Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.949
Peer-review started: October 27, 2016
First decision: December 2, 2016
Revised: December 14, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: February 14, 2017
Processing time: 111 Days and 7.1 Hours
Lymphocytic esophagitis (LE) is a clinicopathologic entity first described by Rubio et al in 2006. It is defined as peripapillary intraepithelial lymphocytosis with spongiosis and few or no granulocytes on esophageal biopsy. This definition is not widely accepted and the number of lymphocytes needed to make the diagnosis varied in different studies. Multiple studies have described potential clinical associations and risk factors for LE, such as old age, female gender and smoking history. This entity was reported in inflammatory bowel disease in the pediatric population but not in adults. Other associations include gastroesophageal reflux disease and primary esophageal motility disorders. The most common symptom is dysphagia, with a normal appearing esophagus on endoscopy, though esophageal rings, webs, nodularities, furrows and strictures have been described. Multiple treatment modalities have been used such as proton pump inhibitors and topical steroids. Esophageal dilation seems to be therapeutic when dysphagia is present along with esophageal narrowing secondary to webs, rings or strictures. The natural history of the disease remains unclear and needs to be better delineated. Overall, lymphocytic esophagitis seems to have a chronic and benign course, except for two cases of esophageal perforation in the literature, thought to be secondary to this entity.
Core tip: Lymphocytic esophagitis has recently been described in 2006 and subsequently, multiple groups have attempted to describe its clinical associations and risk factors with minimal information available on treatment. We performed a PubMed search of all case reports and retrospective studies published in English about lymphocytic esophagitis. The objective of this paper is to present a scientific review of all aspects of this emerging clinical entity known to date.
- Citation: Rouphael C, Gordon IO, Thota PN. Lymphocytic esophagitis: Still an enigma a decade later. World J Gastroenterol 2017; 23(6): 949-956
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/949.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.949
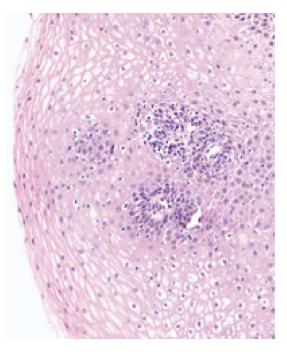
Lymphocytic esophagitis (LE) is a new clinicopathologic entity that was first described by Rubio et al[1] in 2006 and subsequently, more frequently diagnosed and reported by clinicians and pathologists over the last decade. It consists of peripapillary intraepithelial lymphocytes (IELs) with no or rare granulocytes on esophageal biopsies. Despite LE being an emerging entity, esophageal IELs have been described years ago and several studies, as early as the 1970s, suggested a possible association between gastroesophageal reflux disease (GERD) and an increased number of IELs[2]. The co-occurrence of lymphocytes and granulocytes in reflux esophagitis has also been reported[2-4]. IELs with irregular nuclear contours were described on the esophageal biopsy specimens of patients with reflux esophagitis[4]. In the late 1990s, Wang et al[5] found that IELs were not an independent marker of reflux esophagitis as no correlation was noted between the number of neutrophils and T lymphocytes, despite the presence of a correlation between the number of T lymphocytes and eosinophils. Around that time, in a Swedish study looking for Menetrier's disease and varioliform lymphocytic gastritis in baboons, one esophageal biopsy specimen was noted to be infiltrated by lymphocytes with round irregular contours and a lack of granulocytes[6]. The latter study was the basis for Rubio's group to start looking for human biopsy specimens with similar characteristics and describe LE for the first time. They characterized this condition by heavy peripapillary intraepithelial lymphocytic infiltration with no or rare granulocytes[1]. Consequently, multiple studies done on LE noted intercellular edema known as spongiosis[7,8] on pathology slides, a criterion that was later added to Rubio et al definition. The widely accepted definition for lymphocytic esophagitis is increased peripapillary IELs by more than 20 IELs per high power field with little or no granulocytes, along with spongiosis (Figure 1).
The presence of neutrophils, eosinophils or increased lymphocytes on esophageal biopsy indicates an inflammatory process. A normal esophageal mucosa has a small number of dispersed lymphocytes, mostly with irregular nuclear contours, and mainly in the peripapillary epithelium[9]. An acceptable count of IELs in a normal esophagus was reported to be 10 IELs/HPF[10]. The lymphocytic population in the esophagus is part of the gut-associated lymphoid tissue: Cytotoxic CD8+ T cells are normally seen in the squamous epithelium along with Langerhans cells. Helper CD4+ T cells and B lymphocytes reside in the lamina propria[11].
Given that LE is defined as an increased number of IELs, we would expect a peripapillary predominance of cytotoxic CD8+ T cells in the esophageal squamous epithelium. This has been inconsistent however, and a recent study demonstrated a CD4+ T-cell-predominant intraepithelial lymphocytosis in patients with primary esophageal motility disorder (PEMD) raising the possibility of a new clinicopathologic entity that they labeled "dysmotility-associated LE"[12]. In contrast, in cases of LE in patients with normal esophageal motility, the number of CD4+ and CD8+ T lymphocytes varied. Indeed, one study found a CD8+ T-cell predominance when LE was associated with GERD in the absence of PEMD[13]. A better immunophenotypic characterization of LE is hence needed for a superior definition. Another aspect of deficiency in the definition of LE is reflected in the various studies performed with different cut-offs used to demarcate an increased IEL count, ranging between ≥ 10 IEL/HPF[14] and ≥ 50 IEL/HPF[15,16] with a cut off of ≥ 20 IEL/HPF being commonly used[17,18]. Moreover, the acceptable number of a "few granulocytes" needs to be better defined, as granulocytic inflammation is typically seen in GERD.
The incidence of LE has been on the rise in the United States over the past few years. It is unclear whether this is true increase in incidence or simply secondary to the condition being better recognized by pathologists and clinicians. In one study of 81 subjects with LE, 81.5% were diagnosed between 2006 and 2009 as compared to 6.2% diagnosed between 1998 and 2001[18]. It has been published that LE is being detected at a rate of 1 in a 1000 on endoscopies and biopsies performed in the outpatient setting[7]. It is also unclear whether LE is more commonly seen in the western world or developing countries. Despite the fact that a Swedish group first characterized it, most published case reports and studies looking for clinical associations and potential risk factors took place in the United States. To the best of our knowledge, only three case reports in the English literature described cases of LE outside the United States, including Japan[19], Portugal[20] and Australia[21]. In contrast to the findings of Rubio et al[1], it is becoming evident that LE seems to affect older women to a larger extent, in their sixth decade[12,14,17,18], in contrast to eosinophilic esophagitis (EoE) seen in younger men. Smoking was also found to be associated with LE in multiple retrospective studies[14,17]. Patients tend to present with dysphagia as the most common symptom, though reflux/heartburn, chest pain, nausea and abdominal pain have been reported as well[8,12,14,18].
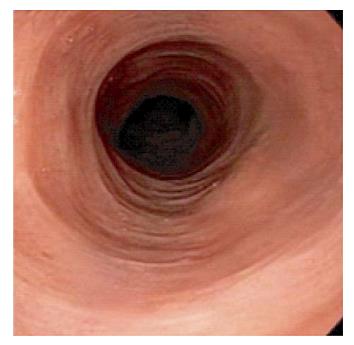
Endoscopic findings vary from normal mucosa in up to one-third of the cases (19.8%-35%)[12,18] to esophageal rings, strictures, furrows and webs (Figure 2). For instance, Cohen et al[18] demonstrated that the esophagus looked normal in approximately 30% of their subjects, with the most common lesions in others being esophageal rings (19.6%), esophagitis (13%) and strictures (8.6%). Erythema, nodularities, furrows and webs were observed to a lesser extent. Purdy et al[8] noted a significant difference in endoscopic findings between LE subjects and controls. Similar to Cohen et al[18] findings, a normal appearance of the esophagus was the most common finding in both groups. It is noteworthy however that when Putra et al[22] compared patients with PEMD to their controls (GERD patients with no dysmotility disorder), patients with LE were more likely to have a normal upper endoscopy than patients with "dysmotility negative-GERD" (P = 0.004). No significant difference was noted however between both groups when looking at rings, furrows, esophagitis, possible Barrett's esophagus, ulcer or stricture, findings that were much less encountered in both groups than a normal esophageal mucosa. The majority of the retrospective studies conducted to look for clinical associations and risk factors for LE, found that LE most commonly presented with a normal esophagus. This observation was in contrast to Pasricha et al[14] findings, who noted that 82% (23/27) of their sample had abnormal endoscopic findings in the esophagus. In addition, all of the case reports published to date, report abnormal esophageal findings. For instance, Zhang et al[23] reported the case of a 66-year-old female with history of dysphagia, who was found to have multiple concentric rings in her mid and lower esophagus with biopsies consistent with LE. Those endoscopic findings are similar to EoE given the lesions and the location. It is hence important to realize that a feline esophagus is not specific for EoE. In addition, the most common presentation of LE seems to be dysphagia with a normal esophageal mucosa on endoscopy, hence the need for a biopsy, not only to rule out EoE, but also to look for LE. The esophageal abnormalities associated with LE have been reported in multiple locations of the esophagus. In a patient with systemic lupus erythematosus and Behçet's disease, multiple rings were noted in the upper third of the esophagus exclusively, with small outpouchings developing in the cervical esophagus years later and requiring dilation[20]. In another patient with lymphoma and esophagitis, presenting with dysphagia and food impaction, endoscopy revealed mid-esophageal rings as well as a distal esophageal stricture[24]. Lesions seem to develop in all 3 parts of the esophagus with the mid esophagus being mostly co-involved with the proximal or distal parts. As a matter of fact, in their study, Xue et al[12] specified that most biopsies were obtained from the mid-esophagus and had a higher IEL count compared to other locations in the esophagus.
Since Rubio et al[1] first described LE, multiple groups throughout the United States attempted to better characterize this entity through retrospective studies trying to identify this emerging entity's clinical features. Numerous potential clinical associations were looked at, including inflammatory bowel disease (IBD)[15,16,25], esophageal dysmotility disorders[22], hypersensitivity and mucosal insults[8,14,17], celiac sprue, common variable immunodeficiency disorder[26] and GERD, as presented below (Table 1). Other groups tried to establish LE as an independent and distinct clinical entity, many of them failing to find any potential correlates[8,12,14]. To date, LE remains a "disease" with no established definition or clinical associations.
| Ref. | Number of Patients with Lymphocytic Esophagitis | Gender Distribution n (%) | Mean age1 of patients with LE (yr) | Associated conditions | Treatment |
| Rubio et al[1], 2006 | 20 | Female: 10; Male: 10 | 31.3 Range: (2-28) | Crohn’s disease, asthma, liver cirrhosis, Sclerosing cholangitis | Not available |
| Purdy et al[8], 2008 | 42 | Not Available | 44 Range: (2-81) | Allergies (drug and non-drug), Helicobacter pylori gastritis, Crohn’s disease | Not available |
| Xue et al[12], 2015 | 45 (21 LE-NG) (24 LE-FG) | LE-NG: Female: 15; Male: 7 LE-FG: Female: 15; Male: 9 | LE-NG: 59 ± 12 LE-FG: 65 ± 13 | Motility abnormalities | Not available |
| Putra et al[22], 2016 | 10/22 with nutcracker esophagus 7/33 with ineffective motility 5/14 with diffuse esophageal spasm | Nutcracker esophagus: Female: 11; Male: 11 Ineffective motility: Female: 20; Male: 13 Ineffective motility: Female: 8; Male: 6 | Nutcracker Esophagus: 54 ± 11 Ineffective motility: 56 ± 14 Diffuse spasm: 62 ± 11 | Nutcracker esophagus, ineffective motility, diffuse esophageal spasm | Diltiazem Proton pump inhibitors Botulinum toxin Peppermint Nitroglycerin sublingual |
| Kissiedu et al[17], 2016 | 33 | Female: 6; Male: 27 | 67 ± 10 | Smoking, hyperlipidemia, cryotherapy, radio- frequency ablation, endoscopic mucosal resection | Not available |
| Pasricha et al[14], 2016 | 27 | Female: 17; Male: 10 | 56 ± 16 | IBD, GERD, Barrett’s esophagus, achalasia, drug allergy, asthma, eczema, IBS, cancer, tobacco, alcohol, drug use | Proton pump inhibitors Fluticasone GI cocktail (maalox, donnatal, lidocaine) Prednisone taper |
| Cohen et al[18], 2012 | 81 | Female: 44; Male: 37 | 51 Range: (19-84) | GERD, achalasia, allergies, asthma, eczema, IBD, hypothyroidism, alcohol use, tobacco use | Proton pump inhibitors Anti-tumor necrosis factor agent |
| Basseri et al[25], 2013 | 4/47 patients with IBD | Female: 23; Male: 24 | 39.3 ± 14.6 | GERD, asthma, IBD, diabetes mellitus, celiac disease, smoking, alcohol use | Not available |
| Sutton et al[16], 2014 | 31 | Female: 15; Male: 16 | 8.9 | Crohn’s disease, GERD, Infectious/inflammatory disorders, Polyps/neoplasms, Immune disorders, mechanical disorders, functional abdominal pain | Not available |
| Ebach et al[15], 2011 | Crohn’s and LymphocyticEsophagitis: 17 out of 60 patients with Crohn’s | Crohn’s disease patients: Female: 17; Male: 43 | Crohn’s disease patients: 13.3 Range: (4.7-20.7) | IBD | Not available |
| Haque et al[7], 2012 | 119 | Female: 72; Male: 47 | 63 (Median) | Helicobacter pylori gastritis, celiac disease, duodenal lymphocytosis, Crohn’s disease | Not available |
Esophageal Crohn's disease (CD) is well recognized and is known to occur in approximately 6.5% of the affected juvenile patients as compared to 0.3%-2% in adults with CD[27]. In severe forms, it is characterized by sharply demarcated ulcers or erosions, and the mucosa surrounding those lesions is typically normal[28]. Of 20 esophageal biopsy cases with histologic LE, 8 cases of CD (including 7 pediatric cases) were identified by Rubio et al[1], suggesting a potential correlation with CD in the juvenile population. Subsequently, Purdy et al[8] confirmed a potential association between CD and LE in the pediatric population. In their study comprising 42 patients with LE from a mixed adult and pediatric database, 38% of the pediatric subset with LE had CD (3 of 8 children). Similarly, Ebach et al[15] studied an exclusively pediatric cohort and found that LE was present in 28% of 60 patients with CD as compared to 4.4% of 68 children without CD. A comparable association was found in the study by Sutton et al[16], where LE was present in 12.2% of 49 patients with CD as compared to 5% of 496 patients without CD. These results were not reproducible in the adult population however, as shown by the work of Basseri et al[25], where only one out of 47 patients with LE had CD. Similarly, results were consistent in the studies conducted by Haque et al[7] and Xue et al[12], where none of the 119 and 45 adult patients with LE respectively, had CD. LE might hence be a manifestation of CD or, possibly, an indicator of IBD activity in the pediatric population, but remains nonspecific and most likely not associated with IBD in adults.
An interesting association of LE with PEMD was noted by Xue et al[12]. In their initial work, they compared the clinical and histological characteristics of 3 groups: LE-No Granulocytes (LE-NG), LE-few granulocytes (LE-FG) and their control group which consisted of patients with "reflux esophagitis with increased IELs" (REIL). Out of the 21 subjects in the LE-NG group, 11 were tested for motility abnormality, which was confirmed in 10 subjects. PEMD were also found in 6/10 patients tested in the LE-FG group (24 patients) and in 6 of 11 tested in the REIL group (28 patients). Interestingly, the prevalence of PEMD was significantly higher in patients with CD4+ T-cell predominant IELs as compared to CD8+ T-cell predominant esophagitis, a finding that suggests a potential association between PEMD and CD4+ T-cell predominant esophagitis. It might hence be necessary to characterize the T- cell subpopulation in patients with LE for diagnostic purposes in some cases.
Reechoing the definition of LE as peripapillary intraepithelial lymphocytosis with spongiosis, one would think of LE as an entity with histopathologic similarities to acute spongiotic dermatitis raising the hypothesis of a possible irritant to the esophageal mucosa resulting in lymphocytosis and epithelial edema. Purdy et al[8] refuted this hypothesis in their work and demonstrated no associations between LE and allergic disorders, asthma or celiac disease. They also looked at GERD as a potential cause of mucosal irritation and injury and found no correlation between reflux and LE, which brings us to a controversy in the present literature: Is GERD associated with LE? A recent study, published by Olson et al[13] in the form of an abstract, concluded that LE could be associated with GERD with CD8+ T-cell predominant IELs. Another study by Kissiedu et al[17] looked at post-ablation surveillance biopsies in patients with Barrett's esophagus. A significantly higher prevalence of LE was noted on surveillance biopsies as compared to pre-ablation specimens, suggesting mucosal injury to be a potential trigger to the development of this condition. Yet, it is noteworthy that all those patients had GERD. A similar mechanism of mucosal injury could hypothetically explain the association noted between LE and smoking.
In addition to the above-mentioned clinical associations, authors have investigated other hypothetical correlations. Pasricha et al[14] excluded patients with lichen planus from their study: according to the authors, lichen planus and LE are two different entities histologically as lichen planus is characterized by lichenoid lymphocytic infiltration, which is absent in LE. The potential link between LE and connective tissue disorders where analyzed in a case report from Portugal[20] where dysphagia secondary to LE was not associated with systemic lupus erythematosus and Behçet's disease flare-ups, as dysphagia and flare-ups would occur at different points in time. LE was also reported in a patient with common variable immune deficiency receiving intravenous immunoglobulin (IVIG) infusions[26]. No other reports in the literature described LE in patients with immune deficiency disorders or in patients receiving IVIG. It is hence unclear whether LE is linked to common variable immune deficiency, IVIG or is simply an independent entity in this specific patient. Some studies also looked at a potential co-occurrence of lymphocytosis in the esophagus and other parts of the gut. One would wonder whether LE would co-occur with lymphocytic colitis for instance. In their study, Purdy et al[8] looked at biopsies from other gastrointestinal sites obtained at the time of esophageal biopsies: Out of the 30 patients with LE, 23 had stomach biopsies which showed normal mucosa (4/23), Helicobacter pylori gastritis (4/23), focally enhanced gastritis (3/23), inactive chronic gastritis (6/23) or other random findings. Small bowel biopsies where obtained in 15 patients and revealed normal mucosa in 8 specimens, CD in one, autoimmune enteropathy in one, and epithelial lymphocytosis in two patients. The other 2 specimens showed nonspecific changes. Six patients had concurrent colon biopsies performed. One was normal, 2 had CD, 2 showed hyperplastic polyps and one had autoimmune enteropathy. The authors comment however that no pattern was noted and that interestingly, these GI tract findings corresponded to known chronic conditions of the patients. In a case report with multiple gastric biopsies taken concurrently with the esophageal biopsies that revealed LE, gastric biopsies were negative for lymphocytosis or eosinophilic gastritis[23]. Haque et al[7] also looked at the co-occurrence of celiac disease and duodenal lymphocytosis with LE and EoE. There was no significant difference in duodenal lymphocytosis co-occurrence between the LE and the EoE groups. On the other hand, celiac disease was more commonly noted in patients with LE as compared to EoE, although this was not statistically significant given the small number of patients (Table 2).
| Ref. | Age (yr) | Gender | Associated conditions | Treatment |
| Figueiredo et al[20], 2014 | 30 | Male | Behçet’s disease | Endoscopic dilation |
| Systemic lupus erythematosus | Swallowed fluticasone | |||
| Mandaliya et al[24], 2012 | 74 | Male | Lymphoma | Endoscopic dilation |
| Esophagitis | Botox injections | |||
| Zhang et al[23], 2016 | 66 | Female | Opioid overdose | Omeprazole 40 mg twice daily |
| Maejima et al[19], 2015 | 68 | Male | Food impaction | Endoscopic dilation |
| Niewiarowski et al[31], 2016 | 82 | Female | Acute food impaction | Not available |
| Vangimalla et al[26], 2016 | 67 | Male | Common variable immune deficiency | Acid suppression |
| Endoscopic dilation | ||||
| Hendy et al[21], 2013 | 35 | Female | Not available | Topical steroids (fluticasone) |
Multiple treatments have been tried including proton pump inhibitors (PPIs), topical steroids, oral prednisone, botox injections and esophageal dilations. Few retrospective studies actually addressed treatment. Cohen et al[18] conducted a study in two stages: they initially performed a retrospective chart review of patients with LE and investigated potential clinical associations with this condition. The second part of the study aimed at exploring the natural history of LE by sending out surveys to their 81 patients with LE with a 3.3-year median follow-up. Out of the 29 patients who completed the survey, approximately 60% reported improvement in their GI symptoms that they thought was as a result of therapy, either with PPIs or after starting an anti-tumor necrosis factor agent for patients with IBD. Esophageal dilation contributed to symptomatic improvement as well. Pasricha et al[14] also collected information on treatment changes after establishing a diagnosis of LE. Out of their 27 patients, one-third had a change in treatment that consisted of either a PPI (6), inhaled fluticasone (1), gastrointestinal cocktail (1) or prednisone taper (1) with improvement noted in patients treated with PPIs or inhaled fluticasone. It is unclear how PPIs result in symptom improvement. Despite the fact that PPIs have actually been associated with lymphocytic and collagenous colitis[29], they actually seem to be therapeutic in LE, most likely secondary to their anti-inflammatory effect. Clinicians have been prescribing PPIs for LE, as LE is thought to be potentially associated with GERD, and given that improvement is being reported. For instance, Zhang et al[23] treated their patient with omeprazole 40 mg twice daily with symptomatic improvement within days. As already mentioned, topical steroids have also been suggested with symptom resolution, assuming LE and EoE belong to the same family. Kasirye et al[30] opted to treat their patient with 220 μg/puff, two puffs three times daily, as their patient was already treated with PPIs and Histamine 2-receptor antagonists with incomplete resolution of their symptoms. Additionally, therapeutic esophageal dilations have been performed in patients presenting with dysphagia (with or without food impaction). Based on the few case reports published, dilation can be repeated as needed[26,31].
Extensive study of the natural history of LE is lacking. We identified one study looking at the clinical course of LE via a survey sent out to patients, which found that 70 of 81 patients with LE (87%) were alive after a 3.3-year median follow up[18]. Of the 29 subjects who completed the survey, 96.5% continued to have GI symptoms, but reported improvement in their symptoms with medical management, which included PPIs or anti-tumor necrosis factor agents (for patients with IBD); 66% were satisfied with their current gastrointestinal health, 22 had repeat endoscopies of which only 2 patients had normal biopsy, 9 had persistent LE and the rest had other forms of esophagitis or CD[18]. Given that 9 out of 22 patients had persistent LE on repeat biopsy, one would hypothesize that LE might be a form of chronic esophagitis independent of other diseases. As a matter of fact, Mandaliya et al[24] described the case of a patient who presented with a 3-year history of dysphagia leading to the diagnosis of LE. Five endoscopies performed four years later, showed persistent LE endoscopically and histologically, requiring serial dilations[24], which supports the possible chronic nature of this entity. It is noteworthy that there are two cases of esophageal perforation in the setting of LE published in the literature[21,24]. In one case, perforation occurred following endoscopic removal of acutely impacted meat. Repeat endoscopy, after the acute episode resolved, showed tight rings and a stricture of the esophagus with pathology consistent with LE[24]. The other case was of a previously healthy 35 year-old female who presented with fever, chest pain and shock, with CT chest showing diffuse thickening of the esophagus and bilateral pleural effusions, exudative in nature with > 60% neutrophils. She was hence resuscitated and started on antibiotics for microperforation. A week later, an upper endoscopy was performed and biopsies from the mid and distal esophagus showed LE, thought to be the cause behind her initial presentation[21].
Overall, LE appears to be a benign entity except for two cases of esophageal perforation. Furthermore, it seems to have a chronic course[20,24], either because appropriate treatment is still not found or because more research is needed to further characterize its mysterious nature. According to the literature published to date, it remains unclear whether LE is associated with any of the clinical entities discussed. Although multiple studies are exploring this entity and trying to attribute it to a disease or investigating its clinical associations, LE might end up being a diagnosis of exclusion. It might also end up being a phenotypic expression of different pathologic processes rather than an actual disease. Prospective studies are needed to depict appropriate treatments of this condition and its possible subsets: CD4+ vs CD8+ T- cell- predominant LE, as well as to follow the clinical course of patients with LE to be able to better characterize the behavior of this new entity with time.
As Haque et al[7] perfectly described it, LE remains an entity "in search of a disease". Increasing in prevalence since it was defined in 2006, it seems to occur more commonly in older females and is associated with smoking. Multiple groups attempted to better characterize this entity and study potential associations with clinical diseases such as IBD, motility disorders, GERD, mucosal injuries and hypersensitivity reactions with inconclusive results and sometimes, conflicting conclusions. PPIs, topical steroids and esophageal dilations are used as effective treatment modalities with good short-term results but unclear long-term outcomes. Prospective studies are needed to define the disease, delineate the disease course, treatment options and long-term outcomes.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Blonski W, Smith RC S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
| 1. | Rubio CA, Sjödahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ismail-Beigi F, Horton PF, Pope CE. Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 444] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Butt AM, Murch SH, Ng CL, Kitching P, Montgomery SM, Phillips AD, Walker-Smith JA, Thomson MA. Upregulated eotaxin expression and T cell infiltration in the basal and papillary epithelium in cows’ milk associated reflux oesophagitis. Arch Dis Child. 2002;87:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Cucchiara S, D’Armiento F, Alfieri E, Insabato L, Minella R, De Magistris TM, Scoppa A. Intraepithelial cells with irregular nuclear contours as a marker of esophagitis in children with gastroesophageal reflux disease. Dig Dis Sci. 1995;40:2305-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wang HH, Mangano MM, Antonioli DA. Evaluation of T-lymphocytes in esophageal mucosal biopsies. Mod Pathol. 1994;7:55-58. [PubMed] |
| 6. | Rubio CA, Hubbard G. Hyperplastic foveolar gastropathy and hyperplastic foveolar gastritis in baboons. In Vivo. 1996;10:507-510. [PubMed] |
| 7. | Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Purdy JK, Appelman HD, Golembeski CP, McKenna BJ. Lymphocytic esophagitis: a chronic or recurring pattern of esophagitis resembling allergic contact dermatitis. Am J Clin Pathol. 2008;130:508-513. [PubMed] [DOI] [Full Text] |
| 9. | Carmack SW, Lash RH, Gulizia JM, Genta RM. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Adv Anat Pathol. 2009;16:290-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (2)] |
| 10. | Resnick MB, Finkelstein Y, Weissler A, Levy J, Yakirevich E. Assessment and diagnostic utility of the cytotoxic T-lymphocyte phenotype using the specific markers granzyme-B and TIA-1 in esophageal mucosal biopsies. Hum Pathol. 1999;30:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 11. | Podolsky DK, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC. Yamada’s Textbook of Gastroenterology, 2 Volume Set. 6th ed. Wiley. 2015;49. |
| 12. | Xue Y, Suriawinata A, Liu X, Li Z, Gabbard S, Rothstein R, Lacy B, Lisovsky M. Lymphocytic Esophagitis With CD4 T-cell-predominant Intraepithelial Lymphocytes and Primary Esophageal Motility Abnormalities: A Potential Novel Clinicopathologic Entity. Am J Surg Pathol. 2015;39:1558-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Olson N, Putra J, Liu X, Suriawinata AA, Lisovsky M. Lymphocytic Esophagitis with CD8 T-cell Predominance may be Associated with Gastroesophageal Reflux Disease. Poster, United States and Canadian Academy of Pathology (USCAP) Annual Meeting, March 12-18, 2016, Seattle, Wa, United States. . |
| 14. | Pasricha S, Gupta A, Reed CC, Speck O, Woosley JT, Dellon ES. Lymphocytic Esophagitis: An Emerging Clinicopathologic Disease Associated with Dysphagia. Dig Dis Sci. 2016;61:2935-2941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn’s disease. Inflamm Bowel Dis. 2011;17:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 16. | Sutton LM, Heintz DD, Patel AS, Weinberg AG. Lymphocytic esophagitis in children. Inflamm Bowel Dis. 2014;20:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 17. | Kissiedu J, Thota PN, Gohel T, Lopez R, Gordon IO. Post-ablation lymphocytic esophagitis in Barrett esophagus with high grade dysplasia or intramucosal carcinoma. Mod Pathol. 2016;29:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Cohen S, Saxena A, Waljee AK, Piraka C, Purdy J, Appelman H, McKenna B, Elmunzer BJ, Singal AG. Lymphocytic esophagitis: a diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46:828-832. [PubMed] [DOI] [Full Text] |
| 19. | Maejima R, Uno K, Iijima K, Fujishima F, Noguchi T, Ara N, Asano N, Koike T, Imatani A, Shimosegawa T. A Japanese case of lymphocytic esophagitis. Dig Endosc. 2016;28:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Figueiredo PC, Pinto-Marques P, Borralho P, Freitas J. Unusual cause for smoldering dysphagia. Lymphocytic esophagitis. Dysphagia. 2014;29:283-285. [PubMed] [DOI] [Full Text] |
| 21. | Hendy PJ, Wong DS, Florin TH. Spontaneous oesophageal perforation: an unreported complication of lymphocytic oesophagitis. Gut. 2013;62:1668-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Putra J, Muller KE, Hussain ZH, Parker S, Gabbard S, Brickley EB, Lacy BE, Rothstein R, Lisovsky M. Lymphocytic Esophagitis in Nonachalasia Primary Esophageal Motility Disorders: Improved Criteria, Prevalence, Strength of Association, and Natural History. Am J Surg Pathol. 2016;40:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Zhang Z, Jain D, Brand M. Ringed Esophagus Secondary to Lymphocytic Esophagitis. Gastroenterol Hepatol (N Y). 2016;12:237-239. [PubMed] |
| 24. | Mandaliya R, Dimarino AJ, Cohen S. Lymphocytic esophagitis mimicking eosinophilic esophagitis. Ann Gastroenterol. 2012;25:355-357. [PubMed] |
| 25. | Basseri B, Vasiliauskas EA, Chan O, Wang HL, Basseri RJ, Pimentel M, Soffer E, Conklin JL. Evaluation of peripapillary lymphocytosis and lymphocytic esophagitis in adult inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:505-511. [PubMed] |
| 26. | Vangimalla S, Gordon I, Thota PN. Image of the Month: Lymphocytic Esophagitis in Common Variable Immune Deficiency. Am J Gastroenterol. 2016;111:170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Feagans J, Victor D, Joshi V. Crohn disease of the esophagus: a review of the literature. South Med J. 2008;101:927-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 28. | Goldstein NS, Amin M. Upper Gastrointestinal Tract in Inflammatory Bowel Disease. Surg Pathol Clin. 2010;3:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Masclee GM, Coloma PM, Kuipers EJ, Sturkenboom MC. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol. 2015;110:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Kasirye Y, John A, Rall C, Resnick J. Lymphocytic esophagitis presenting as chronic dysphagia. Clin Med Res. 2012;10:83-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Niewiarowski TJ, Stoll LM. Recurrent dysphagia in a patient with chronic lymphocytic esophagitis. Gastrointest Endosc. 2016;84:1071-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |