Published online Apr 28, 2018. doi: 10.3748/wjg.v24.i16.1766
Peer-review started: February 13, 2018
First decision: March 9, 2018
Revised: March 14, 2018
Accepted: March 31, 2018
Article in press: March 31, 2018
Published online: April 28, 2018
Processing time: 73 Days and 20.9 Hours
To explore the significance of corticotropin-releasing hormone (CRH)-receptor (R)2 in mucosal healing of dextran sulfate sodium (DSS)-induced colitis and the effect of Tong-Xie-Yao-Fang (TXYF) on CRH-R2 expression and regulation.
Ulcerative colitis was induced in mice by administration of 3% (w/v) DSS for 7 d. Once the model was established, mice were administered urocortin-2 (30 μg/kg), a peptide which binds exclusively to CRH-R2, or various doses of aqueous TXYF extracts (2.8-11.2 g/kg), a CRH-R2 antagonist Astressin (Ast)2B (20 μg/kg), Ast2B + Ucn2, or Ast2B with various doses of aqueous TXYF extracts for 9 d. Colonic mucosal permeability was then evaluated by measuring the fluorescence intensity in serum. The colitis disease activity index (DAI), histology, body weight loss and colon length were assessed to evaluate the condition of colitis. Terminal deoxynucleotidyl transferase dUTP nick-end labeling was used to detect apoptosis of the intestinal epithelial cells. The expression level of Ki-67 represented the proliferation of colonic epithelial cells and was detected by immunohistochemistry. The expression levels of inflammation cytokines IL-6, TNF-α and CXCL-1 were examined in colon tissues using real-time PCR and ELISA kits.
Compared with the DSS group, mice treated with the CRH-R2 antagonist Ast2B showed greater loss of body weight, shorter colon lengths (4.90 ± 0.32 vs 6.21 ± 0.34 cm, P < 0.05), and higher DAI (3.61 ± 0.53 vs 2.42 ± 0.32, P < 0.05) and histological scores (11.50 ± 1.05 vs 8.33 ± 1.03, P < 0.05). Additionally, the Ast2B group showed increased intestinal permeability (2.76 ± 0.11 μg/mL vs 1.47 ± 0.11 μg/mL, P < 0.001), improved secretion of inflammatory cytokines in colon tissue, and reduced colonic epithelial cell proliferation (4.97 ± 4.25 vs 22.51 ± 8.22, P < 0.05). Increased apoptosis (1422.39 ± 90.71 vs 983.01 ± 98.17, P < 0.001) was also demonstrated. The Ucn2 group demonstrated lower DAI (0.87 ± 0.55 vs 2.42 ± 0.32, P < 0.001) and histological scores (4.33 ± 1.50 vs 8.33 ± 1.03, P < 0.05). Diminished weight loss, longer colon length (9.58 ± 0.62 vs 6.21 ± 0.34 cm, P < 0.001), reduced intestinal permeability (0.75 ± 0.07 vs 1.47 ± 0.11 μg/mL, P < 0.001), inhibited secretion of inflammatory cytokines in colon tissue and increased colonic epithelial cell proliferation (90.04 ± 15.50 vs 22.51 ± 8.22, P < 0.01) were all observed. Reduced apoptosis (149.55 ± 21.68 vs 983.01 ± 98.17, P < 0.05) was also observed. However, significant statistical differences in the results of the Ast2B group and Ast2B + Ucn2 group were observed. TXYF was also found to ameliorate symptoms of DSS-induced colitis in mice and to promote mucosal repair like Ucn2. There were significant differences between the Ast2B + TXYF groups and the TXYF groups.
CRH-R2 activates the intestinal mucosal antiinflammatory response by regulating migration, proliferation and apoptosis of intestinal epithelial cells in colitis-induced mice, and plays an important antiinflammatory role. TXYF promotes mucosal repair in colitis mice by regulating CRH-R2.
Core tip: Mucosal healing is a desired therapeutic endpoint in the treatment of inflammatory bowel disease. However, it is difficult to treat inflammatory bowel disease thoroughly, and there are some adverse reactions. Studies have shown that corticotropin-releasing hormone (CRH)-receptor (R)2 can activate the inflammatory response of intestinal mucosa and exert an antiinflammatory effect. Our preliminary study found that Tong-Xie-Yao-Fang could reduce the expression of CRH-R1, increase CRH-R2, and participate in reconstruction of the intestinal barrier. The aim of this study was to explore the significance of CRH-R2 in the mucosal healing of dextran sulfate sodium-induced colitis and study the effect of Tong-Xie-Yao-Fang on CRH-R2 expression and regulation.
- Citation: Gong SS, Fan YH, Wang SY, Han QQ, Lv B, Xu Y, Chen X, He YE. Mucosa repair mechanisms of Tong-Xie-Yao-Fang mediated by CRH-R2 in murine, dextran sulfate sodium-induced colitis. World J Gastroenterol 2018; 24(16): 1766-1778
- URL: https://www.wjgnet.com/1007-9327/full/v24/i16/1766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i16.1766
Inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis (UC), are a group of chronic inflammatory disorders of the gastrointestinal tract, characterized by intestinal inflammation and mucosal damage[1]. In traditional Chinese medicine theory, UC is known as the “changpi” and chronic dysentery[2]. Characterized by chronic mucosal inflammation and damage of the colon, UC presents with bloody diarrhea, tenesmus, abdominal pain, weight loss, anemia, and even toxic megacolon. Intestinal perforation, intestinal obstruction, intestinal bleeding and cancer are also observed, thus affecting an individual’s quality of life[3].
Treatment targets for IBD have changed over the recent years. Previous therapeutic strategies focusing on induction and maintenance of clinical remission have shown no effect on the natural course of the disease[4,5]. However, in the late 1990s, the advent of biologic agents for the treatment of IBD showed that while patients may be in clinical remission, ongoing mucosal inflammation may still be present, resulting in structural damage[6-11].
This finding has led to the concept of mucosal healing as a more meaningful therapeutic target in clinical practice. Indeed, emerging data suggests that mucosal healing is strongly associated with a reduction in steroid use, complications, hospitalizations, and surgeries[12].
Mucosal repair of the intestinal barrier is a tightly coordinated response to injury that preserves homeostasis and limits the adverse effects of inflammation. After damage to the epithelial tissue, intestinal epithelial cells migrate to the site of injury in a critical process known as epithelial restitution[13-15]. Restitution is followed by epithelial cell proliferation and differentiation which is regulated by factors that promote cell viability and limit apoptosis[14,16]. IBD is a chronic relapsing inflammatory disorder that involves a defective epithelial barrier[17].
Corticotropin-releasing hormone (CRH), the primary mediator of the stress response, is expressed in both the central nervous system and the periphery, including the intestine[18]. The CRH family of peptides interacts with a variety of cell types in the intestinal mucosa, including epithelial cells, enteric neurons, and immune cells[19]. In addition to CRH, three distinct peptides known as urocortins (Ucn1, Ucn2, and Ucn3) bind to two types of G protein-coupled receptors to exert their effects, CRH receptor (R)1 and CRH-R2. Yet, Ucn1 has greater affinity for CRH-R2 than CRH-R1, and Ucn2 and Ucn3 bind exclusively to CRH-R2[20]. Interactions between CRH-Rs and their ligands modulate several functional and pathophysiologic responses within the gut, including stress-induced alterations in motility, ion secretion, and visceral pain, and the development and maintenance of intestinal inflammation[21].
Studies from others have found that CRH may be involved in the maintenance of intestinal barrier integrity by regulating autophagy in the intestinal epithelial cells[18]. Our previous studies have also found that CRH could cause an increase in intercellular permeability in the intestinal epithelium[22]. Some studies have found that CRH-R2 can activate the antiinflammatory response of intestinal mucosa and exert an antiinflammatory effect[23]. In addition, activation of CRH-R2 can promote the migration and proliferation of colon cancer cells and gastric mucosa cells[24,25]. Furthermore, the expression of CRH-R2 was found to be down-regulated in the biopsy specimens of UC patients[26] and CRH-deficient mice are unable to initiate healing responses after acute experimental colitis[27], suggesting a role for the CRH peptide family, especially CRH-R2, in mucosal repair mechanisms.
Tong-Xie-Yao-Fang (TXYF) is a prescription in traditional Chinese medicine, used for relieving abdominal pain and diarrhea. TXYF has also been shown to be involved in the reconstruction of the intestinal epithelial barrier and to promote the healing of mucosa in UC[28,29]. While the mechanism is not understood, it is thought to target and intervene with CRH-R2. This regulates the migration, proliferation and apoptosis of epithelial cells, like the role of Ucn2[30,31].
The overall aim of the present investigation was to determine whether CRH-R2 regulates mucosal repair on dextran sulfate sodium (DSS)-induced colitis in mice and to examine the relationship between TXYF and CRH-R2 signaling.
TXYF was prepared with large head atractylodes rhizome (Rhizoma Atractylodis Macrocephalae), white peony root (Radix Paeoniae Alba), dried tangerine peel (Pericarpium Citri Reticulatae) and divaricate saposhnikovia root (Radix Saposhnikoviae)[32], which were used in a 15:12:6:10 proportion. Raw components were soaked in an 8-fold volume of distilled water for 1 h and boiled twice for 0.5 h each time. Two of the boiled ingredients were filtered, mixed together, concentrated at a 1:1 ratio (100% concentration), and stored at 4 °C for later use.
Male CD-1(ICR) mice (8-10 wk old) were purchased from Shanghai Xipuer-bikai Experimental Animal Co., Ltd., (Shanghai, China) and housed 1 wk under a 12 h light/dark cycle at 22-24 °C with 50%-60% humidity and a noise level < 50 d. Prior to experimentation, mice were allowed free access to food and tap water. All the procedures involving animals were conducted in accordance with the ethical principles adopted by the Animal Experimental Center of Zhejiang Chinese Medical University and were approved by the Ethics Committee on Animal Experiments at Zhejiang Chinese Medical University.
Mice (n = 110) were randomized into 11 assigned groups as follows: control group (n = 10), DSS group (n = 10), DSS + Astressin (Ast)2B group (Ast2B group; n = 10), DSS + Ucn2 group (Ucn2 group; n = 10), DSS + Ast2B + Ucn2 group (Ast2B + Ucn2 group; n = 10), DSS + Ast2B + low-dose (2.8 g/kg•d) aqueous TXYF extract group (Ast2B + TXYF-L group; n = 10), DSS + Ast2B + medium-dose (5.6 g/kg•d) aqueous TXYF extract group (Ast2B + TXYF-M group; n = 10), DSS + Ast2B + high-dose (11.2 g/kg•d) aqueous TXYF extract group (Ast2B + TXYF-H group; n = 10), DSS + low-dose (2.8 g/kg•d) aqueous TXYF extract group (TXYF-L group; n = 10), DSS + medium-dose (5.6 g/kg•d) aqueous TXYF extract group (TXYF-M group; n = 10), and DSS + high-dose (11.2 g/kg•d) aqueous TXYF extract group (TXYF-H group; n = 10). Colitis was induced in mice by administering 3% (w/v) DSS (MP Biomedicals, Inc., Aurora, OH, United States) in their drinking water for 7 d. On days 8 to 16, mice were switched to normal water. Additionally, the mice treated with Ast2B were injected daily with the CRH-R2 antagonist Ast2B (Sigma-Aldrich, St. Louis, MO, United States) administered intraperitoneally (20 μg/kg). The mice treated with Ucn2 received an intraperitoneal injection of Ucn2 (Peptide Institute Inc., Osaka, Japan) (30 μg/kg). The mice treated with TXYF were administered the aqueous TXYF extract. The doses of 2.8 g/kg•d, 5.6 g/kg•d, and 11.2 g/kg•d aqueous TXYF extract represented an equivalent of 0.5 ×, 1.0 × and 2.0 × for the human adult dosage.
Intestinal disease activity was assessed based on weight loss, the presence of diarrhea accompanied by blood and mucus, and colonic shortening[33]. DAI was calculated by scoring weight loss, diarrhea and rectal bleeding, based on a previous scoring system (Table 1) described by Murthy et al[34] with little modification. Weight loss was defined as the difference between the initial and final weights. Diarrhea was defined by the absence of fecal pellet formation and the presence of continuous fluid fecal material in the colon. Rectal bleeding was assessed based on the presence of diarrhea containing visible blood and on the presence of gross rectal bleeding, and was scored as diarrhea. Disease activity index (DAI) values were calculated using the following formula: DAI = [(weight loss score) + (diarrhea score) + (rectal bleeding score)]/3. The clinical parameters used in the present study were chosen to represent the subjective clinical symptoms observed in human UC.
| Score | Weight loss, % | Stool consistency | Bloodstain or gross bleeding |
| 0 | None | Normal | Negative |
| 1 | 1-5 | - | - |
| 2 | 5-10 | Loose stool | Positive |
| 3 | 10-15 | - | - |
| 4 | > 15 | Diarrhea | Gross bleeding |
Sections of colon fixed in 10% formalin, paraffin-embedded and stained with hematoxylin and eosin were used for histological scoring. The sections were graded by two blinded investigators, using a range from 0 to 3 as to amount of inflammation (acute and chronic) and depth of inflammation and with a range from 0 to 4 as to the amount of crypt damage or regeneration, as indicated in Table 2[35]. These changes were also quantified as to the percent involvement by the disease process: (1) 1%-25%; (2) 26%-50%; (3) 51%-75%; (4) 76%-100%. Histological score was calculated using the following formula: histological colitis score = inflammation + depth of lesions + destruction of crypt + width of lesions.
| Score | Inflammation | Depth of lesions | Destruction of crypt | Width of lesions, % |
| 0 | None | None | None | |
| 1 | Slight | Mucosa | Basal 1/3 damaged | 1-25 |
| 2 | Moderate | Mucosa and submucosa | Basal 2/3 damaged | 26-50 |
| 3 | Severe | Transmural | Intact epithelium only | 51-75 |
| 4 | - | - | Total crypt and epithelium | 76-100 |
Formalin-fixed, paraffin-embedded colons were sectioned (1 μm) and stained with a Ki-67 antigen (dilution 1:100; AF0198; Affinity Biosciences, Cincinnati, OH, United States) or terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) with the Apop-Tag Plus Peroxidase in situ cell death detection kit, POD (11684817910; Roche, Basel, Switzerland) according to the manufacturer’s instructions. To quantify Ki-67 immunoreactivity and TUNEL, pixel-based quantification of staining intensity was performed with Image-ProPlus 6.0 software. Stained sections were observed under a 40 × objective lens.
The intestinal permeability was measured by determination of the amount of FITC-dextran (molecular weight 4.0 kDa; Sigma-Aldrich) in blood after oral administration, as described previously[36]. Briefly, mice were fasted overnight and FITC-dextran solution (4 kDa, 600 mg/kg) was administered. Blood samples were obtained after 3 h, centrifuged at 10000× rpm for 5 min, and serum was collected. Serum levels of FITC were read at 483 nm and 525 nm on a full wavelength multifunctional enzyme spectrometer (Varioskan Flash, Thermo Fisher Scientific, Waltham, MA, United States).
RNAiso Plus (9108; Takara Bio, Inc., Shiga, Japan) was used to extract RNA from frozen tissue samples, and the concentration of RNA was measured using a trace nucleic acid analyzer (Thermo Fisher Scientific). RNA was reverse-transcribed to cDNA using a PrimeScript RT reverse transcription kit (RR036A; Takara Bio, Inc.). Quantitative real-time PCR was carried out by ABI 7500 real-time PCR system (7500; Applied Biosystems of Thermo Fisher Scientific). Primers were designed and synthesized by Shenggong Biology and Engineering Co., Ltd. (Shanghai, China) (Table 3). β-actin was used as the normalization control, and the 2-ΔΔCT method was used to calculate the relative expression of target genes.
| Gene | Primer sequences | Amplification length |
| TNF-α | Forward: 5’-GCCTATGTCTCAGCCTCTTCTC-3’ | 22 |
| Reverse: 5’-TGGTGGTTTGCTACGACGTG-3’ | 20 | |
| CXCL-1 | Forward: 5’-TCACCTCAAGAACATCCAGAGC-3’ | 22 |
| Reverse: 5’-ACTTGGGGACACCTTTTAGCAT-3’ | 22 | |
| IL-6 | Forward: 5’-TCTCTGCAAGAGACTTCCATCC-3’ | 22 |
| Reverse: 5’-TTCCACGATTTCCCAGAGAACA-3’ | 22 | |
| β-actin | Forward: 5’-AGATCAAGATCATTGCTCCTCC-3’ | 22 |
| Reverse: 5’-GGTGTAAAACGCAGCTCAGTAA-3’ | 22 |
TNF-α, CXCL-1 and IL-6 measurement
CXCL-1 level and IL-6 level were measured by mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit, mouse CXCL-1 ELISA kit and Mouse IL-6 ELISA kit (Shanghai WesTang Bio-Tech Co., Ltd., Shanghai, China), respectively. All assays were conducted by following the manufacturer’s instruction.
All analyses were performed using SPSS 24.0 statistical software (IBM Corp., Armonk, NY, United States). Comparisons between groups were performed using one-way analysis of variance (ANOVA), followed by Scheffe post hoc test for multiple comparisons, otherwise a Dunnett’s T3 method was used. All data are expressed as the mean ± SD. P < 0.05 was considered statistically significant.
We first assessed the involvement of CRH-R2 signaling in mucosal repair after colitis by administering the CRH-R2 antagonist Ast2B to mice after induction of DSS colitis. Mice received an intraperitoneal injection of Ast2B daily for 9 d after withdrawal of DSS, and body weight loss, DAI, colon length and histological score were monitored.
Compared with the DSS group, mice treated with the CRH-R2 antagonist Ast2B showed more body weight loss (P < 0.05) (Figure 1A) and shorter colon lengths (4.90 ± 0.32 cm vs 6.21 ± 0.34 cm, P < 0.05) (Figure 1B). DAI score and histological score were used to evaluate the severity of UC in mice. The mice in the Ast2B group exhibited significantly higher DAI scores (3.61 ± 0.53 vs 2.42 ± 0.32, P < 0.05) (Figure 1D) and histological scores (11.50 ± 1.05 vs 8.33 ± 1.03, P < 0.05) (Figure 1E) compared to the mice in the DSS group.
Interestingly, mice treated with Ucn2 after DSS-induced colitis showed a smaller degree of body weight loss (P < 0.001) (Figure 1A), longer colon length (9.58 ± 0.62 cm vs 6.21 ± 0.34 cm, P < 0.001) (Figure 1B), lower DAI (0.87 ± 0.55 vs 2.42 ± 0.32, P < 0.001) (Figure 1D) and improved histological scores (4.33 ± 1.50 vs 8.33 ± 1.03, P < 0.05) (Figure 1E) compared to the mice in the DSS group. However, a significant statistical difference was found between the Ast2B + Ucn2 group and the Ucn2 group (Figure 1A-F).
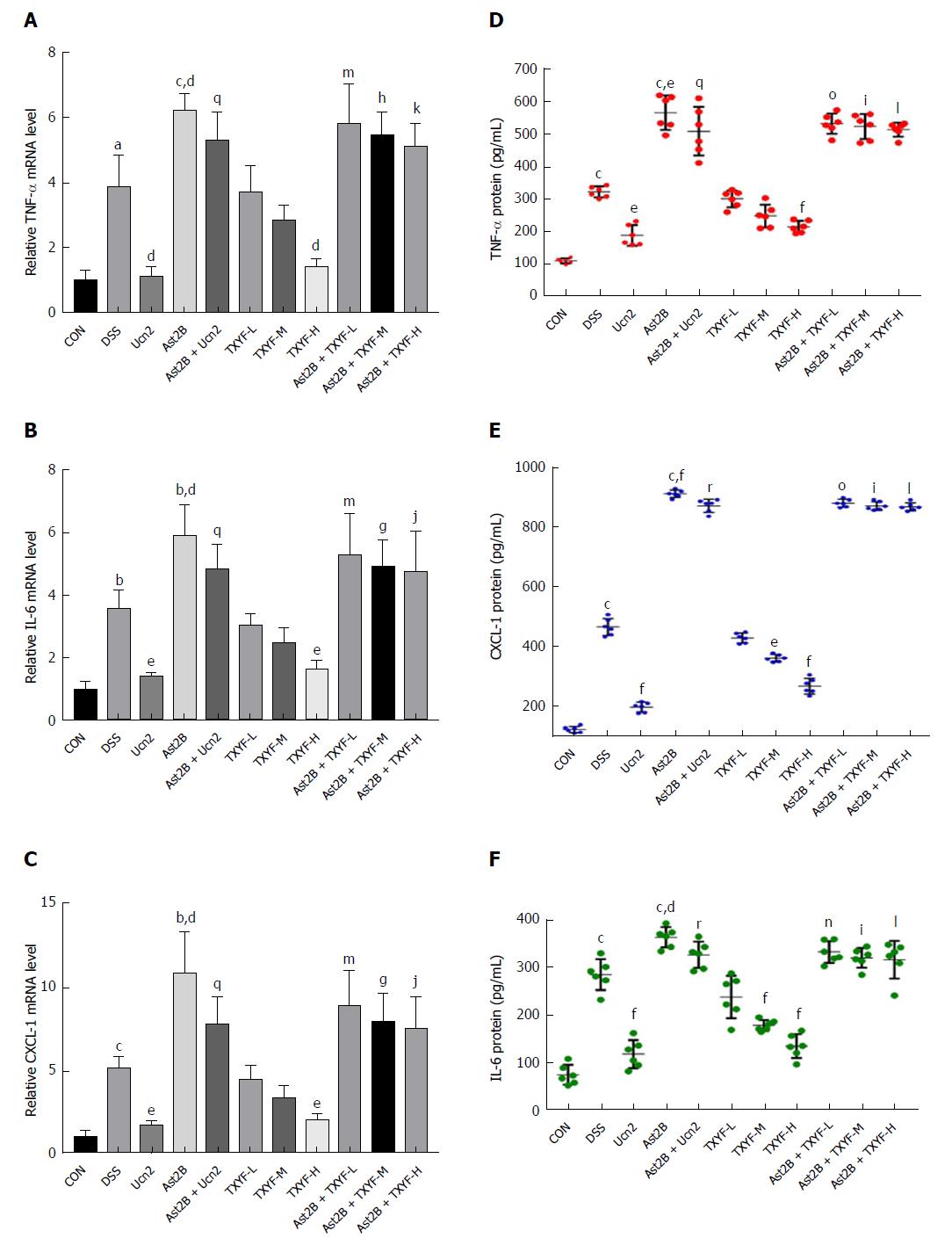
The levels of proinflammatory factors such as TNF-α, CXCL-1 and IL-6 in mouse colon tissues were detected by real time-PCR and ELISA. Compared with the DSS group, the Ast2B group showed significantly up-regulated mRNA expression of TNF-α (6.19 ± 0.51 vs 3.87 ± 0.98, P < 0.05) (Figure 2A), CXCL-1 (10.77 ± 2.55 vs 5.08 ± 0.76, P < 0.05) (Figure 2B),and IL-6 (5.93 ± 0.99 vs 3.55 ± 0.62, P < 0.05) (Figure 2C). Meanwhile, the protein expression levels of TNF-α (Figure 2D), CXCL-1 (Figure 2E) and IL-6 (Figure2F) were increased markedly in the Ast2B group.
However, compared with the DSS group, the Ucn2 group showed significantly decreased mRNA expression of TNF-α (Figure 2A), CXCL-1 (Figure 2B) and IL-6 (Figure 2C). Simultaneously, the Ucn2 group demonstrated reduced protein expression of TNF-α (Figure 2D), CXCL-1 (Figure 2E) and IL-6 (Figure 2F). Interestingly, the Ast2B + Ucn2 group showed drastically increased mRNA and protein expression of TNF-α, CXCL-1 and IL-6 compared with the Ucn2 group (P < 0.05 for all).
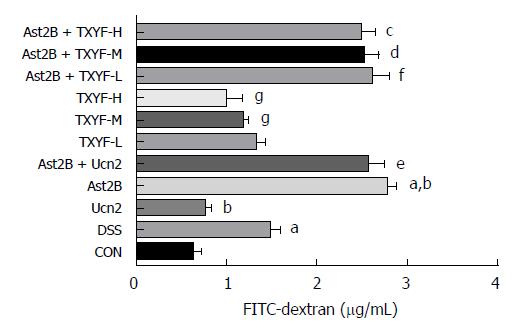
To determine the effect of CRH-R2 signaling on epithelial permeability, we analyzed intestinal permeability in DSS-induced colitis model by measuring the concentration of the serum FITC. The concentration of serum FITC-dextran was higher in the Ast2B group than DSS group (2.76 ± 0.11 μg/mL vs 1.47 ± 0.11 μg/mL, P < 0.05) (Figure 3). However, the concentration of serum FITC-dextran in the Ucn2 group was lower than DSS group (0.75 ± 0.07 μg/mL vs 1.47 ± 0.11 μg/mL, P < 0.05) (Figure 3). An obvious difference was observed between the Ast2B+Ucn2 group and the Ucn2 group.
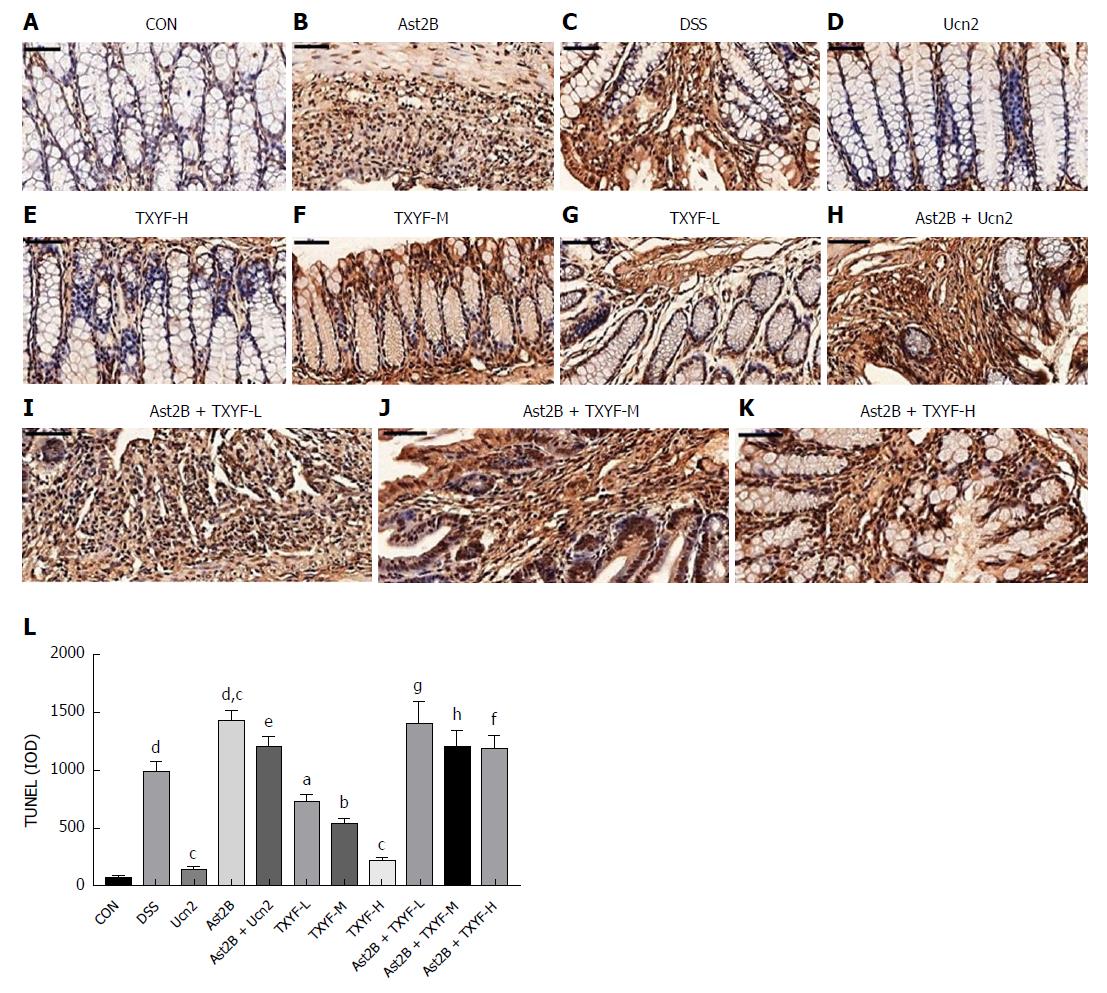
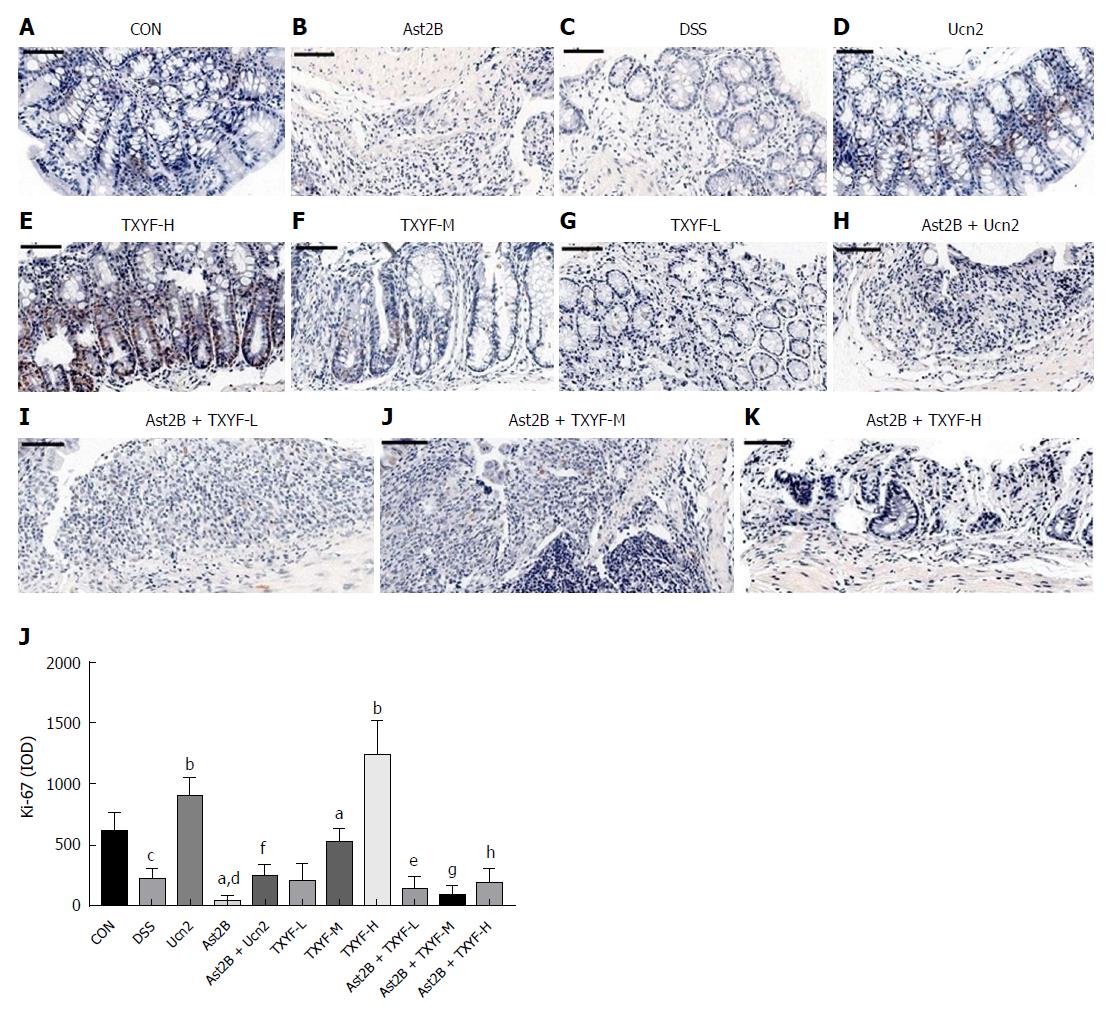
The effect of Ast2B on cell proliferation and cell death was then determined. TUNEL staining was significantly increased in the Ast2B group compared with the DSS group (1422.39 ± 90.71 vs 983.01 ± 98.17, P < 0.001) (Figure 4L). At the same time, the Ast2B group showed significantly decreased cell proliferation (4.97 ± 4.25 vs 22.51 ± 8.22, P < 0.05) (Figure 5L). Interestingly, the Ucn2 group showed promoted colonic epithelial cell proliferation (Figure 5L) and reduced epithelial cell apoptosis (Figure 4L). However, significant statistical differences were found between the Ucn2 group and the Ast2B + Ucn2 group with regards to colonic epithelial cell apoptosis and proliferation (P < 0.01 for both).
To obtain insight into the underlying mechanism responsible for promoting mucosal repair of TXYF, DSS-induced colitis mice were pretreated with the CRH-R2 antagonist Ast2B, and later treated with various doses of aqueous TXYF extracts.
Compared with the DSS group, the TXYF-H groups had lower DAI scores (Figure 1D) and histological scores (Figure 1E), and decreased body weight loss (Figure 1A). TXYF-M,H groups, on the other hand, had longer colon length (Figure 1B) and improved intestinal permeability (Figure 3). Furthermore, TXYF inhibited secretion of inflammatory cytokines in colon tissues (Figure 2A-F) and promoted colonic epithelial cell proliferation (Figure 5L), along with reducing apoptosis (Figure 4L). However, the Ast2B + TXYF groups showed significant statistical difference in DAI, body weight loss, colon length and histological scores, when compared with the TXYF groups. As for inhibiting secretion of inflammatory cytokines, the Ast2B + TXYF groups demonstrated significant differences within the TXYF groups. Additionally, the Ast2B + TXYF groups showed markedly improved intestinal permeability in DSS-induced colitis compared with the TXYF groups, respectively. In addition, the Ast2B + TXYF groups demonstrated significant differences with the TXYF groups in promoting colonic epithelial cell proliferation and reducing epithelial cell apoptosis.
These results further confirm the idea that CRH-R2 signaling is the main mechanism of TXYF-mediated mucosal repair in DSS-induced colitis in mice.
Mucosal healing is a desired therapeutic end-point in the treatment of IBD; interventions that promote restoration of the epithelial barrier are needed to limit inflammation and to prevent future injury. Mucosal healing consists of two processes[15]. Firstly, intact cells in the adjacent region migrate to the injured area; then, the cells compensate for damaged cells by proliferation and help to maintain normal thickness of the intestinal epithelium. Therefore, the migration and proliferation of intestinal epithelial cells are the key mechanisms for the healing of epithelial defects after mucosal injury. In addition, inhibiting apoptosis of intestinal epithelial cells can promote the healing process of mucosa[37]. It is well known that intestinal epithelial barrier defects are characterized by increased intestinal permeability.
In the present study, it was found that selective inhibition of CRH-R2 signaling can aggravate symptoms of DSS-induced colitis, destroy the impaired intestinal barrier function, promote colonic epithelial cell apoptosis and reduce epithelial cell proliferation. After treatment with Ucn2 and TXYF, DSS-induced mice demonstrated ameliorated symptoms of DSS-induced colitis, improved impaired intestinal barrier function, promoted colonic epithelial cell proliferation and reduced epithelial cell apoptosis. Moreover, Ucn2 and TXYF reduced the expression of the proinflammatory factors TNF-α, CXCL-1, and IL-6 in colon tissues.
Cytokines play a central role in the regulation of both intestinal inflammation and mucosal repair mechanisms[38]. Treatments that neutralize the proinflammatory actions of TNF-α promote mucosal healing and are a standard of current IBD treatment paradigms[7,38]. In addition, production of the key proinflammatory cytokine IL-6 correlates with the degree of active intestinal inflammation in IBD patients[39], further supporting the concept that therapeutic interventions that modulate cytokine production and/or release may promote mucosal repair after inflammation. Taken together, these results indicate that Ucn2 and TXYF promote mucosal repair.
Studies from others have found that CRH may be involved in the maintenance of intestinal barrier integrity by regulating autophagy in the intestinal epithelial cells[18]. Our previous studies also found that CRH could induce an increase in intercellular permeability in the intestinal epithelium[22]. Some studies have also found that CRH-R2 can activate the antiinflammatory response of intestinal mucosa and exert an antiinflammatory effect[23]. In addition, activation of CRH-R2 can promote the migration and proliferation of colon cancer cells and gastric mucosa cells[24,25]. Furthermore, the expression of CRH-R2 has been reported as down-regulated in biopsy specimens of UC patients[26] and CRH-deficient mice have been reported as unable to initiate healing responses after acute experimental colitis[27].
These results suggest a role for the CRH peptide family, especially CRH-R2, in mucosal repair mechanisms. It is known that Ucn2 is a peptide which binds exclusively to CRH-R2. Significant statistical differences were found between the Ast2B group and the Ast2B + Ucn2 group. Thus, a conclusion can be made that CRH-R2 activated the intestinal mucosal antiinflammatory response by regulating the migration, proliferation and apoptosis of intestinal epithelial cells in colitis mice.
Subsequently, the efficacy of TXYF was assessed. According to the theory of traditional Chinese medicine, IBD belongs to “diarrhea, dysentery”. The principle of treatment is focused on relieving pain and eliminating dampness and diarrhea. TXYF is a classic formula in the Jing yue quan shu (Jingyue’s Complete Book), which consists of atractylodes rhizome (Rhizoma Atractylodis Macrocephalae) head groups, white peony root (Radix Paeoniae Alba), dried tangerine peel (Pericarpium Citri Reticulatae), and divaricate saposhnikovia root (Radix Saposhnikoviae). TXYF has been believed to be effective in improving disorders of the digestive system and alleviating abdominal pain, diarrhea, and has been used widely as a medication to treat inflammatory bowel syndrome and UC clinically, without inducing hepatomegaly or splenomegaly[40-42].
TXYF has also been shown to improve reconstruction of the intestinal epithelial barrier and promote the healing of mucosa in UC[28,29]. Our previous study found that TXYF down-regulated CRH-R1 and up-regulated CRH-R2. While the mechanism underlying TXYF promotion of mucosal repair is not well understood, it is thought to intervene using CRH-R2 and to regulate the migration, proliferation and apoptosis of epithelial cells, like the role of Ucn2[30,31].
Herein, we describe the selective inhibition of CRH-R2 signaling in the intestinal mucosa of mice after experimental colitis, along with TXYF treatment, leading to exacerbated symptoms of DSS-induced colitis, delayed healing, increased expression of proinflammatory factors TNF-α, CXCL-1 and IL-6 in colon tissues, decreased epithelial cell proliferation and promoted cell apoptosis. These results suggest that TXYF promoted the mucosal repair process of colitis mice by regulating CRH-R2.
In conclusion, CRH-R2 activates the intestinal mucosal antiinflammatory response by regulating the migration, proliferation and apoptosis of intestinal epithelial cells in colitis mice, and exerts an antiinflammatory effect. The effects of TXYF on the mucosal repair process are focused on regulating CRH-R2 in colitis mice.
Mucosal healing is a desired therapeutic end-point in the treatment of inflammatory bowel disease (IBD). However, thorough treatment of IBD is difficult and there are some adverse reactions. According to studies, corticotropin-releasing hormone (CRH)-receptor (R)2 can activate the inflammatory response of intestinal mucosa. Our preliminary study found that Tong-Xie-Yao-Fang could lower CRH-R1, increase the expression of CRH-R2, and participates in reconstruction of the intestinal barrier.
Mucosal healing is a desired therapeutic end-point in the treatment of IBD. However, the mechanism of mucosal healing is still unclear.
To explore the significance of CRH-R2 in the mucosal healing of dextran sulfate sodium (DSS)-induced colitis and study the effect of Tong-Xie-Yao-Fang (TXYF) on CRH-R2.
Ulcerative colitis (UC) was induced in mice by administration of 3% (w/v) DSS for 7 d. Then, mice were administered urocortin (Ucn)-2 or various doses of aqueous TXYF extracts, the CRH-R2 antagonist Astressin (Ast)2B, Ast2B + Ucn2, or Ast2B with various doses of aqueous TXYF extracts for 9 d. The colitis disease activity index (DAI) was assessed to evaluate the condition of colitis. The expression level of Ki-67 represented the proliferation of colonic epithelial cells. The expression levels of inflammation cytokines IL-6, TNF-α and CXCL-1 were examined by PCR and enzyme-linked immunosorbent assay.
Compared with the DSS group, mice treated with the CRH-R2 antagonist Ast2B showed greater loss of body weight, shorter colon lengths, and higher DAI and histological scores. Additionally, the Ast2B group showed increased intestinal permeability, improved secretion of inflammatory cytokines in colon tissue and reduced colonic epithelial cell proliferation. Increased apoptosis was also demonstrated. The Ucn2 group demonstrated lower DAI and histological scores. Diminished weight loss, longer colon length, reduced intestinal permeability, inhibited secretion of inflammatory cytokines in colon tissue and increased colonic epithelial cell proliferation were all observed. Reduced apoptosis was also observed.
CRH-R2 activates the intestinal mucosal antiinflammatory response and plays an important antiinflammatory role. TXYF promotes the mucosal repair process in colitis mice.
The CRH-R2 signaling pathway plays a pivotal role in mucosal healing in experimental UC in mice. Mucosal healing is a desired therapeutic end-point in the treatment of IBD. Thus, the findings of this study indicate a new potential mechanism by which CRH-R2 treats UC. TXYF, which has fewer side effects than other medicines, promotes the mucosal repair process of colitis mice by regulating CRH-R2. Therefore, TXYF can be used in patients with UC to promote their mucosal repair.
We would like to thank our colleagues in the Institute of Digestive Disease affiliated the First Clinical Medical College of Zhejiang Chinese Medical University for their help and support in this research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Gassler N, Ozen H, Tarnawski AS S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Norouzinia M, Chaleshi V, Alizadeh AHM, Zali MR. Biomarkers in inflammatory bowel diseases: insight into diagnosis, prognosis and treatment. Gastroenterol Hepatol Bed Bench. 2017;10:155-167. [PubMed] |
| 2. | Jin F L, Yi-Ping L I. Progress in the traditional Chinese medicine treatment of ulcerative colitis. China Modern Medicine. 2016;. |
| 3. | Ouyang Q, Xue LY. Inflammatory bowel disease in the 21(st) century in China: turning challenges into opportunities. J Dig Dis. 2012;13:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Bernstein CN, Loftus EV Jr, Ng SC, Lakatos PL, Moum B; Epidemiology and Natural History Task Force of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD). Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Magro F, Rodrigues A, Vieira AI, Portela F, Cremers I, Cotter J, Correia L, Duarte MA, Tavares ML, Lago P. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012;18:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2012;18:1634-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995;109:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 731] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 8. | Rutgeerts P, D’Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, Mayer L, Van Hogezand RA, Braakman T, DeWoody KL. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 738] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 9. | Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, Bao W, Patel K, Wolf DC, Safdi M, Colombel JF. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63:433-442; quiz 464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 10. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3047] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 11. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1548] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 12. | Römkens TE, Gijsbers K, Kievit W, Hoentjen F, Drenth JP. Treatment Targets in Inflammatory Bowel Disease: Current Status in Daily Practice. J Gastrointestin Liver Dis. 2016;25:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 467] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 237] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 15. | Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 16. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 17. | Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401-407. [PubMed] |
| 18. | Giannogonas P, Apostolou A, Manousopoulou A, Theocharis S, Macari SA, Psarras S, Garbis SD, Pothoulakis C, Karalis KP. Identification of a novel interaction between corticotropin releasing hormone (Crh) and macroautophagy. Sci Rep. 2016;6:23342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 485] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 20. | Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Hoffman JM, Baritaki S, Ruiz JJ, Sideri A, Pothoulakis C. Corticotropin-Releasing Hormone Receptor 2 Signaling Promotes Mucosal Repair Responses after Colitis. Am J Pathol. 2016;186:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Wang X T, Hu Y, Li M, Lv B. Study on the role of stress through CRF up regulation of CK8 mediated change of close connexin in the pathogenesis of irritable bowel syndrome. The national association of Chinese and western medicine on digestive system disease academic conference. 2015;. |
| 23. | Im E, Rhee SH, Park YS, Fiocchi C, Taché Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010;138:2457-2467, 2467.e1-2467.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Ducarouge B, Pelissier-Rota M, Lainé M, Cristina N, Vachez Y, Scoazec JY, Bonaz B, Jacquier-Sarlin M. CRF2 signaling is a novel regulator of cellular adhesion and migration in colorectal cancer cells. PLoS One. 2013;8:e79335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Chatzaki E, Lambropoulou M, Constantinidis TC, Papadopoulos N, Taché Y, Minopoulos G, Grigoriadis DE. Corticotropin-releasing factor (CRF) receptor type 2 in the human stomach: protective biological role by inhibition of apoptosis. J Cell Physiol. 2006;209:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Chatzaki E, Anton PA, Million M, Lambropoulou M, Constantinidis T, Kolios G, Taché Y, Grigoriadis DE. Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World J Gastroenterol. 2013;19:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Chaniotou Z, Giannogonas P, Theoharis S, Teli T, Gay J, Savidge T, Koutmani Y, Brugni J, Kokkotou E, Pothoulakis C. Corticotropin-releasing factor regulates TLR4 expression in the colon and protects mice from colitis. Gastroenterology. 2010;139:2083-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Stanisic V, Quigley EM. The overlap between IBS and IBD: what is it and what does it mean? Expert Rev Gastroenterol Hepatol. 2014;8:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Quigley EM. Overlapping irritable bowel syndrome and inflammatory bowel disease: less to this than meets the eye? Therap Adv Gastroenterol. 2016;9:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Chao G, Lv B, Meng L, Zhang S, Zahng L, Guo Y. [Influence of tongxie prescription on CRF expression in spinal cord and brain of hypersensitive viscera rats]. Zhongguo Zhong Yao Za Zhi. 2010;35:2012-2016. [PubMed] |
| 31. | Ding Y, Lv B, Meng L N, Fan Y H, Shen Y. Effect of Tongxieyaofang on Colonic Mucosal Protein Expression Profile in Rats with Visceral Hypersensitivity. Chinese Journal of Gastroenterology. 2012;17:660-664. [DOI] [Full Text] |
| 32. | Yang C, Xiong Y, Zhang SS, An FM, Sun J, Zhang QL, Zhan Q. Regulating effect of TongXie-YaoFang on colonic epithelial secretion via Cl- and HCO3- channel. World J Gastroenterol. 2016;22:10584-10591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79-94. [PubMed] |
| 34. | Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722-1734. [PubMed] |
| 35. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] |
| 36. | Nguyen HT, Dalmasso G, Torkvist L, Halfvarson J, Yan Y, Laroui H, Shmerling D, Tallone T, D’Amato M, Sitaraman SV. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest. 2011;121:1733-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Seidelin JB, Larsen S, Linnemann D, Vainer B, Coskun M, Troelsen JT, Nielsen OH. Cellular inhibitor of apoptosis protein 2 controls human colonic epithelial restitution, migration, and Rac1 activation. Am J Physiol Gastrointest Liver Physiol. 2015;308:G92-G99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1955] [Article Influence: 177.7] [Reference Citation Analysis (1)] |
| 39. | Suzuki Y, Saito H, Kasanuki J, Kishimoto T, Tamura Y, Yoshida S. Significant increase of interleukin 6 production in blood mononuclear leukocytes obtained from patients with active inflammatory bowel disease. Life Sci. 1990;47:2193-2197. [PubMed] |
| 40. | Fan H, Qiu MY, Mei JJ, Shen GX, Liu SL, Chen R. Effects of four regulating-intestine prescriptions on pathology and ultrastructure of colon tissue in rats with ulcerative colitis. World J Gastroenterol. 2005;11:4800-4806. [PubMed] |
| 41. | Hu X, Zhang X, Han B, Bei W. The inhibitory effect of tongxieyaofang on rats with post infectious irritable bowel syndrome through regulating colonic par-2 receptor. BMC Complement Altern Med. 2013;13:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Lu X, Zhang S, Yang C, Wang Z, Zhao L, Wu Z, Xie J. Effect of TongXie-YaoFang on Cl(-) and HCO3 (-) Transport in Diarrhea-Predominant Irritable Bowel Syndrome Rats. Evid Based Complement Alternat Med. 2016;2016:7954982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |