Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1725
Peer-review started: November 5, 2016
First decision: December 19, 2016
Revised: January 16, 2017
Accepted: February 7, 2017
Article in press: February 8, 2017
Published online: March 7, 2017
Processing time: 121 Days and 23 Hours
Multiple liver tumors represent a challenging condition for abdominal surgeons both in the selection of technique and the rarity of diagnosis. There are no case reports on co-existence of liver metastases from both intestinal leiomyosarcoma and adenocarcinoma. The patient described in this report successfully underwent resection of both primary lesions and liver metastases in combination with chemotherapy. As for the leiomyosarcoma, the primary cecal lesion was revealed more than three years after the patient's first visit. Peritoneal, lymph-node, and lung recurrences were observed afterward, and thus surgeries on those regions were performed. Pathologically, the peritoneal and lung recurrences comprised leiomyosarcoma and the lymph-node recurrence was diagnosed as adenocarcinoma. Despite newly discovered multiple lung recurrences and regional lymph-node metastases, the patient lived a normal life for 73 mo after the initial operation based on multidisciplinary therapy. He ultimately died of liver failure due to invasive lymph-node recurrence from the rectal adenocarcinoma, in addition to multiple lung recurrences from the leiomyosarcoma. Hepatic recurrence did not occur in this patient's case, which appears to be one reason for his long-term survival.
Core tip: There have been no case reports on co-existence of liver metastases from intestinal leiomyosarcoma and adenocarcinoma. This patient underwent resection of primary lesions and liver metastases in combination with chemotherapy. As for leiomyosarcoma, liver metastasis was discovered three years prior to discovery of the primary lesion. Peritoneal, lymph-node, and lung recurrences were discovered afterward, and therefore surgeries on those regions were performed. Despite newly discovered multiple lung recurrences and regional lymph-node metastases, the patient lived a normal life for 73 mo after the initial operation. He ultimately died of liver failure due to invasive lymph-node recurrence from the rectal adenocarcinoma.
- Citation: Aoki H, Arata T, Utsumi M, Mushiake Y, Kunitomo T, Yasuhara I, Taniguchi F, Katsuda K, Tanakaya K, Takeuchi H, Yamasaki R. Synchronous coexistence of liver metastases from cecal leiomyosarcoma and rectal adenocarcinoma: A case report. World J Gastroenterol 2017; 23(9): 1725-1734
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1725
Since the appearance of gastrointestinal stromal tumor as a distinctly defined entity, the diagnosis of intestinal leiomyosarcoma has not been common. Among the different types of this sarcoma, cecal leiomyosarcoma is extremely rare[1]. Hepatic leiomyosarcoma is also rare, particularly as a primary cancer[2]. Abdominal surgeons often confront multiple liver tumors, making treatment of such cases challenging. This is particularly the case if the diagnoses of hepatic tumors differ from each other. Until now, there had been no case reports regarding co-existence of liver metastases from a combination of intestinal leiomyosarcoma and adenocarcinoma. We hereby report on a patient who underwent successful treatment involving resection of both primary lesions and liver metastases along with chemotherapy. Peritoneal, lymph-node, and lung recurrences were observed afterward, and thus surgeries on those regions were also performed. Multiple lung recurrences and regional lymph-node metastases were newly discovered, but the patient could live a normal life for 73 mo after the initial surgery based on multidisciplinary therapy, which we will discuss in detail in this report.
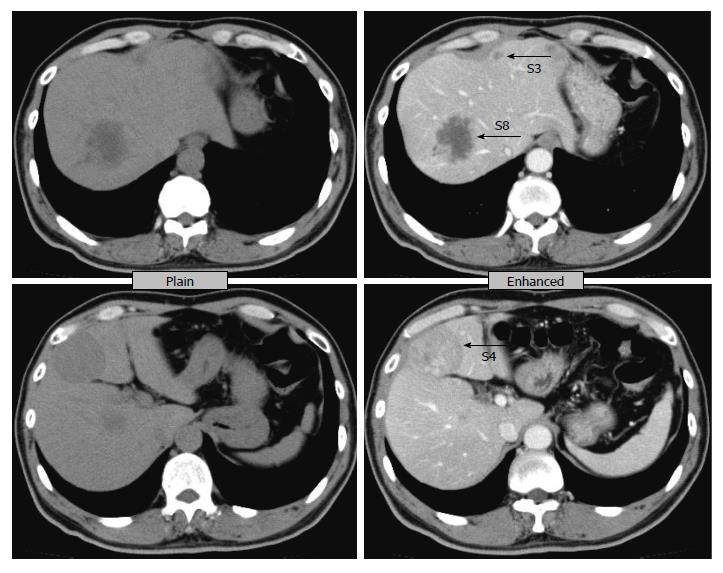
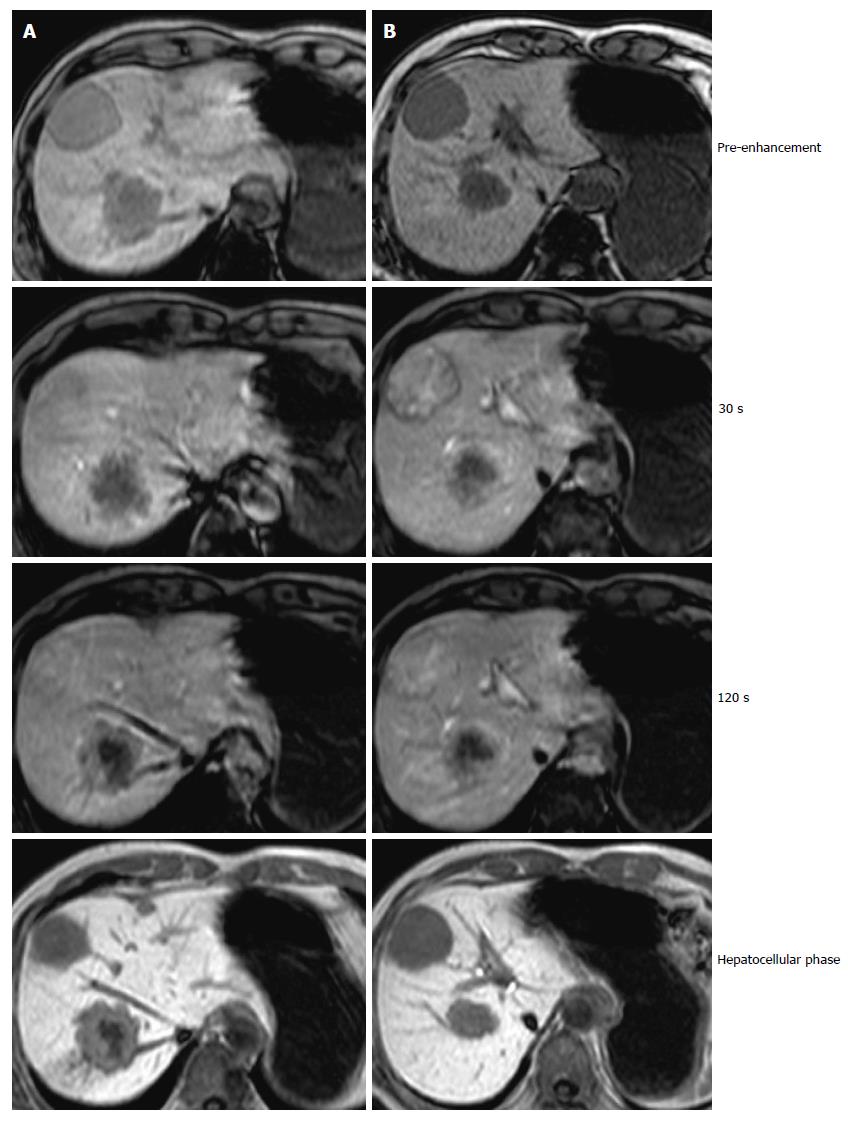
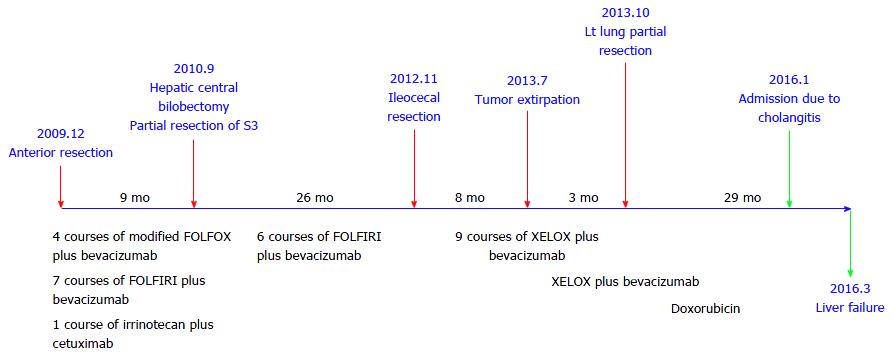
A 61-year-old male visited our hospital with occult blood in stool and multiple liver tumors at an annual medical examination in October 2009. His past medical history involved only hypertension starting at the age of 57. At the examination, he underwent a colonoscopy examination, whereupon type 2 adenocarcinoma was discovered in the rectum. In addition, an abdominal computed tomography (CT) scan and gadoxetic acid enhanced magnetic resonance imaging revealed liver tumors in Segment 3, Segment 4, and Segment 8 (Figures 1 and 2). Anterior rectal resection with regional lymph-node dissection was carried out in December 2009. A pathological examination revealed extra serosal invasion by a moderately differentiated adenocarcinoma and metastases in eight of 14 resected lymph nodes.
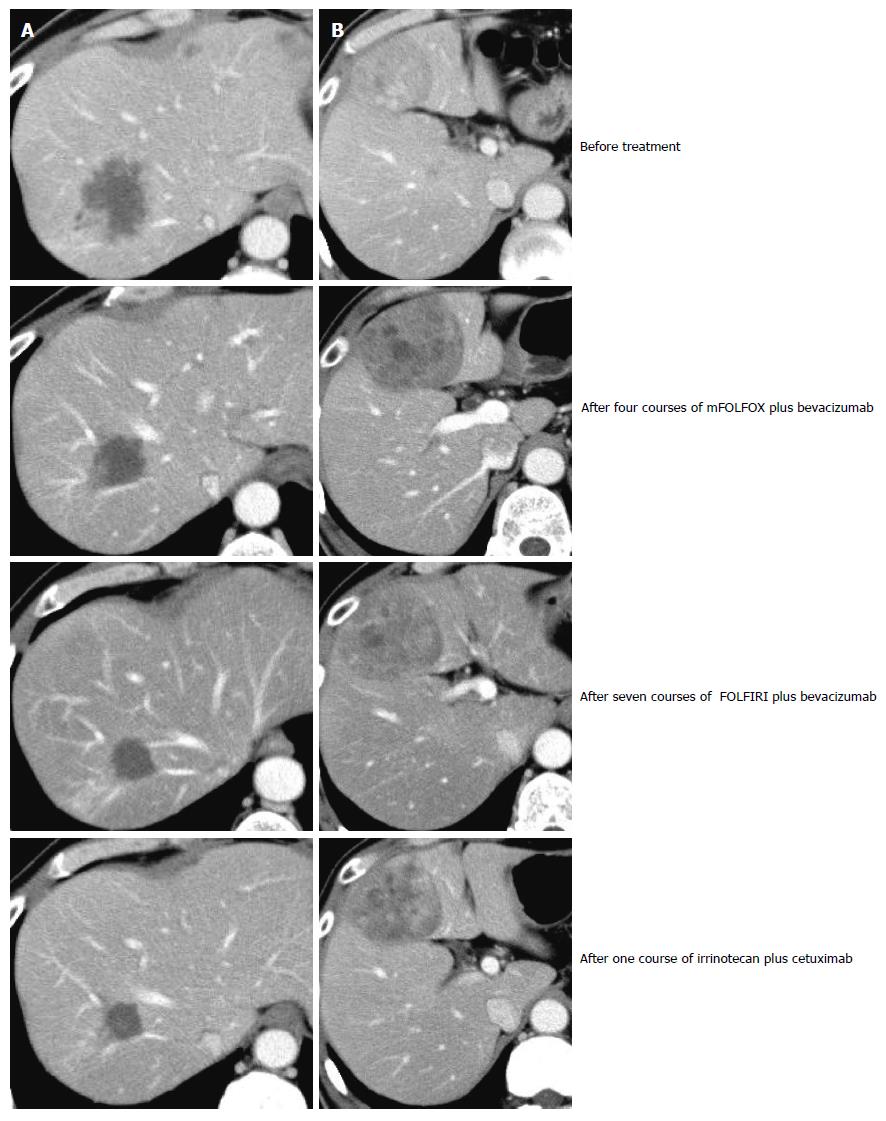
Because genetic analysis confirmed wild-type KRAS, the patient received chemotherapy with modified FOLFOX6 plus bevacizumab after the rectal resection. Following four courses of FOLFOX, the S3 liver tumor disappeared and the S8 tumor decreased in size, but the S4 tumor was found to be enlarged. The patient then underwent seven courses of FOLFIRI plus bevacizumab followed by one course of irrinotecan plus cetuximab; as a result, the S4 and S8 tumors decreased in size (Figure 3). Positron emission tomography-CT after the chemotherapy series showed no significant uptake of fluorodeoxyglucose in the liver. The patient subsequently underwent a central bi-segmentectomy and a partial S3 resection in September 2010 (the second operation).
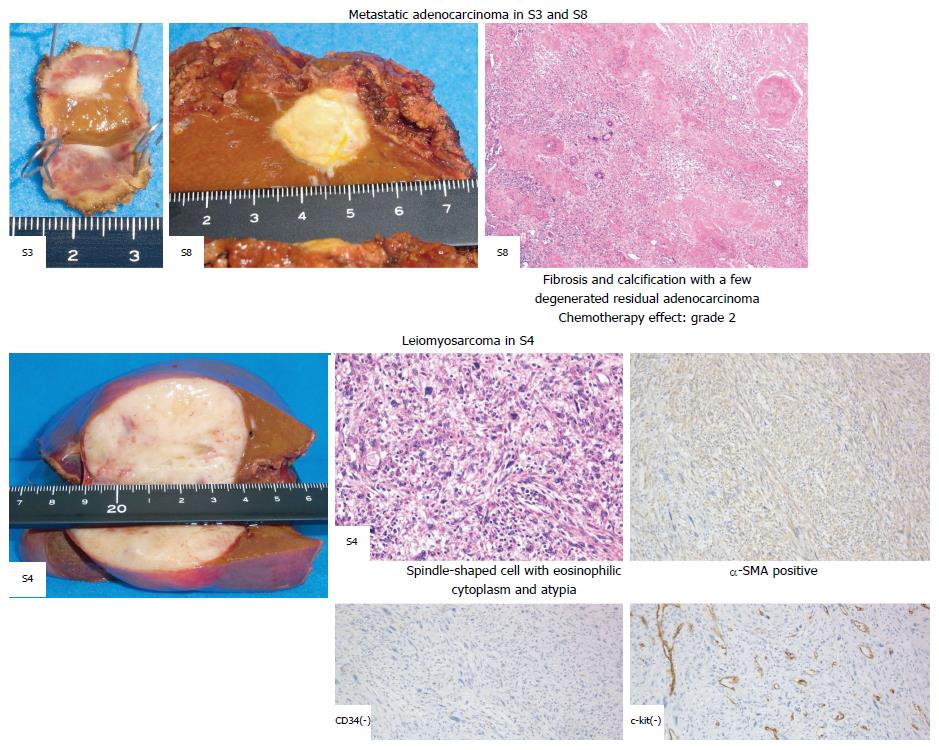
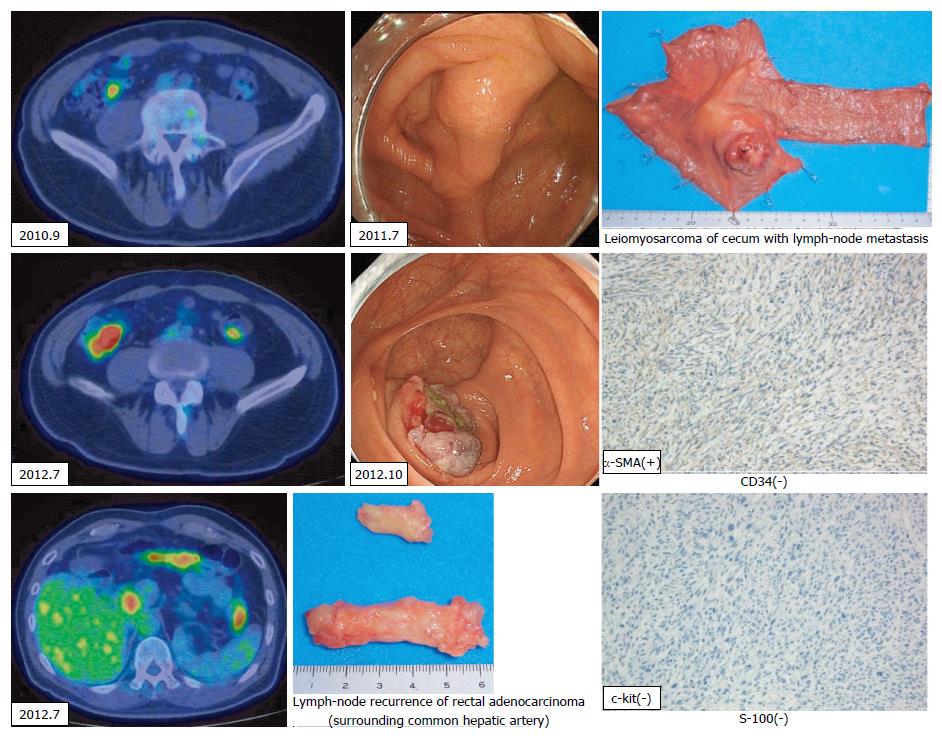
A pathological examination revealed fibrosis and calcification in the S3 and S8 tumors, with a few degenerated residual adenocarcinoma cells, which was compatible with rectal adenocarcinoma metastasis. In contrast, the S4 tumor consisted of irregular fascicles of spindle-shaped cells with eosinophilic cytoplasm and nuclear atypia. An immunohistochemical examination demonstrated that the S4 tumor cells were positive for α-smooth muscle actin and desmin, while negative for CD34, S-100, c-kit, and cytokeratin AE1/3 (Figure 4). Based on these findings, the S4 tumor was designated as leiomyosarcoma. The tumor grade was high according to the classification by Hajdu et al[3].
Histologically, chemotherapy had no apparent effect on this tumor. The patient underwent six courses of FOLFIRI plus bevacizumab as adjuvant chemotherapy after hepatic resection. Twenty-two months later (in July 2012), an abdominal CT scan was performed as part of an annual medical examination. A cecal tumor and lymph-node swelling around the common hepatic artery were discovered. Moreover, accumulation at both sites was discovered in a positron emission tomography (PET)-CT scan (Figure 5). The tumor had not been identified in a colonoscopy. Three months later, a type 2 tumor was discovered and a biopsy via colonoscopy revealed leiomyosarcoma. Ileocecal resection with lymph-node dissection and lymph-node sampling around the common hepatic artery were carried out in November 2012 (the third operation).
A pathological examination revealed cecal leiomyosarcoma with regional lymph-node metastases and metastasis from rectal adenocarcinoma in the lymph node around the common hepatic artery. Retrospectively, accumulation had been observed in the cecum in a PET-CT scan in September 2010, and a submucosal tumor had been suspected in a colonoscopy in July 2011. It is likely that rectal adenocarcinoma and cecal leiomyosarcoma existed synchronously from the beginning, and that both tumors had metastasized to the liver synchronously. The patient was then treated with nine courses of XELOX plus bevacizumab.
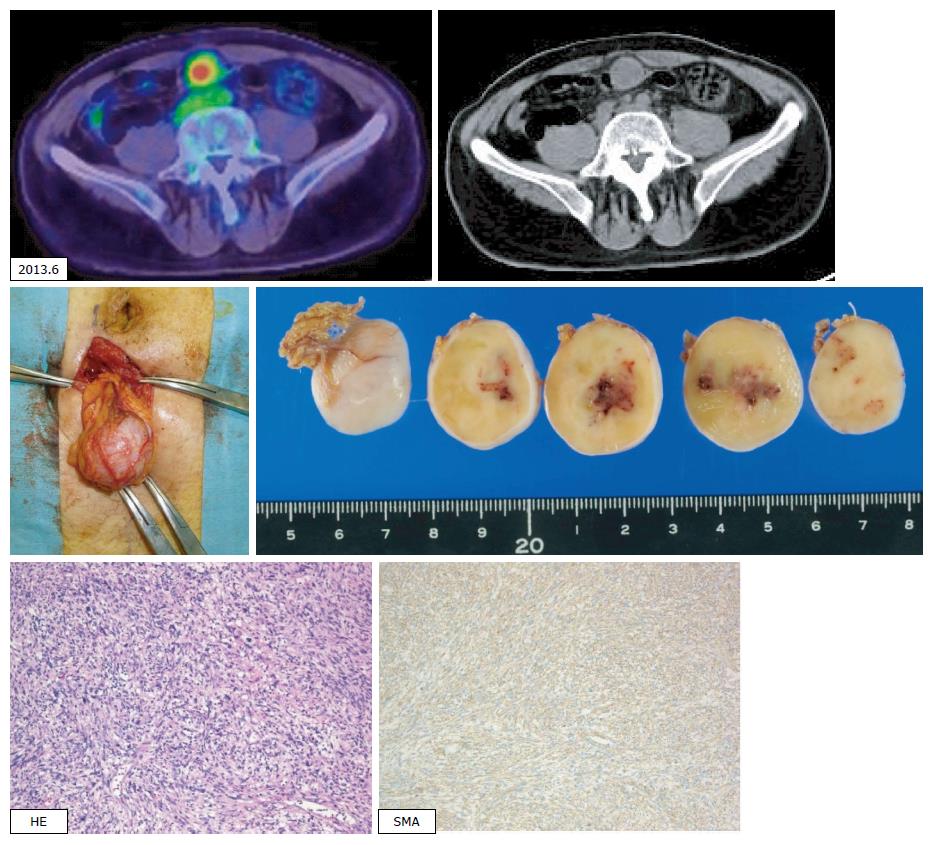
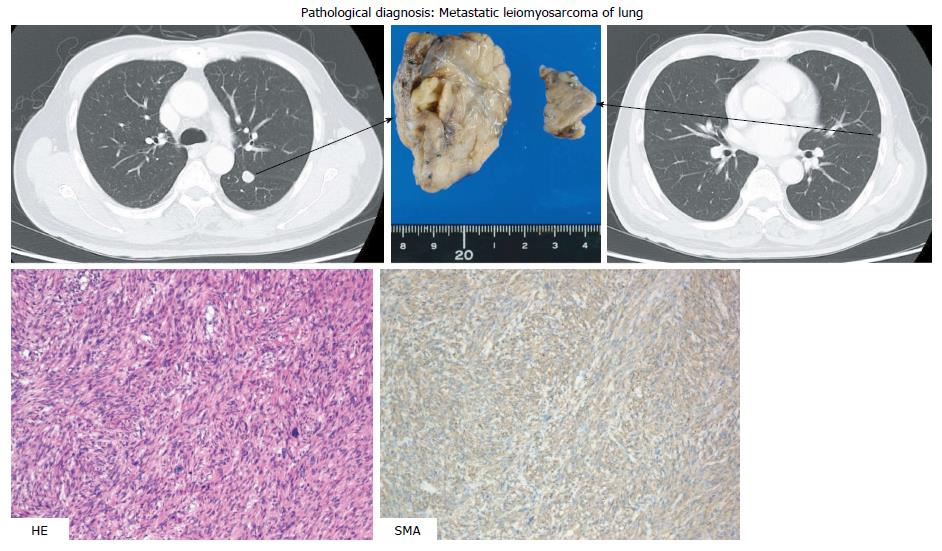
In a follow-up CT scan in June 2013, a tumor just below the peritoneum was discovered. Because this accumulation was identified in a PET-CT scan and no other accumulation was observed, extirpation of the tumor was carried out (the fourth operation). Pathological diagnosis was leiomyosarcoma of the omentum, compatible with recurrence (Figure 6). The patient was treated with XELOX plus bevacizumab following the surgery. Three months later, in a chest CT scan, two coin lesions were discovered in the left lung. There was no indication of accumulation at both lesions in a PET-CT scan, but lung metastases were strongly suspected. In October 2013 (46 mo after the first operation), partial resections of the left upper lobe and left lower lobe were performed (the fifth operation). Pathological diagnosis was metastatic leiomyosarcoma of the lung (Figure 7).
The patient was subsequently treated with XELOX plus bevacizumab again following surgery (the fifth and final operation). The hepatic hilum lymph node was found to be enlarged in July 2014, and multiple lung metastases were newly discovered in November 2014. Chemotherapy was changed to treatment consisting of CPT-11 plus cetuximab, due to neuropathy experienced by the patient. This clinical time course is demonstrated in Figure 8.
Although lymph-node and multiple lung metastases were present, the patient survived for more than six years after the initial operation. Since the left hepatic duct was constricted by lymph-node metastasis, a plastic stent was inserted in October 2015. The patient received chemotherapy with doxorubicin afterward as an outpatient but was hospitalized with cholangitis due to lymph-node metastatic recurrence in January 2016. The patient died from liver failure due to lymph-node invasion from rectal adenocarcinoma in March 2016 (76 mo after the initial operation). The extended treatment proved worthwhile, given that the patient could live a normal life for 73 mo after the initial operation with help from the multidisciplinary therapy we employed.
Hepatic resection for liver metastases from colorectal cancer or neuroendocrine tumor, in combination with chemotherapy, has been established as a safe and standard treatment. The issue in question, however, is how to determine effective treatment for non-colorectal non-neuroendocrine liver metastases (NCNNLM). Hepatic metastasectomy had been thought ineffective for such cases. According to Gladdy's report of 353 patients with primary resectable leiomyosarcoma[4], recurrence occurred in 51% of abdominal and retroperitoneal leiomyosarcoma cases, including 29% of lung, 23% of liver, and 15% of other cases (brain and lymph nodes). Predictive factors for disease-free survival in patients with leiomyosarcoma were size and tumor grade. In addition, DeMatteo et al[5] reported that the rate of recurrence reached as high as 84% even after complete hepatic resection for sarcoma metastasis, although this definition of sarcoma includes gastrointestinal stromal tumors.
Ng et al[6] reviewed 191 cases of gastrointestinal leiomyosarcomas, of which colorectal leiomyosarcoma numbered 22 cases (12%). Without hepatectomy, the median survival time for patients with liver metastases from leiomyosarcoma was no more than 14 mo. Before the 21st century, metastases from leiomyosarcomas were thought to not be sensitive to chemotherapy[7].
Lang et al[8] reported on 26 cases of hepatic metastases from leiomyosarcomas. Only one of these 26 cases (3.8%) originated from the colon. The median survival and five-year survival rate after R0 resection were 32 months and 20%, respectively. The presence of extrahepatic tumor growth should be regarded as a contraindication for liver resection only if an R0 resection does not appear possible. Marudanayagam et al[9] also emphasized the importance of R0 resection for hepatic metastasectomy of soft tissue sarcomas. Among soft tissue sarcomas, leiomyosarcoma was associated with poor prognosis.
Groeschl et al[10] reported on 420 patients who underwent hepatectomy for NCNNLM. The five-year survival rate in recent years was 32% after hepatectomy for liver metastases from sarcomas. Although the rate of recurrence after hepatectomy is as high as 66.5%, NCNNLM can be resected with reasonable survival outcomes when that surgery is appropriately selected as a treatment option. Prognostic factors for NCNNLM are tumor size (greater than 5 cm), lymphovascular invasion, and time-interval to liver metastasis of less than two years. Hepatic recurrence did not occur in the case we present here, which was one of the reasons for the patient's long-term survival.
We reported this patient's case as a primary hepatic leiomyosarcoma with liver metastasis of rectal cancer[2], before our finding of cecal leiomyosarcoma. The cecal leiomyosarcoma was discovered almost three years after the patient's first visit. From a pathological perspective, it was difficult to distinguish the site of primary lesion. Mourra et al[11] published a multi-institutional study on metastatic tumors in the colon and rectum. In that paper, only 35 of 10365 patients with colorectal malignancies (0.338%) were identified as having true metastases to the colon and rectum. Of those 35 metastatic colorectal tumors, leiomyosarcoma was identified in only two cases, with both tumors originating from soft tissue. This indicates only a small probability of the primary hepatic leiomyosarcoma metastasizing to the cecum. In contrast, hepatic metastasis is reported to occur in 20%-66.5% of patients with visceral or retroperitoneal sarcomas[9,10]. Moreover, all 35 patients had a history of metastatic disease in extragastrointestinal sites, with a mean disease-free interval of 10.6 years for sarcomas. We feel that these clinical characteristics were not compatible with our case, and for that reason we concluded that the cecum was the primary lesion site in the patient case we present here. We were aware that primary hepatic leiomyosarcoma is rare, and this patient's case has taught that its diagnosis should be made carefully only after long-term observation. However, rare are cases in which cecal leiomyosarcoma and rectal adenocarcinoma coexisted and metastasized to the liver. Hamai et al[12] reported a case of gastric adenocarcinoma with multiple liver tumors. After 14 mo of chemotherapy, the patient underwent total gastrectomy with partial liver resection, upon which the liver tumors were diagnosed pathologically as leiomyosarcomas. During adjuvant chemotherapy two years and five months after the first visit, a new hepatic tumor appeared and a tumor in the colon was discovered. The patient underwent partial colectomy and partial liver resection. The colon tumor and liver tumors were all immunohistochemically diagnosed as leiomyosarcomas. Seven months later, a third liver resection was carried out for the newly discovered liver tumors. Multiple liver and lung metastases eventually developed, and the patient died four years and 10 mo after the first visit. When a liver tumor is diagnosed as leiomyosarcoma, therefore, careful follow-up is needed to identify the primary site.
Leiomyosarcoma was for some time considered to be a relatively chemo-resistant sarcoma subtype. Recent data have demonstrated a reasonable response rate exhibited by some histological subtypes exposed to specific histology-tailored treatments with doxorubicin-containing chemotherapy[13]. Since without chemotherapy median survival is generally up to 12 mo[14], chemotherapy appears to be necessary for leiomyosarcoma treatment. However, the type of adjuvant therapy appropriate for leiomyosarcoma is an issue requiring debate. Pazopanib was recently reported to be a feasible option for patients who had been heavily pretreated for metastatic sarcoma[15]. In that report, patients with leiomyosarcomas comprised the majority of long-term responders and survivors.
Both leiomyosarcoma and adenocarcinoma recurred after resection of primary lesions and hepatic metastases, making determination of a chemotherapy regimen for the patient in this report difficult. As we are familiar with adenocarcinomas and the distant lymph-node recurrence was adenocarcinoma pathologically, we chose a regimen mainly designed for colorectal adenocarcinoma. After peritoneal and lung tumors were diagnosed as leiomyosarcoma recurrence, we selected the treatment doxorubicin. At the patient's final hospitalization, lymph-node metastases to the hepatic hilum caused liver failure, which proved to be fatal, and chest X-rays showed numerous nodules in the lung field, which had caused a persistent cough in the patient. The former problem derived from adenocarcinoma and the latter from leiomyosarcoma. By that time, chemotherapy was no longer a treatment option.
The patient survived more than six years after initial diagnosis, even though he had both stage IV rectal cancer and cecal leiomyosarcoma with liver metastasis. As for long-term survival, Gladdy et al[4] reported on late disease-specific mortality in primary leiomyosarcoma. In that report, 6% of extremity and 9% of abdominal or retroperitoneal patients developed distant recurrence more than five years after the primary tumor diagnosis. The authors emphasized the need for long-term follow up.
The prognosis for patients with leiomyosarcoma might be prolonged in the future as chemotherapy continues to advance. Long-term follow-up is important and should be considered.
This patient case was extremely rare for the points mentioned below: (1) cecal leiomyosarcoma and rectal adenocarcinoma coexisted; (2) both metastasized to the liver and were resected successfully; (3) primary colon leiomyosarcoma was diagnosed two years after resection of the hepatic metastatic lesion; and (4) long-term survival was attained based on multidisciplinary therapy.
A 61-year-old male patient with occult blood in stool and multiple liver tumors discovered at an annual medical examination.
Rectal adenocarcinoma with multiple liver metastases.
The authors did not at first recognize a differential diagnosis.
All lab measurements were within normal ranges except for slightly elevated serum carcino-embryonic antigen.
An abdominal computed tomography scan and gadoxetic acid enhanced magnetic resonance imaging revealed liver tumors in Segment 3, Segment 4, and Segment 8.
The patient had synchronous liver metastases from both cecal leiomyosarcoma and rectal adenocarcinoma.
The patient successfully underwent resection of both primary lesions and liver metastases in combination with chemotherapy.
There have been no case reports on co-existence of liver metastases from both cecal leiomyosarcoma and rectal adenocarcinoma. Only one report was published regarding a gastric adenocarcinoma with multiple liver tumors, which were diagnosed pathologically as leiomyosarcoma after gastrectomy and hepatectomy. Fifteen months later, a tumor in the colon was discovered, after which a partial colectomy was carried out. The colon tumor was immunohistochemically diagnosed as leiomyosarcoma.
Treatment for multiple liver tumors is challenging, particularly if the diagnoses of hepatic tumors differ from each other.
The main issue regarding diagnosis is whether the hepatic leiomyosarcoma is a primary lesion or a metastasis from the cecum. From a pathological perspective, it is difficult to distinguish which of the sites is a primary lesion. The authors therefore went about trying to make a clinical determination.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Facciorusso A, Lim SC, Seicean R S- Editor: Qi Y L- Editor: A E- Editor: Liu WX
| 1. | Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol. 2000;24:1339-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Takehara K, Aoki H, Takehara Y, Yamasaki R, Tanakaya K, Takeuchi H. Primary hepatic leiomyosarcoma with liver metastasis of rectal cancer. World J Gastroenterol. 2012;18:5479-5484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988;12:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Gladdy RA, Qin LX, Moraco N, Agaram NP, Brennan MF, Singer S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol. 2013;20:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540-557; discussion 540-557;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Ng EH, Pollock RE and Ramsdahl MM. Prognostic implications of patterns of failure for gastrointestinal leiomyosarcomas. Cancer. 1992;69:1334-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, Verweij J, Santoro A, Buesa J, Tursz T. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150-157. [PubMed] |
| 8. | Lang H, Nussbaum KT, Kaudel P, Frühauf N, Flemming P, Raab R. Hepatic metastases from leiomyosarcoma: A single-center experience with 34 liver resections during a 15-year period. Ann Surg. 2000;231:500-505. [PubMed] [DOI] [Full Text] |
| 9. | Marudanayagam R, Sandhu B, Perera MT, Bramhall SR, Mayer D, Buckels JA, Mirza DF. Liver resection for metastatic soft tissue sarcoma: an analysis of prognostic factors. Eur J Surg Oncol. 2011;37:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Groeschl RT, Nachmany I, Steel JL, Reddy SK, Glazer ES, de Jong MC, Pawlik TM, Geller DA, Tsung A, Marsh JW. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg. 2012;214:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Mourra N, Jouret-Mourin A, Lazure T, Audard V, Albiges L, Malbois M, Bouzourene H, Duvillard P. Metastatic tumors to the colon and rectum: a multi-institutional study. Arch Pathol Lab Med. 2012;136:1397-1401. [PubMed] [DOI] [Full Text] |
| 12. | Hamai Y, Hihara J, Emi M, Aoki Y, Kushitani K, Tanabe K, Okada M. Leiomyosarcoma of the sigmoid colon with multiple liver metastases and gastric cancer: a case report. BMC Gastroenterol. 2012;12:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Penel N, Italiano A, Isambert N, Bompas E, Bousquet G, Duffaud F. Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Ann Oncol. 2010;21:1361-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Blay JY, van Glabbeke M, Verweij J, van Oosterom AT, Le Cesne A, Oosterhuis JW, Judson I, Nielsen OS. Advanced soft-tissue sarcoma: a disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer. 2003;39:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Yoo KH, Kim HS, Lee SJ, Park SH, Kim SJ, Kim SH, La Choi Y, Shin KH, Cho YJ, Lee J. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |