | 1.27 | 0.40-4.04 | 0.688 | 1.24 | 0.39-3.99 | 0.716 |
| Histology (before NACT) | ||||||
| Tubular adenocarcinoma vs mixed type | 2.56 | 0.24-26.94 | 0.433 | 2.25 | 0.22-22.56 | 0.491 |
| Mucinous vs mixed type | 3.79 | 0.21-70.55 | 0.372 | 3.12 | 0.18-53.99 | 0.435 |
| Signet ring cell vs mixed type | 5.71 | 0.40-81.22 | 0.199 | 4.99 | 0.37-66.54 | 0.224 |
| Differentiation (before NACT) | ||||||
| Well and moderate vs poor | 2.49 | 0.99-6.24 | 0.052 | 2.45 | 0.98-6.11 | 0.054 |
| Clinical T stage | ||||||
| T2 vs T4 | 1.51 | 0.42-5.39 | 0.524 | 1.67 | 0.48-5.84 | 0.422 |
| T3 vs T4 | 0.99 | 0.31-3.16 | 0.980 | 0.98 | 0.31-3.11 | 0.968 |
| Clinical N stage | ||||||
| Positive vs negative | 0.45 | 0.13-1.62 | 0.221 | 0.49 | 0.14-1.74 | 0.270 |
| Tumor diameter (before NACT) | ||||||
| ≤ 2 cm vs ≥ 5 cm | 3.16 | 0.61-16.45 | 0.171 | 2.88 | 0.57-14.65 | 0.202 |
| 2-5 cm vs ≥ 5 cm | 1.91 | 0.72-5.10 | 0.196 | 1.74 | 0.65-4.65 | 0.267 |
| Tumor location | ||||||
| Upper vs diffuse type | 1.04 | 0.15-7.33 | 0.973 | 0.99 | 0.14-6.98 | 0.989 |
| Middle vs diffuse type | 1.11 | 0.16-7.78 | 0.915 | 1.16 | 0.17-8.05 | 0.879 |
| Lower vs diffuse type | 4.41 | 0.78-25.18 | 0.095 | 3.94 | 0.69-22.50 | 0.123 |
| Surgical procedure | ||||||
| Proximal gastrectomy vs total gastrectomy | 0.69 | 0.17-2.73 | 0.593 | 0.79 | 0.20-3.07 | 0.729 |
| Distal gastrectomy vs total gastrectomy | 0.12 | 0.33-0.42 | 0.001 | 0.13 | 0.36-0.44 | 0.001 |
| ypT stage | ||||||
| T0 vs T4 | 1.04 | 0.15-7.20 | 0.968 | 1.27 | 0.18-9.08 | 0.811 |
| T1 vs T4 | 0.57 | 0.09-4.14 | 0.601 | 0.588 | 0.86-4.04 | 0.589 |
| T2 vs T4 | 1.15 | 0.24-5.53 | 0.858 | 1.29 | 0.26-6.46 | 0.756 |
| T3 vs T4 | 0.60 | 0.15-2.09 | 0.387 | 0.59 | 0.16-2.18 | 0.425 |
| ypN stage | ||||||
| N0 vs N3 | 0.16 | 0.37-0.70 | 0.015 | 0.11 | 024-0.52 | 0.005 |
| N1 vs N3 | 0.14 | 0.02-0.81 | 0.029 | 0.17 | 0.02-0.71 | 0.020 |
| N2 vs N3 | 0.47 | 0.11-1.98 | 0.302 | 0.40 | 0.09-1.67 | 0.208 |
The impact of the NACT-surgery interval on pCR and survival has been proven in rectal cancer and esophageal cancer[8,14]. However, the optimal NACT-surgery interval time and its association with survival, to the best of our knowledge, have never been investigated in GC. Similar to what was found in rectal cancer, the results of the present study suggest that a NACT-surgery interval time > 6 wk had a positive impact on pCR compared with either 4-6 wk or < 4 wk. However, the NACT-surgery interval time did not have an impact on either OS or DFS.
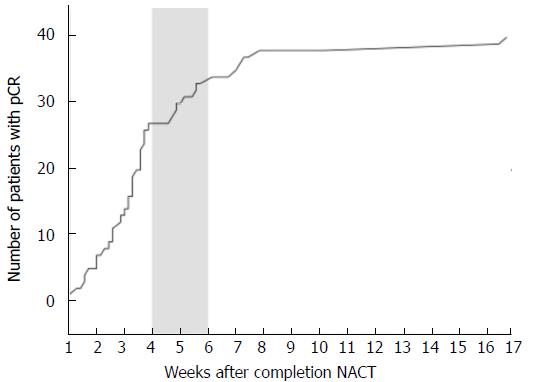
To determine the cutoff level, we plotted a curve of cumulative proportion of pCR by interval weeks (Figure 3). The curve shows that the slope is highest when the interval time is < 4 wk, and 4 and 6 wk are points of inflection. Meanwhile, the NACT-surgery interval time is commonly 4-6 wk, which is what clinicians in China have adopted. Thus, to prove whether a NACT-surgery interval time of 4-6 wk is optimal, after taking all factors into consideration, we divided the population into three groups by the cutoff levels of 4 and 6 weeks.
The impact of NACT-surgery interval time on pCR is the primary objective that we wanted to address. We defined pCR as T0N0M0, and partial response (PR) was not included in this study. This is because PR, which is confirmed using imaging according to RECIST[21], is more subjective and hence, more difficult to confirm than CR. In Table 1, age and tumor differentiation (before NACT) were significantly different among the three groups. The average age is highest in the > 6 wk group and lowest in the < 4 wk group. The result suggests that older patients may need a longer recovery period from NACT. In the subsequent univariate and multivariable analyses, age was shown to have no impact on pCR and long-term outcomes. With respect to tumor differentiation, previous studies showed that the more differentiated a tumor, the higher the pathology response rate when patients were treated with a XELOX regimen[22,23]. However, results from our univariate analysis contradict these previous findings. The NACT-surgery interval time, tumor differentiation (before NACT), clinical T stage, clinical N stage, tumor location, and surgical procedure were significantly different between the pCR group and the no-pCR group. We had not included surgical procedure into univariate analysis, for the reason that the pCR status had been determined before surgery. The subsequent multivariable analysis proved that NACT-surgery interval time and cT stage was independent factors associated with having a pCR. Compared with clinical T4 stage, patients with lower clinical T2 or T3 stage were more likely to achieve a pCR, although there was no significant difference between clinical T2 and T3 stages. This result is consistent with a previous study[24], which showed that lower T and N stages were linked with higher likelihood of pCR. Patients with a NACT-surgery interval time of 4-6 wk had a lower odds of having a pCR than those with an interval time > 6 wk (P = 0.044). Although a NACT-surgery interval time < 4 wk was associated with a 49% lower chance of having a pCR as compared with an interval time > 6 wk, the result was not statistically significant (P = 0.521). From these outcomes and the associations among them, we can conclude that the NACT-surgery interval time > 6 wk was the optimal interval time and had a positive impact on pCR as compared with the other groups.
This result is consistent with those from previous rectal and esophageal cancer studies[25-28], and it may be a common rule in gastrointestinal malignancies. Although many studies have shown that there is a positive impact from delaying the NACT-surgery interval time on pCR rate and short-term outcomes, the underlying mechanism has never been discussed. We speculate that it may be the result of multiple factors, including the ongoing effect of radiochemotherapy, changes in the tumor microenvironment, and recovery of immunity from chemotherapy. Additional basic medical studies may be needed to explain it.
The association between NACT-surgery interval time and long-term outcomes was also investigated. The survival curves of the three groups intersected at certain points and the log-rank test did not find any statistical significance among the curves (Figure 2). For both OS and DFS, Cox regression analysis showed that the NACT-surgery interval time and pCR (reflected by ypT0 status) had no impact on survival. This result is contrary to our expectation because pCR is deemed to have a positive impact on survival. Meredith et al[29] and Abdul-Jalil et al[30] both reported that pCR was an independent factor for OS and DFS. We thought that the small sample size may be the limitation. Regarding the NACT-surgery interval time, many previous studies in esophageal cancer proved that the interval time did not have any effect on survival[13,15,31], while some studies in rectal cancer reached an opposite conclusion[26,28]. Our result is consistent with studies in esophageal cancer. Our finding that ypN stage had a significant impact on OS and DFS aligns with those from previous studies[32,33]. The surgical procedure was found to be also an independent factor that can influence OS and DFS. Patients on whom a distal gastrectomy was performed had a significant difference in survival compared with patients on whom a total gastrectomy was performed. The reason for this result may be that patients who undergo a distal gastrectomy have a greater chance of having a pCR, and also, may be the difference of surgical method itself.
There were some limitations to our study. Its retrospective nature may induce some bias. Our relatively short follow-up time for survival (3-year estimates) and the absence of information regarding diseases not treated at the PLA General Hospital after the operation may have impacted our results. Also, our single institute research cannot avoid sampling bias and may not be representative. The small sample size was the biggest limitation, and the number of patients with interval time > 6 wk was not sufficient to explore more timing groups or the maximum interval time (such as 6-8 wk, 8-12 wk, and > 12 wk). A future multi-center randomized control trial with a larger sample size may be needed to validate our results.
To conclude, the NACT-surgery interval time > 6 wk can increase the chance of a pCR, but the NACT-surgery interval time does not have an impact on long-term survival.
The impact of the interval time from the completion of neoadjuvant chemotherapy (NACT) to surgery on pathological complete response (pCR) and survival has been proved in rectal cancer and esophageal cancer. However, the optimal NACT-surgery interval time and its association with survival, to the best of our knowledge, have never been investigated in gastric cancer. This study can provide evidence for the timing of surgery and patients with neoadjuvant chemotherapy may benefit from it.
To investigate whether the interval time between NACT and surgery have an impact on pCR was our main topic. The investigation lays a foundation for the further RCT research.
There were two objectives in this study. The primary objective was to evaluate the impact of NACT-surgery interval time on pCR rate and the optimal timing of operation. The secondary objective was to determine the association between NACT-surgery interval time and 3-year OS or disease-free survival (DFS). If the impacts are existent, more studies will focus on the investigation of optimal interval time and this evidence will bring a change in treatment plan for GC patients with neoadjuvant chemotherapy.
This is a retrospective study, in which we realized our objectives through data analysis using bivariate analysis, logistic regression analysis, and Cox proportion hazards regression. These methods are routinely used in studies and have high stability.
The impact of the NACT-surgery interval time on pCR has been proved and the interval time > 6 wk can increase the chance of a pCR. Clinical T stage also have an impact on pCR. The independent predictors of long-term survival are ypN stage and surgical procedure. These findings for the first time proved the impact of the NACT-surgery interval time on pCR in gastric cancer and give a reference for the optimal interval time. The further investigations of accurate optimal interval time are needed.
The authors for the first time investigated and found the impact of the NACT-surgery interval time on pCR, and the optimal interval time may be > 6 wk. This result is consistent with those from previous rectal and esophageal cancer studies, and we speculate that it may be the result of multiple factors, including the ongoing effect of radiochemotherapy, changes in the tumor microenvironment, and recovery of immunity from chemotherapy. Additional basic medical studies may be needed to explain it.
Further studies, either retrospective or prospective, are needed to investigate more interval time groups with a large sample size. Also, it is meaningful to investigate the mechanism of this finding through basic medical studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Espinel J, Ilhan E, Tanabe S S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Patel SH, Kooby DA. Gastric adenocarcinoma surgery and adjuvant therapy. Surg Clin North Am. 2011;91:1039-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 3. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 4. | Xiong BH, Cheng Y, Ma L, Zhang CQ. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest. 2014;32:272-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 475] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 6. | Cho H, Nakamura J, Asaumi Y, Yabusaki H, Sakon M, Takasu N, Kobayashi T, Aoki T, Shiraishi O, Kishimoto H. Long-term survival outcomes of advanced gastric cancer patients who achieved a pathological complete response with neoadjuvant chemotherapy: a systematic review of the literature. Ann Surg Oncol. 2015;22:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 558] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval &gt;7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | de Campos-Lobato LF, Geisler DP, da Luz Moreira A, Stocchi L, Dietz D, Kalady MF. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg. 2011;15:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, D’Hoore A. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19:2833-2841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Probst CP, Becerra AZ, Aquina CT, Tejani MA, Wexner SD, Garcia-Aguilar J, Remzi FH, Dietz DW, Monson JR, Fleming FJ; Consortium for Optimizing the Surgical Treatment of Rectal Cancer (OSTRiCh). Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg. 2015;221:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Ruol A, Rizzetto C, Castoro C, Cagol M, Alfieri R, Zanchettin G, Cavallin F, Michieletto S, Da Dalt G, Sileni VC. Interval between neoadjuvant chemoradiotherapy and surgery for squamous cell carcinoma of the thoracic esophagus: does delayed surgery have an impact on outcome? Ann Surg. 2010;252:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Shaikh T, Ruth K, Scott WJ, Burtness BA, Cohen SJ, Konski AA, Cooper HS, Astsaturov I, Meyer JE. Increased time from neoadjuvant chemoradiation to surgery is associated with higher pathologic complete response rates in esophageal cancer. Ann Thorac Surg. 2015;99:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Shapiro J, van Hagen P, Lingsma HF, Wijnhoven BP, Biermann K, ten Kate FJ, Steyerberg EW, van der Gaast A, van Lanschot JJ; CROSS Study Group. Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann Surg. 2014;260:807-813; discussion 813-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Tessier W, Gronnier C, Messager M, Hec F, Mirabel X, Robb WB, Piessen G, Mariette C. Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg. 2014;97:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Lin G, Han SY, Xu YP, Mao WM. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in esophageal cancer: a meta-analysis of published studies. Dis Esophagus. 2016;29:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Hashemzadeh S, Pourzand A, Somi MH, Zarrintan S, Javad-Rashid R, Esfahani A. The effects of neoadjuvant chemotherapy on resectability of locally-advanced gastric adenocarcinoma: a clinical trial. Int J Surg. 2014;12:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Oki E, Emi Y, Kusumoto T, Sakaguchi Y, Yamamoto M, Sadanaga N, Shimokawa M, Yamanaka T, Saeki H, Morita M. Phase II study of docetaxel and S-1 (DS) as neoadjuvant chemotherapy for clinical stage III resectable gastric cancer. Ann Surg Oncol. 2014;21:2340-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 20. | Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 21. | Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, Onaya H, Endo M, Sone M, Arai Y. [New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1)]. Gan To Kagaku Ryoho. 2009;36:2495-2501. [PubMed] |
| 22. | Wu ZF, Cao QH, Wu XY, Chen C, Xu Z, Li WS, Yao XQ, Liu FK. Regional Arterial Infusion Chemotherapy improves the Pathological Response rate for advanced gastric cancer with Short-term Neoadjuvant Chemotherapy. Sci Rep. 2015;5:17516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Sun LB, Zhao GJ, Ding DY, Song B, Hou RZ, Li YC. Comparison between better and poorly differentiated locally advanced gastric cancer in preoperative chemotherapy: a retrospective, comparative study at a single tertiary care institute. World J Surg Oncol. 2014;12:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Al-Sukhni E, Attwood K, Mattson DM, Gabriel E, Nurkin SJ. Predictors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Ann Surg Oncol. 2016;23:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Panagiotopoulou IG, Parashar D, Qasem E, Mezher-Sikafi R, Parmar J, Wells AD, Bajwa FM, Menon M, Jephcott CR. Neoadjuvant Long-Course Chemoradiotherapy for Rectal Cancer: Does Time to Surgery Matter? Int Surg. 2015;100:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Garrer WY, El Hossieny HA, Gad ZS, Namour AE, Abo Amer SM. Appropriate Timing of Surgery after Neoadjuvant ChemoRadiation Therapy for Locally Advanced Rectal Cancer. Asian Pac J Cancer Prev. 2016;17:4381-4389. [PubMed] |
| 27. | Lee A, Wong AT, Schwartz D, Weiner JP, Osborn VW, Schreiber D. Is There a Benefit to Prolonging the Interval Between Neoadjuvant Chemoradiation and Esophagectomy in Esophageal Cancer? Ann Thorac Surg. 2016;102:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Mihmanlı M, Kabul Gürbulak E, Akgün İE, Celayir MF, Yazıcı P, Tunçel D, Bek TT, Öz A, Ömeroğlu S. Delaying surgery after neoadjuvant chemoradiotherapy improves prognosis of rectal cancer. World J Gastrointest Oncol. 2016;8:695-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Meredith KL, Weber JM, Turaga KK, Siegel EM, McLoughlin J, Hoffe S, Marcovalerio M, Shah N, Kelley S, Karl R. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Abdul-Jalil KI, Sheehan KM, Kehoe J, Cummins R, O’Grady A, McNamara DA, Deasy J, Breathnach O, Grogan L, O’Neill BD. The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis. 2014;16:O16-O25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Ranney DN, Mulvihill MS, Yerokun BA, Fitch Z, Sun Z, Yang CF, D’Amico TA, Hartwig MG. Surgical resection after neoadjuvant chemoradiation for oesophageal adenocarcinoma: what is the optimal timing? Eur J Cardiothorac Surg. 2017;52:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, Paty PB, Weiser MR, Klimstra D, Saltz L. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829-836; discussion 836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 33. | Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM, Eng C, Wolff RA, Janjan NA, Delclos ME. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol. 2006;29:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |